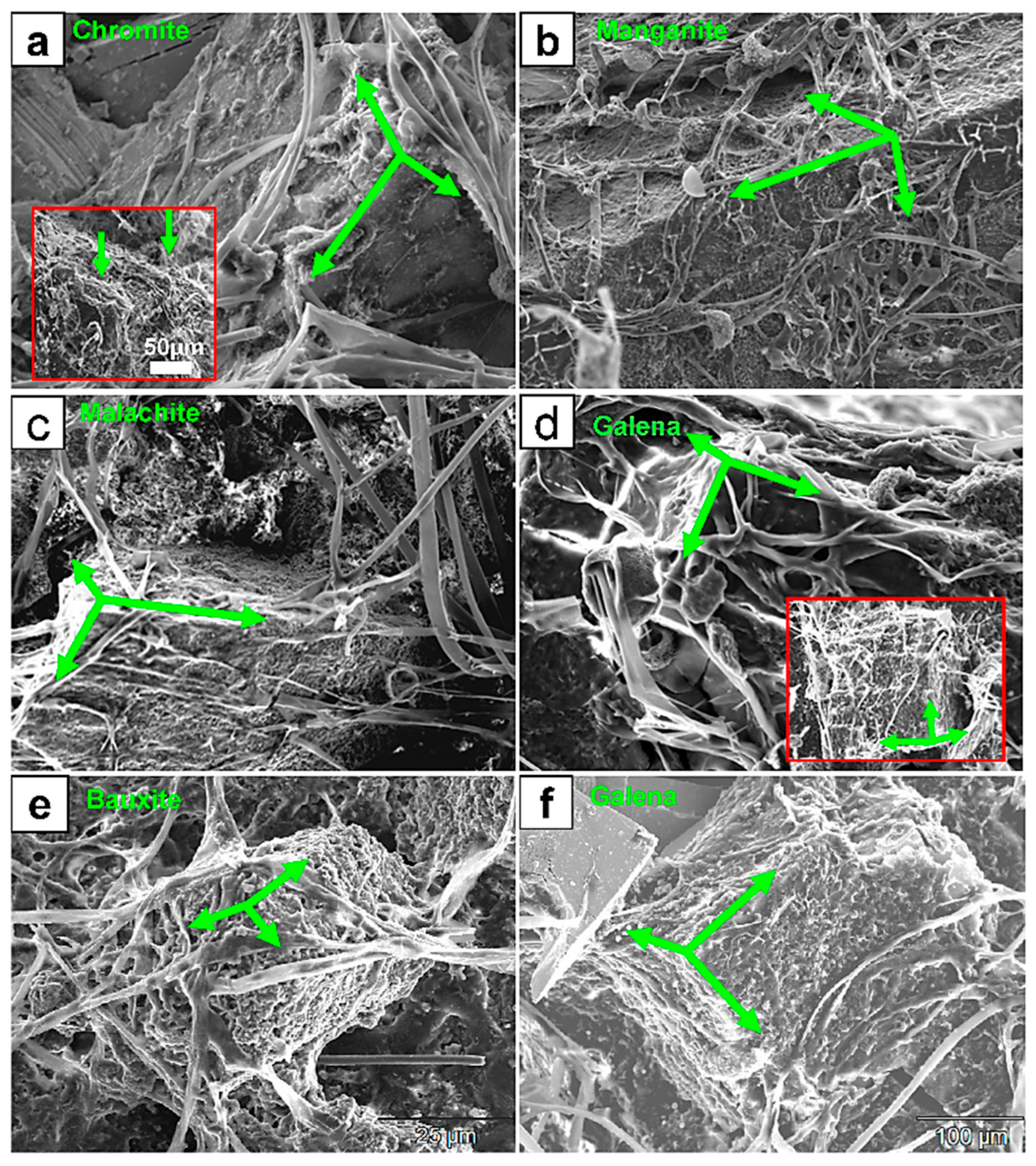
Figure 1.
SEM micrographs showing examples of 3D colonization and thigmotropic behavior of fungi on mineral surfaces of chromite (a), manganite (b), malachite (c), bauxite (e), and galena (d,f). The extensive 3D fungal colonization occurred regardless of the substrate’s mineralogy, suggesting a metallophagous behavior. The intersecting green arrows in (a–f) point to the colonized metal surfaces in 3D. The colonization is not typically a longitudinal crossover but rather tangential, passing from one intersecting surface to another (green arrows) on the same crystal. This pattern even extends from one crystal to another, producing a 3D colonization pattern that envelops all exposed surfaces. The green arrows on all figures indicate the intersection in 3D of minerals surface exposed to fungal colonization. Note how the individual fungal hyphae grow from one surface to another while continuously adhering to those surfaces. The inset in (a) shows ridge-to-ridge 3D colonization and also the fungally digested ridges (green arrows).
Figure 1.
SEM micrographs showing examples of 3D colonization and thigmotropic behavior of fungi on mineral surfaces of chromite (a), manganite (b), malachite (c), bauxite (e), and galena (d,f). The extensive 3D fungal colonization occurred regardless of the substrate’s mineralogy, suggesting a metallophagous behavior. The intersecting green arrows in (a–f) point to the colonized metal surfaces in 3D. The colonization is not typically a longitudinal crossover but rather tangential, passing from one intersecting surface to another (green arrows) on the same crystal. This pattern even extends from one crystal to another, producing a 3D colonization pattern that envelops all exposed surfaces. The green arrows on all figures indicate the intersection in 3D of minerals surface exposed to fungal colonization. Note how the individual fungal hyphae grow from one surface to another while continuously adhering to those surfaces. The inset in (a) shows ridge-to-ridge 3D colonization and also the fungally digested ridges (green arrows).
![Minerals 13 01540 g001 Minerals 13 01540 g001]()
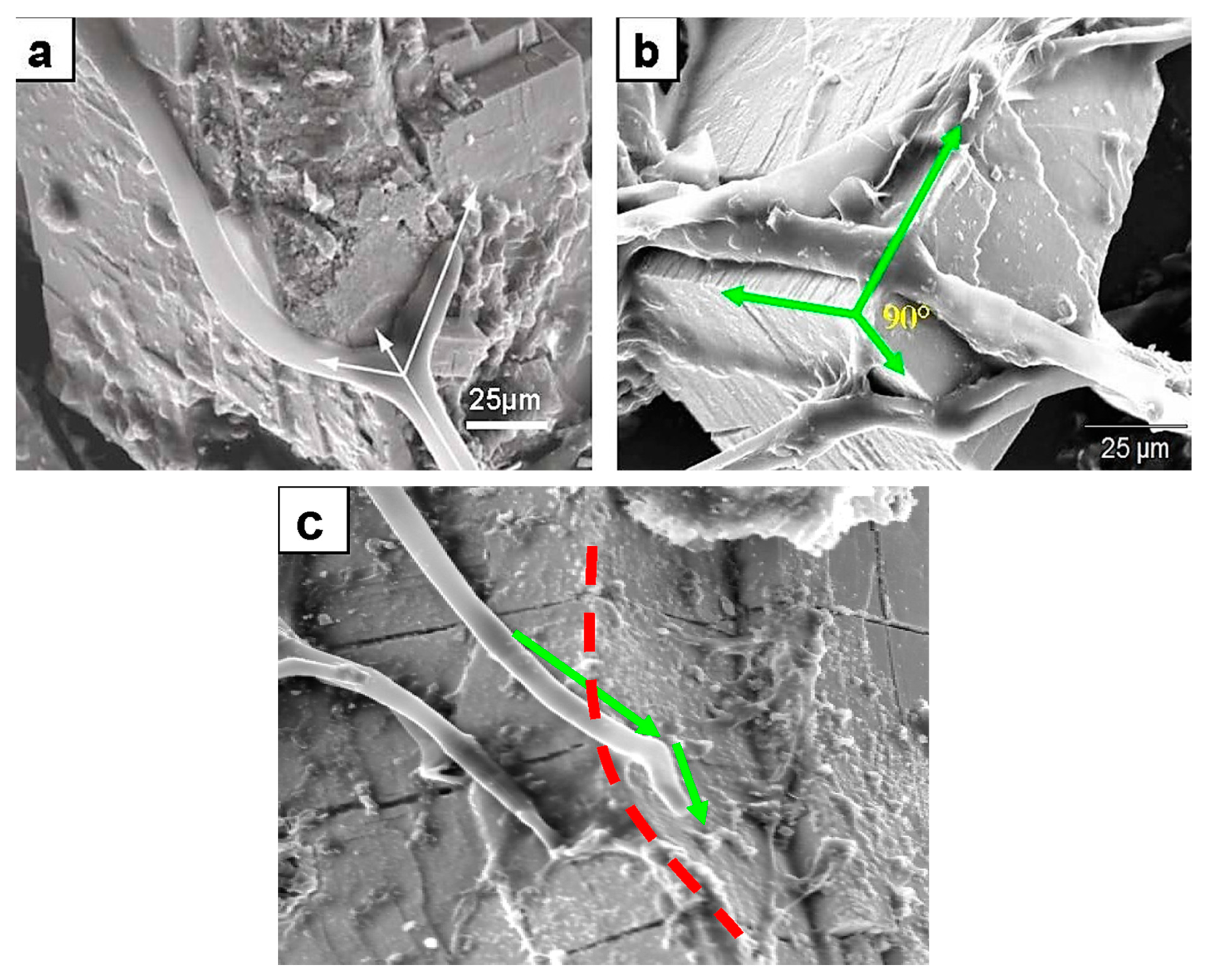
Figure 2.
SEM images showing thigmotropic behavior of fungal hyphae during mineral colonization. (a) Branching of a hypha exactly at the intersecting planes of galena crystals. The dichotomy suggests a surface topographic sensing. The arrows point in the original growth direction before the meeting of the intersecting planes (middle arrow). The two new directional hyphal growths are following crystal planes. (b) Fungal hyphae anchoring on a galena mineral surface by EPS material, and the change of the hyphal growth direction at planes intersecting at 90° (green arrows). This is a quite significant thigmotropic behavior marking a topographic sensing not only of a horizontal plane surface but also of planes intersecting at very sharp angles. (c) Change in growth direction (green arrows) at crystal surfaces of an intersection on chromite (red dotted line). The three cases presented here are strongly suggestive of not only topographic sensing but, importantly, also 3D sensing of a growth environment.
Figure 2.
SEM images showing thigmotropic behavior of fungal hyphae during mineral colonization. (a) Branching of a hypha exactly at the intersecting planes of galena crystals. The dichotomy suggests a surface topographic sensing. The arrows point in the original growth direction before the meeting of the intersecting planes (middle arrow). The two new directional hyphal growths are following crystal planes. (b) Fungal hyphae anchoring on a galena mineral surface by EPS material, and the change of the hyphal growth direction at planes intersecting at 90° (green arrows). This is a quite significant thigmotropic behavior marking a topographic sensing not only of a horizontal plane surface but also of planes intersecting at very sharp angles. (c) Change in growth direction (green arrows) at crystal surfaces of an intersection on chromite (red dotted line). The three cases presented here are strongly suggestive of not only topographic sensing but, importantly, also 3D sensing of a growth environment.
![Minerals 13 01540 g002 Minerals 13 01540 g002]()
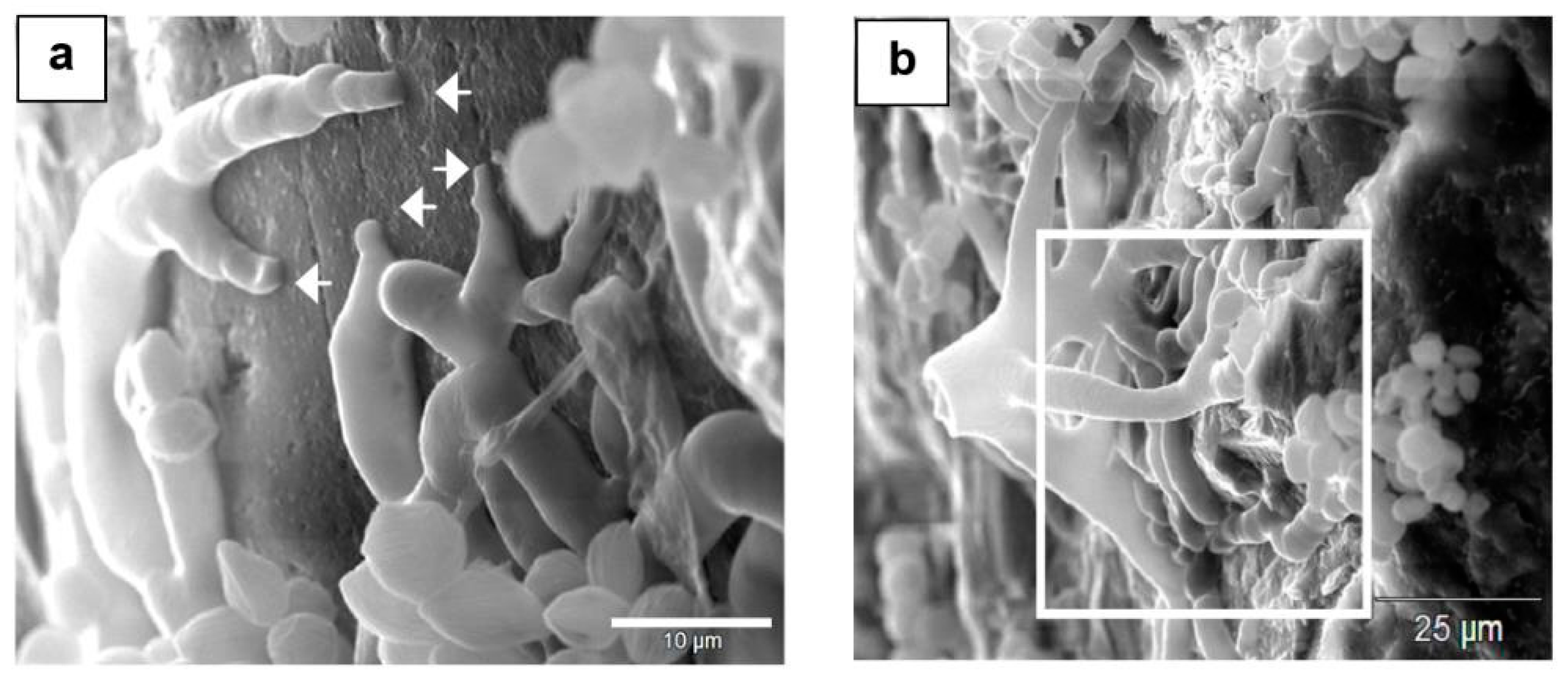
Figure 3.
SEM images of fungal colonization of a mineral surface and their thigmotropic behavior: (a) Rounded nondeflated hyphal tips in a nose-down position growing on a plagioclase surface (short arrows) showing contact sensing. (b) Rhizoidal growth on the plagioclase substrate displaying contact sensing of the surface rugosity and topography (rectangle area).
Figure 3.
SEM images of fungal colonization of a mineral surface and their thigmotropic behavior: (a) Rounded nondeflated hyphal tips in a nose-down position growing on a plagioclase surface (short arrows) showing contact sensing. (b) Rhizoidal growth on the plagioclase substrate displaying contact sensing of the surface rugosity and topography (rectangle area).
