Investigation on Segregation Granulation by Fuel and Flux in Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experimental Design
2.3. Experimental Methods
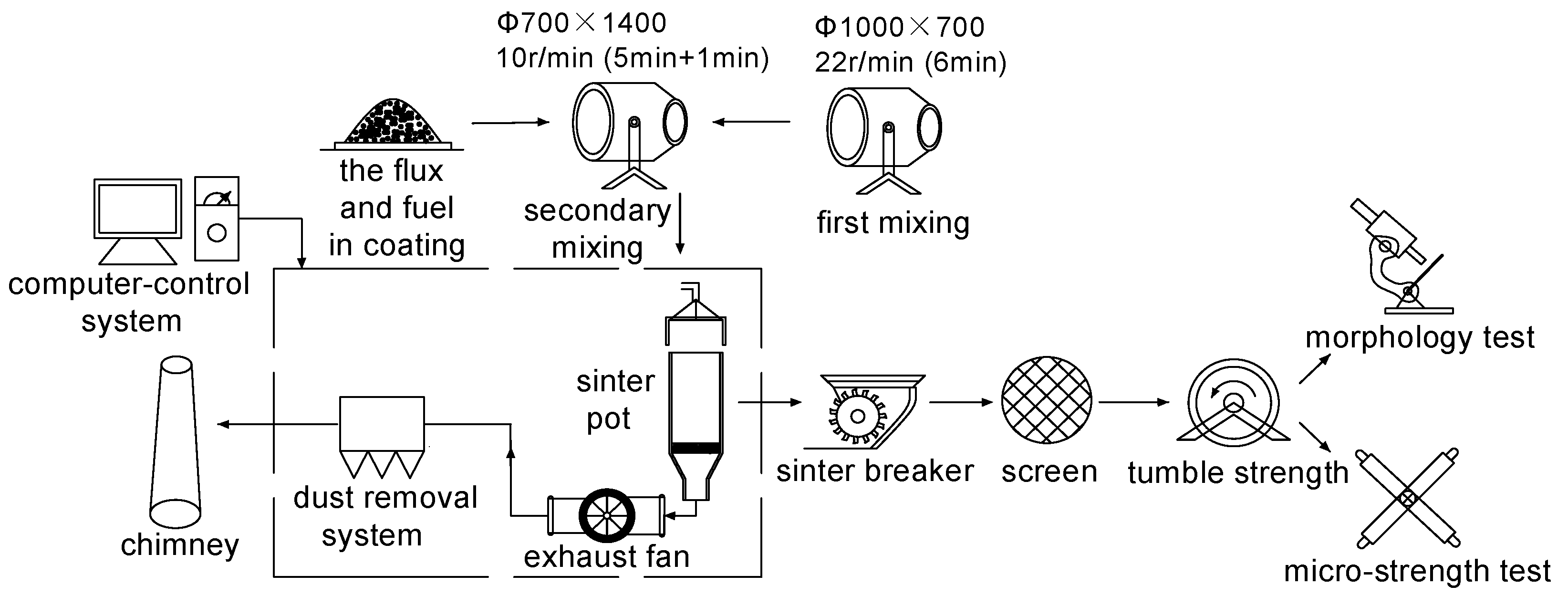
2.3.1. Sinter Pot Experiments
- (1)
- Sintering process
- (2)
- Sintering indices test
- (a)
- Vertical sinter speed ()
- (b)
- Rate of qualified product ()
- (c)
- Tumble strength index (TI)
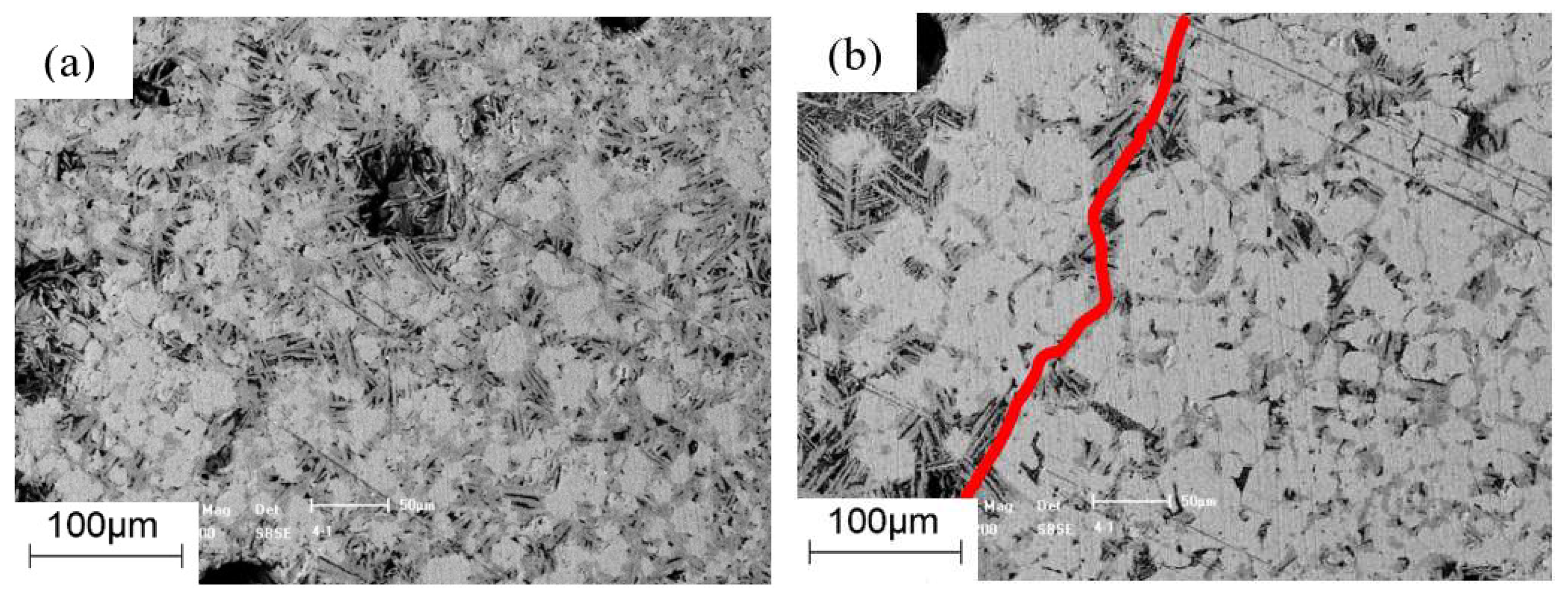
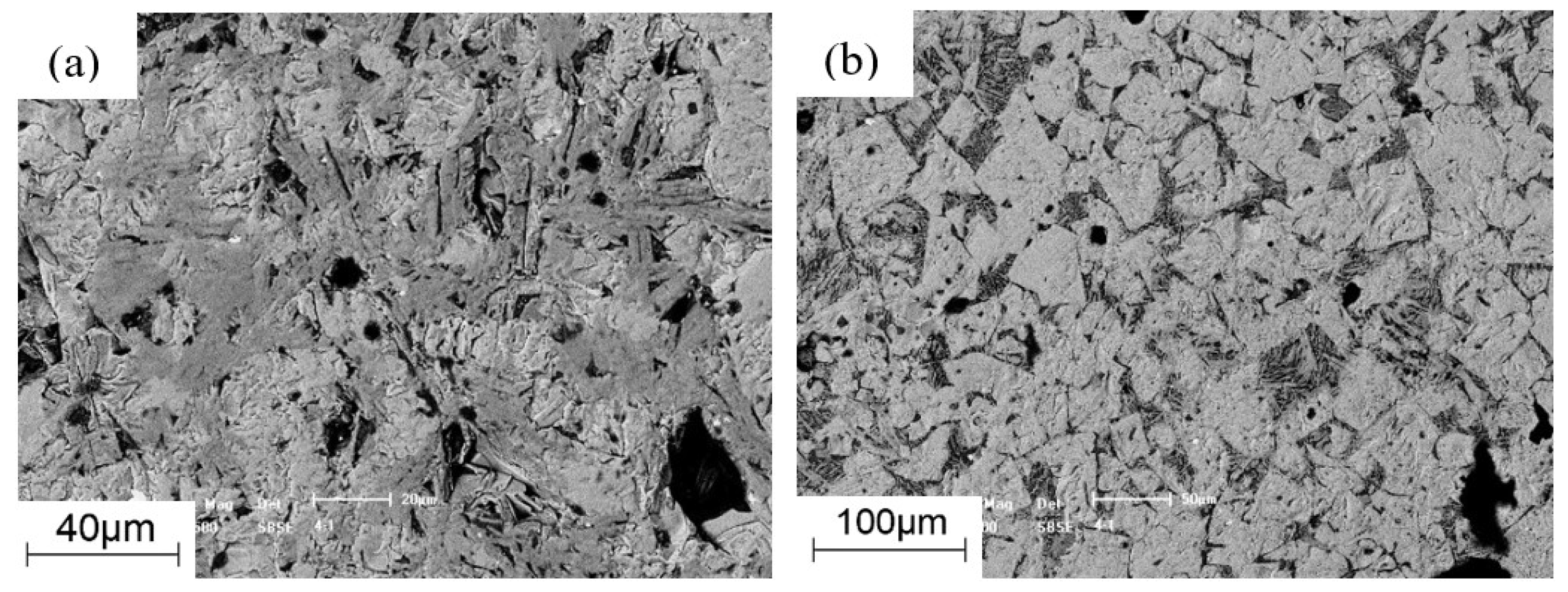
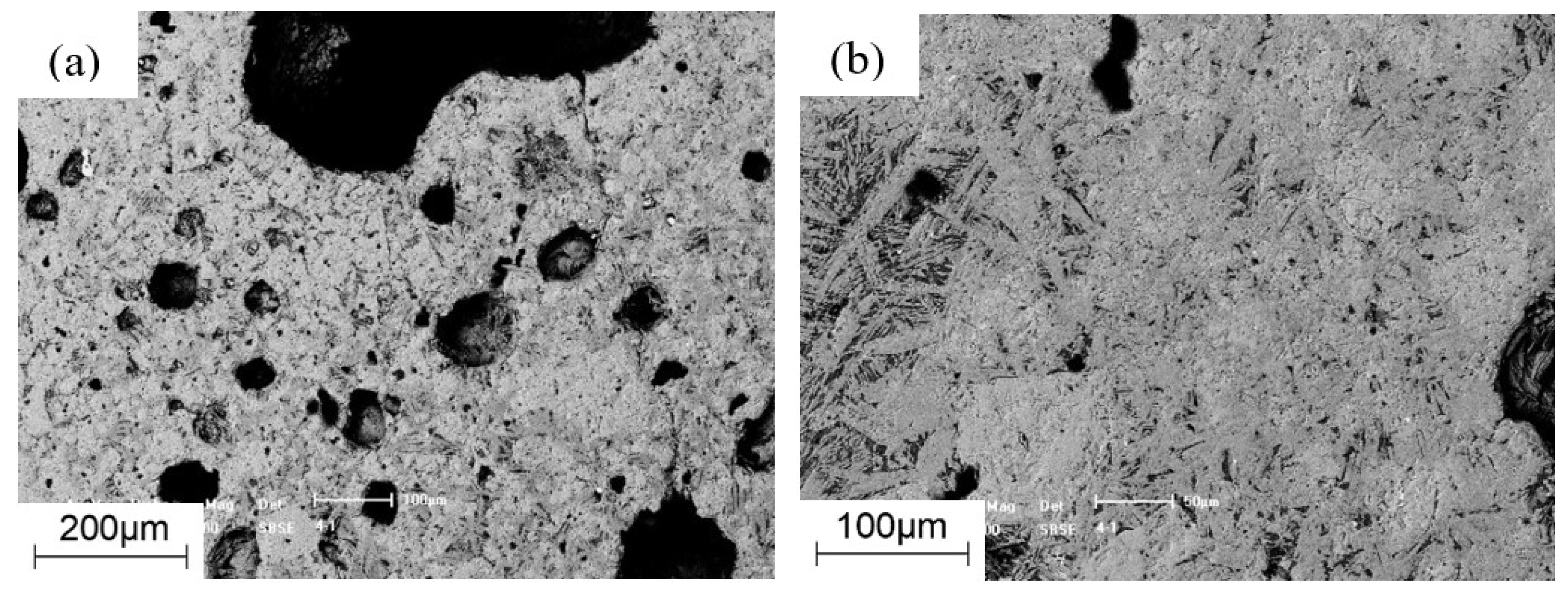
2.3.2. Micro-Strength Tests
2.3.3. Morphology Tests
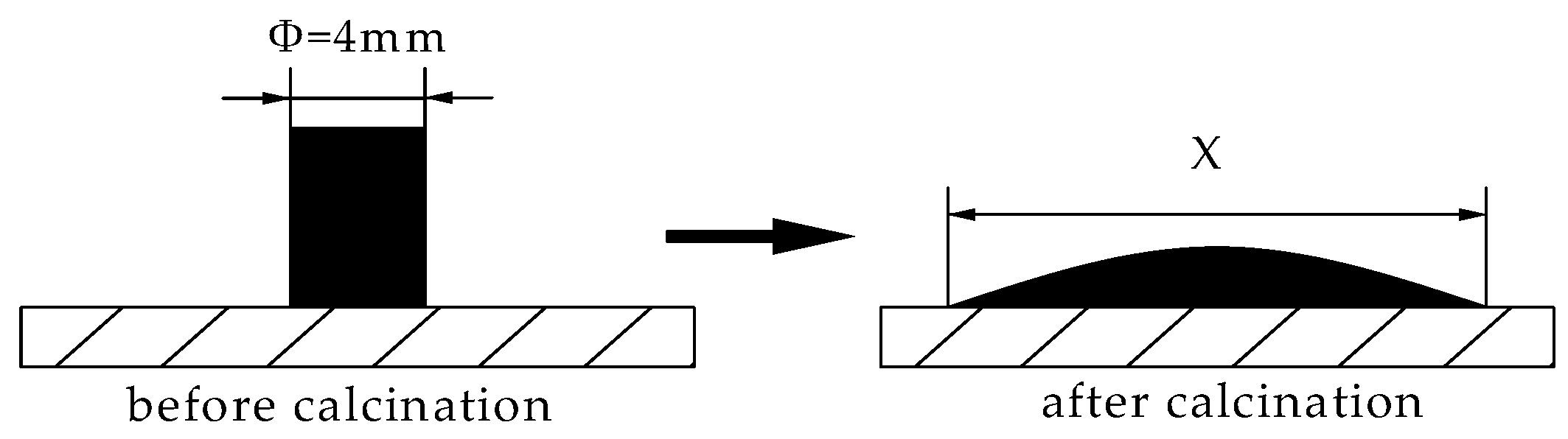
2.3.4. Fluidity Tests
- (1)
- Through calculation, the mixed ore powder with different proportions Fe2O3 and CaO was prepared. Weigh 1 g and put it into a mold with a diameter of 8 mm. The samples were prepared at a pressure of 12 MPa and kept for 3 min.
- (2)
- The preset temperature of the Equipment of melting point was set as 1280 °C. When the temperature rose to 1280 °C, the sample was put into the Equipment of melting point for heating.
- (3)
- The sample temperature also reached 1280 °C, the sample was kept at 1280 °C for 4 min, at which time, the sample would flow on the alumina sheet.
3. Experimental Results and Discussion
3.1. Sintering Indices
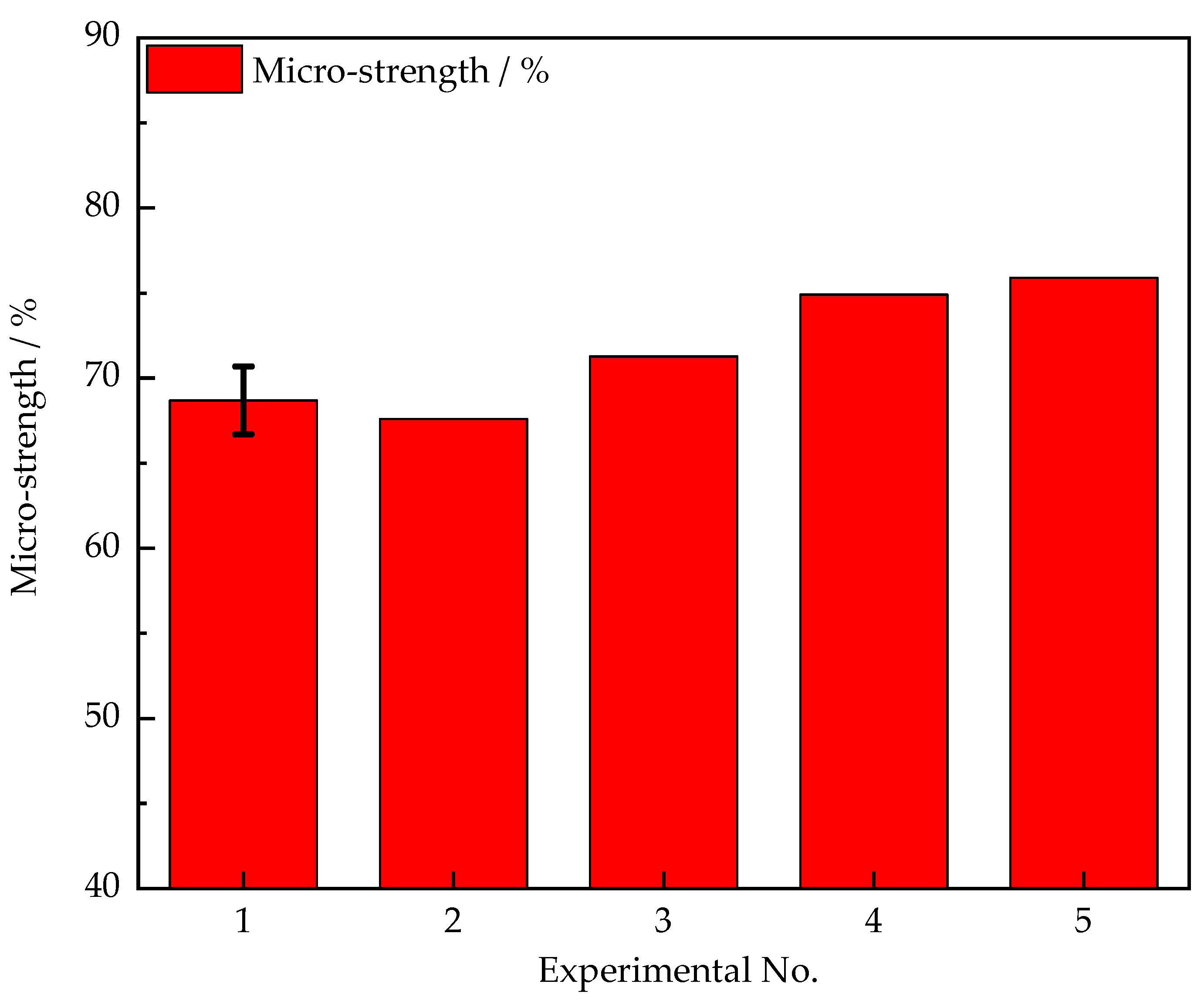
3.2. Micro-Strength
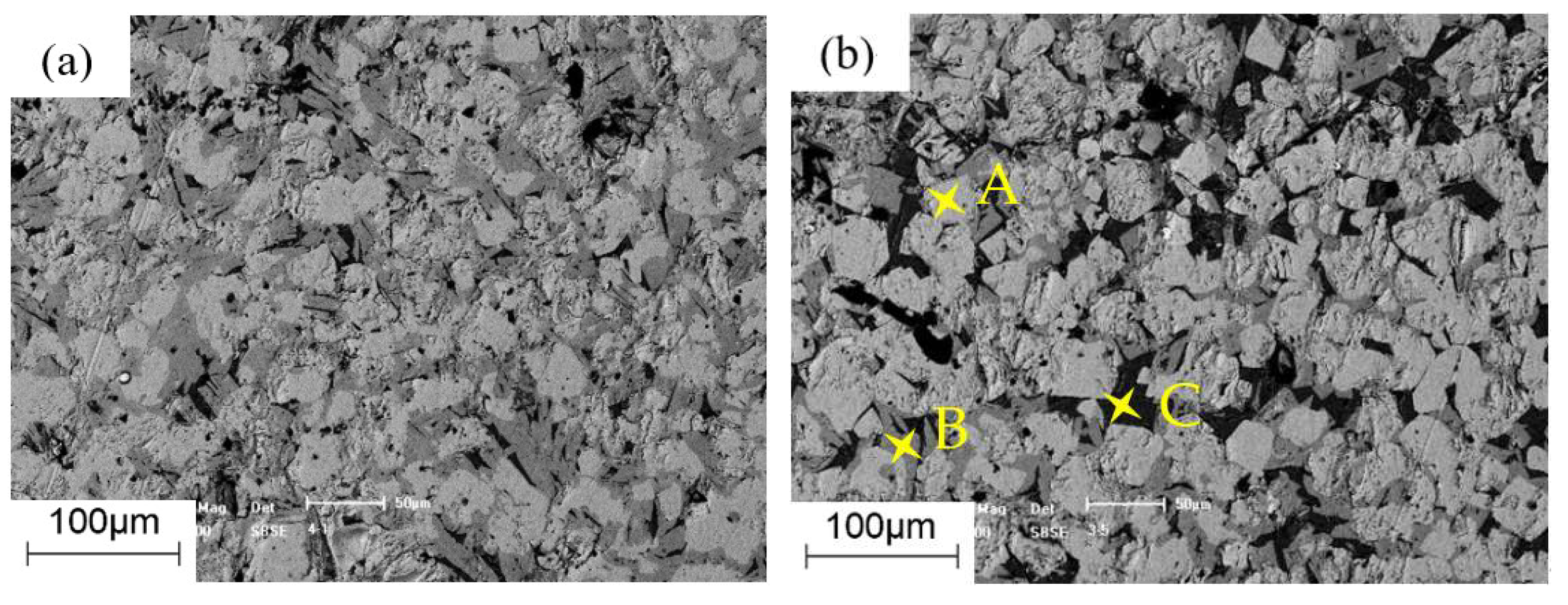
3.3. Morphology Analysis
3.4. Effect of Coating on the Fluidity of Bonding Phase
4. Conclusions
- (1)
