Abstract
In this research, a novel collector cetyl trimethyl ammonium chloride (CTAC) was used to separate hematite from quartz via reverse flotation for the first time. Micro-flotation tests showed that CTAC had a strong ability to selectively collect quartz and that a separation of hematite from quartz could be accomplished with a concentration of 0.00263 mmol/L CTAC. Zeta-potential measurements indicated that the positive CTAC+ species could selectively increase the surface potential of quartz, but that it had rather a weak effect on the hematite. X-ray photoelectron spectroscopy (XPS) detection indicated that CTAC had a stronger binding affinity to oxygen sites on the surface of quartz than on hematite, resulting in a large amount of CTAC being predominantly adsorbed onto quartz. This was supported by the atomic concentration of C1s and N1s of quartz after CTAC treatments were 4.25 and 2.84 times higher than hematite, respectively.
1. Introduction
Metallic iron is the basic supporting metal for human life and social development. It is widely used in steel production, the military industry, machinery, construction, and transportation [1,2]. Therefore, efficient utilization of iron ore resources is crucial to the development of economy and people’s livelihood. With high iron content, hematite (Fe2O3) is an important source of iron metal extraction. In natural deposits, the main gangue minerals associated with hematite are quartz, chlorite, kaolinite, etc. [3]. According to the difference in mineralization conditions and ore properties, various techniques such as magnetic separation, gravity concentration and static separation have been used for the separation and purification of hematite [4]. However, with the depletion of high-grade resources, the above methods can become inefficient in processing low-grade and complex fine-grained hematite ores. In such circumstances, flotation is considered the most effective technical solution for hematite purification.
Today, many studies and industrial practices have confirmed that the reverse cationic flotation route, performed using a cationic collector to float and reject silicate gangue minerals, is the most effective and widely used method for hematite purification [1,5]. In addition, the effects of mineral particle size [6], stirring speed [7], and type of flotation cell [8] on flotation efficiency have been extensively studied in examinations of the separation process of hematite and quartz.
In iron ore beneficiation, the most commonly used collector for removing quartz from hematite was dodecylamine (DDA) [9,10]. However, due to the strong foaming and electrostatic attraction function of DDA, some hematite can be collected using DDA, thus causing inefficient separation. Additionally, DDA has the disadvantage of limited solubility in aqueous solutions. Therefore, a large number of new collectors have been studied, such as bis(2-hydroxy-3-chloropropyl) dodecylamine (N23) [1], tertiary amines [11], amides [12], and polyamines [13], to enhance the separation of hematite and quartz. Despite their effectiveness, the complex synthesis process and high production costs of theses reagents limit their commercial application. Thus, high-selectivity and low-costs collectors for the efficient separation of hematite from quartz are becoming more and more important.
Quaternary ammonium salt cation (QAS) is commonly used as a surfactant due to its excellent water solubility and adaptability in a wide array of pH values [14,15]. In terms of mineral purification, the application of QAS as a cationic collector in oxidized ores was studied. As a QAS-type reagent, cetyl trimethyl ammonium chloride (CTAC) has much better water solubility than DDA, and it is easily obtained commercially with economic feasibility. It was studied as a collector in the flotation of magnesite and quartz and exhibited good collection ability for quartz [16]. However, there is no report regarding the effectiveness of CTAC in iron ore flotation system. Therefore, it would be valuable to investigate the potential use of CTAC in the purification of hematite.
In this study, the flotation experiment studied the effect of CTAC on the floatability of hematite and quartz, and the zeta potential and XPS measurements analyzed the adsorption capacity and intensity of CTAC on the mineral surface, respectively.
2. Materials and Methods
2.1. Materials and Reagents
High purity hematite and quartz samples were obtained from Havre Saint-Pierre iron ore mine in eastern Canada and US SILICA company, respectively. The results of X-ray powder diffraction (XRD) pattern inspection (Figure 1) and X-ray fluorescence chemical (XRF) analysis (Table 1) were determined using Ultima IV and S8 TIGER devices (Laboratory of Chemistry and Materials Engineering, Shenyang Ligong University, China), respectively. The purities of the hematite and quartz samples were 97.12% and 98.51%, respectively.
