Uranium in Fluorite, a Case Study: The La Azul Fluorspar Deposit, Taxco, Guerrero, Mexico
Abstract
1. Introduction
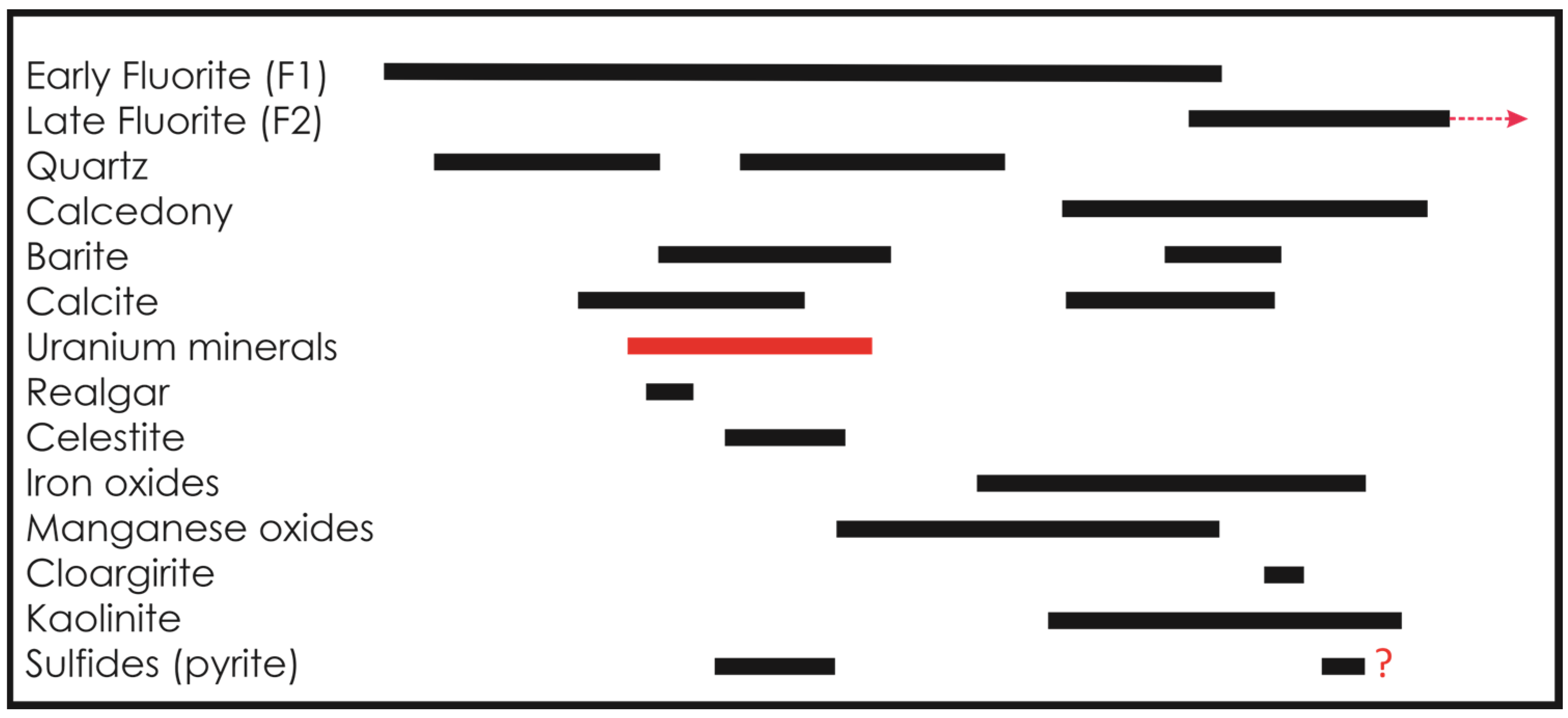
2. Geological Setting and Mineral Paragenesis
3. Materials and Methods
3.1. Bulk Fluorspar Uranium Concentration via ICP-MS
3.2. Alpha-Autoradiography
3.3. Microprobe Analysis of Uranium Inclusions
3.4. Micro-Raman Spectroscopy
3.5. X-ray Diffraction
3.6. Measurement of Fluorine in Volcanic Rocks
3.7. Microthermometry of Fluid Inclusions in Uranium-Rich Fluorite
4. Results
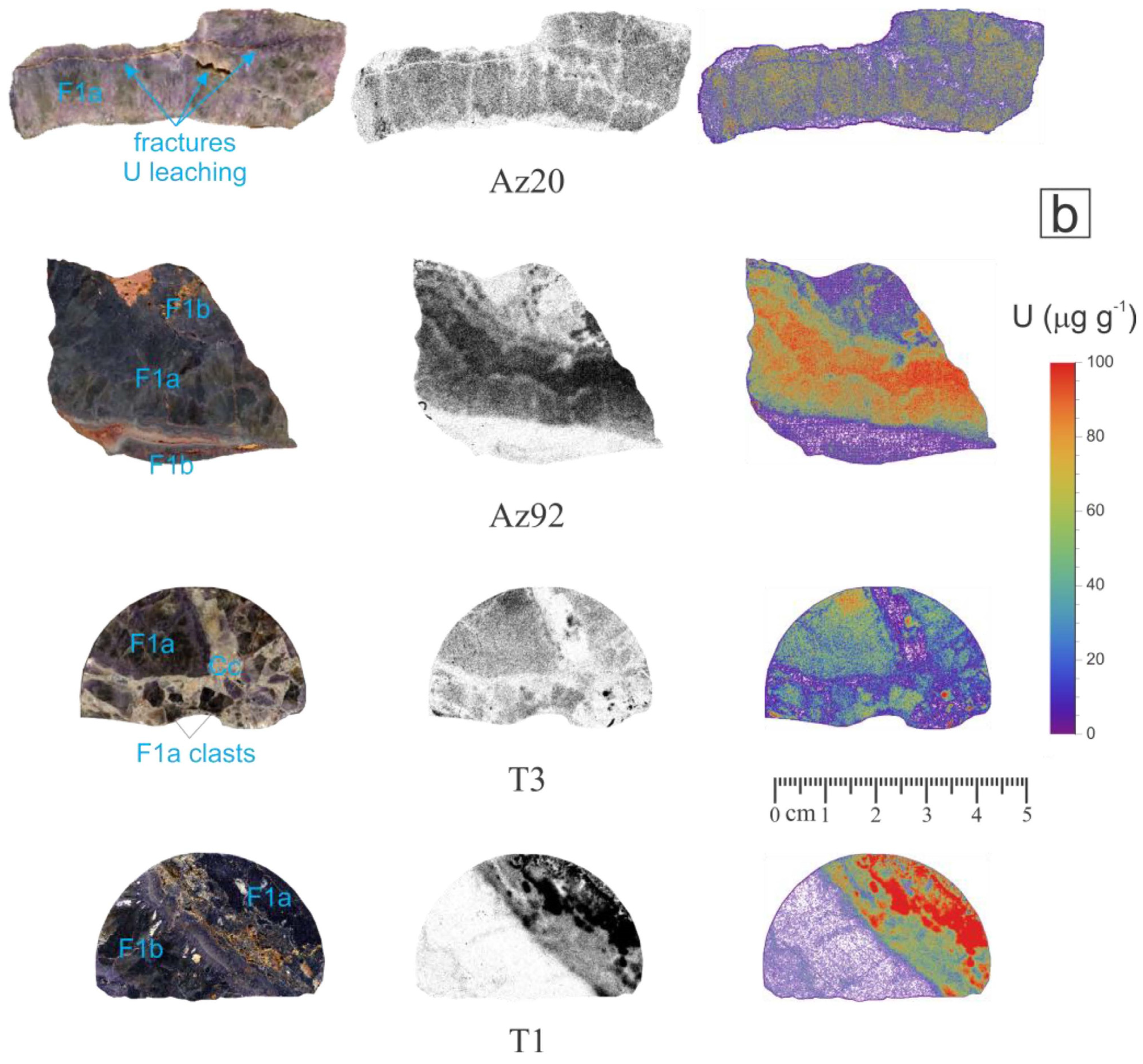
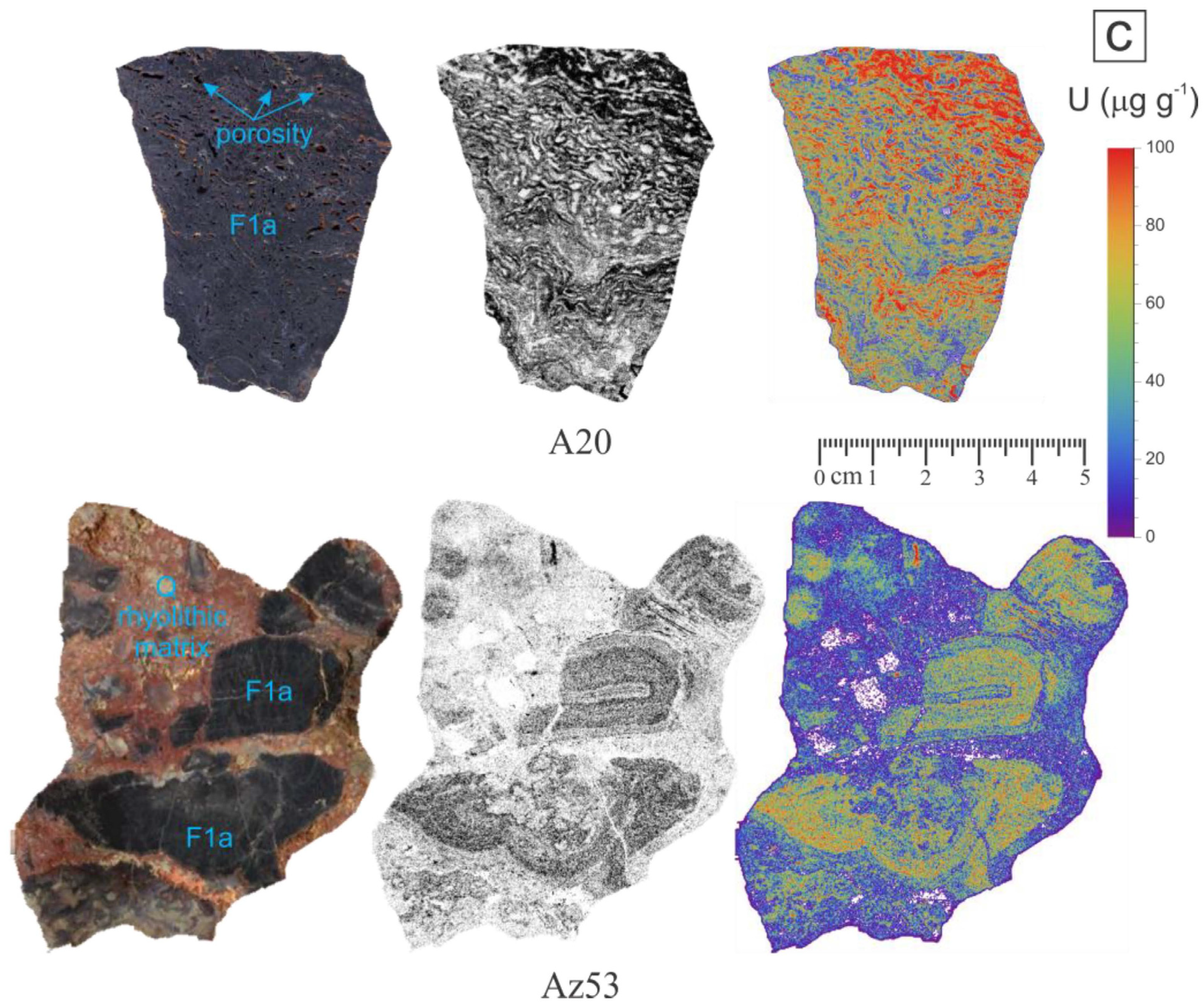
4.1. Uranium Concentration and Distribution in Fluorspar Samples
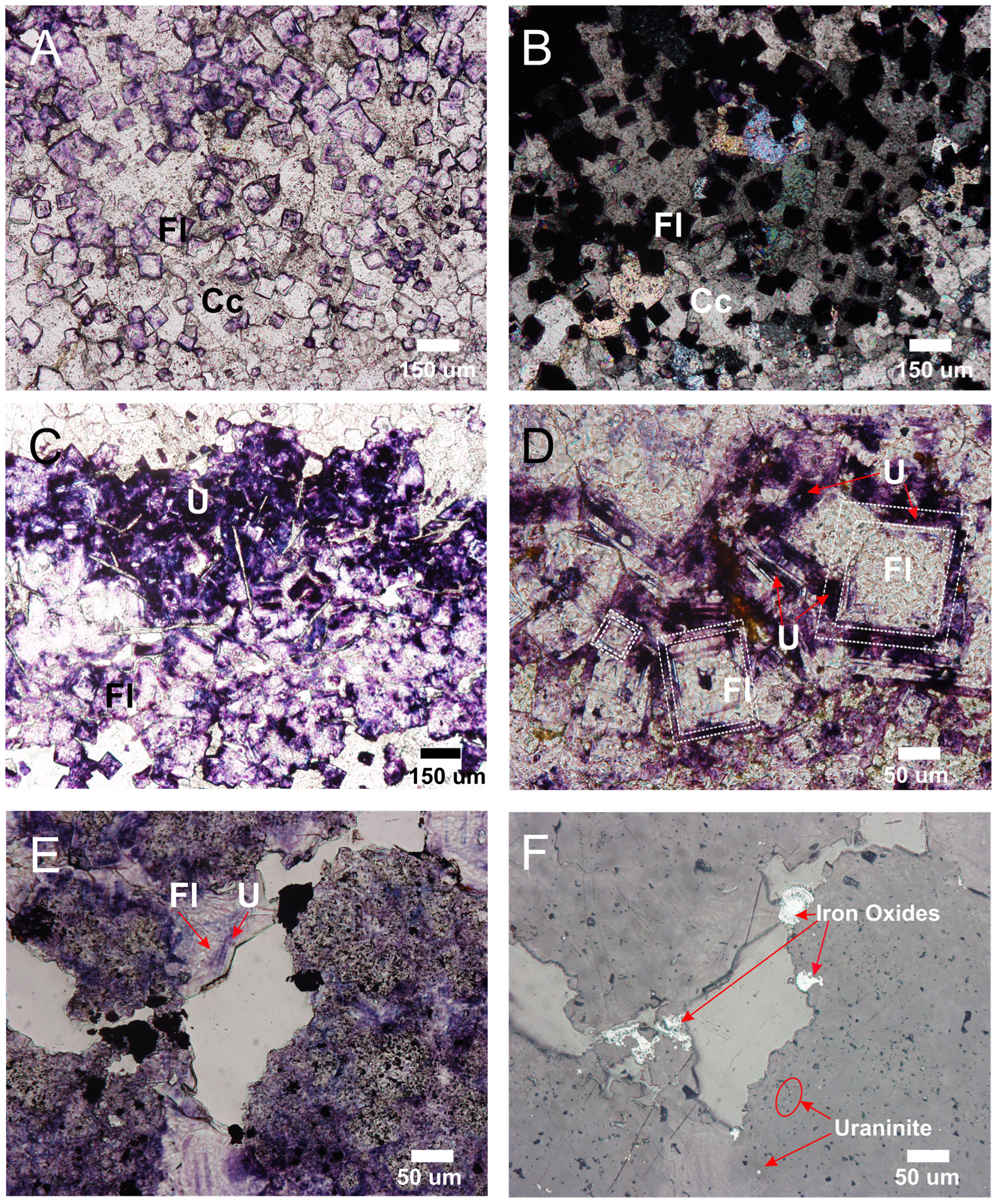
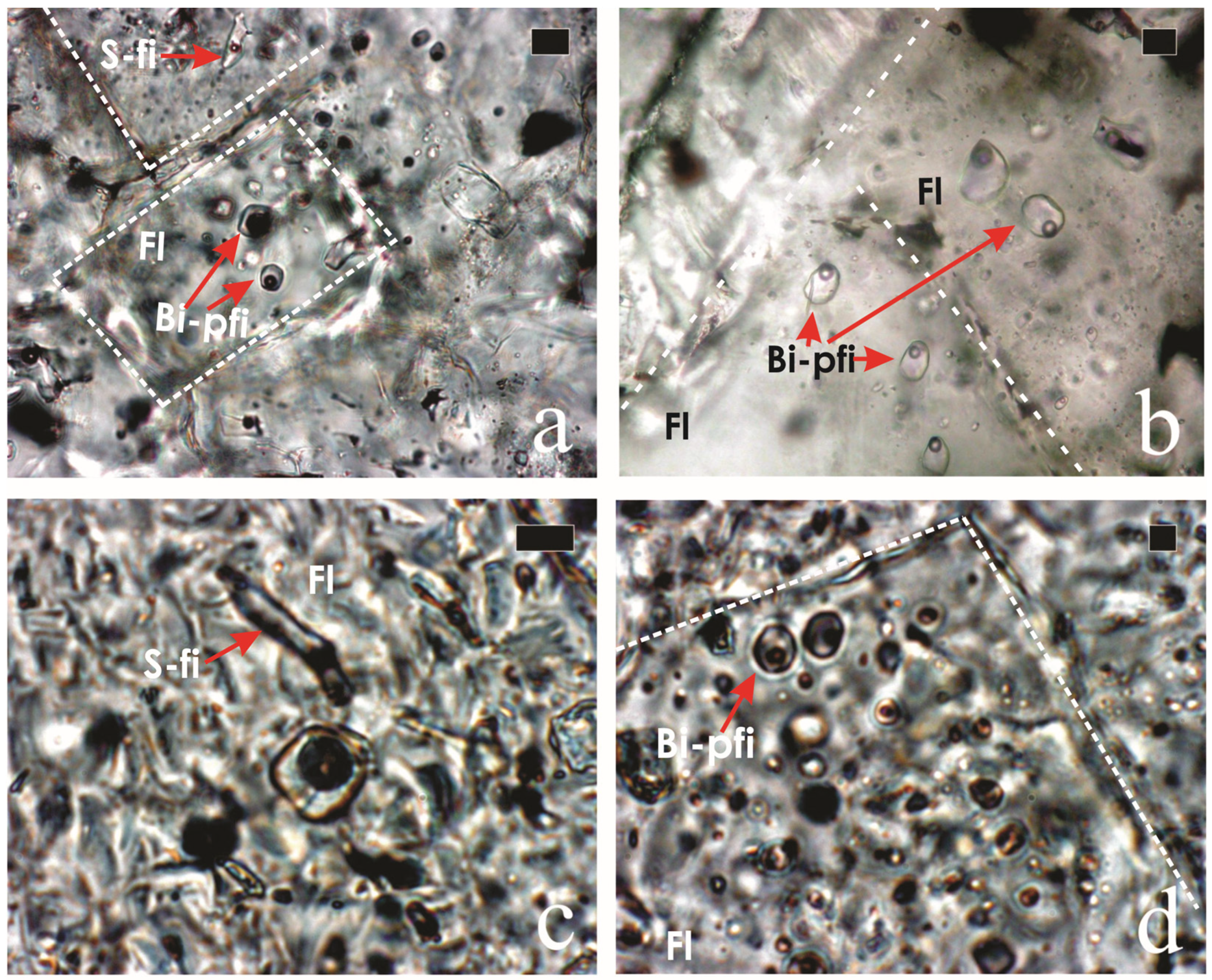
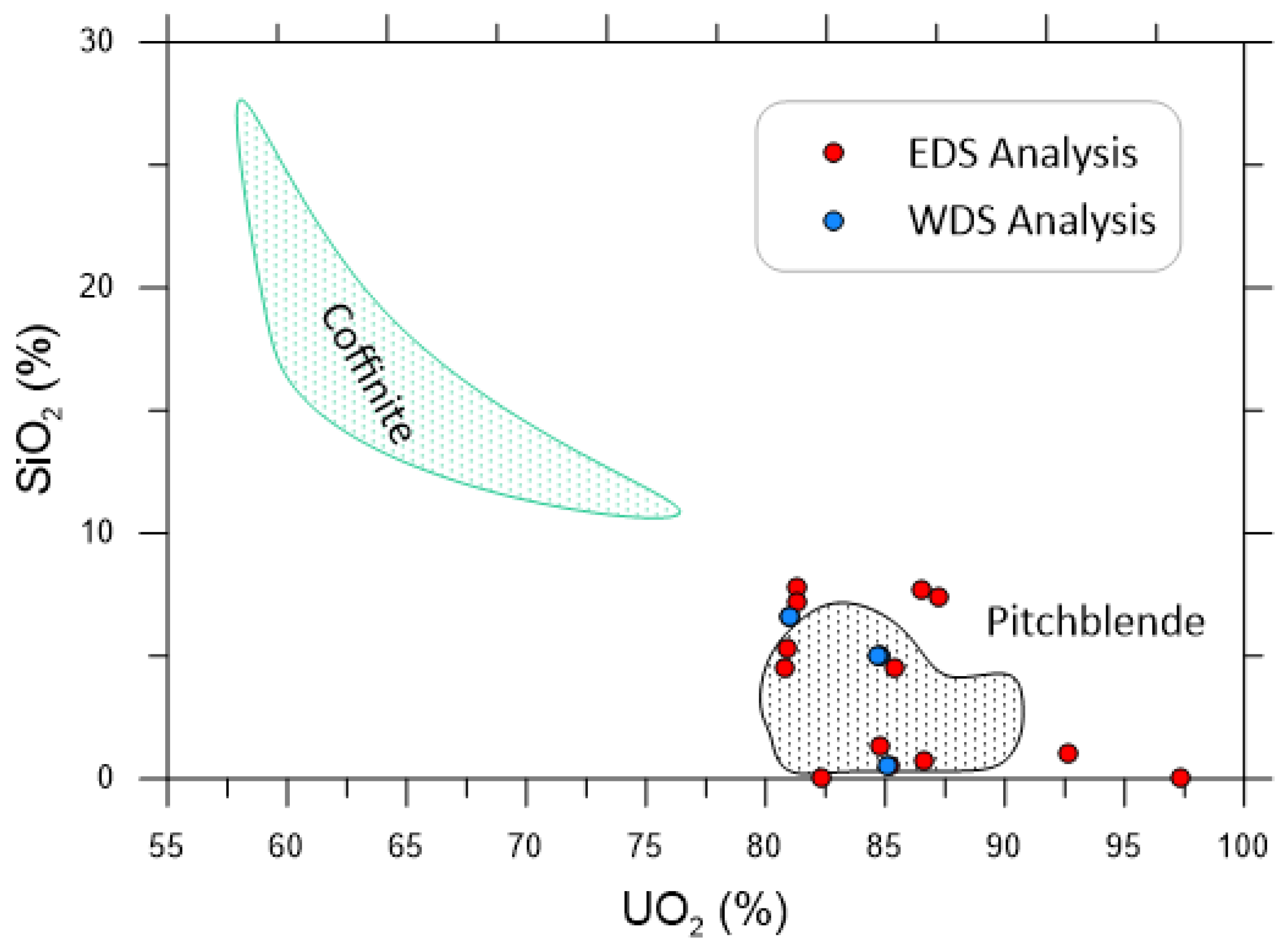
4.2. Composition (EDS and WDS) and Textural Characteristics of Uranium-Rich Inclusions in Fluorite
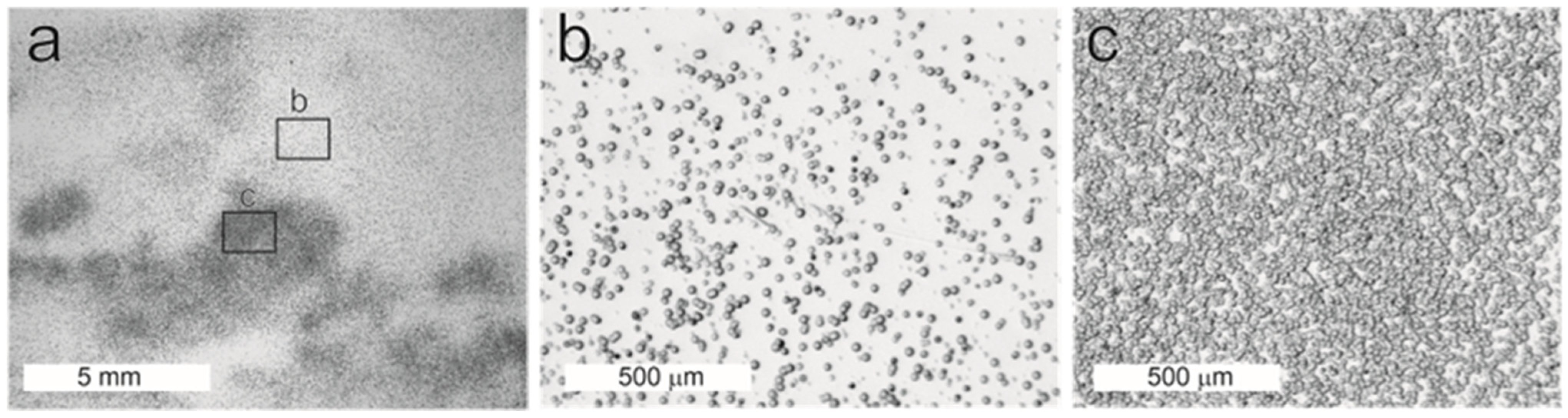
4.3. Alpha-Autoradiography
