Comparison of the Effects of Sodium Oleate and Benzohydroxamic Acid on Fine Scheelite and Cassiterite Hydrophobic Flocculation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mineral Samples and Reagents
2.2. Microflotation Tests
2.3. Hydrophobic Flocculation Tests and Particle Size Measurements
2.4. Laser-Based Particle Size Analysis
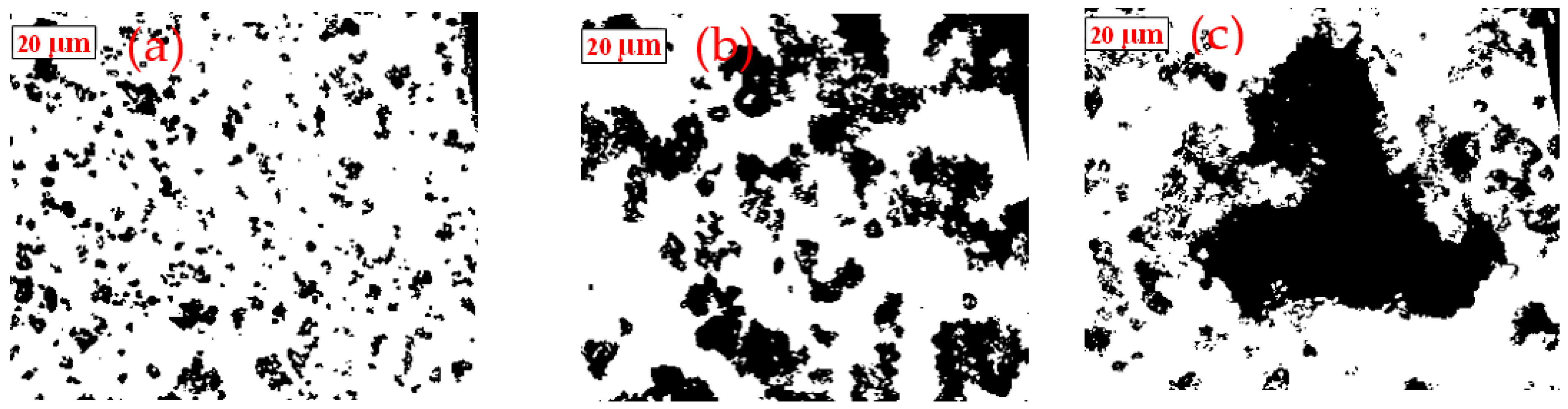
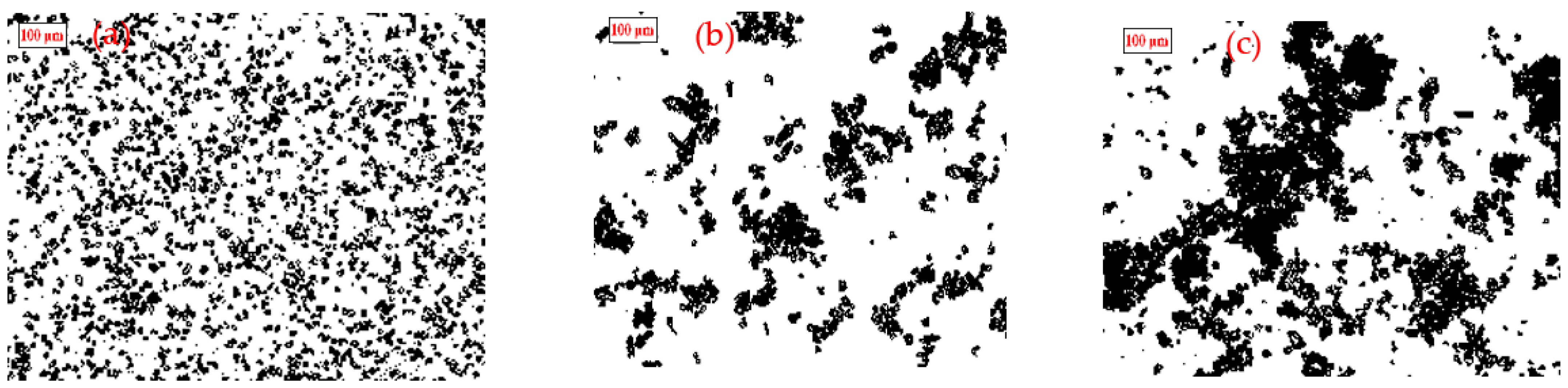
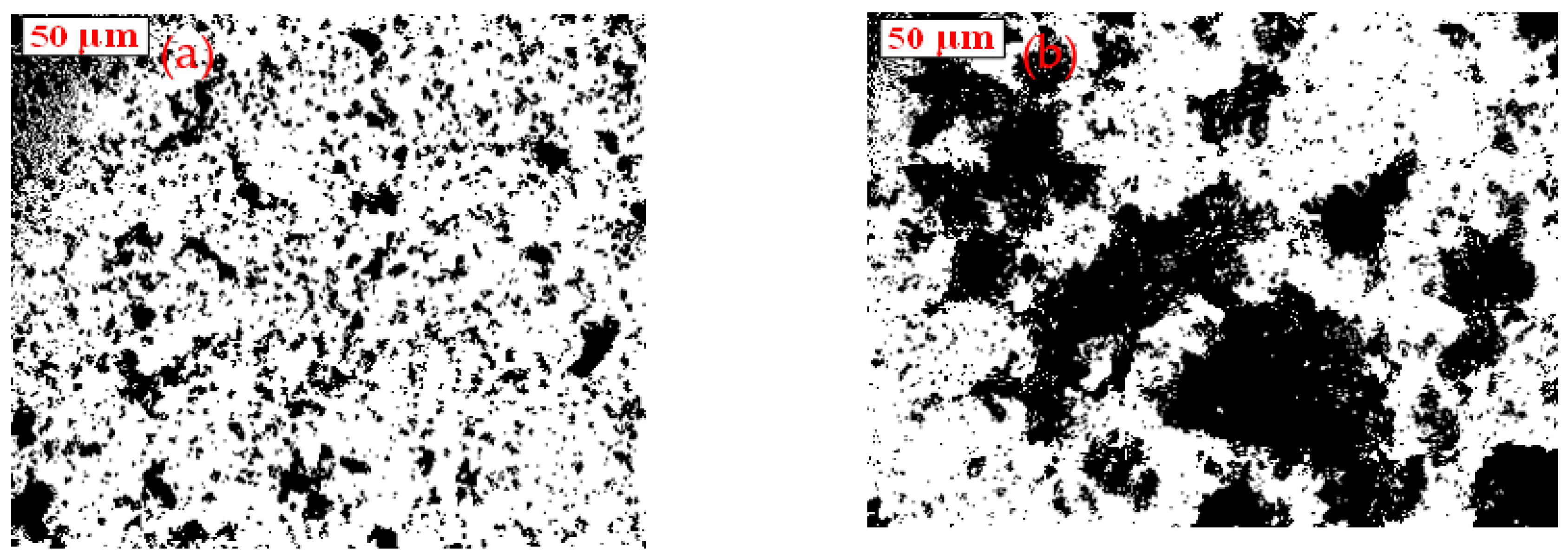
2.5. Optical Microscopy Observation of Aggregate Structures
3. Results
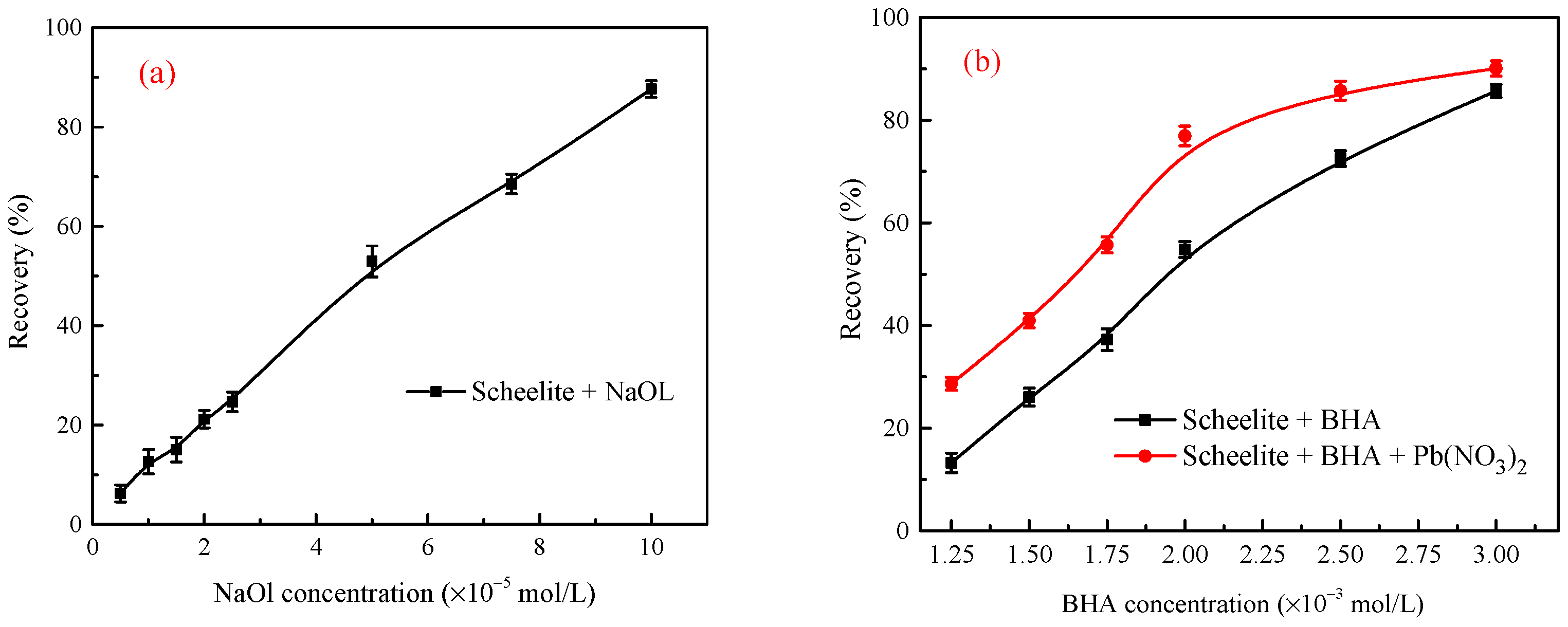
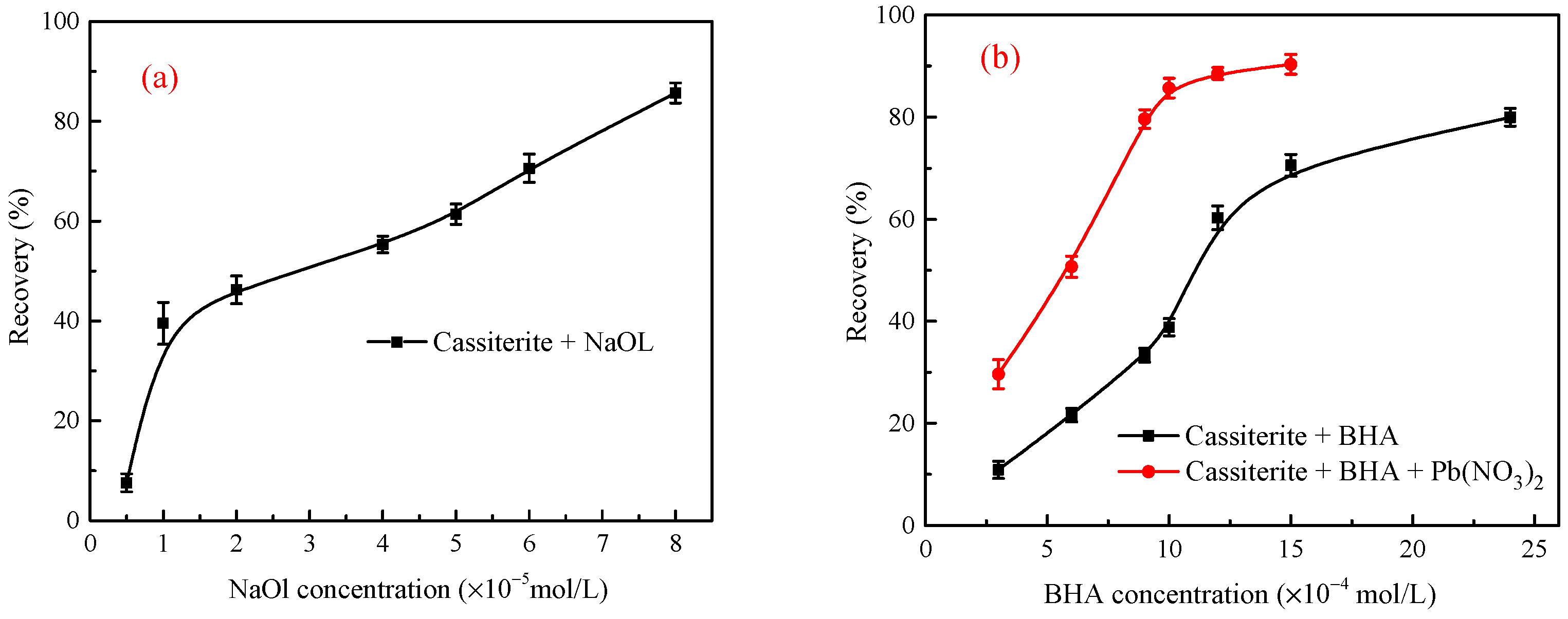
3.1. Conventional Froth Flotation of Single Minerals
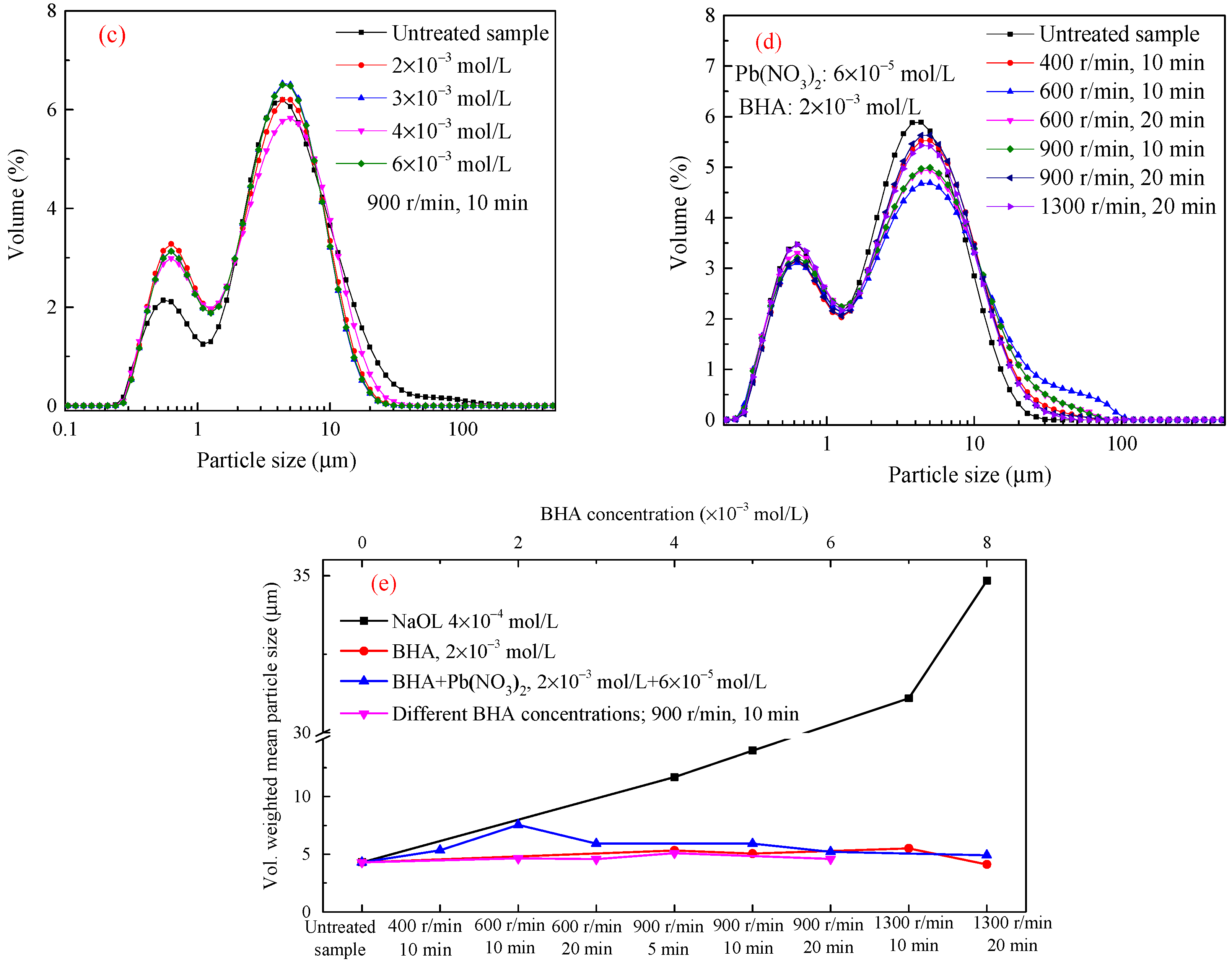
3.2. Shear-Flocculation of Single Minerals Stirred Suspension
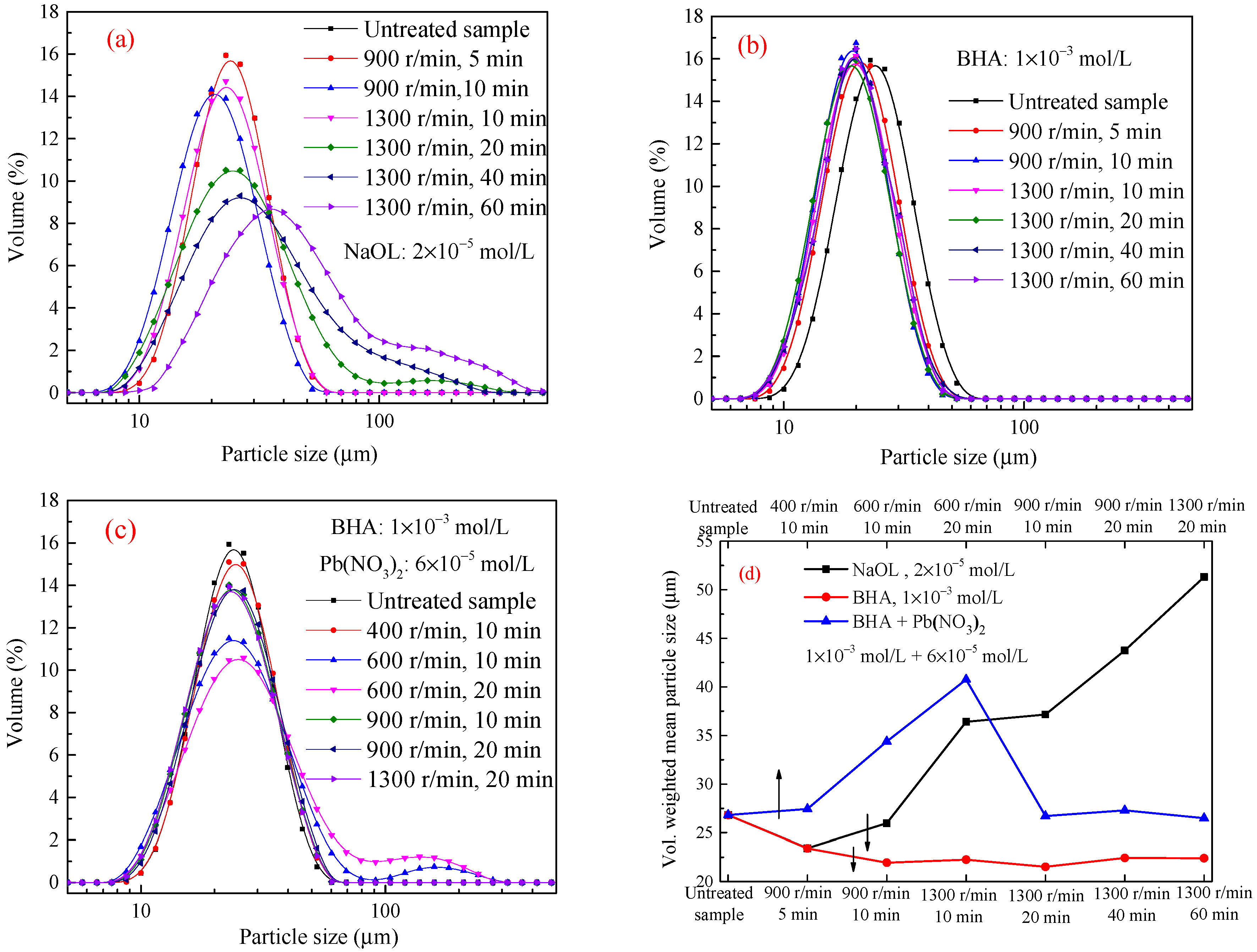
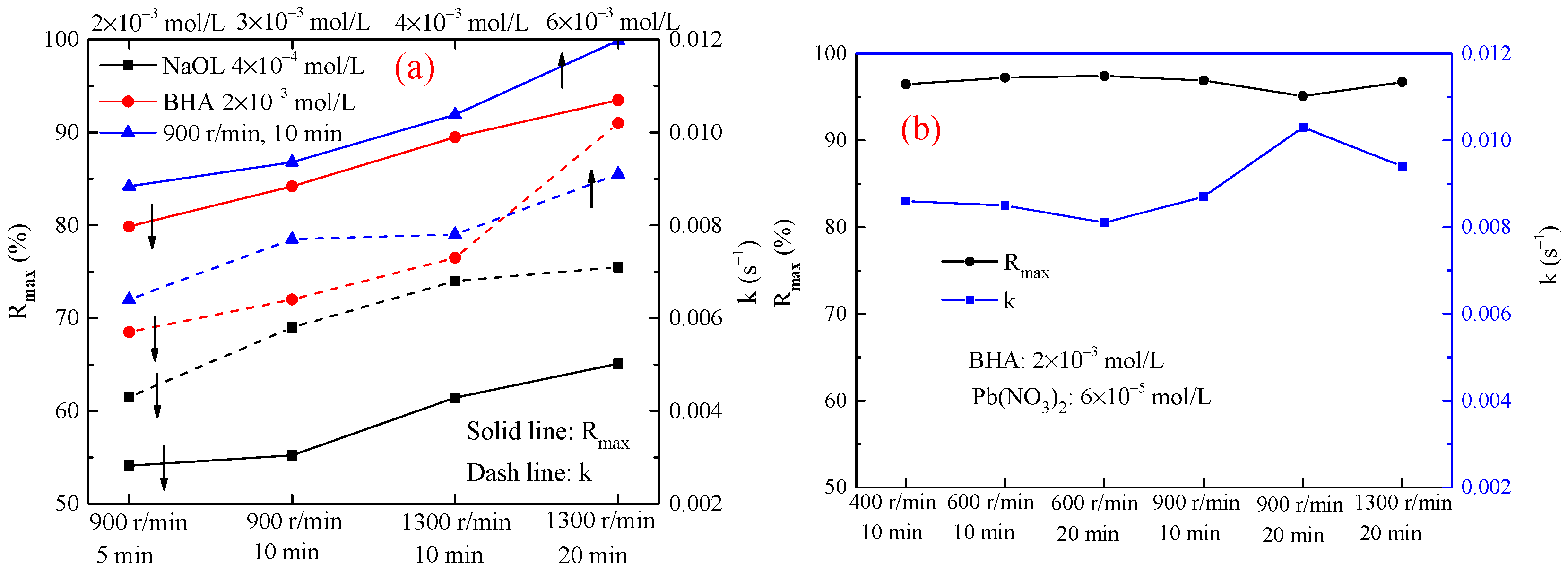
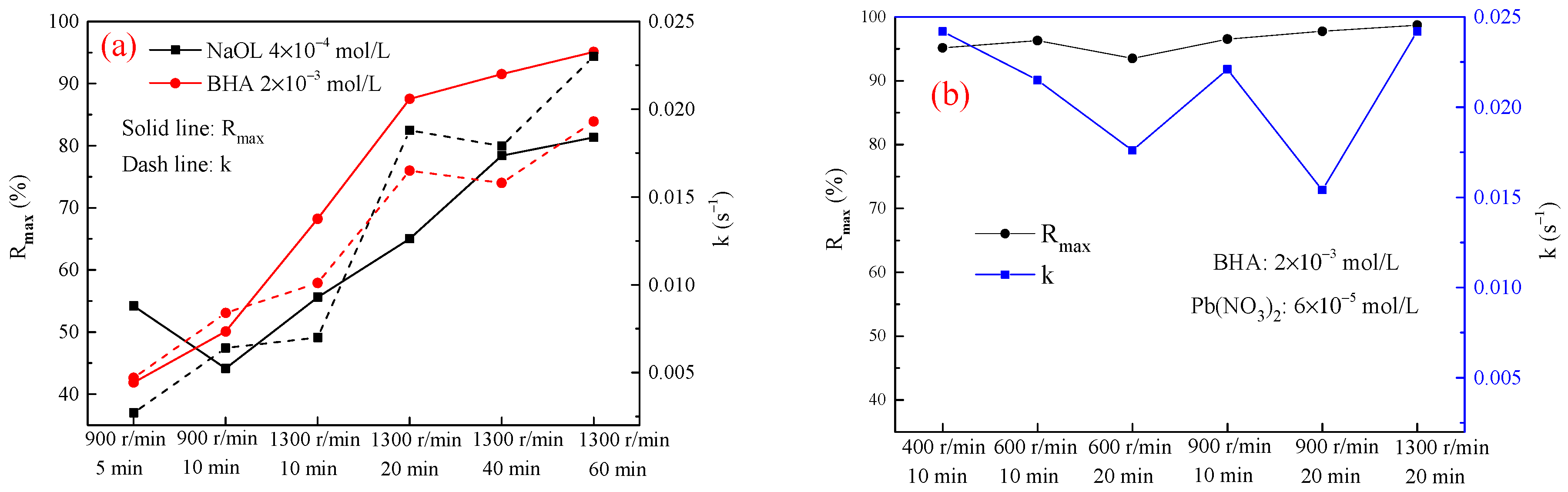
3.2.1. Scheelite
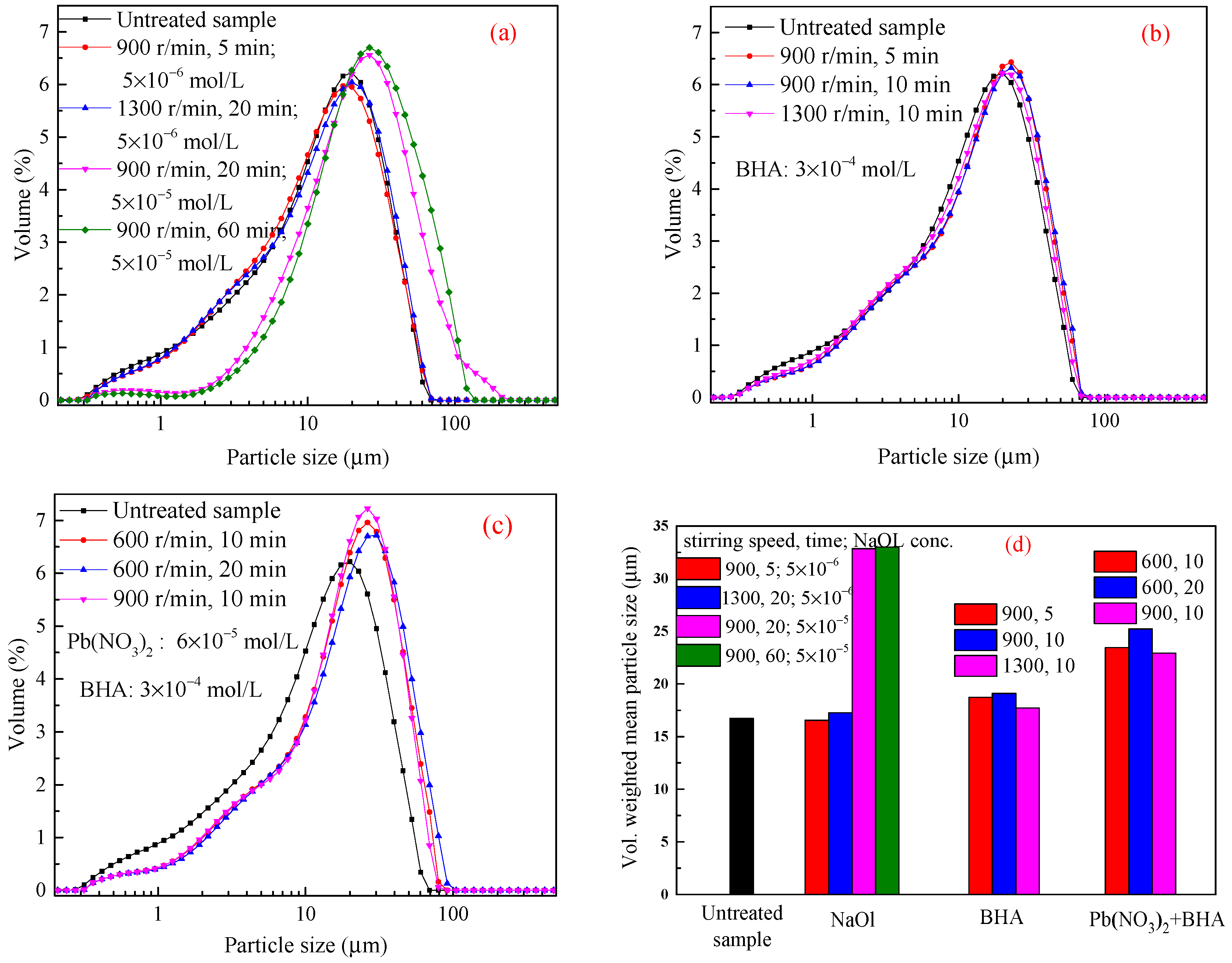
3.2.2. −37 + 10 μm Cassiterite
3.2.3. −23 μm Cassiterite
3.3. Flotation Tests of the Stirred Mineral Suspensions
3.4. The Influence of the Agitation Time of the Actual Ore Suspension on the Flotation Recovery of Cassiterite
