Mineral Weathering and Element Migration in Granite Weathering Pits (Gnammas): A Case Study in Eastern China
Abstract
:1. Introduction
2. Materials and Methods
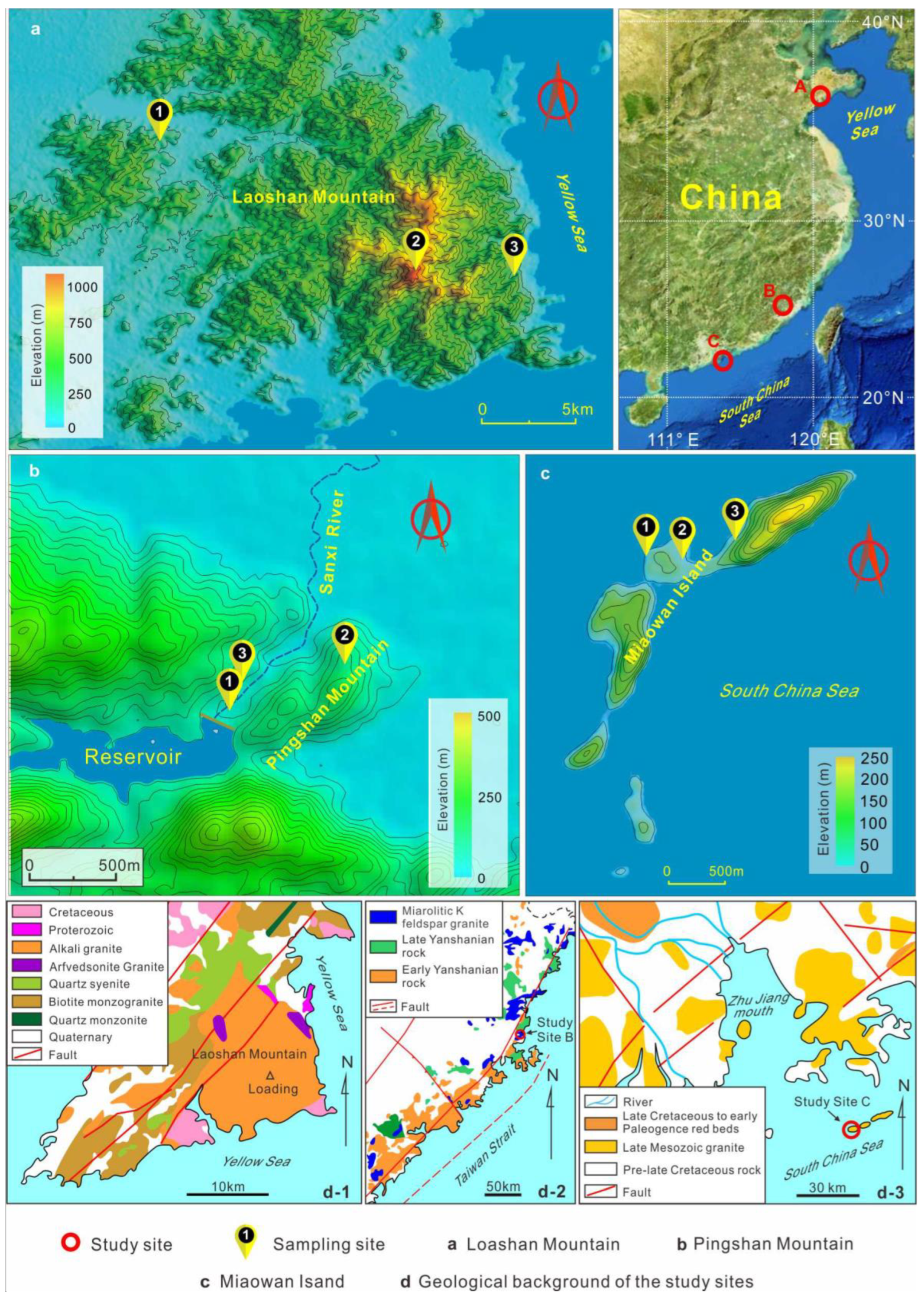
2.1. Study Areas
2.2. Materials and Methods
3. Results
| Sample No. | Al2O3 | CaO | Fe2O3 | K2O | MgO | MnO | Na2O | P2O5 | SiO2 | TiO2 | L.O.I | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study site A (Loashan Mountain) | ||||||||||||
| LD-1-BY | 13.29 | 0.09 | 1.42 | 4.59 | 0.1 | 0.06 | 4.3 | 0.02 | 74.86 | 0.19 | 0.63 | 99.54 |
| LD-1-CJW | 10.83 | 0.98 | 2.08 | 2.81 | 0.62 | 0.05 | 2.46 | 0.05 | 77.24 | 0.52 | 1.93 | 99.58 |
| LD-2-BY | 12.22 | 0.03 | 1.05 | 4.24 | 0.01 | 0.09 | 4.08 | 0.13 | 77.17 | 0.08 | 0.54 | 99.64 |
| LD-2-CJW | 10.78 | 0.22 | 2.12 | 3.54 | 0.36 | 0.1 | 2.8 | 0.05 | 78.29 | 0.29 | 1.02 | 99.55 |
| LD-3-BY | 13.66 | 0.04 | 1.3 | 4.77 | 0.04 | 0.04 | 4.34 | 0.01 | 74.61 | 0.21 | 0.66 | 99.67 |
| LD-3-CJW | 12.29 | 0.3 | 2.68 | 3.64 | 0.51 | 0.07 | 2.75 | 0.06 | 75.02 | 0.37 | 1.84 | 99.55 |
| LYG-BY | 14.12 | 0.46 | 1.36 | 4.98 | 0.18 | 0.07 | 4.07 | 0.05 | 73.49 | 0.22 | 0.53 | 99.54 |
| LYG-CJW | 12.24 | 1.63 | 3.34 | 3.87 | 1.03 | 0.07 | 2.65 | 0.14 | 70.85 | 0.4 | 3.44 | 99.66 |
| YNP-BY | 13.77 | 0.29 | 0.8 | 5.24 | 0.1 | 0.04 | 3.78 | 0.03 | 74.79 | 0.11 | 0.58 | 99.54 |
| YNPG-CJW | 13.03 | 0.38 | 1.26 | 4.89 | 0.21 | 0.03 | 3.29 | 0.07 | 75.29 | 0.17 | 0.93 | 99.55 |
| HL-1-1-BY | 13.97 | 0.35 | 1.27 | 5.09 | 0.13 | 0.05 | 3.98 | 0.05 | 73.94 | 0.2 | 0.53 | 99.54 |
| HL-1-1-CJW | 13.67 | 0.56 | 1.93 | 4.27 | 0.5 | 0.04 | 3.28 | 0.1 | 73.34 | 0.38 | 1.51 | 99.57 |
| BYSK-2 | 23.20 | 0.11 | 9.89 | 2.01 | 1.22 | 0.04 | 0.25 | 0.20 | 46.19 | 1.41 | 15.49 | 100 |
| Study site B (Pingshan Mountain/the Sanxi River) | ||||||||||||
| TLJ-15-BY | 12.4 | 0.16 | 0.68 | 4.58 | 0.07 | 0.02 | 4.64 | 0.02 | 76.33 | 0.11 | 0.42 | 99.44 |
