Abstract
This paper is concerned with the kinetics of stibnite (Sb2S3) dissolution in H2SO4-NaCl-Fe(SO4)1.5-O2 solutions. The experimental results of our study showed a positive effect from ferric ions on stibnite dissolution of up to 0.05 M. An increase in H2SO4 concentration from 0.8 to 3 M and NaCl concentration from 0 to 3 M NaCl improved the stibnite dissolution. In solutions without ferric ions, the leaching occurs by the acid dissolution reaction producing H2S without forming elemental sulfur, as we confirmed by the X-ray diffraction (XRD) analysis of leach residues. In this case, the kinetic data was analyzed using a shrinking core model controlled by a chemical reaction, and the calculated activation energy was 54.6 kJ/mol. Conversely, in the presence of ferric ions, the XRD patterns of the leach residues showed large peaks for elemental sulfur, confirming that the stibnite was oxidized and formed a sulfur porous layer. The mechanism in the presence of ferric ions involved an acid dissolution reaction, with the diffusion of the reactant/product species through the porous product layer, followed by the oxidation of H2S by ferric ions. The kinetic data were analyzed using a diffusion-controlled model, and the activation energy was 123.8 kJ/mol. This high activation value is consistent with the proposed mechanism.
1. Introduction
The mineral stibnite (Sb2S3) is the main source of antimony. Stibnite ores sometimes contain small amounts of native antimony and antimony oxides formed by the decomposition of stibnite as well as minerals containing other valuable metals or impurities. Stibnite ores are mainly treated by froth flotation using standard circuits that include rougher, scavenger and, usually, two-stage cleaner in order to produce concentrates with an antimony content over 60%.
More than 90% of the antimony production worldwide is done by pyrometallurgy, using oxidizing roasting to volatilize Sb2O3, followed by a reduction to antimony metal by carbon [1,2]. However, this pyrometallurgical method has several shortcomings, such as the high energy consumption and environmental problems that are associated with the gaseous emissions of SO2 and with arsenic and antimony compounds [3]. Alternative, less contaminant pyrometallurgical methods have also been investigated, including the carbothermic reduction of stibnite using a sulfur scavenger agent such as CaO [4] or ZnO [5,6] to avoid SO2 emissions. On the other hand, hydrometallurgical processes have also been used to recover antimony from stibnite—among them, alkaline leaching with Na2S-NaOH [7,8]—which have been applied in several countries [2]. Conversely, the acid leaching processes were based on hydrochloric acid media [9,10] and were frequently done in the presence of an oxidant such as ferric chloride [11,12], ozone [13,14], chlorine [15,16] and antimony pentachloride [17]. The reductive leaching of stibnite using metallic iron in hydrochloric acid media to produce metallic antimony has also been studied [18].
Chemistry of the Leaching of Stibnite in Hydrochloric Acid Media
The dissolution of stibnite in hydrochloric acid media is favorable, owing to the formation of very stable Sb(III)-Cl− complex ions from the general formula , with i = 1–6 [19,20].
The dissolution reaction in HCl solutions can be written as:
This Reaction (1) has a small equilibrium constant, which means that its use will be favorable only in solutions with high chloride concentrations and a very low pH [13].
In the presence of an oxidant such as oxygen or ferric ion, the following corresponding oxidative dissolution reactions would occur:
Even though oxygen is a stronger oxidant than ferric, it is well-known that the dissolution of most sulfide minerals is more effective when done with ferric ions than with oxygen [21,22]. Therefore, when a stibnite leaching system contains significant amounts of ferric ion, Reaction (3) should be the dominant leaching reaction. The main role of the oxygen in this case would be to re-oxidize the ferrous ions produced by Reaction (3) according to the following:
By adding Reactions (3) and (4), we can obtain Reaction (2) as the overall reaction.
The acid dissolution Reaction (1) could also occur in oxidizing conditions and play a role in the leaching of stibnite. In this case, the product H2S of Reaction (1) can be subsequently oxidized to elemental sulfur by oxygen or ferric ion according to Reactions (5) or (6):
Again, in the oxidation of H2S, ferric ion has been shown to be more effective than oxygen [23].
In Reactions (3), (4) and (6) for simplicity, the iron ions are written as the free cations Fe3+ and Fe2+, even though they are also present in the solutions as complex ions with chloride [20]. Furthermore, when using stronger oxidants than ferric ion and oxygen, such as chlorine or ozone, the production of sulfate instead of elemental sulfur becomes more likely, and the partial oxidation of the Sb(III) to Sb(V) will also occur [14,15]. On this matter, Guo et al. [14], who studied the leaching of an antimony-bearing refractory gold concentrate in HCl media using ozone as the oxidant, did not find elemental sulfur in the leaching residues. The Sb(V) present in high-potential solutions also exists mostly as complex ions with chloride using the general formula , with i = 1–6. Recently, Li et al. [24] reported that Sb(V)-Cl− complex ions have larger formation constants than Sb(III)-Cl− complexes.
Regarding the kinetics of the stibnite leaching in HCl media, Çopur et al. [9] studied the dissolution of a stibnite mineral sample in HCl solution in the temperature range from 12 to 60 °C, and found that the rate of the acid dissolution process could be represented well by a first-order pseudo-homogeneous kinetic model given by:
In this equation, X is the fraction of antimony dissolved, k is the kinetic constant and t is the reaction time. The authors determined an activation energy value of 51.92 kJ/mol. Later, Çopur et al. [15] used the same kinetics equation to analyze the dissolution kinetics of stibnite ore in HCl solutions saturated with Cl2 gas. For this oxidizing leaching system, the experimental activation energy was found to be 39.87 kJ/mol.
Ko et al. [25] studied the kinetics of stibnite dissolution with HCl-FeCl3 solutions in the temperature range from 30 to 90 °C and found that the rate of reaction was affected positively by an increase in the HCl concentration within the range of 1 to 2.5 M and also by an increase in FeCl3 concentration within the range from 0.015 to 0.075 M. For particle sizes smaller than 270 mesh, the kinetics followed the shrinking core model controlled by the diffusion of ferric ion through the porous sulfur layer on the surface of the particles. The reported activation energy was 71 kJ/mol.
