Preliminary Study on the Surface Modification of Lignite and Bioflotation by White-Rot Fungi Hypocrea lixii AH
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture and Preparation of Biosurfactants
2.2. Lignite Sample and Biotreatment Test
2.3. Surface Property Tests
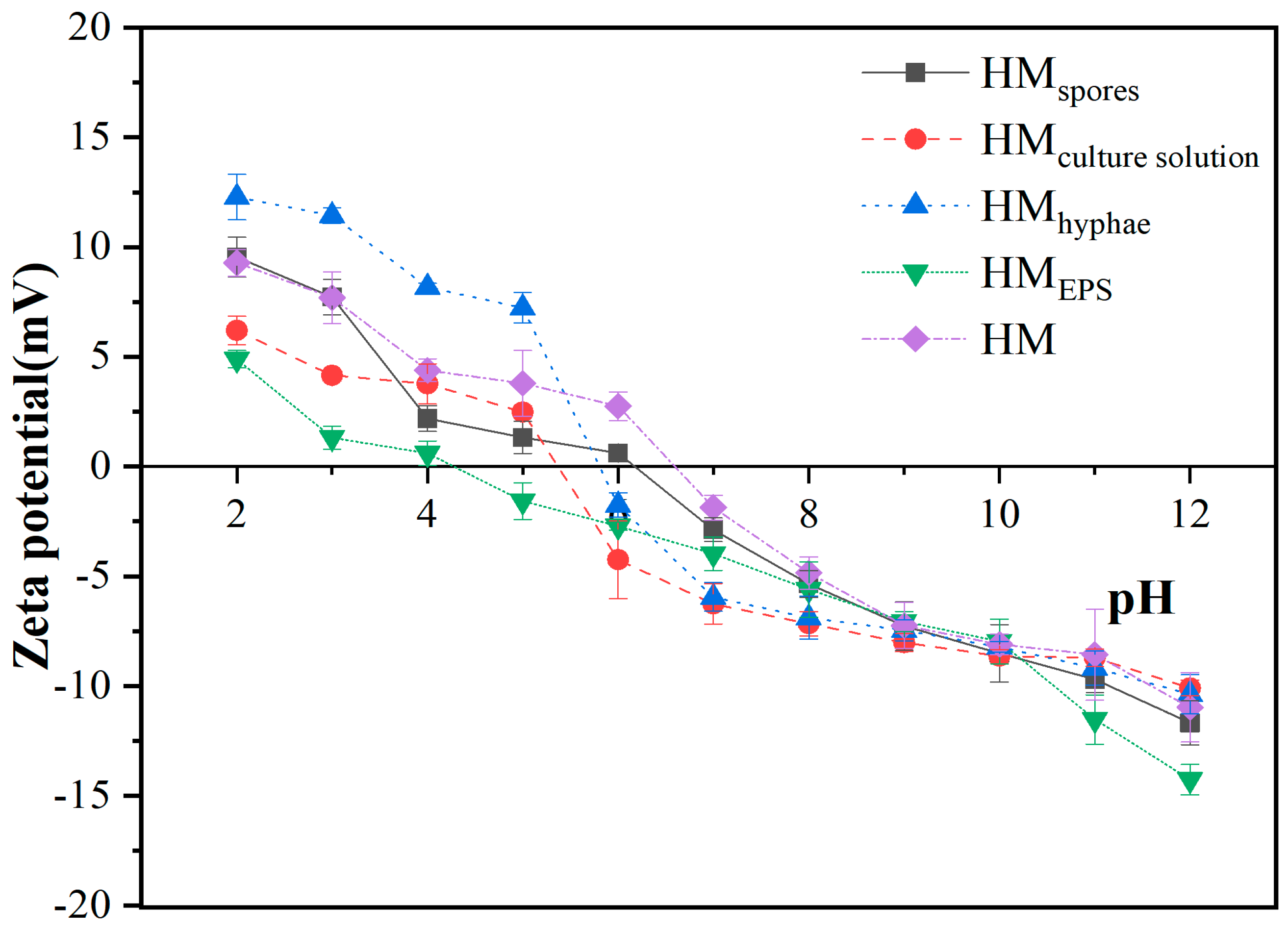
2.3.1. Zeta Potential Analysis
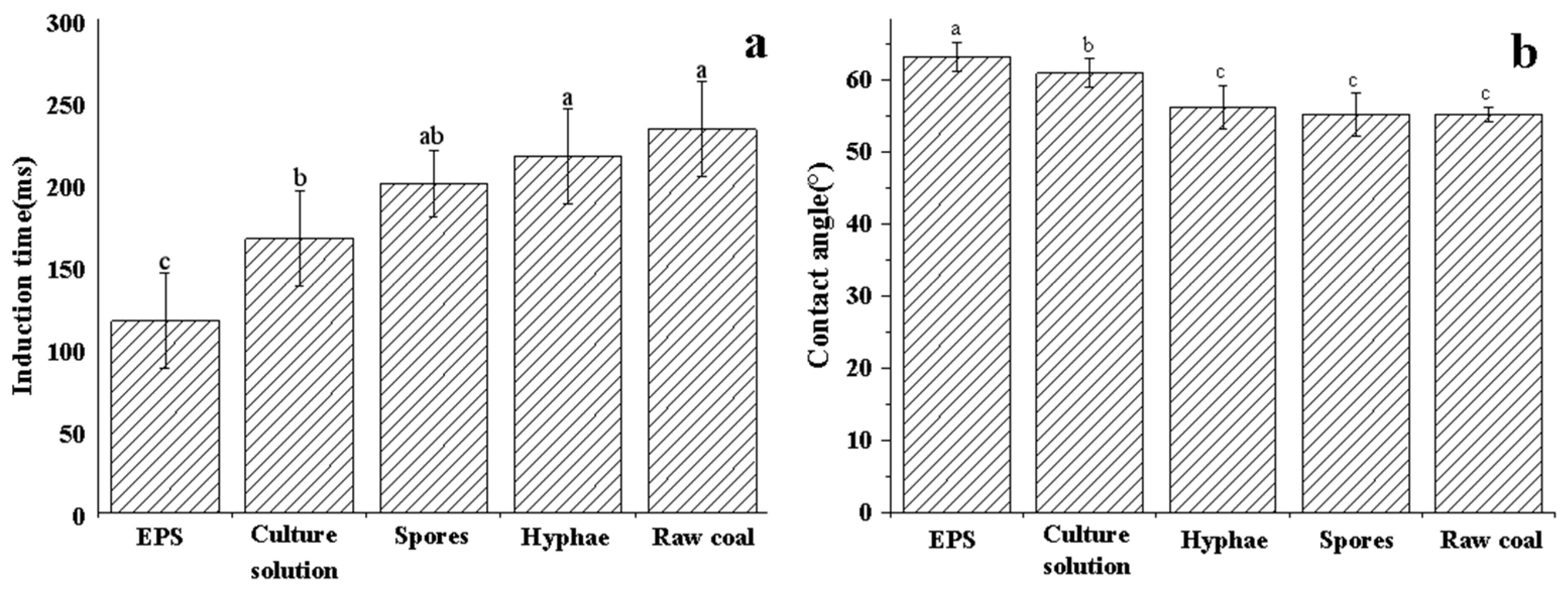
2.3.2. Induction Time Analysis
2.3.3. Contact Angle Analysis
2.3.4. Fourier Transform Infrared Analysis
2.4. Bioflotation Test
3. Results and Discussion
3.1. Zeta Potential Results of Raw and Biotreated Lignite
3.2. Contact Angle and Induction Time Results of Raw and Biotreated Lignite
3.3. FT-IR Spectra of Raw and Biotreated Lignite
3.4. Bioflotation Tests of Raw and Biotreated Lignite
4. Conclusions
- All tested biosurfactants exhibited the ability to enhance the surface properties of lignite, with EPS demonstrating the most pronounced effect, followed by the culture solution, hyphae, and spores. Notably, the most significant change observed was the reduction in induction time when lignite was treated with EPS.
- Lignite displayed poor inherent flotability. While the fungal cells improved the hydrophobicity of the coal, they did not contribute to improved flotation. Conversely, the EPS demonstrated the capacity to either depress the concentrate or facilitate the flotation of impurities present in lignite.
