Interaction Forces between Diaspore and Kaolinite in NaOL Solution Probed by EDLVO Theory and AFM Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Minerals and Reagents
2.2. Zeta Potential Testing
2.3. Contact Angle Measurement
2.4. EDLVO Theory
2.4.1. Van der Waals Interaction Energy
2.4.2. Electrostatic Interaction Energy
2.4.3. Hydrophobic Interaction Energy
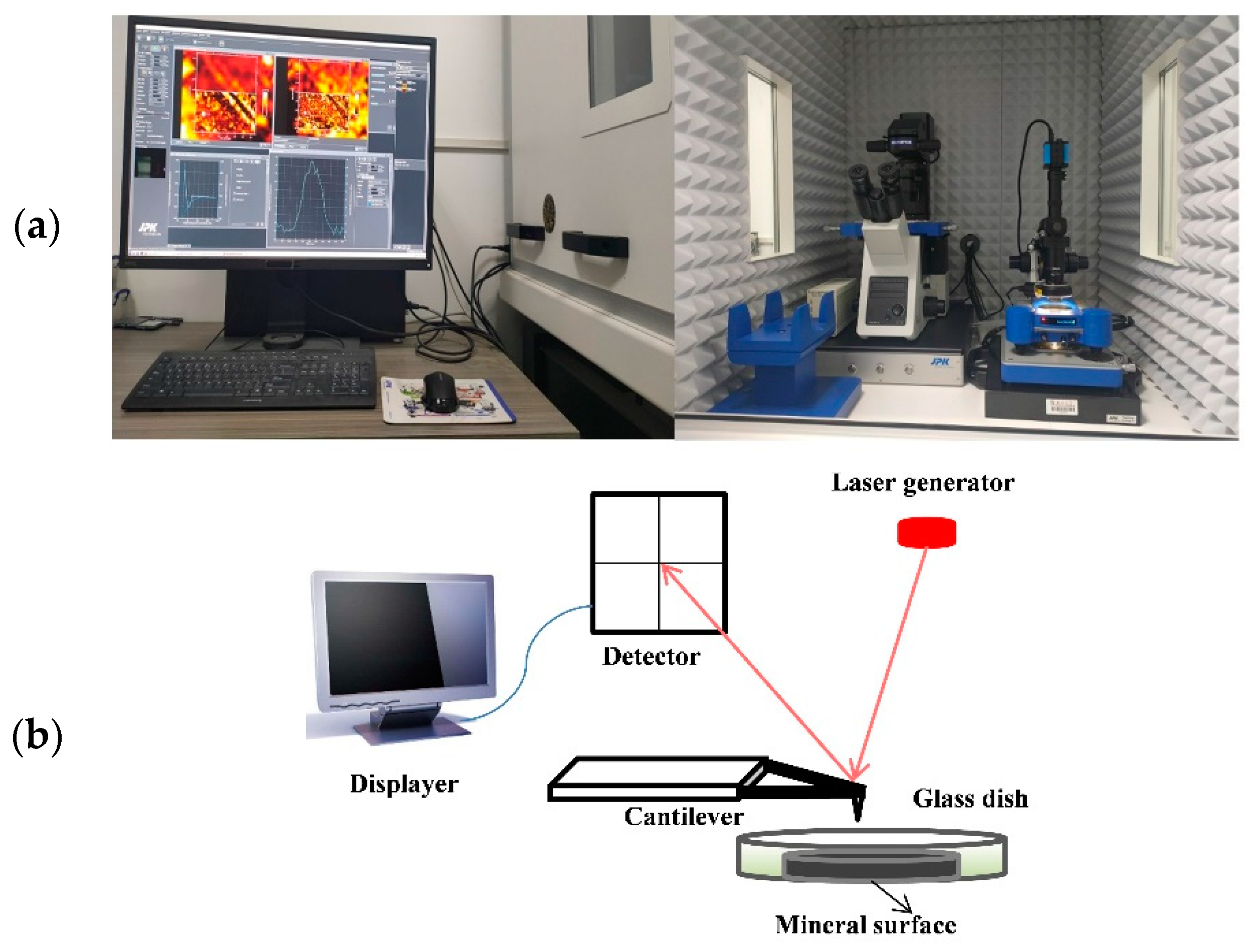
2.5. AFM Measurements
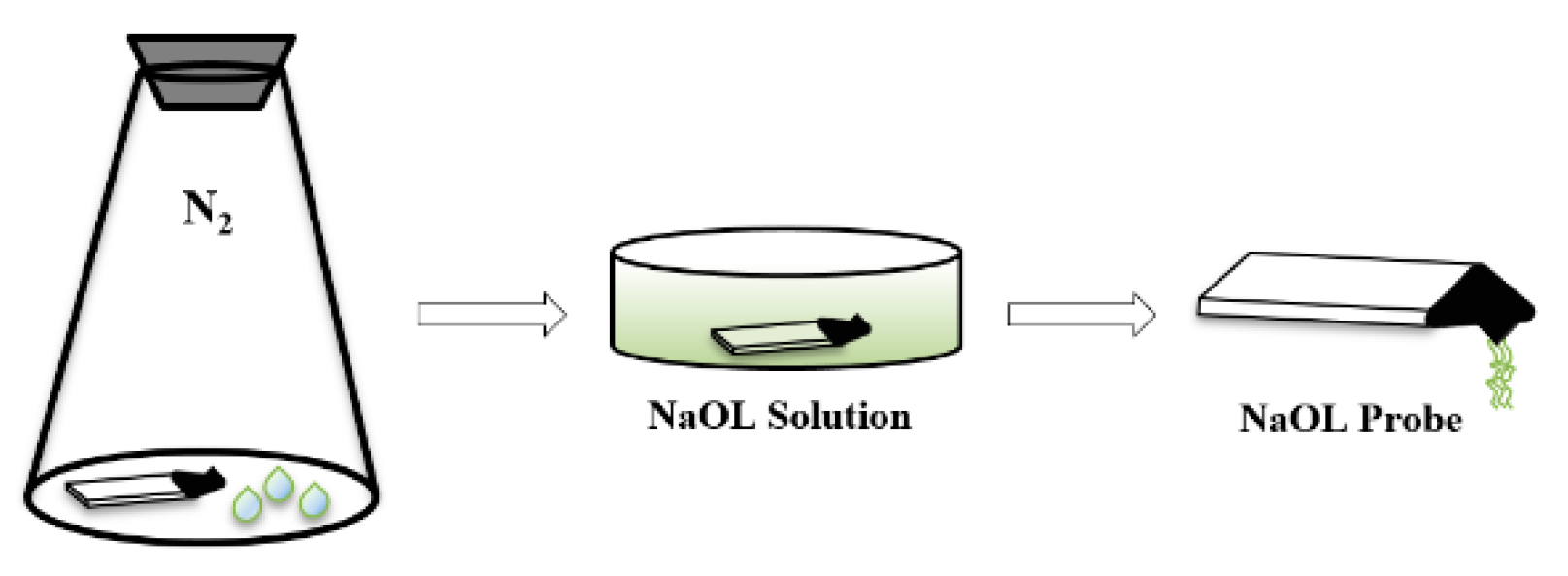
2.5.1. Preparation of Colloid Particle Probe
2.5.2. Preparation of Collector Probe
2.5.3. Interaction Force Measurement
3. Results and Discussion
3.1. Surface Properties of Diaspore and Kaolinite
3.1.1. Zeta Potential Analysis
3.1.2. Wettability Analysis
3.2. Interaction Energy between Mineral Particles
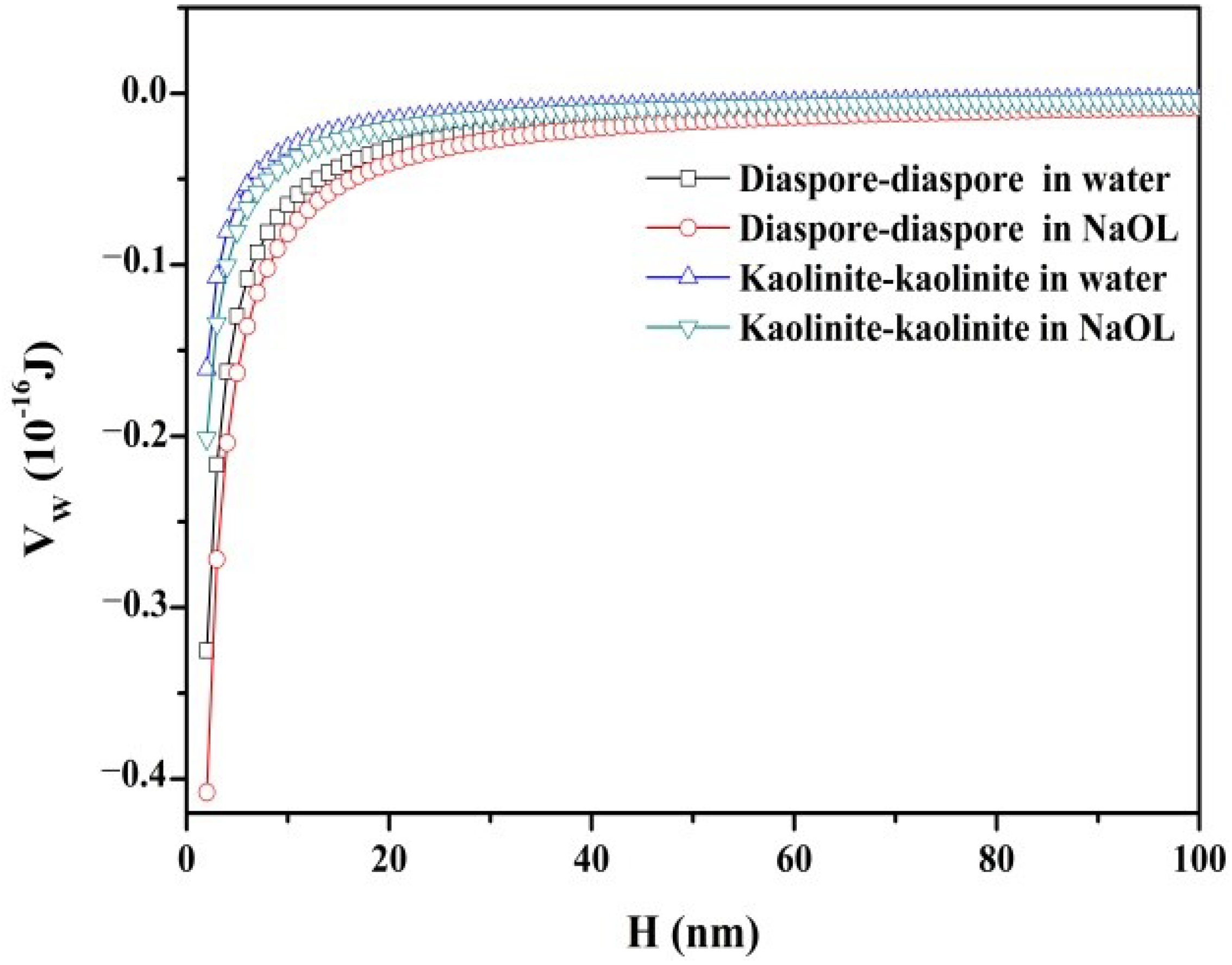
3.2.1. Van der Waals Interaction Energy
3.2.2. Electrostatic Interaction Energy
3.2.3. Hydrophobic Interaction Energy
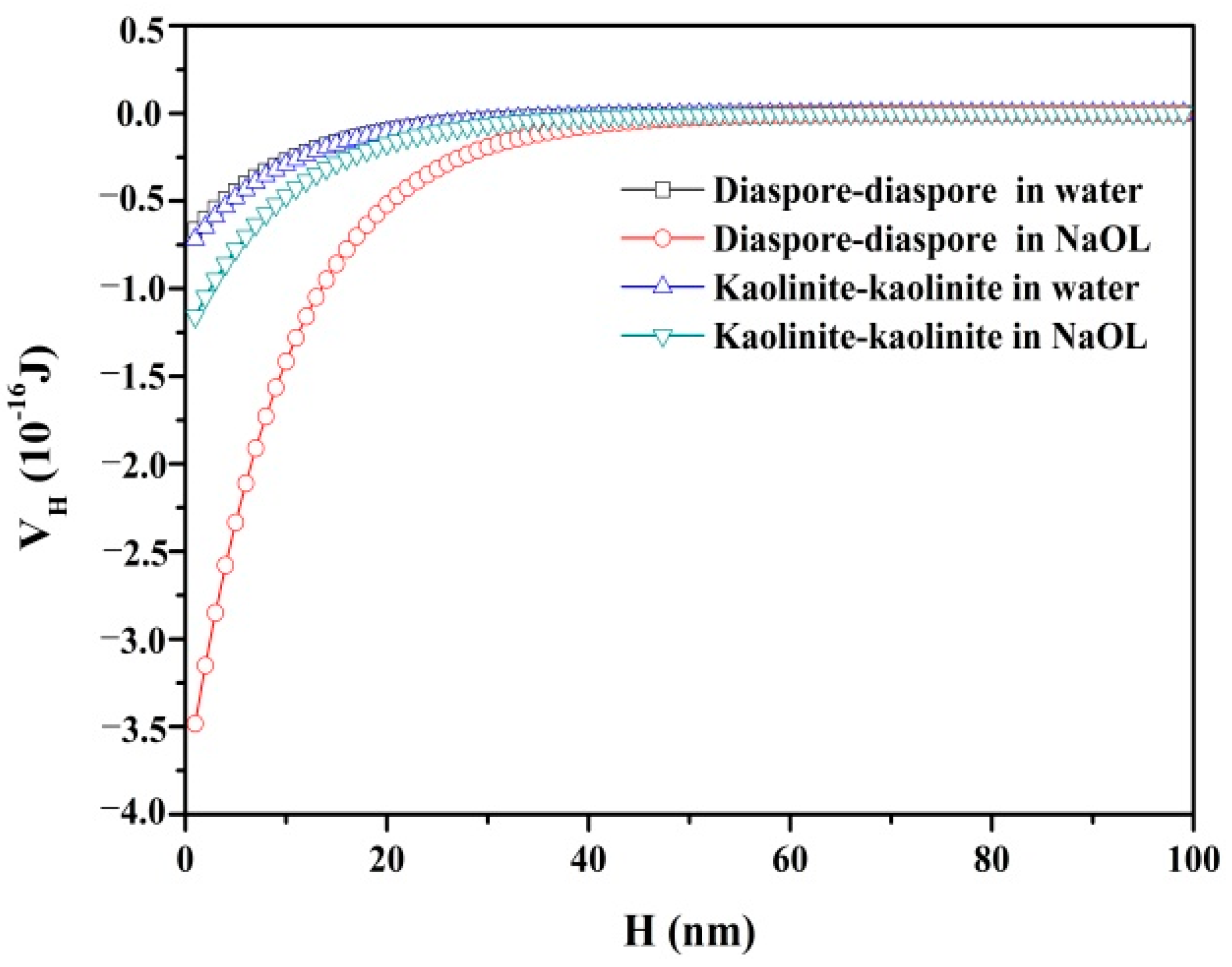
3.2.4. Total Interaction Energy
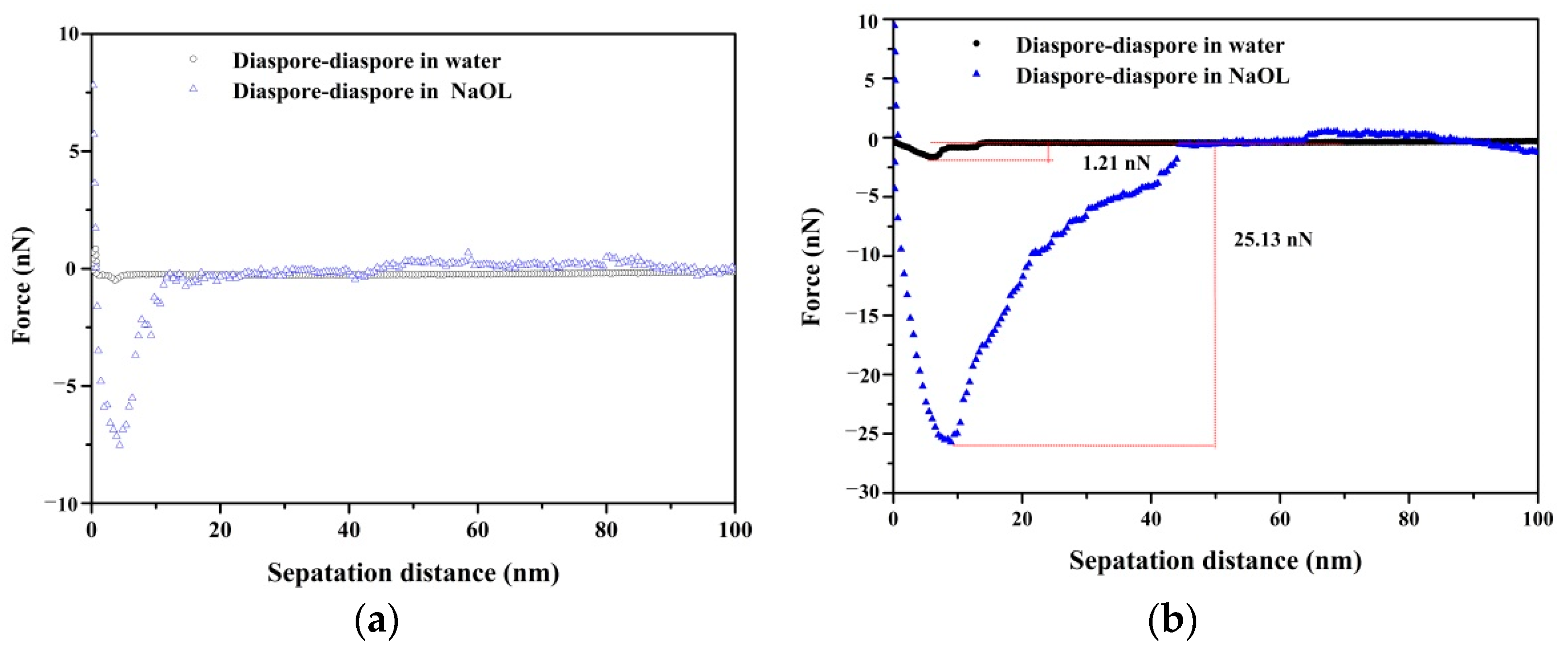
3.3. Adhesion Force between Mineral Particles
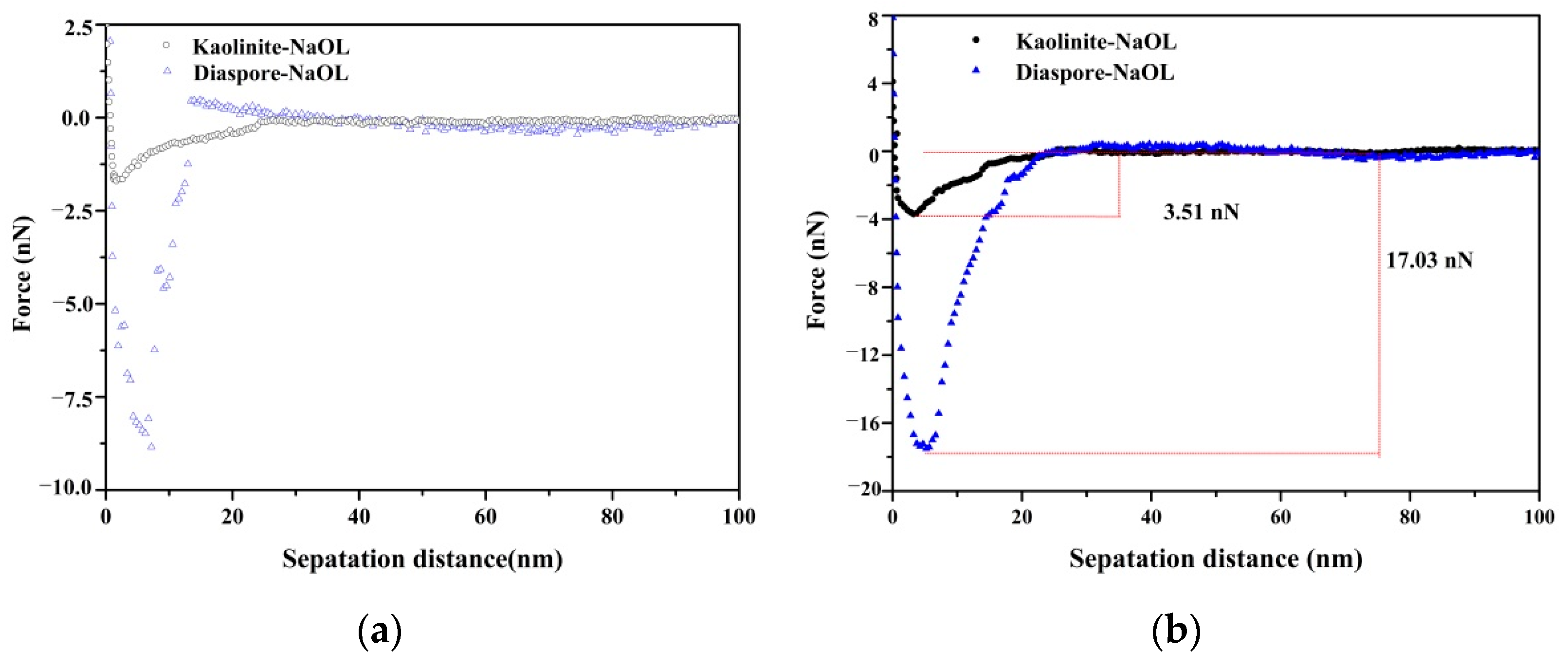
3.4. Adhesion Force between Collector and Mineral Particles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pugh, R.J. The role of the solution chemistry of dodecylamine and oleic acid collectors in the flotation of fluorite. Colloids Surf. 1986, 18, 19–41. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, Z.; Meng, Q.; Zhao, X.; Du, Y. Study on the flotation behavior and interaction mechanism of ilmenite with mixed BHA/NaOL collector. Miner. Eng. 2021, 170, 107034. [Google Scholar] [CrossRef]