In contrast, there is little information on stibnite dissolution in sulfuric acid–chloride systems. Hence, the present paper is concerned with an experimental study on leaching stibnite using H2SO4-NaCl-Fe(SO4)1.5-O2 solutions. The main objectives include the determination of the role of chloride and ferric ions in the stibnite dissolution rate and the determination of the mechanism and dissolution kinetics of stibnite in this media.
2. Experimental Work
2.1. Materials
The present investigation was carried out using two antimony trisulfide samples: a synthetic Sb2S3 obtained from Aldrich Chemicals with 96% Sb2S3 and a high-grade concentrate of stibnite mineral. The synthetic Sb2S3 had a wide range of particle sizes with 100% being lower than 102 μm and 51% lower than 25 μm, as determined by a laser diffraction size analyzer. The stibnite concentrate sample was prepared using hand-sorted natural stibnite crystals. This concentrate was crushed, ground and classified by sieving into narrow size fractions, and each fraction was analyzed for antimony content. The chemical composition of the different size fractions varied very little and the average antimony content was 68.6% Sb, 0.03% Pb and siliceous gangue minerals. Based on this analysis, the Sb2S3 content was calculated to be 96.3%. Thus, most of the kinetic experiments were carried out using the most abundant size fraction −75 + 53 μm (average size 64 µm). The X-ray diffraction analysis of the stibnite sample using a Rigaku (model Geiger Flex) copper anode spectrometer confirmed that the stibnite concentrate was nearly pure antimony trisulfide (Sb2S3). The result is shown in Figure 1.
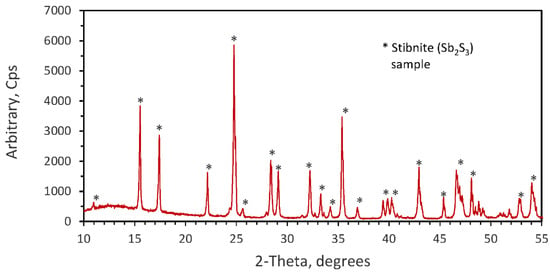
Figure 1.
X-ray diffraction pattern of the natural stibnite mineral an average size of 64 µm.
2.2. Experimental Procedure
The leaching setup consisted of a heating bath with a digital temperature controller, a 2 L cylindrical glass reactor, a mechanical stirrer and a gas condenser system. The cover of the 2 L cylindrical glass reactor had four openings to fit the various accessories needed in the setup. The central opening was used for adjusting the shaft of the mechanical stirrer, the others were fitted with a water glass condenser, a thermometer, and a fritted tube designed for the injection of oxygen and the sampling of the leaching solution at various times. The temperature of the solution was controlled within ±1 K using the thermometer in conjunction with the controller of the heating bath.
A typical leaching experiment consisted of heating 1 L of leaching solution containing predetermined concentrations of H2SO4, NaCl and sometimes, Fe(SO4)1.5 to the desired temperature. Once the set temperature was reached, 1 g of synthetic antimony sulfide or 2 g of stibnite mono-sized sample was charged to the reactor and the leaching was carried out at a constant stirring speed and oxygen flow rate for a selected time with the periodical withdrawing of liquid samples at time intervals. At the end of the experiment, the solids were filtered and washed, and the sampled solutions were analyzed for elemental antimony by atomic absorption spectroscopy (AA).
3. Results
Preliminary tests were carried out using the synthetic stibnite to determine the effect of the stirring speed and the oxygen flow rate on the Sb dissolution in the H2SO4-NaCl-O2 media. The results indicated that, without oxygen injection, the stibnite dissolution was very low, and this improved with oxygen flow rates of up to 1 L/min. The effect of the stirring speed was studied in the range from 300 to 600 rpm, and it was found that the Sb dissolution was nearly independent of the stirring speed in the range studied. Therefore, subsequent experiments were carried out using an injection rate of oxygen of 1 L/min and a stirring speed of 550 rpm to assure the independence of the Sb dissolution from these variables.
The study concerning the influence of the concentration of Fe(SO4)1.5, H2SO4 and NaCl on the stibnite dissolution was also carried out using synthetic stibnite, and natural stibnite was used only to study the effects of the temperature and particle size in order to determine the appropriate kinetic models for the stibnite dissolution with and without ferric sulfate in the leaching solution.
3.1. Effect of Fe(III) in the Sb Dissolution
The effect of adding Fe(SO4)1.5 in the range from 0 to 0.2 M to the system was carried out using a leaching solution comprising 1.5 M in H2SO4 and 1M in NaCl. The results are presented in Figure 2, where it can be seen that an Fe(III) concentration in the range from 0 to 0.05M has a significantly positive effect on the dissolution rate of stibnite. A further increase in the Fe(SO4)1.5 concentration over 0.05 M had a relatively small effect on the stibnite dissolution rate.
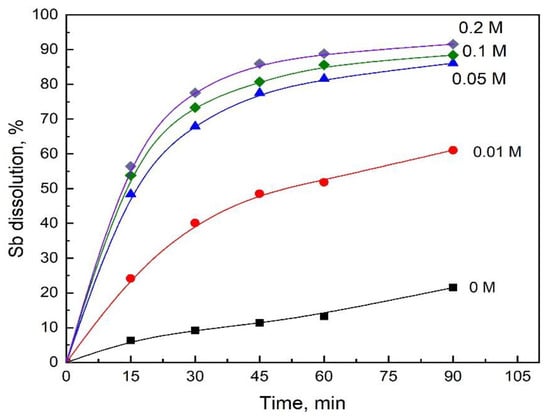
Figure 2.
Effect of Fe(III) concentration on Sb dissolution. Conditions: 1.5 M H2SO4, 1 M NaCl, O2 flowrate 1 L/min, 550 rpm, 1 g/L of synthetic stibnite, and 80 °C.
