Theoretical Research on the Reduction of CO2 with H2S on Pyrite FeS2 Surfaces
Abstract
:1. Introduction
2. Parameters and Models of Theoretical Calculation
3. Results and Discussion
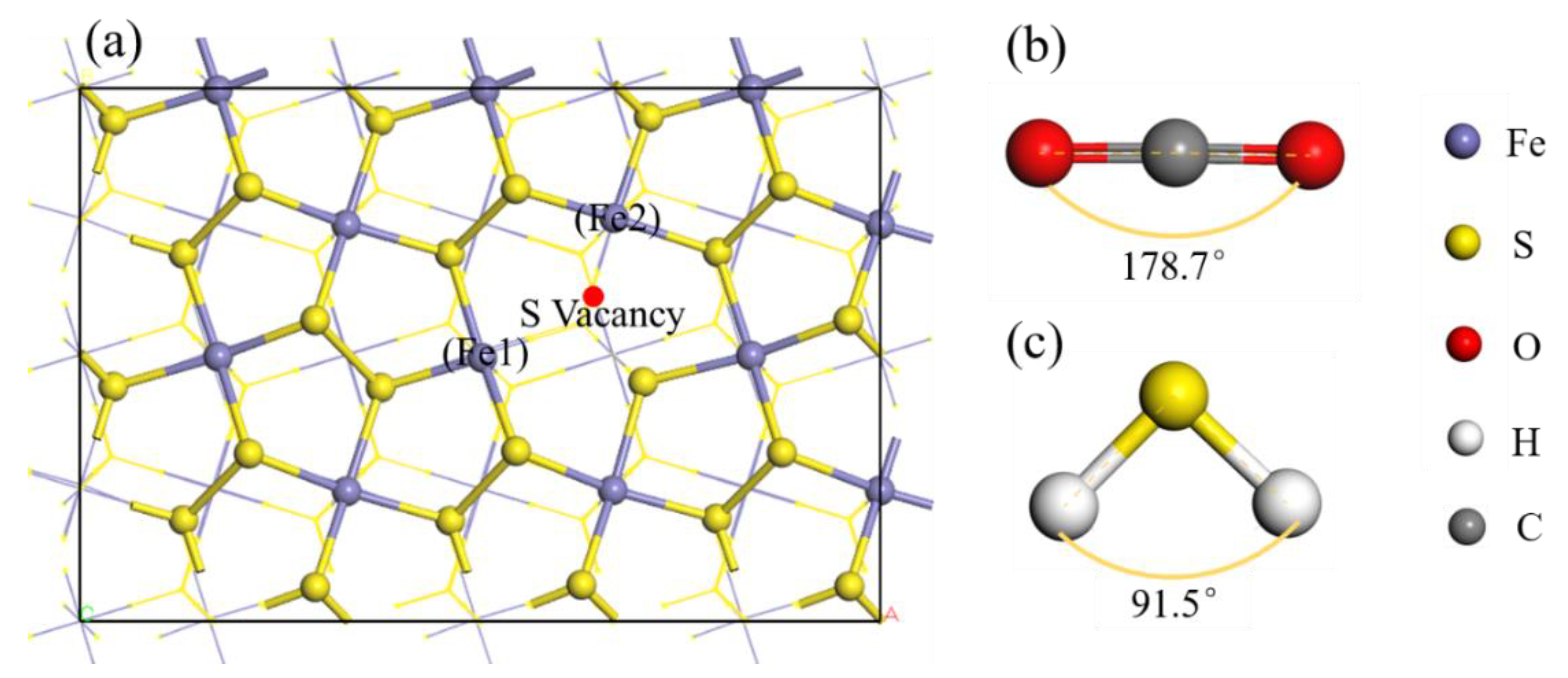
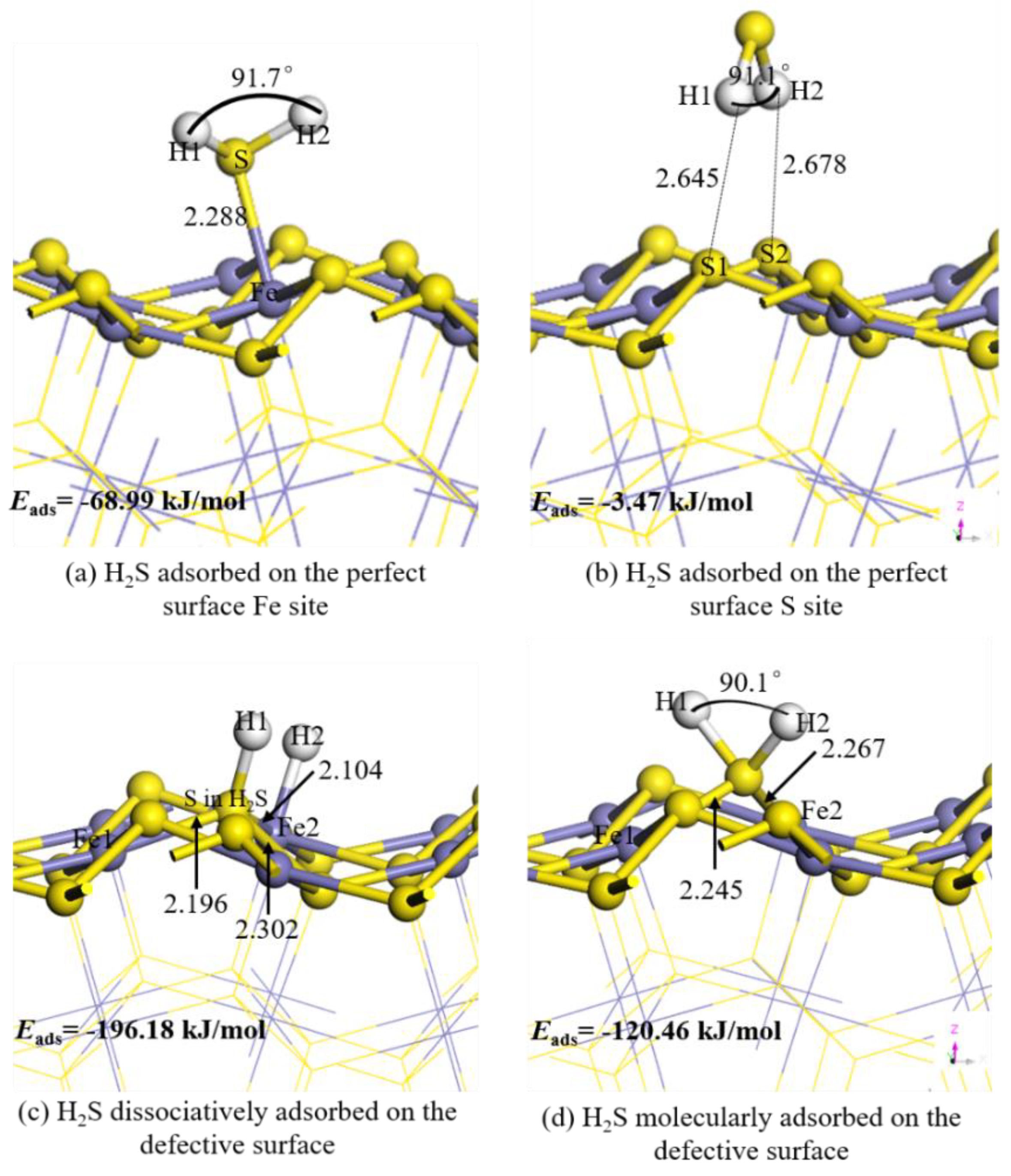
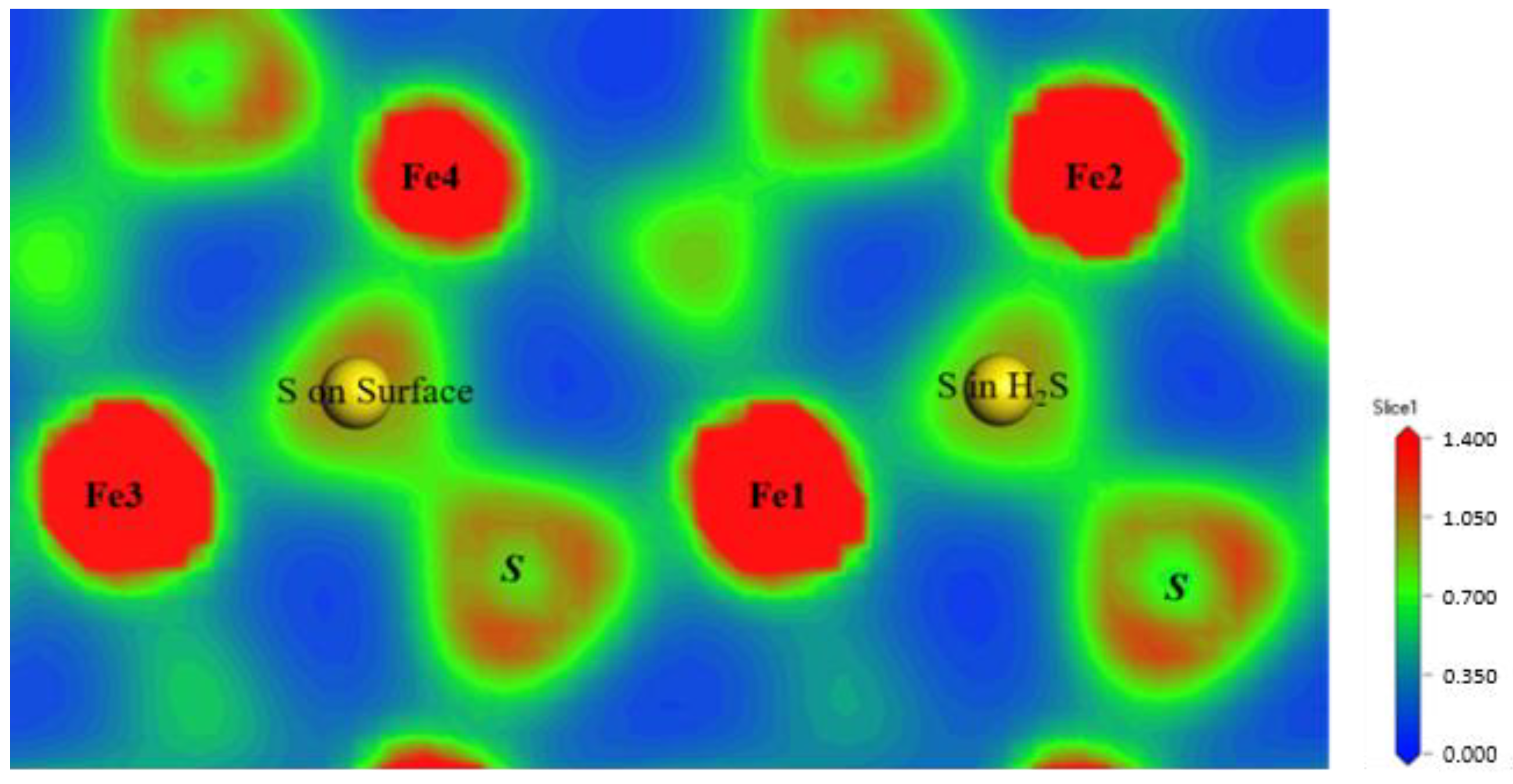
3.1. H2S Molecule Adsorbed on Pyrite Surface
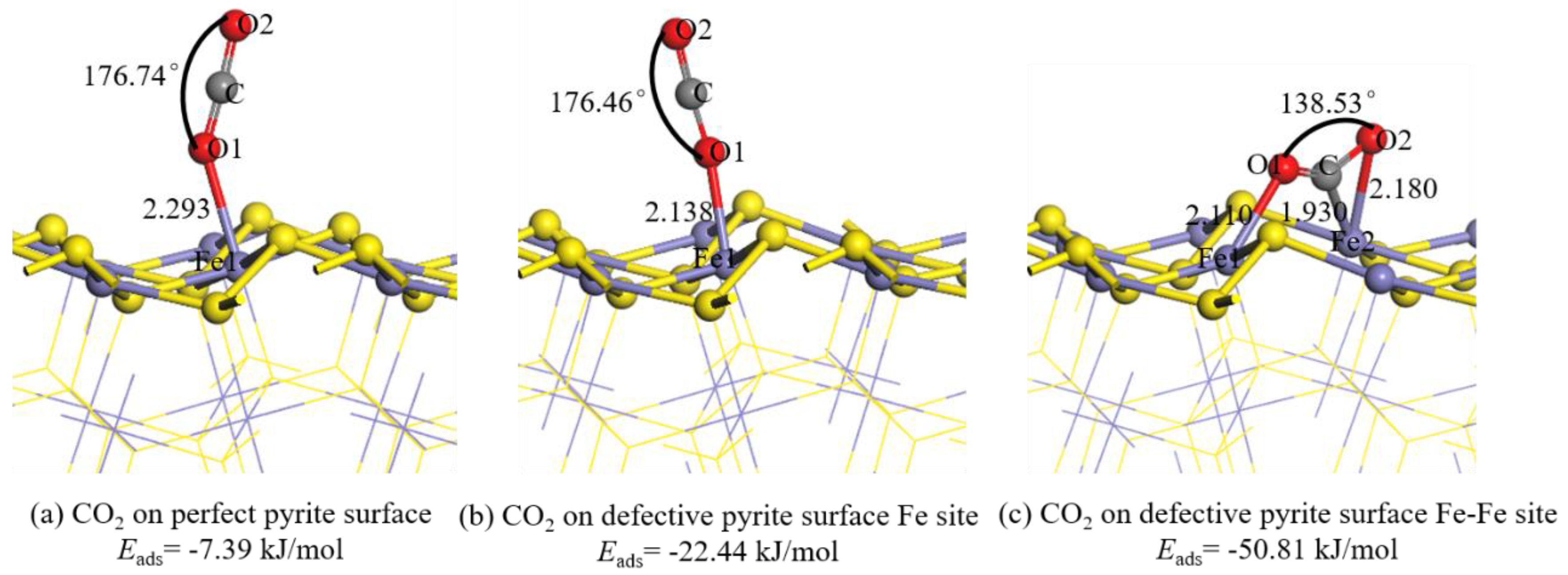
3.2. CO2 Molecule Adsorbed on Pyrite Surface
3.3. CO2 Molecule Adsorbed on the H2S-Preadsorbed Defective Pyrite Surface
3.4. H2S Molecule Adsorbed on the CO2-Preadsorbed Defective Pyrite Surface
3.5. Co-Adsorption of H2S and CO2 Molecules on Pyrite Surface
3.6. General Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.H.; Li, C.X.; Han, L.J.; Li, C.S.; Zhang, S.J. Photocatalytic reduction of CO2 with H2O on CuO/TiO2 catalysts. Energ. Sources Part A 2016, 38, 420–426. [Google Scholar] [CrossRef]
- Mori, K.; Yamashita, H.; Anpo, M. Photocatalytic reduction of CO2 with H2O on various titanium oxide photocatalysts. RSC Adv. 2012, 2, 3165–3172. [Google Scholar] [CrossRef]
- Dong, Y.P.; Nie, R.; Wang, J.X.; Yu, X.G.; Tu, P.C.; Chen, J.Z.; Jing, H.W. Photoelectrocatalytic CO2 reduction based on metalloporphyrin-modified TiO2 photocathode. Chin. J. Catal. 2019, 40, 1222–1230. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, Q.; Simon, M.; Zheng, G.D.; Tan, Y.S. Effect of H2S content on relative permeability and capillary pressure characteristics of acid gas/brine/rock systems: A review. J. Rock Mech. Geotech. 2022, 14, 2003–2033. [Google Scholar] [CrossRef]
