Abstract
The cyclic wetting–drying (W–D) effect as a typical form of weathering causes the engineering properties of rock degradation. Unlike previous research on soft sedimentary rocks, this study sought to investigate the influence of W–D cycles on the physical and mechanical properties of the black sandy dolostone. The results show that the surface hardness and uniaxial compressive strength decreased by 1.5% and 17.2%, respectively, after 12 W–D cycles. The behavior of water absorption of dolostone showed a logarithmic growth with W–D cycles. Analysis of the pH and electrical conductivity values of the soaking solution and microstructure of dolostone revealed that carbonate mineral and feldspar dissolution was the major reason to result in the increase in pore volume and micro-fissure. The oxidation of pyrite contained in the rock was deduced to accelerate the chemical reaction and rock degradation. The obtained results are expected to provide engineering values for rock mechanics studies when compared with in situ conditions.
1. Introduction
The mechanical behaviors and the degradation characteristics of geomaterials have traditionally been met with the effects of environmental factors, which determine the behaviors and responses of samples representative of the physical environments. Due to exposure to a changing ambient environment, the rock is generally suffering various kinds of weathering [1]. One of the most important factors in environmental condition is the moisture of the rock. Any variation of the moisture condition affects its rock conditions. As a typical type of weathering process, the cyclic wetting–drying effect plays a significant important role in influencing the geological and strength properties of rock. A good understanding of the degradation characteristics of rock under the effect of cyclic wetting–drying will facilitate proper design of engineering structures in geohazards mitigation and geotechnical applications [2,3].
There have been several previous studies to investigate the rock mechanics in the laboratory through a series of wetting–drying (W–D) cycles. Mass changes to physical properties have been recorded in laboratory studies [4,5]. The effect of wetting and drying has been found to be an effective agent under a range of simulated environments [6,7,8]. However, most of the studies were performed on relatively weak and soft rocks, in which the influence of water–rock interaction was mainly focused on the water–clay interaction [9]. After experiencing the wetting–drying cycles, the main failure mechanisms for the laminated mudstone start on the microscopic scale by fissures coalescence, exhibiting physico-chemical degradation as well [10]. Compared to the mechanical behaviors of soft rocks subjected to W–D cycles, the process of strength degradation in hard rocks was rather different, with a more pronounced effect of crack development [11]. Subjected to different rock moisture fluctuations ranging from 29% to 63% saturation after 52 W–D cycles, the porosity of the sandstone samples had increased, and water absorption capacities and the saturation coefficients of the samples had decreased [12]. The deterioration of medium-grained sandstone strength and deformation properties is found to be mainly associated with the micro-cracks generated inside the rock in response to cyclic wetting–drying weathering [13].
Some experimental studies of the relationship between water–rock interaction and rock mechanical effects from the perspective of chemical corrosion have been conducted [14,15,16]. Franzoni and Sassoni tested several limestone, sandstone and marble specimens in a simulated acid rain test [17]. It was shown that moisture change that occurs under cyclic wetting and drying can weaken the overlying rock by expansion and contraction. Water is a good solvent and principal agent in physical and chemical weathering.
Some studies have proposed that chemical composition analysis theory and the damage mechanics method can effectively analyze chemical damage mechanisms in water–rock interaction [18,19,20]. The water–rock interaction has a significant time effect on the mechanical parameters of rocks, and its effect varies for different rocks [21,22,23]. It was found that an aqueous environment with higher OH− concentration causes a significant increase in the growth rate of quartz fracture [24,25]. The weathering and dissolution of many mudstone minerals are caused by the reaction of pyrite with acids in the environment during oxidation [26,27,28]. After chemical etching of shale specimens, the soluble mineral composition decreases and clay minerals increase, while mineral cementation becomes loose and mineral edges become blurred. The relative mass loss, secondary porosity and most of the mechanical characteristics of the shale decreased gradually with the decrease in pH and the increase in soaking time [29,30,31]. In addition, due to the existence of pore fractures in the rock mass, if the groundwater level changes, the pore water pressure will also change accordingly, leading to deformation of and damage to the rock mass [32,33]. Although the wetting and drying methods are different among these studies, a consensus can be obtained that the cyclic wetting–drying treatment greatly deteriorates the performance of rock. In order to preserve the integrity of rocks, reducing the moisture content should be taken into consideration [34].
Among the sedimentary rocks, mudstone and sandstone are typical and prevalent research objects under the influence of water–rock interactions. Whereas black sandy dolostone, referred to as a kind of black carbonate rock with a low content of pyrite, is rarely studied. In this study, the free immersion method was experimentally investigated to study the influence of cyclic wetting and drying treatment on the mechanical behavior of the black sandy dolostone. A uniaxial compression test and hardness test on sandy dolostone specimens experiencing different numbers of wetting–drying cycles were conducted, and the variations of physico-mechanical, geochemical and structural properties of the rock are examined and discussed.
2. Materials and Methods
2.1. Chemical Composition of Sample
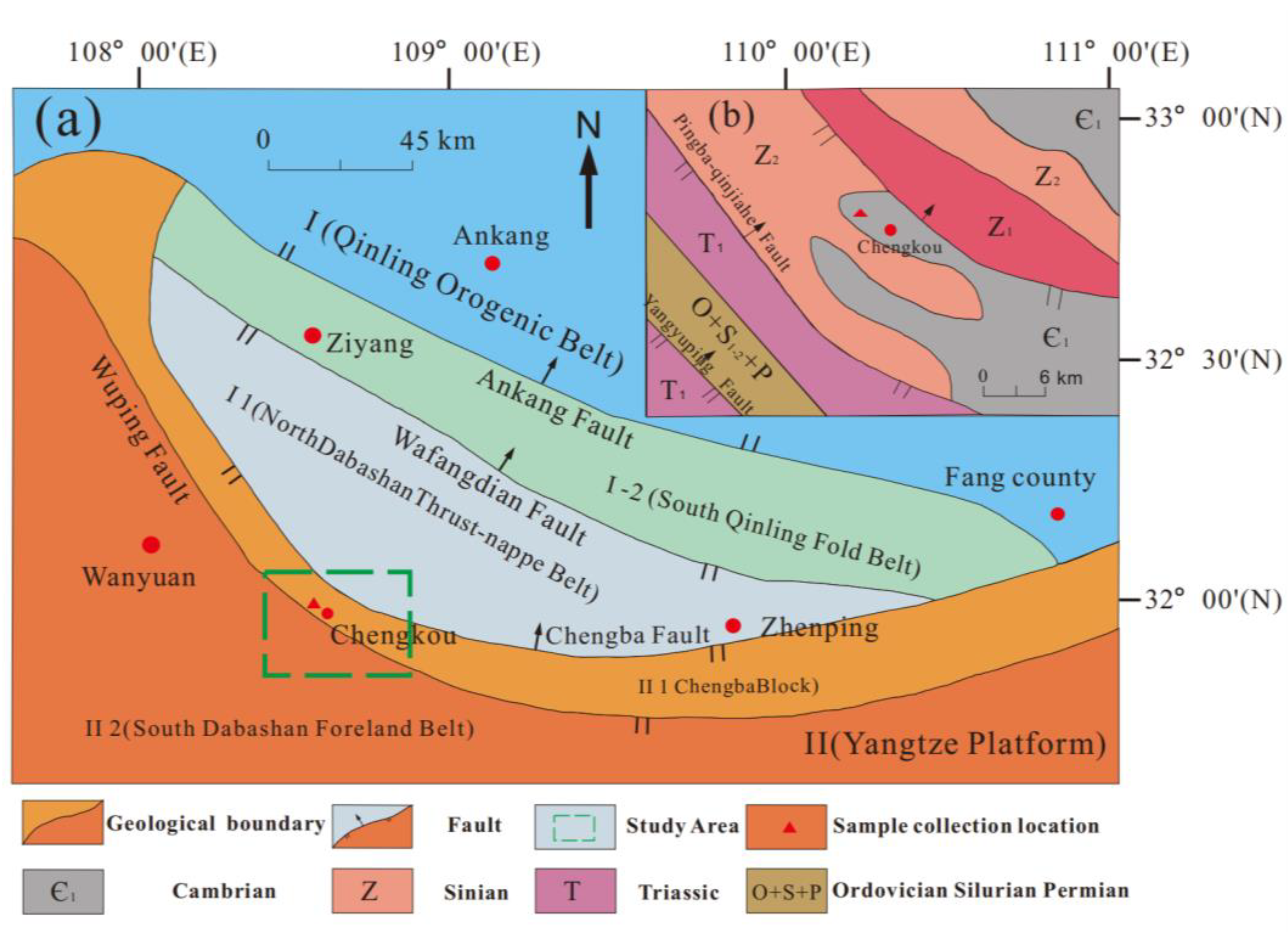
In this paper, the lower Cambrian black sandy dolostone in Chengkou County, Chongqing Municipality, China, was selected as the main object of this study (Figure 1). A simplified structural map of Chengkou County is presented in Figure 1a, and a general geological map of the study area is shown in Figure 1b. The study area is a subtropical monsoon climate zone with sufficient sunshine and rainfall, and the weathering of rocks in the area is mainly in the form of alternating wet and dry effects. Under natural weathering conditions, an acidic water environment is easily formed near the exposed dolostone, which affects the physical and mechanical properties of rock mass.
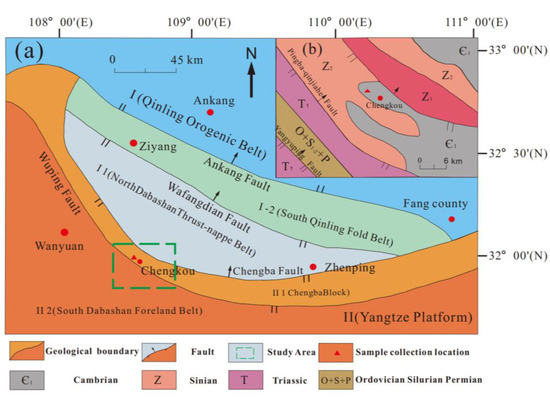
Figure 1.
