Abstract
The issue of heavy metal pollution has gradually emerged as a significant global concern. Microbial fuel cells (MFCs) hold immense potential for clean energy production and pollutant treatment. However, their limited power generation efficiency hampers the large-scale implementation of MFCs. The porous microstructure of biochar and the excellent physical and chemical properties of rutile render both materials promising catalysts with positive potential. In this study, we employed biochar as a carrier for rutile to fabricate a novel rutile–biochar (Rut-B) composite material, investigating its efficacy in enhancing MFC power generation efficiency as a cathode catalyst, as well as its application in heavy metal pollutant degradation. Scanning electron microscopy (SEM) results confirmed the successful preparation of biochar-loaded rutile composites. The MFC achieved maximum current density and power density values of 152.26 mA/m2 and 9.88 mW/m2, respectively—an increase of 102.7% and 224% compared to the control group without the addition of Rut-B. Furthermore, the biochar-loaded rutile MFC exhibited excellent performance in degrading heavy metal pollutants; within 7 h, the Pb2+ degradation rate reached 92.4%, while the Zn2+ degradation rate reached 84%. These rates were significantly higher than those observed in the control group, by factors of 437.2% and 345%, respectively. The cyclic degradation experiments also demonstrated the outstanding stability of the system over multiple cycles. In summary, this study successfully combined natural rutile with biochar to create an efficient electrode catalyst that not only enhances electricity generation performance but also provides an environmentally friendly and cost-effective approach for remediating heavy metal pollution.
1. Introduction
Energy shortage and environmental pollution are major challenges facing the world today. The environmental pollution mainly includes organic pollution and heavy metal pollution [1,2]. Among them, heavy metals not only inflict immeasurable harm upon the ecological environment but also pose a significant threat to human health through direct ingestion, inhalation of air, and skin contact [3,4]. Moreover, heavy metals exhibit characteristics such as high toxicity, prolonged persistence, and limited biodegradability, thereby causing substantial detriment [5,6,7]. Notably, lead (Pb) and zinc (Zn) are common heavy metals, and their high concentrations can cause immeasurable harm to the human nervous system [8]. Therefore, it is imperative to control heavy metal pollution caused by Pb and Zn.
The common heavy metal treatment methods are mainly physical and chemical methods. However, these physicochemical techniques are energy-intensive and cost-effective. In addition, biotreatment technology is economical and environmentally friendly, but it is greatly limited in dealing with heavy metals with low bioavailability [9]. A fuel cell’s function is usually defined as the conversion of organic energy into electrical energy without using any kind of combustion. The microbial fuel cell (MFC) approach is also a form of electrochemical fuel cell [10]. MFCs are a novel technology that converts chemical energy into electricity, providing a low-cost, adaptable, and environmentally friendly method for removing heavy metal pollutants from the environment [11,12]. In MFCs, the bacterial species break down different organic compounds and heavy metal complexes to produce electrons and protons, in order to empower their respiration system while exhibiting the capability of producing a flow of electrons by means of the electrodes [10]. As a new type of bioelectrochemical system capable of simultaneously producing clean energy and degrading pollutants, MFCs have garnered significant attention from researchers [13,14]. At the same time, MFCs have shown great potential in the remediation of heavy metal pollution [15,16,17]. However, low power generation efficiency has always been the bottleneck in MFC development, due to activation losses, concentration/mass transfer limitations, and ohmic losses during operation [18]. Therefore, microbial fuel cells have not been widely developed in practical pollutant degradation applications [19,20]. It is necessary to further develop the MFC technology to a scalable level by using innovative design and cost-effective materials [21].
In recent years, researchers have been striving to identify suitable approaches for enhancing the power generation performance of MFCs [22]. However, the performance of MFCs is subject to various limiting factors, primarily originating from the composition of the electrolyte medium, along with the anode and cathode materials [23,24,25]. Among the numerous strategies for improving MFC efficiency, research on developing efficient electrode catalytic materials has consistently remained a focus [26,27,28]. Some studies have successfully enhanced reaction rates and reduced charge-transfer impedance through modifications to activated carbon cathodes [29,30]. The results obtained with the Li0.95Ta0.76Nb0.19Mg0.15O3 (LTNMg) ferroelectric cathode ceramic in the MFC device process showed a high COD removal of 96% after a working time of 168 h, allowing the biofuel cells to produce a maximum power density of 228 mW/m−2 [31]. Zhao et al. [32] increased the number of active sites on the cathode by oxidizing modified molybdenite. Previous investigations have demonstrated that the physical and chemical properties of cathode materials, such as conductivity, roughness, ductility, and biocompatibility, directly impact the battery’s power generation efficiency [33]. Platinum (Pt) has been widely employed as a cathode catalyst due to its exceptional catalytic performance, which significantly enhances MFCs’ electrical output. However, its high cost restricts its large-scale application. Consequently, researchers are devoting considerable attention towards developing non-noble-metal electrode catalysts. Lu et al. [34] developed a porous nitrogen-doped carbon material encapsulating iron-based nanoparticles (Fe-Nx/C) as an MFC cathode catalyst with superior catalytic activity compared to Pt catalysts. However, the complex manufacturing process poses new challenges for practical implementation. Therefore, it is imperative to discover and prepare more economical and environmentally friendly cathodic catalytic materials.
Compared with high-cost and complex synthetic materials, natural minerals that are widely distributed in the Earth are more environmentally friendly and easy to obtain. Therefore, the use of natural minerals as cathode catalysts to improve the power generation performance of MFCs has received extensive attention from previous studies. Ren et al. [35] used natural hematite as a cathode catalyst to significantly increase the maximum power density of MFCs. Previous studies have shown that TiO2, as a typical semiconductor mineral, has great application prospects as a cathode catalyst [36]. TiO2 has excellent electrochemical properties, able to transfer electrons and enhance the catalytic activity of redox reactions [37]. Based on the abovementioned excellent properties of TiO2, sulfonated titanium dioxide (S-TiO2) was prepared by the grafting of sulfonic groups onto TiO2 and doped into the PVDF-g-PSSA as a highly efficient inorganic additive, thus achieving simultaneous improvement of the proton conductivity and anti-fouling properties of the synthesized S-TiO2/PPSSA proton-exchange membrane (PEM) [38]. Ren et al. [39] developed a one-dimensional titanium-dioxide-doped iron oxide photoanode that can effectively improve the electricity generation performance of MFCs. Chen et al. [40] prepared a CeO2/TiO2/ACF catalytic cathode and combined it with a biological anode to accelerate the cathodic reaction. However, the agglomeration of TiO2 particles limits the further improvement of MFC performance to a certain extent. Biochar is a low-cost, environmentally friendly, porous material. Biochar is rich in various functional groups, with a high specific surface area and porosity, so it is often used as a carrier. At the same time, it also has corrosion resistance, low resistance, and high conductivity [41]. Therefore, biochar is widely used as a carrier material for electrode catalysts [42,43]. Yuan et al. [44] prepared sludge biochar and used it as cathode catalytic material; the MFC with sludge biochar had the highest redox peak current. Based on this, it was necessary to combine nature rutile with biochar and explore its effects on MFC performance.
However, the agglomeration of TiO2 significantly compromises its semiconductor properties. In addition, there have been relatively few studies on the combination of natural minerals and biochar as MFC electrode catalysts. In this study, we successfully constructed a novel rutile–biochar (Rut-B) MFC. The Rut-B composite exhibited remarkable catalytic activity, significantly enhancing the electrical performance and power output of MFCs. Furthermore, we investigated the impact of Rut-B on the degradation efficiency of heavy metal pollution in MFC systems. Our results demonstrated that Rut-B greatly improved the degradation rate of Pb2+ and Zn2+ under high-concentration conditions. Importantly, even after multiple cycles of degradation, the system maintained excellent cyclic stability. We propose that biochar-loaded rutile effectively mitigates particle agglomeration, offering a simple, environmentally friendly, and cost-effective cathode catalyst with immense potential for enhancing MFCs’ power output and remediating heavy metal pollution.
2. Materials and Methods
2.1. Chemical Reagents for the Experiment
Rutile samples were obtained from the Lingshou mining area in Hebei Province, China. Polytetrafluoroethylene, ethanol, tryptophan, diphenylthiocarbazone, sodium chloride, potassium chloride, yeast extract fermentation, and casein tryptone were procured from Tianjin Damao Chemical Reagent Factory. All chemicals used in the experiments were of analytical grade, and deionized water was employed.