| TLJ-15-CJW | 6.67 | 0.07 | 1.17 | 2.69 | 0.05 | 0.06 | 0.74 | 0.02 | 86.66 | 0.28 | 1.03 | 99.45 |
| TLJ-16-BY | 12.17 | 0.15 | 0.82 | 4.89 | 0.07 | 0.04 | 3.06 | 0.02 | 77.68 | 0.11 | 0.42 | 99.44 |
| TLJ-16-2CJW | 5.09 | 0.08 | 1.08 | 1.55 | 0.06 | 0.03 | 0.67 | 0.03 | 89.89 | 0.15 | 0.85 | 99.48 |
| TLJ-16-5-CJW | 6.51 | 0.23 | 1.03 | 1.72 | 0.11 | 0.04 | 0.34 | 0.05 | 87.49 | 0.26 | 1.8 | 99.58 |
| SXHG-BY | 12.52 | 0.18 | 0.9 | 4.54 | 0.09 | 0.05 | 3.71 | 0.01 | 77.08 | 0.15 | 0.29 | 99.54 |
| SXHG-CJW | 9.33 | 0.15 | 1.34 | 2.42 | 0.15 | 0.05 | 0.78 | 0.03 | 82.67 | 0.23 | 2.47 | 99.6 |
| SXHG-R | 18.21 | 0.03 | 4.79 | 0.21 | 0.12 | 0.12 | 0.04 | 0.01 | 68.91 | 0.2 | 7.51 | 100.15 |
| Study site C (Miaowan Island) | ||||||||||||
| MW-WP-BY | 12.66 | 0.44 | 0.68 | 4.8 | 0.1 | 0.03 | 3.16 | 0.02 | 77.34 | 0.06 | 0.43 | 99.72 |
| MW-WP-CJW | 12.86 | 0.36 | 0.64 | 4.84 | 0.09 | 0.02 | 3.36 | 0.02 | 76.92 | 0.07 | 0.55 | 99.72 |
| MW-2-BY | 12.84 | 0.29 | 0.91 | 5.1 | 0.06 | 0.06 | 3.16 | 0.01 | 76.87 | 0.05 | 0.35 | 99.72 |
| MW-2-CJW | 10.51 | 0.23 | 1.43 | 3.83 | 0.15 | 0.04 | 2.14 | 0.03 | 79.12 | 0.12 | 2.2 | 99.78 |
| MW-R | 16.07 | 0.1 | 3.1 | 1.56 | 0.27 | 0.02 | 0.16 | 0.01 | 71.02 | 0.2 | 7.24 | 99.75 |
| Study Site | Sample No. | Quartz | Feldspar | Illite | Montmorillonite | Others |
|---|---|---|---|---|---|---|
| Study site A (Loashan Mountain) | ||||||
| A | LD-1-BY | 20.2 | 65.4 | 14.4 | ||
| LD-1-CJW | 26.2 | 48.5 | 4.3 | 1.1 | 3.9 (Amphibole) | |
| LD-2-BY | 29.4 | 61.8 | 8.7 | |||
| LD-2-CJW | 27.8 | 50 | 22.2 | |||
| LD-3-BY | 24.5 | 67.5 | 8 | |||
| LD-3-CJW | 34.6 | 58.3 | 7.2 | |||
| LD-4-BY | 18.1 | 61 | 20.8 | |||
| LD-4-CJW | 36.7 | 63.3 | ||||
| LYG-BY | 18.3 | 77.9 | 3.8 | |||
| LYG- CJW | 27.9 | 67.1 | 5 | |||
| YNP-BY | 17.9 | 65 | 17.1 | |||
| YNP-CJW | 20.5 | 67.3 | 12.2 | |||
| HL-1-1-BY | 18.6 | 66.8 | 14.6 | |||
| HL-1-1-CJW | 29.6 | 55.2 | 15.2 | |||
| Study site B (Pingshan Mountain/the Sanxi River) | ||||||