(a) Simplified structural map of Chengkou County; (b) simplified general geological map of the study area from the green box in (a).
The seepage water from the slope containing the black sandy dolostone is weakly acidic at the study site. This is due to groundwater acidification by the oxidation of sulfide minerals contained in the black dolostone [30]. The chemical alteration of the rock mineral composition caused by acidic erosion water is an important reason for the structural damage of the rock [31]. Acidic water not only destroys the internal structure of the rock and causes rock alteration but also carries away the loose material at the flow path. The acidic water will accelerate the acidification and hydrolysis inside the rock and even form cavities when it flows deeper into the rock.
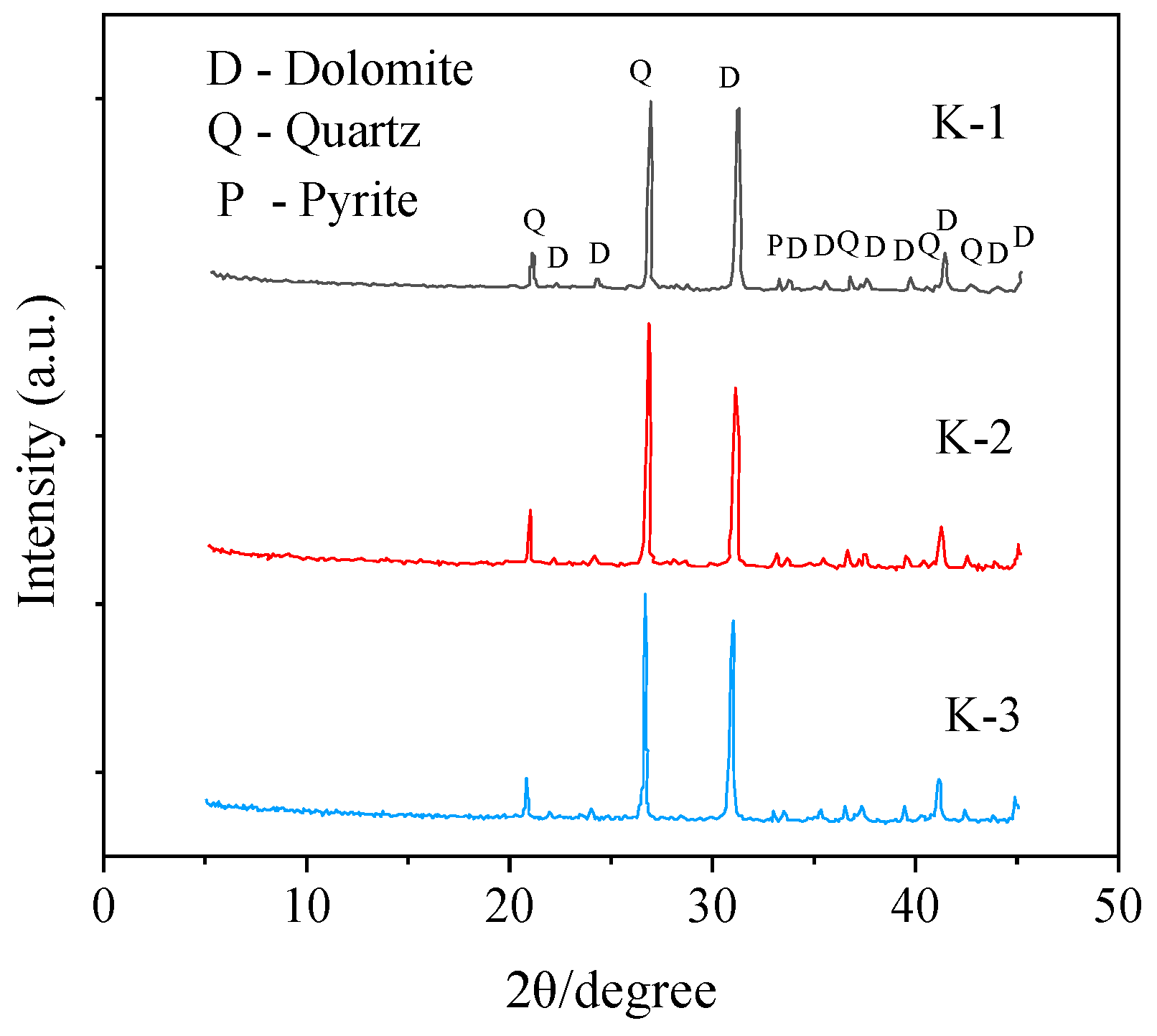
Rock specimens for the experiment were sampled from a depth of 3 m from the outcrop surface. Thirty typical rock samples were collected without significant visual differences. The samples were stored on-site in a sealed bag under dry conditions. Three rocks were selected as specimens for the test, named K-1, K-2 and K-3, respectively. The rock samples (K-1, K-2, K-3) were cleaned on the surface with deionized water and then dried in a drying oven at 60 °C for 24 h. Then, the rock sample was crushed by the crusher with agate mortar and was screened with a 200-mesh nylon sieve (<75 μm). X-ray analyses were widely used to determine the mineralogical and chemical composition of the geological and industrial materials [35]. The following powders are used for mineral composition determination and analysis. Mineral analysis was conducted with X-ray diffraction (XRD; Rigaku Geigerflex RAD-IIB, Japan) of randomly oriented powder mounts using Cu Kα radiation at 20 kV and 50 mA from 3° to 40°. The results of XRD mineral analysis are shown in Table 1 and Figure 2. It can be obtained that the minerals with high contents in the black sandy dolostone are mainly dolomite, quartz, clay minerals, pyrite and plagioclase. Dolomite has the highest content, accounting for 46.6%–52.4%, followed by quartz, clay minerals, pyrite and plagioclase, accounting for 31.7%–36.8%, 8.4%–9.2%, 3.6%–5.3% and 1.6%–2.9%, respectively. Among them, the clay minerals mainly consist of illite, chlorite and kaolinite, accounting for 1.9%–3.6%, 0.4%–0.6% and 0.3%–0.5%, respectively.

Table 1.
Mineral composition of black sandy dolostone (Mass fraction: %).
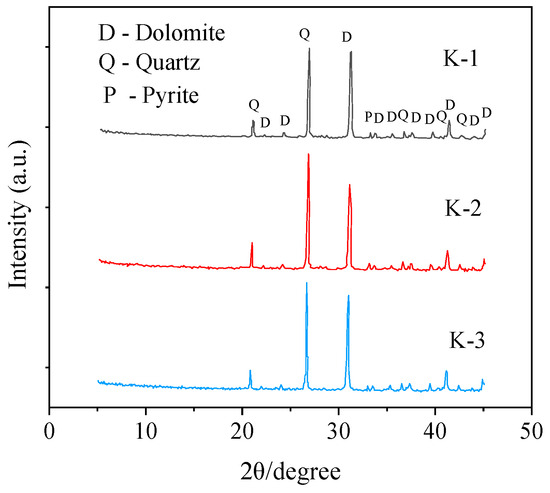
Figure 2.
XRD mineralogical analysis of black sandy dolostone.
The main elemental analysis of the dolostone sample was performed using an X-ray fluorescence spectrometer (ARL Advant’XP). The main chemical compositions are reported in Table 2. It can be seen that SiO2 content is the highest at 32.0%–36.4%, followed by CaO and MgO at 16.5%–18.6% and 11.6%–13.0%, respectively. For the rest, Al2O3 is about 2.2%–3.3%, SO3 is 2.3%–2.8% and TFe2O3 is 1.3%–1.6%. The loss on ignition (LOI) of the rock specimens was relatively high at 25.6%–29.0%.

Table 2.
Main chemical composition of black sandy dolostone.
2.2. Experiments
2.2.1. Sample Preparation and Immersion
According to the International Society of Rock Mechanics (ICRM) recommendations to prepare the corresponding specimen, the shape of the test pieces is a cylinder, with a diameter of 50 mm and a height of 100 mm.