- As the CaO increased from 0% to 40% and coke breeze increased from 0% to 100% (No. 1 to No. 5), the sintering indices were improved, the tumble strength of sinter increased 65.8% from to 68.4%, the rate of qualified products increased from 77.4% to 81.0%, and the micro-strength of sinter increased from 68.7% to 75.9%;
- (2)
- There were two reasons for the high strength of sinter by segregation granulation of fuel and flux in coating, (a) the effective absorption of heat and mineralization of flux, and (b) the improvement of fluidity of bonding phase, as the content of Fe2O3 in Fe2O3-CaO binary system decreased from 84% to 72%, the fluidity index increased from 5.8 to 7.9.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, X.Y. Application Technology Development of Limonite in Sintering of Baogang. Master’s Thesis, Inner Mongolia University of Technology, Inner Mongolia, China, 2021. [Google Scholar]
- Zhu, M.Y. Modern Metallurgical Technology—Iron and Steel Metallurgy Volume, 2nd ed.; Metallurgical Industry Press: Beijing, China, 2016; pp. 33–34. [Google Scholar]
- Liu, L.N.; Han, X.L. A Summary of Factors Affecting Sinter Quality. J. HEBUT 2006, 28, 18–21. [Google Scholar]
- Bi, X.G.; Wu, M.; Zhou, J.D.; Zhang, X.L. Development and Application of Optimized Blast Furnace Burdening. Ironmaking 2017, 36, 10–13. [Google Scholar]
- Zhang, Q.; Liu, R.; Wang, X.A.; Sun, Y.Q.; Lui, X.J. Optimization of the sintering proportioning of rich ore fines. J. CQU 2018, 41, 45–52. [Google Scholar]
