Calcium Carbonate Precipitation Behavior in the System Ca-Me2+-CO3-H2O (Me2+ = Co, Ni, Cu, Fe): Ion Incorporation, Effect of Temperature and Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization Methods
3. Results
3.1. Resulting Phase Composition and Crystal (Micro) Morphology
3.1.1. Calcite
3.1.2. Aragonite
3.1.3. Monohydrocalcite
3.1.4. Amorphous Carbonate (AC)
3.1.5. Malachite
3.1.6. Iron (Hydro)oxides
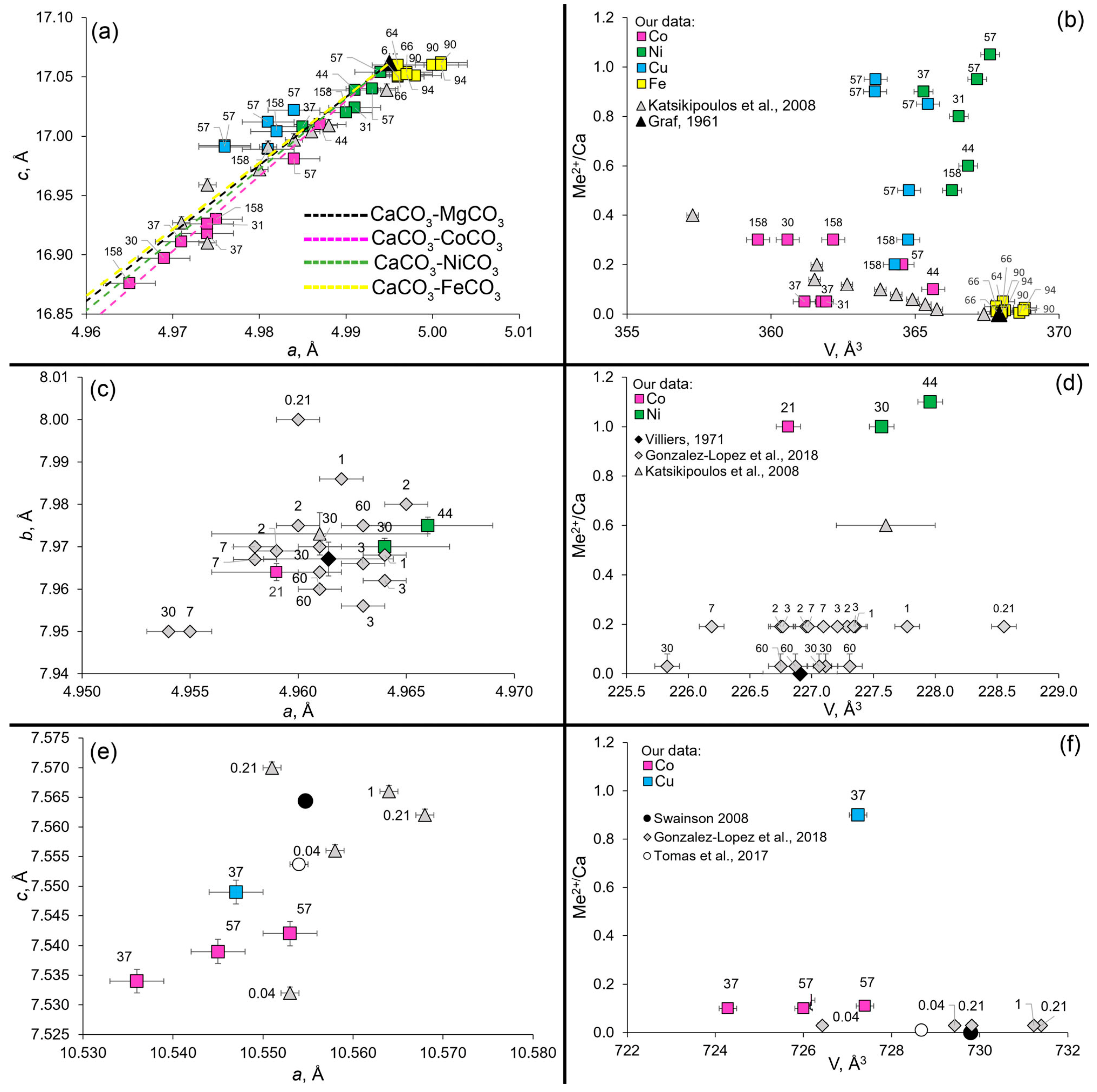
3.2. Unit Cell Parameters and Chemical Composition of Synthesized Calcium Carbonates
3.2.1. Calcite
3.2.2. Aragonite
3.2.3. Monohydrocalcite
3.2.4. Amorphous Carbonate
4. Discussion
4.1. The Effect of Temperature
4.2. The Effect of Aging Time
4.3. Heavy Metal (Me2+ = Co, Ni, Cu, Fe) Incorporation into CCC Lattices
| No. | Sample Name | T, °C | Aging Time, Days | Me2+ | Me2+/Ca, Solution | Main Phase | V, Å3 | Ref. | Δ1, Å3 | Δ2, Å3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | - | natural | - | 0.000 | Calcite (CaCO3) | 367.90(1) | [42] | - | - | ||
| 2 | CAV-49 | 23 | 158 | Co | 0.300 | 362.2(3) | This study | 6.0 | 8.3 | ||
| 3 | CAV-52 | 158 | 0.300 | 359.6(3) | |||||||
| 4 | CAV-46 | 57 | 0.200 | 364.6(3) | |||||||
| 5 | CAV-67 | 37 | 0.050 | 361.2(3) | |||||||
| 6 | CAV-53 | 31 | 0.050 | 361.9(3) | |||||||
| 7 | CME-25 | 3 | 44 | 0.200 | 366.5(3) | ||||||
| 8 | CME-26 | 44 | 0.100 | 365.6(3) | |||||||
| 9 | CAV-74 | 23 | 57 | Ni | 1.050 | 367.6(3) | 1.3 | 1.6 | |||
| 10 | CAV-76 | 57 | 0.950 | 367.2(3) | |||||||
| 11 | CAV-51 | 158 | 0.500 | 366.3(3) | |||||||
| 12 | CME-29 | 3 | 44 | 0.600 | 366.8(3) | ||||||
| 13 | CAV-78 | 23 | 57 | Cu | 0.900 | 363.6(3) | 1.8 | 4.3 | |||
| 14 | CAV-79 | 57 | 0.850 | 365.4(3) | |||||||
| 15 | CAV-44 | 57 | 0.500 | 364.8(3) | |||||||
| 16 | CAV-47 | 158 | 0.300 | 364.8(3) | |||||||
| 17 | CAV-50 | 158 | 0.200 | 364.3(3) | |||||||
| 18 | CAV-150 | 44 | Fe | 0.100 | 368.6(3) | 1.8 | 0.9 | ||||
| 19 | CAV-124 | 66 | 0.050 | 368.0(3) | |||||||
| 20 | CAV-123 | 66 | 0.030 | 367.8(3) | |||||||
| 21 | CAV-138 | 90 | 0.025 | 368.8(3) | |||||||
| 22 | CAV-137 | 90 | 0.015 | 368.1(3) | |||||||
| 23 | CAV-122 | 66 | 0.010 | 367.8(3) | |||||||
| 24 | CAV-136 | 90 | 0.005 | 368.6(3) | |||||||
| 25 | CME-81 | 3 | 64 | Fe | 0.050 | 367.8(3) | |||||
| 26 | CME-80 | 64 | 0.030 | 368.2(3) | |||||||
| 27 | CME-95 | 94 | 0.025 | 367.0(3) | |||||||
| 28 | CME-94 | 94 | 0.015 | 368.8(3) | |||||||
| 29 | CME-79 | 64 | 0.010 | 368.0(3) | |||||||
| 30 | CME-93 | 94 | 0.005 | 368.0(3) | |||||||
| 31 | - | ~222 | 2 | Co | - | Spherocobaltite (CoCO3) | 281.62(1) | [48] | - | 86.3 | |
| 32 | - | ~222 | 2 | Ni | - | Gaspéite (NiCO3) | 271.39(1) | - | 96.5 | ||
| 33 | - | - | - | Fe | - | Siderite (FeCO3) | 293.17 | [8] | - | 74.7 | |
| 34 | - | natural | - | - | Aragonite (CaCO3) | 226.91(1) | [43] | - | - | ||
| 35 | CAV-43 | 23 | 21 | Co | 1.000 | 226.8(3) | This study | 1.2 | 1.1 | ||
| 36 | CME-27 | 3 | 44 | Ni | 1.100 | 228.0(3) | |||||
| 37 | CME-2 | 30 | Ni | 1.000 | 227.6(3) | ||||||
| 38 | - | natural | - | - | Monohydrocalcite (CaCO3·H2O) | 729.79(6) | [44] | ||||
| 39 | - | natural | Cu | - | 728.68(18) | [45] | 1.11 | ||||
| 40 | CAV-71 | 23 | 57 | Co | 0.110 | 727.4(3) | This study | 3.1 | 5.49 | ||
| 41 | CAV-70 | 37 | Co | 0.100 | 724.3(3) | ||||||
| 42 | CAV-72 | 57 | Co | 0.100 | 726.0(3) | ||||||
| 43 | CAV-63 | 37 | Cu | 0.900 | 727.2(3) | ||||||