3.2. Effect of the Concentration of NaCl on the Sb Dissolution
The study concerning the influence of a sodium chloride concentration on the dissolution rate of stibnite was carried out at a constant concentration of H2SO4 equal to 1.5 M, 80 °C and Fe(III) concentrations of 0 and 0.1 M. The results are shown in Figure 3, where it can be observed that, when the leaching system does not contain Fe(III), the stibnite dissolution in the absence of NaCl is very low. However, an increase in the concentration of NaCl increases the dissolution rate of stibnite dramatically. On the other hand, when the leaching solution contains 0.1 M of Fe(III), the antimony dissolution is significant even in the absence of NaCl; nevertheless, an increase in NaCl concentration also improves the antimony dissolution. The positive effect of NaCl on the dissolution rates of other sulfide minerals has also been reported in the literature [23,26,27].
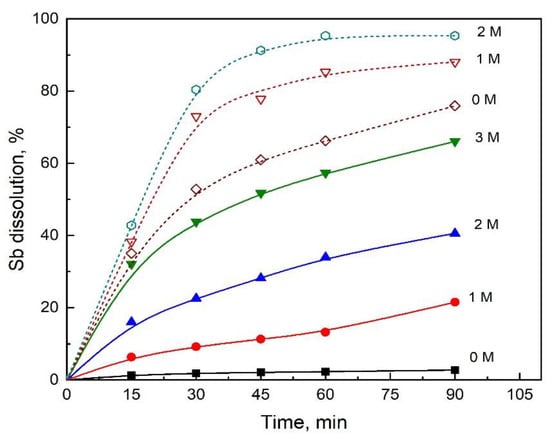
Figure 3.
Effect of NaCl on the Sb dissolution for 0 M Fe(III) (shown with solid lines and solid symbols) and 0.1 M Fe(III) (shown with dash lines and open symbols). Conditions: 1.5 M H2SO4, O2 flowrate 1 L/min, 550 rpm, 1 g/L of synthetic stibnite and 80 °C.
3.3. Effect of the Concentration of Sulfuric Acid
Experiments were carried out using a sulfuric acid concentration in the range from 0.8 to 3 M for two ferric and chloride concentrations: (a) 0 M Fe(III) and 2 M NaCl and (b) 0.1 M Fe(III) and 1 M NaCl. The results are shown in Figure 4, where the large positive effect of the presence of Fe(III) in the leaching system can again be observed. When the solution does not contain ferric ions, the sulfuric acid concentration has a significant effect on the stibnite dissolution, suggesting that acid dissolution is probably the dominant dissolution mechanism of stibnite under these conditions. For a leaching solution containing 0.1 M of Fe(III), the effect of the sulfuric acid concentration on the antimony dissolution was less important, suggesting a more complex dissolution mechanism for stibnite in the presence of ferric ions.
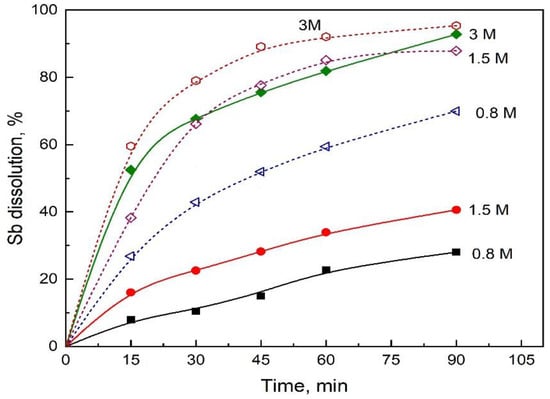
Figure 4.
Effect of H2SO4 on the Sb dissolution. Solid lines and solid symbols are for 2M NaCl and 0 M Fe(III). Short dash lines and open symbols are for 1M NaCl and 0.1 M Fe(III). Conditions: O2 flowrate 1 L/min, 80 °C, 550 rpm and 1 g/L of synthetic stibnite.
3.4. Effect of Temperature and Particle Size
The study concerning the effects of temperature and particle size on the stibnite dissolution consisted of experiments carried out using 2 g/L of the mono-sized natural stibnite samples in order to obtain the kinetic parameters using solutions with and without Fe(III).
3.4.1. H2SO4-NaCl-O2 Leaching Solution
The influence of temperature on the antimony dissolution can be seen in Figure 5 for experiments carried out with the stibnite concentrate sample with an average size of 64 μm, in the temperature range from 60 to 90 °C, with an initial concentration of 3 M H2SO4, 2 M NaCl and an O2 flow rate of 1 L/min. As seen in Figure 5, temperature had a large effect on the Sb dissolution rate. These results suggest a dissolution of Sb that is controlled by a chemical surface reaction.
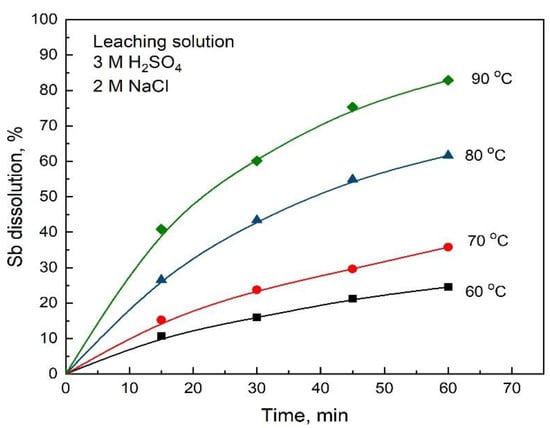
Figure 5.
Effect of temperature on the Sb dissolution from stibnite concentrate with H2SO4-NaCl-O2 solutions. Conditions: 3M H2SO4, 2 M NaCl, 1 L/min O2 flowrate and 2 g stibnite mineral with a particle size of 64 μm.
The study concerning the effect of particle size was carried out using three size fractions comprising 90 µm, 64 µm, and 45 µm of stibnite concentrate. The results are shown in Figure 6, where we can observe, as expected, a clear increasing effect on the dissolution of stibnite with the decreasing size of the stibnite particles due to the increased surface area.
