Study on the Alteration of Pore Parameters of Shale with Different Natural Fractures under Supercritical Carbon Dioxide Seepage
Abstract
:1. Introduction
2. Multi-Scale Natural Fracture Distribution and Filling Characteristics of Shale
2.1. Characteristics of Macroscopic Fractures in Field Shale Outcrops
2.2. Fracture Characteristics of Downhole Shale Core
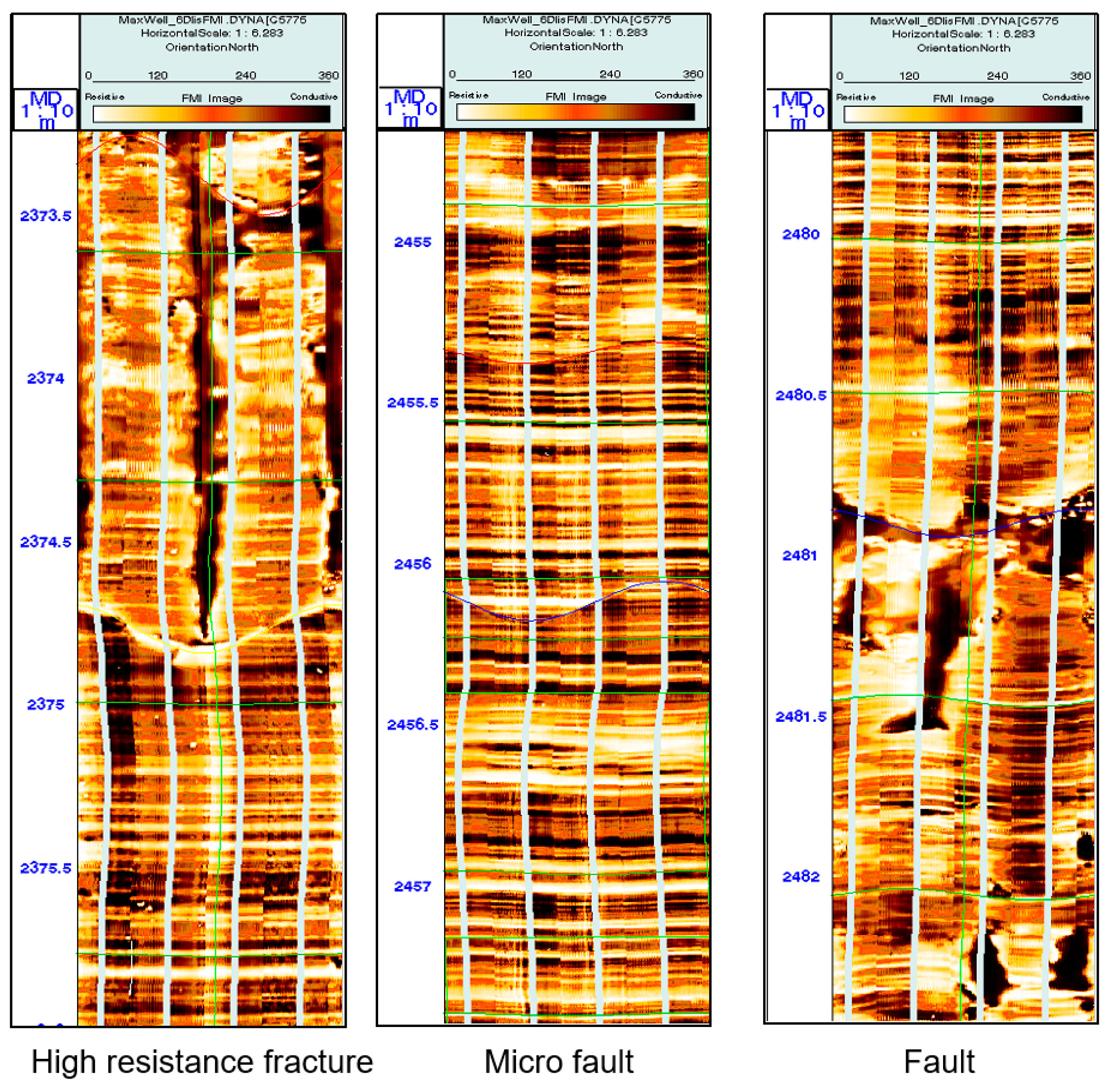
2.3. Downhole Imaging Logging Fracture Characteristics
3. Simulation of Shale CO2 Injection Considering Different Angles of Natural Fracture Filling
3.1. Experimental Conceptual Model
3.1.1. Geometric Model Setup
3.1.2. Initial Parameters of the Simulation Specimen and Fracture Parameters Setting
3.1.3. Core Pore Fluid Geochemical Parameters Setting
3.2. Engineering Parameters Acquisition
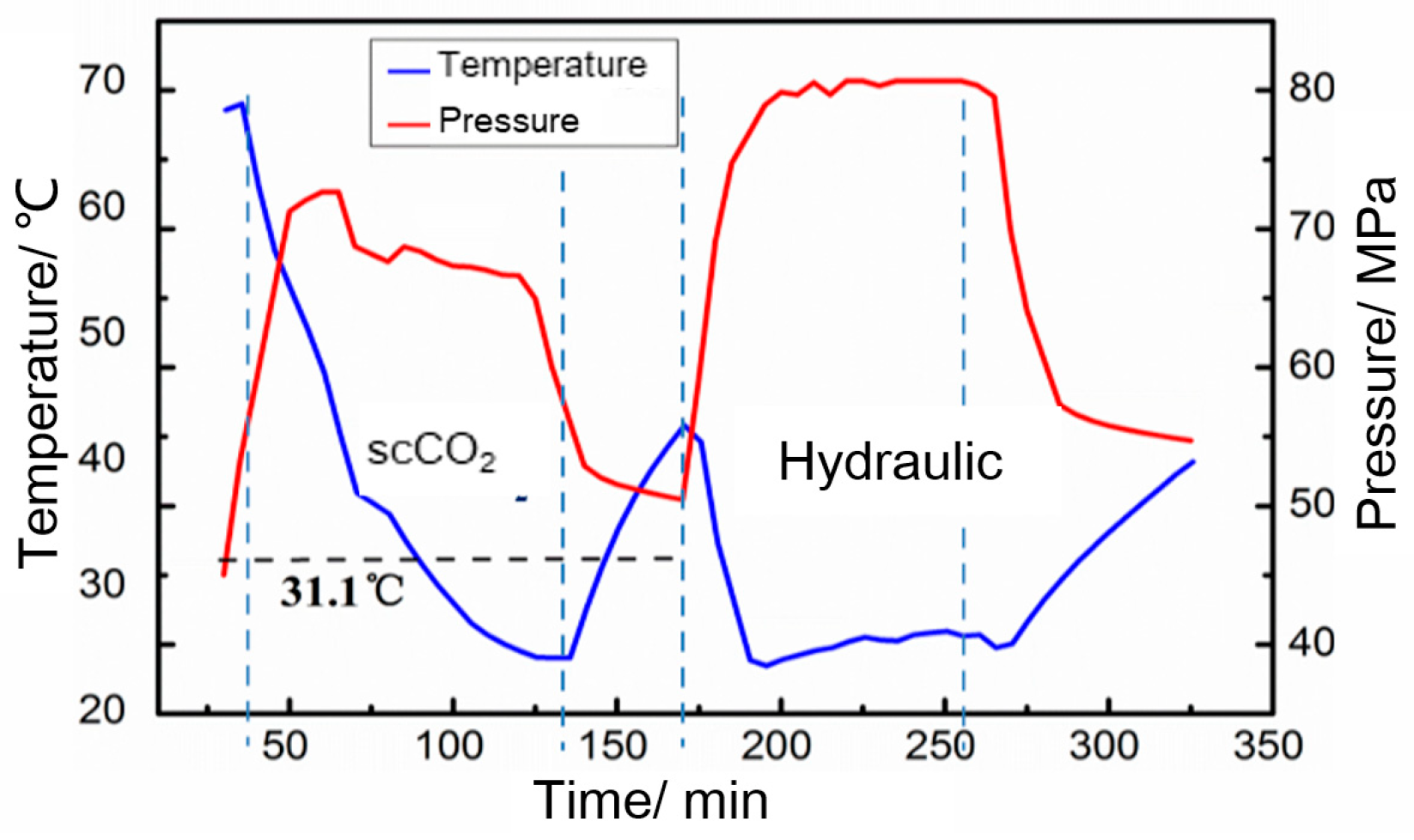
3.2.1. CO2 Fracturing Field Test to Obtain Bottomhole Temperature and Pressure
3.2.2. Simulated Temperature Conditions Setting
3.2.3. Simulated Pressure Conditions Setting
3.2.4. Simulation Calculation Time Setting
3.3. Simulation Scheme Design
3.4. Chemical Kinetic Simulation Software and Solution Methods
3.4.1. TOUGHREACT Software and Solution Process
3.4.2. Software Algorithm Implementation
3.4.3. Software Solution Process
- Multi-phase fluid flow
- Solution flow mass transfer
- Chemical reaction invocation
- Dissolution discrimination and iterative calculation
4. Study of Porosity of Fractured Shale under CO2 Conditions with Temperature and Pressure
4.1. Variation in Porosity with Temperature for Simulation Specimens with Different Natural Fracture Patterns