- Yao, C.Q.; Zhang, J.L.; Zhang, Y.P.; Li, Z.J.; Li, X.Y. Sintering proportioning optimization based on high temperature performance of Tianjin Steel iron ore fines. Sinter. Pelletizing 2015, 40, 15–19. [Google Scholar]
- Zhang, J.L.; Hu, Z.W.; Zhou, H.B.; Lui, Z.J.; Zhao, Z.X.; Yang, T.Z. Ore blending ratio optimization for sintering based on iron ore properties and cost. Ironmak. Steelmak. 2014, 41, 279–285. [Google Scholar] [CrossRef]
- Zhang, D.S.; Gao, X.W.; Wang, Y.J.; Wang, M.S.; Ye, Y.J.; Tong, J.L. The Research of Sintering Ore Blending Based on Profit Maximization. In Proceedings of the 31st Chinese Control and Decision Conference, Nanchang, China, 3–5 June 2019. [Google Scholar]
- Lv, X.W.; Bai, C.G.; Deng, Q.Y.; Huang, X.B.; Qiu, G.B. Behavior of liquid phase formation during iron ores sintering. ISIJ Int. 2011, 51, 722–727. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, J.X.; Wang, L.; Xiang, D.W.; Gao, Q.J.; Zheng, H.Y.; Shen, F.M. Distribution of reformed coke oven gas in shaft furnace. J. Iron Steel Res. Int. 2020, 27, 1382–1390. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, J.D.; Wang, L.; An, H.W.; Gao, Q.J.; Zheng, H.Y.; Shen, F.M. Effects of liquidus temperature and liquid amount on the fluidity of bonding phase and strength of sinter. ISIJ Int. 2021, 61, 86–92. [Google Scholar] [CrossRef]
- Lv, X.F.; Han, H.L.; Wu, S.L. Research on Ore-Proportioning Optimization Technology in Sintering. Appl. Mech. Mater. 2011, 117, 980–983. [Google Scholar] [CrossRef]