- Jong, K.; Han, Y.; Ryom, S. Flotation mechanism of oleic acid amide on apatite. Colloids Surf. A. 2017, 523, 127–131. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. Selective flotation of rare earth oxide from hematite and quartz mixtures using oleic acid as a collector. Int. J. Miner. Process. 2017, 169, 60–69. [Google Scholar] [CrossRef]
- Li, H.; Chai, W.; Cao, Y.; Yang, S. Flotation enhancement of low-grade bauxite using oxalic acid as surface pretreatment agent. Appl. Surf. Sci. 2022, 577, 151964. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, Y.; Cao, X. Synthesis and structure-activity relationships of carboxyl hydroxidoxime in bauxite flotation. Chin. J. Nonferrous Met. 2001, 11, 702–706. [Google Scholar]
- Deng, L.; Wang, S.; Zhong, H.; Liu, G. A novel surfactant 2-amino-6-Decanamidohexanoic acid: Flotation performance and adsorption mechanism to diaspore. Miner. Eng. 2016, 93, 16–23. [Google Scholar] [CrossRef]
- Deng, L.; Wang, S.; Zhong, H.; Liu, G. N-(6-(hydroxyamino)-6-oxohexyl) decanamide collector: Flotation performance and adsorption mechanism to diaspore. Appl. Surf. Sci. 2015, 347, 79–87. [Google Scholar] [CrossRef]
- Liu, S.; Hu, Y.; Qin, W. Study on the accelerant to the sodium oleate during bauxite flotation. Eng. Sci. 2012, 10, 45–48. [Google Scholar]
- Li, H.; Chai, W.; Cao, Y.; Wu, Y.; Yang, S. Synergistic collection mechanism of D-phenylalanineand sodium oleate in flotation of diaspore from kaolinite. Appl. Surf. Sci. 2021, 538, 147937. [Google Scholar] [CrossRef]
- Gao, Y.; Fu, X.; Pan, Z.; Yue, T.; Sui, W. Surface complexation model theory application in NaOL and CTAB collector adsorption differences of diaspore and kaolinite flotation. Sep. Purif. Technol. 2022, 295, 121288. [Google Scholar] [CrossRef]