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Summerfield, M.A. Global Geomorphology: An Introduction to the Study of Landforms; Longman Sc.: New York, NY, USA, 1991. [Google Scholar]
- Sulpis, O.; Jeansson, E.; Dinauer, A.; Lauvset, S.K.; Middelburg, J.J. Calcium Carbonate Dissolution Patterns in the Ocean. Nat. Geosci. 2021, 14, 423–428. [Google Scholar] [CrossRef]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Bindschedler, S.; Cailleau, G.; Verrecchia, E. Role of Fungi in the Biomineralization of Calcite. Minerals 2016, 6, 41. [Google Scholar] [CrossRef]
- Millero, F.J.; Woosey, R.; Ditrolio, B.; Water, J. Effect of Ocean Acidification on the Speciation of Metals in Seawater. Oceanography 2009, 22, 72–85. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Falini, G.; Fermani, S.; Gazzano, M.; Ripamonti, A. Structure and Morphology of Synthetic Magnesium Calcite. J. Mater. Chem. 1998, 8, 1061–1065. [Google Scholar] [CrossRef]
- Effenberger, H. Crystal Structure and Infrared Absorption Spectrum of Synthetic Monohydrocalcite, CaCO3 H2O. Monatshefte Für Chem. 1981, 112, 899–909. [Google Scholar] [CrossRef]
- Caspi, E.N.; Pokroy, B.; Lee, P.L.; Quintana, J.P.; Zolotoyabko, E. On the Structure of Aragonite. Acta Crystallogr. Sect. B Struct. Sci. 2005, 61, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, A.L.; Wyllie, P.J. The Calcite-Aragonite Transition Measured in the System CaO-CO2-H2O. J. Geol. 1968, 3, 314–330. [Google Scholar] [CrossRef]
- Garvie, L.A.J. Seasonal Formation of Ikaite in Slime Flux Jelly on an Infected Tree (Populus fremontii) Wound from the Sonoran Desert. Sci. Nat. 2022, 109, 48. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Kudłacz, K.; Cizer, Ö.; Ruiz-Agudo, E. Formation of Amorphous Calcium Carbonate and Its Transformation into Mesostructured Calcite. CrystEngComm 2015, 17, 58–72. [Google Scholar] [CrossRef]
- Ontl, T.A.; Schulte, L.A. Soil Carbon Storage. Nat. Educ. Knowl. 2012, 3, 35. [Google Scholar]
- Little, S.H.; Wilson, D.J.; Rehkämper, M.; Adkins, J.F.; Robinson, L.F.; van de Flierdt, T. Cold-Water Corals as Archives of Seawater Zn and Cu Isotopes. Chem. Geol. 2021, 578, 120304. [Google Scholar] [CrossRef]
- Défarge, C. Organomineralization. In Encyclopedia of Geobiology; Reitner, J., Thiel, V., Eds.; Springer: Dodrecht, The Netherlands, 2011; pp. 697–701. [Google Scholar]
- Sondi, I.; Mikac, N.; Vdović, N.; Ivanić, M.; Furdek, M.; Škapin, S.D. Geochemistry of Recent Aragonite-Rich Sediments in Mediterranean Karstic Marine Lakes: Trace Elements as Pollution and Palaeoredox Proxies and Indicators of Authigenic Mineral Formation. Chemosphere 2017, 168, 786–797. [Google Scholar] [CrossRef] [PubMed]
- de Nooijer, L.J.; Reichart, G.J.; Duenas-Bohorquez, A.; Wolthers, M.; Ernst, S.R.; Mason, P.R.D.; van der Zwaan, G.J. Copper Incorporation in Foraminiferal Calcite: Results from Culturing Experiments. Biogeosciences 2007, 4, 493–504. [Google Scholar] [CrossRef]
- Raiswell, R.; Brimblecombe, P. The Partition of Manganese into Aragonite between 30 and 60 °C. Chem. Geol. 1977, 19, 145–151. [Google Scholar] [CrossRef]
- Mavromatis, V.; Goetschl, K.E.; Grengg, C.; Konrad, F.; Purgstaller, B.; Dietzel, M. Barium Partitioning in Calcite and Aragonite as a Function of Growth Rate. Geochim. Cosmochim. Acta 2018, 237, 65–78. [Google Scholar] [CrossRef]
- Brazier, J.M.; Mavromatis, V. Effect of Growth Rate on Nickel and Cobalt Incorporation in Aragonite. Chem. Geol. 2022, 600, 120863. [Google Scholar] [CrossRef]