2.2. Preparation of Biochar and Rut-B
Corn cobs were sourced from Gansu, China and sun-dried for 5 days before being ground into powder. The resulting powder was placed in a N2 atmosphere with an intake flow rate of 0.5 L/min to create an anaerobic environment. Biochar was produced by heating the powder in a muffle furnace at a heating rate of 5 °C/min to a temperature of 350 °C for 2 h. The biochar was then sifted through a 200-mesh sieve and stored in a dry glass vessel. The biochar and rutile composites were prepared by subjecting biochar and 75% (mass ratio) rutile to oxygen-limited pyrolysis at 500 °C under the aforementioned preparation conditions. The resulting compounds were subsequently pulverized, sieved through a 200-mesh sieve, and stored in dry glassware for further use.
2.3. Characterization of Composite Materials
By conducting X-ray photoelectron spectroscopy (XPS, Ultima IV, Tokyo, Japan) analysis, the element type characteristics of biochar and Rut-B can be compared. Additionally, this technique enables the determination of the valence state and functional group type of carbon elements in the material itself, which is essential for elucidating the mechanism of heavy metal adsorption by modified biochar materials in adsorption experiments. Scanning electron microscopy (SEM, JSM-6510, Tokyo, Japan) is commonly employed to depict the fundamental microstructure of materials. The micromorphologies of biochar and Rut-B were observed using SEM at 10 kV, with a working distance of 10 mm. The structures and compositions of rutile, biochar, and Rut-B composites were characterized via X-ray diffraction (XRD) utilizing an Ultima IV X-ray diffractometer (Rigaku Corporation, Akishima, Japan). The scanning range was set between 10° and 80°, with a scanning rate of 4° min−1.
2.4. Construction of MFC
The experimental setup used a two-chamber microbial fuel cell (MFC). The anode chamber was sealed with a rubber diaphragm, and a 3.5 cm × 6.0 cm graphite plate was used as the anode. The anode chamber and the cathode chamber were separated by an ion exchange-membrane of the appropriate size. To investigate the catalytic effect of rutile on biochar, we established four MFCs, where a common graphite electrode, natural-rutile-coated electrode, biochar-coated electrode, and biochar-loaded rutile-coated electrode were used as cathodes for each MFC.
The control group consisted of an ordinary graphite electrode, while the other three experimental groups were prepared using a two-step process. In the preparation of the experimental group, 0.3 mL of polytetrafluoroethylene and a few drops of ethanol were added to 0.3 g of rutile, biochar, or Rut-B and stirred until the gel solidified. Subsequently, the gel was coated on a graphite electrode (Hongfeng Carbon, Shanghai, China) with a size of 3.5 × 6 cm2 and a thickness of 5 mm to form a thin mineral film of about 0.2 mm. The film was then dried at room temperature for 12 h before four groups of microbial fuel cells (MFCs) were cultured in an incubator maintained at 35 °C.
Each anode chamber contained 10 g/L tryptophan, 5 g/L yeast extract, and 10 g/L NaCl of Luria-B retention (LB). At the bottom of each anode chamber were soil samples containing microbial communities (collected from Lanzhou University, Gansu Province, Lanzhou, China). Each cathode chamber was equipped with an open mouth with a diameter of 1.7 mm to ensure adequate air contact, and the cathode electrolyte was a 0.1 M KCl solution. In each MFC, a titanium wire was connected to the circuit, and an external circuit was connected to a 1000 Ω resistor for electronic transmission, which could be used to measure the voltage.
2.5. Determination of Pollutant Degradation Performance
After achieving a steady state in the MFCs with four distinct cathodes, solutions containing 300 mg/L Pb2+ and 200 mg/L Zn2+ were introduced to replace the cathode solution individually. Hourly samples were collected from the cathode chamber, and their concentrations were measured. The concentration and degradation efficiency of Pb2+ and Zn2+ were determined using UV–visible spectrophotometry through the standard curve method. Based on the standard curves correlating Pb2+ and Zn2+ concentrations with absorbance values, the sample concentrations were determined. The concentrations of Pb2+ and Zn2+ in the cathode chamber were measured at pH 2.0 at normal room temperature (25 ± 2 °C).
3. Results and Discussion
3.1. Characterization of Biochar, Rutile, and Rut-B
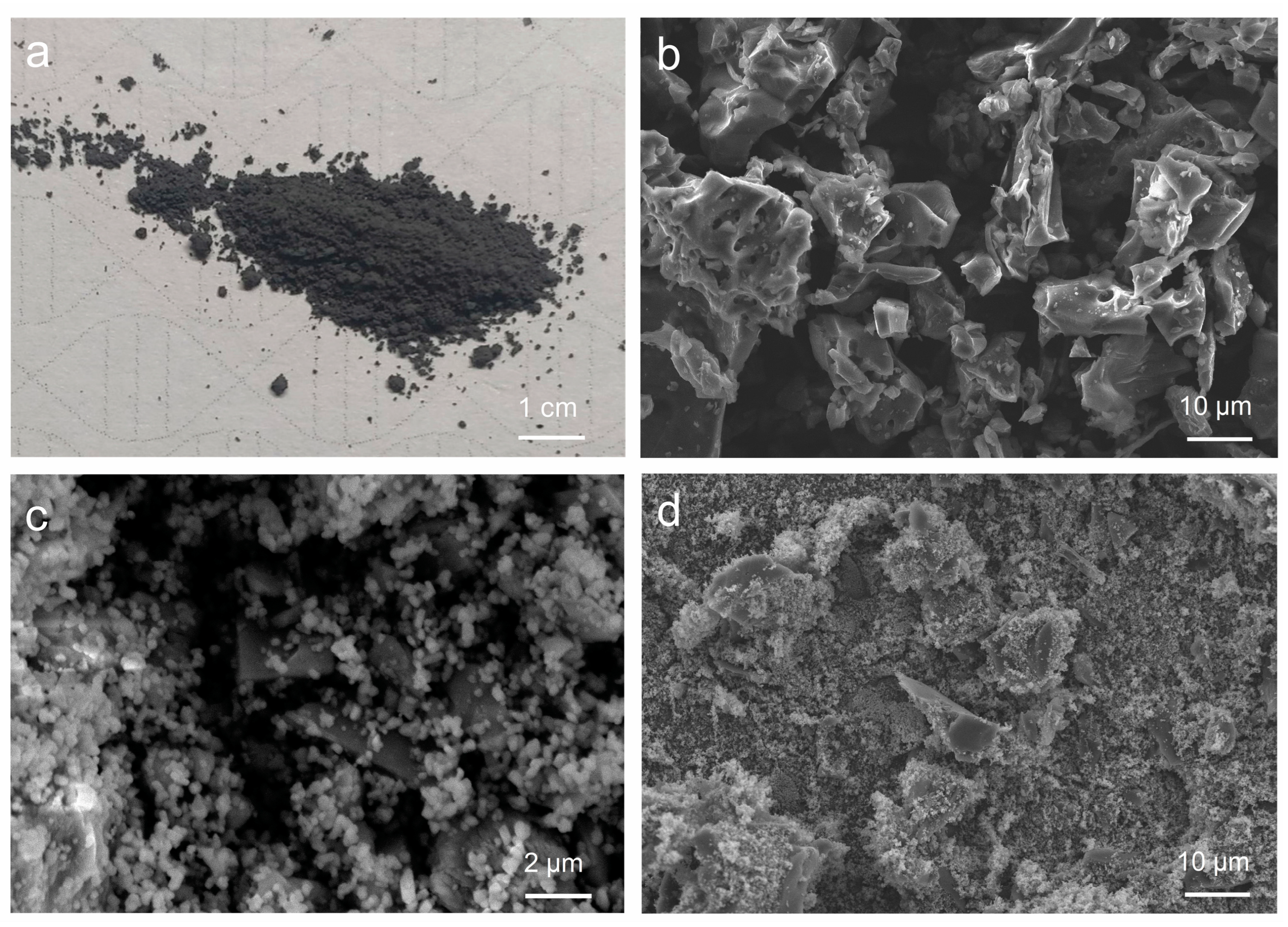
The micromorphology of biochar (Figure 1a) and Rut-B was observed by SEM analysis. Careful observation of Figure 1b shows that the biochar presents a lamellar or fibrous structure. Biochar contains a large number of pore structures of different sizes. Natural rutile, in contrast, is round, oval, and granular (Figure 1c). The whole structure is relatively close and highly ordered. There are almost no pores between the particles. After compounding with rutile, biochar has less deformation and more obvious crushing (Figure 1d). The average particle size of biochar is about 30–40 μm. The average particle size of Rut-B decreased to 10–20 μm. By comparing the original biochar with the composite material, it can be seen that the surface of the original biochar is relatively smooth, and the surface of the biochar in the composite material is rough. This is due to the fact that rutile particles are closely attached to the surface of the biochar, which changes the microstructure of the biochar. The results showed that the composite effect of rutile and biochar was excellent.