- Zhang, N.; Nguyen, A.V.; Zhou, C. Impact of interfacial Al- and Si-active sites on theelectrokinetic properties, surfactant adsorption and floatability of diaspore and kaolinite minerals. Miner. Eng. 2018, 122, 258–266. [Google Scholar] [CrossRef]
- Zhang, N.; Nguyen, A.V.; Zhou, C. A review of the surface features and properties, surfactant adsorption and floatability of four key minerals of diasporic bauxite resources. Adv. Colloid Interface Sci. 2018, 254, 56–75. [Google Scholar] [CrossRef]
- Afekare, D.; Garno, J.; Rao, D. Enhancing oil recovery using silica nanoparticles: Nanoscale wettability alteration effects and implications for shale oil recovery. J. Pet. Sci. Eng. 2021, 203, 108897. [Google Scholar] [CrossRef]
- Afekare, D.; Garno, J.; Rao, D. Insights into Nanoscale Wettability Effects of Low Salinity and Nanofluid Enhanced Oil Recovery Techniques. Energies 2020, 13, 4443. [Google Scholar] [CrossRef]
- Tetteh, J.T.; Brady, P.V.; Ghahfarokhi, R.B. Review of low salinity water flooding in carbonate rocks:mechanisms, investigation techniques, and future directions. Adv. Colloid Interface Sci. 2020, 284, 102253. [Google Scholar] [CrossRef] [PubMed]
- Derjaguin, B.; Landau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog. Surf. Sci. 1941, 43, 30–59. [Google Scholar] [CrossRef]
- Verwey, E.J. Theory of the stability of lyophobic colloids. J. Colloid Sci. 1947, 51, 631. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, L.; Xu, H.; Sun, X.; Zhang, Z.; Ye, G. DLVO theoretical analyses between montmorillonite and fine coal under different pH and divalent cations. Powder Technol. 2018, 330, 147–151. [Google Scholar] [CrossRef]