- Although fungal cells were not found to be effective bio-collectors in microflotation tests, they exhibited the ability to remove or modify minerals in lignite, particularly those containing sulfur. This suggests a potential for the fungal cells to play a role in desulfurization or mineral transformation processes within lignite.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xia, Y.; Zhang, R.; Cao, Y.; Xing, Y.; Gui, X. Role of molecular simulation in understanding the mechanism of low-rank coal flotation: A review. Fuel 2020, 262, 116535. [Google Scholar] [CrossRef]
- Chen, S.; Tang, L.; Tao, X.; He, H.; Yang, Z.; Chen, L. Exploration on the mechanism of oily-bubble flotation of long-flame coal. Fuel 2018, 216, 427–435. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.; Zhang, R.; Xing, Y.; Gui, X. Performance of used lubricating oil as flotation collector for the recovery of clean low-rank coal. Fuel 2019, 239, 717–725. [Google Scholar] [CrossRef]
- Liao, Y.; Hao, X.; An, M.; Yang, Z.; Ma, L.; Ren, H. Enhancing low-rank coal flotation using mixed collector of dodecane and oleic acid: Effect of droplet dispersion and its interaction with coal particle. Fuel 2020, 280, 118634. [Google Scholar] [CrossRef]
- Chen, S.; Tang, L.; Tao, X.; He, H.; Chen, L.; Yang, Z. Enhancing flotation performance of low rank coal by improving its hydrophobicity and the property of oily bubbles using 2-ethylhexanol. Int. J. Miner. Process. 2017, 167, 61–67. [Google Scholar] [CrossRef]
- Hu, H.; Li, M.; Li, L.; Tao, X. Improving bubble-particle attachment during the flotation of low rank coal by surface modification. Int. J. Min. Sci. Technol. 2020, 30, 217–223. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, Y.; Li, H.; Xu, C.; Jiang, S.; Ma, R.; Wang, B. Pretreatment of deep-sea bacteria for reverse flotation of magnesite tailings: Cleaner production, behavior and mechanism. Sep. Purif. Technol. 2023, 307, 122685. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.Y.; Zhu, S.Q. Surface Modification Effects of Microorganism from Lignite on Fine Coal. Adv. Mater. Res. 2014, 868, 423–428. [Google Scholar] [CrossRef]
- Kim, G.; Choi, J.; Silva, R.A.; Song, Y.; Kim, H. Feasibility of bench-scale selective bioflotation of copper oxide minerals using Rhodococcus opacus. Hydrometallurgy 2017, 168, 94–102. [Google Scholar] [CrossRef]
- Fazaelipoor, M.H.; Khoshdast, H.; Ranjbar, M. Coal flotation using a biosurfactant from Pseudomonas aeruginosa as a frother. Korean J. Chem. Eng. 2010, 27, 1527–1531. [Google Scholar] [CrossRef]
- El-Midany, A.A.; Abdel-Khalek, M.A. Influence of bacteria–coal electrostatic interaction on coal cleaning. Int. J. Miner. Process. 2014, 126, 30–34. [Google Scholar] [CrossRef]
- El-Midany, A.A.; Abdel-Khalek, M.A. Reducing sulfur and ash from coal using Bacillus subtilis and Paenibacillus polymyxa. Fuel 2014, 115, 589–595. [Google Scholar] [CrossRef]