- Gutjahr, A.; Dabringhaus, H.; Lacmann, R. Studies of the Growth and Dissolution Kinetics of the CaCO3 Polymorphs Calcite and Aragonite II. The Influence of Divalent Cation Additives on the Growth and Dissolution Rates. J. Cryst. Growth 1996, 158, 310–315. [Google Scholar] [CrossRef]
- Vereshchagin, O.S.; Frank-Kamenetskaya, O.V.; Kuz’mina, M.A.; Chernyshova, I.A.; Shilovskikh, V.V. Effect of Magnesium on Monohydrocalcite Formation and Unit-Cell Parameters. Am. Mineral. 2021, 106, 1294–1305. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Q.; Ren, B.; Luo, J.; Yuan, J.; Ding, X.; Bian, H.; Yao, X. Trends and Health Risks of Dissolved Heavy Metal Pollution in Global River and Lake Water from 1970 to 2017. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, Switzerland, 2020; Volume 251, pp. 1–24. [Google Scholar]
- Liao, Z.; Wu, S.; Zhang, H.; Chen, F. Removal of Aqueous Cu2+ by Amorphous Calcium Carbonate: Efficiency and Mechanism. Minerals 2022, 12, 362. [Google Scholar] [CrossRef]
- Macomber, L.; Hausinger, R.P. Mechanisms of Nickel Toxicity in Microorganisms. Metallomics 2011, 3, 1153–1162. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt Toxicity in Humans—A Review of the Potential Sources and Systemic Health Effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Sun, Z.; Gong, C.; Ren, J.; Zhang, X.; Wang, G.; Liu, Y.; Ren, Y.; Zhao, Y.; Yu, Q.; Wang, Y.; et al. Toxicity of Nickel and Cobalt in Japanese Flounder. Environ. Pollut. 2020, 263, 114516. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; Burgos-Cara, A.; Ruiz-Agudo, E.; Putnis, C.V.; Prieto, M. Effect of Ferrous Iron on the Nucleation and Growth of CaCO3 in Slightly Basic Aqueous Solutions. CrystEngComm 2017, 19, 447–460. [Google Scholar] [CrossRef]
- Katsikopoulos, D.; Fernández-gonzález, Á.; Carmelo, A.; Prieto, M. Co-Crystallization of Co (II) with Calcite: Implications for the Mobility of Cobalt in Aqueous Environments. Chem. Geol. 2008, 254, 87–100. [Google Scholar] [CrossRef]
- González-López, J.; Fernández-González, Á.; Jiménez, A. Precipitation Behaviour in the System Ca2+-Co2+-CO32−-H2O at Ambient Conditions—Amorphous Phases and CaCO3 Polymorphs. Chem. Geol. 2018, 482, 91–100. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, J.; Wu, S.; Chen, F.; Yang, Y. Divalent Heavy Metals and Uranyl Cations Incorporated in Calcite Change Its Dissolution Process. Sci. Rep. 2020, 10, 16864. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, Y. Experimental Study on the Relationship between Metal Ions and Formation of CaCO3 Crystalline Fouling under Boiling Scaling System. Heat Mass Transf. Stoffuebertragung 2019, 55, 3077–3085. [Google Scholar] [CrossRef]
- Lin, P.Y.; Wu, H.M.; Hsieh, S.L.; Li, J.S.; Dong, C.; Chen, C.W.; Hsieh, S. Preparation of Vaterite Calcium Carbonate Granules from Discarded Oyster Shells as an Adsorbent for Heavy Metal Ions Removal. Chemosphere 2020, 254, 126903. [Google Scholar] [CrossRef]
- Rosenberg, P.E. Subsolidus Relations in the System CaCO3-FeCO3. J. Sci. 1963, 261, 683–689. [Google Scholar] [CrossRef]
- Goldsmith, J.R.; Graf, D.L.; Witters, J.; Northrop, D.A. Studies in the System CaCO3-MgCO3-FeCO3: 1. Phase Relations; 2. A Method for Major-Element Spectrochemical Analysis; 3. Compositions of Some Ferroan Dolomites. J. Geol. 1962, 70, 659–688. [Google Scholar] [CrossRef]
- Neerup, R.; Løge, I.A.; Fosbøl, P.L. FeCO3 Synthesis Pathways: The Influence of Temperature, Duration, and Pressure. ACS Omega 2023, 8, 3404–3414. [Google Scholar] [CrossRef] [PubMed]