- Hartmann, R.; Kinnunen, P.; Illikainen, M. Cellulose-mineral interactions based on the DLVO theory and their correlation with flotability. Miner. Eng. 2018, 122, 44–52. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, Y.; Wang, Y. Effect of metallic ions on dispersibility of fine diaspore. Trans. Nonferrous Met. Soc. China. 2011, 21, 1166–1171. [Google Scholar] [CrossRef]
- Ducker, W.A.; Mastropietro, D. Forces between extended hydrophobic solids: Is there a long-range hydrophobic force? Curr. Opin. Colloid Interface Sci. 2016, 22, 51–58. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Van der Waals Forces between Particles and Surfaces. In Intermolecular and Surface Forces, 3rd ed.; Academic Press: New York, NY, USA, 2011; pp. 253–289. [Google Scholar]
- Yan, X.; Wei, L.; Meng, Q.; Wang, J.; Yang, Q.; Zhai, S.; Lu, J. A study on the mechanism of calcium ion in promoting the sedimentation of illite particles. J. Water Process Eng. 2021, 42, 102153. [Google Scholar] [CrossRef]
- Jong, K.; Paek, I.; Kim, Y.; Li, I.; Jang, D. Flotation mechanism of a novel synthesized collector from Evodiaefructus onto fluorite surfaces. Miner. Eng. 2020, 146, 106017. [Google Scholar] [CrossRef]
- Jiao, F.; Cui, Y.; Wang, D.; Hu, C. Research of the replacement of dichromate with depressants mixture in the separation of copper-lead sulfides by flotation. Sep. Purif. Technol. 2022, 278, 119330. [Google Scholar] [CrossRef]
- Li, Z.; Yoon, R.H. AFM force measurements between gold and silver surfaces treated in ethyl xanthate solutions: Effect of applied potentials. Miner. Eng. 2012, 36–38, 126–131. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, M.; Gui, X.; Cao, Y.; Babel, B.; Rudolph, M.; Weber, S.; Kappl, M.; Butt, H.J. The application of atomic force microscopy in mineral flotation. Adv. Colloid Interface Sci. 2018, 256, 373–392. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Zhu, H.; Jia, W. Activation effect of lead ions on scheelite flotation: Adsorption mechanism, AFM imaging and adsorption model. Sep. Purif. Technol. 2019, 209, 955–963. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Wei, Q. New insights into the depressive mechanism of citric acid in the selective flotation of scheelite from fluorite. Miner. Eng. 2021, 171, 107117. [Google Scholar] [CrossRef]
- Lv, X.; Fan, W.; Wang, J.; Liang, M.; Qian, C.; Luo, H.; Nan, G.; Yao, B. Study on adhesion of asphalt using AFM tip modified with mineral particles. Constr. Build. Mate. 2019, 207, 422–430. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Hu, S.; Wang, J.; Guo, F.; Li, S. Effects of solution pH and polyethylene oxide concentrations on molybdenite–molybdenite, molybdenite–kaolinite, and molybdenite–quartz interaction forces: AFM colloidal probe study. Sep. Purif. Technol. 2022, 280, 119926. [Google Scholar] [CrossRef]
- Liu, S.; Xie, L.; Liu, J.; Zeng, H. Probing the interactions of hydroxamic acid and mineral surfaces: Molecular mechanism underlying the selective separation. Chem. Eng. J. 2019, 374, 123–132. [Google Scholar] [CrossRef]
- Fa, K.; Nguyen, A.V.; Miller, J.D. Interaction of calcium dioleate collector colloids with calcite and fluorite surfaces as revealed by AFM force measurements and molecular dynamics simulation. Int. J. Miner. Process. 2006, 81, 166–177. [Google Scholar] [CrossRef]
- Xie, L.; Wang, J.; Lu, Q.; Hu, W.; Zeng, H. Surface interaction mechanisms in mineral flotation: Fundamentals, measurements, and perspectives. Adv. Colloid Interface Sci. 2021, 295, 102491. [Google Scholar] [CrossRef]