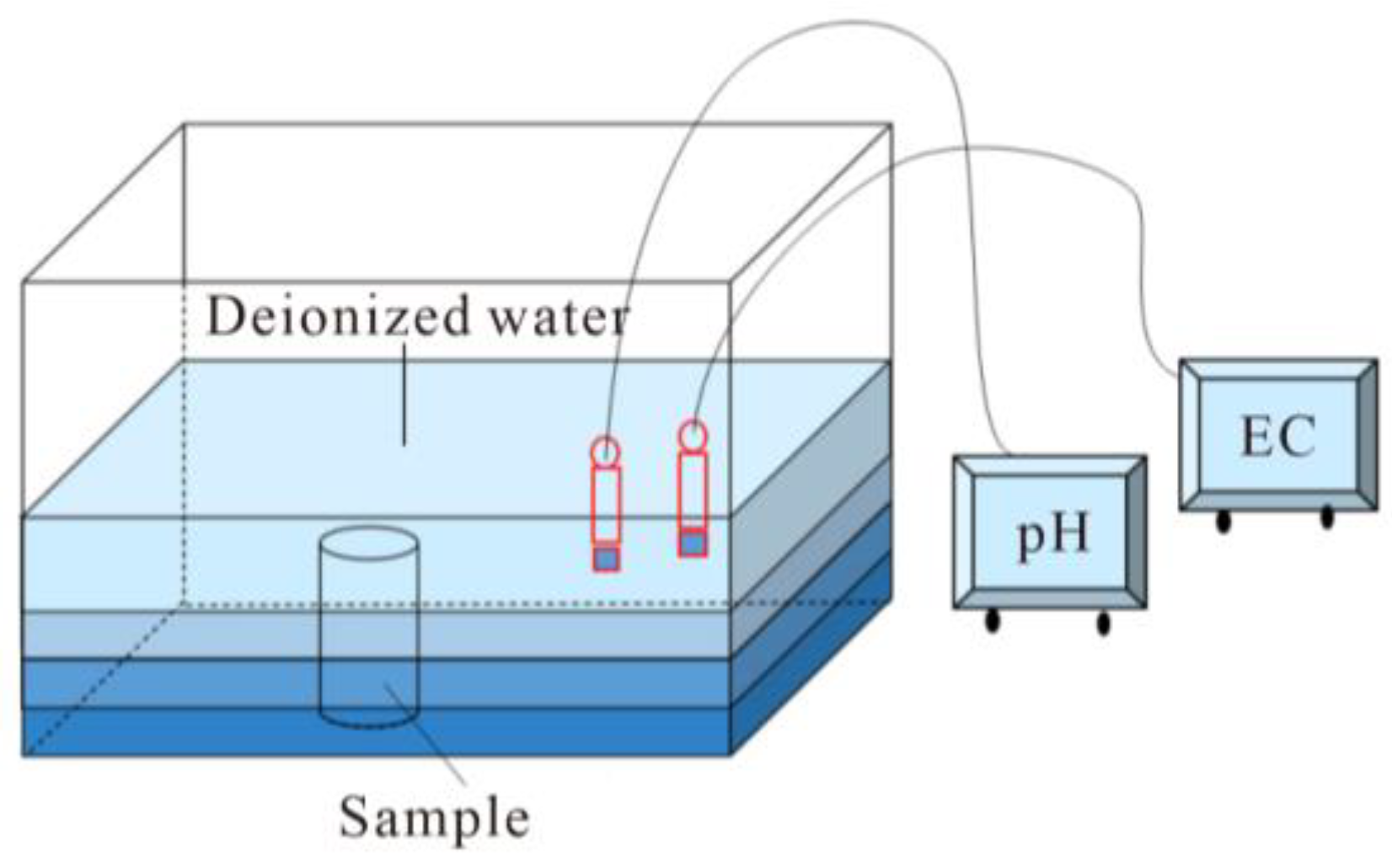
A schematic of the wetting procedure is presented in Figure 3. The water absorption process adopted the free immersion method. The specimen was placed vertically in the immersion tank. The deionized water was added to 1/4 of the sample for the first time, and then water was added to 1/2 and 3/4 of the sample every 2 h. After 6 h, all the specimens were submerged in water, and the water level was 20 cm higher than the sample. During the immersion process, the Electrical Conductivity (EC) and pH values of the solution were measured with an EC meter and pH meter.
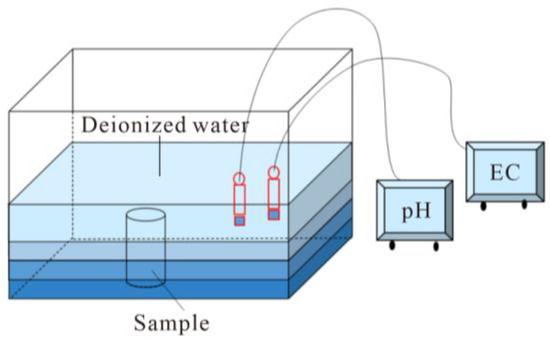
Figure 3.
Schematic illustration of the immersion process of black sandy dolostone.
The rock sample was removed, and the water absorption process of the rock specimen was completed after immersion for 48 h. After that, the sample was placed in a drying oven with a temperature of 60 °C for 24 h. The sample was then cooled to room temperature and subjected to relevant testing.
To study the effect of wetting–drying cycles on the deterioration of dolostone, standard samples of dolostone (A to H) were used for different physical and mechanical tests. Group A was in the natural state, group B was in the saturated state, group C was in the dry state and groups D to H referred to 1, 3, 6, 9 and 12 times of W–D cycles, respectively.
2.2.2. Water Absorption Test
Water absorption is one of the most important hydrologic properties, which reflects the degree of fissure development in rock mass. The water absorption test was completed during the W–D cycles, and the quality of the dolostone samples was recorded in each W–D cycle. The natural water absorption of rock is calculated as follows:
where ωi is the water absorption of the samples, mw is the mass of the sample after immersion in water and ms is the mass of the sample after drying.
2.2.3. Surface Hardness Test
In order to determine whether the W–D alternating environment affects the surface hardness of the black sandy dolomite samples, the surface hardness tests of the samples under the W–D state after different cycles were carried out with the Richter Leeb hardness tester (MH680).
2.2.4. Uniaxial Compression Test
Uniaxial compression tests are conducted by using a YAW4106 computer-controlled electro-hydraulic servo pressure tester. During the tests, the dolostone specimen is placed in the center of the bearing plate of the testing machine, so that the two end surfaces of the specimen and the upper and lower end surfaces of the bearing plate of the testing machine are contacted evenly. The loading speed was 1.0 MPa/sec.
To study the extent of the samples’ deterioration in each stage, the total deterioration degree (∆σi) is defined as the percentage of uniaxial compressive strength loss of the dolostone sample under the action of W–D cycles. At the same time, the stage deterioration degree (∆σsi) is defined as the increase in the percentage of rock strength loss under different W–D cycles. The rock sample without the W–D cycle is regarded as the benchmark, and the stage deterioration degree is 0. Their calculation formula is as follows:
where σi (MPa) is the uniaxial compressive strength of the i cycle and σ0 (MPa) is the uniaxial compressive strength without experiencing W–D cycles.
2.2.5. BET and BJH Test
Gas physisorption is a laboratory method that relies on the balance of Van der Waals forces between gas molecules and solid particles. This technique is used to measure characteristics such as the specific surface area (SSA), the distribution of pore sizes (PSD) and the volume of pores within solid materials and powders [36,37]. This technique depends on an equilibrium adsorption isotherm, which is typically measured at the normal boiling point of the adsorbate, such as 77 K for nitrogen (N2) or 87 K for argon (Ar). The variation of pore characteristics can reflect the deterioration mechanism of black sandy dolomite to a certain extent. The pore characteristics of rock were tested by nitrogen adsorption test, using a BSD-PS1-type nitrogen adsorption test instrument. The test results include the specific surface area and pore size of the rock sample. The BET multipoint method and BJH method were used to test specific surface area and pore size of rock.
3. Results
3.1. Effect of Wetting–Drying Cycles on Water Absorption
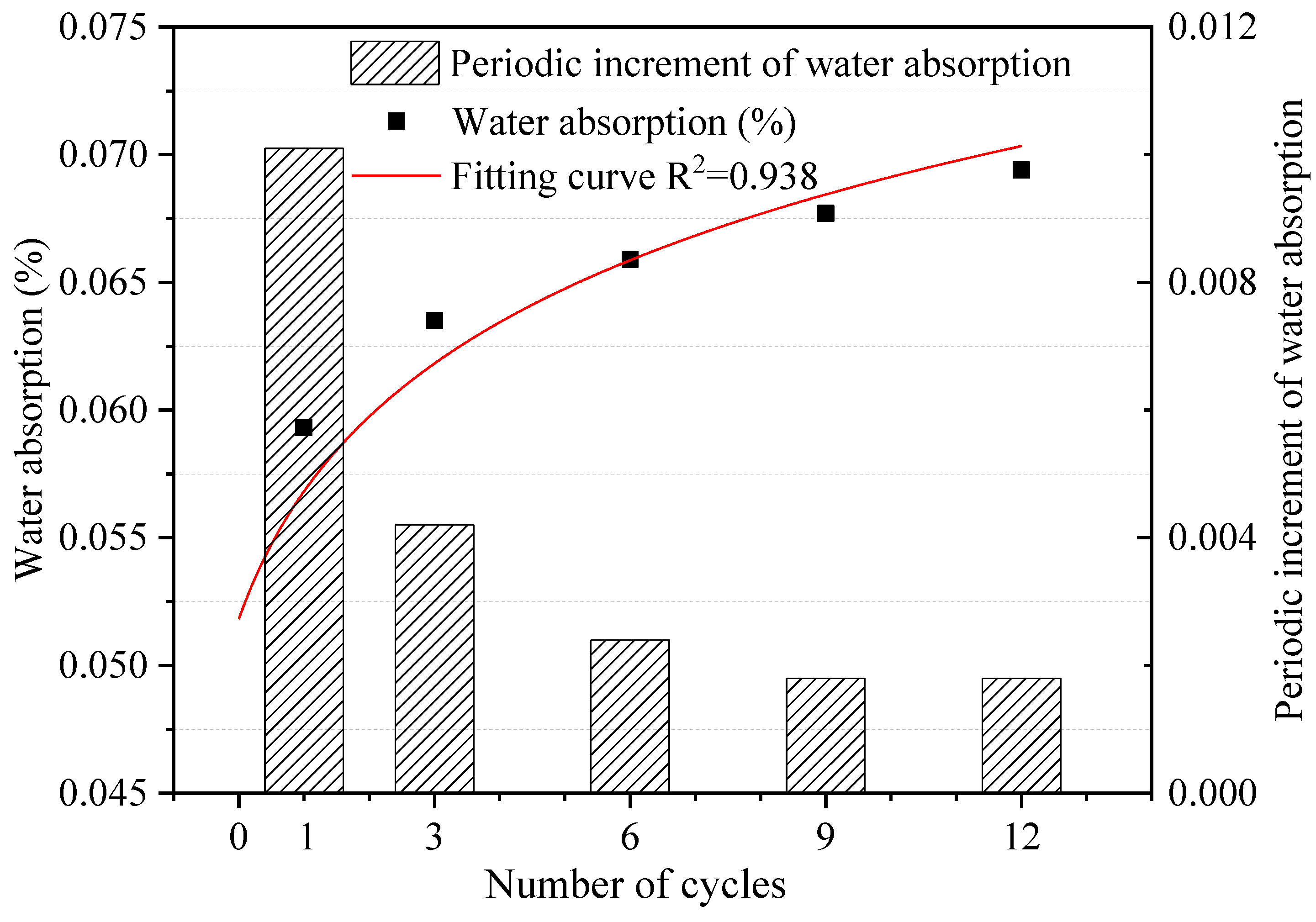
The water absorption of dolostone samples under different W–D cycles was tested, and the effect of W–D cycles on the water absorption rate of dolostone was further investigated to derive the variation patterns involved. Figure 4 shows the test results of the water absorption test. The periodic increment of water absorption was the amount of change between two adjacent test results. Water absorption of samples was one of the most important hydrologic properties, and it can be seen that there is not much increase in water absorption, which may be related to the very dense rocks themselves [38]. The fitting curve equation is shown in Equation (4).
where N is the number of W–D cycles. The water absorption of the dolostone samples was positively correlated with the number of cycles. This indicates that the W–D cycling process causes changes in the water absorption of the rocks, which leads to deterioration of the rock properties. As the water absorption of the rocks increased, the water–rock interaction also strengthened. The periodic increment of water absorption decreased gradually with the number of W–D cycles increased.