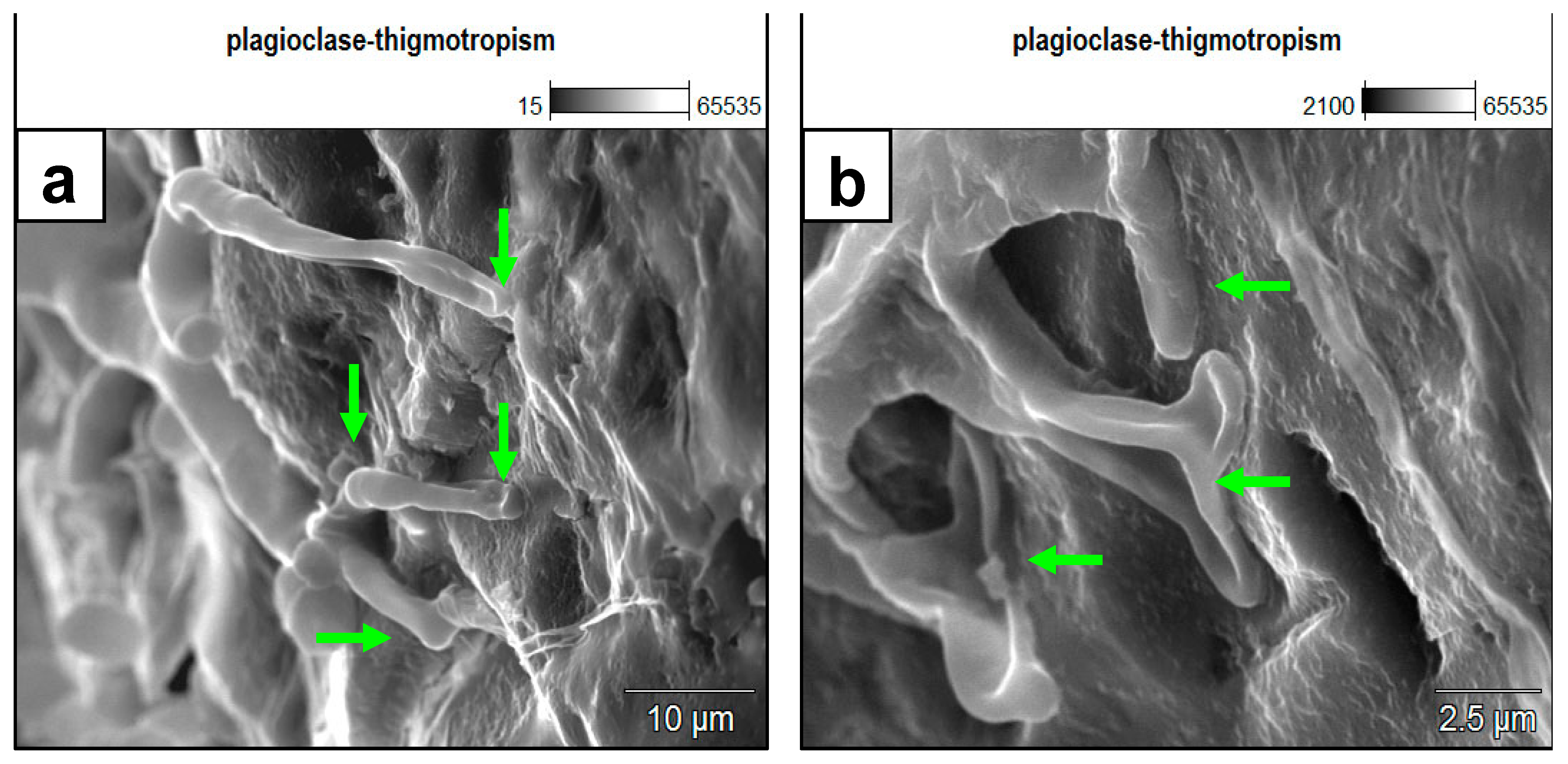
Figure 4.
SEM images of rhizoids growth on plagioclase surfaces, displaying topographic and rugosity sensing behavior. Remarkably, the growth appears not to bypass the depressions on the mineral surface but rather fills in the depression. (a) The branching of a rhizoidal foot exactly at an elevated topographic barrier and filling the adjacent depressions further indicate thigmotropic behavior. (b) A detailed microscale (~2.5 um) thigmotropic-sensing behavior of the fungal rhizoid responding to several topographic features of depressions and elevations on the plagioclase substrate surface. The arrows indicate points in contact with the substrate surface. The middle arrow is pointing to the characteristically significant dichotomy of the rhizoid foot at exactly an elevated feature separating two depressions.
Figure 4.
SEM images of rhizoids growth on plagioclase surfaces, displaying topographic and rugosity sensing behavior. Remarkably, the growth appears not to bypass the depressions on the mineral surface but rather fills in the depression. (a) The branching of a rhizoidal foot exactly at an elevated topographic barrier and filling the adjacent depressions further indicate thigmotropic behavior. (b) A detailed microscale (~2.5 um) thigmotropic-sensing behavior of the fungal rhizoid responding to several topographic features of depressions and elevations on the plagioclase substrate surface. The arrows indicate points in contact with the substrate surface. The middle arrow is pointing to the characteristically significant dichotomy of the rhizoid foot at exactly an elevated feature separating two depressions.
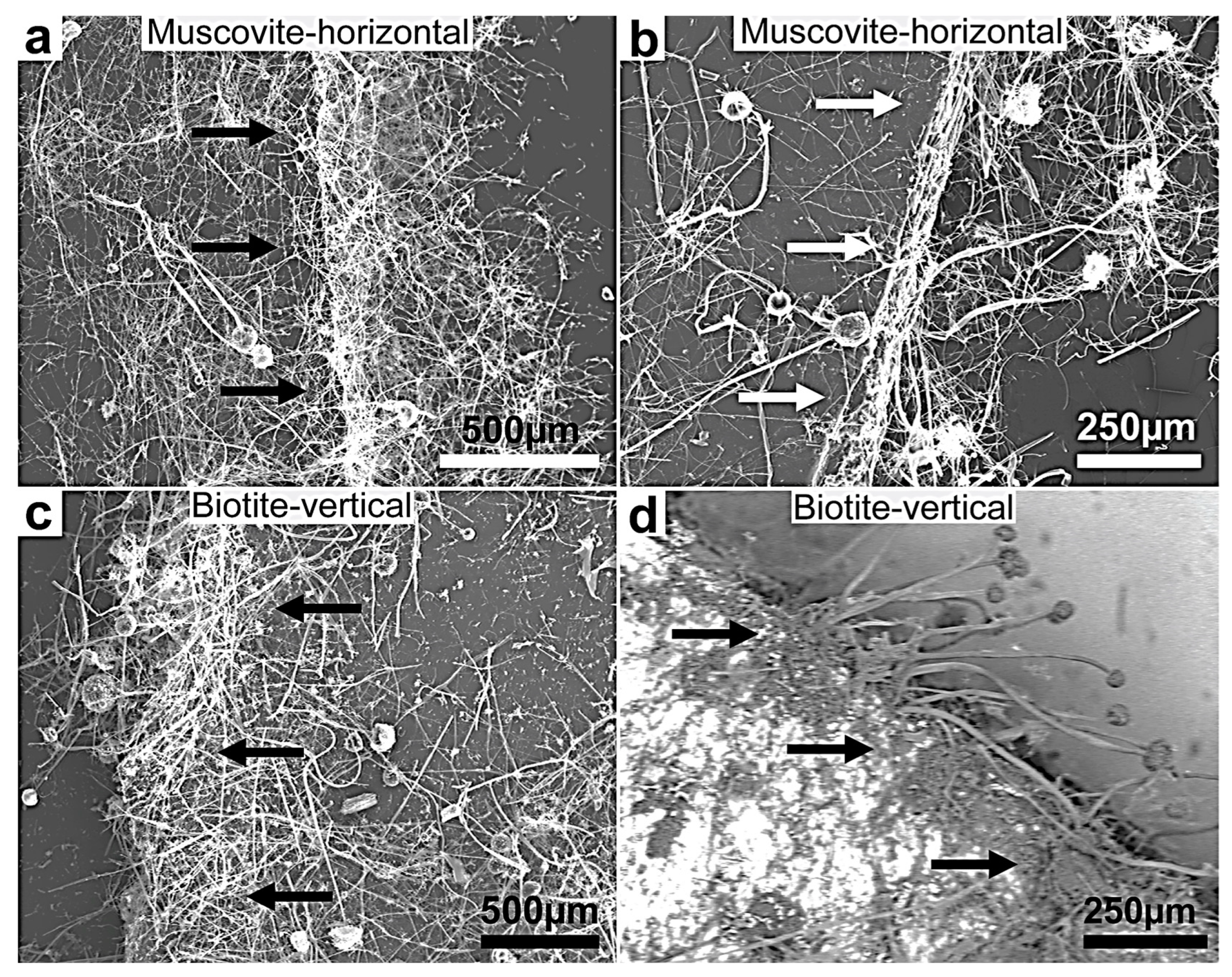
Figure 5.
(a–d) SEM images of fungal growth and attack on muscovite and biotite showing more intensive growth on the two minerals’ multilamellar faces compared to the plane surfaces (the black and white arrows point in the direction of the preferential and intense fungal colonization growth at the biotite edges). This behavior shows a selective thigmotropic preference to attack and invade the points of weakness represented by the interlamellar bundle faces over the single plane surfaces irrespective of the position of the experimental lamellae, horizontally or vertically positioned. The growing hyphae showed a marked dense colonization of the substrata margin. (d) A backscattered image showing a clear contrast between the mineral surface (bright) and the organic biomass (dark grey). This selective growth indicates exceptional thigmotropic and metalophagus behavior.
Figure 5.
(a–d) SEM images of fungal growth and attack on muscovite and biotite showing more intensive growth on the two minerals’ multilamellar faces compared to the plane surfaces (the black and white arrows point in the direction of the preferential and intense fungal colonization growth at the biotite edges). This behavior shows a selective thigmotropic preference to attack and invade the points of weakness represented by the interlamellar bundle faces over the single plane surfaces irrespective of the position of the experimental lamellae, horizontally or vertically positioned. The growing hyphae showed a marked dense colonization of the substrata margin. (d) A backscattered image showing a clear contrast between the mineral surface (bright) and the organic biomass (dark grey). This selective growth indicates exceptional thigmotropic and metalophagus behavior.
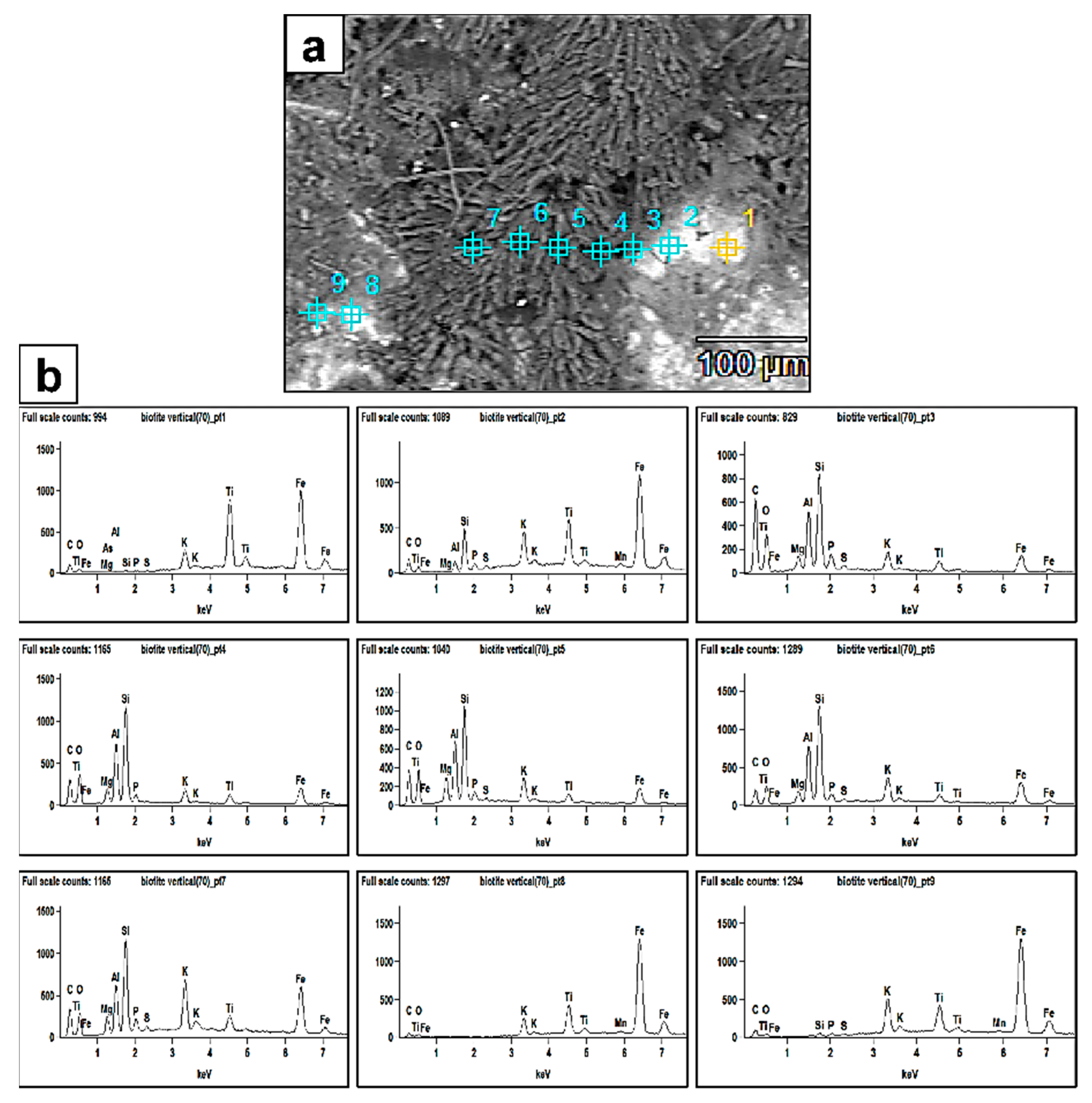
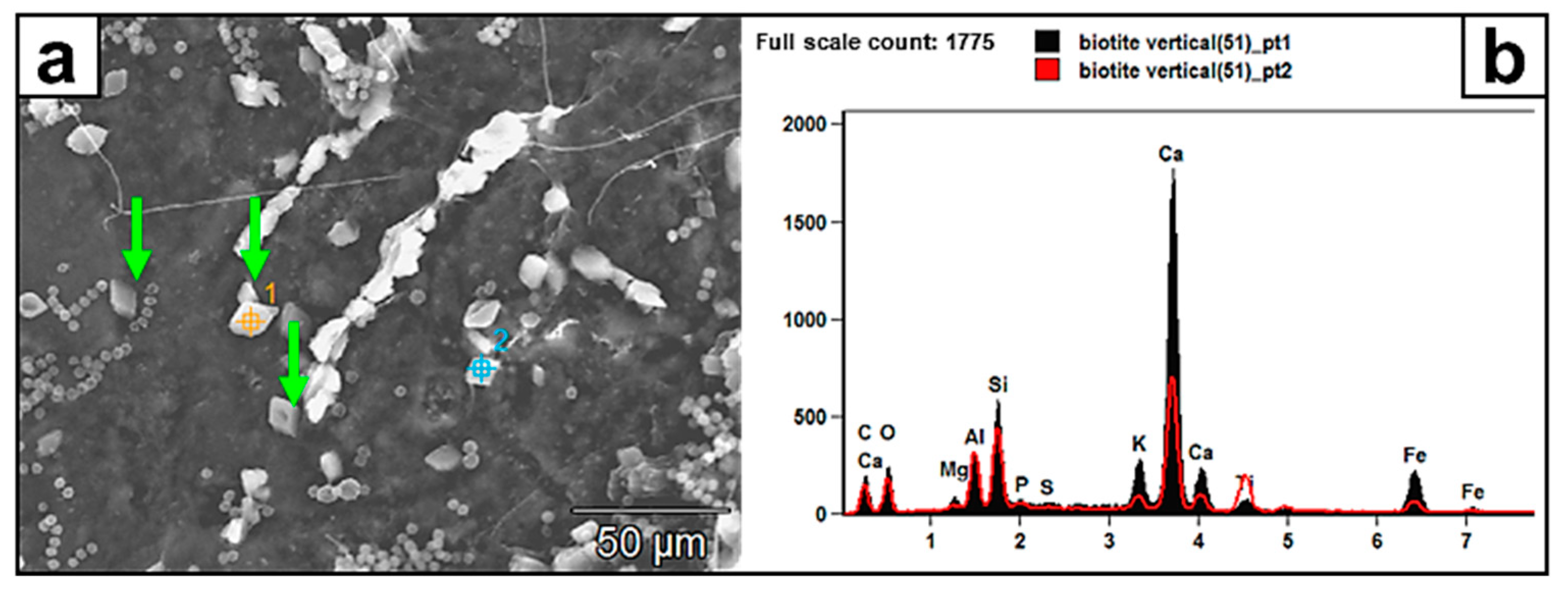
Figure 6.