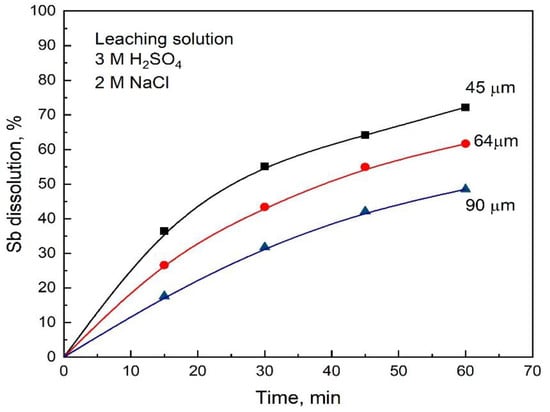
Figure 6.
Effect of particle size on the rate of dissolution of antimony sulfide for the H2SO4-NaCl-O2 leaching system. Conditions: 3 M H2SO4, 2 M NaCl, 1 L/min O2 flowrate, 2 g sample and 80 °C.
3.4.2. H2SO4-NaCl-Fe(SO4)1.5-O2 Leaching Solution
The influence of temperature on the antimony dissolution from the stibnite concentrate with an average size of 64 μm in leaching solution containing 1.5 M H2SO4, 1 M NaCl and 0.1 M Fe(III) can be seen in Figure 7 for the experiments carried out in the temperature range from 60 to 80 °C. It should be noted that in the presence of Fe(III), concentrated leaching solutions are not necessary to dissolve the stibnite effectively. For example, similar conversions to those obtained in leaching without Fe(III) that are shown in Figure 6 can also be obtained using half-concentrated solutions (1.5 M H2SO4 and 1 M NaCl) as shown in Figure 7.
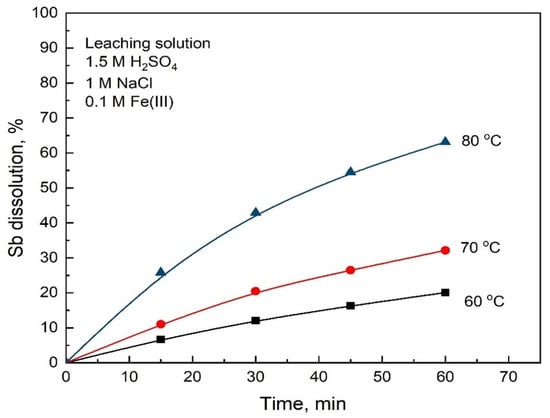
Figure 7.
Effect of temperature on Sb dissolution from stibnite with an average particle size of 64 μm. Conditions: 1.5 M H2SO4, 1 M NaCl, 0.1 M Fe(III), oxygen flow rate 1 L/min, 550 rpm and 2 g of stibnite mineral.
The effect of particle size for this leaching system can be seen in Figure 8. As expected, higher conversions are shown in this figure as compared to the data shown in Figure 6.
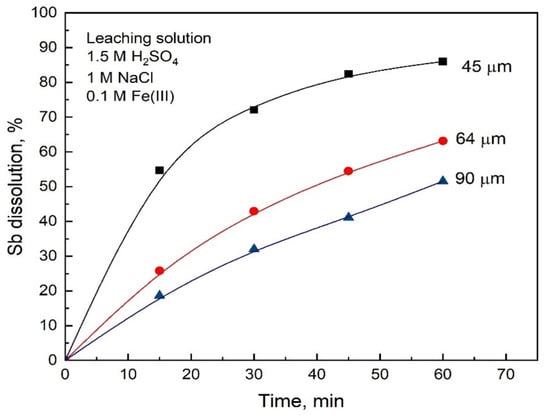
Figure 8.
Effect of particle size on the Sb dissolution from stibnite in solutions containing 0.1 M Fe(III). Other conditions: 1.5 M H2SO4, 1 M NaCl, oxygen flow rate 1 L/min, 550 rpm and 80 °C.
3.5. Characterization of Solid Residues from Leaching
Partially reacted samples were analyzed by X-ray diffraction spectroscopy in order to characterize the reaction products formed during the leaching of stibnite mineral. The results at 15 min (pattern a), and 90 min (pattern b) for the residues obtained by leaching at 80 °C in the solutions containing H2SO4, NaCl and O2 are presented in Figure 9. In this figure, we can identify in both patterns, (a) and (b), only strong diffraction lines for stibnite (Sb2S3). The absence of elemental sulfur in these patterns suggests again that the acid dissolution reaction is the main dissolution reaction taking place in this leaching media. On the other hand, for the leaching solutions containing Fe(SO4)1.5, the XRD results shown in Figure 10, pattern (a) for 15 min, and pattern (b) for 90 min, show diffraction lines in both patterns for stibnite and elemental sulfur. It is evident that for 90 min the production of crystalline sulfur was higher, as indicated by the strong diffraction lines for sulfur resulting from the higher conversion for stibnite. These results indicate that the leaching of stibnite occurred in the presence of ferric ions, producing elemental sulfur as a reaction product.
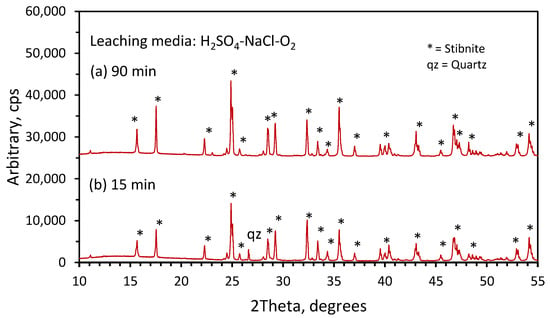
Figure 9.
XRD results for solid residues obtained in the stibnite dissolution. (a) 15 min leaching time (b) 90 min leaching time; other conditions: 3 M H2SO4, 2 M NaCl, 1 L/min O2 and 80 °C.