- Zhang, W.H.; Liu, H.L.; Sheng, H.L.; Wei, R.R.; Chen, N.G. Optimization of Sinter Ore Blending and Coping Practice of Blast Furnace. Met. World 2022, 1, 45–49. [Google Scholar]
- Ou, D.M.; Sun, Q.; Shen, H.B.; Yan, L.J.; Shi, H.Z. Effect of Coke Size on Iron Ore Sintering. Iron. Steel. 2008, 43, 8. [Google Scholar]
- Roberts, D.G.; Harris, D.J. A Kinetic Analysis of Coal Char Gasification Reactions at High Pressures. Energ. Fuel. 2006, 20, 2314–2320. [Google Scholar] [CrossRef]
- Roberts, D.G.; Harris, D.J. Char Gasification in Mixtures of CO2 and H2O Competition and Inhibition. Fuel 2007, 86, 2672–2678. [Google Scholar] [CrossRef]
- Loo, C.E. Role of Coke Size in Sintering of a Hematite Ore Blend. Ironmak. Steelmak. 1991, 18, 33. [Google Scholar]
- Wu, S.L.; Chen, D.F.; Zhao, C.X.; Han, H.L.; Xue, F.; Zhang, L.H. Study on Improving Combustion. Iron. Steel. 2010, 45, 16–21. [Google Scholar]
- Du, X.Y.; Goplakrishnan, C.; Annamalai, K. Ignition and Combustion of Coal particle Stream. Fuel 1995, 74, 487–494. [Google Scholar] [CrossRef]
- Lockwood, F.C.; Mahmud, T.; Yehia, M.A. Simulation of Pulverized Coal Test Furnace Performance. Fuel 1998, 77, 1329–1337. [Google Scholar] [CrossRef]
- Zhai, J.N. Economic Analysis of Baosteel’s deep bed sintering Technology Innovation. Master’s Thesis, Central South University, Hunan, China, 2005. [Google Scholar]