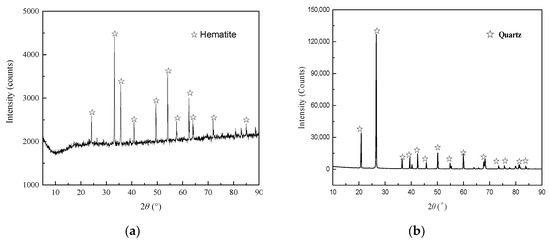
Figure 1.
XRD patterns of (a) hematite and (b) quartz.

Table 1.
XRF analysis of samples (wt%).
In total, 80 mL of water and 200 g of high-purity ore with a particle size of less than 5 mm were ground in a XMQ 150 × 50 ball mill for 582 s. Grinding products were manually screened to obtain products with particle sizes ranging from 45 to 90 μm and dried for micro-flotation experiments, and the samples were further ground in an agate mortar to obtain the −5 μm particle fraction for zeta-potential and XPS measurements. Cetyl trimethyl ammonium chloride (CTAC) with a purity of 99.71% and hydrochloric acid were purchased from Thermo Fisher Scientific and used as the collector and pH regulator, respectively.
2.2. Micro-Flotation Tests
Micro-flotation tests for the single or synthetic mixed minerals (the mass ratio of hematite and quartz was 1:1) were carried out in a custom-made glass flotation tube modified according to the recommendations of Siwek, shown schematically in Figure 2 [17]. For each test, 2 g of sample was added to the sintered glass container installed at the bottom of the tube with a diameter of 1.6 µm. Subsequently, add 180 mL of water with a pH value of 5.0 from the inlet and agitated at 2000 rpm for 2 min. After stirring this pulp, the collector CTAC was added to the flotation tube after water dissolution. This was followed by 5 min of stirring and a subsequent injection 5.33 kPa of gas from the nitrogen inlet of the flotation tube. Finally, 5-min flotation was performed, and the concentrate was scraped, dried, and weighed, and the chemical compositions (Fe and SiO2) were determined via the S8 TIGER device.
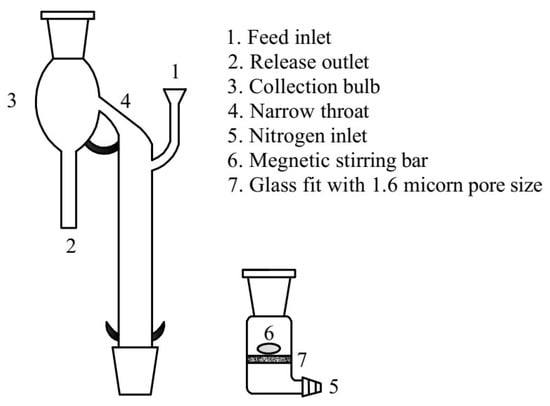
Figure 2.
Schematic illustration of the glass tube for micro-flotation test.
The recoveries of single and artificially mixed mineral flotation were determined using Equations (1) and (2), respectively.
where ε represents the recovery (%); mT and mc represents the sample (g) and concentrate mass (g), respectively; βi represents the Fe2O3 or SiO2 grade (%) of this mineral in the concentrate; and mi represents the Fe2O3 or SiO2 mass (g) of this mineral in the sample.
2.3. Zeta-Potential Measurements
The surface potential of the hematite and quartz under different CTAC concentrations was measured at pH 5.0 using the FZT 500F (Laboratory of Chemistry and Materials Engineering, Shenyang Ligong University, China). In total, 90 mg of sample particles were suspended in 180 mL of 1 × 10−3 mol/L KCl solution and conditioned at 2000 rpm for 5 min [9,15]. Afterwards, the dosages of CTAC from 0.00 to 43.75 ×10−4 mmol/L were added and stirred for 5 min. Eventually, the pulp was left to stand for 5 min, and the supernatant was detected. Five tests were conducted for each condition, and average and standard deviation values were calculated and reported as the final results.