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Warren, L.J. Shear-flocculation of ultrafine scheelite in sodium oleate solutions. J. Colloid Interface Sci. 1975, 50, 307–318. [Google Scholar] [CrossRef]
- Warren, L.J. Slime coating and shear-flocculation in the scheelite-sodium oleate system. Trans. Inst. Min. Metall. 1975, 84, C99–C104. [Google Scholar]
- Chen, W.; Feng, Q.M.; Zhang, G.F.; Li, L.F.; Jin, S.Z. Effect of energy input on flocculation process and flotation performance of fine scheelite using sodium oleate. Miner. Eng. 2017, 112, 27–35. [Google Scholar] [CrossRef]
- Akdemir, Ü. Shear flocculation of fine hematite particles and correlation between flocculation, flotation and contact angle. Powder Technol. 1997, 94, 1–4. [Google Scholar] [CrossRef]
- Akdemir, Ü.; Hiçyilmaz, C. Shear flocculation of chromite fines in sodium oleate solutions. Colloids Surf. A 1996, 110, 87–93. [Google Scholar] [CrossRef]
- Song, S.X. Hydrophobic Flocculation Theory and Separation Technology; China Coal Industry Publishing House: Beijing, China, 1993; p. 54. [Google Scholar]
- Warren, L.J. Flocculation of stirred suspensions of cassiterite and tourmaline. Colloids Surf. 1982, 5, 301–319. [Google Scholar] [CrossRef]
- Baldauf, H.; Schoenherr, J.; Schubert, H. Alkane dicarboxylic acids and aminonaphthol-sulfonic acids—A new reagent regime for cassiterite flotation. Int. J. Miner. Process. 1985, 15, 117–133. [Google Scholar] [CrossRef]