- Mejri, W.; Ben Salah, I.; Tlili, M.M. Speciation of Fe(II) and Fe(III) Effect on CaCO3 Crystallization. Cryst. Res. Technol. 2015, 50, 236–243. [Google Scholar] [CrossRef]
- Zeppenfeld, K. Prevention of CaCO3 Scale Formation by Trace Amounts of Copper (II) in Comparison to Zinc (II). Desalination 2010, 252, 60–65. [Google Scholar] [CrossRef]
- Iglikowska, A.; Bełdowski, J.; Chełchowski, M.; Chierici, M.; Kędra, M.; Przytarska, J.; Sowa, A.; Kukliński, P. Chemical Composition of Two Mineralogically Contrasting Arctic Bivalves’ Shells and Their Relationships to Environmental Variables. Mar. Pollut. Bull. 2017, 114, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Blanco, J.D.; Shaw, S.; Bots, P.; Roncal-Herrero, T.; Benning, L.G. The Role of Mg in the Crystallization of Monohydrocalcite. Geochim. Cosmochim. Acta 2014, 127, 204–220. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Graf, D.L. Crystallographic Tables for the Rhombohedral Carbonates. Am. Mineral. 1961, 46, 1283–1316. [Google Scholar]
- De Villiers, J.P.R. Crystal Structures of Aragonite, Strontianite, and Witherite. Am. Mineral. 1971, 56, 758–767. [Google Scholar]
- Swainson, I.P. The Structure of Monohydrocalcite and the Phase Composition of the Beachrock Deposits of Lake Butler and Lake Fellmongery, South Australia. Am. Mineral. 2008, 93, 1014–1018. [Google Scholar] [CrossRef]
- Mikuš, T.; Patúš, M.; Luptáková, J.; Bancík, T.; Biroň, A. Mineralogical Characteristics of the Secondary Calcium Carbonates Association from the Špania Dolina-The First Occurrence of Monohydrocalcite in Ore Deposits in Slovakia. Bull. Miner. Petrol. 2017, 25, 318–326. [Google Scholar]
- Hazen, R.M.; Hystad, G.; Golden, J.J.; Hummer, D.R.; Liu, C.; Downs, R.T.; Morrison, S.M.; Ralph, J.; Grew, E.S. Cobalt Mineral Ecology. Am. Mineral. 2017, 102, 108–116. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, W.; Zhang, D.; Liu, M.; Wang, J.; Meng, K.; Yang, C.; Jin, X.; Zhang, G. Recent Advances on Synthesis of CoCO3 with Controlled Morphologies. Chem. Rec. 2022, 22, e202200021. [Google Scholar] [CrossRef] [PubMed]
- Pertlik, F. Structures of Hydrothermally Synthesized Cobalt (II) Carbonate and Nickel (II) Carbonate. Acta Crystallogr. 1986, C42, 4–5. [Google Scholar] [CrossRef]
- Li, H.; Duan, X.; Ma, J.; Zheng, W. A Controllable Ionic Liquid-Assisted Hydrothermal Route to Prepare CoCO3 Crystals and Their Conversion to Porous Co3O4. Cryst. Res. Technol. 2012, 47, 25–30. [Google Scholar] [CrossRef]
- Bermanec, V.; Sijarić, G.; Kniewald, G.; Mandarino, J.A. Gaspéite and Associated Ni-Rich Minerals from Veins in Altered Ultrabasic Rocks from Duboštica, Bosnia and Herzegovina. Can. Mineral. 2000, 38, 1371–1376. [Google Scholar] [CrossRef]
- Seguin, M.K. The Stability of Gaspeite in Inert Atmospheres and in Air. Can. Mineral. 1973, 12, 26–32. [Google Scholar]
- Gaines, A.M.; Goldsmith, J.Κ. Crystal Chcmistry and Stability Relations in the System MgCO3—NiCO3. Z. Für Krist.-Cryst. Mater. 1971, 133, 432–444. [Google Scholar] [CrossRef]
- Seidel, H.; Ehrhardt, H.; Viswanathan, K.; Johannes, W. Darstellung, Struktur Und Eigenschaften von Kupfer(II)-Carbonat. ZAAC 1974, 410, 138–148. [Google Scholar] [CrossRef]
- Holme, E.A.; Henkes, G.A.; Tosca, N.J.; Rasbury, E.T.; Young, J.M.; Schaub, D.R.; Nekvasil, H.; Hurowitz, J.A. Experimental Constraints on Siderite Clumped Isotope Thermometry. Geochim. Cosmochim. Acta 2023, 343, 323–340. [Google Scholar] [CrossRef]