2.4. XPS Measurement
The effect of CTAC on mineral surface elements was detected using a Kratos AXIS 165 spectrometer (Laboratory of Minerals Processing Engineering, Northeastern University, Shenyang, China) at pH 5.0. In this research, C1s peak at 280. Four was taken as the standard on the basis of which the XPS spectra were corrected [18,19]. A total of 2 g of sample mixed with 180 mL water was added to the glass tube, centrifuged at 2000 rpm for 5 min, and subjected to vacuum filtration. The solid substances were analyzed after filtering, washing, and drying.
3. Results
3.1. Micro-Flotation Tests
Numerous studies have shown that the zero electrical points of hematite and quartz were around pH 2.2 and 4.9, respectively [1,9,15]. Therefore, in order to ensure a significant difference in surface electrical properties between hematite and quartz, micro-flotation was carried out under the natural suspension pH, which was set to approximately 5.0. In order to observe the floatability of both minerals by CTAC, flotation recovery was examined as a function of CTAC concentration as shown in Figure 3.
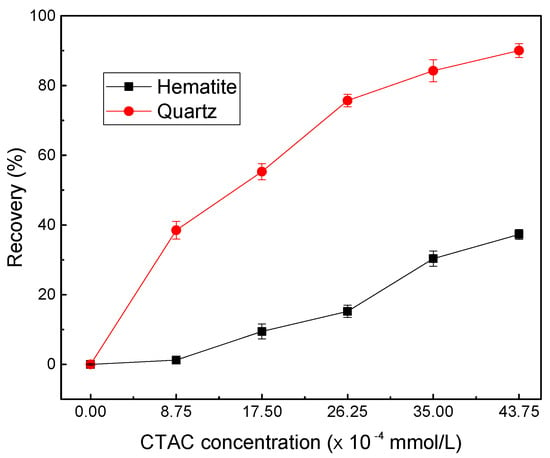
Figure 3.
Effect of CTAC concentration on mineral recovery.
As seen in Figure 3, a similar increasing floatability trend was observed for quartz and hematite. With increasing CTAC dosages from 0.00 to 43.75 × 10−4 mmol/L, the hematite and quartz recovery increased from 0.21 to 37.29% and 0.13 to 90.04%, respectively. Moreover, the recoveries of the two minerals indicated that CTAC exhibited much better collection ability towards quartz than hematite. A significant recovery difference (~60.47%) between them was obtained when the concentration of CTAC was 26.25 × 10−4 mmol/L.
To further investigate the selectivity of CTAC between hematite and quartz, the mixed minerals separation tests were conducted at a mass ratio of 1:1, and the assay results of the froth products are shown in Table 2.

Table 2.
Mixed-mineral flotation test results.
From Table 2, it can be seen that the yield and hematite recovery of froth product increased from 51.27 to 71.12% and from 26.44 to 54.38%, respectively, with CTAC concentration increasing from 17.50 to 35.00 × 10−4 mmol/L. Additionally, the quartz recovery increased from 77.26 to 94.13% with CTAC dosages from 17.50 to 26.25 × 10−4 mmol/L, and a stable trend was observed as the concentration increased further. Compared with single-mineral flotation results, the quartz recovery rarely changed. However, the hematite recovery rate in artificial mixed minerals was about 1.8 times higher than that in the single hematite flotation under the same concentration of CTAC. The reason for the increase in hematite recovery was likely due to the partial hetero-aggregation and entrapment between positively charged hematite and quartz with negative surface charge in suspension. They floated together into the froth phase, especially in the presence of higher dosages of CTAC. Overall, hematite and quartz still had a recovery difference of 45.48%, with a CTAC concentration of 26.25 × 10−4 mmol/L, indicating that the CTAC remained a high selective collection capacity for quartz in mixed minerals.
3.2. Zeta-Potential Measurements
CTAC dissociates in water and exists in the form of positively charged C19H42N+, the adsorption of which onto a mineral surface can change the electrical properties of the mineral/liquid interface. Here, the adsorption capacity of CTAC on the surface of hematite and quartz was analyzed by measuring surface potential changes of minerals before and after the interaction with CTAC. The zeta-potential measurements results are presented in Figure 4.