(a) SEM backscattered image revealing the extent of fungal biomass (dark grey) colonizing the biotite surface and also the biomineralized crystal aggregates (prismatic–tabular) associated with the biomass. The numbers represent the EDX analyses points. The bright areas are the biotite surface. (b) EDX spectra of points 1–9. The peaks clearly show more bioleaching of the Al and Si elements from the biotite surface and enrichment towards the fungal biomass and newly formed authigenic mineral phases.
Figure 6.
(a) SEM backscattered image revealing the extent of fungal biomass (dark grey) colonizing the biotite surface and also the biomineralized crystal aggregates (prismatic–tabular) associated with the biomass. The numbers represent the EDX analyses points. The bright areas are the biotite surface. (b) EDX spectra of points 1–9. The peaks clearly show more bioleaching of the Al and Si elements from the biotite surface and enrichment towards the fungal biomass and newly formed authigenic mineral phases.
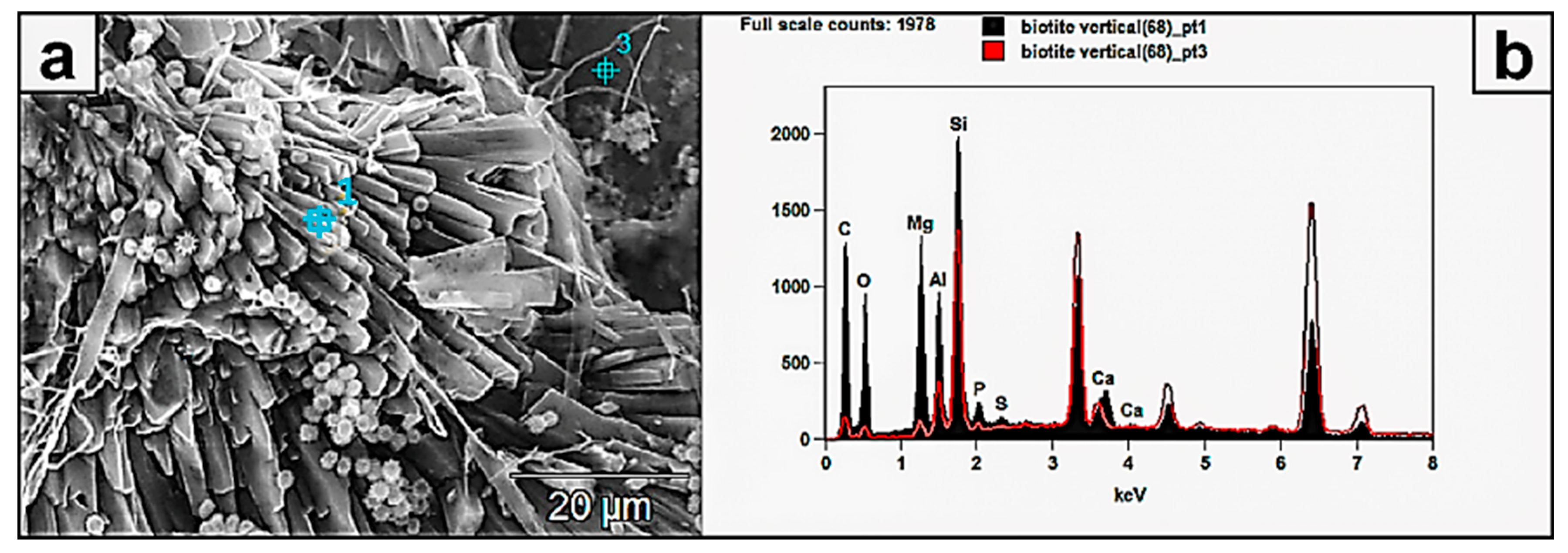
Figure 7.
(a) SEM image showing the details of biomineralized prismatic–tabular crystals formed by fungal interaction with a biotite surface. Their composition reflects the major bioleached elements Si, Al, K, Fe, and Ti. (b) The spectra show relative mobility/leachability of Si and Al, because their peaks show higher intensity in the authigenic minerals than in the leached biotite substrate.
Figure 7.
(a) SEM image showing the details of biomineralized prismatic–tabular crystals formed by fungal interaction with a biotite surface. Their composition reflects the major bioleached elements Si, Al, K, Fe, and Ti. (b) The spectra show relative mobility/leachability of Si and Al, because their peaks show higher intensity in the authigenic minerals than in the leached biotite substrate.
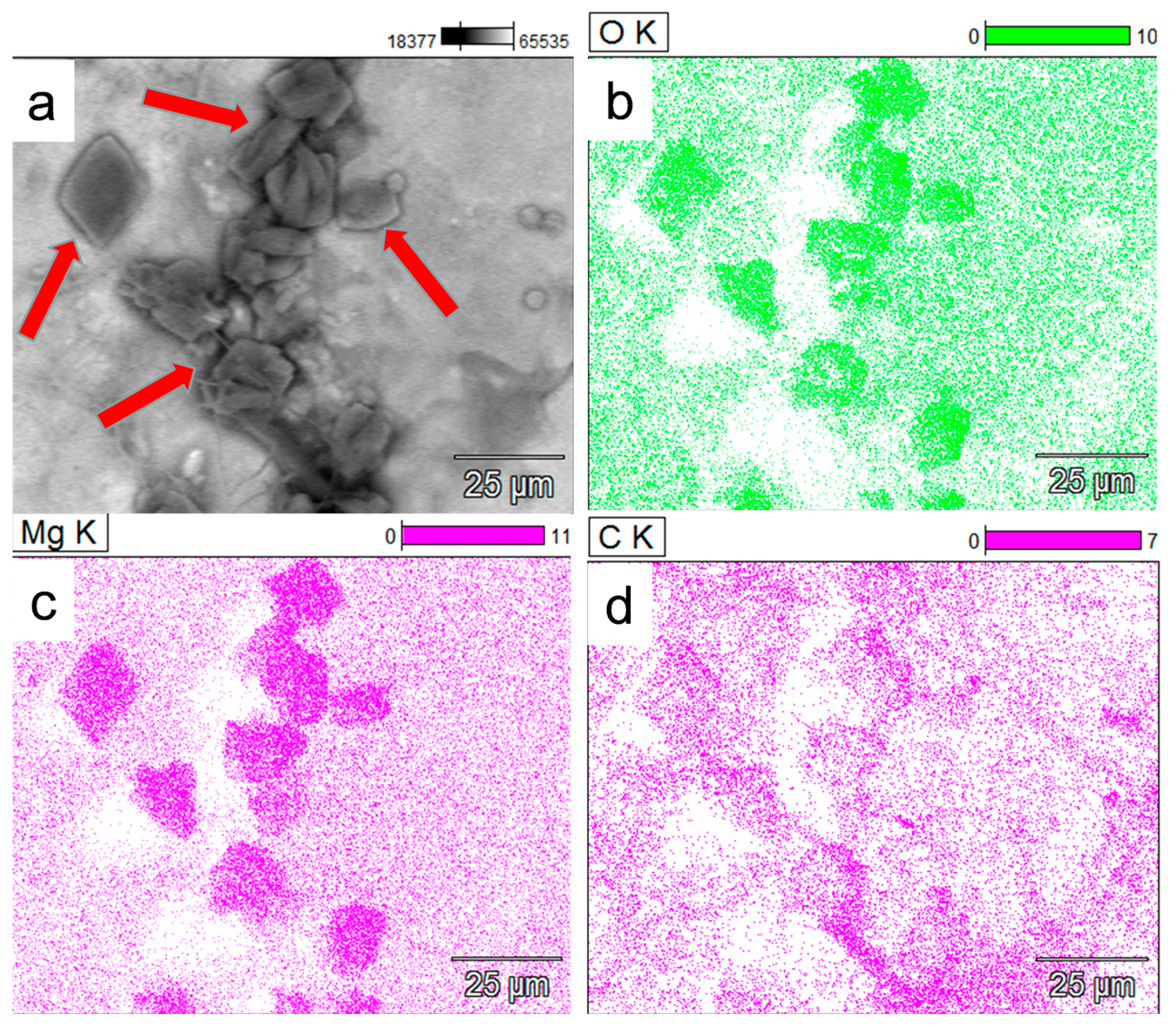
Figure 8.
(a) SEM X-ray mapping image (backscattering mode) of authigenic minerals (black-greyish crystal forms) formed in response to fungal interaction with the biotite substrate (red arrows). (b–d) The new mineral crystals (the high color intensity shapes) depicted by the analysis to be mainly composed of O, Mg, and C, indicating the formation of Mg-oxalate mineral species MgC2O4. The Mg is bioleached from the biotite mineral substrate.
Figure 8.
(a) SEM X-ray mapping image (backscattering mode) of authigenic minerals (black-greyish crystal forms) formed in response to fungal interaction with the biotite substrate (red arrows). (b–d) The new mineral crystals (the high color intensity shapes) depicted by the analysis to be mainly composed of O, Mg, and C, indicating the formation of Mg-oxalate mineral species MgC2O4. The Mg is bioleached from the biotite mineral substrate.
Figure 9.
(a) SEM image of authigenic Ca-rich rhombohedral crystals (green arrows) deposited on the biotite surface and (b) the corresponding EDX pattern. These crystals, in absence of a mineralogical analysis, are likely poly-metal oxalates. Spot points 1 and 2 represent the EDX analyses of two crystals. The biotite surface appears scarred with pits and tunnels created by fungi.
Figure 9.
(a) SEM image of authigenic Ca-rich rhombohedral crystals (green arrows) deposited on the biotite surface and (b) the corresponding EDX pattern. These crystals, in absence of a mineralogical analysis, are likely poly-metal oxalates. Spot points 1 and 2 represent the EDX analyses of two crystals. The biotite surface appears scarred with pits and tunnels created by fungi.
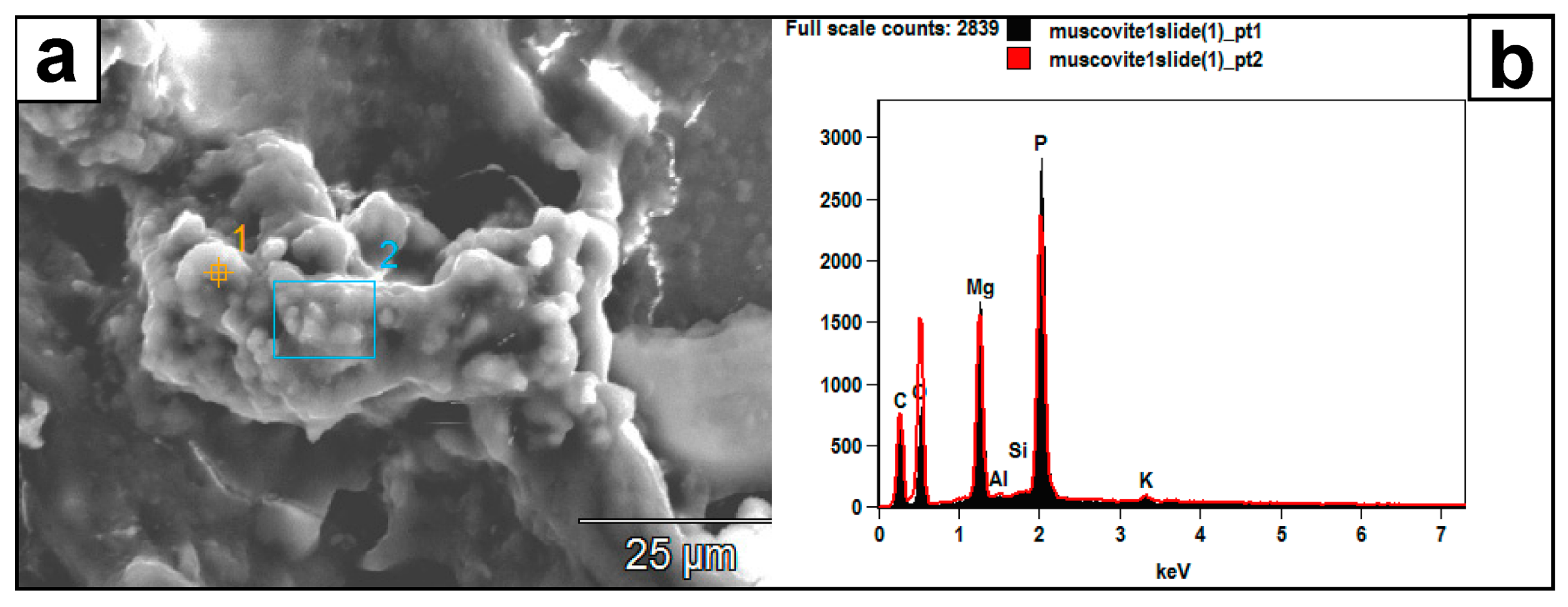
Figure 10.
(a) SEM image of authigenic crystal aggregates formed on a muscovite surface and within a mass of EPS material. (b) Their EDX spectrum (pts. 1 and 2) reveals a composition of P, Mg, C, and O, which the muscovite substrate did not likely contribute. Instead, this composition might represent struvite: (NH4) MgPO4·6H2O.
Figure 10.
(a) SEM image of authigenic crystal aggregates formed on a muscovite surface and within a mass of EPS material. (b) Their EDX spectrum (pts. 1 and 2) reveals a composition of P, Mg, C, and O, which the muscovite substrate did not likely contribute. Instead, this composition might represent struvite: (NH4) MgPO4·6H2O.
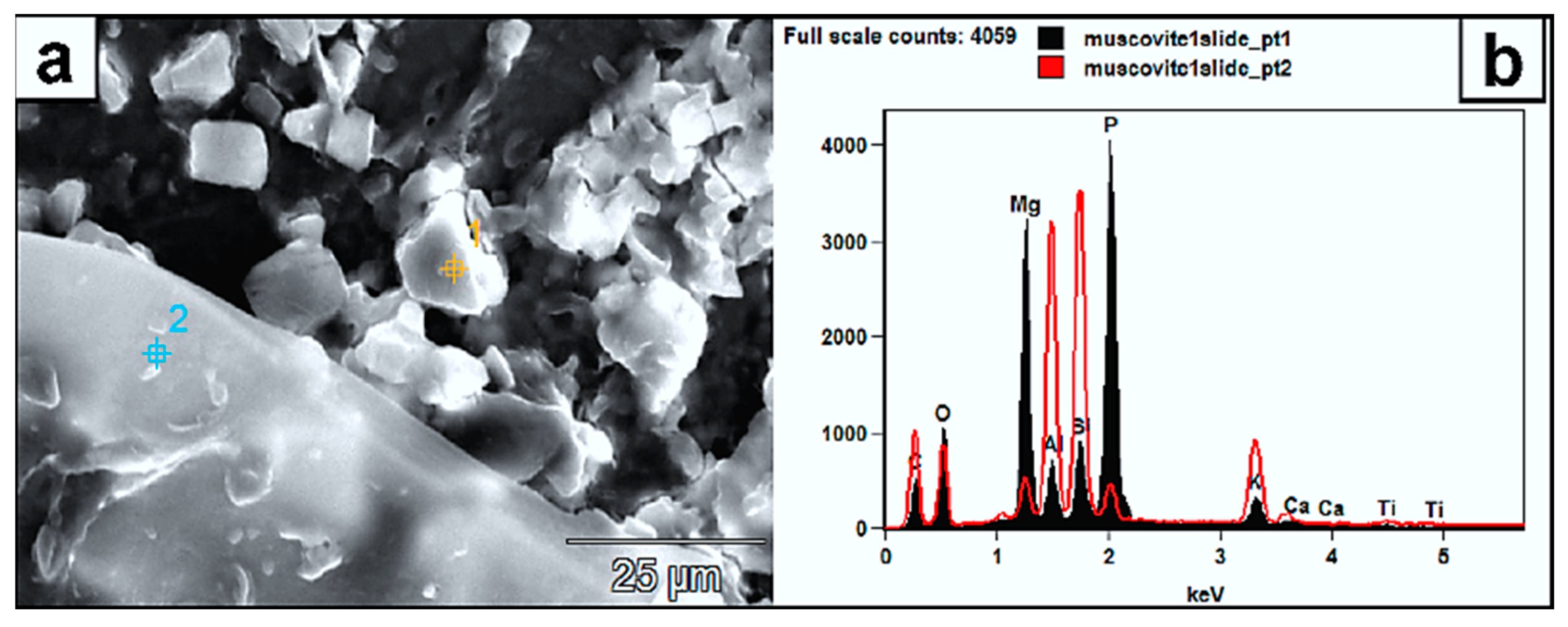
Figure 11.