- Martín, F.S.; Aguilar, C. Study of the Adhesion Mechanism of Acidithiobacillus ferrooxidans to Pyrite in Fresh and Saline Water. Minerals 2019, 9, 306. [Google Scholar] [CrossRef]
- Kim, G.; Park, K.; Choi, J.; Gomez-Flores, A.; Han, Y.; Choi, S.Q.; Kim, H. Bioflotation of malachite using different growth phases of Rhodococcus opacus: Effect of bacterial shape on detachment by shear flow. Int. J. Miner. Process. 2015, 143, 98–104. [Google Scholar] [CrossRef]
- Olivera, C.A.C.E.; Merma, A.G.; Puelles, J.G.S.; Torem, M.L. On the fundamentals aspects of hematite bioflotation using a Gram positive strain. Miner. Eng. 2016, 106, 55–63. [Google Scholar] [CrossRef]
- Raichur, A.; Misra, M.; Bukka, K.; Smith, R. Flocculation and flotation of coal by adhesion of hydrophobic Mycobacterium phlei. Colloids Surf. B Biointerfaces 1996, 8, 13–24. [Google Scholar] [CrossRef]
- Bumpus, J.A.; Tien, M.; Wright, D.; Aust, S.D. Oxidation of persistent environmental pollutants by a white rot fungus. Science 1985, 228, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- González, C.; Wu, Y.; Zuleta-Correa, A.; Jaramillo, G.; Vasco-Correa, J. Biomass to value-added products using microbial consortia with white-rot fungi. Bioresour. Technol. Rep. 2021, 16, 100831. [Google Scholar] [CrossRef]
- Shi, K.; Liu, Y.; Chen, P.; Li, Y. Contribution of Lignin Peroxidase, Manganese Peroxidase, and Laccase in Lignite Degradation by Mixed White-Rot Fungi. Waste Biomass Valorization 2021, 12, 3753–3763. [Google Scholar] [CrossRef]
- Haider, R.; Ghauri, M.A.; Sanfilipo, J.R.; Jones, E.J.; Orem, W.H.; Tatu, C.A.; Akhtar, K.; Akhtar, N. Fungal degradation of coal as a pretreatment for methane production. Fuel 2013, 104, 717–725. [Google Scholar] [CrossRef]
- Haider, R.; Ghauri, M.A.; Akhtar, K. Isolation of Coal Degrading Fungus from Drilled Core Coal Sample and Effect of Prior Fungal Pretreatment on Chemical Attributes of Extracted Humic Acid. Geomicrobiol. J. 2015, 32, 944–953. [Google Scholar] [CrossRef]
- Park, S.; Liang, Y. Bioleaching of trace elements and rare earth elements from coal fly ash. Int. J. Coal Sci. Technol. 2019, 6, 74–83. [Google Scholar] [CrossRef]
- Ghani, M.J.; Rajoka, M.I.; Akhtar, K. Investigations in fungal solubilization of coal: Mechanisms and significance. Biotechnol. Bioprocess Eng. 2015, 20, 634–642. [Google Scholar] [CrossRef]
- Ralph, J.P.; Catcheside, D.E.A. Recovery and analysis of solubilised brown coal from cultures of wood-rot fungi. J. Microbiol. Methods 1996, 27, 1–11. [Google Scholar] [CrossRef]
- Deska, M.; Głodniok, M.; Ulfig, K. Coal Enrichment Methods by Using Microorganisms and Their Metabolites. J. Ecol. Eng. 2018, 19, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mishra, A. Optimization of laccase production from WRF-1 on groundnut shell and cyanobacterial biomass: By application of Box-Behnken experimental design. J. Microbiol. Biotechnol. Res. 2017, 1, 33–53. [Google Scholar]
- Guan-Qun, G.; Xiu-Xiang, T. Research on white decay bacterium flotation and leaching for coal biological desulphurization. Coal Sci. Technol. 2006, 2, 49–51. [Google Scholar]