- Kvamme, B.; Iden, E.; Tveit, J.; Veland, V.; Zarifi, M.; Qorbani, K. Effect of H2S Content on Thermodynamic Stability of Hydrate Formed from CO2/N2 Mixtures. J. Chem. Eng. Data 2017, 62, 1645–1658. [Google Scholar] [CrossRef]
- Lu, A.H.; Li, Y.; Li, Y.Z.; Ding, H.R.; Wang, C.Q. The role of mineral photoelectron energy in the origin and evolution of early life on the Earth. Geol. Rev. 2023, 69, 234–246. (In Chinese) [Google Scholar] [CrossRef]
- Li, Y.Z.; Li, Y.; Liu, Y.; Wu, Y.F.; Wu, J.Q.; Wang, B.; Ye, H.; Jia, H.N.; Wang, X.; Li, L.H.; et al. Photoreduction of inorganic carbon(+IV) by elemental sulfur: Implications for prebiotic synthesis in terrestrial hot springs. Sci. Adv. 2020, 6, 3687. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Upadhyay, R.; Rangarajan, S.; Baltrusaitis, J. Inhibitor, Co-Catalyst, or Co-Reactant? Probing the Different Roles of H2S during CO2 Hydrogenation on the MoS2 Catalyst. ACS Catal. 2019, 9, 10044–10059. [Google Scholar] [CrossRef]
- Ennaoui, A.; Fiechter, S.; Pettenkofer, C.; Alonso-Vante, N.; Büker, K.; Bronold, M.; Höpfner, C.; Tributsch, H. Iron Disulfide for Solar-Energy Conversion. Sol. Energy Mater. Sol. Cells 1993, 29, 289. [Google Scholar] [CrossRef]
- Zhang, X.V.; Ellery, S.P.; Friend, C.M.; Holland, H.D.; Michel, F.M.; Schoonen, M.A.A.; Martin, S.T. Photodriven reduction and oxidation reactions on colloidal semiconductor particles: Implications for prebiotic synthesis. J. Photochem. Photobiol. 2007, 185, 301–311. [Google Scholar] [CrossRef]