- Wang, D.J.; Wu, S.L.; Li, C.J.; Zhu, J. Efficient and Clean Production Practice of Large-Scale Sintering Machine. ISIJ Int. 2013, 53, 1665–1672. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, M.S.; Wu, F.D.; Zhang, H.; Xu, L.B.; Zhai, L.W.; Gao, W.; Zhong, Q. Study of the Double-Layer Sintering Process with Stand-Support. Metal 2022, 12, 629. [Google Scholar] [CrossRef]
- Sato, S.; Kawaguchi, T.; Kato, M. Lower limit of the energy consumption and the double ignition process for iron ore sintering. ISIJ 1988, 28, 705–713. [Google Scholar] [CrossRef][Green Version]
- Zhou, M.S.; Wang, Y.D.; Han, S.F.; Zhao, D.M.; Zhu, J.W. Research and industrial test on ultra-deep bed double-layer pre-sintering process. Sinter. Pelletizing 2019, 44, 23–27. [Google Scholar]
- Jiang, X.; An, H.W.; Han, H.S.; Ding, X.; Li, L.S.; Shen, F.M. Reaction characteristics between sinter and serpentine. Metall. Mater. Trans. B 2020, 51, 937–944. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, H.Y.; Zheng, H.Y.; Gao, Q.J.; Shen, F.M. Three-segment control theory of MgO/Al2O3 ratio based on viscosity experiments and phase diagram analyses at 1500 °C. J. Iron Steel Res. Int. 2020, 27, 624–630. [Google Scholar] [CrossRef]
- Balat, M. Influence of coal as an energy source on environmental pollution. Energy Sources 2007, 29, 581–589. [Google Scholar] [CrossRef]