- Glynn, P. Solid-Solution Solubilities and Thermodynamics: Sulfates, Carbonates and Halides. Rev. Mineral. Geochem. 2000, 40, 481–511. [Google Scholar] [CrossRef]
- Gonzalez-Lopez, J.; Ruiz-Hernandez, S.E.; Fernandez-Gonzalez, A.; Jimenez, A.; Leeuw, N.H.D.; Grau-Crespo, R. Cobalt Incorporation in Calcite: Thermochemistry of (Ca,Co)CO3 Solid Solutions from Density Functional Theory Simulations. Geochim. Cosmochim. Acta 2014, 142, 205–216. [Google Scholar] [CrossRef]
- Maksimovic, Z.J. Resultats Preliminaires de l’examen Des Affleurements de Nickel Du Village de Ba, Pres de Ljig, Dans La Serbie Occidentale. Recl. Trav. Acad. Derbe Sci. 1952, 23, 21–48, 49–52. [Google Scholar]
- Maksimovic, Z.J.; Stupar, J. La Calcite Nickelomagnesienne et l’aragonite de Rujevac (Village de Ba, Serbie Occidentale). Recl. Trav. Acad. Derbe Sci. 1953, 33, 220–221. [Google Scholar]
- Ciurej, A.; Struska, M.; Wolska, A.; Szczerba, M.; Olszak, J. Copper-Bearing Mineralisation in the Upper Devonian Limestones: A Case Study from the Historical Teresa Adit in the Świętokrzyskie Mountains, Poland. Minerals 2023, 13, 54. [Google Scholar] [CrossRef]
- Rosenberg, P.E. Subsolidus Relations in the System CaCO3-MgCO3-FeCO3 between 350° and 550 °C. Am. Mineral. 1967, 52, 787–796. [Google Scholar]
- Radha, A.V.; Navrotsky, A. Thermodynamics of Carbonates. Rev. Mineral. Geochem. 2013, 77, 73–121. [Google Scholar] [CrossRef]
- Fosbøl, P.L.; Thomsen, K.; Stenby, E.H. Review and Recommended Thermodynamic Properties of FeCO3. Corros. Eng. Sci. Technol. 2010, 45, 115–135. [Google Scholar] [CrossRef]
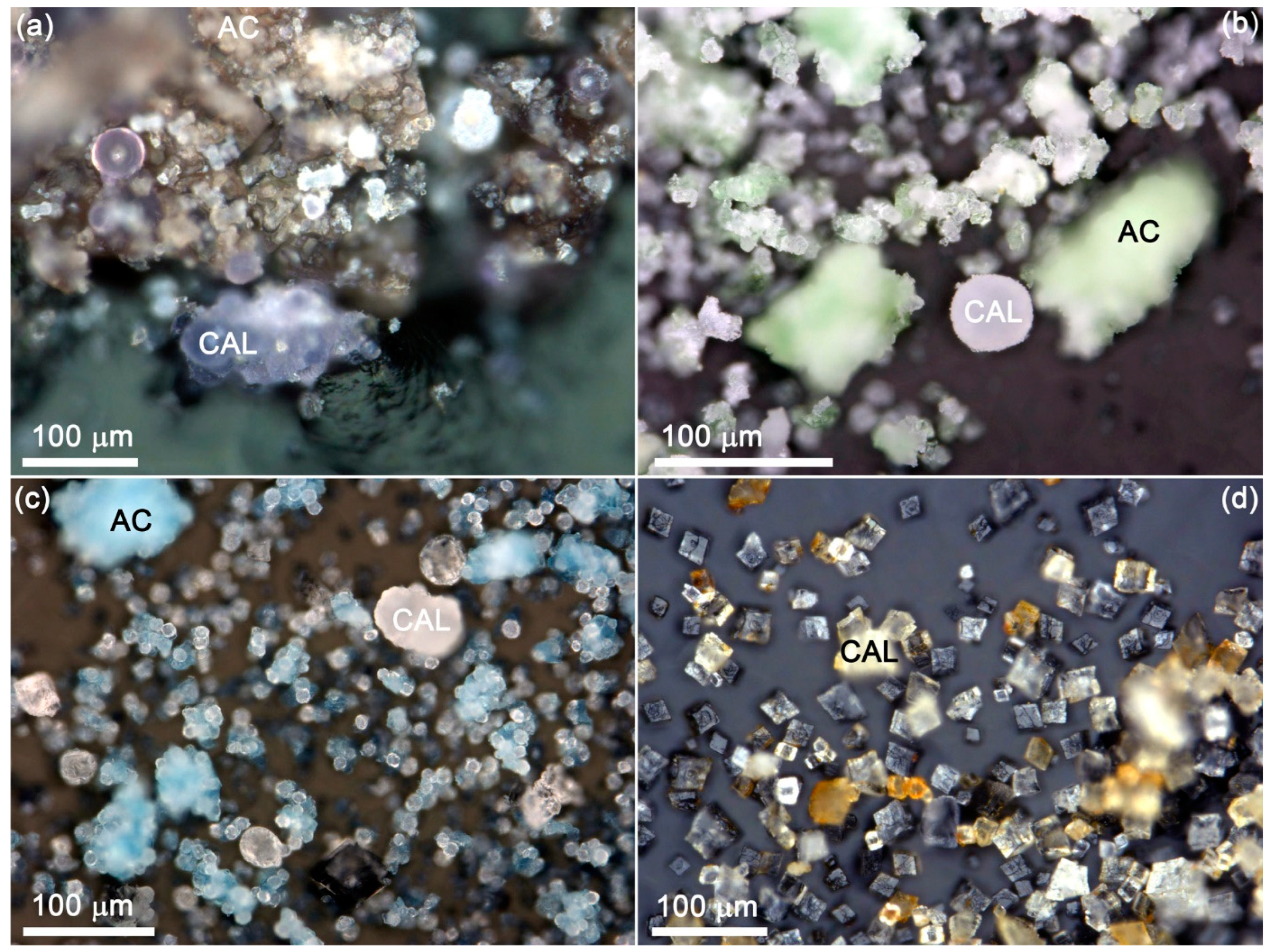
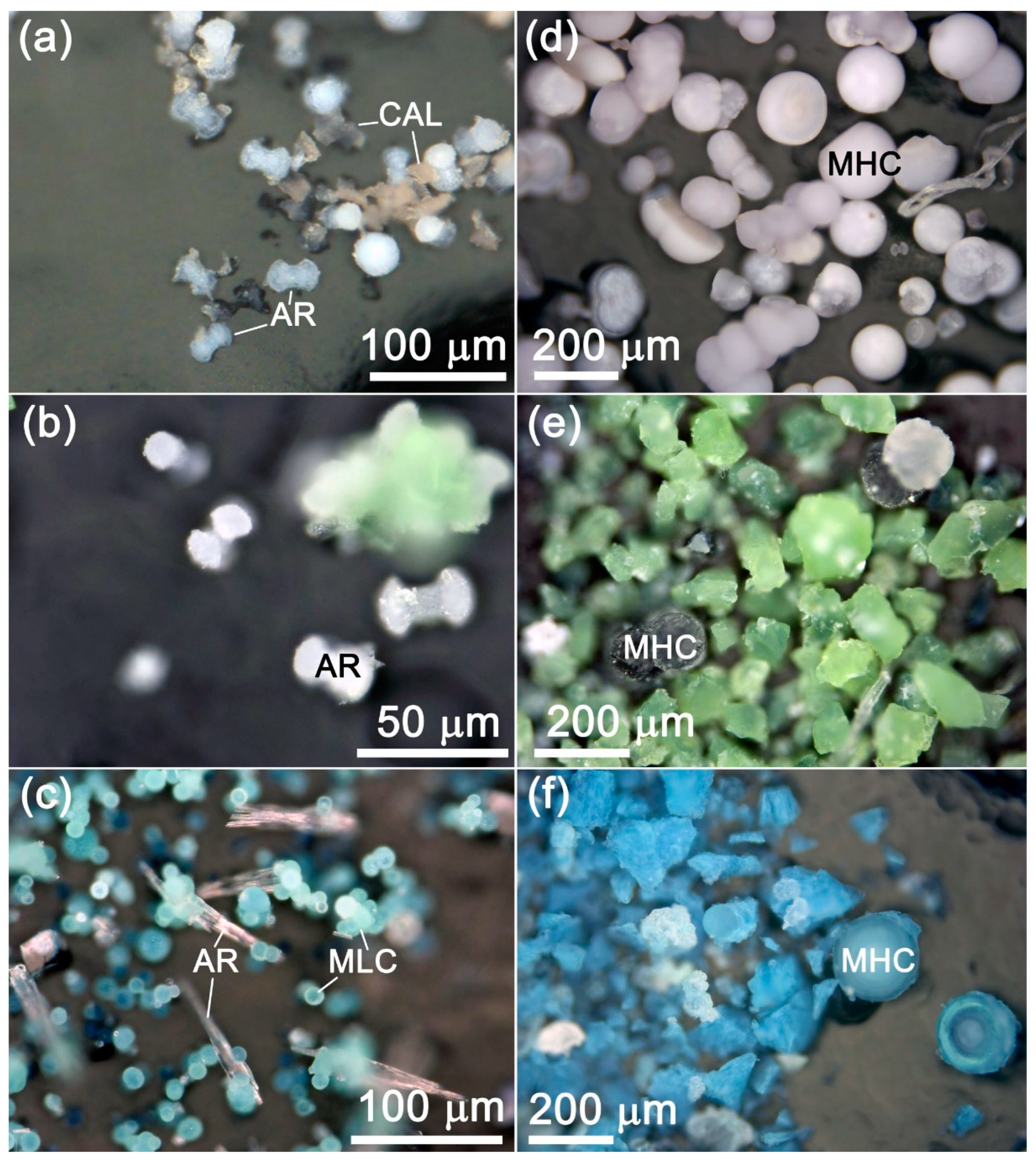
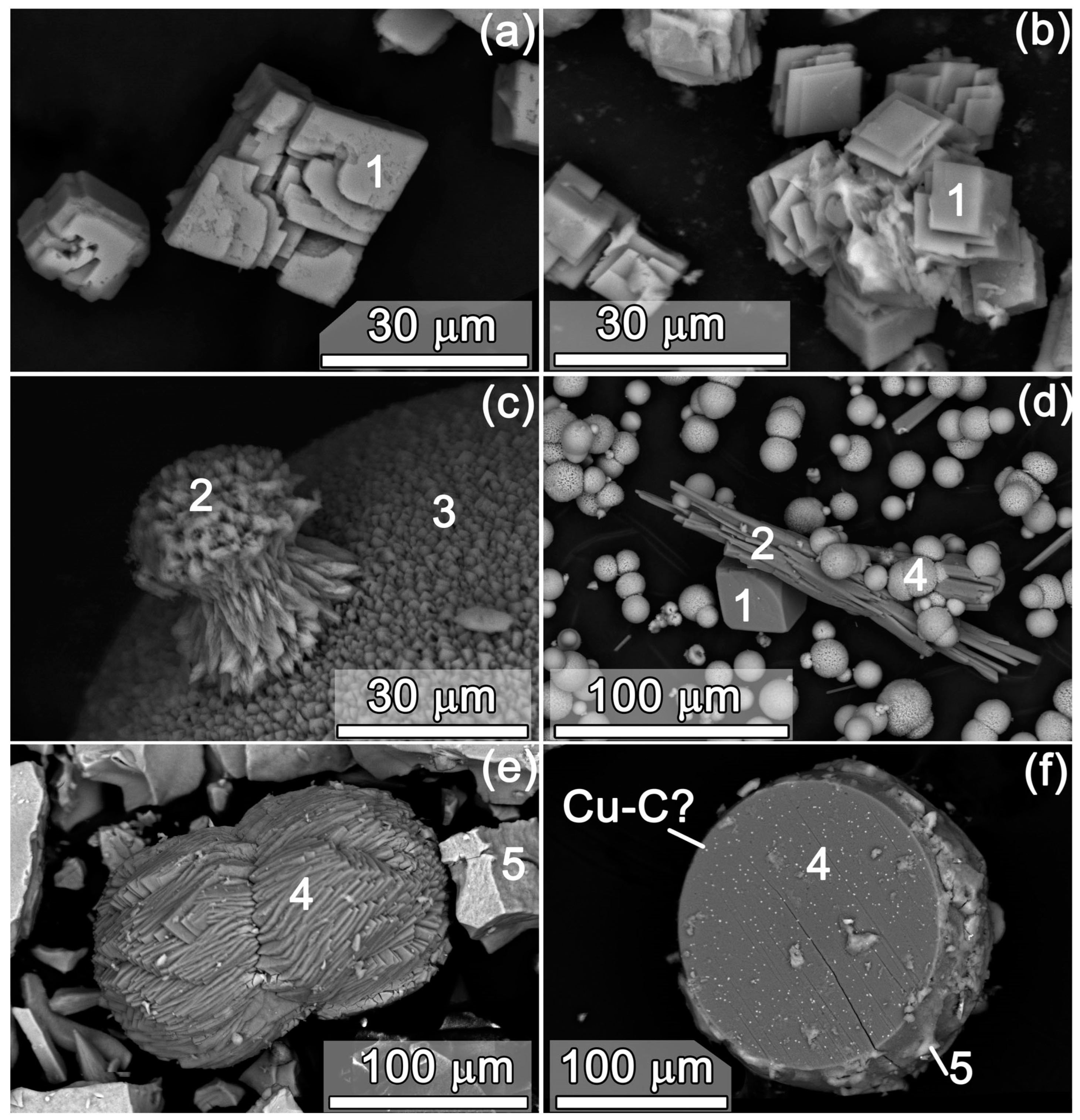
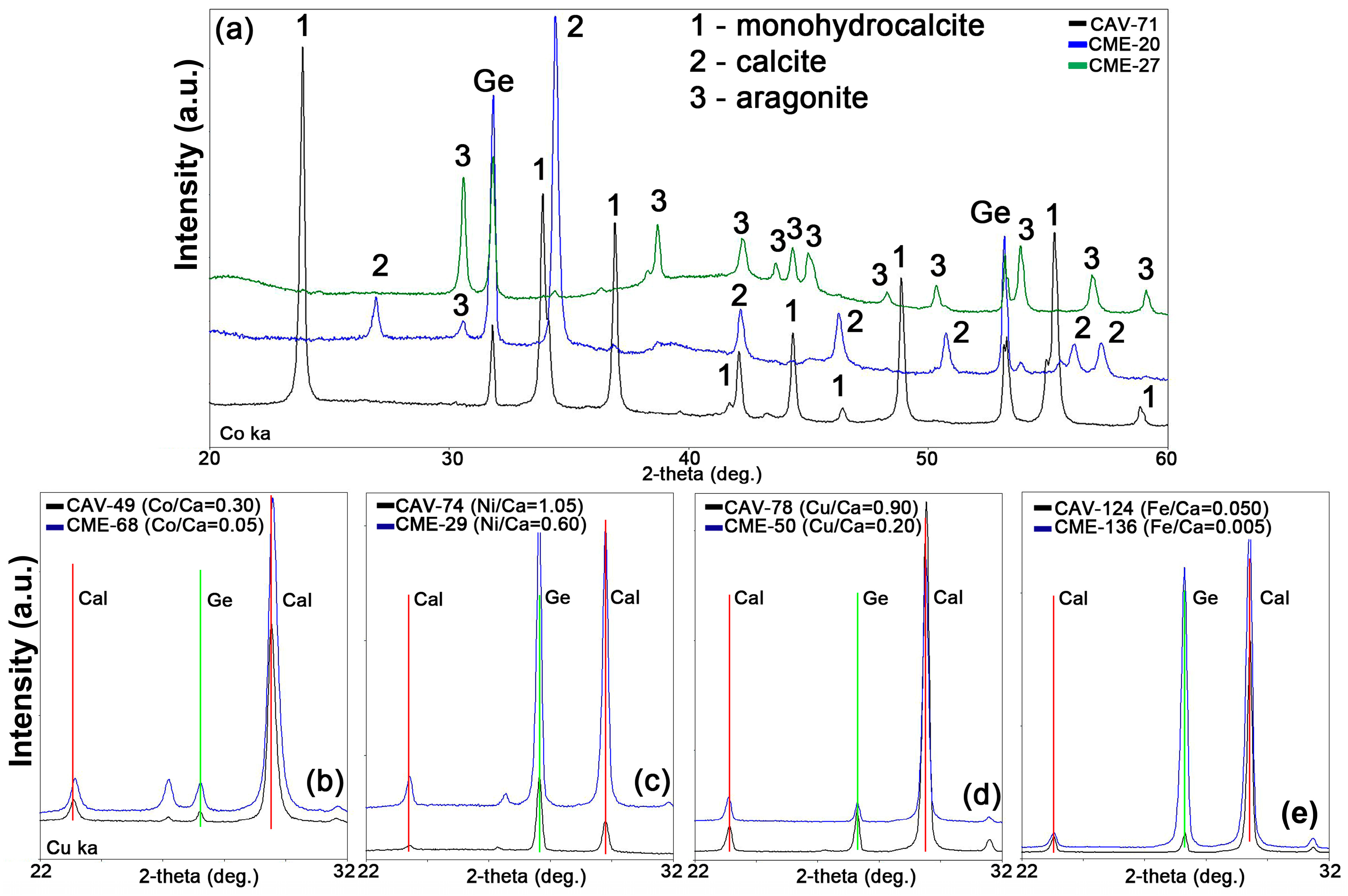
| No. | Sample Name (CAV) | Exposure Time, Day | Me2+, mmol/L | Ca, mmol/L | CO3, mmol/L | Me2+/Ca, Solution | pH Initial | pH Final |
|---|---|---|---|---|---|---|---|---|
| Me2+ = Co | ||||||||
| 1 | 43 | 21 | 10.000 | 10.000 | 20.000 | 1.000 | 7.50 | 7.95 |
| 2 | 42 | 21 | 5.000 | 10.000 | 20.000 | 0.500 | 8.55 | 8.68 |
| 3 | 49 | 158 | 3.320 | 10.000 | 20.000 | 0.300 | 8.75 | 8.73 |
| 4 | 52 | 158 | 4.640 | 14.000 | 28.000 | 0.300 | 10.05 | 9.90 |
| 5 | 46 | 57 | 2.000 | 10.000 | 20.000 | 0.200 | 8.60 | 8.20 |
| 6 | 54 | 31 | 2.800 | 14.000 | 28.000 | 0.200 | 9.44 | 9.33 |
| 7 | 55 | 31 | 2.100 | 14.000 | 28.000 | 0.150 | 9.54 | 8.80 |
| 8 | 41A | 34 | 2.800 | 14.000 | 28.000 | 0.120 | 8.50 | 8.32 |
| 9 | 97 | 103 | 1.612 | 14.000 | 28.000 | 0.115 | 9.38 | 9.65 |
| 10 | 71 | 57 | 1.540 | 14.000 | 28.000 | 0.110 | 9.80 | 9.73 |
| 11 | 96 | 103 | 1.472 | 14.000 | 28.000 | 0.105 | 10.03 | 9.75 |
| 12 | 69 | 37 | 1.400 | 14.000 | 28.000 | 0.100 | 9.23 | 8.41 |
| 13 | 70 | 37 | 1.400 | 14.000 | 28.000 | 0.100 | 8.77 | 8.54 |
| 14 | 72 | 57 | 1.400 | 14.000 | 28.000 | 0.100 | 9.73 | 9.53 |
| 15 | 56 | 31 | 1.400 | 14.000 | 28.000 | 0.100 | 9.61 | 9.58 |
| 16 | 95 | 103 | 1.332 | 14.000 | 28.000 | 0.095 | 9.96 | 9.84 |
| 17 | 73 | 57 | 1.260 | 14.000 | 28.000 | 0.090 | 9.50 | 9.53 |
| 18 | 40A | 34 | 1.200 | 14.000 | 28.000 | 0.080 | 9.15 | 8.93 |
| 19 | 68 | 37 | 0.700 | 14.000 | 28.000 | 0.050 | 8.98 | 8.52 |
| 20 | 67 | 37 | 0.700 | 14.000 | 28.000 | 0.050 | 9.37 | 8.62 |
| 21 | 53 | 31 | 0.700 | 14.000 | 28.000 | 0.050 | 9.63 | 9.76 |
| Me2+ = Ni | ||||||||
| 22 | 57 | 31 | 16.000 | 10.000 | 20.000 | 1.600 | 7.55 | 7.82 |