- Scribano, V.; Simakov, S.K.; Finocchiaro, C.; Correale, A.; Scirè, S. Pyrite and Organic Compounds Coexisting in Intrusive Mafic Xenoliths (Hyblean Plateau, Sicily): Implications for Subsurface Abiogenesis. Origins Life Evol. B 2019, 49, 19–47. [Google Scholar] [CrossRef] [PubMed]
- He, R.T.; Hu, B.Y.; Zhong, H.; Jin, F.M.; Fan, J.J.; Hu, Y.H.; Jing, Z.Z. Reduction of CO2 with H2S in a simulated deep-sea hydrothermal vent system. Chem. Commun. 2019, 55, 1056. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Hydrogenation of CO2 Promoted by Silicon Activated H2S: Origin and Implications. Molecules 2021, 26, 50. [Google Scholar] [CrossRef]
- Li, Y.Q.; Chen, J.H.; Chen, Y.; Zhao, C.; Zhang, Y.B.; Ke, B.L. Interactions of Oxygen and Water Molecules with Pyrite Surface: A New Insight. Langmuir 2018, 34, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Sit, P.H.L.; Cohen, M.H.; Selloni, A. Interaction of Oxygen and Water with the (100) Surface of Pyrite: Mechanism of Sulfur Oxidation. J. Phys. Chem. Lett. 2012, 3, 2409–2414. [Google Scholar] [CrossRef]
- Chen, J.H.; Long, X.H.; Chen, Y. Comparison of Multilayer Water Adsorption on the Hydrophobic Galena (PbS) and Hydrophilic Pyrite (FeS2) Surfaces: A DFT Study. J. Phys. Chem. C 2014, 118, 11657–11665. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Law, M.; Wu, R.Q. First-Principles Studies of the Electronic Properties of Native and Substitutional Anionic Defects in Bulk Iron Pyrite. Phys. Rev. B 2012, 85, 085203. [Google Scholar] [CrossRef]
- Nair, N.N.; Schreiner, E.; Marx, D. Glycine at the Pyrite-Water Interface: The Role of Surface Defects. J. Am. Chem. Soc. 2006, 128, 13815–13826. [Google Scholar] [CrossRef]