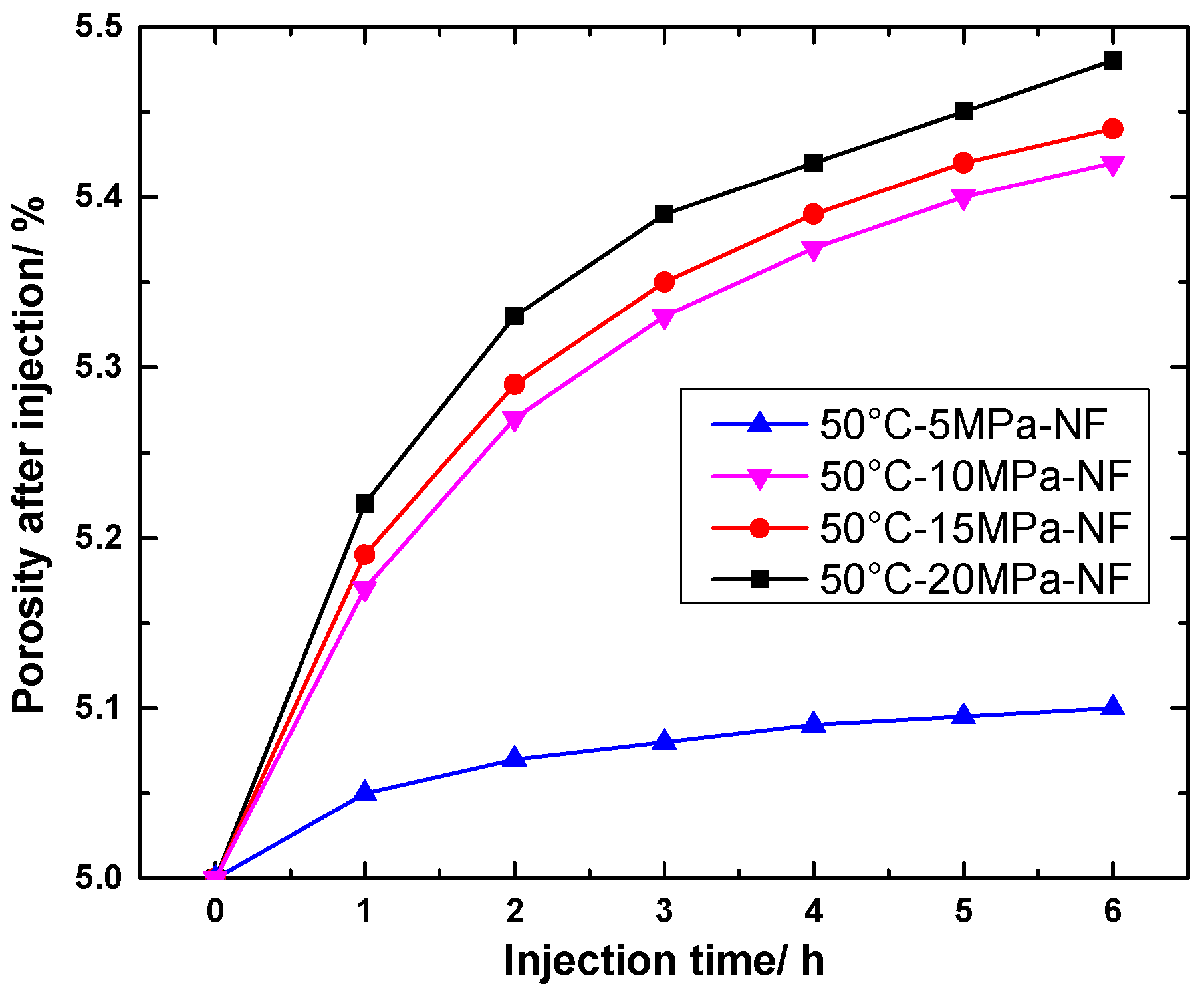
4.1.1. Change in Porosity of NF Specimens
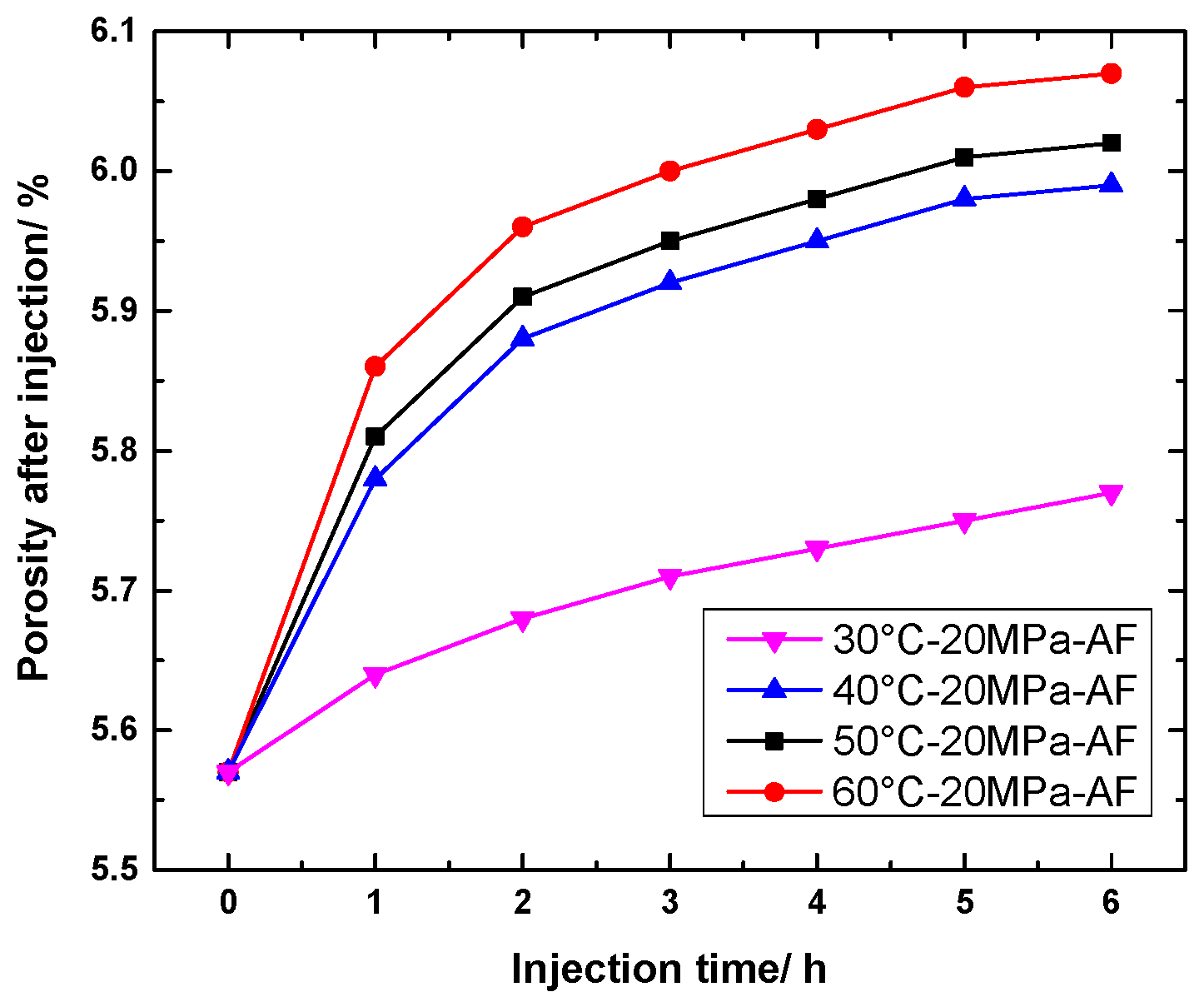
4.1.2. Change in Porosity of AF Specimen
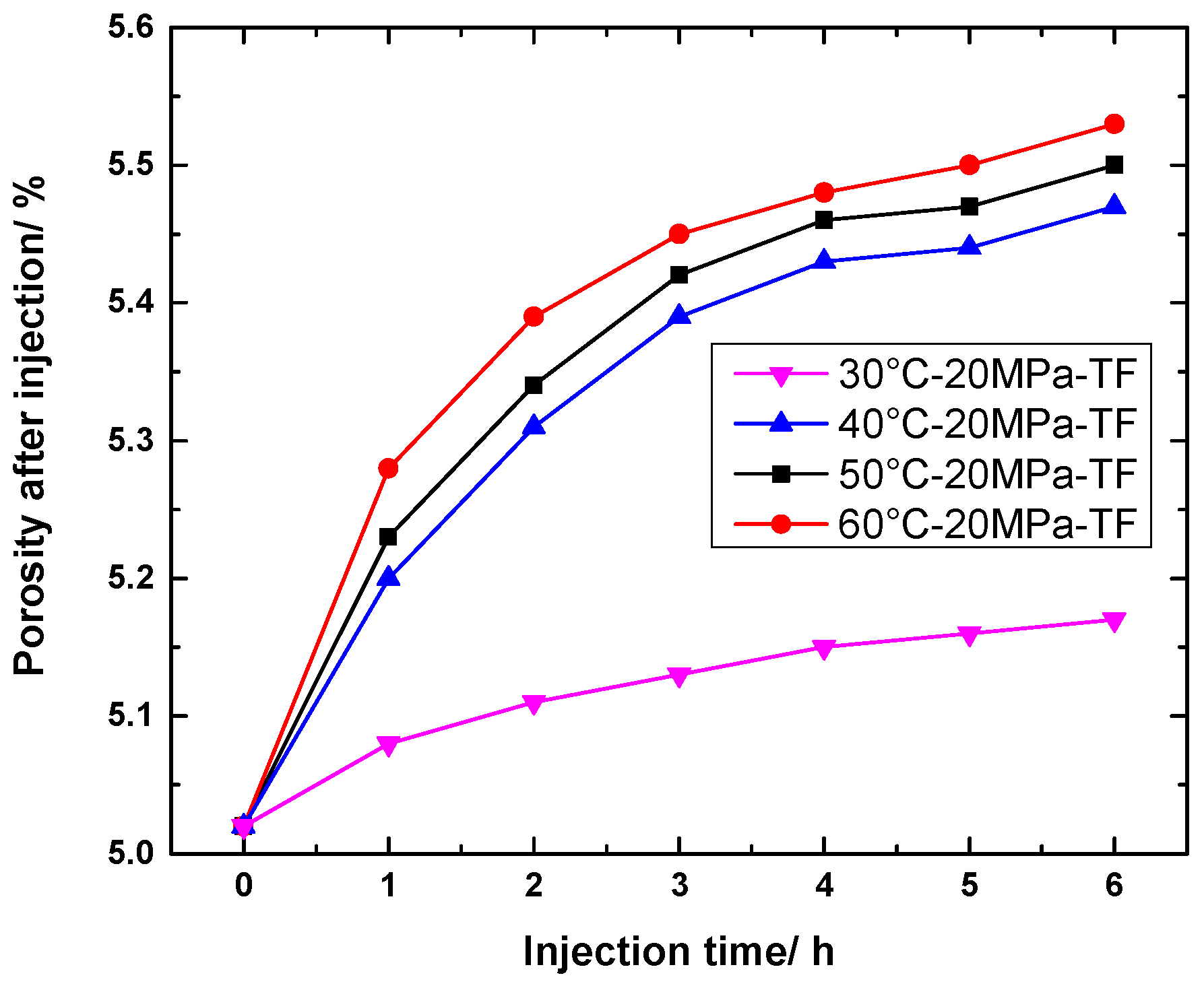
4.1.3. Change in Porosity of TF Specimens
4.1.4. Change in Relative Porosity Growth with Temperature
4.2. Variation in Porosity with Pressure for Specimens with Different Natural Fracture Patterns
4.2.1. Change in Porosity of NF Specimens
4.2.2. Change in Porosity of AF Specimens
4.2.3. Change in Porosity of TF Specimens
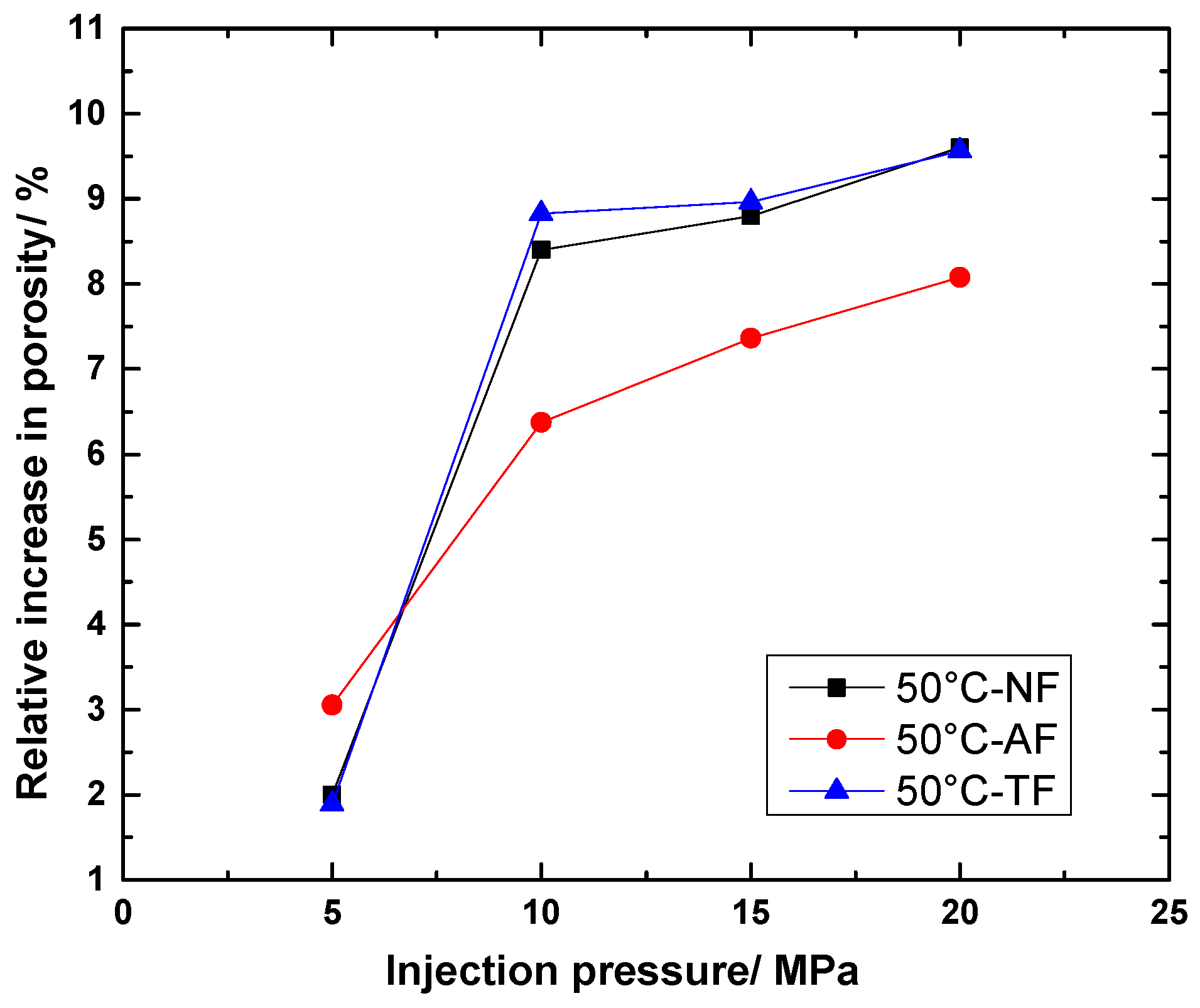
4.2.4. Change in Relative Porosity Growth with Pressure
5. Experimental Verification of Multi-Field Coupling of Supercritical CO2 with Shale
5.1. Experimental Samples and Scheme
5.2. Main Experimental Steps
5.2.1. Core Pretreatment and Initial Porosity Testing
- The cores were drilled and cut according to the relevant standards, dried and tested for basic geometric parameters, and small samples were kept for later scanning electron microscopy microscopic morphology testing (Figure 14).
- Considering the influence of thermal expansion of the specimen and the deformation of the rock skeleton and pore joints under the surrounding pressure and pore pressure on the measured porosity, the same conditions of external temperature, surrounding pressure and pore pressure as those of the scCO2 injection experiment were used to test the initial porosity before the experiment. The porosity of the cores under the design temperature, pore pressure and surrounding pressure was measured according to the petroleum industry standard “pulse decay method” as the initial porosity before the reaction using the pressure pulse measurement rock porosity device.