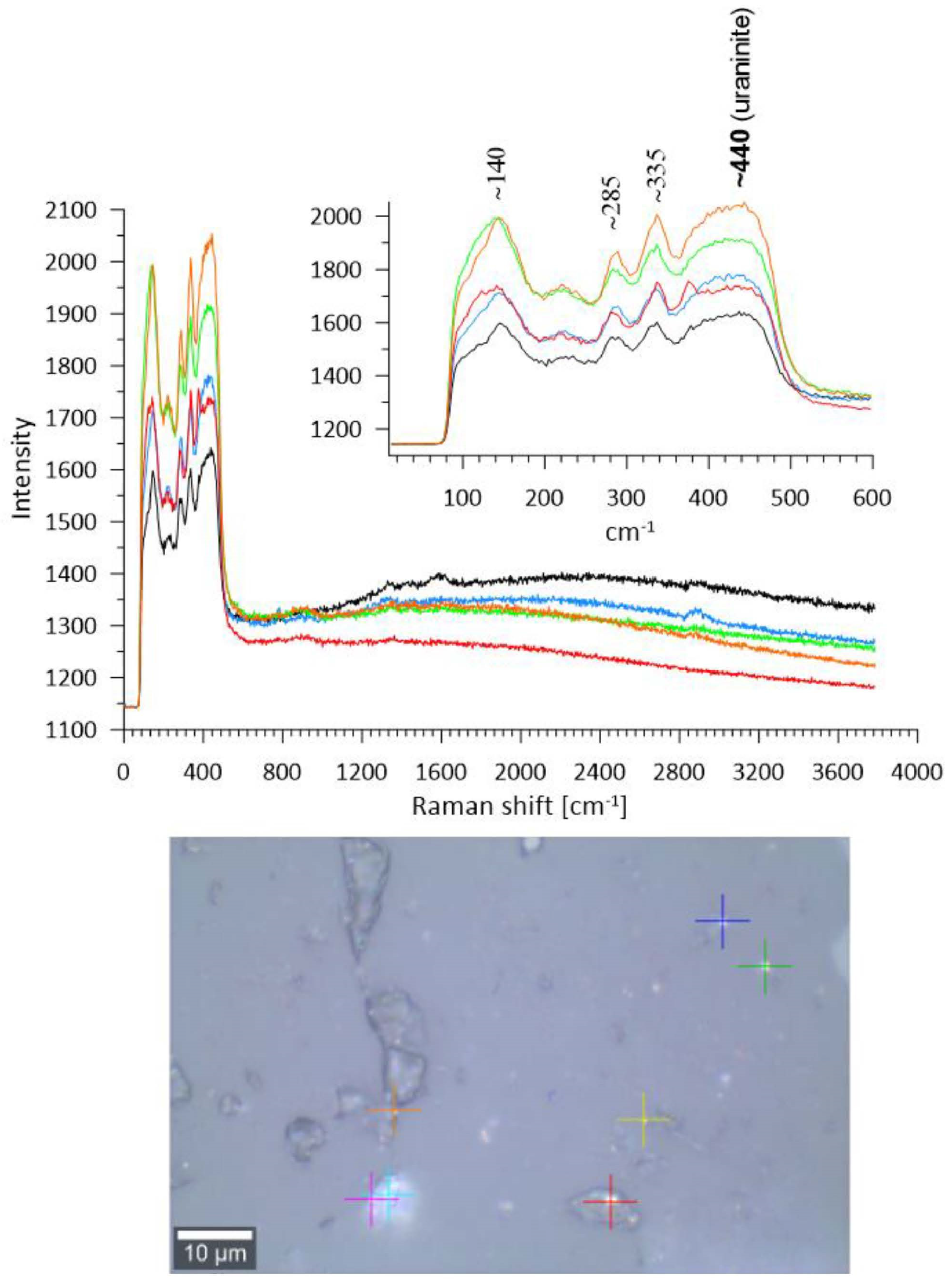
4.4. Micro-Raman Spectroscopy
4.5. X-ray Diffraction
4.6. Fluorine Contents in Volcanic Rocks
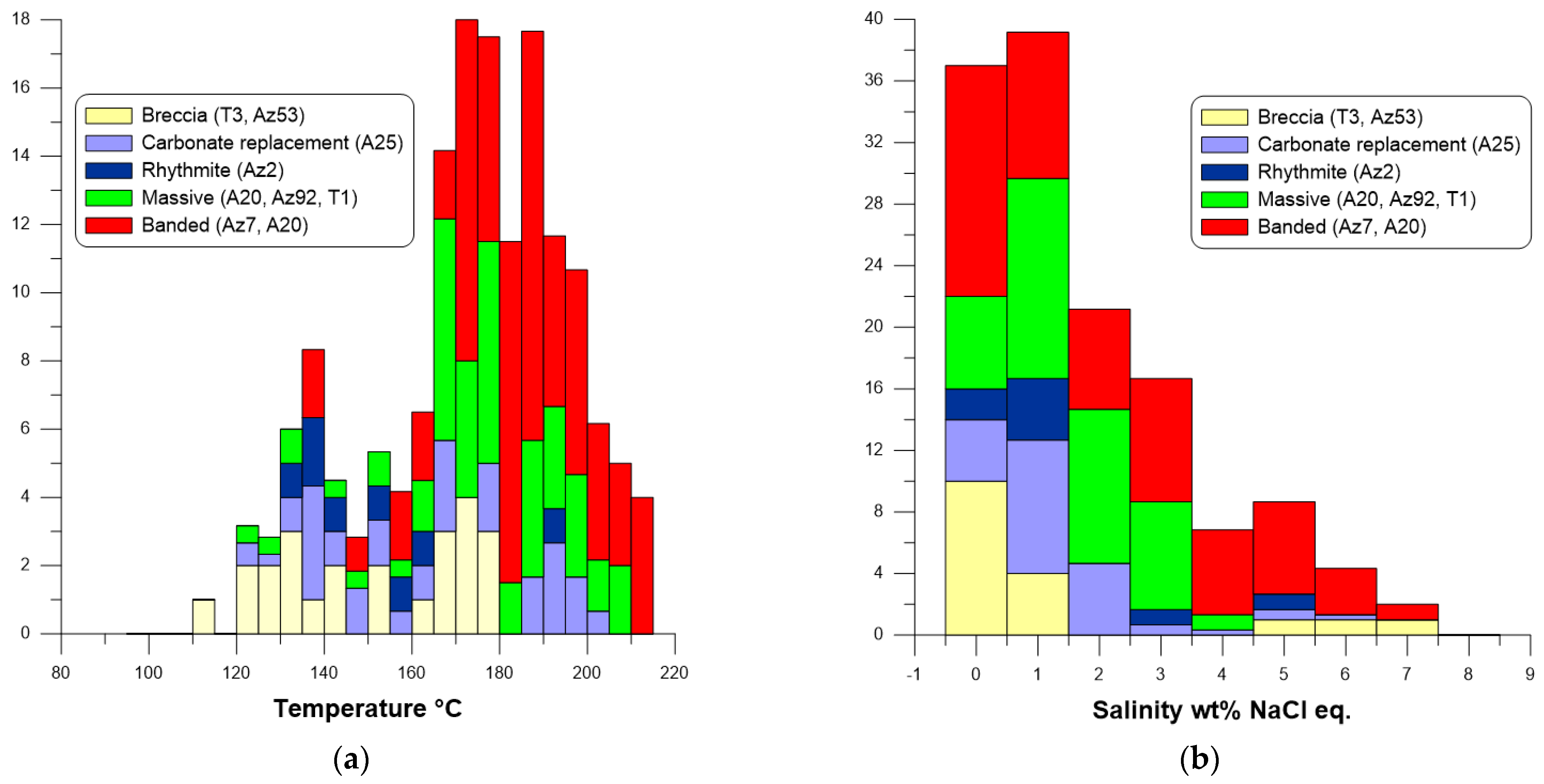
4.7. Microthermometric Data of Fluid Inclusions
5. Discussion
5.1. Distribution of U in Fluorite Samples
5.2. Uranium Mineralogy
5.3. Uraninite as a Geochemical Indicator
5.4. Chemical Age of Uraninite
5.5. Transport and Precipitation of Uranium in Fluorite Samples
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Condie, K.C. Chemical composition and evolution of the upper continental crust: Contrasting results from surface samples and shales. Chem. Geol. 1993, 104, 1–37. [Google Scholar] [CrossRef]
- Frondel, C. Systematic mineralogy of uranium and thorium. US Geol. Surv. Bull. 1958, 1064, 400. [Google Scholar]
- Frondel, J.W.; Fleischer, M.; Jones, R.S. Glossary of uranium and thorium bearing minerals 4th edition. US Geol. Surv. Bull. 1967, 1250, 69. [Google Scholar]