| B | SXHG-CJW | 78.4 | 21.6 | |||
| SXHG-BY | 16.3 | 74.7 | 3.5 | 5.5 | ||
| TLJ-16-BY | 32 | 64.4 | 3.6 | |||
| TLJ-16-2-CJW | 76 | 14 | 9.9 | |||
| TLJ-16-5-CJW | 76 | 14 | 9.9 | |||
| TLJ-15-BY | 41.1 | 55.2 | 3.7 | |||
| TLJ-15-CJW | 79.6 | 20.4 | ||||
| Study site C (Miaowan Island) | ||||||
| C | MW-2-BY | 16.5 | 63.4 | 9.2 | 10.9 | |
| MW-2-CJW | 30 | 48.8 | 7.8 | 13.4 | ||
| MW-WP1-BY | 22.8 | 54.7 | 14.5 | 8.1 | ||
| MW-WP1-CJW | 33.8 | 59 | 7.2 (Mica) | |||
| Study Site | Sample No. | CIA | Q/F | Na2O/K2O |
|---|---|---|---|---|
| Study site A (Laoshan Mountain) | ||||
| A | LD-1-BY | 52.13 | 0.31 | 0.94 |
| LD-1-CJW | 54.98 | 0.54 | 0.88 | |
| LD-2-BY | 51.84 | 0.48 | 0.96 | |
| LD-2-CJW | 54.99 | 0.56 | 0.79 | |
| LD-3-BY | 52.48 | 0.36 | 0.91 | |
| LD-3-CJW | 57.67 | 0.59 | 0.76 | |
| LYG-BY | 52.21 | 0.24 | 0.82 | |
| LYG- CJW | 51.55 | 0.42 | 0.68 | |
| YNP-BY | 52.56 | 0.27 | 0.72 | |
| YNP-CJW | 53.34 | 0.31 | 0.67 | |
| HL-1-1-BY | 52.42 | 0.28 | 0.78 | |
| HL-1-1-CJW | 55.35 | 0.54 | 0.77 | |
| BYSK-2 | 84.73 | - | 0.06 | |
| Study site B (Pingshan Mountain/the Sanxi River) | ||||
| B | TLJ-15-BY | 49.16 | 0.75 | 1.01 |
| TLJ-15-CJW | 61.29 | 3.9 | 0.28 | |
| TLJ-16-BY | 53.58 | 0.5 | 0.63 | |
| TLJ-16-2-CJW | 64.08 | 3.12 | 0.43 | |
| TLJ-16-5-CJW | 70.37 | 5.43 | 0.20 | |
| SXHG-144F-CJW | 72.04 | 3.63 | 0.25 | |
| SXHG-143F-CJW | 69.53 | 1.41 | 0.32 | |
| SXHG-BY | 52.52 | 0.22 | 0.82 | |
| SXHG-R | 98.26 | - | 0.19 | |
| C | Study site C (Miaowan Island) | |||
| MW-2-BY | 53.35 | 0.26 | 0.19 | |
| MW-2-CJW | 56.84 | 0.61 | 0.62 | |
| MW-WP-BY | 52.35 | 0.42 | 0.66 | |
| MW-WP-CJW | 53.07 | 0.57 | 0.69 | |
| MW-R | 88.28 | - | 0.10 | |
4. Discussion
4.1. Difference in CIA Values inside and outside Weathering Pits
4.2. Differences in Mineral Composition inside and outside Weathering Pits
4.3. Major Element Migrations during the Formation of Weathering Pits