- Gruner, H.; Bilsing, U. Cassiterite flotation using styrene phosphonic acid to produce high-grade concentrates at high recoveries from finely disseminated ores- comparison with other collectors and discussion of effective circuit configurations. Miner. Eng. 1992, 5, 429–434. [Google Scholar] [CrossRef]
- Huang, K.H.; Huang, X.P.; Jia, Y.; Wang, S.; Cao, Z.F.; Zhong, H. A novel surfactant styryl phosphonate mono-iso-octyl ester with improved adsorption capacity and hydrophobicity for cassiterite flotation. Miner. Eng. 2019, 142, 105895. [Google Scholar] [CrossRef]
- Zhou, W.G.; Chen, H.; Ou, L.; Shi, Q. Aggregation of ultra-fine scheelite particles induced by hydrodynamic cavitation. Int. J. Miner. Process. 2016, 157, 236–240. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Tong, X.; Song, S.X.; Wang, X.; Deng, Z.B.; Xie, X. Beneficiation of cassiterite fines from a tin tailing slime by froth flotation. Sep. Sci. Technol. 2014, 49, 458–463. [Google Scholar] [CrossRef]
- Leistner, T.; Embrechts, M.; Leißner, T.; Chelgani, S.C.; Osbahr, I.; Möckel, R.; Peuker, U.A.; Rudolph, M. A study of the reprocessing of fine and ultrafine cassiterite from gravity tailing residues by using various flotation techniques. Miner. Eng. 2016, 96–97, 94–98. [Google Scholar] [CrossRef]