(a) SEM micrograph of authigenic mineral aggregates (white rhombs) formed on muscovite. (b) Their EDX spectra “pt1” reveals a composition of P, Mg, C, and O. In this single instance, Al, Si, and K are also mobilized from the substrate into the crystals. Point 2 is the muscovite EDX (red spectrum) and clearly shows the overall difference in elemental composition. Higher peaks are recorded here from the substrate for C and O due to the possible fungal material on the spot. The high intensity peak of P from the formed mineral can be sourced from the organic media; thus, compared to the muscovite EDX signature (point 2), the P is very low, and its existence is related to organic media as well. The high Mg signature in the newly formed mineral could reflect a higher mobility from the substrate.
Figure 11.
(a) SEM micrograph of authigenic mineral aggregates (white rhombs) formed on muscovite. (b) Their EDX spectra “pt1” reveals a composition of P, Mg, C, and O. In this single instance, Al, Si, and K are also mobilized from the substrate into the crystals. Point 2 is the muscovite EDX (red spectrum) and clearly shows the overall difference in elemental composition. Higher peaks are recorded here from the substrate for C and O due to the possible fungal material on the spot. The high intensity peak of P from the formed mineral can be sourced from the organic media; thus, compared to the muscovite EDX signature (point 2), the P is very low, and its existence is related to organic media as well. The high Mg signature in the newly formed mineral could reflect a higher mobility from the substrate.
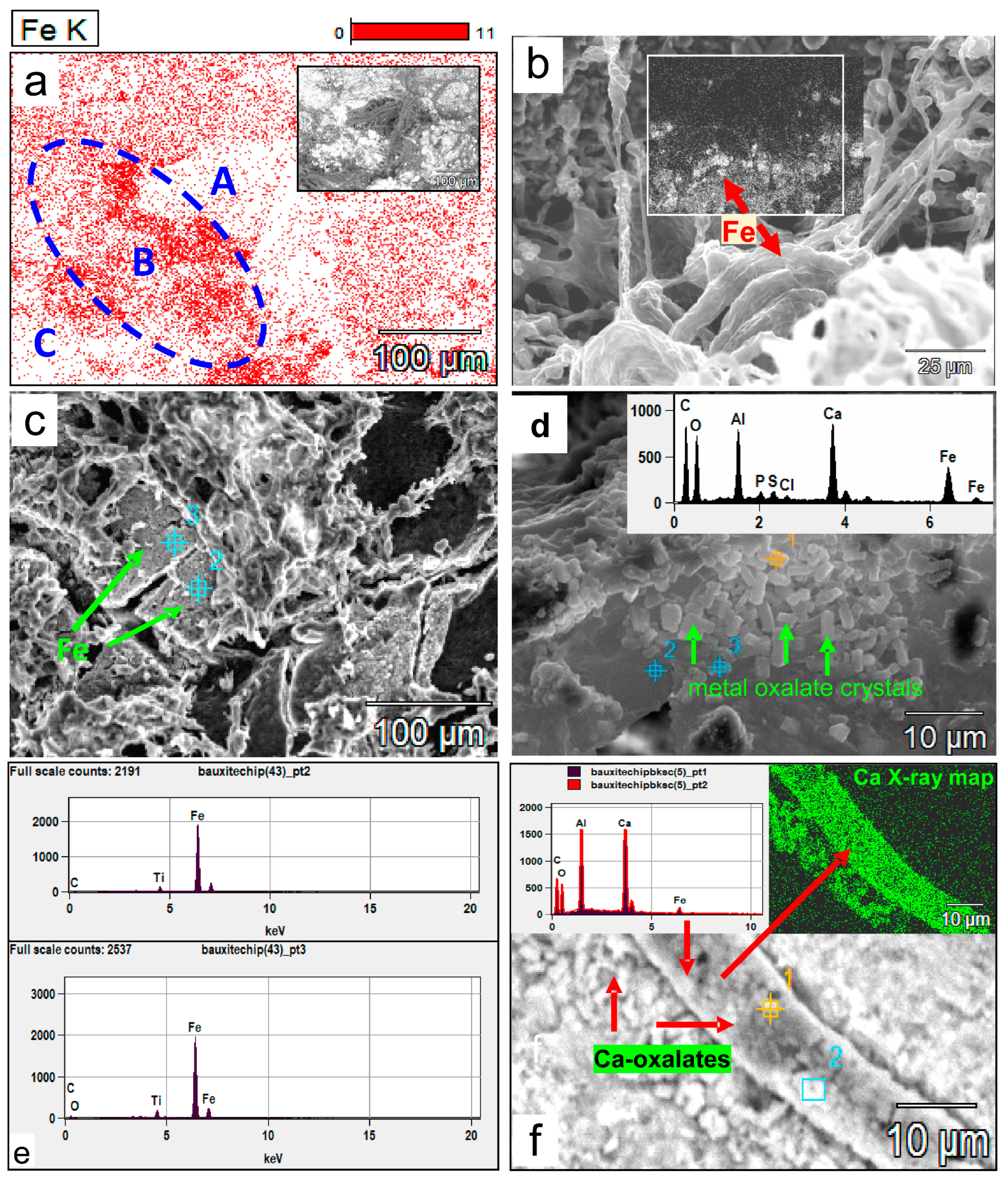
Figure 12.
(a) SEM X-Ray mapping of Fe on a bauxite surface densely colonized by fungi (zones A and C and inset figure for the corresponding areas), showing that high Fe concentrations in zone B are related to the presence of a dense fungal biomass in contrast to the lower Fe concentration associated with the rest of the map. The white areas indicate fungal biomass, as is the SEM backscattered inset figure. (b) A high concentration of Fe (inset backscattering map, the red arrow indicating the same iron enriched area) is contained within the fungal mass itself compared to the surroundings. (c) Bioleached Fe (red arrows) forms a separate layer of spongy and porous Fe mineral within the fungal biomass. The EDX spectra of points 1 and 2 (e) indicate Fe makes up to 87–93 wt% of this layer. (d) Prismatic tetragonal bipyramidal crystal aggregates (arrows) formed in or within fungal mucilaginous material that contain Al and Fe; metals presumably bioleached from the substrate bauxite and now incorporated into a Ca-Al-Fe-oxalate complex shown in the inset EDX spectrum of spot point 1. (e) EDX spectra of spot points 2 and 3 on the iron-rich layer bioleached from bauxite in (c). (f) The role of fungal hyphae in bioleaching. Biomineralized crystals encrusting a hypha yielded an EDX spectrum with strong Al and Ca peaks and low Fe. The high Ca crystals on hyphae and deposited parallel to the hyphae are likely Ca- and Al-oxalates (red arrows point to the EDX spectra of spot points 1 and 2 on the X-ray map of Ca in crystals encrusting the fungal hypha).
Figure 12.
(a) SEM X-Ray mapping of Fe on a bauxite surface densely colonized by fungi (zones A and C and inset figure for the corresponding areas), showing that high Fe concentrations in zone B are related to the presence of a dense fungal biomass in contrast to the lower Fe concentration associated with the rest of the map. The white areas indicate fungal biomass, as is the SEM backscattered inset figure. (b) A high concentration of Fe (inset backscattering map, the red arrow indicating the same iron enriched area) is contained within the fungal mass itself compared to the surroundings. (c) Bioleached Fe (red arrows) forms a separate layer of spongy and porous Fe mineral within the fungal biomass. The EDX spectra of points 1 and 2 (e) indicate Fe makes up to 87–93 wt% of this layer. (d) Prismatic tetragonal bipyramidal crystal aggregates (arrows) formed in or within fungal mucilaginous material that contain Al and Fe; metals presumably bioleached from the substrate bauxite and now incorporated into a Ca-Al-Fe-oxalate complex shown in the inset EDX spectrum of spot point 1. (e) EDX spectra of spot points 2 and 3 on the iron-rich layer bioleached from bauxite in (c). (f) The role of fungal hyphae in bioleaching. Biomineralized crystals encrusting a hypha yielded an EDX spectrum with strong Al and Ca peaks and low Fe. The high Ca crystals on hyphae and deposited parallel to the hyphae are likely Ca- and Al-oxalates (red arrows point to the EDX spectra of spot points 1 and 2 on the X-ray map of Ca in crystals encrusting the fungal hypha).
![Minerals 13 01540 g012 Minerals 13 01540 g012]()
Figure 13.
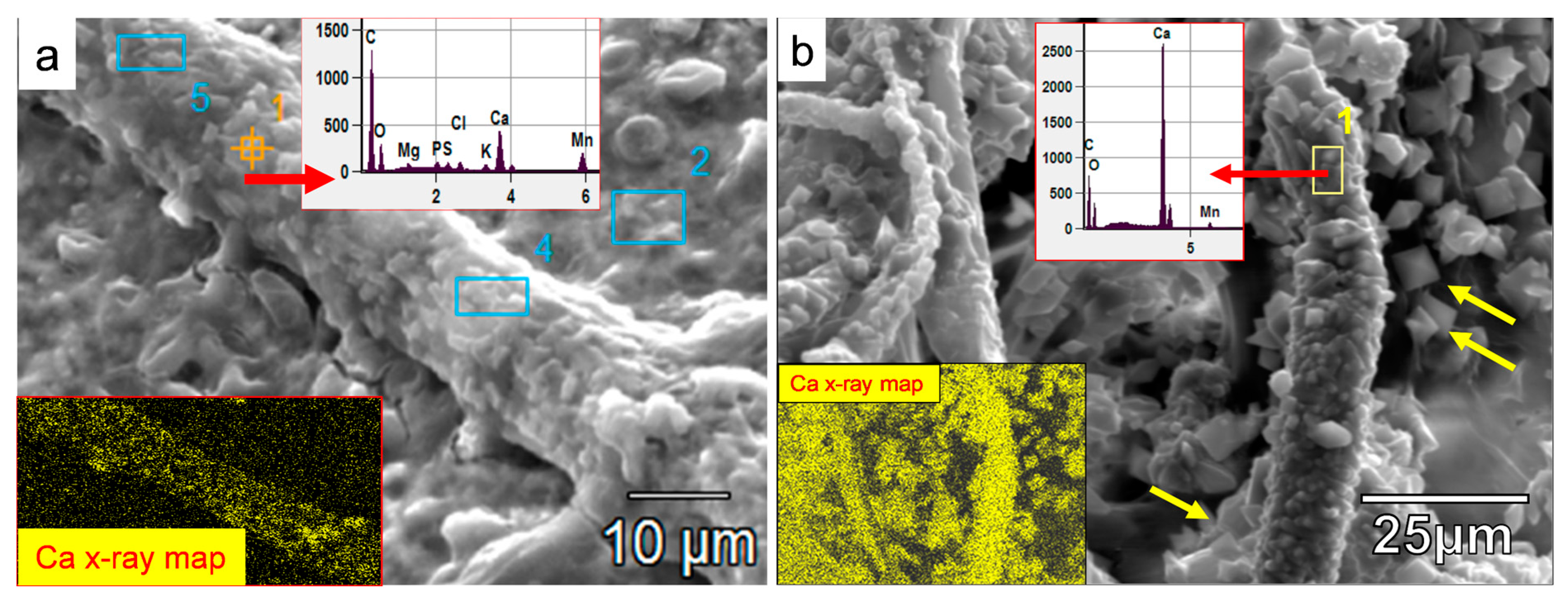
SEM images of authigenic crystals densely encrusting the fungal hyphae in (a,b) and littering the surface of the manganite substrate. (a) The substrate, as well as the hypha, are intermixed with EPS material. The hypha is densely encrusted with mineral crystals formed during the interaction with the substrate. The typical EDX signature (inset, above and arrow of analyzed spot 1) shows a mainly Ca-dominated composition with minor contributions from other elements, specifically Mn. The lower inset shows the Ca makeup of those encrusting minerals. (b) EPS material is not clearly visible. The fungal hyphae are more densely encrusted, with well-formed Ca-oxalates with a very minor contribution of fungally leached Mn (EDX spectrum inset and area analyzed in rectangle 1) from the substrate and subsequently incorporated in the Ca-rich crystals, which represent a mixed metal oxalate (CaMnC2O4·2H2O). The X-ray map of Ca shows the extent of the dense encrustation and of the fungal hyphae by the Ca-oxalates. The yellow arrows are pointing to typical prismatic bipyramidal tetragonal crystal shapes of Ca-oxalates. (Blue rectangles in (a) are other EDX analyzed areas of similar spectrum as for spot point 1).
Figure 13.
SEM images of authigenic crystals densely encrusting the fungal hyphae in (a,b) and littering the surface of the manganite substrate. (a) The substrate, as well as the hypha, are intermixed with EPS material. The hypha is densely encrusted with mineral crystals formed during the interaction with the substrate. The typical EDX signature (inset, above and arrow of analyzed spot 1) shows a mainly Ca-dominated composition with minor contributions from other elements, specifically Mn. The lower inset shows the Ca makeup of those encrusting minerals. (b) EPS material is not clearly visible. The fungal hyphae are more densely encrusted, with well-formed Ca-oxalates with a very minor contribution of fungally leached Mn (EDX spectrum inset and area analyzed in rectangle 1) from the substrate and subsequently incorporated in the Ca-rich crystals, which represent a mixed metal oxalate (CaMnC2O4·2H2O). The X-ray map of Ca shows the extent of the dense encrustation and of the fungal hyphae by the Ca-oxalates. The yellow arrows are pointing to typical prismatic bipyramidal tetragonal crystal shapes of Ca-oxalates. (Blue rectangles in (a) are other EDX analyzed areas of similar spectrum as for spot point 1).
![Minerals 13 01540 g013 Minerals 13 01540 g013]()
Figure 14.
SEM micrographs and EDX analyses. (a) Area 1 and (b) spot 1 of fine-grained crystals (2–3 μm) formed on fungal hyphae and displaying high Cu% that was leached from the malachite substrate and adsorbed onto the hyphal walls and encrusting crystals. (Blue spot points and rectangles in (a,b) are additional EDX analyses of similar spectra).
Figure 14.
SEM micrographs and EDX analyses. (a) Area 1 and (b) spot 1 of fine-grained crystals (2–3 μm) formed on fungal hyphae and displaying high Cu% that was leached from the malachite substrate and adsorbed onto the hyphal walls and encrusting crystals. (Blue spot points and rectangles in (a,b) are additional EDX analyses of similar spectra).