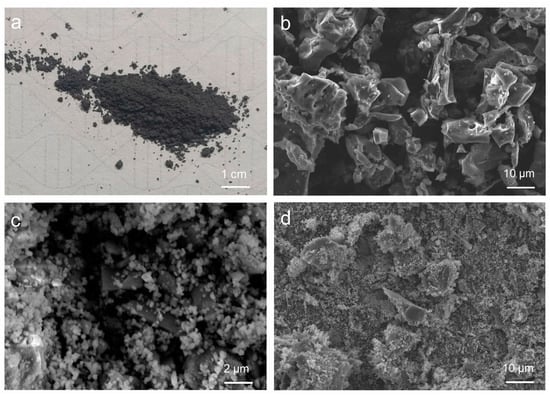
Figure 1.
The physical diagram of biochar (a) and the SEM images of biochar (b), rutile (c), and Rut-B (d).
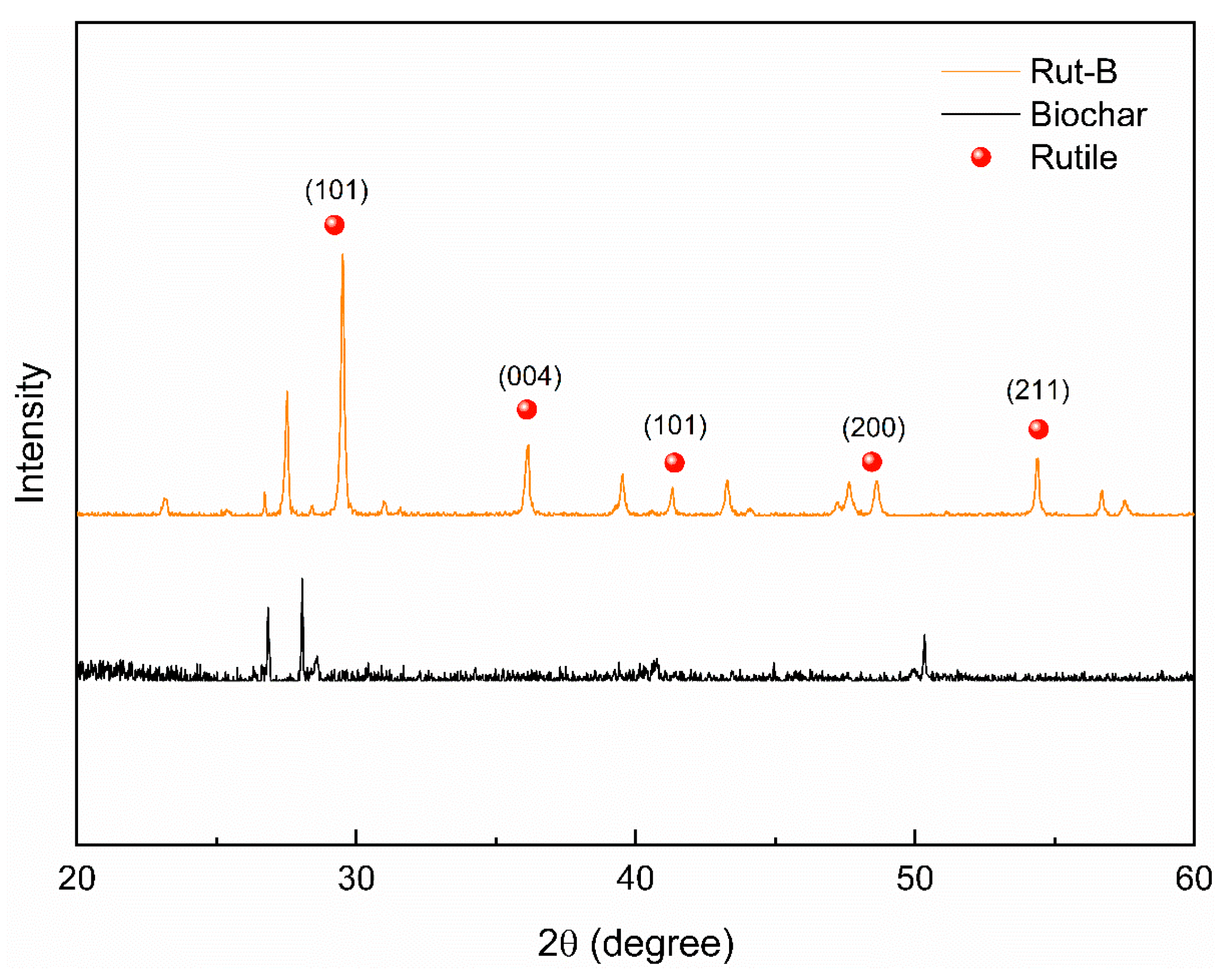
The biochar and Rut-B composites were characterized by XRD. The XRD spectrum of biochar showed that the biochar had three broad and slow amorphous diffraction peaks. At the same time, there were more sharp, small diffraction peaks, indicating that the biochar was being carbonized (Figure 2). The crystalline compound was carbonized into microcrystalline carbon with a fine-particle graphitized structure. After high-temperature carbonization, the structure of the biochar in Rut-B did not change much, so the diffraction peak did not change much. After the composite, the TiO2 peak representing rutile appeared in Rut-B, indicating that the preparation of the composite was very successful. The composite structure of biochar and rutile is relatively stable.
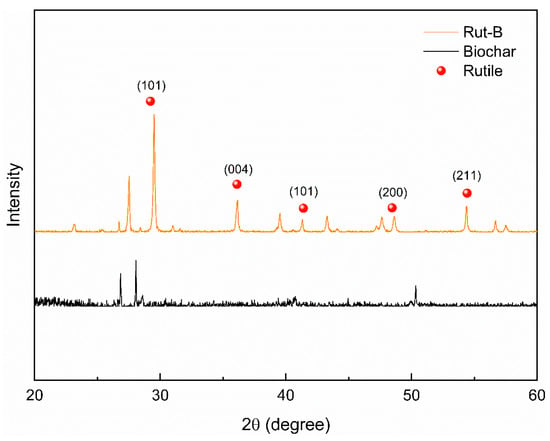
Figure 2.
XRD patterns of biochar and Rut-B.
The BET results showed that the adsorption capacity of Rut-B on N2 was significantly higher than that of the rutile, biochar, and blank groups, indicating that biochar co-doping did improve the porosity of the rutile. With the increase in pyrolysis temperature, the BET surface area of Rut-B increased from 25.47 m2/g to 218.85 m2/g, and the total pore volume increased from 0.09 cm3/g to 0.26 cm3/g, which was significantly higher than that of rutile and biochar. Therefore, the specific surface area and adsorption properties of Rut-B composites are significantly increased compared with rutile and biochar.