- Liu, Y.C.; Li, Y.Q.; Chen, J.H.; Kang, D.; Yang, X. Influence of sulfur vacancy on pyrite oxidization by water and oxygen molecules. Colloid Surface A 2022, 634, 127954. [Google Scholar] [CrossRef]
- Stirling, A.; Bernasconi, M.; Parrinello, M. Ab initio simulation of H2S adsorption on the (100) surface of pyrite. J. Chem. Phys. 2003, 119, 4934–4939. [Google Scholar] [CrossRef]
- Stirling, A.; Bernasconi, M.; Parrinello, M. Ab initio simulation of water interaction with the (100) surface of pyrite. J. Chem. Phys. 2003, 118, 8917–8926. [Google Scholar] [CrossRef]
- Živković, A.; Somers, M.; Camprubi, E.; King, H.E.; Wolthers, M.; de Leeuw, N.H. Changes in CO2 Adsorption Affinity Related to Ni Doping in FeS Surfaces: A DFT-D3 Study. Catalysts 2021, 11, 486. [Google Scholar] [CrossRef]
- Kolos, M.; Tunega, D.; Karlicky, F. Theoretical study of adsorption on iron sulfides towards nanoparticles modeling. Phys. Chem. Chem. Phys. 2020, 22, 23258–23267. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.; Muscat, J.; Yarovsky, I.; Russo, S.P. Density-functional theory studies of pyrite FeS2 (100) and (110) surfaces. Surface Sci. 2002, 513, 511–524. [Google Scholar] [CrossRef]
- Silva, J.C.M.; dos Santos, E.C.; Heine, T.; Abreu, H.A.D.; Duarte, H.A. Pyrite oxidation mechanism by Oxygen in Aqueous Mediun. J. Phys. Chem. C 2016, 120, 2760–2768. [Google Scholar] [CrossRef]