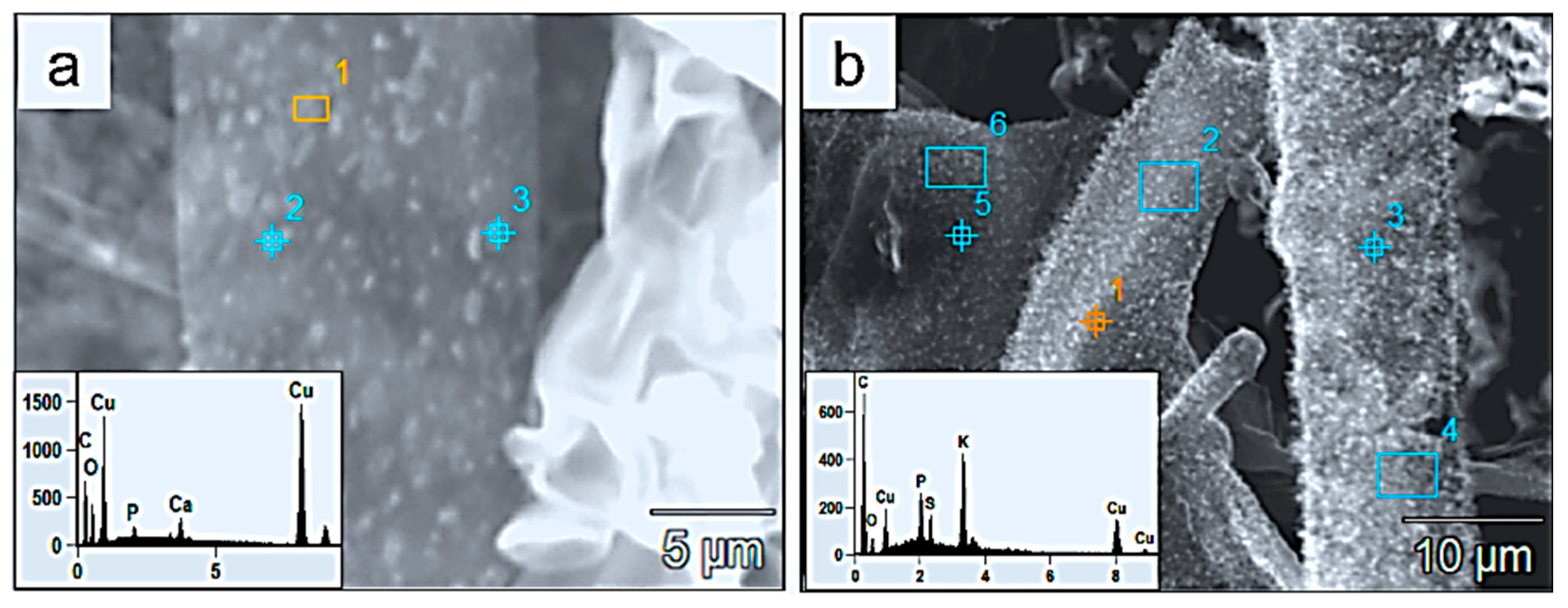
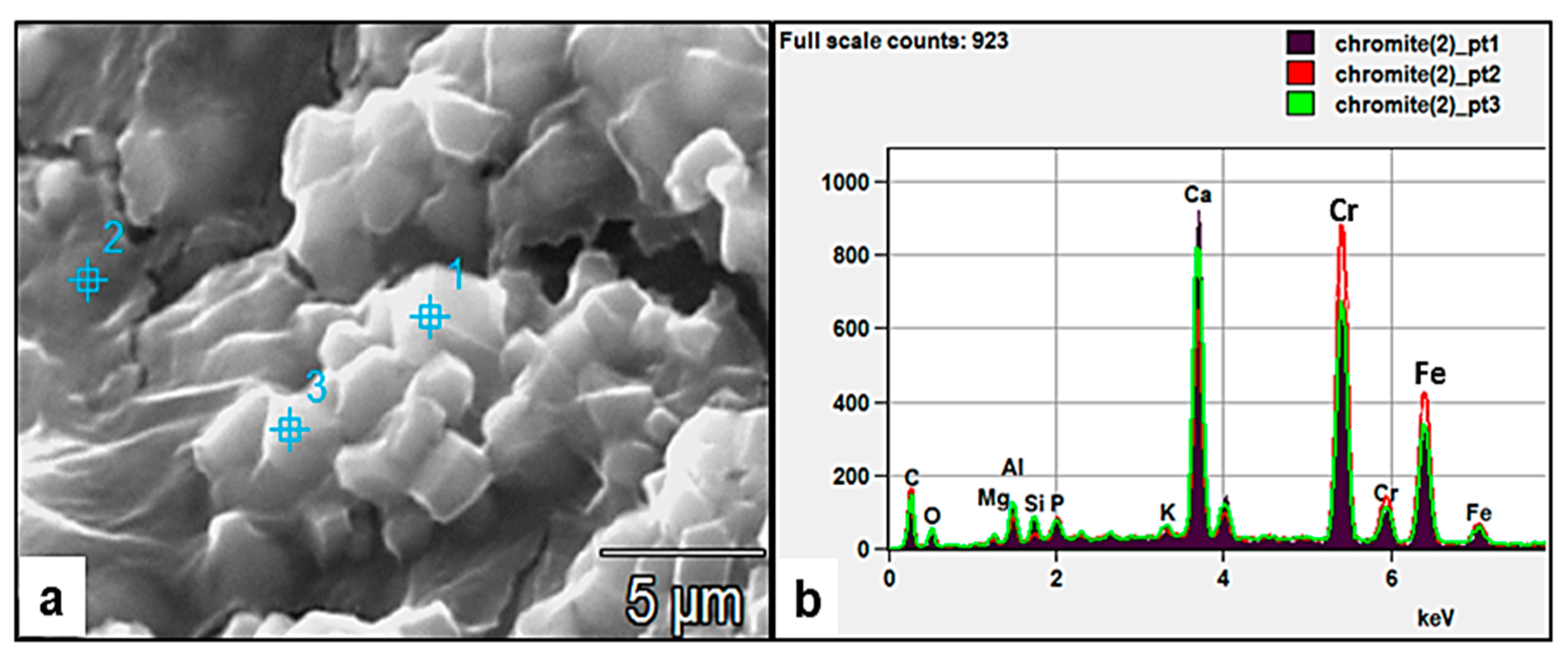
Figure 15.
(a) SEM images of clusters of fungally produced rhombic crystals embedded in EPS and comprising Cr, Fe, and Ca (as evident in the EDX spectra (b) of spot points analyses 1–3 in (a). The association of the crystals with the fungal EPS (point 1 and 3) material suggests the preferential sorption of Cr to the biomass.
Figure 15.
(a) SEM images of clusters of fungally produced rhombic crystals embedded in EPS and comprising Cr, Fe, and Ca (as evident in the EDX spectra (b) of spot points analyses 1–3 in (a). The association of the crystals with the fungal EPS (point 1 and 3) material suggests the preferential sorption of Cr to the biomass.
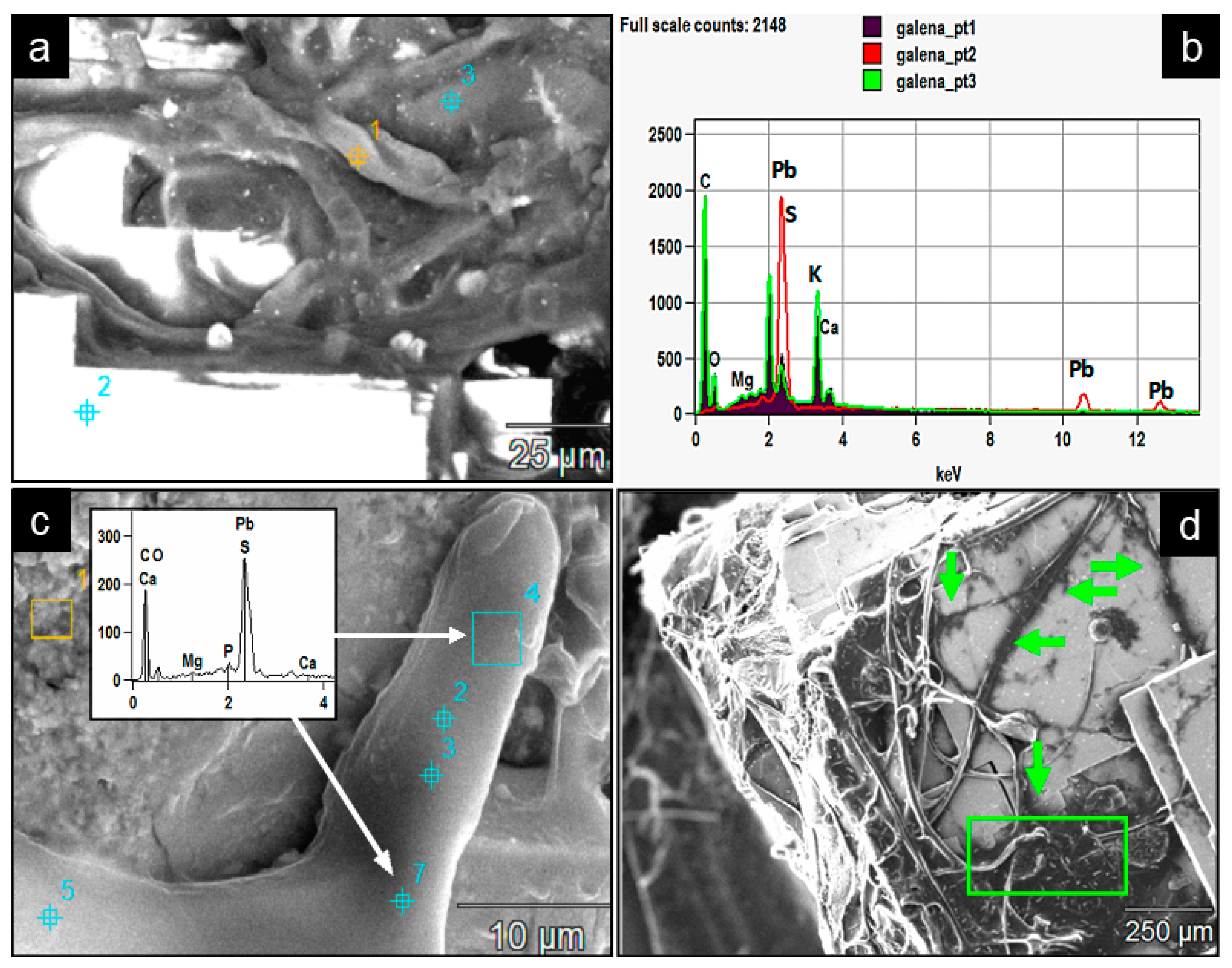
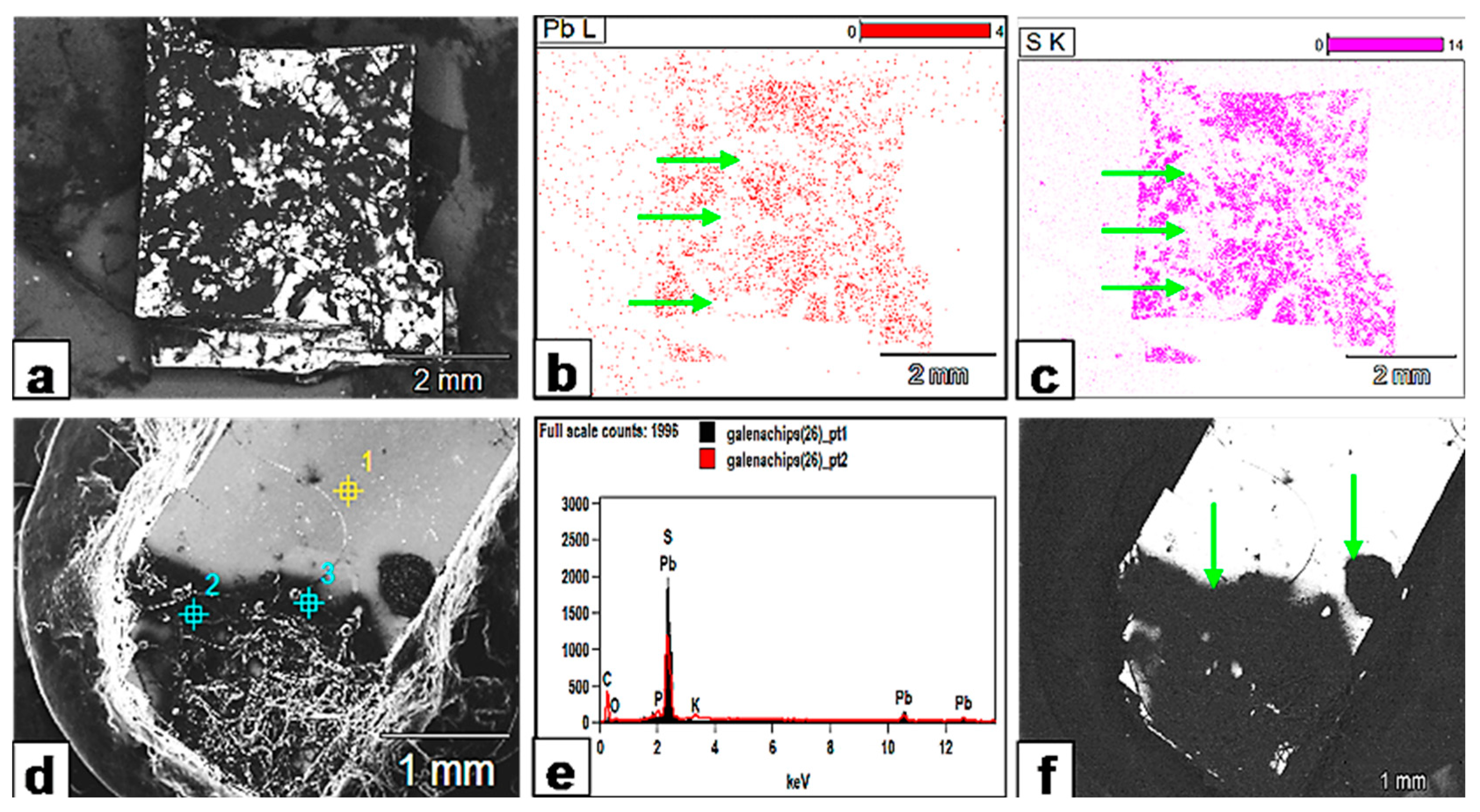
Figure 16.
(a) SEM backscattered image of a fungal biomass (dark grey) colonizing galena crystal surface (bright white). (b) The EDX spectra of the three analyzed spot points in (a) within the fungal mass (1 and 3) and galena crystal surface (2) show Pb, S enrichment and the original galena elemental composition respectively. (c) A detailed EDX analysis of the hypha from two representative analyzed area 4 (blue rectangle) and spot point 7. The spectrum strongly suggests the leaching of Pb and S from colonized galena surface into the hypha. (d) Fungal hyphae are embedded in black areas (green arrows and the rectangle), suggesting a possible reaction–depletion process around the fungal hyphae colonizing the galena surface.
Figure 16.
(a) SEM backscattered image of a fungal biomass (dark grey) colonizing galena crystal surface (bright white). (b) The EDX spectra of the three analyzed spot points in (a) within the fungal mass (1 and 3) and galena crystal surface (2) show Pb, S enrichment and the original galena elemental composition respectively. (c) A detailed EDX analysis of the hypha from two representative analyzed area 4 (blue rectangle) and spot point 7. The spectrum strongly suggests the leaching of Pb and S from colonized galena surface into the hypha. (d) Fungal hyphae are embedded in black areas (green arrows and the rectangle), suggesting a possible reaction–depletion process around the fungal hyphae colonizing the galena surface.
Figure 17.
Metal bioleaching from galena. (a) SEM backscattered image showing the extent of the fungally affected areas (black) on galena. (b,c) XR mapping of the galena crystal surface shows that the black areas in (a) (fungally attacked) are quite depleted in both Pb and S and appear as white zones (green arrows) on the XR maps (compare the same areas with the black counterparts in (a)). (d) SEM image showing that the fungally attacked areas (black) extend beyond the fungal hypha or biomass and create a halo around it. (e) The EDX analysis of spot points 1 (yellow) and 2 (blue) on the crystal surface (d) confirms Pb and S depletion. (f) SEM backscattered image reveals how the whole area (green arrows) is affected by fungal exudates and appears as one reaction zone.
Figure 17.
Metal bioleaching from galena. (a) SEM backscattered image showing the extent of the fungally affected areas (black) on galena. (b,c) XR mapping of the galena crystal surface shows that the black areas in (a) (fungally attacked) are quite depleted in both Pb and S and appear as white zones (green arrows) on the XR maps (compare the same areas with the black counterparts in (a)). (d) SEM image showing that the fungally attacked areas (black) extend beyond the fungal hypha or biomass and create a halo around it. (e) The EDX analysis of spot points 1 (yellow) and 2 (blue) on the crystal surface (d) confirms Pb and S depletion. (f) SEM backscattered image reveals how the whole area (green arrows) is affected by fungal exudates and appears as one reaction zone.