3.2. System Efficiency and Potential Characteristics of MFCs
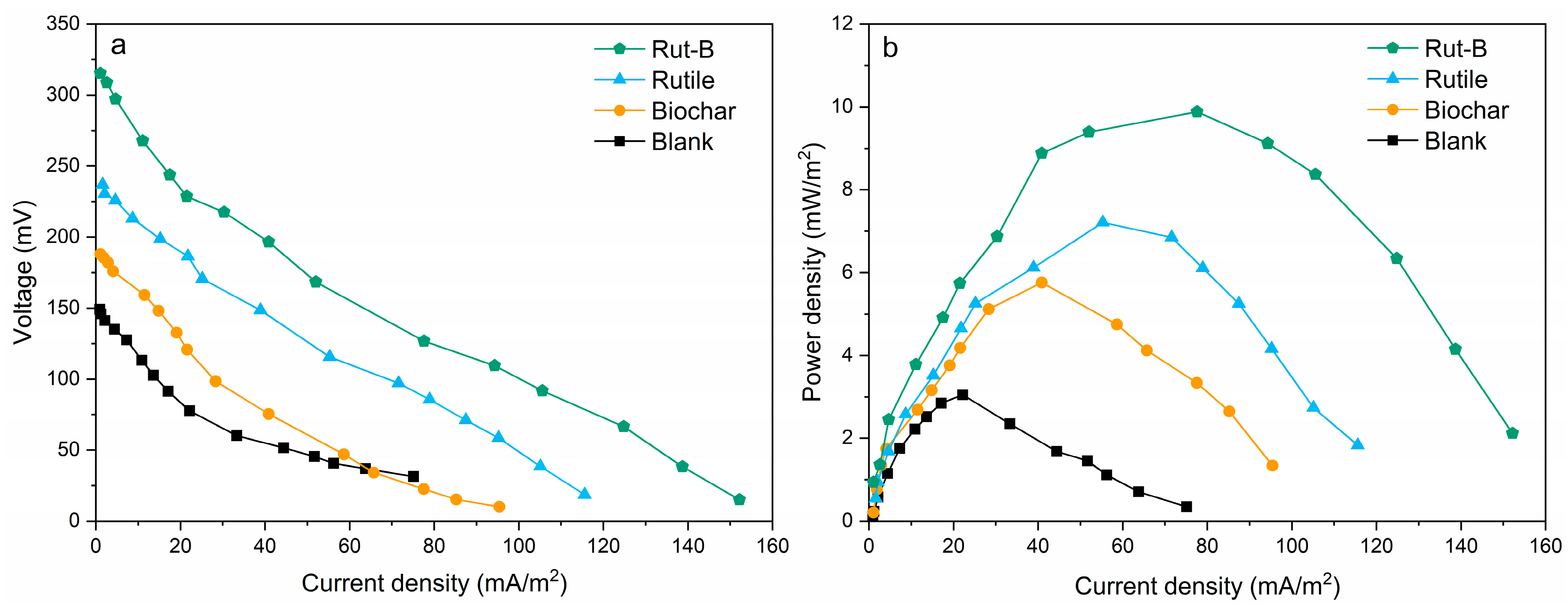
The energy conversion efficiency and current density in MFCs mainly depend on the material used in the electrode. Previous studies have shown that the material composition and the physical and chemical properties of the cathode can significantly improve the energy conversion efficiency and current density of MFCs [45]. We explored the electricity generation performance of MFCs with four different cathodes, and we compared the polarization curves and power density curves of MFCs (Figure 3). The limiting current densities of Rut-B, rutile, and biochar reached 152.26 mA/m2, 115.67 mA/m2, and 95.41 mA/m2, respectively, which were 102.7%, 53.9%, and 27% higher than that of the blank group, respectively (Table 1). Meanwhile, Rut-B had the maximum limiting voltage (315.27 mV), which was 33.1% and 67.3% higher than that of rutile (236.91 mV) and biochar (188.43 mV), respectively. When the external resistance of the MFC system is close to the internal resistance, the power density reaches the maximum. The maximum power densities of Rut-B, rutile, and biochar were 9.88 mW/m2, 7.21 mW/m2, and 5.76 mW/m2, respectively. The maximum power density of the blank group was only 3.05 mW/m2. Rut-B increased by 224% compared with the blank. The above results indicate that Rut-B had the best power generation performance among the four MFCs. This is because the surface of the biochar-loaded rutile composite has abundant functional groups, which further improve its electron transfer ability [46]. At the same time, the growth of crystalline TiO2 and the epitaxial carbonization of biochar occur simultaneously, which contributes to the highly dispersed distribution and embedding of spherical TiO2 particles on the surface of the biochar [47].
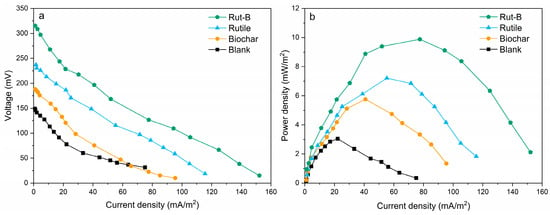
Figure 3.
Polarization curves (a) and power density curves (b) of different MFCs.

Table 1.
Polarization curve and power density curve parameters.
The ohm polarization region of the polarization curve was fitted linearly, where the slope reflected the internal resistance. The internal resistance of Rut-B, rutile, and biochar was 3223 Ω, 3397 Ω, and 3600 Ω, respectively. The systems with lower internal resistance had stronger electron transfer and showed capable microbial degradation of heavy metal pollutants.
The polarization curves and power density curves show that the electrode performance of Rut-B is higher than that of rutile, biochar, and the blank. Therefore, the application of Rut-B composite as cathode catalyst is beneficial to improve the system power output of MFCs. Compared with previous studies, the power generation efficiency of MFC using Rut-B composite as a cathode catalyst was significantly improved. Compared with the power density of an Fe-Mn-Mg/CF composite cathode MFC (5.47 mW/m2), the maximum power density of Rut-B increased by 80.62% [45]. The Rut-B MFC was significantly enhanced compared to an Fe-anode- and bio-cathode-based MFC device, and its maximum power density was 1.1 mW/m2 [48]. This comparative study shows that the power generation efficiency of the Rut-B MFC was significantly improved compared with previous studies, indicating that this efficient MFC may have higher pollutant removal performance. It is worth noting that the oxygen reduction reaction process on the MFC cathode is slow, and the efficient electrode catalyst helps to accelerate the reaction process, thereby improving the battery performance [49].
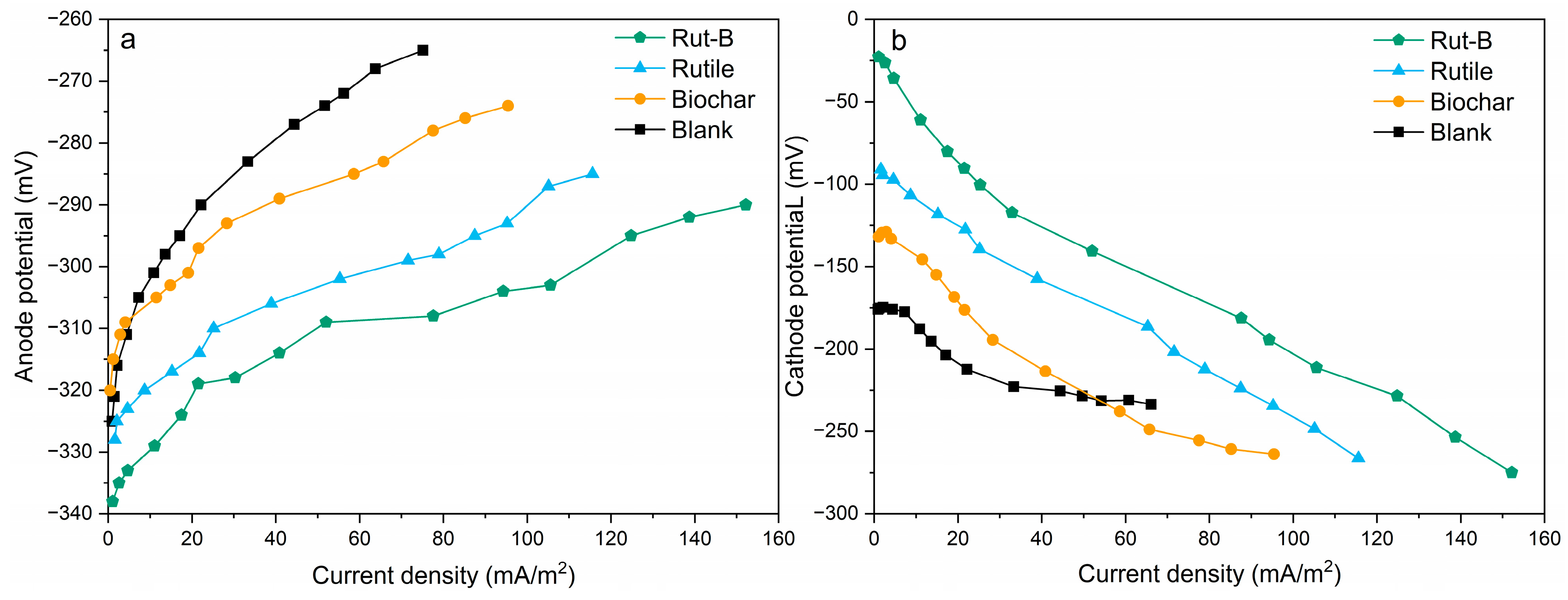
In order to further explore the effect of Rut-B on the electrode potential of MFC systems, the electrode potential curves of the anode and cathode were measured individually (Figure 4). The slope of the anodic potential curve of Rut-B was lower than that of other systems, indicating that the anode can maintain a lower potential for a long time (Figure 4a). At the same time, the initial anode potential of Rut-B (−338 mV) was much lower than that of other systems. In other words, the Rut-B-cathode-catalyzed MFC system always maintained a high potential, its degree of polarization was low, and the anode showed a good ability to transfer electrons. Furthermore, the cathode potential curve showed that the initial cathode potential of Rut-B was up to −22.8 mV (Figure 4b), and the slope of the cathode potential curve of the Rut-B-cathode-catalyzed MFC system was the lowest, indicating that this system has low polarizability and excellent electron acceptance ability. The results showed that the Rut-B composite, as a cathode catalyst, effectively increased the electrode potential difference of the MFC, thereby increasing its electron transfer rate. A higher electron transfer rate is also conducive to the redox reaction [50].