- Using the drying box, precision electronic scales, measuring cylinder, dropper and other devices, the formation water prepared was saturated according to Table 1 into the shale core; the size of the core water content saturation was adjusted by continuous drying, saturation, and weighing, so that it reached the design value (the simulation part of this paper is set to 40%); cling film was used to seal and wrap the water content specimens; conditions of no light and airtight storage were used; and then preparations were made for scCO2 long time flow experiments.
5.2.2. The scCO2 Injection Experiment
- The multi-field coupling experimental system and related basic experimental operation steps were adopted, the sealed specimen was installed to the specimen table inside the enclosure cylinder, and the fluid injection line and sensor line were connected inside the enclosure, as shown in Figure 15.
- In order to prevent scCO2 from flowing too fast and to ensure that the upstream and downstream pressures of the core are basically the same, a regulator was installed at the gas outlet end of the enclosing pressure chamber, and its limit pressure was set to the upstream injection pressure during the experiment, and with the upstream pressurization injection, the excess gas exceeding the limit pressure was discharged through the pressure relief port of the regulator. The reliability of the seal was tested after installation.
- Temperature, circumferential pressure and axial pressure was applied to the specimen until the required value according to the experimental plan, scCO2 was injected, and the injection experiment was officially begun.
- After completing the injection for 6 h, close the upstream injection device of the core was closed and the pressure relief valve was opened to drain the saturated scCO2 in the core. After completing the reduction of temperature and surrounding pressure, the surrounding pressure chamber was opened to remove the cores.
5.2.3. Testing the Porosity of the Injected Cores
5.2.4. Comparison of Microscopic Morphology before and after the Experiment
5.3. Experimental Results Analysis Verification
5.3.1. Porosity Comparison
5.3.2. Scanning Electron Microscopy Microscopic Morphology Verification
6. Conclusions
- Considering the field shale outcrop field study and core observation, it is found that high-angle natural fractures are mostly developed in the Longmaxi–Wufeng Formation shale in Sichuan, accounting for more than 60%; high-angle fractures are mostly seen in all calcite filled fractures; and calcite filled fractures account for more than 90% of the total number of filled fractures.
- The numerical simulation found that the porosity of shale gradually increased with the injection of CO2 under the same pressure (5, 10, 15, 20 MPa). Additionally, the higher the temperature, the more obvious the increase in porosity (from a 5% increase to more than 5.2%). However, when the temperature is lower than the critical point temperature, the porosity does not change obviously because the CO2 is no longer in the supercritical state and the reaction rate is low.
- The numerical simulation shows that the porosity of AF specimen grows faster than TF specimen and NF specimen in the same time under the same pressure and different temperatures, but the growth of porosity gradually slows down afterwards. The porosity change pattern of TF specimens is not different from that of NF. The reason is related to the dominant pooling effect of axial calcite fracture and the premature reaction with scCO2.
- Numerical simulations found that at the same temperature with different pressures, the effect of pressure variation on the porosity of specimens is more obvious than that of temperature. This is because high gas pressure can increase the concentration of reactants, while high temperature only increases the rate of reaction, but cannot change the degree of final reaction.
- Experimental verification revealed that the porosity of all three shale specimens under the same temperature and pressure increased after the CO2 injection flow, and the relative increase in porosity between the three samples measured experimentally was basically consistent with the numerical simulation results. Scanning electron microscopy (SEM) before and after the experiments showed that the microstructure of the shale was more severely damaged by scCO2 at a higher temperature and pressure, producing more tiny fragments and microfractures, while the microstructure of the specimens that did not reach the supercritical state condition did not change obviously before and after the CO2 injection; this verified the accuracy of the simulation results.