- Hida, Y.; Sasaki, M.; Enokido, T.; Umezu, Y.; Iida, T.; Uno, S. Effect of Existing State of Coke Breeze in Quasi-Particles of Raw Mix on Coke Combustion in the Sintering Process. Tetsu-to-Hagang 1982, 68, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Hida, Y.; Miyazaki, T.; Sasaki, M.; Soma, K.; Naito, H.; Kagawa, M. Study on sintering of iron ore with advanced analyzers (production of high-reducibility sinter with low fuel consumption). Overseas 1987, 35, 59–67. [Google Scholar]
- Oyama, N.; Igawa, K.; Takena, J.; Ariyama, T.; Jinno, T. Influence of Limestone and Coke Breeze Distribution in the Quasi-particle on Permeability during Sintering and Sinter Quality. Tetsu-to-Hagang 2004, 90, 546–553. [Google Scholar] [CrossRef]
- Umadevi, T.; Deodhar, A.V.; Mahapatra, P.C.; Prabhu, M.; Ranjan, M. Influence of Coating Granulation Process on Iron Ore Sinter Quality and Productivity. Steel Res. Int. 2010, 81, 717–723. [Google Scholar] [CrossRef]
- Yang, C.C.; Zhu, D.; Pan, J.; Lu, L. Granulation Effectiveness of Iron Ore Sinter Feeds: Effect of Ore Properties. ISIJ Int. 2018, 58, 1427–1436. [Google Scholar] [CrossRef]
- Han, C.; Chen, M.; Zhang, W.; Zhao, Z.; Evans, T.; Nguyen, A.T.; Zhao, B.J. Viscosity model for iron blast furnace slags in SiO2-Al2O3-CaO-MgO System. Steel Res. Int. 2015, 86, 678–685. [Google Scholar] [CrossRef]
- Iida, T.; Sakai, H.; Kita, Y. An Equation for accurate prediction of the viscosities of blast furnace type slags form chemical composition. ISIJ Int. 2000, 40, 110–114. [Google Scholar] [CrossRef] [PubMed]




| No. | Raw Material | TFe | SiO2 | CaO | MgO | Al2O3 | LOI | Proportion |