- Subrahmanyam, T.V.; Forssberg, K. Fine particles processing: Shear-flocculation and carrier flotation—A review. Int. J. Miner. Process. 1990, 30, 265–286. [Google Scholar] [CrossRef]
- Yin, W.Z.; Tang, Y. Interactive effect of minerals on complex ore flotation: A brief review. Int. J. Miner. Metall. Mater. 2020, 27, 571–583. [Google Scholar] [CrossRef]
- Hu, Y.H.; Yang, F.; Sun, W. The flotation separation of scheelite from calcite using a quaternary ammonium salt as collector. Miner. Eng. 2011, 24, 82–84. [Google Scholar] [CrossRef]
- Luo, L.P.; Wu, H.Q.; Xu, L.H.; Meng, J.H.; Lu, J.H.; Zhou, H.; Huo, X.M.; Huang, L.Y. An in situ ATR-FTIR study of mixed collectors BHA/DDA adsorption in ilmenite-titanaugite flotation system. Int. J. Min. Sci. Technol. 2021, 31, 689–697. [Google Scholar] [CrossRef]
- Wu, X.Q.; Zhu, J.G. Selective flotation of cassiterite with benzohydroxamic acid. Miner. Eng. 2006, 19, 1410–1417. [Google Scholar] [CrossRef]
- Han, H.S.; Xiao, Y.; Hu, Y.H.; Sun, W.; Nguyen, A.V.; Tang, H.H.; Gui, X.H.; Xing, Y.W.; Wei, Z.; Wang, J.J. Replacing Petrov’s process with atmospheric flotation using Pb-BHA complexes for separating scheelite from fluorite. Miner. Eng. 2020, 145, 106053. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, W.; Han, H.S.; Cao, J.; Gui, X.H.; Xing, Y.W.; Zhang, C.Y.; Zou, J.X. Enhanced electronic effect improves the collecting efficiency of benzohydroxamic acid for scheelite flotation. Miner. Eng. 2020, 152, 106308. [Google Scholar] [CrossRef]
- Tian, M.J.; Gao, Z.Y.; Khoso, S.A.; Sun, W.; Hu, Y.H. Understanding the activation mechanism of Pb2+ ion in benzohydroxamic acid flotation of spodumene: Experimental findings and DFT simulations. Miner. Eng. 2019, 143, 106006. [Google Scholar] [CrossRef]
- Cao, M.; Gao, Y.D.; Bu, H.; Qiu, X.Y. Study on the mechanism and application of rutile flotation with benzohydroxamic acid. Miner. Eng. 2019, 134, 275–280. [Google Scholar] [CrossRef]
- Tian, M.J.; Zhang, C.Y.; Han, H.S.; Liu, R.Q.; Gao, Z.Y.; Chen, P.; He, J.Y.; Hu, Y.H.; Sun, W.; Yuan, D.D. Novel insights into adsorption mechanism of benzohydroxamic acid on lead (II)-activated cassiterite surface: An integrated experimental and computational study. Miner. Eng. 2018, 122, 327–338. [Google Scholar] [CrossRef]
- Jin, S.Z.; Zhang, P.Y.; Ou, L.M.; Zhang, Y.B.; Chen, J.H. Flotation of cassiterite using alkyl hydroxamates with different carbon chain lengths: A theoretical and experimental study. Miner. Eng. 2021, 170, 107025. [Google Scholar] [CrossRef]