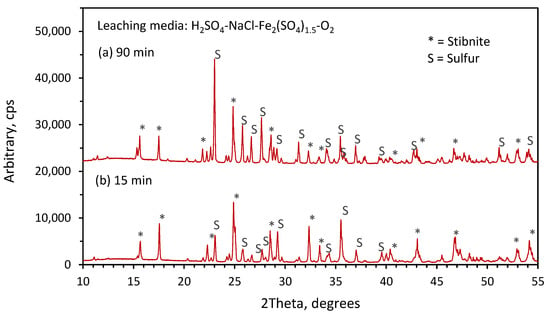
Figure 10.
XRD results for solid residues obtained in the stibnite dissolution. 1 L/min (a) 15 min leaching time (b) 90 min leaching time; other conditions: 1.5 M H2SO4, 1 M NaCl, 0.1 M Fe3+,O2 and 80 °C.
3.6. Kinetics of Stibnite Leaching in Sulfuric Acid-Sodium Chloride Solutions
For the leaching experimental data obtained using 2 g of mono-sized nonporous natural stibnite particles, the shrinking core kinetic model can be applied, and simple kinetic equations, depending on the rate-controlling step, can be determined. Therefore, the kinetic equation for the case of surface chemical reaction control and the kinetic equation for control by diffusion through a porous product layer can be written as Equations (8) and (9), respectively.
1 − (1 − X)1/3 = kt
1 − (2/3) X − (1 − X)2/3 = k′t
In these kinetic models, X is the fractional conversion, k and k′ are apparent rate constants, and t is the reaction time. In these models, k should be inversely proportional to the initial particle size and k′ should be inversely proportional to the square of the initial particle size.
3.7. Dissolution Kinetics of Antimony from Stibnite in H2SO4-NaCl-O2 Media
As already mentioned, the large effect of the sulfuric acid concentration on stibnite dissolution (Figure 4) and the absence of elemental sulfur in the leaching residues suggested that the acid dissolution reaction, Reaction (1), is the main reaction occurring in this leaching media and that the oxidizing dissolution with oxygen, Reaction (2), is not significant. However, to clarify the positive effect from injecting oxygen on the rate of stibnite dissolution observed in the preliminary tests, additional leaching experiments were carried out using a flowrate of 1 L/min of N2 instead of O2. The results, as presented in Figure 11, show that the Sb dissolution remained the same whether the gas flowrate was in O2 or N2, confirming that the leaching of stibnite in H2SO4-NaCl-O2 occurs mainly by acid dissolution, Reaction (1). Thus, the observed effect of the oxygen flowrate on Sb dissolution was due to the mechanical removal of the H2S product, which would allow the acid dissolution reaction to proceed without being arrested by the buildup of H2S near the surface of the stibnite particles.
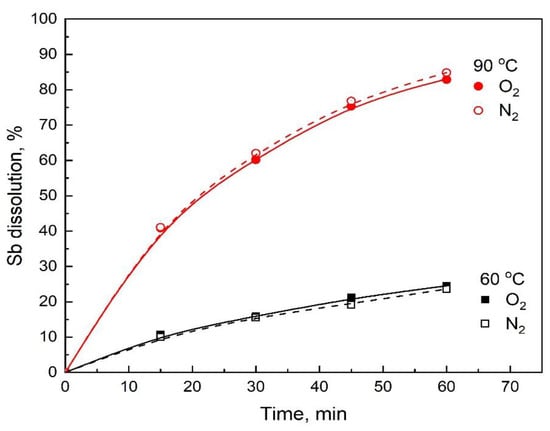
Figure 11.
Comparison of the Sb dissolution when injecting N2 or O2. Conditions: 3 M H2SO4, 2 M NaCl, 80 °C, gas flowrate 1 L/min and 550 rpm.
Consequently, the rate Equations (8) and (9) were tested with the experimental data, and as expected, Equation (8) fitted well with the data obtained in the sulfuric acid-sodium chloride-oxygen media, as evidenced by the straight lines (R2 range 0.984 to 0.992) shown in Figure 12, indicating that, for this leaching media, the dissolution rate was controlled by the surface chemical reaction.
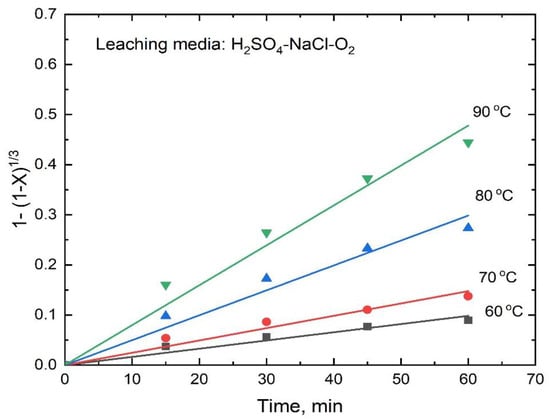
Figure 12.
Dissolution kinetics of antimony from stibnite with an average particle size of 63 μm (other conditions same as in Figure 5).
The values of the apparent rate constants k at various temperatures were obtained from the slopes of the straight lines shown in Figure 12, which were used to draw an Arrhenius plot, as shown in Figure 13. The calculated activation energy for the dissolution of stibnite was 54.6 kJ/mol for the temperature range from 60 to 90 °C; in this case, R2 was 0.989. This activation energy is consistent with a process controlled by a surface chemical reaction. The experimental data obtained for the various particle sizes also fitted well with the kinetic model expressed by Equation (8) as can be seen in Figure 14. Thus, the k values obtained from the slopes of the lines in Figure 14 were plotted as a function of the inverse of the initial particle radius, as shown in Figure 15. The good linear correlation between k and ro−1 (R2 = 0.999) in Figure 15 confirms the suitability of the chemical reaction controlled kinetic model used for this leaching media.
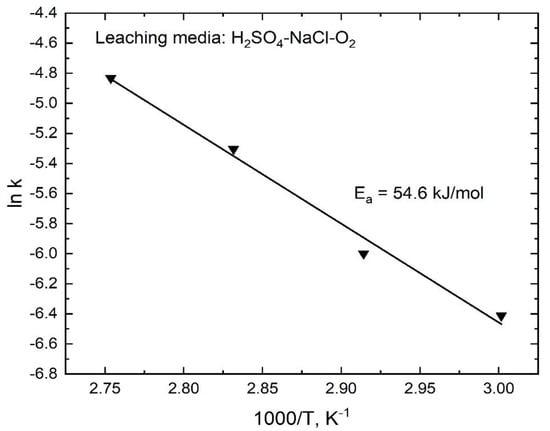
Figure 13.