- Shi, K. Research on Mechanisms of Bio-Liquefaction of Fushun Long Flame Coal and Its Model Compounds by White Rot Fungi Hypocrealixii AH. Ph.D. Thesis, China University of Mining and Technology, Xuzhou, China, 2011. (In Chinese). [Google Scholar]
- Kai-Yi, S.; Xiu-Xiang, T.; Su-Dong, Y.; Ying, D.; Zuo-Peng, L. Bio-liquefaction of Fushun lignite: Characterization of newly isolated lignite liquefying fungus and liquefaction products. Procedia Earth Planet. Sci. 2009, 1, 627–633. [Google Scholar] [CrossRef][Green Version]
- Shi, K.Y.; Yin, S.D.; Tao, X.X.; Du, Y.; He, H.; Lv, Z.P.; Xu, N. Quantitative Measurement of Coal Bio-solubilization by Ultraviolet-visible Spectroscopy. Energy Sources 2013, 35, 1456–1462. [Google Scholar] [CrossRef]
- Hao, B.; Fan, M.; Li, Z.; Li, H. Study on the mechanism of adsorption and hydrophobic modification of glycerol monooleate/kerosene mixed collector on low-rank coal surface. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131960. [Google Scholar] [CrossRef]
- Li, H.R. The Research Progress of White Rot Fungi. Environ. Sci. Technol. 1996, 6, 69–77. (In Chinese) [Google Scholar]
- Harneit, K.; Göksel, A.; Kock, D.; Klock, J.H.; Gehrke, T.; Sand, W. Adhesion to metal sulfide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans. Hydrometallurgy 2006, 83, 245–254. [Google Scholar] [CrossRef]
- Govender, Y.; Gericke, M. Extracellular polymeric substances (EPS) from bioleaching systems and Its application in bioflotation. Miner. Eng. 2011, 24, 1122–1127. [Google Scholar] [CrossRef]
- Laskowski, L.Y.Z.S. The adsorption of polysaccharides onto mineral surfaces: An acid/base interaction. Int. J. Miner. Process. 2000, 60, 229–245. [Google Scholar]
- Dorobantu, L.S.; Bhattacharjee, S.; Foght, J.M.; Gray, M.R. Analysis of force interactions between AFM tips and hydrophobic bacteria using DLVO theory. Langmuir ACS J. Surf. Colloids 2009, 25, 6968–6976. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J.; Dewil, R.; Heyder, B.D. Advanced sludge treatment affects extracellular polymeric substances to improve activated sludge dewatering. J. Hazard. Mater. 2004, 106, 83–92. [Google Scholar] [CrossRef]
- Yesankar, P.J.; Pal, M.; Patil, A.; Qureshi, A. Microbial exopolymeric substances and biosurfactants as ‘bioavailability enhancers’ for polycyclic aromatic hydrocarbons biodegradation. Int. J. Environ. Sci. Technol. 2022, 20, 5823–5844. [Google Scholar] [CrossRef]
- Dwyer, R.; Bruckard, W.; Rea, S.; Holmes, R. Bioflotation and bioflocculation review: Microorganisms relevant for mineral beneficiation. Miner. Process. Extr. Metall. 2012, 121, 65–71. [Google Scholar] [CrossRef]
- Vijayalakshmi, S.; Raichur, A. Bioflocculation of high-ash Indian coals using Paenibacillus polymyxa. Int. J. Miner. Process. 2002, 67, 199–210. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Bao, X.; Hao, Y.; Gui, X.; Xing, Y. New insight into the role of the emulsified diesel droplet size in low rank coal flotation. Fuel 2023, 338, 127388. [Google Scholar] [CrossRef]