5. Conclusions
- (1)
- CIA values for debris within weathering pits are higher than those for adjacent rock surfaces, indicating that the weathering pits resulted from chemical weathering. However, the CIA values of weathering pits in different climatic zones cannot be used as an indicator to identify local climate types. CIA values of different weathering pits differ only because of differences in chemical weathering times.
- (2)
- When assessing the chemical characteristics of weathering pits, the rock surfaces adjacent to the pits cannot be regarded as the parent rocks of the pits. This is because the rock surface next to the pits is also weathered and generally has higher CIA values than local parent rock.
- (3)
- The Q/F ratios of debris in weathering pits and surrounding rock surfaces may also indicate the chemical origin of the weathering pits. It is because the ratio is generally lower at the surface of the rock and higher in the debris in the pits. CIA is more effective than Na2O/K2O in indicating the degree of chemical weathering in weathering pits.
- (4)
- The magnitude of mass transfer intensities of the rock samples relative to their parent rocks coincides with that of CIA values of the samples, also indicating the chemical origin of the weathering pits.
- (5)
- Based on the mass transfer between debris in weathering pits and nearby rock surfaces, only elements Na and K always have τj < 0. This indicates that Na and K are continuously leached during the formation of weathering pits, regardless of whether they are situated in valleys, mountains or coastal regions.
- (6)
- Clay mineral assemblages are more complicated and less effective than the major minerals in assessing the degree of chemical weathering in weathering pits.
- (7)
- Chemical weathering conditions in coastal areas differ from those in inland areas, and migration patterns of elements in weathering pits are also different.