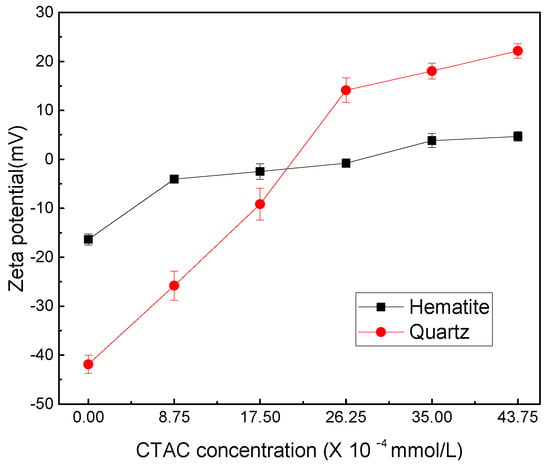
Figure 4.
Zeta potentials of hematite and quartz as functions of CTAC concentration.
From Figure 4, it can be seen the mineral surface potentials of hematite and quartz were −16.37 and −41.87 mV, respectively, without any reagents. It can be observed that as the CTAC concentration increased from 0 to 26.25 × 10−4 mmol/L, the sample surface potential of hematite and quartz positively increased by 15.58 and 55.97 mV, respectively. These results indicated that the positively charged C19H42N+ cation was adsorbed onto the surface of hematite and quartz, increasing the minerals potential value of the two mineral surfaces. In addition, the increase in surface potential of quartz was significantly higher (about 3.59 times) than that of hematite, indicating that the adsorption amount of C19H42N+ on quartz surface was far greater compared with hematite. This observation may be attributed to two main reasons. First, CTAC as a quaternary ammonium salt could completely dissolve in water and generated positively charged C19H42N+ and negatively charged Cl− with the following equation [16].
C19H42NCl = C19H42N+ + Cl−
Second, isoelectric points of hematite and quartz were approximately pH 4.8 and pH 2.5, respectively [20,21], and the surface potential of quartz was more negative than that of hematite at pH 5.0. Therefore, C19H42N+ with cationic functional groups exhibited stronger affinity towards quartz than hematite due to the same charge will repel, different charges will attract.
3.3. XPS Measurements
In order to further reveal the adsorption difference and mechanism of CTAC on the mineral surface, the XPS analysis was detected with a CTAC concentration of 26.25 × 10−4 mmol/L at pH 5. The XPS spectra of hematite and quartz before and after treatment with CTAC are shown in Figure 5 and Figure 6, respectively.
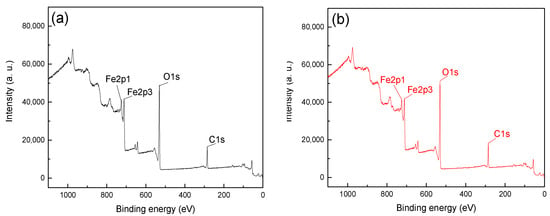
Figure 5.
XPS spectra of hematite (a) before and (b) after treatment with CTAC.
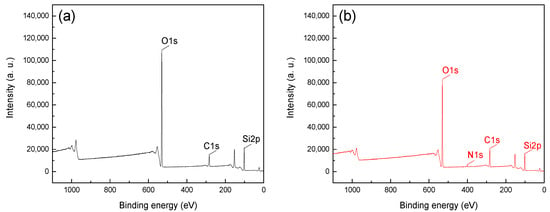
Figure 6.
XPS spectra of quartz (a) before and (b) after treatment with CTAC.