| 23 | 58 | 31 | 14.000 | 14.000 | 28.000 | 1.400 | 7.83 | 7.75 |
| 24 | 59 | 31 | 12.000 | 10.000 | 20.000 | 1.200 | 7.94 | 8.06 |
| 25 | 66 | 37 | 11.000 | 10.000 | 20.000 | 1.100 | 8.08 | 8.20 |
| 26 | 74 | 57 | 10.500 | 10.000 | 20.000 | 1.050 | 8.90 | 9.33 |
| 27 | 45 | 57 | 10.000 | 10.000 | 20.000 | 1.000 | 8.75 | 9.10 |
| 28 | 98 | 114 | 12.000 | 12.000 | 24.000 | 1.000 | 8.85 | 9.31 |
| 29 | 75 | 57 | 10.000 | 10.000 | 20.000 | 1.000 | 8.45 | 9.02 |
| 30 | 76 | 57 | 9.500 | 10.000 | 20.000 | 0.950 | 8.50 | 9.09 |
| 31 | 65 | 37 | 9.000 | 10.000 | 20.000 | 0.900 | 8.41 | 7.75 |
| 32 | 60 | 31 | 8.000 | 10.000 | 20.000 | 0.800 | 8.63 | 8.33 |
| 33 | 51 | 158 | 7.000 | 14.000 | 28.000 | 0.500 | 8.80 | 8.46 |
| Me2+ = Cu | ||||||||
| 34 | 62 | 31 | 15.000 | 10.000 | 20.000 | 1.500 | 5.44 | 7.49 |
| 35 | 64 | 37 | 11.000 | 10.000 | 20.000 | 1.100 | 6.35 | 7.87 |
| 36 | 61 | 31 | 10.000 | 10.000 | 20.000 | 1.000 | 6.56 | 8.10 |
| 37 | 77 | 57 | 9.500 | 10.000 | 20.000 | 0.950 | 6.60 | 9.06 |
| 38 | 63 | 37 | 9.000 | 10.000 | 20.000 | 0.900 | 6.93 | 7.74 |
| 39 | 99 | 114 | 10.800 | 12.000 | 24.000 | 0.900 | 7.50 | 9.34 |
| 40 | 78 | 57 | 9.000 | 10.000 | 20.000 | 0.900 | 6.75 | 9.25 |
| 41 | 79 | 57 | 8.500 | 10.000 | 20.000 | 0.850 | 6.80 | 9.26 |
| 42 | 44 | 57 | 5.000 | 10.000 | 20.000 | 0.500 | 6.95 | 8.50 |
| 43 | 47 | 158 | 3.000 | 10.000 | 20.000 | 0.300 | 7.48 | 8.87 |
| 44 | 50 | 158 | 2.800 | 14.000 | 28.000 | 0.200 | 7.55 | 9.03 |
| Me2+ = Fe | ||||||||
| 45 | 154 | 44 | 5.000 | 10.000 | 20.000 | 0.500 | 9.30 | 10.49 |
| 46 | 153 | 44 | 4.000 | 10.000 | 20.000 | 0.400 | 9.50 | 10.40 |
| 47 | 152 | 44 | 3.000 | 10.000 | 20.000 | 0.300 | 10.15 | 9.84 |
| 48 | 151 | 44 | 2.000 | 10.000 | 20.000 | 0.200 | 10.35 | 10.81 |
| 49 | 150 | 44 | 1.000 | 10.000 | 20.000 | 0.100 | 10.50 | 10.69 |
| 50 | 124 | 66 | 0.500 | 10.000 | 20.000 | 0.050 | 11.15 | 10.40 |
| 51 | 123 | 66 | 0.300 | 10.000 | 20.000 | 0.030 | 11.40 | 11.02 |
| 52 | 138 | 90 | 0.252 | 10.000 | 20.000 | 0.025 | 11.05 | 9.70 |
| 53 | 137 | 90 | 0.152 | 10.000 | 20.000 | 0.015 | 11.11 | 9.99 |
| 54 | 122 | 66 | 0.100 | 10.000 | 20.000 | 0.010 | 11.20 | 10.09 |
| 55 | 136 | 90 | 0.052 | 10.000 | 20.000 | 0.005 | 11.05 | 9.78 |
| No. | Sample Name (CME) | Exposure Time, Day | Me2+, mmol/L | Ca, mmol/L | CO3, mmol/L | Me2+/Ca, Solution | pH Initial | pH Final |
|---|---|---|---|---|---|---|---|---|
| Me2+ = Co | ||||||||
| 1 | 20 | 42 | 5.000 | 10.000 | 20.000 | 0.500 | 9.74 | 8.21 |
| 2 | 21 | 42 | 4.000 | 10.000 | 20.000 | 0.400 | 9.92 | 9.53 |
| 3 | 1 | 30 | 5.000 | 15.000 | 30.000 | 0.300 | 9.50 | 8.40 |
| 4 | 25 | 44 | 2.000 | 10.000 | 20.000 | 0.200 | 10.36 | 9.74 |
| 5 | 26 | 44 | 1.000 | 10.000 | 20.000 | 0.100 | 10.45 | 8.98 |
| Me2+ = Ni | ||||||||
| 6 | 22 | 42 | 13.000 | 10.000 | 20.000 | 1.300 | 7.93 | 7.82 |
| 7 | 27 | 44 | 11.000 | 10.000 | 20.000 | 1.100 | 8.18 | 8.24 |
| 8 | 2 | 30 | 15.000 | 15.000 | 30.000 | 1.000 | 8.20 | 8.00 |
| 9 | 28 | 44 | 9.000 | 10.000 | 20.000 | 0.900 | 8.40 | 7.95 |
| 10 | 23 | 42 | 8.000 | 10.000 | 20.000 | 0.800 | 8.31 | 7.89 |
| 11 | 29 | 44 | 6.000 | 10.000 | 20.000 | 0.600 | 9.34 | 8.52 |
| Me2+ = Cu | ||||||||
| 12 | 30 | 44 | 12.000 | 10.000 | 20.000 | 1.200 | 6.27 | 8.13 |
| 13 | 31 | 44 | 9.000 | 10.000 | 20.000 | 0.900 | 6.91 | 8.06 |
| 14 | 32 | 44 | 5.400 | 10.000 | 20.000 | 0.700 | 8.80 | 7.99 |
| 15 | 24 | 42 | 6.000 | 10.000 | 20.000 | 0.600 | 8.72 | 7.74 |
| Me2+ = Fe | ||||||||