- Finch, R.; Murakami, T. Systematic and paragenesis of uranium minerals. In Uranium: Mineralogy, Geochemistry and the Environment, Reviews in Mineralogy; Burns, P.C., Finch, R., Eds.; Walter de Gruyter GmbH & Co. KG.: Berlin, Germany, 1999; Volume 38, pp. 9–179. [Google Scholar]
- Lauf, R.J. Mineralogy of Uranium and Thorium; Schiffer Publishing, Ltd.: Atglen, PA, USA, 2016; 352p. [Google Scholar]
- Deer, W.A. Rock-Forming Minerals: Framework Silicates; Longmans: Harlow, UK, 1962; Volume 4. [Google Scholar]
- Berzina, I.G.; Melnikov, I.V.; Popenko, D.P. Determination of content and spatial distribution of uranium in fluorites from tracks of fission fragments of uranium. Sov. At. Energy 1972, 32, 237–241. [Google Scholar] [CrossRef]
- Nassau, K.; Prescott, B.E. Smoky, blue, greenish yellow, and other irradiation-related colors in quartz. Mineral. Mag. 1977, 41, 301–312. [Google Scholar] [CrossRef][Green Version]
- Akyüz, T.; Boical, C.; Tretyakova, S.P.; Güzel, T.; Saritepe, P.; Oganiessian, Y.T. Investigation of uranium and thorium concentrations in the fluorite beds of Anatolia by using solid state nuclear track detectors. J. Radioanal. Nucl. Chem. 1997, 220, 259–261. [Google Scholar] [CrossRef]
- El-Mansi, M.M. Colouration of fluorite and its relation to radioactivity. J. Mineral. Soc. Egypt 2000, 12, 93–106. [Google Scholar]
- Raslan, M.F. Mineralogical and geochemical characteristics of uranium-rich fluorite in El-Missikat mineralized granite, Central Eastern Desert, Egypt. Geologija 2009, 52, 213–220. [Google Scholar] [CrossRef]
- Richardson, C.K.; Holland, H.D. Fluorite deposition in hydrothermal systems, an experimental study. Geochim. Cosmochim. Acta 1979, 43, 1313–1325. [Google Scholar] [CrossRef]
- Narayan Das, G.R.; Bhatnagar, G.S. Uraninite-fluorite-bearing sheared granites of Brijrani Gad in Bhilangana Valley. J. Geol. Soc. India 1981, 22, 247. [Google Scholar]
- Cathelineau, M. Les Gisements D’uranium Lies Spatialement aux Leucogranites Sud-Armoricains et a Leur Encaissant Metamorphique, Relations et Interactions Entre les Mineralisations et Divers Contextes Geologiques et Structuraux; Volum 42 de Sciences de la terre, Mémoires, ed.; Fondation Scientifique de la Géologie et de ses Applications: Nancy, France, 1982; 375p. [Google Scholar]
- George-Aniel, B.; Poty, B.; Leroy, J. Uranium deposits of the Sierra Peña Blanca: Three examples of mechanisms of ore deposit formation in a volcanic environment. In Uranium Deposits in Volcanic Rocks. Proceedings of the IAEA Vienna Symp Uranium Deposits in Volcanic Rocks, El Paso, TX, USA, 2–5 April 1984; International Atomic Energy Agency: Vienna, Austria, 1985; pp. 175–186. [Google Scholar]
- Kaiser, E.P.; Page, L.R. Distribution of Uranium Deposits in the United States; Kaiser, P., Kiag, R.U., Stogard, V.R., Stogard, F., Jr., Wyaat, D.G., Gott, G.B., Eds.; Department of the Interior, U.S. Geological Survey: Reston, VA, USA, 1952; p. 5.