- In scCO2 fracturing construction, this conclusion can be used to optimize the pressure and time interval of CO2 injection, so that the CO2 can fully exchange heat with the formation to achieve the supercritical state and achieve better effect of shale gas production enhancement.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, H.; Tang, X.; Liu, Q.; Li, K.; Xiao, J.; Jiang, S.; McLennan, J.D. 4D multi-physical stress modelling during shale gas production: A case study of Sichuan Basin shale gas reservoir, China. J. Pet. Sci. Eng. 2018, 167, 929–943. [Google Scholar] [CrossRef]
- Zhu, H.; Tang, X.; Song, Y.; Li, K.; Xiao, J.; Dusseault, M.; McLennan, J.D. An infill well fracturing model and its microseismic events barrier effect: A case in fuling shale gas reservoir. SPE J. 2021, 26, 113–134. [Google Scholar] [CrossRef]
- Bennion, B.; Bachu, S. Relative permeability characteristics for supercritical CO2 displacing water in a variety of potential sequestration zones. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 9–12 October 2005. [Google Scholar] [CrossRef]
- Schremp, F.W.; Roberson, G.R. Effect of supercritical carbon dioxide (CO2) on construction materials. SPE J. 1975, 15, 227–233. [Google Scholar] [CrossRef]
- Raventós, M.; Duarte, S.; Alarcón, R. Application and Possibilities of Supercritical CO2 Extraction in Food Processing Industry: An Overview. Food Sci. Technol. Int. 2002, 8, 269–284. [Google Scholar] [CrossRef]
- Lashkarbolooki, M.; Vaferi, B.; Rahimpour, M.R. Comparison the capability of artificial neural network (ANN) and EOS for prediction of solid solubilities in supercritical carbon dioxide. Fluid Phase Equilibria 2011, 308, 35–43. [Google Scholar] [CrossRef]
- Rezaei, T.; Nazarpour, V.; Shahini, N.; Bahmani, S.; Shahkar, A.; Abdihaji, M.; Ahmadi, S.; Shahdost, F.T. A universal methodology for reliable predicting the non-steroidal anti-inflammatory drug solubility in supercritical carbon dioxide. Sci. Rep. 2022, 12, 1043. [Google Scholar] [CrossRef]
- Vaferi, B.; Karimi, M.; Azizi, M.; Esmaeili, H. Comparison between the artificial neural network, SAFT and PRSV approach in obtaining the solubility of solid aromatic compounds in supercritical carbon dioxide. J. Supercrit. Fluids 2013, 77, 44–51. [Google Scholar] [CrossRef]
- Middleton, R.S.; Carey, J.W.; Currier, R.P.; Hyman, J.D.; Kang, Q.; Karra, S.; Jiménez-Martínez, J.; Porter, M.L.; Viswanathan, H.S. Shale gas and non-aqueous fracturing fluids: Opportunities and challenges for supercritical CO2. Appl. Energy 2015, 147, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Tao, L.; Zhu, H.; Lei, Z.; Jiang, S.; McLennan, J.D. Macroscale mechanical and microscale structural changes in Chinese Wufeng shale with supercritical carbon dioxide fracturing. SPE J. 2018, 23, 691–703. [Google Scholar] [CrossRef]
- Xiao, T.; Dai, Z.; McPherson, B.; Viswanathan, H.; Jia, W. Reactive transport modeling of arsenic mobilization in shallow groundwater: Impacts of CO2 and brine leakage. Geomech. Geophys. Geo-Energy Geo-Resour. 2017, 3, 339–350. [Google Scholar] [CrossRef]
- Xiao, T.; McPherson, B.; Pan, F.; Esser, R.; Jia, W.; Bordelon, A.; Bacon, D. Potential chemical impacts of CO2 leakage on underground source of drinking water assessed by quantitative risk analysis. Int. J. Greenh. Gas Control. 2016, 50, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Xiao, T.; Kweon, H.; McPherson, B.; Deo, M. Wormhole Generations in Indiana limestone with CO2 intrusion: Numerical simulations based on core flooding experiments. Energy Fuels 2017, 31, 12487–12499. [Google Scholar] [CrossRef]
- Lahann, R.; Mastalerz, M.; Rupp, J.A.; Drobniak, A. Influence of CO2 on New Albany Shale composition and pore structure. Int. J. Coal Geol. 2013, 108, 2–9. [Google Scholar] [CrossRef]
- Alemu, B.L.; Aagaard, P.; Munz, I.A.; Skurtveit, E. Caprock interaction with CO2: A laboratory study of reactivity of shale with supercritical CO2 and brine. Appl. Geochem. 2011, 26, 1975–1989. [Google Scholar] [CrossRef]
- Wang, X.; Alvarado, V.; Swoboda-Colberg, N.; Kaszuba, J.P. Reactivity of dolomite in water-saturated supercritical carbon dioxide: Significance for carbon capture and storage and for enhanced oil and gas recovery. Energy Convers. Manag. 2013, 65, 564–573. [Google Scholar] [CrossRef]
- Wang, T.; Wang, H.; Zhang, F.; Xu, T. Simulation of CO2-water-rock interactions on geologic CO2 sequestration under geological conditions of China. Mar. Pollut. Bull. 2013, 76, 307–314. [Google Scholar] [CrossRef]
- Jung, H.B.; Um, W.; Cantrell, K.J. Effect of oxygen co-injected with carbon dioxide on Gothic shale caprock- CO2-brine interaction during geologic carbon sequestration. Chem. Geol. 2013, 354, 1–14. [Google Scholar] [CrossRef]