- Chen, J.H. Coordination Principle of Minerals Flotation; Science Press Beijing: Beijing, China, 2022; pp. 157–213. [Google Scholar]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Krist. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Segall, M.; Lindan, P.J.D.; Probert, M.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C.J. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Mater. 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Jiang, H. Accurate Prediction of Band Structure of FeS2: A Hard Quest of Advanced First-Principles Approaches. Front. Chem. 2021, 9, 747972. [Google Scholar] [CrossRef]
- Halgren, T.A.; Lipscomb, W.N. The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem. Phys. Lett. 1977, 49, 225–232. [Google Scholar] [CrossRef]
- Li, Y.Q.; Chen, J.H.; Chen, Y.; Guo, J. Density functional theory calculation of surface properties of pyrite (100) with implications for flotation. Chin. J. Nonferrous Met. 2011, 21, 919–926. [Google Scholar]
- Guevremont, J.M.; Strongin, D.R.; Schoonen, M.A.A. Thermal chemistry of H2S and H2O on the (100) plane of pyrite: Unique reactivity of defect sites. Am. Mineral. 1998, 83, 1246–1255. [Google Scholar] [CrossRef]
- Li, S.F.; Guo, Z.X. CO2 activation and total reduction on titanium (0001) surface. J. Phys. Chem. C 2010, 114, 11456–11459. [Google Scholar] [CrossRef]
- Mishra, A.K.; Roldan, A.; de Leeuw, N.H. CuO surfaces and CO2 activation: A dispersion-corrected DFT+ U study. J. Phys. Chem. C 2016, 120, 2198–2214. [Google Scholar] [CrossRef]
- Khaledialidusti, R.; Mishra, A.K.; Barnoush, A. CO2 Adsorption and Activation on the (110) Chalcopyrite Surfaces: A Dispersion-Corrected DFT + U Study. ACS Omega 2019, 4, 15935–15946. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.W.; Zhang, N.; Wang, H.M.; Hong, S.G. Adsorption of CO2 on Cu2O (111) oxygen-vacancy surface: First-principles study. Chem. Phys. Lett. 2013, 568–569, 84–89. [Google Scholar] [CrossRef]