- (8)
- As a result of wet conditions in river valleys, chemical weathering processes are more likely to occur in the valley than on the top of mountains.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turkington, A.V.; Paradise, T.R. Sandstone weathering: A century of research and innovation. Geomorphology 2005, 67, 229–253. [Google Scholar] [CrossRef]
- Norwick, A.S. Lessons from a Mixed Deterministic Stochastic Model of Periglacial Gnamma Development. Available online: https://scholarworks.calstate.edu/concern/publications/f4752h512 (accessed on 28 November 2022).
- Moses, C.; Robinson, D.; Barlow, J. Methods for measuring rock surface weathering and erosion: A critical review. Earth-Sci. Rev. 2014, 135, 141–161. [Google Scholar] [CrossRef]
- Paradise, T.R. Tafoni and other rock basins. In Treatise on Geomorphology; Shroder, J.F., Ed.; Academic: San Diego, CA, USA, 2013; pp. 111–126. [Google Scholar] [CrossRef]
- Twidale, C.R.; Vidal Romaní, J.R. Landforms and Geology of Granite Terrains; Taylor & Francis: London, UK, 2005. [Google Scholar]
- Domínguez-Villar, D. Early formation of gnammas (weathering pits) in a recently glaciated area of Torres del Paine, southern Patagonia (Chile). Geomorphology 2006, 76, 137–147. [Google Scholar] [CrossRef]
- Hall, A.M.; Phillips, W.M. Weathering pits as indicators of the relative age of granite surfaces in the Cairngorm Mountains, Scotland. Geogr. Ann. Ser. A Phys. Geogr. 2006, 88, 135–150. [Google Scholar] [CrossRef]
- Migoń, P. Granite Landscapes of the World; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Domínguez-Villar, D.; Jennings, C.E. Multi-phase evolution of gnammas (weathering pits) in a Holocene deglacial granite landscape, Minnesota (USA). Earth Surf. Process. Landf. 2007, 33, 165–177. [Google Scholar] [CrossRef]
- Domínguez-Villar, D.; Arteaga, C.; Garcia-Giménez, R.; Smith, E.A.; Pedraza, J. Diurnal and seasonal water variations of temperature, pH, redox potential and conductivity in gnammas (weathering pits): Implications for chemical weathering. Catena 2008, 72, 37–48. [Google Scholar] [CrossRef]
- Domínguez-Villar, D.; Razola, L.; Carrasco, R.; Jennings, C.; Pedraza, J. Weathering phases recorded by gnammas developed since last glaciation at Serra da Estrela, Portugal. Quat. Res. 2009, 72, 218–228. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, S.Z.; Li, B.Y.; Xie, P.; Feng, X.Z. Queries about the glacial potholes of Mountain Laoshan, China. Quat. Sci. 2011, 31, 917–932, (In Chinese with English abstract). [Google Scholar]
- Wang, W.; Lin, Z.H.; Liu, Z.P.; Huang, R.H.; Liu, Y.; Lai, Y.X. Evidences for the formation of the weathering pits and the stream potholes at Changle, Fujian Province of China. Acta Geogr. Sin. 2013, 68, 328–342, (In Chinese with English abstract). [Google Scholar]
- Wang, W.; Xu, L.B.; Lin, Z.H.; Liu, Z.P.; Duan, R.P.; Cheng, K.J.; Huang, R.H.; Liu, Y.; Lai, Y.X. Evidences for the origin of coastal weathering pits and marine potholes on the coast of Guangdong—A case study in Shapa Town and Miaowan Island, China. Quat. Sci. 2013, 33, 1016–1033, (In Chinese with English abstract). [Google Scholar]
- Timms, B.V.; Rankin, C. The geomorphology of gnammas (weathering pits) of northwestern Eyre Peninsula, South Australia: Typology, influence of haloclasty and origins. Trans. R. Soc. South Aust. 2015, 140, 28–45. [Google Scholar] [CrossRef]