- Bolonin, A.V.; Nikiforov, A.V.; Lykhin, D.A.; Sugorakova, A.M. The Chailag-Khem fluorite-barium-strontium rare earth carbonatite occurrence, the Western Sayan Range, Russia. Geol. Ore Depos. 2009, 51, 17–32. [Google Scholar] [CrossRef]
- Wilmarth, V.R.; Bauer, H.L., Jr.; Staatz, M.H.; Wyant, D.G. Uranium in fluorite deposits. US Geol. Surv. Circ. 1952, 220, 13. [Google Scholar]
- Sherborne, J.E., Jr.; Buckovic, W.A.; Dewitt, D.B.; Hellinger, T.S.; Pavlak, S.J. Major uranium discovery in volcaniclastic sediments, basin and range province, Yavapai County, Arizona. Am. Assoc. Pet. Geol. Bull. 1979, 63, 621–646. [Google Scholar]
- Cunningham, C.G.; Steven, T.A.; Rasmussen, J.D. Volcanogenic uranium deposits associated with the Mount Belknap volcanics, Marysvale volcanic field, west-central Utah (abstract). Energy exploration in the 80’s. In Proceedings of the Annual Meeting of the Southwest Section, American Association of Petroleum Geologists, El Paso, TX, USA, 25–27 February 1980; p. 22. [Google Scholar]
- Lindsey, D.A. Volcanism and uranium mineralization at Spor Mountain, Utah. AAPG Stud. Geol. 1981, 13, 89–98. [Google Scholar]
- Duex, T.W.; Henry, C.D. Uranium mobility in late magmatic and hydrothermal processes. Evidence from fluorite deposits, Texas and México. In Uranium Deposits in Volcanic Rocks; International Atomic Energy Agency: Vienna, Austria, 1985. [Google Scholar]
- Kesler, S.E. Metallogenic evolution of convergent margins; selected ore deposits models. Ore Geol. Rev. 1997, 12, 153–171. [Google Scholar] [CrossRef]
- Langmuir, D. Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochim. Cosmochim. Acta 1978, 42, 547–569. [Google Scholar] [CrossRef]
- Parks, G.A.; Pohl, D.C. Hydrothermal solubility of uraninite. Geochim. Cosmochim. Acta 1988, 52, 863–875. [Google Scholar] [CrossRef]
- Xing, Y.; Mei, Y.; Etschmann, B.; Liu, W.; Brugger, J. Uranium Transport in F-Cl-Bearing Fluids and Hydrothermal Upgrading of U-Cu Ores in IOCG Deposits Hindawi. Geofluids 2018, 2018, 6835346. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.; Xu, D. Relationship between Uranium Minerals and Pyrite and Its Genetic Significance in the Mianhuakeng Deposit, Northern Guangdong Province. Minerals 2021, 11, 73. [Google Scholar] [CrossRef]
- Holland, H.D.; Malinin, S.D. The solubility and occurrence of non-ore minerals. In Geochemistry of Hydrothermal Ore Deposits, 2nd ed.; Barnes, H.L., Ed.; Wiley: New York, NY, USA, 1979; pp. 461–501. [Google Scholar]
- Cunningham, C.G.; Rasmussen, J.D.; Steven, T.A.; Rye, R.O.; Rowley, P.D.; Romberger, S.B.; Selverstone, J. Hydrothermal uranium deposits containing molybdenum and fluorite in the Marysvale volcanic field, west-central Utah. Miner. Depos. 1998, 33, 477–494. [Google Scholar] [CrossRef]
- Henley, R.W. Hydrolysis reactions in hydrothermal fluids, Fluid-mineral equilibria in hydrothermal systems. Rev. Econ. Geol. 1984, 1, 45–56. [Google Scholar]
- Reed, M.; Spycher, N. Calculation of pH and mineral equilibria in hydrothermal waters with application to geothermometry and studies of boiling and dilution. Geochim. Cosmochim. Acta 1984, 48, 1479–1492. [Google Scholar] [CrossRef]
- Spirakis, C.S.; Heyl, A.V. Evaluation of proposed precipitation mechanisms for Mississippi Valley-type deposits. Ore Geol. Rev. 1995, 10, 1–17. [Google Scholar] [CrossRef]
- Campa-Uranga, M.F. Early Cretaceous protolith ages for metavolcanic rocks from Taxco and Taxco Viejo in Southern Mexico. In Proceedings of the Cordilleran Section 99th Annual Meeting, Puerto Vallarta, Mexico, 1–3 April 2003. [Google Scholar]
- De Cserna, Z.; Fries, C., Jr. Carta Geológica de México, Hoja Taxco 14Q-h(7), Serie 1:100,000 y Resumen de la Geología de la Hoja de Taxco. Estados de Guerrero, México y Morelos; Instituto de Geología de la UNAM: Ciudad de México, Mexico, 1981. [Google Scholar]
- Fries, C., Jr. Geología del Estado de Morelos y de Partes Adyacentes de México y Guerrero, Región Central-Meridional de México; Universidad Nacional Autónoma de México, Instituto de Geología, Boletín: Ciudad de México, Mexico, 1960; Volume 60, 236p. [Google Scholar]
- Morán-Zenteno, D.J.; Alba-Aldave, L.A.; Solé, J.; Iriondo, A. A major resurgent caldera in southern Mexico: The source of the late Eocene Tilzapotla ignimbrite. J. Volcanol. Geotherm. Res. 2004, 136, 97–119. [Google Scholar] [CrossRef]
- Hernández-Vargas, J.J.; Martiny, B.M.; Morán-Zenteno, D.J.; Pérez-Gutiérrez, R.; López-Martínez, M. 40Ar/39Ar geochronology and revised stratigraphy of the late Eocene Taxco volcanic field, southern Mexico. J. S. Am. Earth Sci. 2017, 79, 40–56. [Google Scholar] [CrossRef]
- Pi, T.; Solé, J.; Taran, Y. (U–Th)/He dating of fluorite: Application to the La Azul fluorspar deposit in the Taxco mining district, Mexico. Miner. Depos. 2005, 39, 976–982. [Google Scholar] [CrossRef]
- Pi, T. Estudio Mineralógico, Gequímico y Geocronológico de los Yacimientos de Fluorita de Taxco (Guerrero) y Zacualpan (Edo. De México). Ph.D. Thesis, Universidad Nacional Autónoma de México, Ciudad de México, Mexico, 2005; 420p. [Google Scholar]