From Figure 5, it can be seen the Fe2p1, Fe2p2 and O1s peaks were detected at approximately 724, 711 and 531eV, which was attributable to the iron and oxygen elements on the surface of hematite [22]. Figure 6 shows that O1s and Si2p peaks were detected at binding energies of 102 and 531 eV, respectively, which indicated that SiO2 was the main component of quartz and that no significant contaminant was detected [15,16]. The C1s peaks in spectra were positioned at around 284.8 eV, which was mainly attributable to the presence of carbon dioxide in the air and the CTAC molecules [23,24,25]. The peak intensities of C1s in Figure 5a,b were 16,516.7 and 18,096.7, respectively, and the offset value was 1580 after treatment with CTAC. In contrast, the peak intensities of C1s in Figure 6a,b were 14,933.3 and 20,283.3, respectively, with a much higher offset value of 5350 achieved using CTAC. The above results indicated that the adsorption of CTAC could enhance peak intensities of C1s for both materials. However, the increment of quartz was 3.39 times higher than that of hematite, suggesting a stronger affinity between CTAC and the quartz surface. In addition, N1s peaks were detected in 400.23 eV (Figure 6b) of quartz after CTAC treatment, and there was no obvious change in the spectrum of hematite before and after treatment with CTAC. These results indicate that, in addition to carbon dioxide in the air, CTAC was also adsorbed onto the surface of minerals and the adsorption density of CTAC onto the quartz surface was far greater than that onto hematite.
To further quantify the impact of CTAC on mineral surfaces, the relative content of mineral surface elements under different conditions was analyzed and the results are shown in Table 3.

Table 3.
Effect of CTAC on the relative content of mineral surface elements.
As can be seen, there was a slight change in the atomic concentration of the main element on hematite surface after interaction with CTAC, indicating weak adsorption. This was supported by the low offsets of C1s (2.40%) N1s (0.43%). However, for quartz + CTAC, the atomic concentrations of C1s and N1s were 23.74% and 1.22%, respectively. In contrast with the hematite sample, the concentration offsets of C1s and N1s of quartz after CTAC treatment were 4.25 and 2.84 times higher, respectively. This proved that CTAC adsorbed onto quartz rather than hematite, thus achieving the efficient removal of quartz from hematite via reverse flotation.
As an amine cationic collector, CTAC was mainly adsorbed onto the mineral surface via electrostatic adsorption [15,16]. In order to obtain the difference in the adsorption strength of CTAC on the surface of hematite and quartz, the anions (i.e., O1s) on the surface of the two minerals were fitted and analyzed before and after treatment with CTAC, and the results are shown in Figure 7 and Figure 8.
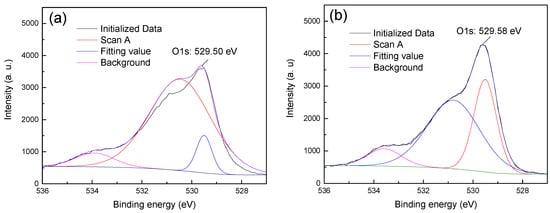
Figure 7.
The O1s XPS spectra of hematite: (a) hematite (b) hematite + CTAC.
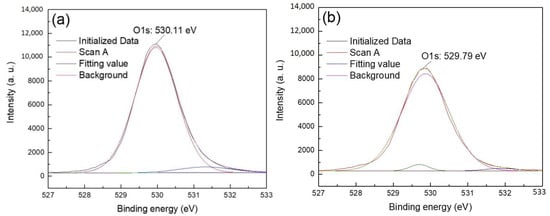
Figure 8.
The O1s XPS spectra of quartz (a) quartz (b) quartz + CTAC.
Figure 7 shows that the O1s XPS spectra of hematite before and after treatment with CTAC shifted from 529.50 (Figure 7a) to 529.58 eV (Figure 7b). This was an offset of 0.08 eV, indicating that the interaction between CTAC and oxygen sites on the surface of hematite was weak. However, after treating quartz with CTAC, the binding energy of O1s shifted from 530.11 (Figure 8a) to 529.79 eV (Figure 8b), which was an offset of 0.32 eV. After treatment with CTAC, the deviation of the binding energy of O1s on the surface of quartz was four times that of hematite, which indicated that the adsorption capacity of CTAC onto the surface of quartz was much greater than that seen for hematite.
4. Conclusions
As a quaternary ammonium salt cationic surfactant, CTAC was used for the first time in the research of flotation separation of hematite and quartz. The main conclusions are as follows:
- Micro-flotation experiments found that there was at least a 45.48% difference between hematite and quartz as the concentration of CTAC was 26.25 × 10−4 mmol/L under pH 5.0.