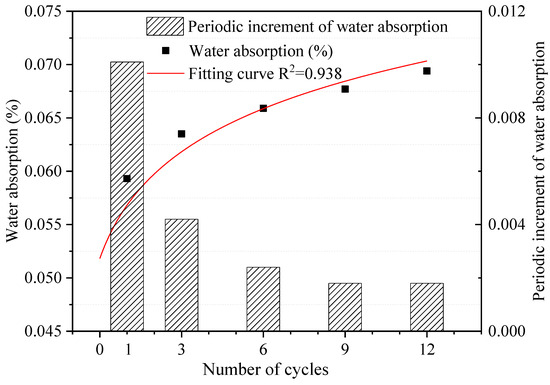
Figure 4.
Water absorption of dolostone samples under different W–D cycles.
3.2. Effect of Wetting–Drying Cycles on Surface Hardness
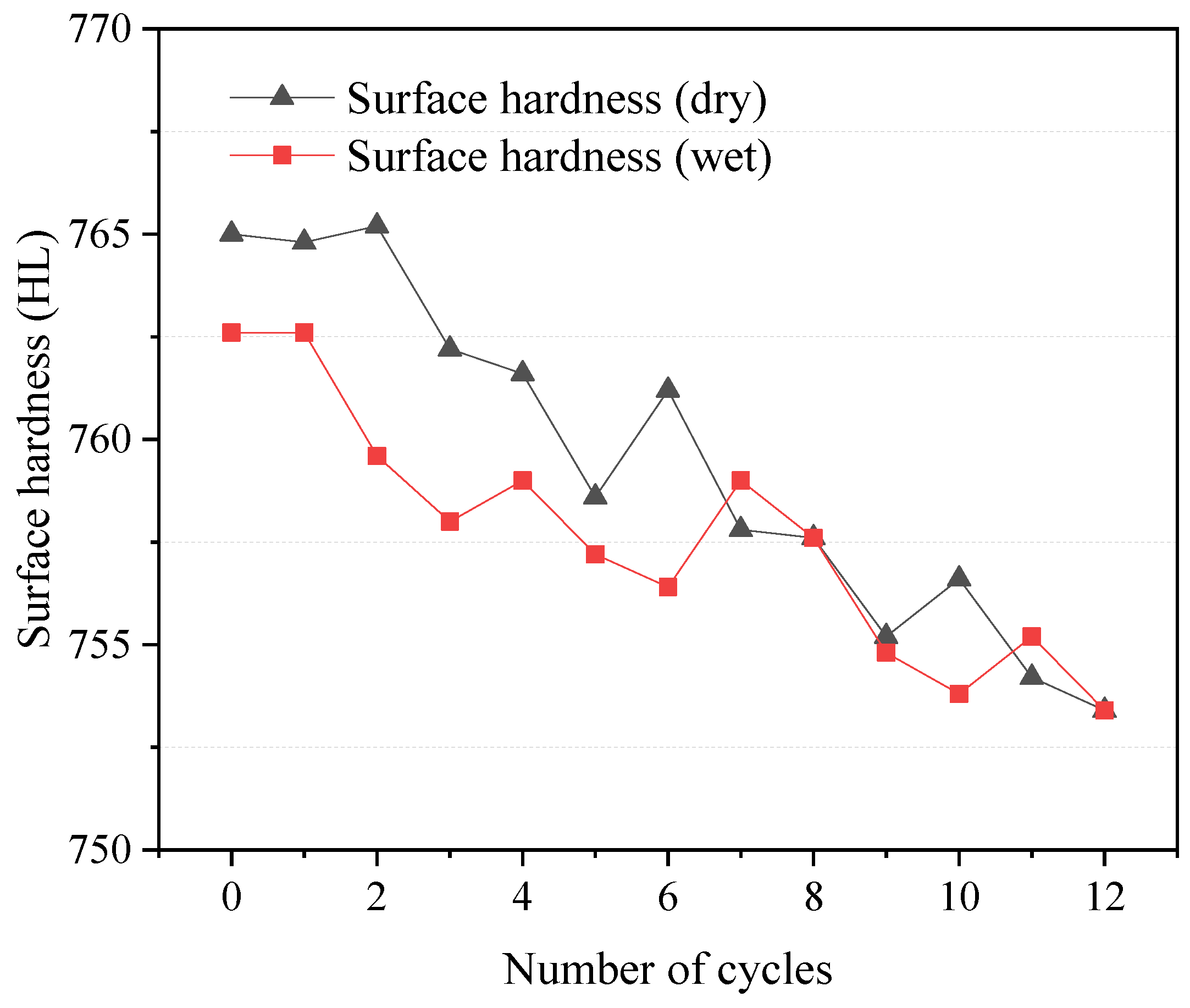
The relationship between W–D cycles and the surface hardness of dolostone was investigated by measuring the surface hardness of specimens. The experimental results of the surface hardness test are shown in Figure 5. The surface hardness of the samples was 765.0 and 762.6 in the dry and wet states, respectively. The surface hardness of the sample did not change significantly during the whole experiment process when the total hardness degradation was less than 2%. However, as the number of cycles increased, the overall trend decreased, which means that the W–D cycles affected the hardness of the rock and led to a reduction in rock properties. Other things being equal, the surface hardness of the dolostone in the wet condition was slightly lower than that in the dry condition. This may be due to the hydrolysis of some minerals in dolostone during the W–D cycles, which leads to the alteration of the surface hardness of the rocks.
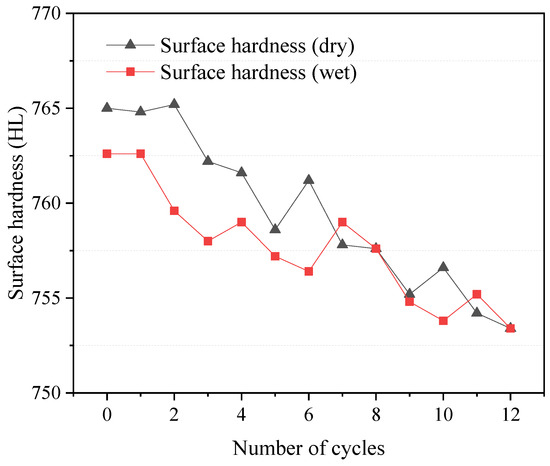
Figure 5.
Relationship between surface hardness and W–D cycles.
3.3. Unconfined Compressive Strength of Dolostone Subjected to W–D Cycles
The mechanical parameters of dolostone samples after different numbers of W–D cycles were obtained by uniaxial compression tests. The damage patterns of two samples are shown in Figure 6. It can be seen that brittle damage is the main damage type of the rocks.

Figure 6.
Damage of K-1 and K-2 samples.