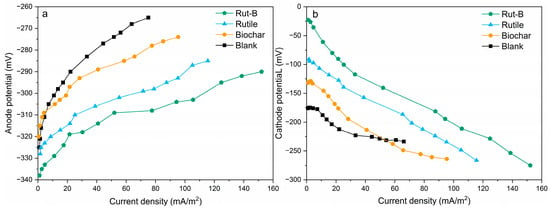
Figure 4.
Anode potential curves (a) and cathode potential curves (b) of different MFCs.
3.3. Study on the Degradation of Heavy Metal Pollutants by MFCs
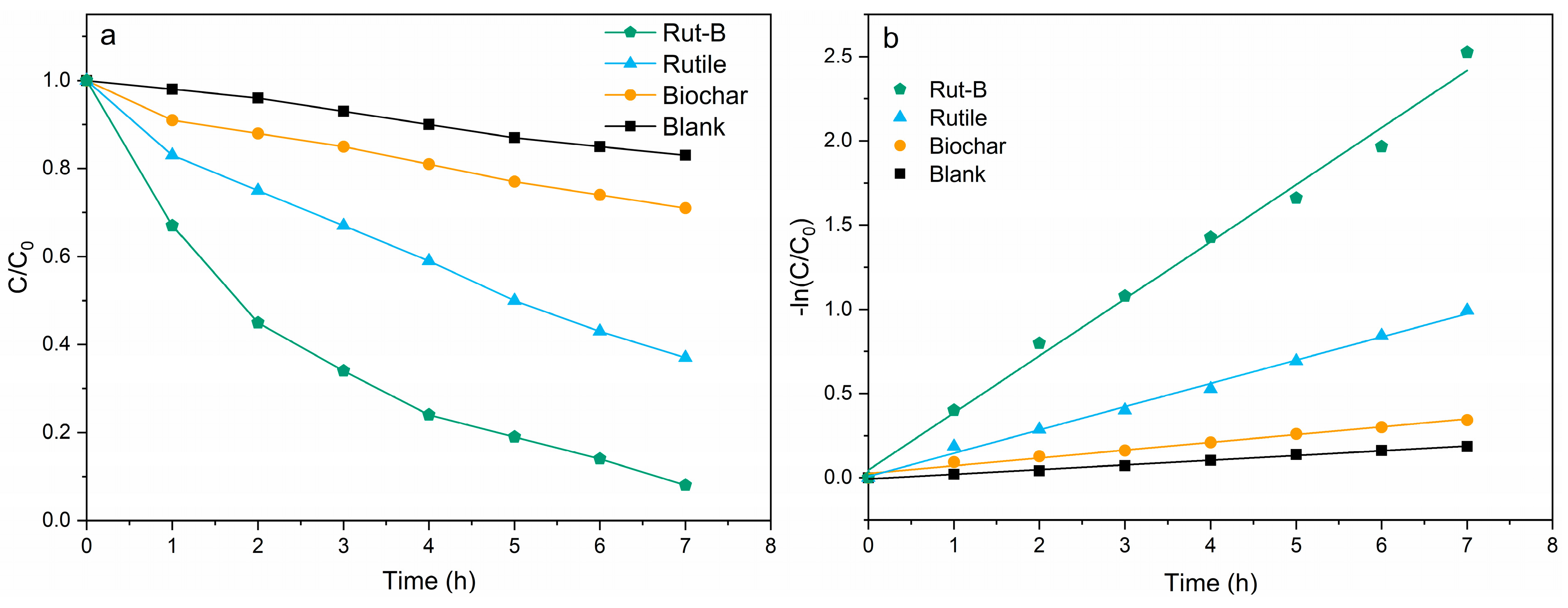
Studies have shown that the electricity generation performance of MFCs is positively correlated with their pollutant degradation efficiency [32]. Therefore, the use of Rut-B composite as a cathode catalyst with excellent performance also has great potential in environmental remediation. With the significant enhancement of the electricity generation performance of the MFC by Rut-B, we used Pb2+ and Zn2+ as indicators of heavy metal pollution to further explore its effect on the treatment efficiency of heavy metal pollution. In order to eliminate the possible errors and contingencies of the experiment, each degradation experiment was repeated three times under the same experimental conditions. The degradation curves of Pb2+ by the different MFCs are shown in Figure 5a. The initial concentration of the Pb2+ solution was 300 mg/L, and the degradation rates of Rut-B, rutile, and biochar within 7 h were 92.4%, 63.1%, and 29.7%, respectively, while that of the blank group was only 17.2%. According to the calculation, the degradation rate of Rut-B was 46.4% and 211.1% higher than that of rutile and biochar, respectively. There is no doubt that Rut-B has the fastest degradation efficiency.
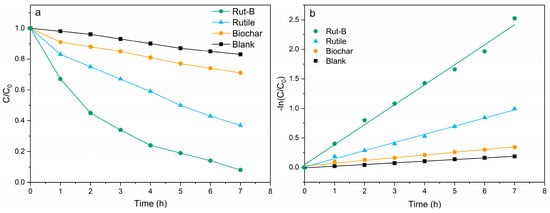
Figure 5.
Degradation curve (a) and kinetic fitting curve (b) of Pb2+.
The kinetic fitting of the degradation process of Pb2+ was further carried out to better explore the adsorption mechanism. The kinetic fitting curves of different MFCs are shown in Figure 5b. C0 is the initial Pb2+ concentration in the aqueous solution, C is the Pb2+ concentration at the specified reaction time, and k is the degradation rate constant. It can be seen that Rut-B has the highest reaction rate constant, which further indicates that this system has the fastest degradation rate for Pb2+ (Table 2).

Table 2.
Reaction dynamics fitting parameters of the system (Pb2+).
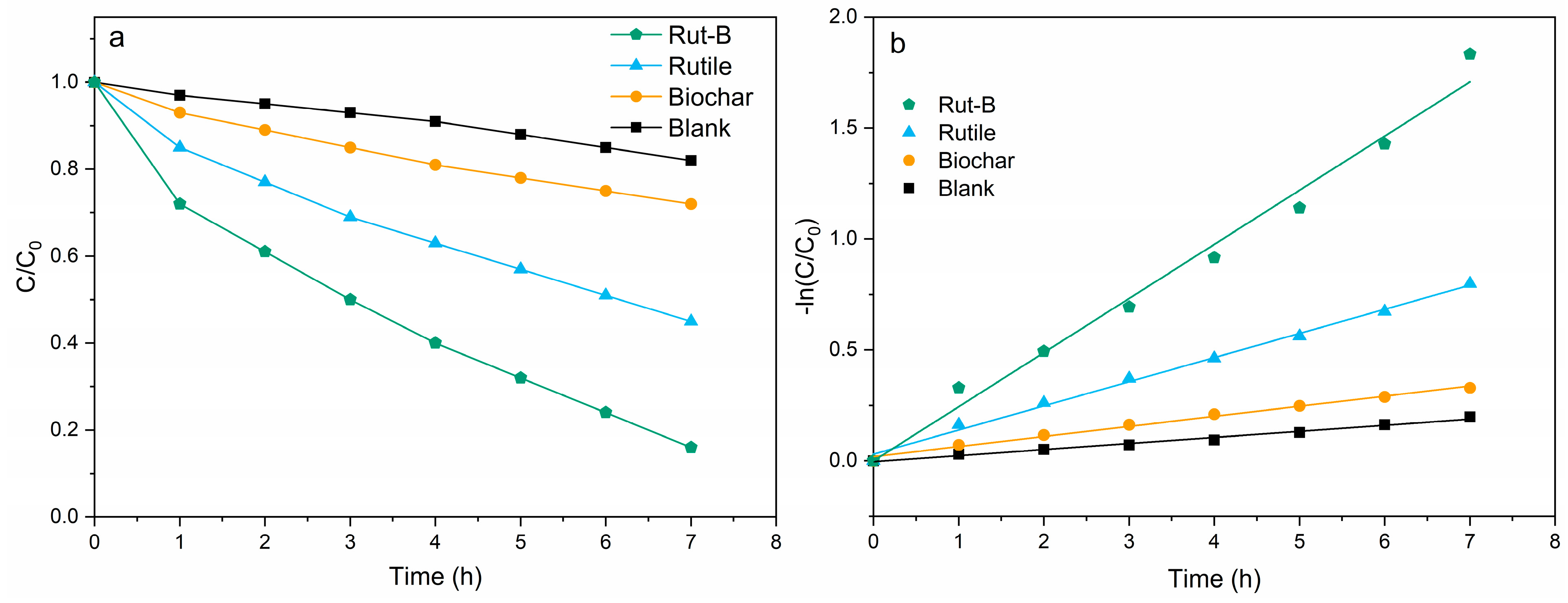
At the same time, we also carried out degradation experiments on Zn2+, which is common in heavy-metal-contaminated wastewater (Figure 6). The degradation curves of Zn2+ by different MFCs are shown in Figure 6a. The initial concentration of Zn2+ solution was 200 mg/L, and the degradation rates of Rut-B, rutile, and biochar within 7 h were 84.1%, 55.4%, and 28.7%, respectively. Compared with the blank group (18.9%), this represented increases of 345.0%, 193.1%, and 51.8%, respectively. Notably, the degradation rate was 3.3 times higher than that found in previous studies [51]. In addition, compared with previous studies, our degradation cycle was only 1/7, and the degradation rate of Zn was increased by 55% [52]. There is no doubt that the degradation performance of Rut-B on Zn2+ was the most excellent among the four MFCs, and its degradation rate reached 24 mg·L−1·h−1.