- Qin, W.Q.; Ren, L.Y.; Wang, P.P.; Yang, C.R.; Zhang, Y.S. Electro-flotation and collision-attachment mechanism of fine cassiterite. Trans. Nonferr. Met. Soc. China 2012, 22, 917–924. [Google Scholar] [CrossRef]
- Assis, S.M.; Montenegro, L.; Peres, A. Utilisation of hydroxamates in minerals froth flotation. Miner. Eng. 1996, 9, 103–114. [Google Scholar] [CrossRef]
- Sreenivas, T.; Padmanabhan, N.P.H. Surface chemistry and flotation of cassiterite with alkyl hydroxamates. Colloids Surf. A 2002, 205, 47–59. [Google Scholar] [CrossRef]
- Hu, Y.H.; Gao, Z.Y.; Wei, S.; Liu, X.W. Anisotropic surface energies and adsorption behaviors of scheelite crystal. Colloids Surf. A 2012, 415, 439–448. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2017, 28, 1–12. [Google Scholar] [CrossRef]
- Yoon, R.H.; Luttrell, G.H. Development of the Selective Coagulation Process; Virginia Center for Coal and Minerals Processing: Blacksburg, VA, USA, 1992; pp. 64–66. [Google Scholar]
- Xu, Z.H. A Study of Hydrophobic Interaction in Fine Particle Coagulation. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 1990; p. 228. [Google Scholar]