Arrhenius plot for the dissolution of stibnite in H2SO4-NaCl-Fe(SO4)1.5-O2 media.
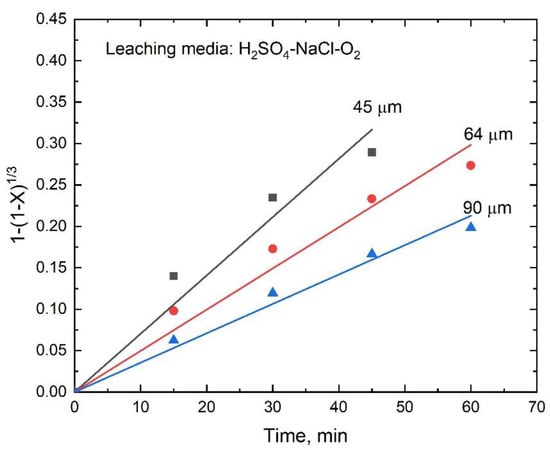
Figure 14.
Dissolution kinetics of a stibnite sample with various particle sizes (other conditions same as in Figure 7).
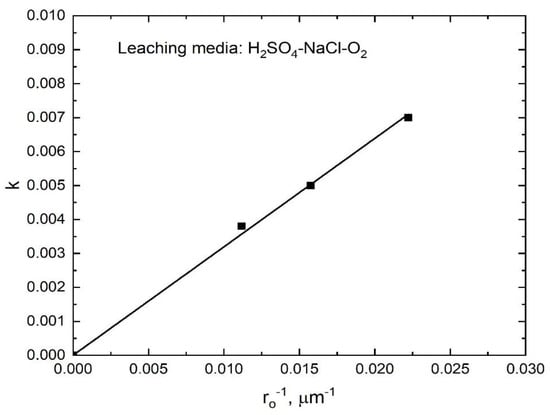
Figure 15.
Rate constant dependency on the inverse of the initial particle size in the dissolution of antimony from stibnite, calculated from the data shown in Figure 14.
3.8. Dissolution Kinetics of Antimony from Stibnite in H2SO4-NaCl-Fe(SO4)1.5-O2 Media
From the experimental data (Figure 2 and Figure 3), the dissolution of stibnite is considerably faster when there are ferric ions present in the solution; the formation of elemental sulfur in this case is also clear from the XRD results (Figure 10). Considering these facts, the ferric dissolution of stibnite represented by Reaction (3) is the overall leaching reaction in this case. Nevertheless, the effect of a sulfuric acid concentration on the dissolution rate, shown in Figure 4, suggests that the non-oxidative dissolution by sulfuric acid also has a role in the dissolution mechanism. Therefore, the dissolution of stibnite occurs by two parallel reaction paths, namely, (a) the direct ferric dissolution Reaction (3) and (b) the acid dissolution Reaction (1). This produces H2S, which is subsequently oxidized by ferric ions in order to produce elemental sulfur, Reaction (6). Since Reaction (1) is limited by a small equilibrium constant, the subsequent H2S oxidation by the inward diffusing ferric ions at the reaction interphase will allow Reaction (1) to proceed at a faster rate. By adding Reactions (1) and (6), we are able to obtain the overall reaction, Reaction (3), which produces elemental sulfur.
Therefore, for the leaching of stibnite in the presence of ferric ions, Equation (9) fitted the experimental data well, as evidenced by the straight lines (R2 ranging from 0.976 to 0.988) shown in Figure 16. These results indicate that the dissolution rate is controlled by the diffusion of reagents/products through a sulfur porous product layer. Accordingly, for the stibnite dissolution in the presence of ferric ions, the Arrhenius plot was drawn by using the apparent rate constants k′ that were determined from the slopes of the lines in Figure 16 in order to obtain an activation energy of 123.8 kJ/mol (R2 = 0.986), as shown in Figure 17. This high activation energy is not typical for a diffusion-controlled process. However, if the overall stibnite reaction included the acid dissolution reaction with a small equilibrium constant producing H2S at the surface of the mineral, coupled with the mass transfer of reactants/products through the porous sulfur layer, the apparent activation energy would be high, because it would include the standard enthalpy of the acid dissolution reaction. Therefore, the common premise that a diffusion-controlled reaction has a small activation energy would be incorrect in this case [28]. A similar dissolution mechanism was recently reported by Padilla et al. [23] in the marmatite (Zn,Fe)S leaching in sulfuric acid-sodium chloride-ferric sulfate-O2 media.
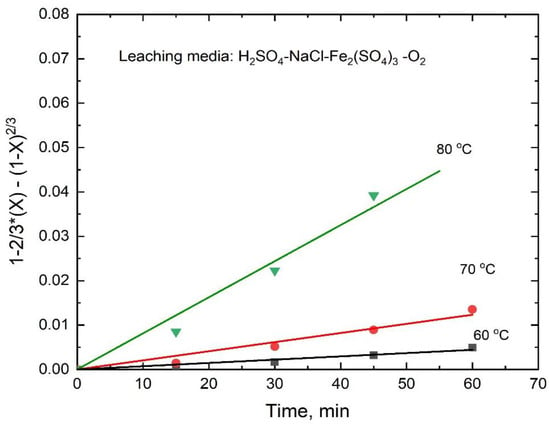
Figure 16.
Diffusion-control kinetics for the dissolution of antimony from stibnite in H2SO4-NaCl-Fe(SO4)1.5-O2 as a function of time.