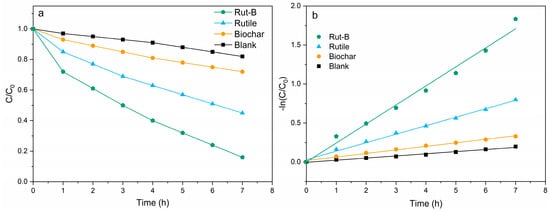
Figure 6.
Degradation curve (a) and kinetic fitting curve (b) of Zn2+.
The kinetics of Zn2+ degradation by different MFCs was further fitted (Figure 6b). C0 is the initial Zn2+ concentration in the aqueous solution, C is the Zn2+ concentration at the specified reaction time, and k is the degradation rate constant. It is obvious that the slope of the fitting curve of Rut-B is larger, indicating that it has the highest reaction rate constant. The reaction rate constant of Rut-B reached 0.2438, far exceeding that of rutile (0.1088), biochar (0.0455), and the blank (0.0274) (Table 3). This indicates that the use of Rut-B composite as a cathode catalyst can not only greatly improve the power generation and output power of MFCs, but also significantly enhance the degradation efficiency of Zn2+ by MFCs.

Table 3.
Reaction dynamics fitting parameters of the system (Zn2+).
3.4. Study on the Cyclic Degradation Performance of MFCs
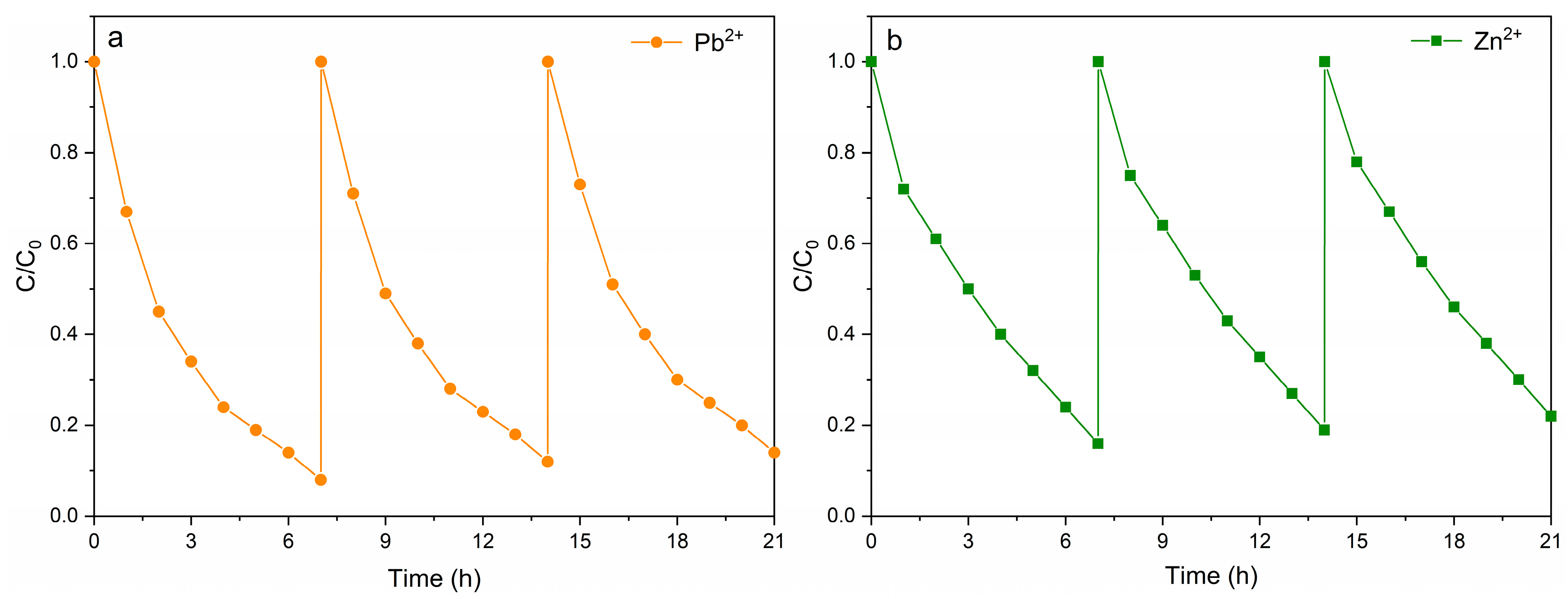
The experimental results of the degradation of simulated heavy-metal-contaminated wastewater show that using the Rut-B composite material as a cathode catalyst can significantly enhance the degradation efficiency of Pb2+ and Zn2+ in MFCs. In order to further explore its wide applicability and cycle stability in the actual remediation of heavy metal pollution, we set up three rounds of cycle experiments. After each round of degradation, the pollutants were supplemented to restore their concentrations to their initial values, and then the next round of degradation experiments was carried out. The results of the loop experiment are shown in Figure 7. Obviously, whether for Pb2+ or Zn2+, the time of each cycle was almost unchanged. It is worth noting that after two cycles of degradation, although the degradation rate of the third round of degradation decreased, the overall degradation rate of the system remained essentially unchanged. This shows that the use of the Rut-B composite material as a cathode catalyst significantly enhances the performance of MFCs in the degradation of heavy metal pollutants, and it also has excellent cyclic degradation stability. The relatively stable cyclic degradation performance indicates that this material can be recycled and reused many times in actual wastewater treatment. This provides a low-cost and environmentally friendly new method for the remediation of heavy metal pollution.
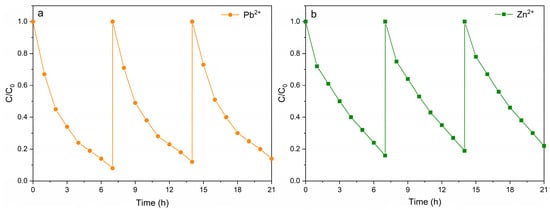
Figure 7.
Cyclic degradation curves of Pb2+ (a) and Zn2+ (b).
3.5. Mechanism of Rut-B Cathode Catalysis
In this study, biochar-supported rutile was used as an MFC’s cathode catalyst to effectively improve its power generation performance. TiO2 is a kind of semiconductor mineral with a band gap of about 3.0–3.2 eV [36]. The Ti(IV) site on the surface of the TiO2 crystal captures the conduction electrons and is reduced to the Ti(III) site. The oxygen () molecules adsorbed on the cathode surface of the MFC react with the Ti(III) center to form superoxide free radicals (), which enhance the oxygen reduction reaction in the cathode center and promote the power generation of the MFC [53]. In addition, compared with other metal oxide semiconducting materials, rutile has higher Ti-O bond polarity, and water molecules adsorbed on the surface can easily become polarized and dissociated to form hydroxyl groups [54,55]. In the liquid medium, the surface hydroxyl group can improve the performance of TiO2 and promote the particles to adsorb the charged molecules through static electricity, forming a diffused electric bilayer, which increases the electron holding capacity [56].
Biochar-loaded rutile avoids rutile agglomeration and effectively improves the catalytic performance of the cathode. Meanwhile, biochar has a graphite structure and quinoline-like redox active groups, so biochar has a large number of active sites and unsaturated electron pairs [57], enabling it to accelerate electron transfer and pollutant reduction/transformation, thus improving the MFC’s cathode reaction rate and pollutant removal efficiency [58]. In addition, biochar is made under high-temperature conditions, so it has high contents of pyrrolic, graphitic, and pyridinic nitrogen, which can be used in cathode catalysts to further accelerate the cathode electron transfer [59].