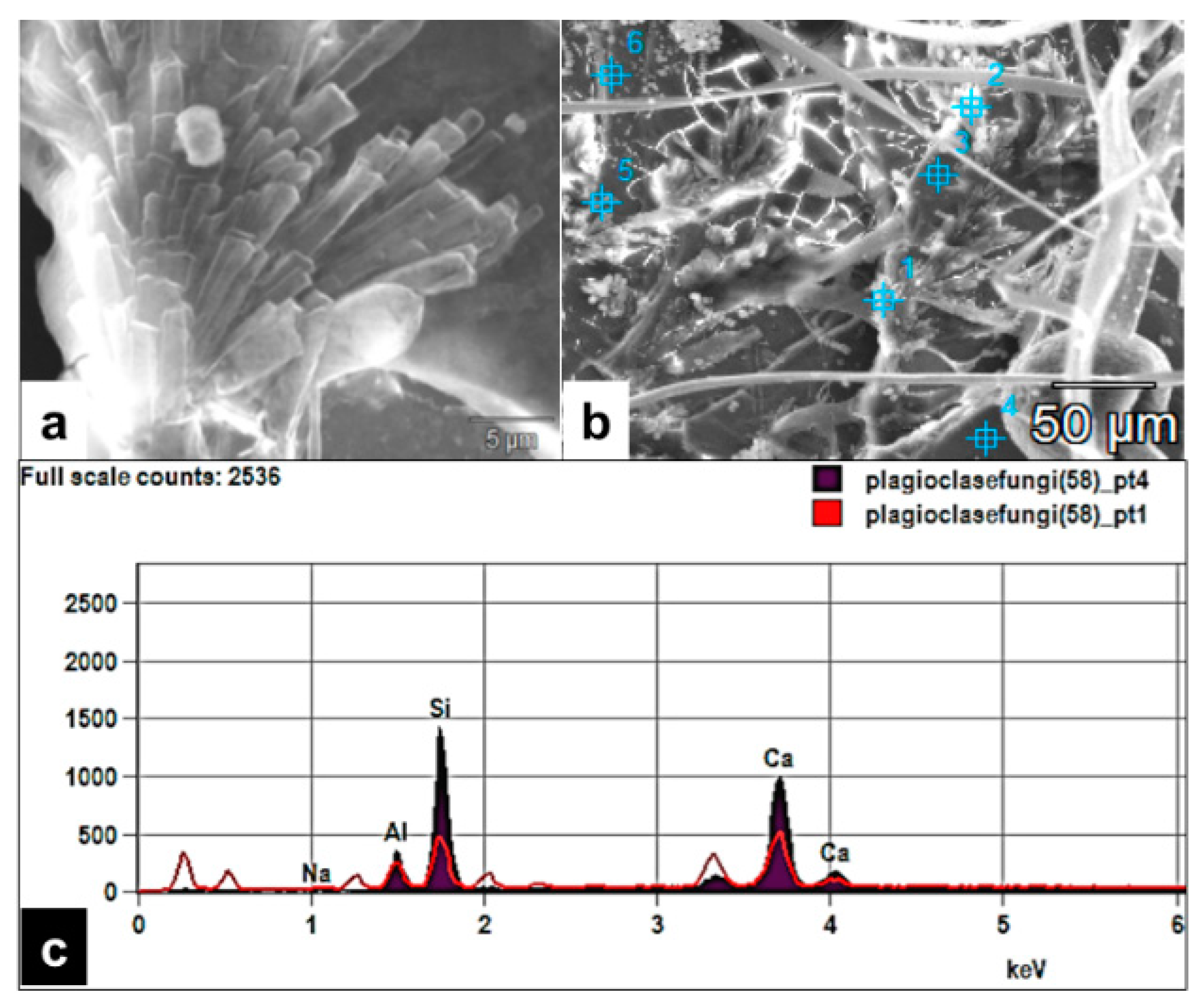
Figure 18.
Bioleaching of plagioclase. (a,) Prismatic–tabular crystals are formed on the plagioclase surface within the colonizing fungal biomass (b). (b) The fungal mass colonizing the plagioclase with different EDX spot analyses (blues points 1–6). (c) EDX spot analysis spectra of points 1 and 4 in (b) depicting the bioleaching of Al, Si and Ca from the plagioclase substrate into the fungal mass. Their chemical composition reflects the main components of plagioclase: Al, Si, Ca, and K.
Figure 18.
Bioleaching of plagioclase. (a,) Prismatic–tabular crystals are formed on the plagioclase surface within the colonizing fungal biomass (b). (b) The fungal mass colonizing the plagioclase with different EDX spot analyses (blues points 1–6). (c) EDX spot analysis spectra of points 1 and 4 in (b) depicting the bioleaching of Al, Si and Ca from the plagioclase substrate into the fungal mass. Their chemical composition reflects the main components of plagioclase: Al, Si, Ca, and K.
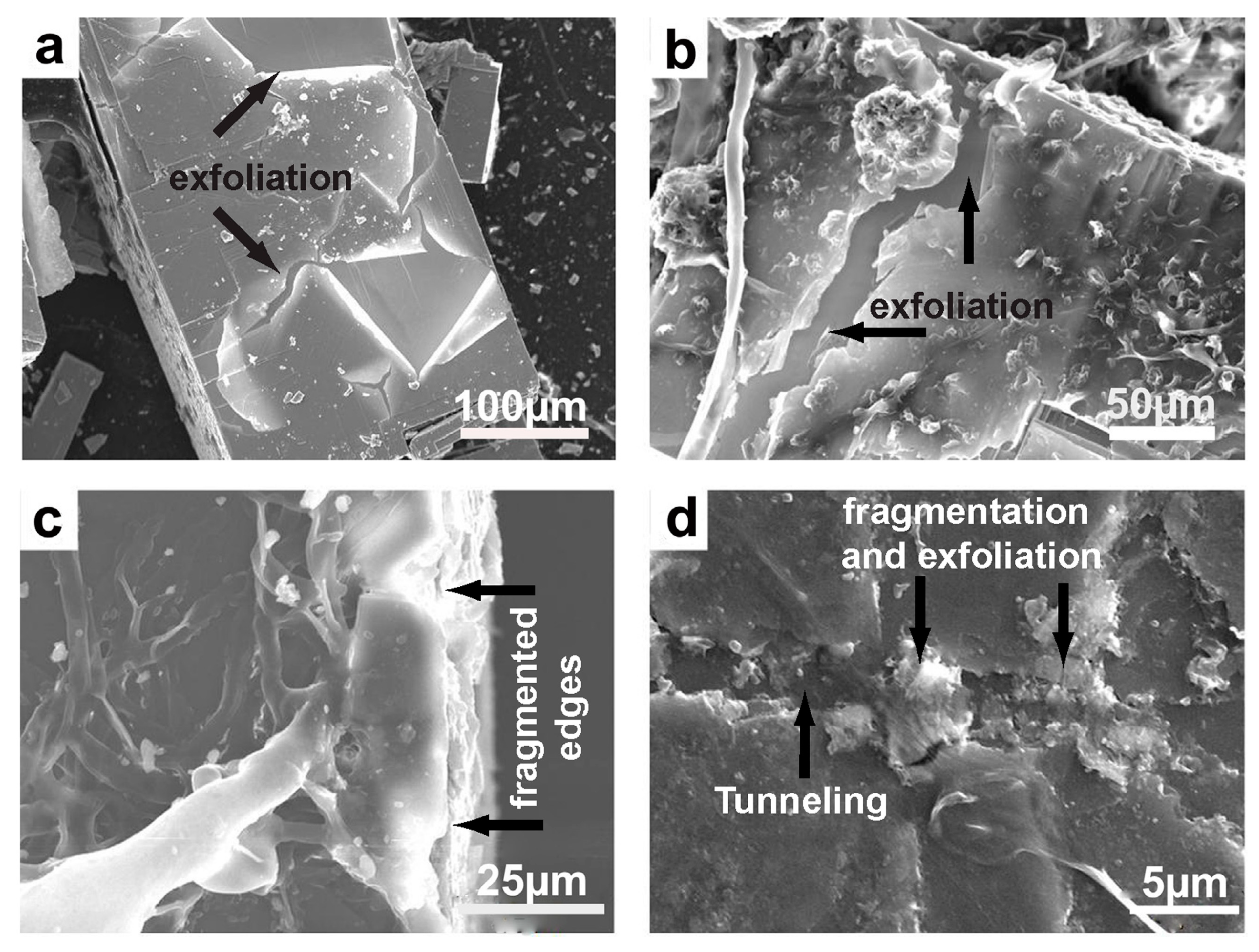
Figure 19.
SEM images of exfoliation and fragmentation of the mineral substrata. (a,b) Galena crystals displaying pronounced exfoliation and parting along the cleavage planes following fungal colonization. (c) Muscovite lamellae displaying severe fragmentation on the edges. (d) Biotite surface showing tunneling and fragmentation.
Figure 19.
SEM images of exfoliation and fragmentation of the mineral substrata. (a,b) Galena crystals displaying pronounced exfoliation and parting along the cleavage planes following fungal colonization. (c) Muscovite lamellae displaying severe fragmentation on the edges. (d) Biotite surface showing tunneling and fragmentation.
Figure 20.
SEM images showing fragmentation of the mineral substrata. (a) Severely fragmented manganite substrate still showing the invading fungal hyphae. (b) The densely colonized malachite is grooved and scarred by fungal hyphae and, on the surface, the secondary precipitation of authigenic mineral phases (inset figure). (c) The densely colonized chromite substrate displays strong degradation at its corner. (d) Similar degradation and fragmentation on chromite shows a powdery material as a result of fungal attack. The single vertical hyphae appear to have perforated the chromite crystal. (c,d) The crystal lineaments are obscured by the fungal interaction with the substrate. (e) Plagioclase showing pitting and fragmentation following the fungal colonization. Its surface is littered with prismatic crystal that formed as secondary minerals with elements leached from the substrate.
Figure 20.
SEM images showing fragmentation of the mineral substrata. (a) Severely fragmented manganite substrate still showing the invading fungal hyphae. (b) The densely colonized malachite is grooved and scarred by fungal hyphae and, on the surface, the secondary precipitation of authigenic mineral phases (inset figure). (c) The densely colonized chromite substrate displays strong degradation at its corner. (d) Similar degradation and fragmentation on chromite shows a powdery material as a result of fungal attack. The single vertical hyphae appear to have perforated the chromite crystal. (c,d) The crystal lineaments are obscured by the fungal interaction with the substrate. (e) Plagioclase showing pitting and fragmentation following the fungal colonization. Its surface is littered with prismatic crystal that formed as secondary minerals with elements leached from the substrate.
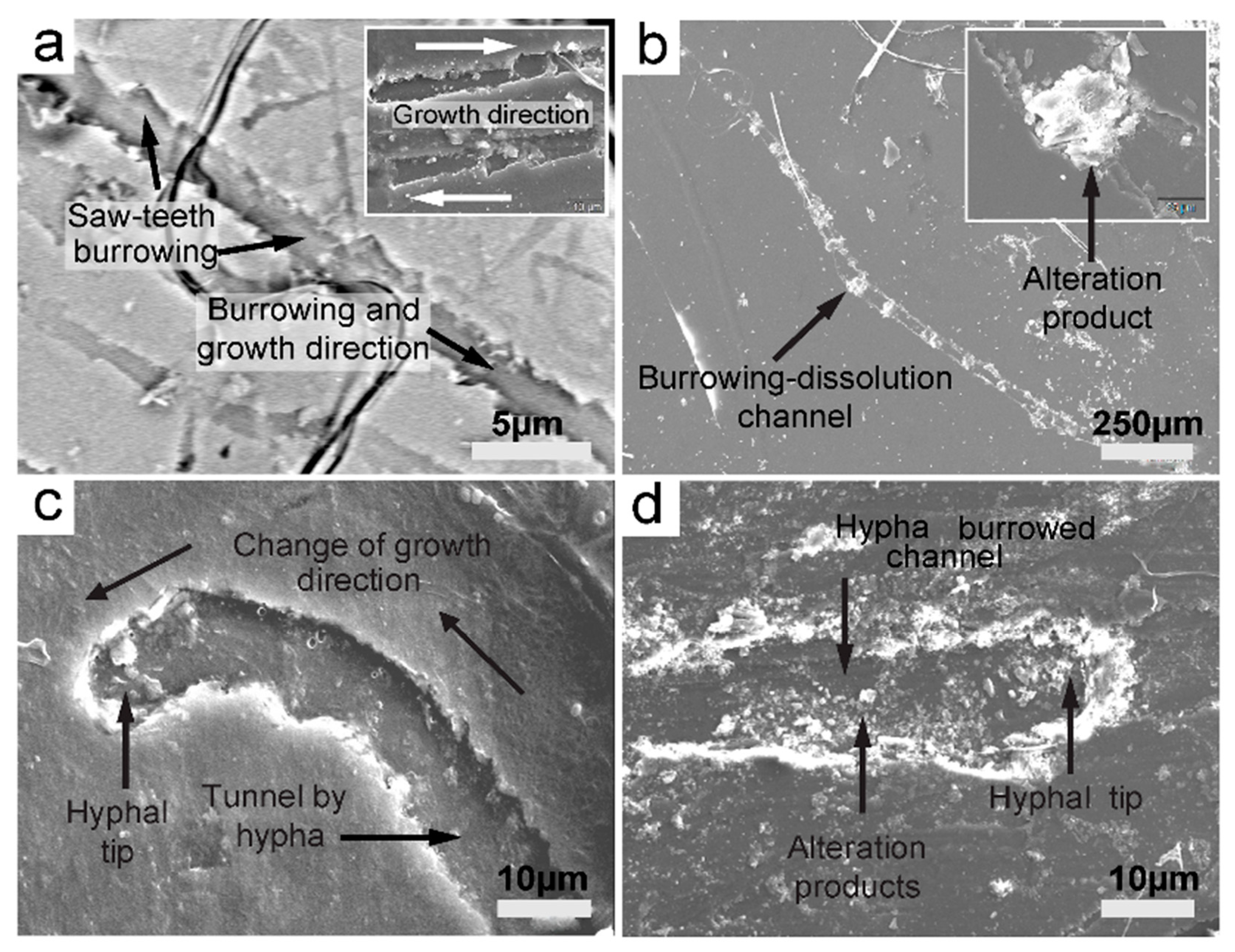
Figure 21.
SEM images of fungal hyphae displaying saw teeth tunneling into various mineral substrata. (a) Fungal sawtooth burrows into biotite, creating a network of channels. (b) A burrowed channel showing alteration products at equal length segments, probably septation locations on the fungal hyphae on biotite. (c) A clear case of fungal tunneling of a biotite surface displaying a hyphal tip and, importantly, a change in growth direction: a thigmotropic response. (d) Burrowed channel with rich alternation products and a hyphal tip on muscovite. (a) Backscattered mode.
Figure 21.
SEM images of fungal hyphae displaying saw teeth tunneling into various mineral substrata. (a) Fungal sawtooth burrows into biotite, creating a network of channels. (b) A burrowed channel showing alteration products at equal length segments, probably septation locations on the fungal hyphae on biotite. (c) A clear case of fungal tunneling of a biotite surface displaying a hyphal tip and, importantly, a change in growth direction: a thigmotropic response. (d) Burrowed channel with rich alternation products and a hyphal tip on muscovite. (a) Backscattered mode.