- Pan, Y.X.; Liu, C.J.; Mei, D.H.; Ge, Q.F. Effects of hydration and oxygen vacancy on CO2 adsorption and activation on β-Ga2O3 (100). Langmuir 2010, 26, 5551–5558. [Google Scholar] [CrossRef]
- Xiong, X.L.; Hua, X.M.; Zheng, Y.F.; Lu, X.G.; Li, S.G.; Cheng, H.W.; Xu, Q. Oxidation mechanism of chalcopyrite revealed by X-ray photoelectron spectroscopy and first principles studies. Appl. Surf. Sci. 2018, 427, 233–241. [Google Scholar] [CrossRef]
- Wei, Z.L.; Li, Y.B.; Gao, H.M.; Zhu, Y.G.; Qian, G.J.; Yao, J. New insights into the surface relaxation and oxidation of chalcopyrite exposed to O2 and H2O: A first-principles DFT study. Appl. Surf. Sci. 2019, 492, 89–98. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Hu, J.; Law, M.; Wu, R.Q. Effect of surface stoichiometry on the band gap of the pyrite FeS2(100) surface. Phys. Rev. B 2012, 85, 085314. [Google Scholar] [CrossRef]
- Cheng, W.; Cheng, C.; Ke, B.L. The Effect of Carbon Defects in the Coal–Pyrite Vacancy on the Electronic Structure and Optical Properties: A DFT + U Study. Minerals 2020, 10, 815. [Google Scholar] [CrossRef]





| Adsorption State | s | p | d | Charge | |
|---|---|---|---|---|---|
| Before | 0.35 | 0.44 | 7.15 | 7.93 | Perfect surface |
| After | 0.33 | 0.45 | 7.12 | 7.90 | |
| Before | 0.35 | 0.37 | 7.14 | 7.86 | Defective surface |
| After | 0.33 | 0.38 | 7.04 | 7.75 |
| Perfect Surface | Defective Surface | |