- Tian, M.J.; Liu, R.Q.; Gao, Z.Y.; Chen, P.; Han, H.S.; Wang, L.; Zhang, C.Y.; Sun, W.; Hu, Y.H. Activation mechanism of Fe (III) ions in cassiterite flotation with benzohydroxamic acid collector. Miner. Eng. 2018, 119, 31–37. [Google Scholar] [CrossRef]




| Size Range (μm) | Weight (%) | Assay (% Sn) | Distribution (% Sn) | Cumulative Oversize (%) | Cumulative Distribution (% Sn) |
|---|---|---|---|---|---|
| +74 | 4.18 | 0.08 | 1.40 | 4.18 | 1.40 |
| −74 + 38 | 34.07 | 0.17 | 23.02 | 38.25 | 24.42 |
| −38 + 19 | 48.93 | 0.30 | 59.06 | 87.18 | 83.48 |
| −19 + 10 | 8.69 | 0.31 | 10.66 | 95.87 | 94.14 |
| −10 + 5 | 2.60 | 0.50 | 5.17 | 98.47 | 99.31 |
| −5 | 1.53 | 0.11 | 0.69 | ||
| 100.00 | 0.25 | 100.00 |
| T (min) | Concentrate | Tailing | ||||
|---|---|---|---|---|---|---|
| Yield (%) | Assay (%) | Recovery (%) | Yield (%) | Assay (%) | Recovery (%) | |
| 3 | 11.12 | 1.54 | 60.37 | 88.88 | 0.13 | 39.96 |
| 10 | 8.59 | 2.00 | 55.65 | 91.41 | 0.15 | 44.35 |
| 20 | 6.17 | 2.50 | 48.90 | 93.83 | 0.17 | 51.10 |
| 30 | 7.22 | 2.05 | 48.79 | 92.78 | 0.17 | 51.21 |
| 40 | 2.77 | 3.95 | 36.12 | 97.23 | 0.20 | 63.88 |
| Sample | N (r/min), T (min) | RI | R (s−1) | Sample | N (r/min), T (min) | RI | R (s−1) |
|---|---|---|---|---|---|---|---|
| Scheelite | 900, 5 | 1.72 | 0.34 | −37 + 10 μm cassiterite | 900, 5 | −0.13 | −0.025 |