- Twidale, C.R.; Bourne, J.A. Rock basins (gnammas) revisited. Géomorphologie Relief Process. Environ. 2018, 24, 139–149. [Google Scholar] [CrossRef]
- Wang, W.; Huang, R.H.; Feng, J. A new method for determining weathering rates in weathering pits. Earth Surf. Process. Landf. 2020, 45, 1262–1272. [Google Scholar] [CrossRef]
- Smith, B.J.; Gomez-Heras, M.; Meneely, J.; McCabe, S.; Viles, H.A. High resolution monitoring of surface morphological change of building limestones in response to simulated salt weathering. In 11th International Congress on Deterioration and Conservation of Stone; Lukaszewicz, J.W., Niemcewicz, P., Eds.; Wydawnictwo Naukowe Universytetu Mikolaja Kopernika: Torun, Poland, 2008; Volume 2. [Google Scholar]
- Dahl, R. Block fields, weathering pits and tor-like forms in the Narvik Mountains, Nordland, Norway. Geogr. Ann. 1966, 48A, 55–85. [Google Scholar] [CrossRef]
- Roberts, D. Occurrences of weathering pits from Söröy, northern Norway. Geogr. Ann. 1968, 50A, 60–63. [Google Scholar] [CrossRef]
- Watls, S.H. Weathering processes and products under arid arctic conditions. Geogr. Ann. 1983, 65A, 85–98. [Google Scholar] [CrossRef]
- Fahey, B.D. Weathering pit development in the Central Otago Mountains of Southern New Zealand. Arct. Alp. Res. 1986, 18, 337. [Google Scholar] [CrossRef]
- Netoff, D.I.; Chan, M.A. Aeolian activity at a giant sandstone weathering pit in arid south-central Utah. Earth Surf. Process. Landf. 2009, 34, 99–108. [Google Scholar] [CrossRef]
- Cui, Z.J.; Li, H.J.; Nan, L.; Li, D.W. The discovery and environmental significance of Chifeng wind route and huge pots of Inner Mongolia and Hebei Province. Chin. Sci. Bull. 1999, 44, 1429–1434. (In Chinese) [Google Scholar] [CrossRef]
- Twidale, C.R. Distribution and Morphology of the Bedrock Basins Known as Pans in a Granitic Inselberg Landscape. J. Geol. 2021, 130, 311–324. [Google Scholar] [CrossRef]
- Kusky, T.; Guo, L.; Xiang, S.B.; Guo, X.; Xu, X.Y. A critical examination of evidence for a Quaternary glaciation in Mt. Laoshan, Eastern China. J. Asian Earth Sci. 2011, 40, 403–416. [Google Scholar] [CrossRef]
- Han, T.L. Moulin Discovered; Huaxia Press: Beijing, China, 2004. [Google Scholar]
- Lü, H.B.; Ren, X.H.; Xu, M.; Ouyang, J.Y. An argument on the genesis of potholes formed by differential weathering or wind deflation. Geol. Rev. 2008, 54, 192–198, (In Chinese with English abstract). [Google Scholar]
- Zhao, S.L. The Paleo-Glaciation Remains at Low Altitudes in East China; China Ocean Press: Beijing, China, 2010. [Google Scholar]
- Han, Y.S.; Wang, S.Q.; Mang, G.L. The forming-storing environment and abundance conditions of quaternary underground brine in the embayed coast of Qingdao. Chin. J. Ocean. Limnol. 1997, 15, 332–341. [Google Scholar] [CrossRef]
- Zhao, G.T.; Wang, D.Z.; Cao, Q.C.; Yu, L.S. Thermal evolution, and its significance of A type granitoid complex—The Laoshan granitoid as an example. Sci. China (Ser. D) 1998, 28, 296–302. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Z.Z.; Jin, J.h.; Zhu, S.Y.; Hu, F.G.; Zhang, H. Cliamte change indicated by Quaternary red earht trace elements at Changle in Fujian Province. J. Chongqing Norm. Univ. (Nat. Sci.) 2012, 29, 93–100. (In Chinese) [Google Scholar] [CrossRef]