|---|---|---|
| dx2-y2 | 1.35 | 1.56 |
| dxy | 1.53 | 1.71 |
| dzx | 1.75 | 1.78 |
| dzy | 1.69 | 1.76 |
| dz2 | 1.65 | 1.68 |
| Adsorption State | s | p | d | Charge | ||
|---|---|---|---|---|---|---|
| Without HCOOH | Before | 0.35 | 0.44 | 7.15 | 7.93 | Perfect surface |
| After | 0.34 | 0.48 | 7.17 | 7.99 | ||
| With HCOOH | Before | 0.35 | 0.37 | 7.14 | 7.86 | Defective surface |
| After | 0.34 | 0.44 | 7.15 | 7.94 |
| Surface | Band Gap/eV |
|---|---|
| Perfect surface | 0.667 |
| Defective surface | 0.301 |
| Defective surface where the H2S molecule is adsorbed (Figure 2c) | 0.587 |
| Defective surface where the H2S molecule is adsorbed (Figure 2d) | 0.451 |
| Defective surface where the CO2 molecule is adsorbed | 0.199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, Y.; Feng, Y.; Chen, J.; Zhao, C. Theoretical Research on the Reduction of CO2 with H2S on Pyrite FeS2 Surfaces. Minerals 2023, 13, 1292. https://doi.org/10.3390/min13101292
Liu Y, Li Y, Feng Y, Chen J, Zhao C. Theoretical Research on the Reduction of CO2 with H2S on Pyrite FeS2 Surfaces. Minerals. 2023; 13(10):1292. https://doi.org/10.3390/min13101292
Chicago/Turabian StyleLiu, Yingchao, Yuqiong Li, Yao Feng, Jianhua Chen, and Cuihua Zhao. 2023. "Theoretical Research on the Reduction of CO2 with H2S on Pyrite FeS2 Surfaces" Minerals 13, no. 10: 1292. https://doi.org/10.3390/min13101292
APA StyleLiu, Y., Li, Y., Feng, Y., Chen, J., & Zhao, C. (2023). Theoretical Research on the Reduction of CO2 with H2S on Pyrite FeS2 Surfaces. Minerals, 13(10), 1292. https://doi.org/10.3390/min13101292