- Xu, T.; Apps, J.A.; Pruess, K. Mineral sequestration of carbon dioxide in a sandstone–shale system. Chem. Geol. 2005, 217, 295–318. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, L.; Tian, H. Reactive transport modeling for CO2 geological sequestration. J. Pet. Sci. Eng. 2011, 78, 765–777. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Pan, F.; Xu, T.; McPherson, B.J.; Yue, G.; Mandalaparty, P. Impacts of hydrological heterogeneities on caprock mineral alteration and containment of CO2 in geological storage sites. Int. J. Greenh. Gas Control. 2014, 24, 30–42. [Google Scholar] [CrossRef]
- Tian, H.; Xu, T.; Li, Y.; Yang, Z.; Wang, F. Evolution of sealing efficiency for CO2 geological storage due to mineral alteration within a hydrogeologically heterogeneous caprock. Appl. Geochem. 2015, 63, 380–397. [Google Scholar] [CrossRef]
- Gherardi, F.; Xu, T.; Pruess, K. Numerical modeling of self-limiting and self-enhancing caprock alteration induced by CO2 storage in a depleted gas reservoir. Chem. Geol. 2007, 244, 103–129. [Google Scholar] [CrossRef] [Green Version]
- Dalkhaa, C.; Shevalier, M.; Nightingale, M.; Mayer, B. 2-D reactive transport modeling of the fate of CO2 injected into a saline aquifer in the Wabamun Lake Area, Alberta, Canada. Appl. Geochem. 2013, 38, 10–23. [Google Scholar] [CrossRef]
- Emberley, S.; Hutcheon, I.; Shevalier, M.; Durocher, K.; Mayer, B.; Gunter, W.D.; Perkins, E.H. Monitoring of fluid–rock interaction and CO2 storage through produced fluid sampling at the Weyburn CO2-injection enhanced oil recovery site, Saskatchewan, Canada. Appl. Geochem. 2005, 20, 1131–1157. [Google Scholar] [CrossRef]
- Liu, F.; Lu, P.; Griffith, C.; Hedges, S.W.; Soong, Y.; Hellevang, H.; Zhu, C. CO2–brine–caprock interaction: Reactivity experiments on Eau Claire shale and a review of relevant literature. Int. J. Greenh. Gas Control. 2012, 7, 153–167. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Li, G.; Tian, S.; Wang, H.; Shen, Z.; Li, J. SEM analysis on rock failure mechanism by supercritical CO2 jet impingement. J. Pet. Sci. Eng. 2016, 146, 111–120. [Google Scholar] [CrossRef]
- Cai, C.; Kang, Y.; Wang, X.; Hu, Y.; Huang, M.; Liu, Y.; Liu, J.; Chen, H.; Li, X. Experimental study on shale fracturing enhancement by using multi-times pulse supercritical carbon dioxide (SC-CO2) jet. J. Pet. Sci. Eng. 2019, 178, 948–963. [Google Scholar] [CrossRef]
- Cai, C.; Kang, Y.; Yang, Y.; Wang, X.; Li, Y.; Huang, M.; Wu, J. The effect of shale bedding on supercritical CO2 jet fracturing: An experimental study. J. Pet. Sci. Eng. 2020, 195, 107798. [Google Scholar] [CrossRef]
- Okamoto, I.; Li, X.; Ohsumi, T. Effect of supercritical CO2 as the organic solvent on cap rock sealing performance for underground storage. Energy 2005, 30, 2344–2351. [Google Scholar] [CrossRef]
- Davila, G.; Cama, J.; Luquot, L.; Soler, J.M.; Ayora, C. Experimental and modeling study of the interaction between a crushed marl caprock and CO2-rich solutions under different pressure and temperature conditions. Chem. Geol. 2017, 448, 26–42. [Google Scholar] [CrossRef]
- Edlmann, K.; Haszeldine, S.; McDermott, C.I. Experimental investigation into the sealing capability of naturally fractured shale caprocks to supercritical carbon dioxide flow. Environ. Earth Sci. 2013, 70, 3393–3409. [Google Scholar] [CrossRef] [Green Version]
- Wollenweber, J.; Alles, S.; Busch, A.; Krooss, B.M.; Stanjek, H.; Littke, R. Experimental investigation of the CO2 sealing efficiency of caprocks. Int. J. Greenh. Gas Control. 2010, 4, 231–241. [Google Scholar] [CrossRef]
- Bourg, I.C.; Beckingham, L.E.; DePaolo, D.J. The nanoscale basis of CO2 trapping for geologic storage. Environ. Sci. Technol. 2015, 49, 10265–10284. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, J.; Jiang, Y.; Xian, X.; Liu, Q. Physical and structural changes in shale associated with supercritical CO2 exposure. Fuel 2016, 184, 289–303. [Google Scholar] [CrossRef]
- Zhou, J.; Yin, H.; Tan, J.; Jiang, Y.; Xian, X.; Liu, Q.; Ju, Y. Pore structural characterization of shales treated by sub-critical and supercritical CO2 exposure. J. Nanosci. Nanotechnol. 2017, 17, 6603–6613. [Google Scholar] [CrossRef]
- Zhu, H.; Tao, L.; Liu, Q.; Lei, Z.; Jiang, S.; McLennan, J.D. Fracture characteristics and change of permeability under the influence of natural fractures: Experimental study of Wufeng-Longmaxi shale. SPE Reserv. Eval. Eng. 2017, 21, 225–237. [Google Scholar] [CrossRef]
- Gale, J.F.W.; Reed, R.M.; Holder, J. Natural fractures in the Barnett Shale and their importance for hydraulic fracture treatments. Am. Assoc. Pet. Geol. Bull. 2007, 91, 603–622. [Google Scholar] [CrossRef]
- Wu, K. Pore Fracture Characterization of Typical Shale Gas Reservoirs in Eastern Sichuan; Southwest Petroleum University: Chengdu, China, 2015. (In Chinese) [Google Scholar]