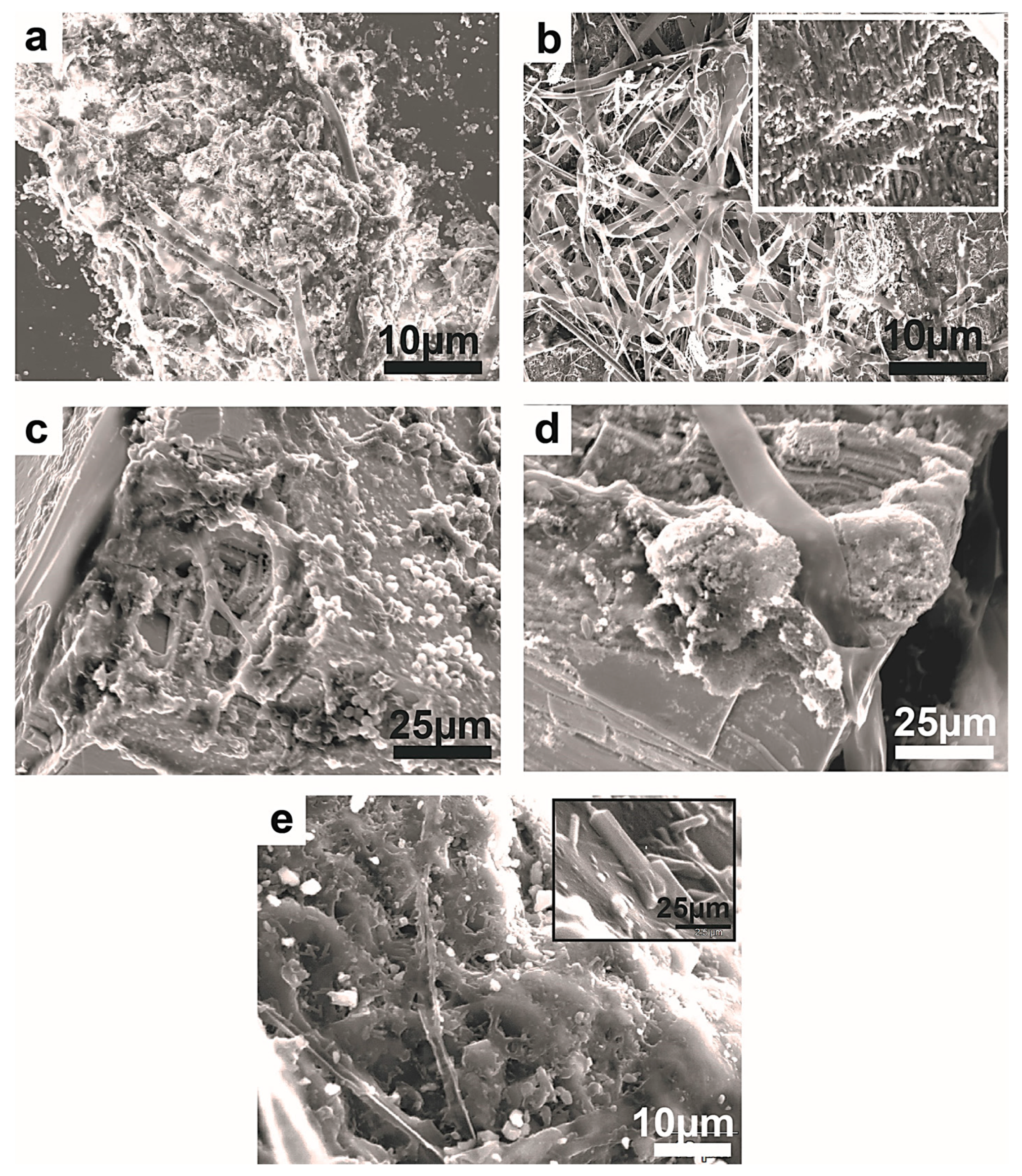
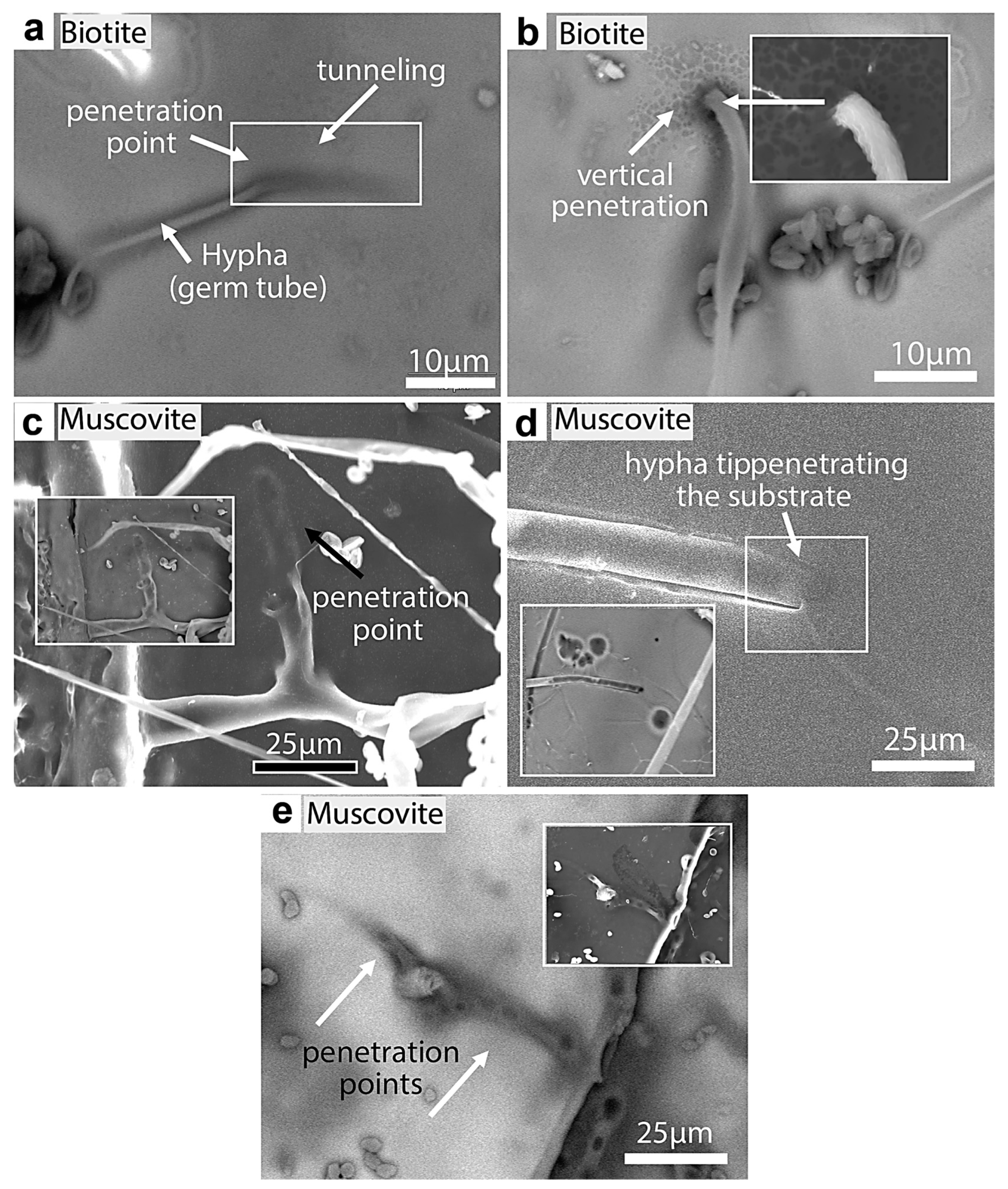
Figure 22.
SEM images of fungal tunneling into biotite and muscovite. (a) A germ tube (Rhizopus oryzae) tunneling into a biotite substrate at an inclined angle. (b) Hypha vertically penetrating biotite. (c) Hypha branching and tunneling into muscovite (shadow traces of penetration). (d) Hypha showing gradual tunneling and the disappearance of the hyphal tip below the muscovite surface (BKSC image shows the black traces of the entrance). (e) Hypha tunneled into muscovite, which shows possible penetration points (black circles). The white edge (inset) of the substrate clearly shows one of the penetration sites (round opening).
Figure 22.
SEM images of fungal tunneling into biotite and muscovite. (a) A germ tube (Rhizopus oryzae) tunneling into a biotite substrate at an inclined angle. (b) Hypha vertically penetrating biotite. (c) Hypha branching and tunneling into muscovite (shadow traces of penetration). (d) Hypha showing gradual tunneling and the disappearance of the hyphal tip below the muscovite surface (BKSC image shows the black traces of the entrance). (e) Hypha tunneled into muscovite, which shows possible penetration points (black circles). The white edge (inset) of the substrate clearly shows one of the penetration sites (round opening).
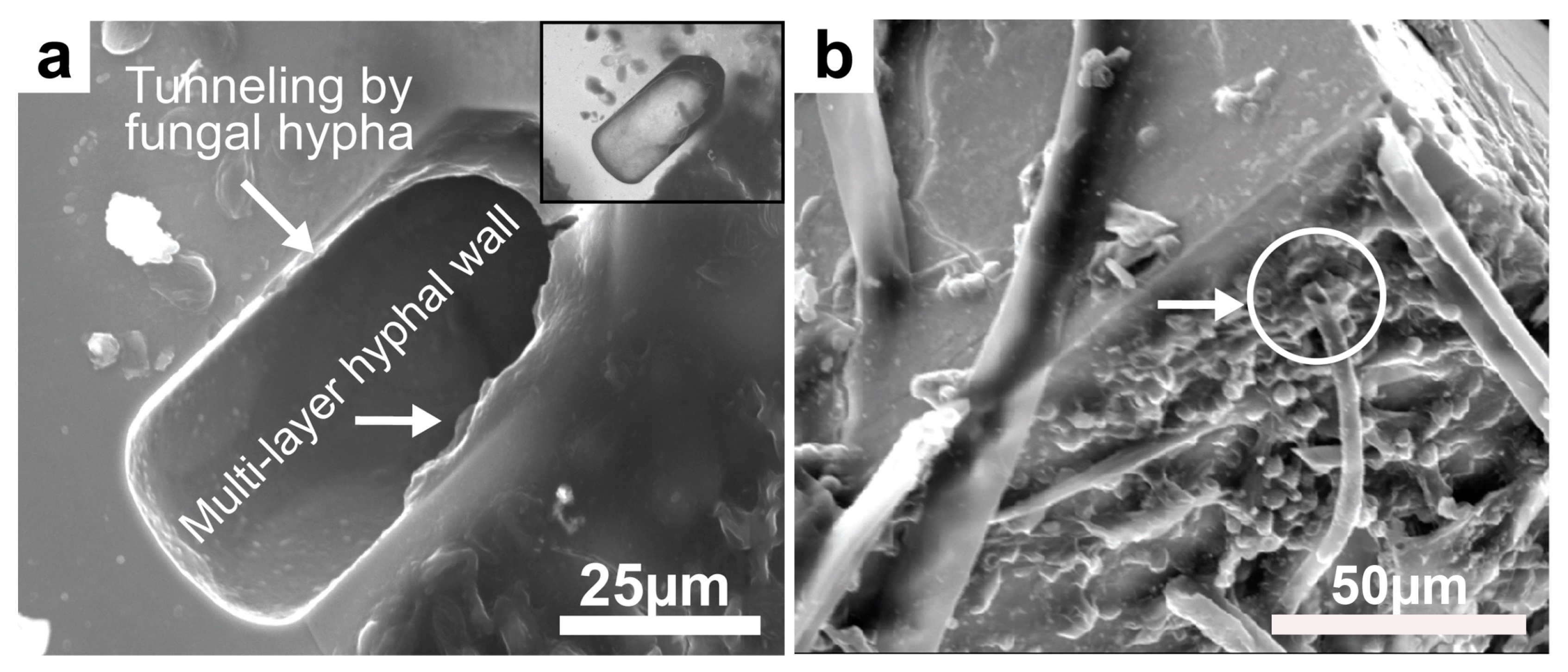
Figure 23.
(a) SEM image of hypha tunneling into galena. The broken hollow hypha appears to have perforated into a galena crystal. The hypha clearly displays a multilayer wall (an indication of an organic wall), and the backscattered SEM image (inset) shows the wall in black, also an indication of its organic material. (b) Rigid and hollow hyphae are also found here. The broken end of the hypha (white arrow and encircled hyphal end) shows a hollow “tube” of the hypha which normally should be collapsed, but the acquired rigidity prevented it. The rigidity is attributed to the mineral encrustation on the hyphae and possible intracellular uptake of metals.
Figure 23.
(a) SEM image of hypha tunneling into galena. The broken hollow hypha appears to have perforated into a galena crystal. The hypha clearly displays a multilayer wall (an indication of an organic wall), and the backscattered SEM image (inset) shows the wall in black, also an indication of its organic material. (b) Rigid and hollow hyphae are also found here. The broken end of the hypha (white arrow and encircled hyphal end) shows a hollow “tube” of the hypha which normally should be collapsed, but the acquired rigidity prevented it. The rigidity is attributed to the mineral encrustation on the hyphae and possible intracellular uptake of metals.
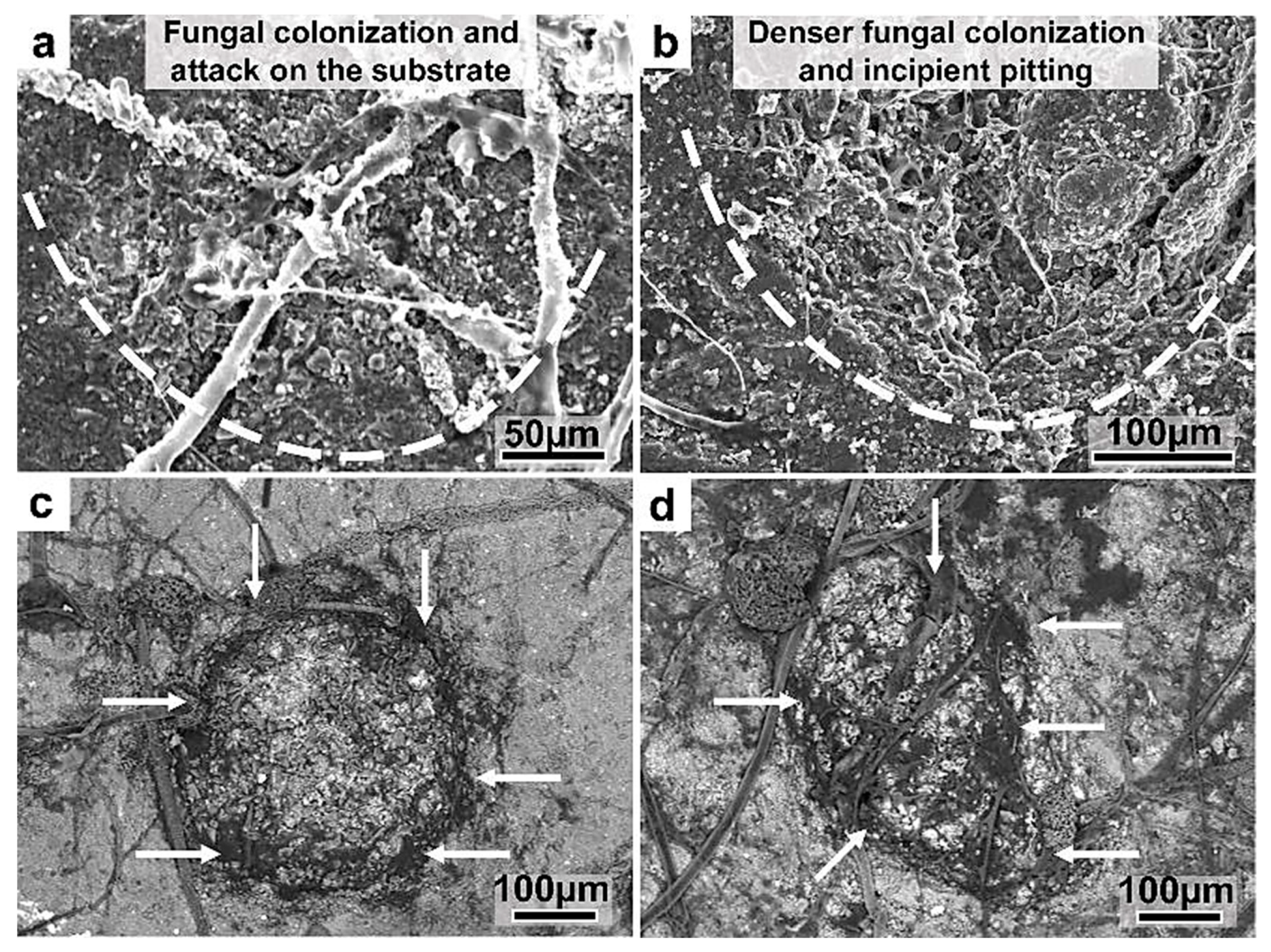
Figure 24.
SEM micrographs of the fungal colonization of bauxite and the development of incipient pitting. (a) The fungi colonize and attack certain zones (area encircled by the dotted line) on bauxite, which produces de-coloration and fragmentation of the substrate. (b) A circular and deeper shape develops following the aggressive colonization, fragmentation, and dissolution of the substrate. (c,d) These backscattered images clearly show the denser fungal colonization (arrows, black-dark gray material) surrounding the attacked zones.