4. Conclusions
In this study, a Rut-B composite was prepared as a green and efficient MFC cathode catalyst. The use of Rut-B as a cathode catalyst significantly improved the power generation performance and power output of the MFC system. The maximum current density of Rut-B is 152.26 mA/m2, and its limiting voltage is 315.27 mV. At the same time, its maximum power density also reached 9.88 mW/m2, which was 224% of the blank’s. While the electrical performance was significantly enhanced, the degradation efficiency of heavy metal pollutants by the MFC was also improved. When the initial concentration of Pb2+ was 300 mg/L, the degradation rate of Rut-B reached 92.4% in 7 h, while that of the blank was only 17.2%. The degradation rate of the best experimental group reached 5.4 times that of the blank control group. When the concentration of Zn2+ was 200 mg/L, Rut-B could degrade 84.1% in 7 h, and the degradation rate was 24 mg·L−1·h−1. Based on the excellent degradation performance of Rut-B, the cyclic degradation experiment further verified its good cyclic degradation stability. In the three rounds of cyclic degradation experiments, the degradation rate of Rut-B essentially did not change, and 90% of the pollutants could still be degraded within 7 h. This is a kind of mineral–biochar composite material with high economic benefit and green environmental protection. As an efficient positive catalytic material, it is of great significance to solve the problem of poor electricity generation performance of MFCs. At present, the treatment of heavy metal pollution in high-concentration complex systems is a major bottleneck of Rut-B MFCs. Considering that Rut-B is a photocatalyst, further use of Rut-B as a photocatalytic material for cathodes will be the target of our next research.
Author Contributions
Conceptualization, J.Z. and L.W.; methodology, Z.W. (Zhe Wang) and Y.F.; validation, G.L. and Z.W. (Zhiyang Wang); formal analysis, J.W. and P.H.; investigation, J.Z., L.W. and P.H.; writing—original draft preparation, J.Z., L.W., Z.W. (Zhe Wang), Y.F. and G.L.; writing—review and editing, Z.W. (Zheyang Wang), J.W. and P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Gansu Nonferrous Geological Bureau Research Project (Grant No. YSJD2022-11) and the Technical Innovation Center of Mine Geological Environment Restoration Engineering in Alpine Arid Area (Grant No. HHGCKK2201).
Data Availability Statement
All relevant data are within the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gao, Q.; Tao, D.W.; Qi, Z.B.; Liu, Y.F.; Guo, J.; Yu, Y. Amidoxime functionalized PVDF-based chelating membranes enable synchronous elimination of heavy metals and organic contaminants from wastewater. J. Environ. Manage. 2022, 318, 115643. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Ghosh, N.; Mandal, C.; Das, K.; Dey, N.; Adak, M.K. Responses of the maize plant to chromium stress with reference to antioxidation activity. Braz. J. Plant Physiol. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Shaikh, A.; Mishra, S.P.; Mohapatra, P.; Parida, S. One-step solvothermal synthesis of TiO2-reduced graphene oxide nanocomposites with enhanced visible light photoreduction of Cr(VI). J. Nanopart. Res. 2017, 19, 206. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Dytlow, S.; Gorka-Kostrubiec, B. Concentration of heavy metals in street dust: An implication of using different geochemical background data in estimating the level of heavy metal pollution. Environ. Geochem. Health 2021, 43, 521–535. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Pandiyan, J.; Mahboob, S.; Govindarajan, M.; Al-Ghanim, K.A.; Ahmed, Z.; Al-Mulhm, N.; Jagadheesan, R.; Krishnappa, K. An assessment of level of heavy metals pollution in the water, sediment and aquatic organisms: A perspective of tackling environmental threats for food security. Saudi J. Biol. Sci. 2021, 28, 1218–1225. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, Y.; Moon, H.; Ra, K. Characteristics of metal pollution and multi-isotopic signatures for C, cu, zn, and pb in coastal sediments from special management areas in korea. Mar. Pollut. Bull. 2023, 188, 114642. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, T.A.K.; Khoo, K.S.; Hoang, T.K.A.; Ng, H.S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.K. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere. 2021, 287, 132369. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Guerrero-Barajas, C. Modern trend of anodes in microbial fuel cells (MFCs): An overview. Environ. Technol. Innov. 2021, 23, 101579. [Google Scholar] [CrossRef]
- Daud, N.N.M.; Ahmad, A.; Yaqoob, A.A.; Ibrahim, M.N.M. Application of rotten rice as a substrate for bacterial species to generate energy and the removal of toxic metals from wastewater through microbial fuel cells. Environ. Sci. Pollut. Res. 2021, 28, 62816–62827. [Google Scholar] [CrossRef]
- Patel, A.; Choi, Y.; Sim, S. Emerging prospects of mixotrophic microalgae: Way forward to sustainable bioprocess for environmental remediation and cost-effective biofuels. Bioresour. Technol. 2020, 300, 122741. [Google Scholar] [CrossRef]
- AlSayed, A.; Soliman, M.; Eldyasti, A. Microbial fuel cells for municipal wastewater treatment: From technology fundamentals to full-scale development. Renew. Sustain. Energy Rev. 2020, 134, 110367. [Google Scholar] [CrossRef]
- Idris, S.A.; Esat, F.N.; Abd Rahim, A.A.; Rizzqi, W.A.Z.; Ruzlee, W.; Razali, W.M.Z. Electricity generation from the mud by using microbial fuel cell. Matec Web Conf. 2016, 69, 02001. [Google Scholar] [CrossRef]
- Lovley, D. Bug juice: Harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006, 4, 487–508. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, H.; Zhen, H. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energ. Environ. Sci. 2014, 7, 911–924. [Google Scholar] [CrossRef]
- Mian Chen, B.Y.; Tobias, M.B.; Rakan, M.A.; Peter, G.P. Composition Dependence of Ethanol Oxidation at Ruthenium. J. Electrochem. Soc. 2018, 165, J3019–J3025. [Google Scholar] [CrossRef]
- Aelterman, P.; Rabaey, K.; Pham, H.T.; Boon, N.; Verstraete, W. Continuous electricity generation at high voltages and currents using stacked microbial fuel cells. Environ. Sci. Technol. 2006, 40, 3388–3394. [Google Scholar] [CrossRef]
- Shi, L.; Dong, H.L.; Reguera, G.; Beyenal, H.; Lu, A.H.; Liu, J.; Yu, H.Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef]
- Katuri, K.P.; Kalathil, S.; Ragab, A.; Bian, B.; Alqahtani, M.F.; Pant, D.; Saikaly, P.E. Dual-function electrocatalytic and macro-porous hollow-fiber cathode for converting waste streams to valuable resources using microbial electrochemical systems. Adv. Mater. 2018, 30, e1707072. [Google Scholar] [CrossRef]
- Pandit, S.; Savla, N.; Jung, S. Recent advancements in scaling up microbial fuel cells. In Integrated Microbial Fuel Cells for Wastewater Treatment; Butterworth-Heinemann: Oxford, UK, 2020; pp. 349–368. [Google Scholar]
- Badi, N.; Theodore, A.M.; Alghamdi, S.A.; Al-Aoh, H.A.; Lakhouit, A.; Roy, A.S.; Ignatiev, A. Fabrication and Characterization of Flexible Solid Polymers Electrolytes for Supercapacitor Application. Polymers 2022, 14, 3837. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Mench, M.M.; Regan, J.M.; Oak Ridge National Lab (ORNL); Oak Ridge, TN (United States). Impedance characteristics and polarization behavior of a microbial fuel cell in response to short-term changes in medium pH. Environ. Sci. Technol. 2011, 45, 9069–9074. [Google Scholar] [CrossRef] [PubMed]
- Jung, S. Impedance analysis of geobacter sulfurreducens PCA, shewanella oneidensis MR-1, and their coculture in bioeletrochemical systems. International Int. J. Electrochem. Sci. 2012, 7, 11091–11100. [Google Scholar] [CrossRef]
- Kang, H.; Jeong, J.; Gupta, P.L.; Jung, S.P. Effects of brush-anode configurations on performance and electrochemistry of microbial fuel cells. Int. J. Hydrog. Energy 2017, 42, 27693–27700. [Google Scholar] [CrossRef]
- Ogan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar]
- Zhou, M.; Chi, M.; Luo, J. An overview of electrode materials in microbial fuel cells. J. Power Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]