- Wu, Y.; Chai, W.; Cao, Y. Collection and selectivity contrast of propyl gallate and sodium oleate for diaspore and kaolinite flotation. In Proceedings of the Light Metals 2021, Orlando, FL, USA, 14–18 March 2021. [Google Scholar]
- Li, D.; Yin, W.; Liu, Q.; Cao, S.; Sun, Q.; Zhao, C.; Yao, J. Interactions between fine and coarse hematite particles in aqueous suspension and their implications for flotation. Miner. Eng. 2017, 114, 74–81. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, S.; Li, J.; Weng, L.; Wu, C.; Liu, K. Improvement on combustible matter recovery in coal slime flotation with the addition of sodium silicate. Colloids Surf. A. 2020, 603, 125220. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.; Bu, X.; Ni, C.; Xie, G.; Peng, Y. Investigation on interaction behavior between coarse and fine particles in the coal flotation using focused beam reflectance measurement (FBRM) and particle video microscope (PVM). Sep. Sci. Technol. 2021, 56, 1418–1430. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Xie, S.; Duan, W.; Chen, W. Roles and influences of kerosene on chalcopyrite flotation in MgCl2 solution: EDLVO and DFT Approaches. Minerals 2021, 12, 48. [Google Scholar] [CrossRef]
- Afekare, D.A.; Radonjic, M. From mineral surfaces and coreflood experiments to reservoir implementations: Comprehensive review of low-salinity water flooding (LSWF). Energ. Fuel. 2017, 31, 13043–13062. [Google Scholar] [CrossRef]