- The positive CTAC+ species could selectively increase the surface potential of quartz, but had rather a weak effect on the hematite.
- More quantities of CTAC were adsorbed onto quartz than hematite owing to the stronger binding ability between CTAC and oxygen elements of the quartz surface.
Author Contributions
Validation and data curation, H.S.; methodology and conceptualization, Y.W. and W.Y.; writing—original draft preparation, J.Y.; Writing—review & editing, S.Y. and D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (52374271, 52174239 and 51974064), Key Development Plan for Applied Basic Research Project of Liaoning Province (2022JH2/101300111), General Project of the Educational Department of Liaoning Province (LJKMZ20220588), and Project of Shenyang Bureau of Science and Technology (22–322–3–03).
Data Availability Statement
No new data were created.
Acknowledgments
The authors would like to express their sincere gratitude to the editors and the anonymous reviewers for their helpful remarks and constructive comments, which have improved the quality of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, W.G.; Liu, W.B.; Dai, S.J.; Wang, B.Y. Adsorption of bis(2-hydroxy-3-chloropropyl) dodecylamine on quartz surface and its implication on flotation. Results Phys. 2018, 9, 1096–1101. [Google Scholar] [CrossRef]
- Filippov, L.O.; Severov, V.V.; Filippova, I.V. An overview of the beneficiation of iron ores via reverse cationic flotation. Int. J. Miner. Process. 2014, 127, 62–69. [Google Scholar] [CrossRef]
- Wu, H.; Qiu, T.; Zhao, G.; Zhu, D.; Li, X.; Feng, B. Investigations on the reverse cationic flotation separation of quartz from hematite using polyaspartic acid as depressant. Appl. Surf. Sci. 2023, 614, 156143. [Google Scholar] [CrossRef]
- Han, W.; Zhu, Y.; Liu, J.; Li, Y. A novel depressant HPAM of the hematite in reverse cationic flotation of iron ore. Colloids Surf. A 2022, 641, 128547. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, S.; Guo, Z.; Sun, W.; Zhang, C. Selective separation mechanism of hematite from quartz by anionic reverse flotation: Implications from surface hydroxylation. Appl. Surf. Sci. 2023, 614, 156056. [Google Scholar] [CrossRef]
- Safari, M.; Hoseinian, F.; Deglon, D.; Filho, L.; Souza, P. Impact of flotation operational parameters on the optimization of fine and coarse Itabirite iron ore beneficiation. Powder Technol. 2022, 408, 117772. [Google Scholar] [CrossRef]
- Francisco, G.; Mehdi, S.; David, D.; Laurindo, D. Influence of agitation intensity on flotation rate of apatite particles. Mining REM Int. Eng. J. 2017, 70, 491–495. [Google Scholar]
- Safari, M.; Hoseinian, F.; Deglon, D.; Filho, L.; Souza, P. Investigation of the reverse flotation of iron ore in three different flotation cells: Mechanical, oscillating grid and pneumatic. Miner. Eng. 2020, 150, 106283. [Google Scholar] [CrossRef]
- Sun, H.; Yang, B.; Zhu, Z.; Yin, W.; Sheng, Q.; Hou, Y.; Yao, J. New insights into selective-depression mechanism of novel depressant EDTMPS on magnesite and quartz surfaces: Adsorption mechanism, DFT calculations, and adsorption model. Miner. Eng. 2021, 160, 106660. [Google Scholar] [CrossRef]
- Han, C.; Zhang, H.; Tan, R.; Shen, Y.; Wei, D.; Liu, W. Effects of monohydric alcohols of varying chain lengths and isomeric structures on magnesite and dolomite flotation by dodecylamine. Powder Technol. 2020, 374, 233–240. [Google Scholar] [CrossRef]