- Dong, X.; Wang, F.; Guo, L.; Han, T. Study on the Influence of Coal Structure and Oxidation Performance by Endogenous Bacterium. Fire 2023, 6, 339. [Google Scholar] [CrossRef]
- Olawale, J.T.; Edeki, O.G.; Cowan, A.K. Bacterial degradation of coal discard and geologically weathered coal. Int. J. Coal Sci. Technol. 2020, 7, 405–416. [Google Scholar] [CrossRef]
- Gonsalvesh, L.; Marinov, S.; Stefanova, M.; Yürüm, Y.; Dumanli, A.; Dinler-Doganay, G.; Kolankaya, N.; Sam, M.; Carleer, R.; Reggers, G. Biodesulphurized subbituminous coal by different fungi and bacteria studied by reductive pyrolysis. Part 1: Initial coal. Fuel 2008, 87, 2533–2543. [Google Scholar] [CrossRef]
- Gonsalvesh, L.; Marinov, S.; Stefanova, M.; Carleer, R.; Yperman, J. Organic sulphur alterations in biodesulphurized low rank coals. Fuel 2012, 97, 489–503. [Google Scholar] [CrossRef]
- Xingxiang, C.; Jie, M.A. Study on the white-rot fungus’s flocculation processing slime water. Jiangsu Vocat. Inst. Archit. Technol. 2016, 16, 6–11. (In Chinese) [Google Scholar]

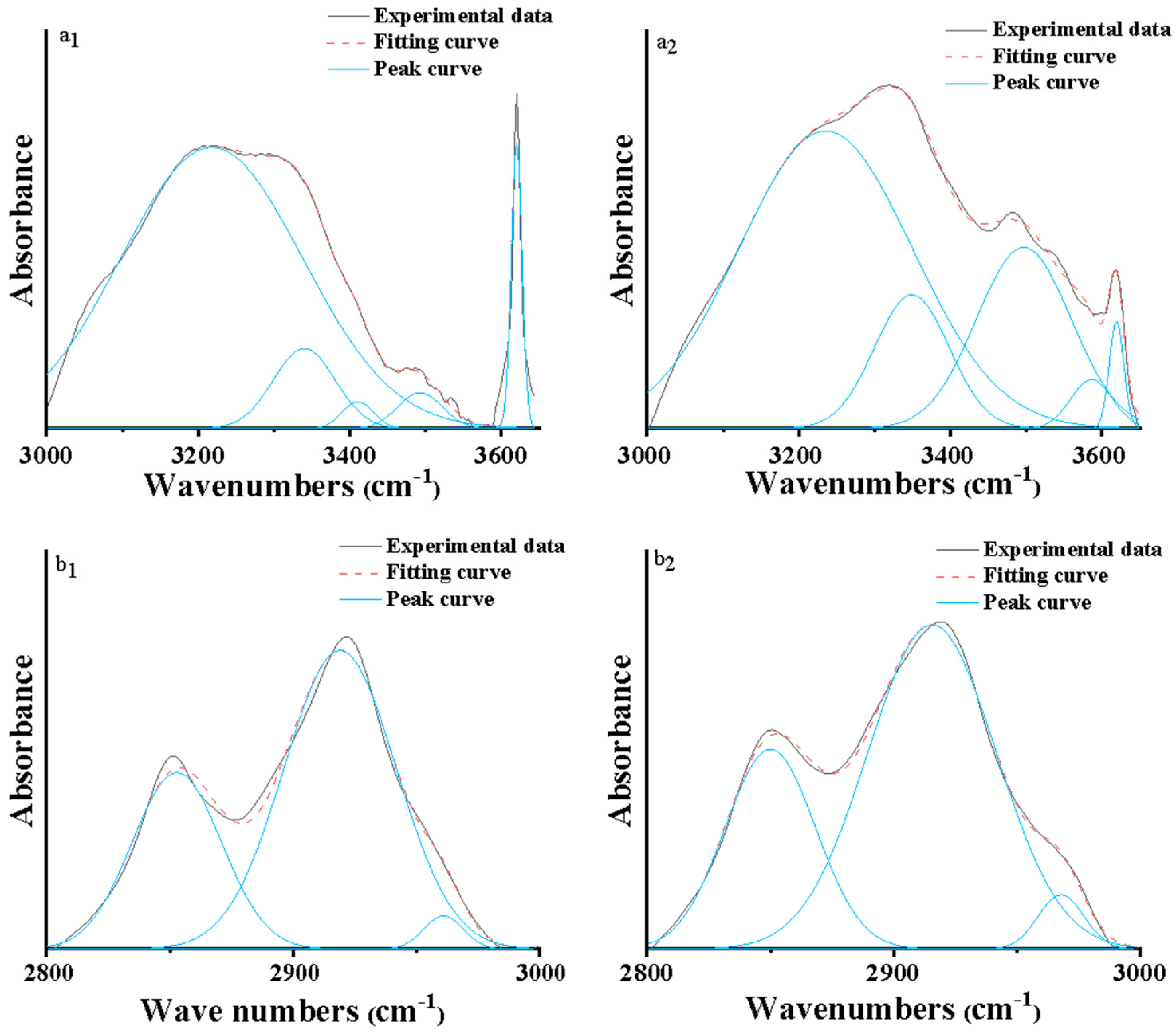

| Samples | Antisymmetric Stretching Vibration of CH3 (%) | CH Stretching Vibration (%) | Symmetric Stretching Vibration of CH2 (%) |
|---|---|---|---|
| Raw coal | 66.22 | 2.60 | 31.18 |
| Treated by EPS | 66.91 | 3.99 | 29.10 |
| Tests | Ash Content of Concentrate (%) | Ash Content of Tailings (%) | Recovery of Concentrate (%) | Recovery of Combustible (%) |
|---|---|---|---|---|
| L* | 14.72 | 15.67 | 16.39 | 16.58 |
| L* + diesel oil | 15.52 | 16.01 | 31.95 | 32.14 |
| L* + EPS | 19.82 | 15.45 | 11.92 | 11.30 |
| L* + spores | 15.10 | 15.67 | 3.96 | 3.99 |
| L* + culture solution | 16.18 | 15.73 | 9.66 | 9.60 |
| Liquefied L* + diesel oil | 11.04 | 15.30 | 43.65 | 45.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Cao, M.; Zhan, D.; Xia, W.; Chen, S.; Tao, X.; Huang, Z. Preliminary Study on the Surface Modification of Lignite and Bioflotation by White-Rot Fungi Hypocrea lixii AH. Minerals 2023, 13, 1492. https://doi.org/10.3390/min13121492
He H, Cao M, Zhan D, Xia W, Chen S, Tao X, Huang Z. Preliminary Study on the Surface Modification of Lignite and Bioflotation by White-Rot Fungi Hypocrea lixii AH. Minerals. 2023; 13(12):1492. https://doi.org/10.3390/min13121492
Chicago/Turabian StyleHe, Huan, Mingjun Cao, Di Zhan, Wencheng Xia, Songjiang Chen, Xiuxiang Tao, and Zaixing Huang. 2023. "Preliminary Study on the Surface Modification of Lignite and Bioflotation by White-Rot Fungi Hypocrea lixii AH" Minerals 13, no. 12: 1492. https://doi.org/10.3390/min13121492
APA StyleHe, H., Cao, M., Zhan, D., Xia, W., Chen, S., Tao, X., & Huang, Z. (2023). Preliminary Study on the Surface Modification of Lignite and Bioflotation by White-Rot Fungi Hypocrea lixii AH. Minerals, 13(12), 1492. https://doi.org/10.3390/min13121492