- Fayziyev, A.R.; Koplus, A.V. Uranium and thorium in fluorite from endogenous deposits in the USSR. Geochem. Int. 1988, 25, 80–87. [Google Scholar]
- Jaffey, A.H.; Flynn, K.F.; Glendenin, L.E.; Bentley, B.C.; Esslin, A.M. Precision measurements of half-lives and specific activities of 235U and 238U. Phys. Rev. C 1971, 4, 1889–1906. [Google Scholar] [CrossRef]
- Sohrabi, M.; Shirazi, K.M. Optimization of etchants for electro-chemical etching of traces in policarbonate neutron personnel dosimeters. Nucl. Tracks Radiat. Meas. 1984, 8, 117–120. [Google Scholar]
- Espinosa, G. Trazas Nucleares en Sólidos; Universidad Nacional Autónoma de México, Instituto de Física: Valencia, Spain, 1994; 127p. [Google Scholar]
- Roedder, E. Volume 12: Fluid inclusions. Rev. Mineral. 1984, 12, 644. [Google Scholar]
- Goldstein, R.H. Fluid inclusions in sedimentary and diagenetic systems. Lithos 2001, 55, 159–193. [Google Scholar] [CrossRef]
- Bodnar, R.J. The system H2O-NaCl. Fourth biennial Pan-American conference on research on fluid inclusions. Program Abstr. 1992, 4, 108–111. [Google Scholar]
- Brown, P.E. FLINCOR: A microcomputer program for the reduction and investigation of fluid inclusion data. Am. Miner. 1989, 74, 1390–1393. [Google Scholar]
- Lenoir, L.; Blaise, T.; Somogyi, A.; Brigaud, B.; Barbarand, J.; Boukari, C.; Nouet, J.; Brézard-Oudot, A.; Pagel, M. Uranium incorporation in fluorite and exploration of U–Pb dating. Geochronology 2021, 3, 199–227. [Google Scholar] [CrossRef]
- Wolff, R.; Dunkl, I.; Kempe, U.; Stockli, D.; Wiedenbeck, M.; von Eynatten, H. Variable helium diffusion characteristics in fluorite, Geochim. Cosmochim. Acta 2016, 188, 21–34. [Google Scholar] [CrossRef]
- Piccione, G.; Rasbury, E.T.; Elliott, B.A.; Kyle, J.R.; Jaret, S.J.; Acerbo, A.S.; Lanzirotti, A.; Northrup, P.; Wooton, K.; Parrish, R.R. Vein fluorite U-Pb dating demonstrates post 6.2 Ma rare-earth element mobilization associated with Rio Grande rifting. Geosphere 2019, 15, 1958–1972. [Google Scholar] [CrossRef]
- Piccione, G.; Pi-Puig, T.; Rasbury, T. Solé, Concordant U-Pb and (U_Th)/He fluorite ages from Taxco mining district, México. J. GSA Annu. Meet. 2015, 47, 379. [Google Scholar]
- Janeczek, J.; Ewing, R.C. Structural formula of uraninite. J. Nucl. Mater. 1992, 190, 128–132. [Google Scholar] [CrossRef]
- Biwer, B.M.; Elbert, W.L.; Bates, J.K. The raman spectra of several uranyl-containing minerals using microprobe. J. Nucl. Matter 1990, 175, 188–193. [Google Scholar] [CrossRef]
- Allen, G.C.; Butler, I.S.; Anh Tuan, N. Characterization of uranium oxides by micro-raman spectroscopy. J. Nucl. Mater. 1987, 144, 17–19. [Google Scholar] [CrossRef]
- Palacios, M.L.; Taylor, S.H. Characterization of Uranium Oxides Using in Situ Micro-Raman. Spectrosc. Appl. Spectrosc. 2000, 54, 1373–1378. [Google Scholar] [CrossRef]
- Bonales, L.J.; Menor-Salván, C.; Cobos, J. Study of the alteration products of a natural uraninite by Raman spectroscopy. J. Nucl. Mater. 2015, 462, 296–303. [Google Scholar] [CrossRef]
- Stefaniak, E.A.; Alsecz, A.; Sajó, I.E.; Worobiec, A.; Máthé, Z.; Török, S.; Van Grieken, R. Recognition of uranium oxides in soil particulate matter by means of l-Raman spectrometry. J. Nucl. Mater. 2008, 381, 278–283. [Google Scholar] [CrossRef]
- Stefaniak, E.A.; Alsecz, A.; Frost, R.; Máthé, Z.; Sajó, I.E.; Török, S.; Worobiec, A.; Van Grieken, R. Combined SEM/EDX and micro-Raman spectroscopy analysis of uranium minerals from a former uranium mine. J. Hazard. Mater. 2009, 168, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, R.J.P.; Wolverson, D.; Mitchels, J.M.; Skelton, J.M.; Parker, S.C.; Molinari, M.; Khan, I.; Geeson, D.; Allen, G.C. A Raman spectroscopic study of uranyl minerals from Cornwall, UK. RSC Adv. 2014, 4, 59137–59149. [Google Scholar] [CrossRef]
- Crawford, M.L. Phase equilibria in aqueous fluid inclusions. Fluid Inclusions applications to petrology. In Short Course Handbook; Mineralogical Association of Canada: Québec, QC, Canada, 1981; pp. 75–100. [Google Scholar]
- Hall, D.L.; Sterner, S.M.; Bodnar, R.J. Freezing point depression of NaCl-KCl-H2O solutions. Econ. Geol. 1988, 83, 197–202. [Google Scholar] [CrossRef]
- Doynikova, O.A.; Sidorenko, G.A. To mineralogy of tetravalent uranium. New Data Min. 2010, 45, 79–90. [Google Scholar]
- Zhong, F.; Yan, J.; Wang, K.; Pan, J.; Xia, F.; Liu, G.; Liu, W. Mineralogy and geochemistry of pitchblende in the Changjiang U ore field, Guangdong Province, South China: Implications for its mineralization. Geochem. J. 2022, 56, 74–95. [Google Scholar] [CrossRef]
- McMillan, R.H. Genetics aspects and classification of important Canadian Uranium Deposits. In Mineralogical Society of Canada, Short Course Handbook 3; Kimberly, M.M., Ed.; Uranium Deposits: Their Mineralogy and Origin; Mineralogical Society of Canada: Québec city, QC, Canada, 1978; pp. 187–204. [Google Scholar]
- Plant, J.A.; Simpson, P.R.; Smith, B.; Windley, B.F. Uranium ore deposits: Products of the radioactive Earth. In Reviews in Mineralogy and Geochemistry; Uranium: Mineralogy, Geochemistry and the Environment; Burns, P.C., Finch, R., Eds.; Mineralogical Society of America: Chantilly, VA, USA, 1999; Volume 38, pp. 255–319. [Google Scholar]