| 16 | 111 | 41 | 5.000 | 10.000 | 20.000 | 0.500 | 9.20 | 9.66 |
| 17 | 110 | 40 | 4.000 | 10.000 | 20.000 | 0.400 | 9.50 | 10.00 |
| 18 | 109 | 40 | 3.000 | 10.000 | 20.000 | 0.300 | 10.20 | 10.37 |
| 19 | 108 | 40 | 2.000 | 10.000 | 20.000 | 0.200 | 10.40 | 10.60 |
| 20 | 107 | 40 | 1.000 | 10.000 | 20.000 | 0.100 | 10.60 | 10.99 |
| 21 | 81 | 64 | 0.500 | 10.000 | 20.000 | 0.050 | 10.75 | 11.15 |
| 22 | 80 | 64 | 0.300 | 10.000 | 20.000 | 0.030 | 10.50 | 11.07 |
| 23 | 95 | 94 | 0.252 | 10.000 | 20.000 | 0.025 | 11.20 | 11.14 |
| 24 | 94 | 94 | 0.152 | 10.000 | 20.000 | 0.015 | 11.03 | 10.77 |
| 25 | 79 | 64 | 0.100 | 10.000 | 20.000 | 0.010 | 10.95 | 11.36 |
| 26 | 93 | 94 | 0.052 | 10.000 | 20.000 | 0.005 | 11.15 | 11.39 |
| No. * | Sample Name (CAV) | Me2+/Ca, Solution | Crystalline Phase Composition, % | Amorphous Phase | Unit Cell Parameters, Å | ||
|---|---|---|---|---|---|---|---|
| a | b | c | |||||
| Me2+ = Co | |||||||
| 1 | 43 | 1.000 | Arg 91 Cal 9 | □ | 4.959(2) | 7.964(3) | 5.743(2) |
| 2 | 42 | 0.500 | Cal 81 Arg 19 | + | |||
| 3 | 49 | 0.300 | Cal 90 Arg 10 | - | 4.970(3) | =a | 16.930(2) |
| 4 | 52 | 0.300 | Cal 97 Arg 3 | - | 4.960(3) | =a | 16.876(2) |
| 5 | 46 | 0.200 | Cal 100 | - | 4.979(3) | =a | 16.981(2) |
| 6 | 54 | 0.200 | Cal 78 Arg 22 | + | |||
| 7 | 55 | 0.150 | Cal 63 Arg 37 | + | |||
| 8 | 41A | 0.120 | Mhcal 86 Arg 14 | + | |||
| 9 | 97 | 0.115 | Cal 72 Arg 28 | ± | |||
| 10 | 71 | 0.110 | Mhcal 100 | - | 10.553(3) | =a | 7.542(2) |
| 11 | 96 | 0.105 | Mhcal 70 Cal 27 Arg 3 | ± | |||
| 12 | 69 | 0.100 | Arg 65 Mhcal 23 Cal 12 | + | |||
| 13 | 70 | 0.100 | Mhcal 98 Arg 2 | ± | 10.536(4) | =a | 7.534(2) |
| 14 | 72 | 0.100 | Mhcal 100 | ± | 10.545(3) | =a | 7.539(2) |
| 15 | 56 | 0.100 | Cal 66 Arg 34 | + | |||
| 16 | 95 | 0.095 | Cal 77 Arg 23 | - | |||
| 17 | 73 | 0.090 | Mhcal 52 Cal 28 Arg 20 | □ | |||
| 18 | 40A | 0.080 | Cal 60 Arg 35 Mhcal 5 | + | |||
| 19 | 68 | 0.050 | Cal 81 Arg 19 | - | |||
| 20 | 67 | 0.050 | Cal 95 Arg 5 | - | 4.966(2) | =a | 16.911(3) |
| 21 | 53 | 0.050 | Cal 94 Arg 6 | - | 4.969(2) | =a | 16.926(3) |
| Me2+ = Ni | |||||||
| 22 | 57 | 1.600 | - | ■ | |||
| 23 | 58 | 1.400 | - | ■ | |||
| 24 | 59 | 1.200 | Arg 10 Cal 90 | ■ | |||
| 25 | 66 | 1.100 | Arg 66 Cal 34 | ■ | |||
| 26 | 74 | 1.050 | Cal 90 Arg 10 | + | 4.989(3) | =a | 17.054(4) |
| 27 | 45 | 1.000 | Mhcal 64 Cal36 | ■ | |||
| 28 | 98 | 1.000 | Cal 85 Mhcal 15 | ± | |||
| 29 | 75 | 1.000 | Cal 100 | ■ | |||
| 30 | 76 | 0.950 | Cal 99 Arg 1 | ± | 4.987(3) | =a | 17.040(4) |
| 31 | 65 | 0.900 | Cal 84 Arg 16 | + | |||
| 32 | 60 | 0.800 | Cal 69 Arg 31 | ± | |||
| 33 | 51 | 0.500 | Cal 96 Mhcal 4 | ± | 4.985(3) | =a | 17.020(3) |
| Me2+ = Cu | |||||||
| 34 | 62 | 1.500 | Mlc 100 | ± | 9.536(2) | 11.870(4) | 3.270(1) |
| 35 | 64 | 1.100 | Mlc 78 Arg 22 | + | |||
| 36 | 61 | 1.000 | Amorphous/Mhcal | ■ | |||
| 37 | 77 | 0.950 | Cal 87 Mhcal 7 Arg 6 | + | |||
| 38 | 63 | 0.900 | Mhcal 100 | □ | 10.547(4) | =a | 7.549(2) |
| 39 | 99 | 0.900 | Cal 54 Mhcal 46 | □ | |||
| 40 | 78 | 0.900 | Cal 96 Arg 4 | - | 4.971(3) | =a | 16.991(3) |
| 41 | 79 | 0.850 | Cal 86 Arg 14 | - | 4.979(3) | =a | 17.022(4) |
| 42 | 44 | 0.500 | Cal 100 | - | 4.976(2) | =a | 17.012(3) |
| 43 | 47 | 0.300 | Cal 100 | - | 4.977(2) | =a | 17.004(3) |
| 44 | 50 | 0.200 | Cal 100 | - | 4.976(2) | =a | 16.989(3) |
| Me2+ = Fe | |||||||
| 45 | 154 | 0.500 | Cal 72 Gth 28 | ± | |||
| 46 | 153 | 0.400 | Cal 78 Gth 22 | ± | |||