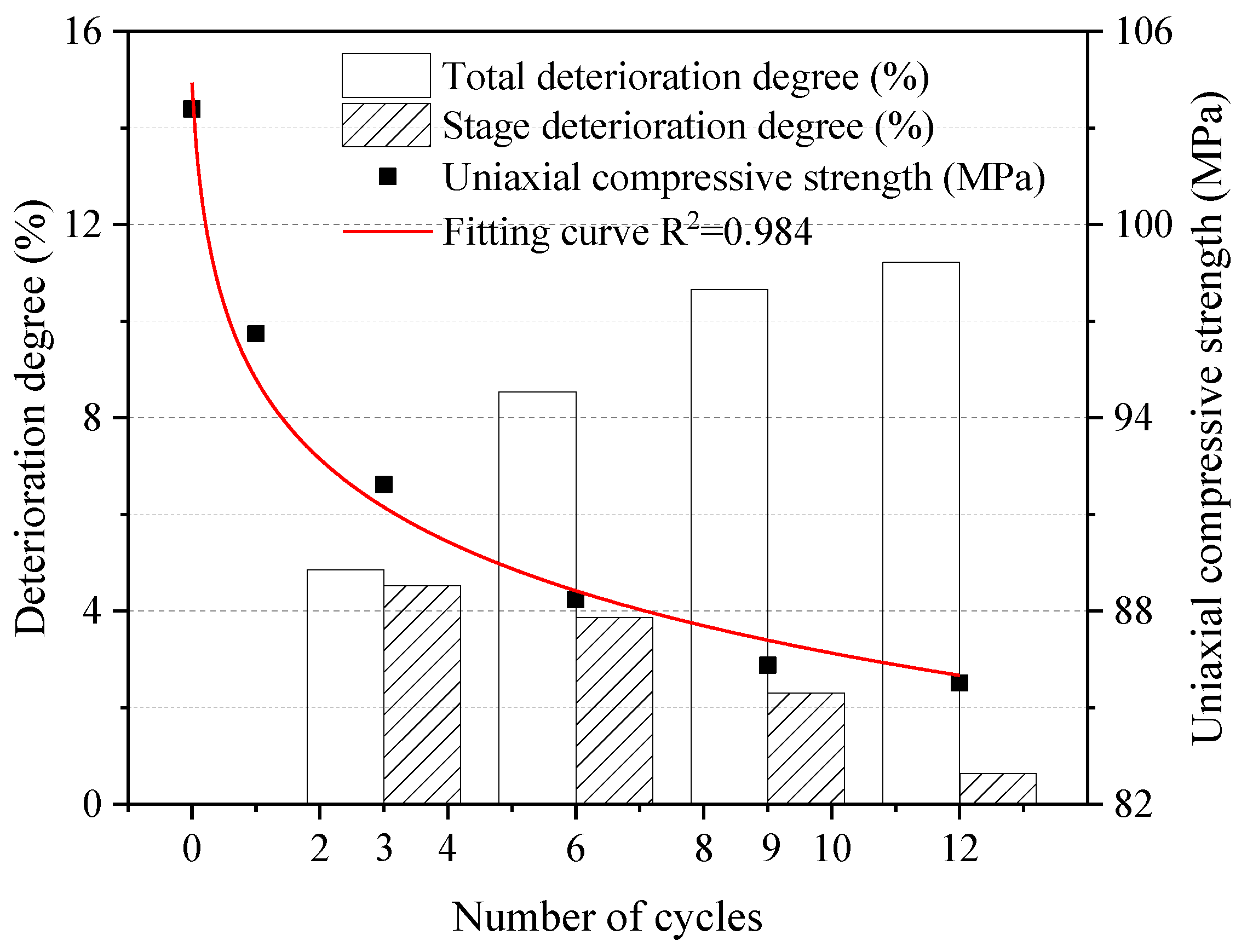
Figure 7 shows the results of the unconfined compressive strength test. The total and stage deterioration degrees of the samples in the test process are calculated from Equations (2) and (3). As can be seen, the uniaxial compressive strength of dolostone showed a general decreasing trend. The deterioration was faster in the early stage of the experiment and slowed down until it stabilized in the later stage of the experiment. The total uniaxial compressive strength deterioration of the dolostone formation samples increased with the number of cycles. The stage deterioration of uniaxial compressive strength was 4.9%, 3.7%, 2.1% and 0.6%, respectively, which showed a significant decreasing trend. It is indicated that the deterioration of early rock properties was more significant by W–D cycles. The unconfined compressive strength values of the samples were fitted, and the fitting curve was as follows.
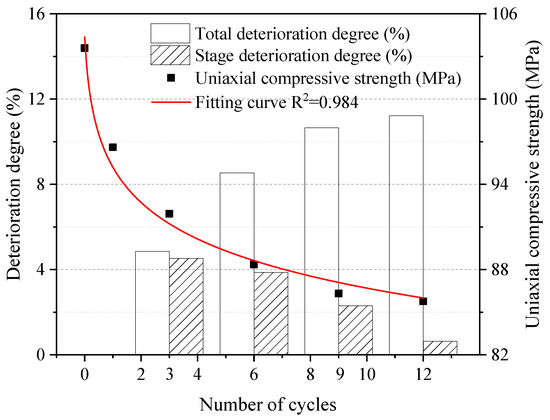
Figure 7.
Uniaxial compressive strength and deterioration degree under different cycles.
4. Discussion
Through the test results of water absorption, surface hardness and uniaxial compressive strength, it can be found that the physical and mechanical properties of black sandy dolostone deteriorate as the number of W–D cycles increases. In order to further investigate the mechanism of water–rock interaction during dolostone degradation, this paper further analyzed the rock pore characteristics and changes of water solution before and after the test and analyzed the rock degradation mechanism caused by water–rock interaction in dolostone under W–D cycles at the microscopic level.
As shown in Table 2, the black sandy dolomite samples used in the test contain carbonate minerals, which are less resistant to water and acid erosion. This results in deterioration of the structural and mechanical properties of the rock. At the same time, sulfide minerals, such as pyrite, in the samples accelerate the weathering and oxidation of rocks. The resulting solution soaks and dissolves the minerals that fill the pores, increasing the porosity inside the rock. The increase in rock void will provide weathering and oxidation channels and reaction sites for the internal structure of rock mass. The sample contains clay minerals, such as illite and montmorillonite. These minerals easily swell and soften in contact with water. These variations will affect the structure and mechanical properties of the rock.
4.1. Effect of Wetting–Drying Cycles on Microstructure
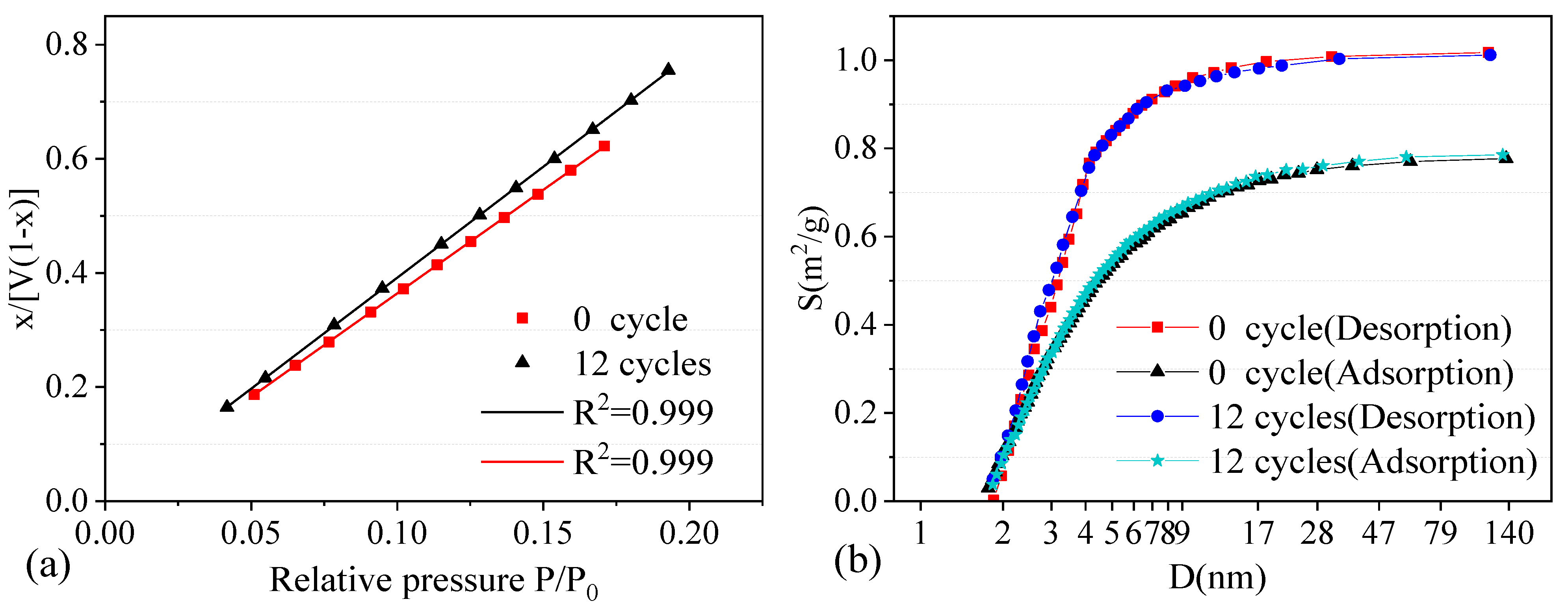
Two groups of samples with 0 and 12 W–D cycles were selected for the nitrogen adsorption test, and the variation trend of pores features was determined through comparative analysis. Nitrogen adsorption test results are shown in Figure 8.
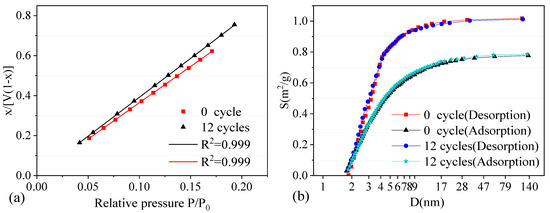
Figure 8.
Nitrogen adsorption test results. (a) Fitting line by BET method. (b) Distribution curve by BJH method.
In Figure 8a, the nitrogen adsorption of the specimen increased continuously with the relative pressure. However, because the specific surface area of the specimen decreased after a certain number of cycles, the corresponding maximum nitrogen adsorption amount also decreased. Figure 8b shows the distribution curve of pore area and pore size by the BJH method. The change in nitrogen adsorption corresponded to the specific surface area of the mesopore (pore diameter ranging from 2 to 100 nm). When the sample exhibits good adsorptivity in the test, it indicates that the sample has abundant mesopores [39].
The pore size distribution result showed that the black sandy dolostone samples are mainly mesopores. The specific surface area of the samples after 12 cycles was obviously smaller than that of the samples without cycles, while the average pore size was larger. The specific surface area and pore size data of the nitrogen adsorption test is shown in Table 3. The pore space of the dolostone samples increased with the number of W–D cycles, while the specific surface area decreased accordingly. The variation of microscopic pores influences the physical and mechanical properties of rock to a certain extent. The relationship between rock water absorption and uniaxial compressive strength was studied to analyze the specific influence law.

Table 3.
Nitrogen adsorption test results of dolostone samples.