- Bowles, J.F.W. Age dating of individual grains of uraninite in rocks from electron microprobe analyses. Chem. Geol. 1990, 83, 47–53. [Google Scholar] [CrossRef]
- Pavshukov, V.V.; Komlev, L.V.; Anderson, E.B.; Smyslova, I.G. On the state of the uranium-lead system in uranium ores (from the data of X-ray microprobe analysis). Geokhimiya 1975, 603–613. [Google Scholar]
- Greenwood, N.; Earnshaw, A. Chemistry of the Elements; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Spirakis, C.S. Discussion of “Fluorite deposits at Encantada-Buenavista, México; products of Mississippi Valley Type Processes (Ore Geology Reviews 23:107-124)”. Ore Geol. Rev. 2004, 25, 327–328. [Google Scholar] [CrossRef]
- Garrels, R.M.; Larsen, E.S., 3rd. Geochemistry and mineralogy of the Colorado Plateau uranium ores. In US Geological Survey Professional Paper; United States Geological Survey: Reston, VA, USA, 1959; Volume 320, 236p. [Google Scholar]
- Tugarinov, A.I.; Naumov, G.B. Die Migrations-und Absatzverhältnisse des Urans bei der Erzbildung. Zeitschr. Angew. Geol. 1974, 20, 410–413. [Google Scholar]
- Spirakis, C.S. The roles of organic matter in the formation of uranium deposits in sedimentary rocks. Ore Geol. Rev. 1996, 11, 53–69. [Google Scholar] [CrossRef]
- Landais, P. Organic geochemistry of sedimentary uranium ore deposits. Ore Geol. Rev. 1996, 11, 33–51. [Google Scholar] [CrossRef]
- Nakashima, S.; Disnar, J.R.; Perruchot, A. Precipitation kinetics of uranium by sedimentary organic matter under diagenetic and hydrothermal conditions. Econ. Geol. 1999, 94, 993–1006. [Google Scholar] [CrossRef]



| Sample | Color | Description | Srbulk µg g−1 | Ubulk µg g−1 | Thbulk µg g−1 | Smbulk µg g−1 | (U-Th-Sm)-He Age (Ma) |
|---|---|---|---|---|---|---|---|
| A20 | Purple | Purple fluorite with irregularly banded texture. Open spaces (porosity) are filled by late fluorite and oxides. Optical microscopy shows the presence of small amounts of quartz. | 43 | 68.5 | 0.68 | 0.17 | 32.0 ± 1.7 |
| Az2 | Purple | Rhythmical purple fluorite and quartz. | 87 | 4.02 | 0.09 | 0.05 | – |
| Az5 | Purple | Deep purple and brown fluorite replacing carbonate. Late calcite fills cavities. Minor quartz. | 143 | 3.63 | 0.10 | 0.16 | 31.5 ± 1.5 |
| Az20 | Pink/blue | Crust formed by elongated fluorite crystals that grow perpendicular to the crust surfaces. Minor amounts of calcite and oxides. | 214 | 21.0 | <0.05 | 0.13 | 31.8 ± 1.7 |
| Az53 | Purple/black | Rhyolite breccia containing purple fluorite clasts in a red matrix composed of quartz, kaolinite, iron oxides, and altered biotite. | 68 | 28.1 | 0.22 | 0.56 | – |
| Az7 | White | Alternate bands of pure white and gray fluorite. Minor amounts of calcite are also present. | 380 | 4.7 | <0.05 | 0.11 | 32.7 ± 1.7 |
| Gray | 488 | 5.4 | <0.05 | 0.11 | 29.8 ± 1.7 | ||
| Az92 | Purple | Massive fluorite of purple and brown hues distributed irregularly. | 409 | 94.4 | <0.05 | 0.05 | 32.0 ± 1.7 |
| Brown | 956 | 51.5 | 0.06 | 0.26 | 32.6 ± 1.8 | ||
| T1 | Purple | Fine-grained massive purple and brown fluorite divided by an alteration band. White points are barite crystals. Minor amounts of quartz and oxides. | 225 | 8.91 | 0.06 | 0.12 | – |
| T3 | Purple | Breccia with clasts of brown/purple fluorite in a calcite matrix with minor amounts of quartz. | 380 | 26.9 | 0.06 | 0.45 | 30.7 ± 1.6 |
| Weight (%) | EDS1 | EDS2 | EDS3 | EDS4 | EDS5 | WDS1 | WDS2 | WDS3 | WDS4 |
|---|---|---|---|---|---|---|---|---|---|
| ThO2 | 2.042 | ||||||||
| UO2 | 86.50 | 92.67 | 87.18 | 85.40 | 97.35 | 81.00 | 84.70 | 85.10 | 84.24 |
| MgO | 0.00 | 2.01 | 1.33 | 0.37 | 1.49 | ||||
| K2O | 1.64 | 0.34 | 1.31 | 0.30 | |||||
| Na2O | 0.29 | 0.05 | 1.78 | 0.49 | |||||
| CaO | 9.55 | 9.42 | 11.77 | 9.62 | |||||
| Al2O3 | 1.31 | 1.13 | 0.31 | ||||||
| SiO2 | 7.76 | 1.00 | 7.43 | 4.56 | 0.07 | 6.63 | 5.01 | 0.57 | 4.87 |
| Y2O3 | |||||||||
| PbO | 1.20 | 2.03 | 3.22 | 0.32 | 0.38 | 0.79 | 0.37 | ||
| P2O5 | 0.88 | 1.52 | |||||||
| FeO | 2.07 | 0.64 | 1.64 | 0.72 | |||||
| F | |||||||||
| Total | 100 | 100 | 100 | 100 | 100 | 97.50 | 99.51 | 98.23 | 99.1 |
| Chemical age (Ma) | 30 | 34 | 69 | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pi-Puig, T.; Solé, J.; Alba-Aldave, L. Uranium in Fluorite, a Case Study: The La Azul Fluorspar Deposit, Taxco, Guerrero, Mexico. Minerals 2023, 13, 1495. https://doi.org/10.3390/min13121495
Pi-Puig T, Solé J, Alba-Aldave L. Uranium in Fluorite, a Case Study: The La Azul Fluorspar Deposit, Taxco, Guerrero, Mexico. Minerals. 2023; 13(12):1495. https://doi.org/10.3390/min13121495
Chicago/Turabian StylePi-Puig, Teresa, Jesús Solé, and Leticia Alba-Aldave. 2023. "Uranium in Fluorite, a Case Study: The La Azul Fluorspar Deposit, Taxco, Guerrero, Mexico" Minerals 13, no. 12: 1495. https://doi.org/10.3390/min13121495
APA StylePi-Puig, T., Solé, J., & Alba-Aldave, L. (2023). Uranium in Fluorite, a Case Study: The La Azul Fluorspar Deposit, Taxco, Guerrero, Mexico. Minerals, 13(12), 1495. https://doi.org/10.3390/min13121495