|---|---|---|---|---|---|---|---|---|
| 1 | Meishan | 56.90 | 5.63 | 3.61 | 1.30 | 1.02 | 8.20 | 12.50 |
| 2 | Newman | 61.64 | 4.43 | 0.017 | 0.06 | 2.17 | 6.16 | 6.00 |
| 3 | Yandi | 57.9 | 4.56 | 0.29 | 0.11 | 1.85 | 10.28 | 13.00 |
| 4 | Hamersley | 62.00 | 3.54 | 0.09 | 0.034 | 2.28 | 3.33 | 15.20 |
| 5 | Chile ore | 66.26 | 2.06 | 0.46 | 0.59 | 0.60 | 1.02 | 3.00 |
| 6 | CVRD | 63.70 | 3.00 | 0.05 | 0.19 | 1.61 | 2.21 | 25.00 |
| 7 | Return fines | 57.00 | 4.86 | 8.90 | 1.90 | 1.22 | 0.29 | 10.00 |
| 8 | Magnesite | 0.00 | 3.80 | 2.38 | 36.37 | 0.50 | 47.22 | 4.60 |
| 9 | Brunt lime | 0.00 | 0.73 | 94.04 | 2.37 | 1.04 | 2.20 | 2.60 |
| 10 | Limestone | 0.00 | 3.50 | 50.98 | 0.69 | 0.80 | 41.10 | 3.50 |
| 11 | Coke breeze | 0.00 | 6. 09 | 0.74 | 0.45 | 3.90 | 85.17 | 4.60 |
| No. | Raw Material | Particle Size Distribution (Mass%) | ||
|---|---|---|---|---|
| −0.2 mm | 0.2–1 mm | +1 mm | ||
| 1 | Meishan | 99.82 | 0.1 | 0.08 |
| 2 | Newman | 19.75 | 24.27 | 55.98 |
| 3 | Yandi | 7.76 | 33.57 | 58.67 |
| 4 | Hamersley | 27.17 | 25.34 | 47.49 |
| 5 | Chile ore | 19.16 | 20.29 | 60.55 |
| 6 | CVRD | 15.25 | 24.23 | 60.52 |
| 7 | Return fines | −5 mm (100%) | ||
| 8 | Magnesite | −3 mm (100%) | ||
| 9 | Brunt lime | −0.074 mm (100%) | ||
| 10 | Limestone | −3 mm (100%) | ||
| 11 | Coke breeze | −3 mm (100%) | ||
| No. | Burnt Lime | Limestone | CaO in Coating | Coke Breeze in Coating | ||
|---|---|---|---|---|---|---|