| 47 | 152 | 0.300 | Cal 82 Mgt 18 | ± | |||
| 48 | 151 | 0.200 | Cal 84 Mgt 16 | - | |||
| 49 | 150 | 0.100 | Cal 100 | - | 4.993(2) | =a | 17.051(3) |
| 50 | 124 | 0.050 | Cal 100 | - | 4.992(2) | =a | 17.054(3) |
| 51 | 123 | 0.030 | Cal 100 | - | 4.991(2) | =a | 17.050(3) |
| 52 | 138 | 0.025 | Cal 100 | - | 4.996(3) | =a | 17.060(4) |
| 53 | 137 | 0.015 | Cal 100 | - | 4.992(2) | =a | 17.051(3) |
| 54 | 122 | 0.010 | Cal 100 | - | 4.991(2) | =a | 17.051(3) |
| 55 | 136 | 0.005 | Cal 100 | - | 4.995(2) | =a | 17.060(3) |
| No * | Sample Name (CME) | Me2+/Ca, Solution | Crystalline Phase Composition, % | Amorphous Phase | Unit Cell Parameters, Å | ||
|---|---|---|---|---|---|---|---|
| a | b | c | |||||
| Me2+ = Co | |||||||
| 1 | 20 | 0.500 | Cal 88 Arg 12 | ± | |||
| 2 | 21 | 0.400 | Cal 89 Arg 11 | ± | |||
| 3 | 1 | 0.300 | Cal 88 Arg 12 | ± | |||
| 4 | 25 | 0.200 | Cal 97 Arg 3 | ± | 4.987(3) | =a | 17.016(2) |
| 5 | 26 | 0.100 | Cal 95 Arg 5 | - | 4.982(3) | =a | 17.010(3) |
| Me2+ = Ni | |||||||
| 6 | 22 | 1.300 | - | ■ | |||
| 7 | 27 | 1.100 | Arg 100 | □ | 4.966(2) | 7.975(4) | 5.756(2) |
| 8 | 2 | 1.000 | Arg 90 Cal 10 | □ | 4.964(2) | 7.970(3) | 5.752(2) |
| 9 | 28 | 0.900 | Arg 78 Cal 22 | + | |||
| 10 | 23 | 0.800 | Cal 61 Arg 39 | + | |||
| 11 | 29 | 0.600 | Cal 91 Arg 9 | - | 4.986 (3) | =a | 17.039(4) |
| Me2+ = Cu | |||||||
| 12 | 30 | 1.200 | Mlc 88 Arg 12 | - | |||
| 13 | 31 | 0.900 | Mlc 65 Arg 35 | ± | |||
| 14 | 32 | 0.700 | Mlc 47 Cal 38 Arg 15 | - | |||
| 15 | 24 | 0.600 | Cal 60 Mlc 34 Arg 6 | ± | |||
| Me2+ = Fe | |||||||
| 16 | 111 | 0.500 | Cal 63 Gth 37 | ± | |||
| 17 | 110 | 0.400 | Cal 83 Gth 17 | - | |||
| 18 | 109 | 0.300 | Cal 84 Mgt 16 | - | |||
| 19 | 108 | 0.200 | Cal 79 Mgt 21 | - | |||
| 20 | 107 | 0.100 | Cal 82 Mgt 18 | - | |||
| 21 | 81 | 0.050 | Cal 100 | + | 4.989(3) | =a | 17.058(4) |
| 22 | 80 | 0.030 | Cal 100 | + | 4.991(3) | =a | 17.047(4) |
| 23 | 95 | 0.025 | Cal 100 | + | 4.986(4) | =a | 17.048(3) |
| 24 | 94 | 0.015 | Cal 100 | - | 4.996(4) | =a | 17.058(4) |
| 25 | 79 | 0.010 | Cal 100 | - | 4.991(4) | =a | 17.060(4) |
| 26 | 93 | 0.005 | Cal 100 | - | 4.992(3) | =a | 17.052(4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vereshchagin, O.S.; Chernyshova, I.A.; Kuz’mina, M.A.; Frank-Kamenetskaya, O.V. Calcium Carbonate Precipitation Behavior in the System Ca-Me2+-CO3-H2O (Me2+ = Co, Ni, Cu, Fe): Ion Incorporation, Effect of Temperature and Aging. Minerals 2023, 13, 1497. https://doi.org/10.3390/min13121497
Vereshchagin OS, Chernyshova IA, Kuz’mina MA, Frank-Kamenetskaya OV. Calcium Carbonate Precipitation Behavior in the System Ca-Me2+-CO3-H2O (Me2+ = Co, Ni, Cu, Fe): Ion Incorporation, Effect of Temperature and Aging. Minerals. 2023; 13(12):1497. https://doi.org/10.3390/min13121497
Chicago/Turabian StyleVereshchagin, Oleg S., Irina A. Chernyshova, Maria A. Kuz’mina, and Olga V. Frank-Kamenetskaya. 2023. "Calcium Carbonate Precipitation Behavior in the System Ca-Me2+-CO3-H2O (Me2+ = Co, Ni, Cu, Fe): Ion Incorporation, Effect of Temperature and Aging" Minerals 13, no. 12: 1497. https://doi.org/10.3390/min13121497
APA StyleVereshchagin, O. S., Chernyshova, I. A., Kuz’mina, M. A., & Frank-Kamenetskaya, O. V. (2023). Calcium Carbonate Precipitation Behavior in the System Ca-Me2+-CO3-H2O (Me2+ = Co, Ni, Cu, Fe): Ion Incorporation, Effect of Temperature and Aging. Minerals, 13(12), 1497. https://doi.org/10.3390/min13121497