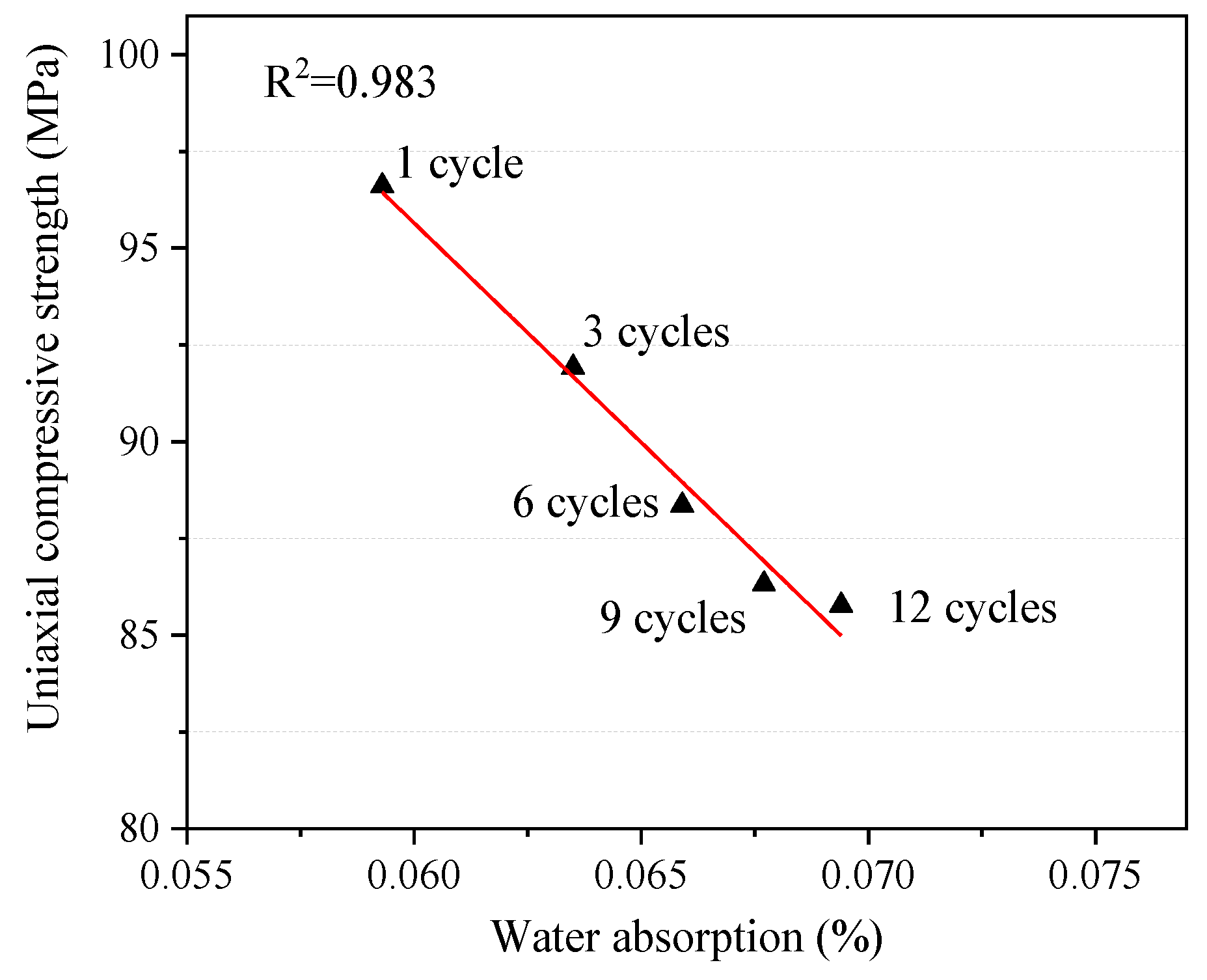
It can be clearly seen from Figure 9 that there is a negative correlation between the water content inside the rock and the uniaxial compressive strength, and the mechanical properties of the rock decrease with the water content. Relevant studies have shown that the mechanical deterioration of rock mass containing clay minerals is more obvious because such rocks have strong water absorption and expansion properties [40,41]. It has been proved in the previous sections that rock water absorption increases with the increase in the number of W–D cycles, while the uniaxial compressive strength of rock decreases on the contrary. It is speculated that the main reason for this trend is that some minerals will be dissolved and altered by water–rock interaction after the rock absorbs water, which leads to the increase in pore size and secondary fractures in the rock. Water is more likely to soak into the rock, breaking the bonds between the mineral grains and weakening it [42].
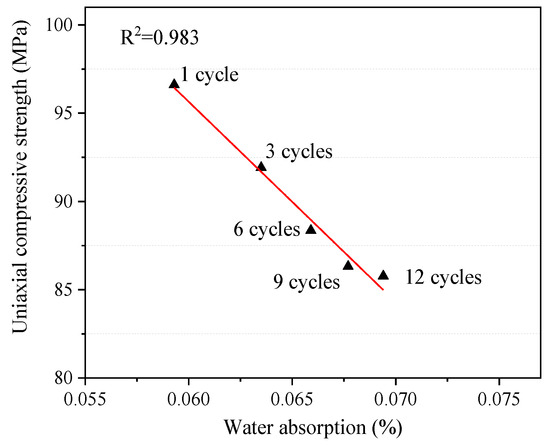
Figure 9.
Relationship between water absorption and uniaxial compressive strength.
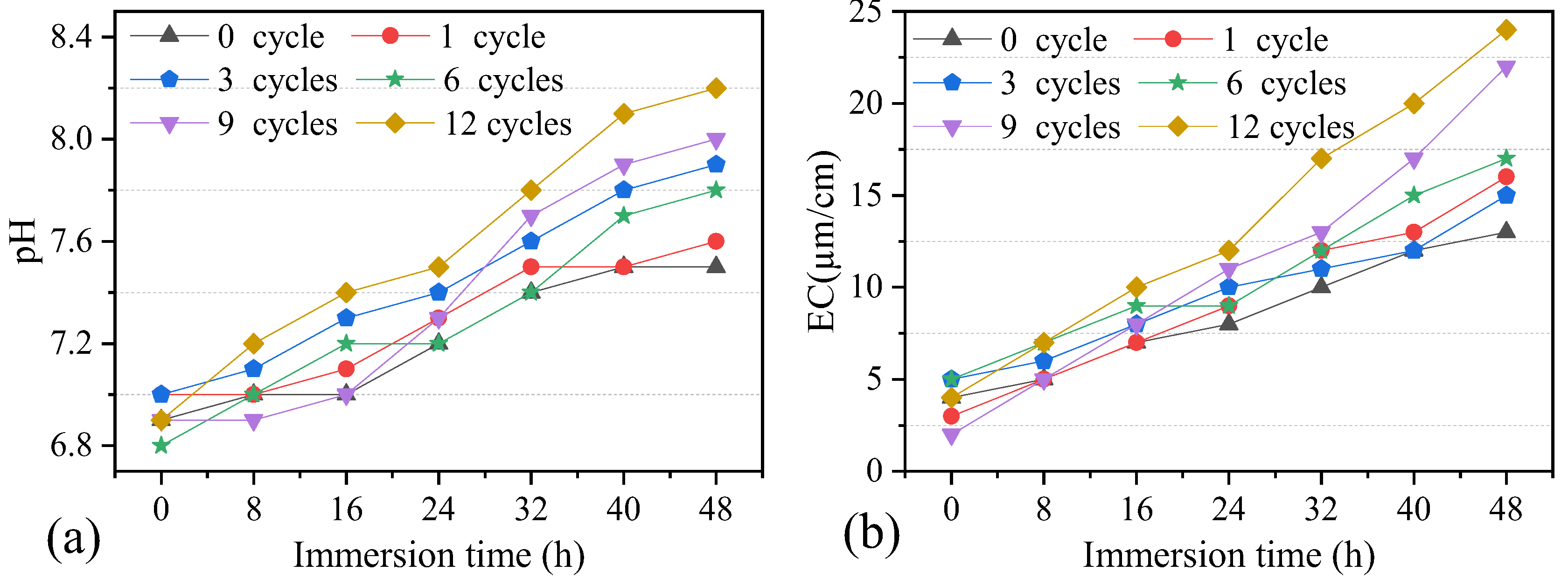
4.2. Effect of Wetting–Drying Cycles on pH and EC of Solution
Chemical interactions play an important role in the deterioration of dolostone. The chemical changes during the W–D cycles were investigated by testing the soaking solutions. The soaking solution was tested every 8 h using the pH meter and EC meter. The test results showed that the sample solution was weakly alkaline, with a pH value of 6.8–8.2 and an EC value of 2–24. In order to better analyze the changes in EC and pH values in a cycle, the variation of pH and EC values with immersion time was plotted (Figure 10). It shows that pH and EC values increased with the soaking time. This is because, in the early stage of the experiment, a small amount of hydrogen ions reacted with some minerals on the surface of the dolostone sample through a cation exchange reaction, resulting in the increasing concentration of soluble salts. EC values remained low throughout the water absorption process, although they showed an increasing trend.
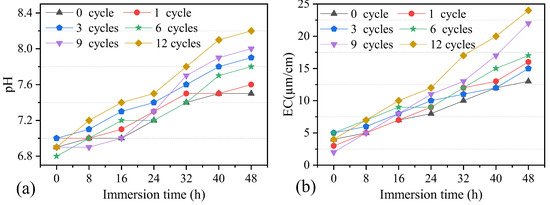
Figure 10.
Variation of pH and EC values of solution during immersion. (a) pH. (b) EC.