- Evans, K.; Toth, R.; Tobi, O.; Jarrett, S.; Natalia, B.; Tim, C.; Ghahfarokhi, P. Fracture Analysis before and after hydraulic fracturing in the marcellus shale using the mohr-coulomb failure criteria. In Proceedings of the SPE/AAPG/SEG Unconventional Resources Technology Conference, Denver, CO, USA, 22–24 July 2019. [Google Scholar] [CrossRef]
- Dai, F. 2018 Shale Pores and Shale Gas Occurrence Mechanism of Longmaxi and Niutitang Formations in Eastern Sichuan-Western Hunan; China University of Geosciences: Wuhan, China, 2018. [Google Scholar]
- Wu, Y.; Fan, T.; Jiang, S.; Yang, X.; Ding, H.; Meng, M.; Wei, D. Methane adsorption capacities of the lower paleozoic marine shales in the Yangtze platform, South China. Energy Fuels 2015, 29, 4160–4167. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.L.; He, M.; Mei, X. Characteristics of Shale Gas Produced Water. Environ. Monit. Manag. Technol. 2018, 30, 50–53. (In Chinese) [Google Scholar]
- Hu, Y. Mechanism and Experimental Research of Supercritical Carbon Dioxide Fracturing Shale; Wuhan University: Wuhan, China, 2017. (In Chinese) [Google Scholar]
- Xu, T.; Sonnenthal, E.; Spycher, N.; Pruess, K. TOUGHREACT—A simulation program for non-isothermal multiphase reactive geochemical transport in variably saturated geologic media: Applications to geothermal injectivity and CO2 geological sequestration. Comput. Geosci. 2006, 32, 145–165. [Google Scholar] [CrossRef]
- Xu, T.; Feng, G.; Shi, Y. On fluid–rock chemical interaction in CO2-based geothermal systems. J. Geochem. Explor. 2014, 144, 179–193. [Google Scholar] [CrossRef]
- Plampin, M.R.; Neuzil, C.E. Multiphase Flow and Underpressured Shale at the Bruce Nuclear Site, Ontario, Canada; Geological Society, London, Special Publications: Bath, UK, 2018; SP482.5. [Google Scholar] [CrossRef] [Green Version]
- Oldenburg, C.M.; Law, D.H.-S.; LeGallo, Y.; White, S.P. Mixing of CO2 and CH4 in gas reservoirs: Code comparison studies. Greenh. Gas Control. Technol. 2003, 1, 443–448. [Google Scholar]
- Narasimhan, T.N.; Witherspoon, P.A. An integrated finite difference method for analyzing fluid flow in porous media. Water Resour. Res. 1976, 12, 57–64. [Google Scholar] [CrossRef] [Green Version]












| Test Type | A Area Test Result | C Area Test Result | Average Result |
|---|---|---|---|
| pH | 6.41~7.08 | 6.61~6.96 | 6.50~7.04 |
| K+ (mg/L) | 240~388 | 261~370 | 250~379 |
| Na+ (mg/L) | 10,800~21,300 | 10,800~12,900 | 10,800~17,100 |
| Ca2+ (mg/L) | 942~1458 | 1308~1970 | 1125~1714 |
| Mg2+ (mg/L) | 112~188 | 114~145 | 113~167 |
| Ba2+ (mg/L) | 1000~1346 | 128~148 | 564~747 |
| Sr2+ (mg/L) | 432~566 | 98~259 | 265~421 |
| Cl− (mg/L) | 18,500~36,800 | 16,200~27,700 | 17,350~32,250 |
| Group No. | Temperature/°C | Injection Pore Pressure/MPa | Remarks |
|---|---|---|---|
| 1 | 50 | 5 |
|
| 2 | 10 | ||
| 3 | 15 | ||
| 4 | 20 | ||
| 5 | 30 | ||
| 6 | 40 | ||
| 7 | 60 |
| No. | Specimen No. | Temperature/°C | Injection Pressure/MPa | External Pressure/MPa | Conditions | Remarks |
|---|---|---|---|---|---|---|
| 1 | 3–12 | 50 | 20 | 25 | Fracturing typical filed working conditions | |
| 2 | 3–13 | 40 | 20 | 25 | Typical working conditions in temperature change. | Comparison with typical working condition of group No. 1 |
| 3 | 3–14 | 50 | 5 | 10 | Not reaching the supercritical state | Compared with group No. 1 and No. 2 which reach the supercritical state |
| No. | Temperature/°C | Injection Pressure/MPa | Porosity at High Temperature and Pressure before Injection/% | Porosity at High Temperature and Pressure after Injection/% | Relative Increase in Final Porosity/% | Relative Increase in Porosity Obtained from Simulation/% |
|---|---|---|---|---|---|---|
| 1 | 50 | 20 | 3.16 | 3.36 | 6.2 | 9.6 |
| 2 | 40 | 20 | 3.19 | 3.38 | 5.8 | 9.2 |
| 3 | 50 | 5 | 3.42 | 3.46 | 1.2 | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, L.; Han, J.; Feng, Y.; McLennan, J.D. Study on the Alteration of Pore Parameters of Shale with Different Natural Fractures under Supercritical Carbon Dioxide Seepage. Minerals 2022, 12, 660. https://doi.org/10.3390/min12060660
Tao L, Han J, Feng Y, McLennan JD. Study on the Alteration of Pore Parameters of Shale with Different Natural Fractures under Supercritical Carbon Dioxide Seepage. Minerals. 2022; 12(6):660. https://doi.org/10.3390/min12060660
Chicago/Turabian StyleTao, Lei, Jian Han, Yanjun Feng, and John D. McLennan. 2022. "Study on the Alteration of Pore Parameters of Shale with Different Natural Fractures under Supercritical Carbon Dioxide Seepage" Minerals 12, no. 6: 660. https://doi.org/10.3390/min12060660
APA StyleTao, L., Han, J., Feng, Y., & McLennan, J. D. (2022). Study on the Alteration of Pore Parameters of Shale with Different Natural Fractures under Supercritical Carbon Dioxide Seepage. Minerals, 12(6), 660. https://doi.org/10.3390/min12060660