| 900, 10 | 2.25 | 0.23 | 900, 10 | −0.03 | −0.003 | ||
| 1300, 10 | 6.23 | 0.62 | 1300, 10 | 0.36 | 0.036 | ||
| 1300, 20 | 7.10 | 0.36 | 1300, 20 | 0.39 | 0.019 | ||
| −23 μm cassiterite | 900, 5 # | −0.01 | −0.002 | 1300, 40 | 0.63 | 0.016 | |
| 1300, 20 # | 0.03 | 0.002 | 1300, 60 | 0.91 | 0.015 | ||
| 900, 20 * | 0.96 | 0.048 | |||||
| 900, 60 * | 0.97 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Ou, L. Comparison of the Effects of Sodium Oleate and Benzohydroxamic Acid on Fine Scheelite and Cassiterite Hydrophobic Flocculation. Minerals 2022, 12, 687. https://doi.org/10.3390/min12060687
Jin S, Ou L. Comparison of the Effects of Sodium Oleate and Benzohydroxamic Acid on Fine Scheelite and Cassiterite Hydrophobic Flocculation. Minerals. 2022; 12(6):687. https://doi.org/10.3390/min12060687
Chicago/Turabian StyleJin, Saizhen, and Leming Ou. 2022. "Comparison of the Effects of Sodium Oleate and Benzohydroxamic Acid on Fine Scheelite and Cassiterite Hydrophobic Flocculation" Minerals 12, no. 6: 687. https://doi.org/10.3390/min12060687
APA StyleJin, S., & Ou, L. (2022). Comparison of the Effects of Sodium Oleate and Benzohydroxamic Acid on Fine Scheelite and Cassiterite Hydrophobic Flocculation. Minerals, 12(6), 687. https://doi.org/10.3390/min12060687