- Wu, K.L.; Yan, P.Q.; Lu, Z.Q.; Liu, J.Q. The general features of the miarolitie (potash) granites in Fujian Province and the preliminary discussion on their origin. Geol. Fujian 1982, 1, 1–28. (In Chinese) [Google Scholar]
- Huang, R.H.; Wang, W. Microclimatic, chemical, and mineralogical evidence for tafoni weathering processes on the Miaowan Island, South China. J. Asian Earth Sci. 2017, 134, 281–292. [Google Scholar] [CrossRef]
- Zou, G.Q.; Chen, P.Q. Geology of Guangdong Islands; Guangdong Science and Technology Press: Guangzhou, China, 1994. [Google Scholar]
- Li, X.H.; Qi, C.S.; Liu, Y.; Liang, X.R.; Tu, X.L.; Xie, L.W.; Yang, Y.H. Petrogenesis of the Neoproterozoic bimodal volcanic rocks along the western margin of the Yangtze Block: New constraints from Hf isotopes and Fe/Mn ratios. Chin. Sci. Bull. 2005, 50, 2481–2486. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Young, G.M. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Eynatten, H.V.; Barceló-Vidal, C.; Pawlowsky-Glahn, V. Modelling compositional change: The example of chemical weathering of granitoid rocks. Math. Geol. 2003, 35, 231–251. [Google Scholar] [CrossRef]
- McLennan, S.M. Weathering and global denudation. J. Geol. 1993, 101, 295–303. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Liu, L.W. Chemical composition and characterization of chemical weathering of late Tertiary red clay in Xifeng, Gansu Province. J. Geomech. 2001, 7, 167–175. (In Chinese) [Google Scholar]
- Chen, J.; Ji, J.F.; Qiu, G.; Lu, H.Y. Geochemical studies on the intensity of chemical weathering in Luochuan loess-paleosol sequence, China. Sci. China (Ser. D) 1998, 41, 235–241. [Google Scholar] [CrossRef]
- Brimhall, G.H.; Dietrich, W.E. Constitutive mass balance relations between chemical composition, volume, density, porosity, and strain in metasomatic hydrochemical systems: Results on weathering and pedogenesis. Geochim. Cosmochim. Acta 1987, 51, 567–587. [Google Scholar] [CrossRef]
- Han, Z.Z.; Sheng, X.T.; Zhao, G.T. A study on mineral chemistry of alkali granite at Laoshan Mountain area. Trans. Oceanol. Limnol. 1990, 3, 30–35. (In Chinese) [Google Scholar]
- Cícera Neysi de Almeida; Ignez de Pinho Guimarães; Adejardo Francisco da Silva Filho. A-Type Post-Collisional Granites in the Borborema Province—NE Brazil: The Queimadas Pluton. Gondwana Res. 2002, 5, 667–681. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Young, G.M. Prediction of some weathering trends of plutonic and volcanic rocks based on thermodynamic and kinetic considerations. Geochim. Cosmochim. Acta 1984, 48, 1523–1534. [Google Scholar] [CrossRef]
- White, A.F.; Blum, A.E.; Stonestrom, D.A.; Bullen, T.D.; Schulz, M.S.; Huntington, T.G.; Peters, N.E. Differential rates of feldspar weathering in granitic regolths. Geochim. Cosmochim. Acta 2001, 65, 847–869. [Google Scholar] [CrossRef]
- Jenny, H. Behavior of Potassium and Sodium during the Process of Soil Formation; University of Missouri: Columbia, MO, USA, 1931. [Google Scholar]
- Ruxton, B.P. Measures of the Degree of Chemical Weathering of Rocks. J. Geol. 1968, 76, 518–527. [Google Scholar] [CrossRef]
- Oliva, P.; Viers, J.; Dupre, B. Chemical weathering in granitic environments. Chem. Geol. 2003, 202, 225–256. [Google Scholar] [CrossRef]
- Goldich, S.S. A study in rock-weathering. J. Geol. 1938, 46, 17–58. [Google Scholar] [CrossRef]