| In Quasi-Particle | In Coating | In Quasi-Particle | In Coating | |||
| 1 | 100 | 0 | 100 | 0 | 0 | 0 |
| 2 | 100 | 0 | 75 | 25 | 10 | 25 |
| 3 | 100 | 0 | 50 | 50 | 20 | 50 |
| 4 | 100 | 0 | 25 | 75 | 30 | 75 |
| 5 | 100 | 0 | 0 | 100 | 40 | 100 |
| Parameters | Values | Parameters | Values |
|---|---|---|---|
| Diameter of sinter pot | 300 mm | Ignition temperature | 1000 °C~1100 °C |
| Height of sintering bed | 750 mm | Ignition time | 2 min |
| Basicity of sinter | 1.86 | Pressure in ignition | 7.0 kP |
| Moisture in the granulation | 6.6%~6.8% | Pressure in sintering process | 13.5 kP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Jiang, X.; Wang, Q.; Ai, M.; Yin, X.; Liu, J.; Shen, F. Investigation on Segregation Granulation by Fuel and Flux in Coating. Minerals 2023, 13, 134. https://doi.org/10.3390/min13020134
Wang L, Jiang X, Wang Q, Ai M, Yin X, Liu J, Shen F. Investigation on Segregation Granulation by Fuel and Flux in Coating. Minerals. 2023; 13(2):134. https://doi.org/10.3390/min13020134
Chicago/Turabian StyleWang, Lin, Xin Jiang, Qingyu Wang, Mingxing Ai, Xiaowei Yin, Jintao Liu, and Fengman Shen. 2023. "Investigation on Segregation Granulation by Fuel and Flux in Coating" Minerals 13, no. 2: 134. https://doi.org/10.3390/min13020134
APA StyleWang, L., Jiang, X., Wang, Q., Ai, M., Yin, X., Liu, J., & Shen, F. (2023). Investigation on Segregation Granulation by Fuel and Flux in Coating. Minerals, 13(2), 134. https://doi.org/10.3390/min13020134