4.3. Mechanism of Water–Rock Interaction Subjected to Wetting–Drying Cycles
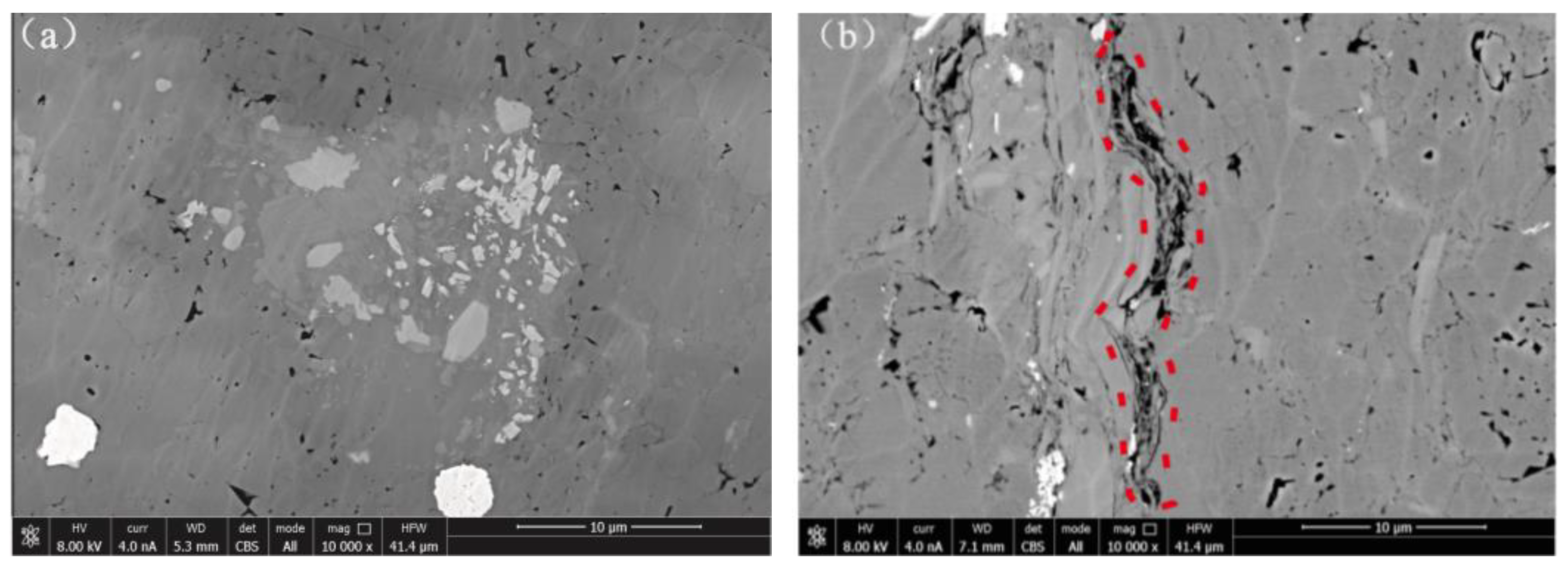
Pore fractures within the black sandy dolomite were observed, as well as some specific mineral morphologies using Scanning Electron Microscopy (SEM) (Figure 11). The figure shows the microscopic structure of the specimens before and after 12 cycles.
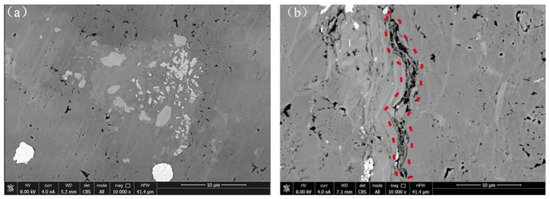
Figure 11.
SEM images of black sandy dolomite before and after W–D cycles. (a) Before W–D cycles. (b) After W–D cycles.
Figure 11a shows the microscopic images of the specimens before W–D cycles. It can be seen that the overall structure of the black sandpaper dolomite was very tight. The darker color was mostly dolomite and quartz, which together formed the overall structural framework of the rock. The clay minerals, on the other hand, showed a sheet-like structure, embedded in the framework of dolomite and quartz, or filled in the pores inside the rock. Figure 11b shows the microscopic images of the specimens after W–D cycles. Rocks that have undergone W–D cycles are significantly more fractured than the initial state. This is because the pore water interacts with mineral particles as it flows through the fractures. These minerals and ions are carried to the fracture openings for deposition and generation of secondary minerals. Secondary minerals continue to accumulate and squeeze the primary pore space, resulting in increasing primary pore space and secondary fractures. The cementation of the mineral particles is altered so that the mechanical properties are reduced. The original cementation surface disappears, and the mineral particles are corroded to be finer and more loosely interconnected [43].
To further investigate the chemical reactions that cause microstructural changes during water–rock interaction, the ion concentration of the immersion solution of H-1 specimens after one cycle was examined. The water quality analysis of the soaking solution was performed on the specimens after a W–D cycle, and the test results are shown in Table 4.

Table 4.
Water quality measurement of soaking water (unit: mg/L).
Table 4 shows that the calcium and magnesium ions were relatively high in the aqueous solution after the first immersion of the rocks, and the concentration of cations such as sodium, potassium and iron was low. Carbonate ions and bicarbonate ions were present in large quantities, while sulfate ions were also measured. It is presumed that some of the carbon dioxide in the air dissolved in the water and some physical dissolution of the carbonate minerals occurred.
The main cause of rock deterioration under the interaction of W–D cycles is the chemical reaction between the rock and the water environment resulting in pore changes. During the whole process of water–rock interaction, the physical and chemical water–rock interactions will exist simultaneously. Previous studies have found that in the original sedimentary environment of black carbonate rocks, carbonate minerals such as dolomite are relatively developed, filling and cementing the pore channels of rock mass, but carbonate minerals are very sensitive to the environment [44]. In a neutral environment, carbonate minerals are mainly physically dissolved by W–D cycles. While in an acidic environment, carbonate minerals tend to chemically react and reduce the overall stability of the dolostone. The reaction equation is shown in Equations (6) and (7).
The dolostone samples contained a small amount of feldspar minerals, but its physical dissolution and chemical weathering process cannot be ignored. In the process of long-term physical weathering and transportation, the grain size of feldspar minerals will constantly decrease. It is easier to dissolve and contact with other minerals in the process of water–rock chemical interaction. The feldspar minerals in dolostone are easily changed into clay minerals such as kaolinite and illite by leaching and dissolution. Clay minerals have strong swelling properties. When encountering water, clay minerals swell, disintegrate and fall off, which will weaken the mechanical properties of rock and also aggravate the weathering of surrounding rock mass [45]. The reaction equation is shown in Equations (8) and (9).
Pyrite was present in relatively higher amounts in dolostone samples. During the process of W–D cycles, divalent iron will be oxidized to trivalent iron, which in turn will form iron hydroxide and finally be decomposed to iron oxide. This reaction could lead to a decrease in the strength of the rock structure and erosion damage to the surrounding rock and concrete structures in actual engineering.
The internal structure characteristics of different rocks determine their different weathering resistance. The process of water–rock interaction is influenced by minerals dissolution and pyrite oxidation subjected to W–D cycles. Meanwhile, the weathering behavior of dolostone changes the development of rock pores. When there are more joints, fractures and pores, the contact area between water and rock is greater, which leads to a greater influence of water–rock interaction on the dolostone [46].
5. Conclusions
In this study, the influence of cyclic wetting–drying weathering on the mechanical behavior of black sandy dolostone was experimentally investigated. The main specific conclusions may be drawn as follows:
- (1)
- The water absorption, surface hardness and uniaxial compressive strength of the rock generally decrease with increasing wetting–drying cycle in the treatment. The relationships between the deterioration degree (water absorption and uniaxial compressive strength) and the number of W–D cycles were obtained from the laboratory experiment.
- (2)
- The average pore size of the dolostone increased, while the specific surface area decreased accordingly after 12 W–D cycles. The results permit water to enter into the pore and react with the rock sufficiently and cause the strength and structure of dolostone deterioration from the micro perspective.
- (3)
- It was found that the pH and EC values increased during the cyclic W–D treatment. Combined with the mineral and water chemistry analysis, it contributed to the feldspar and carbonate minerals dissolution during the water–rock interaction.
Author Contributions
Conceptualization, L.Z. and X.L. (Xin Liao); methodology, Z.L.; software, X.L. (Xinyu Luo); validation, Z.L.; formal analysis, Q.T.; investigation, H.L.; resources, X.L. (Xin Liao); data curation, Z.L.; writing—original draft preparation, L.Z. and H.L.; writing—review and editing, X.L. (Xin Liao) and Q.T.; supervision, X.L. (Xin Liao); project administration, X.L. (Xin Liao); funding acquisition, L.Z., X.L. (Xin Liao) and Q.T. All authors have read and agreed to the published version of the manuscript.
Funding
The research is supported by the National Natural Science Foundation of China (52078317), Natural Science Foundation of Jiangsu Province for Excellent Young Scholars (BK20211597), Natural Science Foundation of Sichuan for Young Scholars (2022 NSFSC1117) and the Research Program of China 19th Metallurgical Group Co., Ltd. (Grant No. FGC-CK-20220475).