- Tetteh, J.T.; Richard, B.; Korsah, P.K. Ionic interactions at the crude oil-brine-rock interfaces using different surface complexation models and DLVO theory: Application to carbonate wettability. ACS Omega 2022, 7, 7199–7212. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, J.; Faghihnejad, A.; Zeng, H.; Liu, Y. Understanding the molecular interactions of lipopolysaccharides during E. coli initial adhesion with a surface forces apparatus. Soft Matter. 2011, 7, 9366–9379. [Google Scholar] [CrossRef]
- Lu, Q.; Huang, J.; Maan, O.; Liu, Y.; Zeng, H. Probing molecular interaction mechanisms of organic fouling on polyamide membrane using a surface forces apparatus: Implication for wastewater treatment. Sci. Total Environ. 2018, 622–623, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhong, H.; Wang, S.; Xia, L.; Zhao, G.; Liu, G. Gemini trisiloxane surfactant: Synthesis and flotation of aluminosilicate minerals. Miner. Eng. 2014, 56, 145–154. [Google Scholar] [CrossRef]
- Guan, F.; Zhong, H.; Liu, G.; Zhao, S.; Xia, L. Flotation of aluminosilicate minerals using alkylguanidine collectors. Trans. Nonferr. Met. Soc. China 2009, 19, 228–234. [Google Scholar] [CrossRef]
- Liu, G.; Huang, Y.; Qu, X.; Xu, Z.; Yang, X. Understanding the hydrophobic mechanism of 3-hexyl-4-amino-1,2,4-triazole-5-thione to malachite by ToF-SIMS, XPS, FTIR, contact angle, zeta potential and micro-flotation. Colloids Surf. A. 2016, 503, 34–42. [Google Scholar] [CrossRef]
- Xia, Y.; Xing, Y.; Gui, X.; Cao, Y. Probing the interactions between collector molecules and hydrophobic graphite surfaces using chemical force microscopy. Appl. Surf. Sci. 2022, 597, 153760. [Google Scholar] [CrossRef]
- Xia, Y.; Xing, Y.; Li, M.; Liu, M.; Tan, J.; Cao, Y.; Gui, X. Studying interactions between undecane and graphite surfaces by chemical force microscopy and molecular dynamics simulations. Fuel 2020, 269, 117367. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, X.; Gui, X.; Cao, Y.; Xu, M. Effect of kaolinite and montmorillonite on fine coal flotation. Fuel 2017, 195, 284–289. [Google Scholar] [CrossRef]