- DiPietro, J.A. Landscape Evolution in the United States. In An Introduction to the Geography, Geology, and Natural History; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Jiménez-Espinosa, R.; Vázquez, M.; Jiménez-Millán, J. Differential weathering of granitic stocks and landscape effects in a Mediterranean climate, Southern Iberian Massif (Spain). Catena 2007, 70, 243–252. [Google Scholar] [CrossRef]
- Lu, F.X.; Chang, L.K.; Wu, J.H.; Lao, Q.A. Petrology; Geological Press: Beijing, China, 2001. [Google Scholar]
- Rich, C.L. Mineralogy of Soil Potassium. In The Role of Potassium in Agriculture, Kilmer, E.J., Younts, S.E., Brady, B.C., Eds.; The American Society of Agronomy: Madison, USA, 1968; pp. 79–108. [Google Scholar] [CrossRef]
- Ren, X.P.; Nie, J.S.; Saylor, J.E.; Li, H.; Bush, M.A.; Horton, B.K. Provenance control on chemical weathering index of fluvio-lacustrine sediments: Evidence from the Qaidam Basin, NE Tibetan Plateau. Geochem. Geophys. Geosyst. 2019, 20, 3216–3224. [Google Scholar] [CrossRef]
- Li, W.D.; Wang, W.B.; Cheng, Z.F. Geochemistry of Lateritization Process and the Possibility of Forming Lateritic Type Gold Deposits in Southern China; Geological Publishing House: Beijing, China, 1995. [Google Scholar]
- White, A.F.; Schulz, M.S.; Vivit, D.V.; Blum, A.E.; Stonestrom, D.A.; Anderson, S.P. Chemical weathering of a marine terrace chronosequence, Santa Cruz, California I: Interpreting rates and controls based on soil concentration-depth profiles. Geochim. Cosmochim. Acta 2008, 72, 36–68. [Google Scholar] [CrossRef]
- Brantley, S.L.; Chesley, J.T.; Stillings, L. Isotopic ratios and release of strontium measured from weathering feldspars. Geochim. Cosmochim. Acta 1998, 62, 1493–1500. [Google Scholar] [CrossRef]
- Liu, S.M.; Huang, W.W.; Zhang, J.; Wang, J.H.; Jing, X.W.; Wang, J.Y. Study on chemical composition of atmospheric deposition at Qingdao. Mar. Environ. Sci. 1993, 12, 89–98. (In Chinese) [Google Scholar]
- Peng, Y.H.; Chen, H.R.; Li, S.F. PH and alkalinity in the water body of Pearl River Mouth. J. Trop. Oceanogr. 1991, 10, 49–55. (In Chinese) [Google Scholar]
- Mustoe, G.E. The origin of honeycomb weathering. Geol. Soc. Am. Bull. 1982, 93, 108–115. [Google Scholar] [CrossRef]
- Mottershead, D.N.; Pye, K. Tafoni on coastal slopes, South Devon, UK. Earth Surf. Proc. Land. 1994, 19, 543–563. [Google Scholar] [CrossRef]
- Maynard, J.B. Treatise of Geochemistry, v. 9, Sediments, Diagenesis, and Sedimentary Rocks; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Feng, J.; Qui, M. Mineral Weathering and Element Migration in Granite Weathering Pits (Gnammas): A Case Study in Eastern China. Minerals 2023, 13, 70. https://doi.org/10.3390/min13010070
Wang W, Feng J, Qui M. Mineral Weathering and Element Migration in Granite Weathering Pits (Gnammas): A Case Study in Eastern China. Minerals. 2023; 13(1):70. https://doi.org/10.3390/min13010070
Chicago/Turabian StyleWang, Wei, Jing Feng, and Mingkun Qui. 2023. "Mineral Weathering and Element Migration in Granite Weathering Pits (Gnammas): A Case Study in Eastern China" Minerals 13, no. 1: 70. https://doi.org/10.3390/min13010070
APA StyleWang, W., Feng, J., & Qui, M. (2023). Mineral Weathering and Element Migration in Granite Weathering Pits (Gnammas): A Case Study in Eastern China. Minerals, 13(1), 70. https://doi.org/10.3390/min13010070