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank the Analytical and Testing Center of Southwest Jiaotong University for XRD and XRF analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Trenhaile, A. Tidal wetting and drying on shore platforms: An experimental study of surface expansion and contraction. Geomorphology 2006, 76, 316–331. [Google Scholar] [CrossRef]
- Peng, J.; Rong, G.; Cai, M.; Wang, X.J.; Zhou, C.B. An empirical failure criterion for intact rocks. Rock Mech. Rock. Eng. 2014, 47, 347–356. [Google Scholar] [CrossRef]
- Zhao, S.; Dai, F.; Deng, J.; Wen, H.; Li, H.; Chen, F. Insights into landslide development and susceptibility in extremely complex alpine geoenvironments along the western Sichuan-Tibet Engineering Corridor, China. Catena 2023, 227, 107105. [Google Scholar] [CrossRef]
- Hale, P.D.; Shakoor, A. A laboratory investigation of the effects of cyclic heating and cooling, wetting and drying, and freezing and thawing on the compressive strength of selected sandstones. Environ. Eng. Geosci. 2003, 9, 117–130. [Google Scholar] [CrossRef]
- Hall, K.J.; Hall, A. Weathering by wetting and drying: Some experimental results. Earth Surf. Proc. Land. 1996, 21, 365–376. [Google Scholar] [CrossRef]
- Wells, T.; Binning, P.; Willgoose, G. The role of moisture cycling in the weathering of a quartz chlorite schist in a tropical environment: Findings of a laboratory simulation. Earth Surf. Proc. Land. 2005, 30, 413–428. [Google Scholar] [CrossRef]
- Kanyaya, J.I.; Trenhaile, A.S. Tidal wetting and drying on shore platforms: An experimental assessment. Geomorphology 2005, 70, 129–146. [Google Scholar] [CrossRef]
- Cantón, Y.; Solé-Benet, A.; Queralt, I.; Pini, R. Weathering of a gypsum-calcareous mudstone under semi-arid environments at Tabernas, SE Spain: Laboratory and field-based experimental approaches. Catena 2001, 44, 111–132. [Google Scholar] [CrossRef]
- Gokceoglu, C.; Ulusay, R.; Sonmez, H. Factors affecting the durability of selected weak and clay-bearing rocks from Turkey, with particular emphasis on the influence of the number of drying and wetting cycles. Eng. Geol. 2000, 57, 215–237. [Google Scholar] [CrossRef]
- Torres-Suarez, M.; Alarcon-Guzman, A.; Moya, R. Effects of loading–unloading and wetting–drying cycles on geomechanical behaviors of mudrocks in the Colombian Andes. J. Rock Mech. Geotech. Eng. 2014, 6, 257–268. [Google Scholar] [CrossRef]
- Gratchev, I.; Pathiranagei, S.V.; Kim, D.H. Strength properties of fresh and weathered rocks subjected to wetting-drying cycles. Geomech. Geophy. Geo-Energy Geo-Resour. 2019, 5, 211–221. [Google Scholar] [CrossRef]
- Sumner, P.D.; Loubser, M.J. Experimental sandstone weathering using different wetting and drying moisture amplitudes. Earth Surf. Proc. Land. 2008, 33, 985–990. [Google Scholar] [CrossRef]
- Li, X.; Peng, K.; Peng, J.; Hou, D. Experimental investigation of cyclic wetting-drying effect on mechanical behavior of a medium-grained sandstone. Eng. Geol. 2021, 293, 106335. [Google Scholar] [CrossRef]
- Liao, X.; Chigira, M.; Matsushi, Y.; Wu, X. Investigation of water–rock interactions in Cambrian black shale via a flow-through experiment. Appl. Geochem. 2014, 51, 65–78. [Google Scholar] [CrossRef]
- Thewes, M.; Hollmann, F. Assessment of clay soils and clay-rich rock for clogging of TBMs. Tunn. Undergr. Sp. Technol. 2016, 57, 122–128. [Google Scholar] [CrossRef]
- Fransson, Å.; Viola, G. Bentonite rock interaction experiment: A hydro-structural-mechanical approach. Eng. Geol. 2021, 28, 105985. [Google Scholar] [CrossRef]
- Franzoni, E.; Sassoni, E. Correlation between microstructural characteristics and weight loss of natural stones exposed to simulated acid rain. Sci. Total Environ. 2011, 412, 278–285. [Google Scholar] [CrossRef]
- Li, J.; Kaunda, R.B.; Zhou, K. Experimental investigations on the effects of ambient freeze-thaw cycling on dynamic properties and rock pore structure deterioration of sandstone. Cold Reg. Sci. Technol. 2018, 154, 133–141. [Google Scholar] [CrossRef]
- Park, J.; Choi, B.Y.; Lee, M.; Yang, M. Porosity changes due to analcime in a basaltic tuff from the Janggi Basin, Korea: Experimental and geochemical modeling study of CO2-water-rock interactions. Environ. Earth Sci. 2021, 80, 81. [Google Scholar] [CrossRef]
- Cuisinier, O.; Masrouri, F.; Mehenni, A. Alteration of the Hydromechanical performances of a stabilized compacted soil exposed to successive wetting–drying cycles. J. Mater. Civ. Eng. 2020, 32, 04020349. [Google Scholar] [CrossRef]
- Soltani, A.; Deng, A.; Taheri, A.; Mirzababaei, M.; Vanapalli, S.K. Swell–shrink behavior of rubberized expansive clays during alternate wetting and drying. Miner 2019, 9, 224. [Google Scholar] [CrossRef]
- Estabragh, A.R.; Soltani, A.; Javadi, A.A. Effect of pore water chemistry on the behaviour of a kaolin-bentonite mixture during drying and wetting cycles. Eur. J. Environ. Civ. Eng. 2019, 24, 895–914. [Google Scholar] [CrossRef]
- Kholghifard, M. Effective stress and compressibility of unsaturated clayey soil under drying and wetting cycles. Period. Polytech. Civ. Eng. 2020, 64, 999–1006. [Google Scholar]
- Király, C.; Varga, G.; Falus, G.; Szalai, Z. Effect of water-rock interaction on particle shapes in sandstone samples (Pannonian Basin, Hungary). Geophys. Res. Abstr. 2019, 21, 1. [Google Scholar]
- Medina, F.G.C.; Ventura-Houle, R.; Rodríguez, L.H.; Lara, G.N.R.; Mansilla, O.G.; Ramírez, E.N. Water–rock interactions in a karst aquifer located in southwestern Tamaulipas, Mexico. Carbonates Evaporites 2021, 36, 59. [Google Scholar] [CrossRef]
- Chigira, M.; Oyama, T. Mechanism and effect of chemical weathering of sedimentary rocks. Eng. Geol. 1999, 55, 3–14. [Google Scholar] [CrossRef]
- Helson, O.; Eslami, J.; Beaucour, A.L.; Noumowe, A.; Gotteland, P. Durability of soil mix material subjected to wetting/drying cycles and external sulfate attacks. Constr. Build. Mater. 2018, 192, 416–428. [Google Scholar] [CrossRef]
- Jerman, M.; Scheinherrova, L.; Medve, I.; Krejsová, J.; Černý, R. Effect of cyclic wetting and drying on microstructure, composition and length changes of lime-based plasters. Cem. Concr. Compos. 2019, 104, 103411. [Google Scholar] [CrossRef]
- Guo, J.; Liu, P.; Wu, C.; Wang, K. Effect of dry–wet cycle periods on properties of concrete under sulfate attack. Appl. Sci. 2021, 11, 888. [Google Scholar] [CrossRef]
- Ling, S.; Wu, X.; Zhao, S.; Liao, X. Evolution of porosity and clay mineralogy associated with chemical weathering of black shale: A case study of Lower Cambrian black shale in Chongqing, China. J. Geochem. Explor. 2018, 188, 326–339. [Google Scholar] [CrossRef]
- Ling, S.; Wu, X.; Sun, C.; Liao, X.; Ren, Y. Mineralogy and geochemistry of three weathered Lower Cambrian black shale profiles in Northeast Chongqing, China. Geosci. J. 2016, 20, 793–812. [Google Scholar] [CrossRef]
- Noor-E-Khuda, S.; Albermani, F. Flexural strength of weathered granites under wetting-drying cycles: Implications to steel structures. Adv. Steel Constr. 2019, 15, 225–231. [Google Scholar]
- Tian, H.; Kempka, T.; Yu, S.; Ziegler, M. Mechanical properties of sandstones exposed to high temperature. Rock Mech. Rock. Eng. 2015, 49, 321–327. [Google Scholar] [CrossRef]
- Kovalski, E.R.; Kongar-Syuryun, C.B.; Petrov, D.N. Challenges and prospects for several-stage stoping in potash minining. Sustain. Dev. Mt. Territ. 2023, 15, 349–364. [Google Scholar] [CrossRef]
- Kaung, P.F.; Semikin, A.A.; Khayrutdinov, A.M.; Dekhtyarenko, A.A. Recycling of industrial waste is a paradigm of resource provision for sustainable development. Sustain. Dev. Mt. Territ. 2023, 15, 385–397. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Hayati-Ashtiani, M. Characterization of nano-porous bentonite (montmorillonite) particles using FTIR and BET-BJH analyses. Part. Part. Syst. Charact. 2013, 28, 71–76. [Google Scholar] [CrossRef]
- Ling, S.; Wu, X.; Sun, C.; Liao, X.; Ren, Y.; Li, X. Experimental study on chemical damage and mechanical deterioration of black shale caused by water-rock chemical action. Exp. Mech. 2016, 31, 511–524. [Google Scholar]
- Feng, X.; Haimson, B.; Li, X.; Chang, C.; Ma, X.; Zhang, X.; Ingraham, M.; Suzuki, K. ISRM suggested method: Determining deformation and failure characteristics of rocks subjected to true triaxial compression. Rock Mech. 2019, 54, 1553–1566. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, Y.; Gao, Y.; Gu, F. Use of cement-chelated solidified MSWI fly ash for pavement material: Mechanical and Environmental Evaluations. Can. Geotech. J. 2017, 54, 1553–1566. [Google Scholar] [CrossRef]
- Chen, J.; Benson, C.H.; Edil, T.B. Hydraulic conductivity of geosynthetic clay liners with sodium bentonite to coal combustion product leachates. J. Geotech. Geoenviron. Eng. 2018, 144, 04018008. [Google Scholar] [CrossRef]
- Gautam, T.P.; Shakoor, A. Slaking behavior of clay-bearing rocks during a one-year exposure to natural climatic conditions. Eng. Geol. 2013, 166, 17–25. [Google Scholar] [CrossRef]
- Ma, T.; Wei, C.; Yao, C.; Yi, P. Microstructural evolution of expansive clay during drying-wetting cycle. Acta. Geotech. 2020, 15, 2355–2366. [Google Scholar] [CrossRef]
- Liu, N.; Cheng, J. Geochemical effects of cement mineral variations on water–rock–CO2 interactions in a sandstone reservoir as an experiment and modeling study. Greenh. Gases Sci. Technol. 2019, 9, 789–810. [Google Scholar] [CrossRef]
- Fan, L.; Gao, J.; Du, X. Thermal cycling effects on micro-property variation of granite by a spatial micro-observation. Rock Mech. 2021, 53, 2921–2928. [Google Scholar] [CrossRef]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Hammes, U. Spectrum of pore types and networks in mudrocks and a descriptive classification for matrix-related mudrock pores. AAPG Bull. 2012, 96, 1071–1098. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).