- Weng, X.; Mei, G.; Zhao, T.; Zhu, Y. Utilization of novel ester-containing quaternary ammonium surfactant as cationic collector for iron ore flotation. Sep. Purif. Technol. 2013, 103, 187–194. [Google Scholar] [CrossRef]
- Liu, W.; Wei, D.; Wang, B.; Ping, F.; Wang, X.; Cui, B. A new collector used for flotation of oxide minerals. Trans. Nonferr. Metal. Soc. 2009, 19, 1326–1330. [Google Scholar] [CrossRef]
- Sahoo, H.; Sinha, N.; Rath, S.S.; Das, B. Ionic liquids as novel quartz collectors: Insights from experiments and theory. Chem. Eng. J. 2015, 273, 46–54. [Google Scholar] [CrossRef]
- Tahani, A.; Karroua, M.; Van, H.; Damme, P.; Levitz, F. Adsorption of a cationic surfactant on Na–montmorillonite: Inspection of adsorption layer by X-ray and fluorescence spectroscopies. J. Colloid Interface Sci. 1999, 216, 242–249. [Google Scholar] [CrossRef]
- Sun, H.; Yin, W.; Yang, B.; Chen, K.; Sheng, Q. Efficiently separating magnesite from quartz using N-hexadecyltrimethylammonium chloride as a collector via reverse flotation. Miner. Eng. 2021, 166, 106899. [Google Scholar] [CrossRef]
- Sun, H.; Yin, W. Selective flotation separation of magnesite from quartz by palmitoyl trimethylammonium chloride. Sep. Purif. Technol. 2022, 295, 121201. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Q. Influence of aggregation/dispersion state of hydrophilic particles on their entrainment in fine mineral particle flotation. Miner. Eng. 2021, 166, 106835. [Google Scholar] [CrossRef]
- Sheng, Q.; Yin, W.; Yang, B.; Chen, K.; Sun, H. Promotion of oxidation pretreatment on sulfidation of cuprite surface and its contribution to flotation. Miner. Eng. 2021, 174, 107256. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, Z.; Yin, W.; Sun, Q.; Sun, H.; Han, H.; Sheng, Q.; Yao, J. Selective adsorption of an eco-friendly and efficient depressant PBTCA onto dolomite for effective flotation of fluorapatite from dolomite. Chem. Eng. J. 2020, 400, 125780. [Google Scholar] [CrossRef]
- Yang, Z.; Han, Y.; Teng, Q.; Zhang, G.; Liu, S. Aggregation process of fine hematite particles suspension using xanthan gum in the presence of Fe(III). Arab. J. Chem. 2023, 16, 104539. [Google Scholar] [CrossRef]
- Liu, W.; Peng, X.; Liu, W.; Tong, K.; Shen, Y.; Zhao, Q.; Zhao, S.; Sun, W. Novel polyhydroxy cationic collector N-(2,3-propanediol)-N-dodecylamine: Synthesis and flotation performance to hematite and quartz. Int. J. Min. Sci. Technol. 2023, 33, 115–122. [Google Scholar] [CrossRef]
- Neitzke, P.; Dantas, T.; Peres, M.; Neto, A. Depressants in nanoemulsion systems applied to quartz and hematite microflotation. J. Mater. Res. Technol. 2019, 8, 5529–5535. [Google Scholar] [CrossRef]
- Mičušík, M.; Šlouf, M.; Stepura, A.; Soyka, Y.; Ovodok, E.; Procházka, M.; Omastová, M. Aging of 2D MXene nanoparticles in air: An XPS and TEM study. Appl. Surf. Sci. 2023, 610, 155351. [Google Scholar] [CrossRef]
- Zhidkov, I.; Maksimov, R.; Kukharenko, A.; Finkelstein, L.; Cholakh, S.; Osipov, E.V. Effect of post-annealing in air on optical and XPS spectra of Y2O3 ceramics doped with CeO2. Mendeleev Commun. 2019, 29, 102–104. [Google Scholar] [CrossRef]
- Dobrovolsky, V.D.; Khyzhun, O.Y.; Sinelnichenko, A.K.; Ershova, O.G.; Solonin, Y.M. XPS study of influence of exposure to air on thermal stability and kinetics of hydrogen decomposition of MgH2 films obtained by direct hydrogenation from gaseous phase of metallic Mg. J. Electron Spectrosc. 2017, 215, 28–35. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).