- Rahman, S.; Balushi, N.J.; Nayak, J.K.; Al-Mamun, A.; Al-Abri, M.; Alawi, M.; Sana, A. A review on semiconductor photocathode in bio electrochemical systems: Mechanism, limitation, and environmental application. Mater. Today Sustain. 2023, 22, 100349. [Google Scholar] [CrossRef]
- Koo, B.; Jung, S.P. Improvement of air cathode performance in microbial fuel cells by using catalysts made by binding metal-organic framework and activated carbon through ultrasonication and solution precipitation. Chem. Eng. J. 2017, 424, 130388. [Google Scholar] [CrossRef]
- Koo, B.; Lee, S.; Oh, S.; Kim, E.J.; Hwang, Y.; Seo, D.; Kim, J.Y.; Kahng, Y.H.; Lee, Y.W.; Chung, S.; et al. Addition of reduced graphene oxide to an activated-carbon cathode increases electrical power generation of a microbial fuel cell by enhancing cathodic performance. Electrochim. Acta 2019, 297, 613–622. [Google Scholar] [CrossRef]
- Touach, N.; Benzaouak, A.; Toyir, J.; El Hamidi, A.; El Mahi, M.; Lotfi, E.M.; Kacimi, M.; Liotta, L.F. Bioenergy Generation and Wastewater Purification with Li0.95Ta0.76Nb0.19Mg0.15O3 as New Air-Photocathode for MFCs. Catalysts 2022, 12, 1424. [Google Scholar] [CrossRef]
- Zhao, X.; Ke, Z.; Wang, Q. Efficient organic contaminant and Cr(VI) synchronous removing by one-step modified molybdenite cathode microbial fuel cells. Env. Sci. Pollut. R 2023, 30, 4423–4434. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.T.; Wang, L.G.; Wen, Q.; Chen, Y.; Qi, L.J.; Huang, J.X.; Tang, Z.S. A 3D porous NCNT sponge anode modified with chitosan and polyaniline for high-performance microbial fuel cell. Bioelectrochemistry 2019, 129, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.L.; Zhu, Y.L.; Lu, L.; Xu, K.L.; Wang, H.M.; Jin, Y.H.; Ren, Z.J.; Liu, Z.N.; Zhang, W. Iron-rich nanoparticle encapsulated, nitrogen doped porous carbon materials as efficient cathode electrocatalyst for microbial fuel cells. J. Power Sources 2016, 315, 302–307. [Google Scholar] [CrossRef]
- Ren, G.P.; Ding, H.R.; Li, Y.; Lu, A.H. Natural hematite as a low-cost and earth-abundant cathode material for performance improvement of microbial fuel cells. Catalysts 2016, 6, 157. [Google Scholar] [CrossRef]
- Lu, A.H.; Li, Y.; Jin, S.; Wang, X.; Wu, X.L.; Zeng, C.P.; Li, Y.; Ding, H.R.; Hao, R.X.; Lv, M.; et al. Growth of non-phototrophic microorganisms using solar energy through mineral photocatalysis. Nat. Commun. 2012, 3, 768. [Google Scholar] [CrossRef]
- Zlámalová, M.; Lásková, P.; Vinarčíková, M.; Zukalová, M. Inherent electrochemical activity of TiO2 (anatase, rutile) enhances the charge capacity of cathodes of lithium-sulfur batteries. J. Solid State Electrochem. 2022, 26, 639–647. [Google Scholar] [CrossRef]
- Li, C.; Song, Y.; Wang, X.; Zhang, Q. Synthesis, characterization and application of S-TiO2/PVDF-g-PSSA composite membrane for improved performance in MFCs. Fuel 2020, 264, 116847. [Google Scholar] [CrossRef]
- Ren, G.P.; Sun, Y.; Lu, A.H.; Li, Y.; Ding, H.R. Boosting electricity generation and Cr(VI) reduction based on a novel silicon solar cell coupled double-anode (photoanode/bioanode) microbial fuel cell. J. Power Sources 2018, 408, 46–50. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, L.; Liu, L.; Zhang, Y. A novel UV-assisted PEC-MFC system with CeO2/TiO2/ACF catalytic cathode for gas phase VOCs treatment. Chemosphere 2020, 255, 126930. [Google Scholar] [CrossRef]
- Cai, T.; Huang, M.H.; Huang, Y.X.; Zheng, W. Enhanced performance of microbial fuel cells by electrospinning carbon nanofibers hybrid carbon nanotubes composite anode. Int. J. Hydrog. Energy. 2019, 44, 3088–3098. [Google Scholar] [CrossRef]
- Chang, H.; Gustave, W.; Yuan, Z. One-step fabrication of binder-free air cathode for microbial fuel cells by using balsa wood biochar. Environ. Technol. Innov. 2020, 18, 100615. [Google Scholar] [CrossRef]
- Ramya, M.; Harsha, V.K.; Senthil, K.P. Metal mixed biochar electrodes for the generation of electricity with high power density in microbial fuel cell. Sustain. Energy Technol. Assess. 2022, 53 Pt B, 102549. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, T.; Wang, D.; Tang, J.H.; Zhou, S.G. Sewage sludge biochar as an efficient catalyst for oxygen reduction reaction in a microbial fuel cell. Bioresour. Technol. 2013, 144, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Zhao, Q.L.; Ding, J.; Wang, K.; Jiang, J.Q. Development of an MFC-powered BEF system with novel Fe–Mn–Mg/CF composite cathode to degrade refractory pollutants. J. Clean. Prod. 2021, 326, 129348. [Google Scholar] [CrossRef]
- Qu, K.J.; Huang, L.; Hu, S.Y.; Liu, C.; Yang, Q.Y.; Liu, L.H.; Li, K.; Zha, Z.P.; Wang, Z.X. TiO2 supported on rice straw biochar as an adsorptive and photocatalytic composite for the efficient removal of ciprofloxacin in aqueous matrices. J. Environ. Chem. Eng. 2023, 11, 109430. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Li, X.; Huang, R.; Liu, Q.; Zhang, Y.; Gan, T.; Huang, Z.; Hu, H. Mutually supportive growth strategy to engineer a hollow biochar sphere-supported TiO2 composite with improved interfacial compatibility for efficient visible light-driven photocatalysis. J. Environ. Chem. Eng. 2023, 11, 110327. [Google Scholar] [CrossRef]
- Wang, R.; Wan, S.; Liu, B. Denitrification in perspective of carbon neutralization: CO2 emission reduction and electricity generation by Fe-anode and bio-cathode MFC. J. Water Process Eng. 2022, 48, 102868. [Google Scholar] [CrossRef]
- Singh, H.; Zhuang, S.Q.; Ingis, B.J.; Nunna, B.B.; Lee, E.S. Carbon-based catalysts for oxygen reduction reaction: A review on degradation mechanisms. Carbon 2019, 151, 160–174. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Luo, M.; Cao, J.; Fang, F.; Feng, Q.; Luo, J.; Hao, L.; Wang, C. Enhancing simultaneous electrosynthesis of CO2 and nitrogen removal in microbial fuel cell (MFC) cathode compartment by adding Fe–C/biochar compound substrates. J. Power Sources 2023, 560, 232707. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, B.K.; Jia, W.F.; Liang, J.H.; Sun, G.X. Can electrokinetic removal of metals from contaminated paddy soils be powered by microbial fuel cells? Environ. Technol. Innov. 2015, 3, 63–67. [Google Scholar]
- Song, T.S.; Zhang, J.; Hou, S.; Wang, H.; Zhang, D.; Li, S.; Xie, J. In situ electrokinetic remediation of toxic metal-contaminated soil driven by solid phase microbial fuel cells with a wheat straw addition. J. Chem. Technol. Biotechnol. 2018, 93, 2860–2867. [Google Scholar] [CrossRef]
- Bhowmick, G.D.; Das, S.; Ghangrekar, M.M.; Mitra, A.; Banerjee, R. Improved wastewater treatment by combined system of microbial fuel cell with activated carbon/TiO2 cathode catalyst and membrane bioreactor. J. Inst. Eng. Ser. A 2019, 100, 675–682. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Yang, F. Destruction of tetracycline hydrochloride antibiotics by FeOOH/TiO2 granular activated carbon as expanded cathode in low-cost MBR/MFC coupled system. J. Membr. Sci. 2017, 525, 202–209. [Google Scholar] [CrossRef]
- Liu, C.; Min, Y.; Zhang, A.; Si, Y.; Chen, J.; Yu, H.Q. Electrochemical treatment of phenol-containing wastewater by facet-tailored TiO2: Efficiency, characteristics and mechanisms. Water Res. 2019, 165, 114980. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, L.; Wang, J.; Liu, Y.Y.; Yang, J.; Xu, Q.Z.; Chen, R.S.; Ni, H.W. Heteroatom-doped carbon nanofilm embedded in highly ordered TiO2 nanotube arrays by thermal nitriding with enhanced electrochemical activity. J. Electroanal. Chem. 2019, 852, 113513. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Zhu, Z.Y.; Shen, B.X.; Liu, L.N. Insights into biochar and hydrochar production and applications: A review. Energy. 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Huang, W. Research progress on the biochar production and its applications in enhancing electron transport and catalysis performance. Res. Environ. Sci. 2021, 34, 1157–1167. [Google Scholar]
- Liang, B.; Li, K.; Liu, Y.; Kang, X. Nitrogen and phosphorus dual-doped carbon derived from chitosan: An excellent cathode catalyst in microbial fuel cell. Chem. Eng. J. 2019, 358, 1002–1011. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).