Figure 24.
SEM micrographs of the fungal colonization of bauxite and the development of incipient pitting. (a) The fungi colonize and attack certain zones (area encircled by the dotted line) on bauxite, which produces de-coloration and fragmentation of the substrate. (b) A circular and deeper shape develops following the aggressive colonization, fragmentation, and dissolution of the substrate. (c,d) These backscattered images clearly show the denser fungal colonization (arrows, black-dark gray material) surrounding the attacked zones.
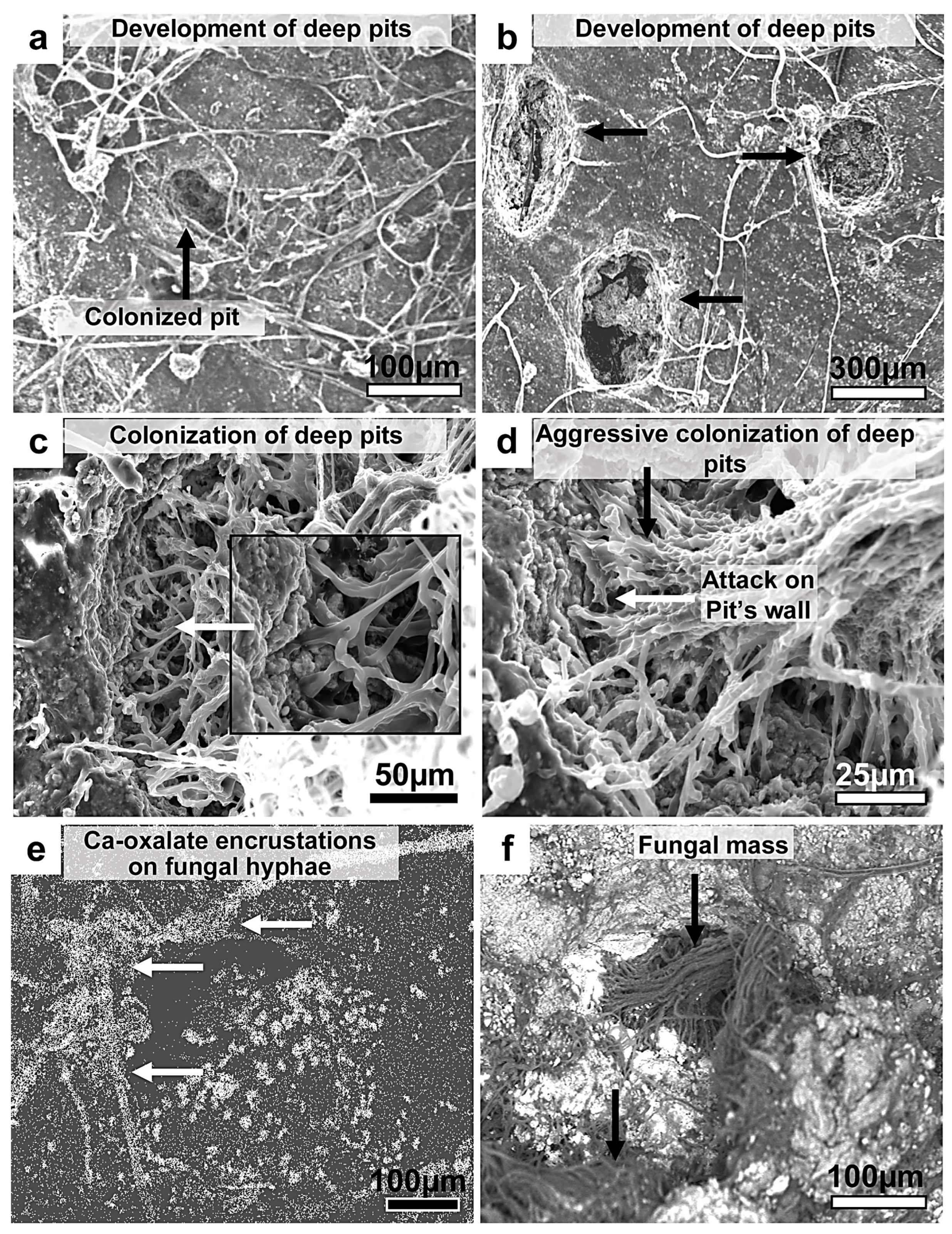
Figure 25.
SEM micrographs showing the advanced pitting of bauxite. (a,b) The formation of deep oval-circular pits on bauxite, which subsequently became densely colonized by fungi. (c,d) The pit’s inner walls are typically attacked by colonizing hyphae, creating an interwoven network of fungal mass lining the walls. The walls are usually degraded and fragmented. Authigenic minerals are deposited (Ca-oxalates: arrows in (e)), and the elements from the substrate are bioleached and concentrated in the fungal surroundings (arrows in (d)). (e,f) Ca SEM X-ray mapping and SEM backscattered images, respectively.
Figure 25.
SEM micrographs showing the advanced pitting of bauxite. (a,b) The formation of deep oval-circular pits on bauxite, which subsequently became densely colonized by fungi. (c,d) The pit’s inner walls are typically attacked by colonizing hyphae, creating an interwoven network of fungal mass lining the walls. The walls are usually degraded and fragmented. Authigenic minerals are deposited (Ca-oxalates: arrows in (e)), and the elements from the substrate are bioleached and concentrated in the fungal surroundings (arrows in (d)). (e,f) Ca SEM X-ray mapping and SEM backscattered images, respectively.
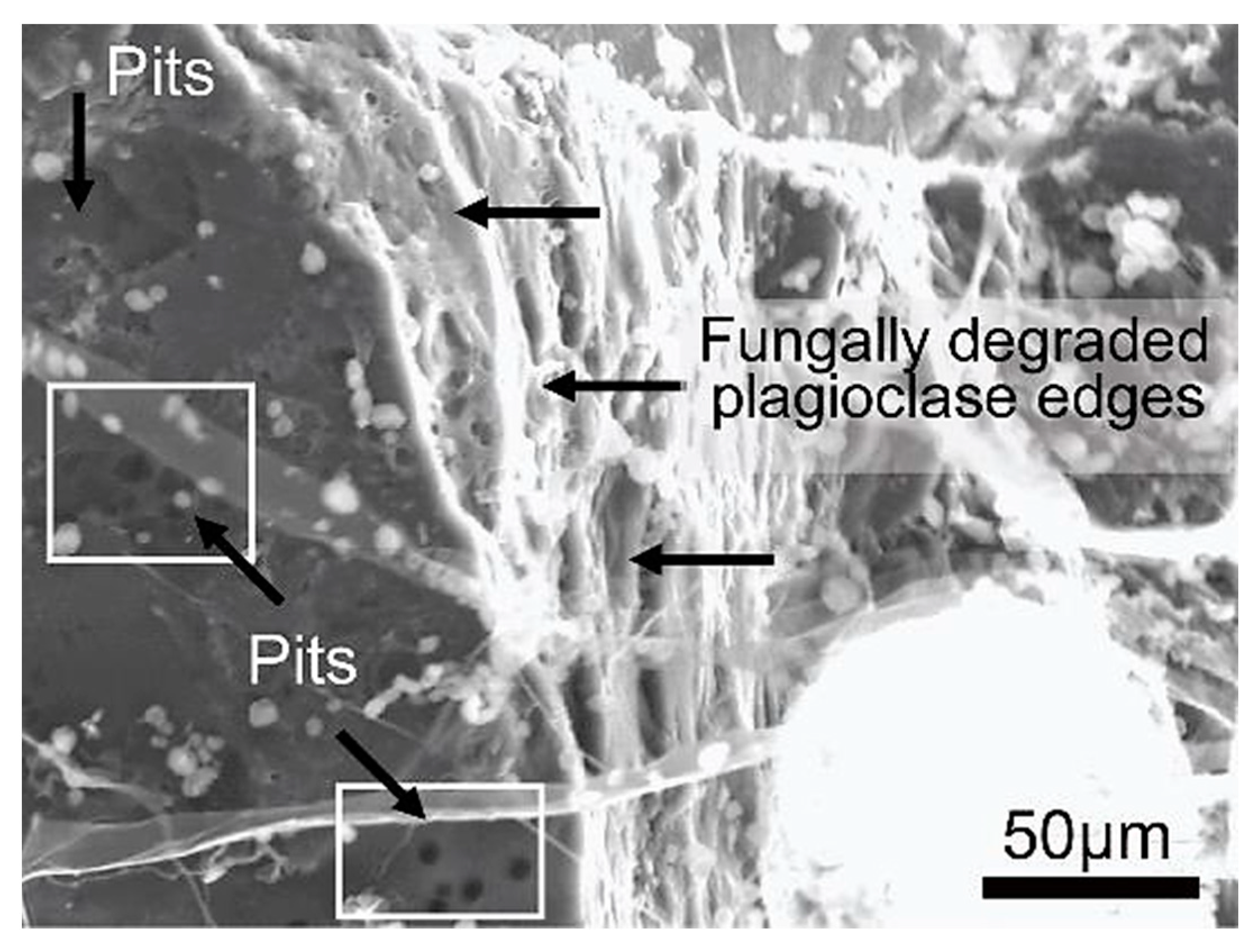
Figure 26.
SEM image of a plagioclase crystal displaying round pits on its surface and degraded bio-weathered edges formed by the fungal interaction.
Figure 26.
SEM image of a plagioclase crystal displaying round pits on its surface and degraded bio-weathered edges formed by the fungal interaction.
Figure 27.
SEM and X-ray mapping micrographs of Ca (green) and S (red) of newly formed minerals. (a) A discoid of radially arranged crystal aggregates comprising Ca, S, and O and presumed gypsum. (b,c) XR maps of the same discoid, revealing the Ca and S composition of the crystal aggregates, which also extends to the composition of the much finer crystal aggregates embedded in the EPS material to the right. (d) Dense authigenic mineral formation as hyphal encrustations and free crystals that litter the background. The typical prismatic bipyramidal crystals are Ca-oxalates. The inset XR map shows their Ca composition.
Figure 27.
SEM and X-ray mapping micrographs of Ca (green) and S (red) of newly formed minerals. (a) A discoid of radially arranged crystal aggregates comprising Ca, S, and O and presumed gypsum. (b,c) XR maps of the same discoid, revealing the Ca and S composition of the crystal aggregates, which also extends to the composition of the much finer crystal aggregates embedded in the EPS material to the right. (d) Dense authigenic mineral formation as hyphal encrustations and free crystals that litter the background. The typical prismatic bipyramidal crystals are Ca-oxalates. The inset XR map shows their Ca composition.
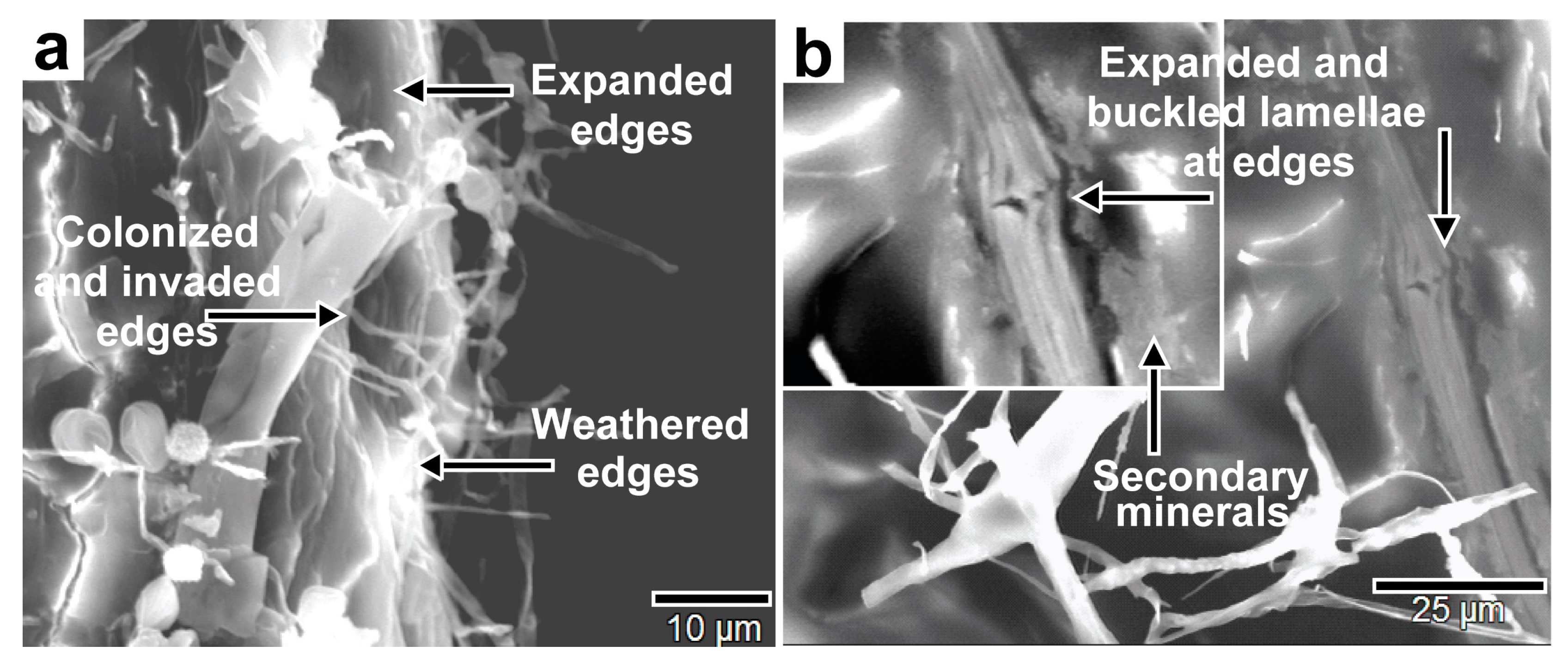
Figure 28.
SEM images of fungal bioweathering of biotite lamellae edges showing (a) expansion, dissolution, and hyphal invasion of the edges, and (b) the expansion, swelling, and buckling of lamellae edges and secondary mineral deposition as bioweathering products.
Figure 28.
SEM images of fungal bioweathering of biotite lamellae edges showing (a) expansion, dissolution, and hyphal invasion of the edges, and (b) the expansion, swelling, and buckling of lamellae edges and secondary mineral deposition as bioweathering products.
Table 1.
The fungal strains isolated from the experimental mineral surfaces.
Table 1.
The fungal strains isolated from the experimental mineral surfaces.
| Mineral Sample | Fungal Strains |
|---|
| Manganite | Rhizopus oryzae | |
| Muscovite/Biotite | Rhizopus oryzae | |
| Bauxite | Rhizopus oryzae | |
| Malachite | Rhizopus oryzae | Aspergillus flavus |
Chromite
Galena | Rhizopus oryzae
Rhizopus oryzae | |
| Plagioclase | Rhizopus oryzae | |
Table 2.
Quantitative EDX analysis of points 1 and 2 on precipitated iron.
Table 2.
Quantitative EDX analysis of points 1 and 2 on precipitated iron.
| Weight % | C | O | Al | Si | P | K | Ti | Fe |
|---|
| Bauxite_pt2 | 2.48 | | 0.28 | 0.16 | | 0.51 | 3.14 | 93.44 |
| Bauxite_pt3 | 8.34 | 0.92 | | 0.09 | 0.21 | 0.28 | 3.61 | 86.56 |
Table 3.
EDX analysis of the prismatic crystal aggregates (point 1) and plagioclase substrate (point 4) in the figure (image).
Table 3.
EDX analysis of the prismatic crystal aggregates (point 1) and plagioclase substrate (point 4) in the figure (image).
| Weight % | C | O | Mg | Al | Si | P | S | K | Ca |
|---|
| EDX of Prismatic crystals aggregate: point 1 | 24.08 | 68.81 | 0.5 | 0.85 | 1.45 | 0.39 | 0.1 | 1.32 | 2.5 |
| EDX Plagioclase substrate point 4 | 18.17 | 6.91 | | 7.02 | 29.79 | | | 2.37 | 32.4 |