- He, J.; Sun, W.; Zeng, H.; Fan, R.; Hu, W.; Gao, Z. Unraveling roles of lead ions in selective flotation of scheelite and fluorite from atomic force microscopy and first-principles calculations. Miner. Eng. 2022, 179, 107424. [Google Scholar] [CrossRef]
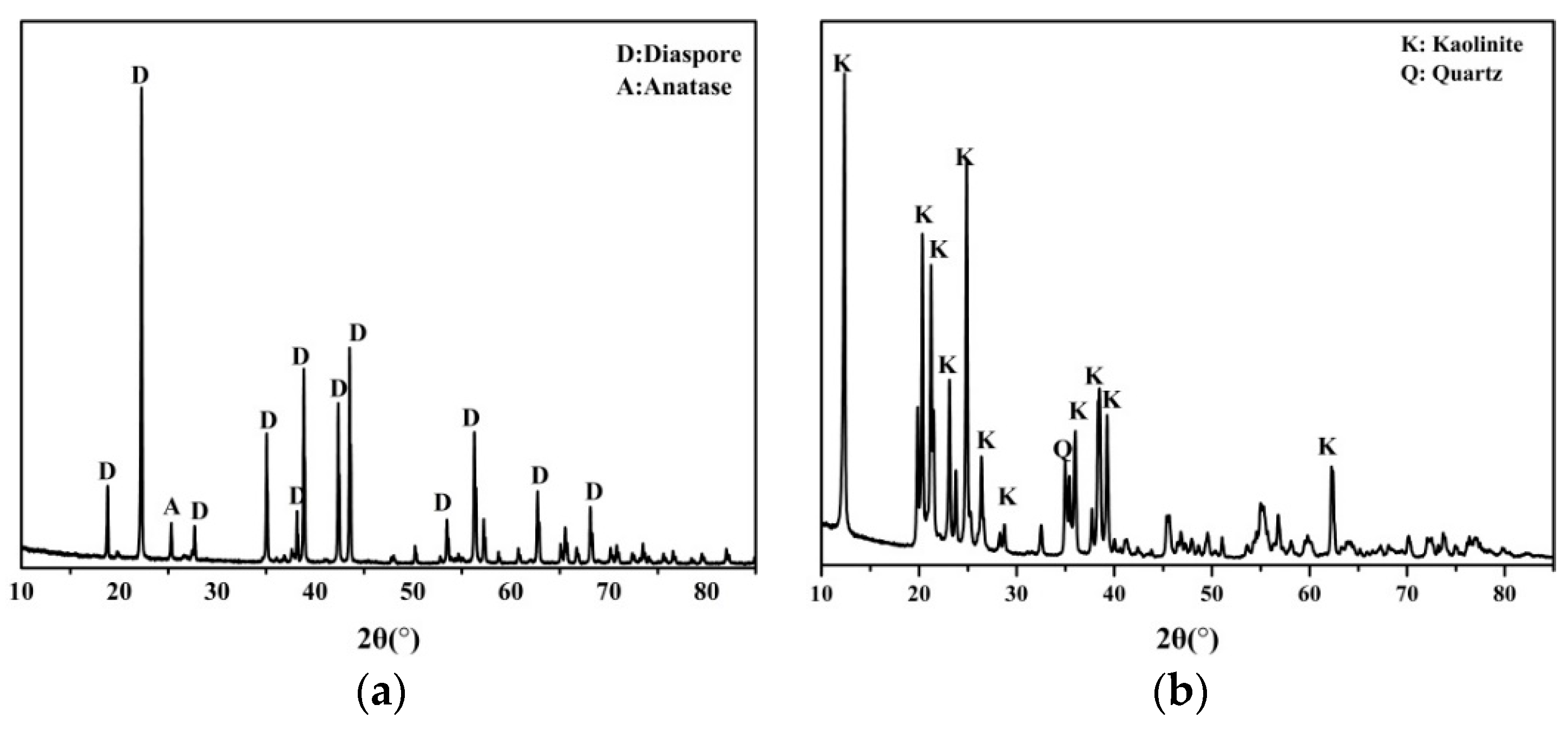






| Minerals | Al2O3 1 | SiO2 1 | TiO2 1 | Fe2O3 | K2O | Na2O | CaO | MgO | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Diaspore | 76.57 | 1.57 | 2.94 | 1.09 | 0.48 | 0.041 | 0.067 | 0.068 | 14.43 |
| Kaolinite | 37.96 | 47.19 | 0.24 | 0.38 | 0.02 | 0.074 | 0.16 | 0.14 | 13.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Yang, S.; Chai, W.; Cao, Y. Interaction Forces between Diaspore and Kaolinite in NaOL Solution Probed by EDLVO Theory and AFM Analysis. Minerals 2022, 12, 1123. https://doi.org/10.3390/min12091123
Wu Y, Yang S, Chai W, Cao Y. Interaction Forces between Diaspore and Kaolinite in NaOL Solution Probed by EDLVO Theory and AFM Analysis. Minerals. 2022; 12(9):1123. https://doi.org/10.3390/min12091123
Chicago/Turabian StyleWu, Yankun, Shichong Yang, Wencui Chai, and Yijun Cao. 2022. "Interaction Forces between Diaspore and Kaolinite in NaOL Solution Probed by EDLVO Theory and AFM Analysis" Minerals 12, no. 9: 1123. https://doi.org/10.3390/min12091123
APA StyleWu, Y., Yang, S., Chai, W., & Cao, Y. (2022). Interaction Forces between Diaspore and Kaolinite in NaOL Solution Probed by EDLVO Theory and AFM Analysis. Minerals, 12(9), 1123. https://doi.org/10.3390/min12091123