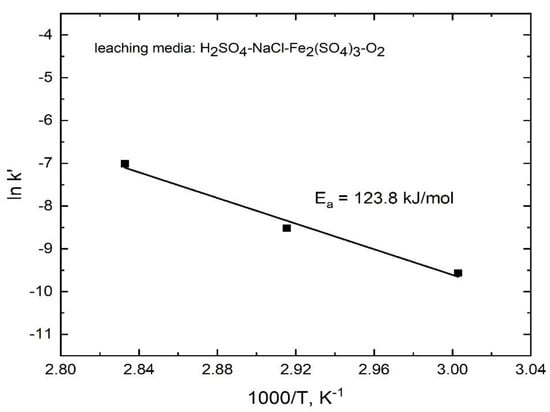
Figure 17.
Arrhenius plot for the dissolution of antimony from stibnite in H2SO4-NaCl-Fe(SO4)1.5-O2 in the temperature range from 60 to 90 °C.
The stibnite dissolution rate for the different particle sizes (shown in Figure 8) also fitted well with the kinetic model expressed by Equation (9) (R2 range from 0.971 to 0.999) as seen in Figure 18, and the k′ values obtained from the slopes of the lines in Figure 18 were plotted as a function of the inverse of the square of the initial particle radius, as shown in Figure 19. The good linear correlation between k′ and ro−2 shown in Figure 19 (R2 = 0.985) confirms the applicability of the diffusion-controlled kinetic model used for this system.
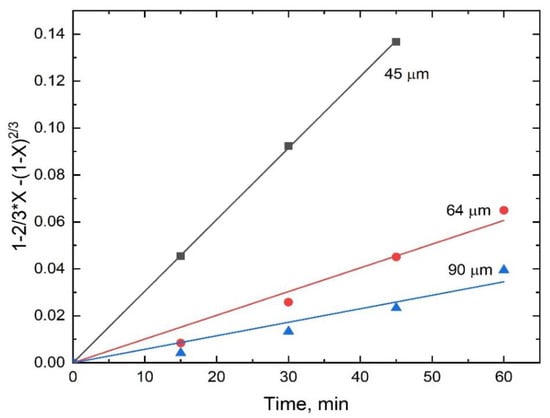
Figure 18.
Diffusion-control kinetics for the dissolution of antimony from stibnite in H2SO4-NaCl-Fe(SO4)1.5-O2 as a function of initial particle size.
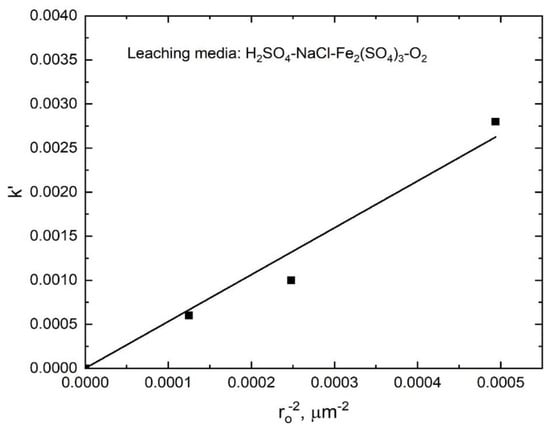
Figure 19.
Rate constants as functions of the inverse of the square stibnite particle size.
4. Conclusions
From the experimental data obtained from the stibnite leaching in H2SO4-NaCl-Fe(SO4)1.5-O2 solutions, the following conclusions can be reached.
The dissolution of antimony in H2SO4-O2 solutions without NaCl and Fe(III) was very low. However, the addition of sodium chloride increased the dissolution rate moderately. Furthermore, the addition of ferric ions into the solution significantly improved the dissolution rate of the stibnite. The effect of the ferric ions was substantial up to 0.05 M, over this concentration, the effect was less pronounced.
In the H2SO4-NaCl-O2 leaching solution, the dissolution of stibnite occurred by the nonoxidative acid dissolution of the mineral, which produced H2S as the only product, as confirmed by the XRD patterns of leach residues showing only diffraction lines for stibnite. In this case, the kinetic data fitted well with the shrinking core model controlled by the process of chemical reaction, and the calculated apparent activation energy of 54.6 kJ/mol is consistent with the chemical reaction control.
Conversely, in H2SO4-NaCl-Fe(SO4)1.5-O2, the X-ray diffraction patterns of the partially reacted leach residues showed a large quantity of crystalline sulfur as a product, indicating that the sulfide sulfur of the mineral was oxidized into elemental sulfur and formed a porous layer on the surface of the stibnite particles. The mechanism, in this case, involved the acid dissolution reaction with the diffusion of the reactant/product species through the porous layer and the oxidation of H2S by ferric ions. The kinetic data, in this case, fitted well with the diffusion-controlled shrinking core model, for which an apparent activation energy of 123.8 kJ/mol was obtained. This high activation energy is consistent with the proposed mechanism.
Author Contributions
Conceptualization, R.P.; Formal analysis, R.P. and M.C.R.; Funding acquisition, R.P.; Investigation, O.C.; Methodology, R.P., O.C. and M.C.R.; Resources, R.P.; Supervision, D.V.-G.; Validation, D.V.-G. and M.C.R.; Visualization, D.V.-G. and M.C.R.; Writing—original draft, R.P.; Writing—review & editing, M.C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lager, T.; Forssberg, K.S.E. Current processing technology for antimony-bearing ores a review, Part 2. Miner. Eng. 1989, 2, 543–556. [Google Scholar] [CrossRef]
- Anderson, C.G. The metallurgy of antimony. Chem. Der Erde 2012, 72, 3–8. [Google Scholar] [CrossRef]
- Ren, B.; Zhou, Y.; Ma, H.; Deng, R.; Zhang, P.; Hou, B. Sb release characteristics of the solid waste produced in antimony mining smelting process. J. Mater. Cycles Waste Manag. 2018, 20, 193–200. [Google Scholar] [CrossRef]
- Padilla, R.; Chambi, L.C.; Ruiz, M.C. Antimony production by carbothermic reduction of stibnite in the presence of Lime. J. Min. Metall. Sect. B-Metall. Sect. B 2014, 50, 5–13. [Google Scholar] [CrossRef]
- Yang, J.G.; Tang, C.B.; Chen, Y.M.; Tang, M.T. Separation of antimony from a stibnite concentrate through a low-temperature smelting process to eliminate SO2 emission. Metall. Mater. Trans. B 2011, 42, 30–36. [Google Scholar] [CrossRef]
- Ye, L.; Ouyang, Z.; Chen, Y.; Chen, Y.; Xiao, L. Sulfur fixation and reduction roasting of stibnite for clean extraction of Sb by a combined metallurgy and beneficiation process. Miner. Eng. 2019, 144, 106049. [Google Scholar] [CrossRef]
- Ubaldini, S.; Veglio, F.; Fornari, P.; Abbruzzese, C. Process flow-sheet for gold and antimony recovery from stibnite. Hydrometallurgy 2000, 57, 187–199. [Google Scholar] [CrossRef]
- Awe, S.A.; Sandström, Å. Electrowinning of antimony from model sulphide alkaline solutions. Hydrometallurgy 2013, 137, 60–67. [Google Scholar] [CrossRef]
- Çopur, M.; Çolak, S.; Yapici, S. Solubility of stibnite ore in HCl solutions. Ind. Eng. Chem. Res. 1995, 34, 3995–4002. [Google Scholar] [CrossRef]
- Çopur, M.; Pekdemir, T.; Çelik, C.; Çulak, S. Determination of the optimum conditions for the dissolution of stibnite in HCl solutions. Ind. Eng. Chem. Res. 1997, 36, 682–687. [Google Scholar] [CrossRef]
- Bhatti, M.A.; Adnan, A.; Ahad, A.; Kazmi, K.R.; Akram, A. Oxidative chlorination leaching of stibnite using acidic ferric chloride lixiviant. J. Chem. Soc. Pak. 2018, 40, 1035–1045. [Google Scholar]
- Ye, L.; Ouyang, Z.; Chen, Y.; Chen, Y. Ferric chloride leaching of antimony from stibnite. Hydrometallurgy 2019, 186, 210–217. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, H.; Xin, Y.; Li, D.; Guo, X. Ozonation leaching of a complex sulfidic antimony ore in hydrochloric acid solution. Hydrometallurgy 2016, 159, 126–131. [Google Scholar] [CrossRef]
- Guo, X.; Xin, Y.; Wang, H.; Tian, Q. Leaching kinetics of antimony-bearing complex sulfides ore in hydrochloric acid solution with ozone. Trans. Nonferrous Met. Soc. China 2017, 27, 2073–2081. [Google Scholar] [CrossRef]
- Çopur, M.; Yartaşi, C.; Özmetin, C.; Kocakerim, M.M. Solubility of stibnite ore in HCl solutions saturated with Cl2 gas. Chem. Biochem. Eng. Q. 2001, 15, 25–28. [Google Scholar]
- Yang, J.G.; Wu, Y.T. A hydrometallurgical process for the separation and recovery of antimony. Hydrometallurgy 2014, 143, 68–74. [Google Scholar] [CrossRef]
- Yang, J.G.; Yang, S.H.; Tang, C.B. The Membrane Electrowinning Separation of Antimony from a Stibnite Concentrate. Metall. Mater. Trans. B 2010, 41, 527–534. [Google Scholar] [CrossRef]
- Mahlangu, T.; Gudyanga, F.P.; Simbi, D.J. Reductive leaching of stibnite (Sb2S3) flotation concentrate using metallic iron in a hydrochloric acid medium I: Thermodynamics. Hydrometallurgy 2006, 84, 192–203. [Google Scholar] [CrossRef]
- Zhao, R.; Shi, X.; Hanying, J. Thermodynamic equilibrium of Sb-Cl-H2O system. Trans. Non Ferrous Met. Soc. China 1997, 7, 123–128. [Google Scholar]
- Senanayake, G.; Muir, D.M. Speciation and Reduction Potentials of Metal Ions in Concentrated Chloride and Sulfate Solutions Relevant to Processing Base Metal Sulfides. Metall. Trans. B 1988, 19, 37–45. [Google Scholar] [CrossRef]
- O′Malley, M.L.; Lidell, K.C. Leaching of CuFeS2 by aqueous FeCl2, HCl and NaCl. Effect of solution composition and limited oxidant. Metall. Trans. B 1987, 18, 505–510. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Gallardo, E.; Padilla, R. Copper extraction from white metal by pressure leaching in H2SO4-FeSO4-O2. Hydrometallurgy 2009, 100, 50–55. [Google Scholar] [CrossRef]
- Padilla, R.; Copa, M.E.; Ruiz, M.C. Dissolution kinetics of marmatite in sulfuric acid-ferric sulfate-sodium chloride-oxygen media at atmospheric pressure. Hydrometallurgy 2022, 208, 105801. [Google Scholar] [CrossRef]
- Li, G.; Xin, Y.T.; Lü, X.D.; Tian, G.H.; Yang, K.; Ye, L.G. Stability constants of Sb5+ with Cl− and thermodynamics of Sb−S−Cl−H2O system involving complex behavior of Sb with Cl. Trans. Nonferrous Met. Soc. China 2020, 30, 3379–3389. [Google Scholar] [CrossRef]
- Ko, I.Y.; Kim, D.J.; Oh, J.H. Leaching kinetics of stibnite in ferric chloride solution-On the leaching behavior of stibnite, 2. Taehan Kumsak Hakhoe China 1981, 19, 410–417. [Google Scholar]
- Cheng, C.Y.; Lawson, F. The kinetics of leaching covellite in acidic oxygenated sulphate-chloride solutions. Hydrometallurgy 1991, 27, 269–284. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Montes, K.S.; Padilla, R. Chalcopyrite leaching in sulfate-chloride media at ambient pressure. Hydrometallurgy 2011, 109, 37–42. [Google Scholar] [CrossRef]
- Sohn, H.Y. The influence of chemical equilibrium on fluid-solid reaction rates and the falsification of activation energy. Metall. Mater. Trans. B Process. Metall. Mater. Process. Sci. 2004, 35, 121–131. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).