Development of Ceramic Tiles from Philippine Nickel Laterite Mine Waste by Ceramic Casting Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation of the Nickel Laterite Mine Waste
2.2. Raw Material Characterization
2.3. Preparation of Slips
2.4. Production of Green Bodies and Sintered Test Bars
3. Results and Discussion
3.1. Raw Material Characterization
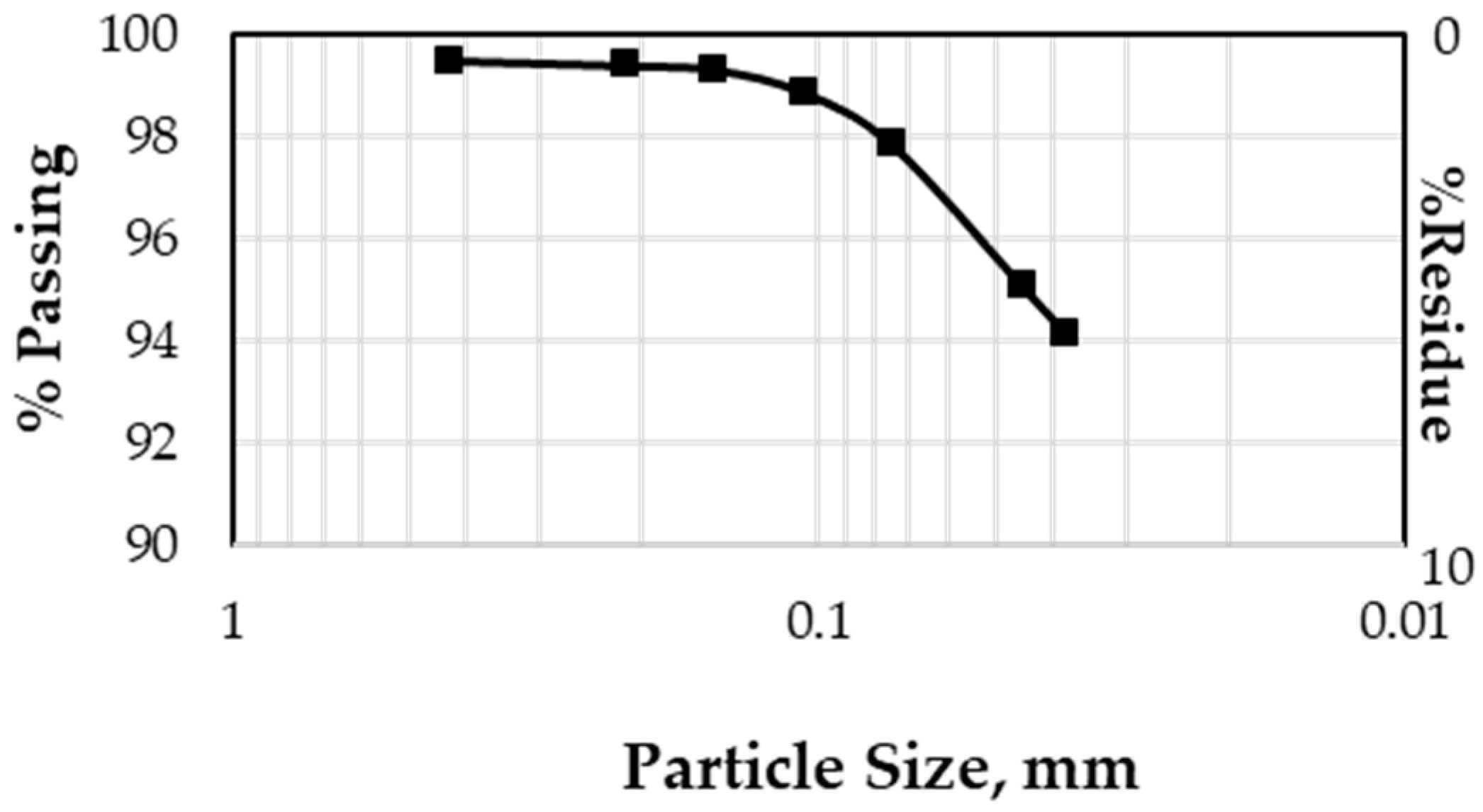
3.1.1. Particle Size Distribution
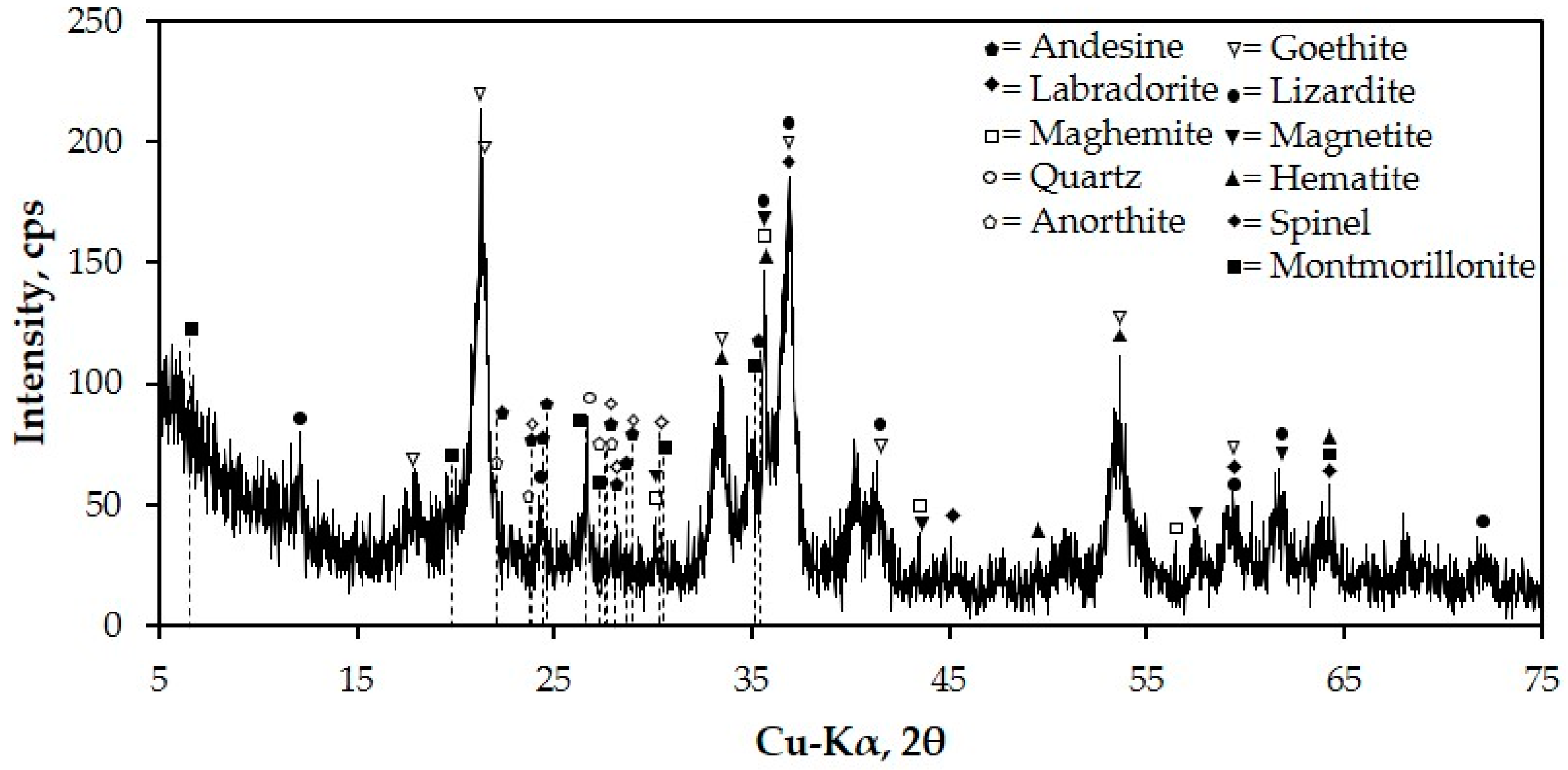
3.1.2. Chemical and Mineralogical Characterization of NMW
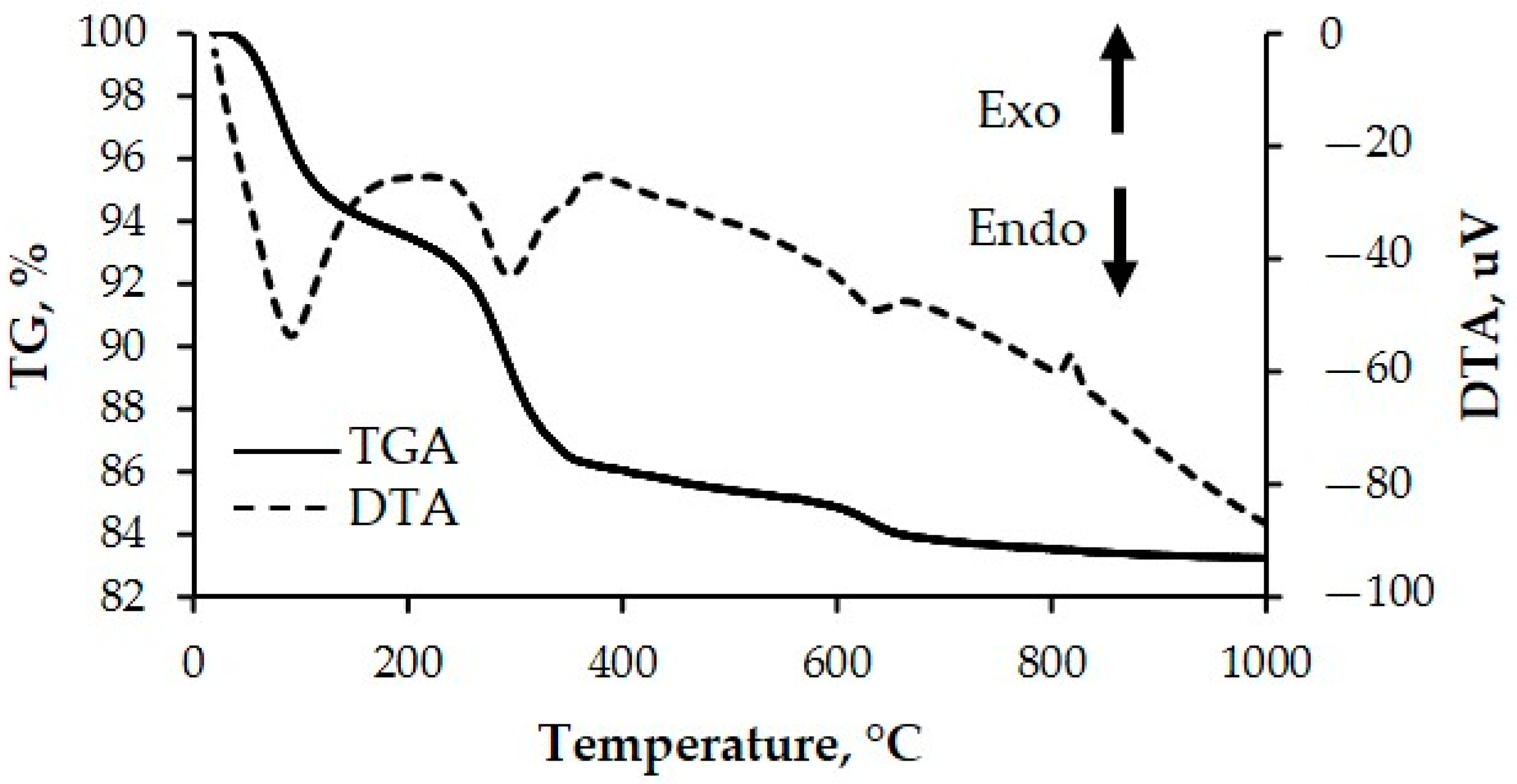
3.1.3. Thermal Stability Test of NMW
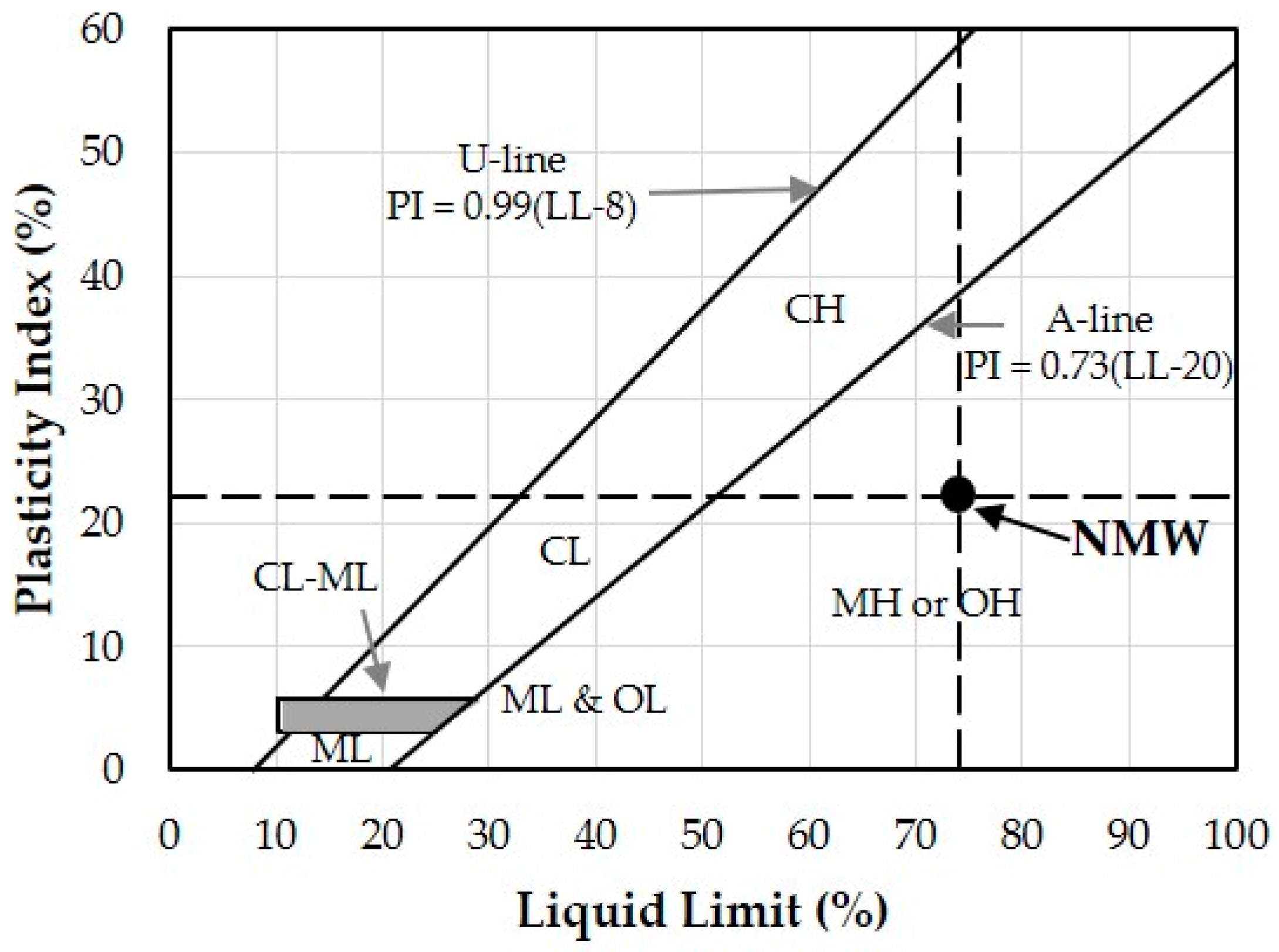
3.1.4. Atterberg Limits
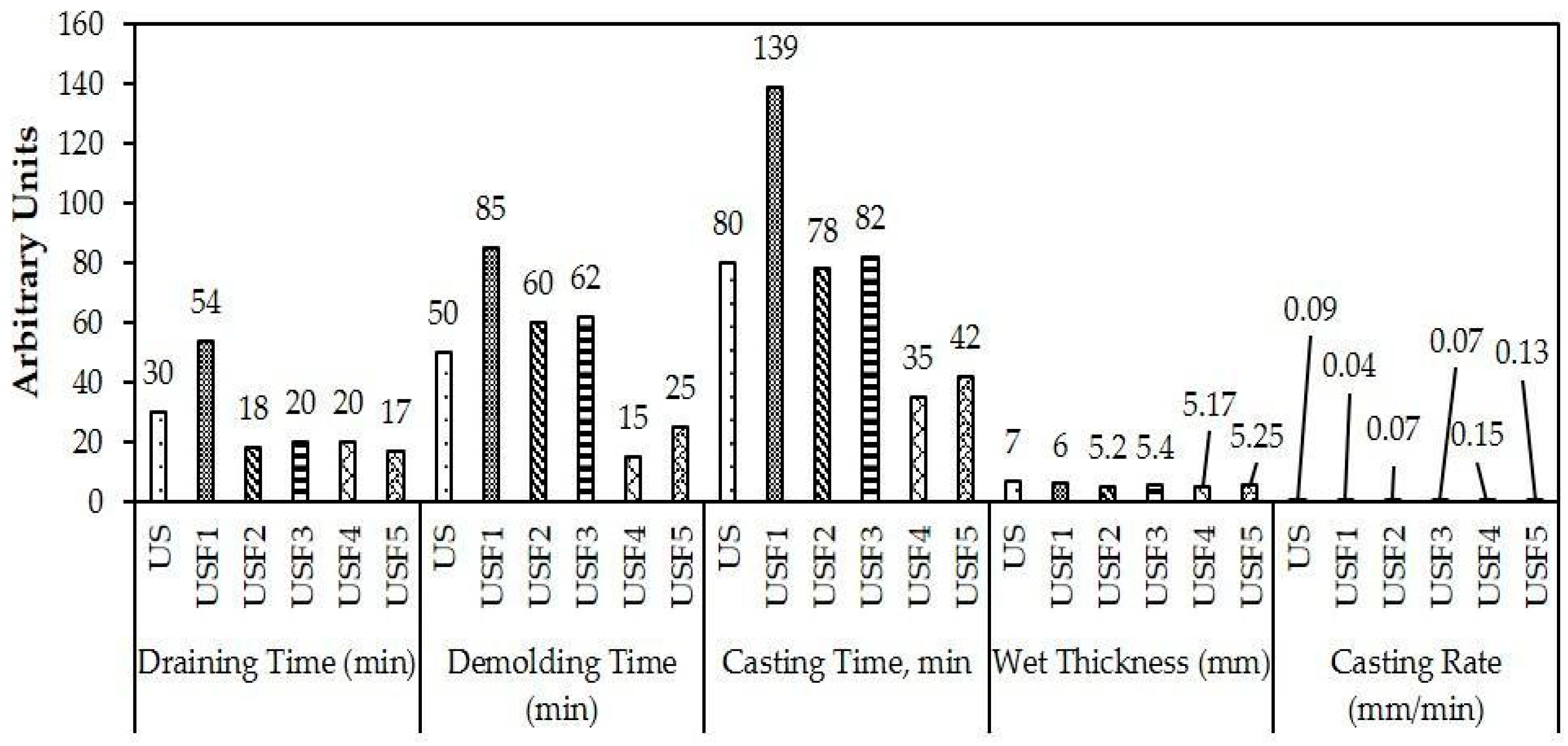
3.2. Characterization of the Formulated Slips
3.3. Physical and Mechanical Characterization of the Ceramic Tiles
3.4. Quality classification
4. Conclusions
- Nickel laterite mine waste is composed of very fine particles with 94.16% 38 micron particles.
- The chemical composition of NMW reveals that it has low alumina and silica contents but with high iron oxide content of 46.26%. The results coincide with XRD results wherein most of the minerals contain Fe such as goethite, maghemite, magnetite and hematite. It also contains phyllosilicates such as montmorillonite, lizardite and other silicates that have potential for slip casting production.
- NMW is classified as high plasticity clay with respect to its Atterberg limits, which is suitable for brick tile production.
- The viscosity of pure and formulated slips decreases with decreasing NMW content in the formulation with USF5 having the least viscosity. Among the formulations, USF4 showed stable viscosity along time. Casting properties showed that formulated slips have cast thickness ranging from 5 to 7 mm and, therefore, can cast a thick tile about 10–14 mm.
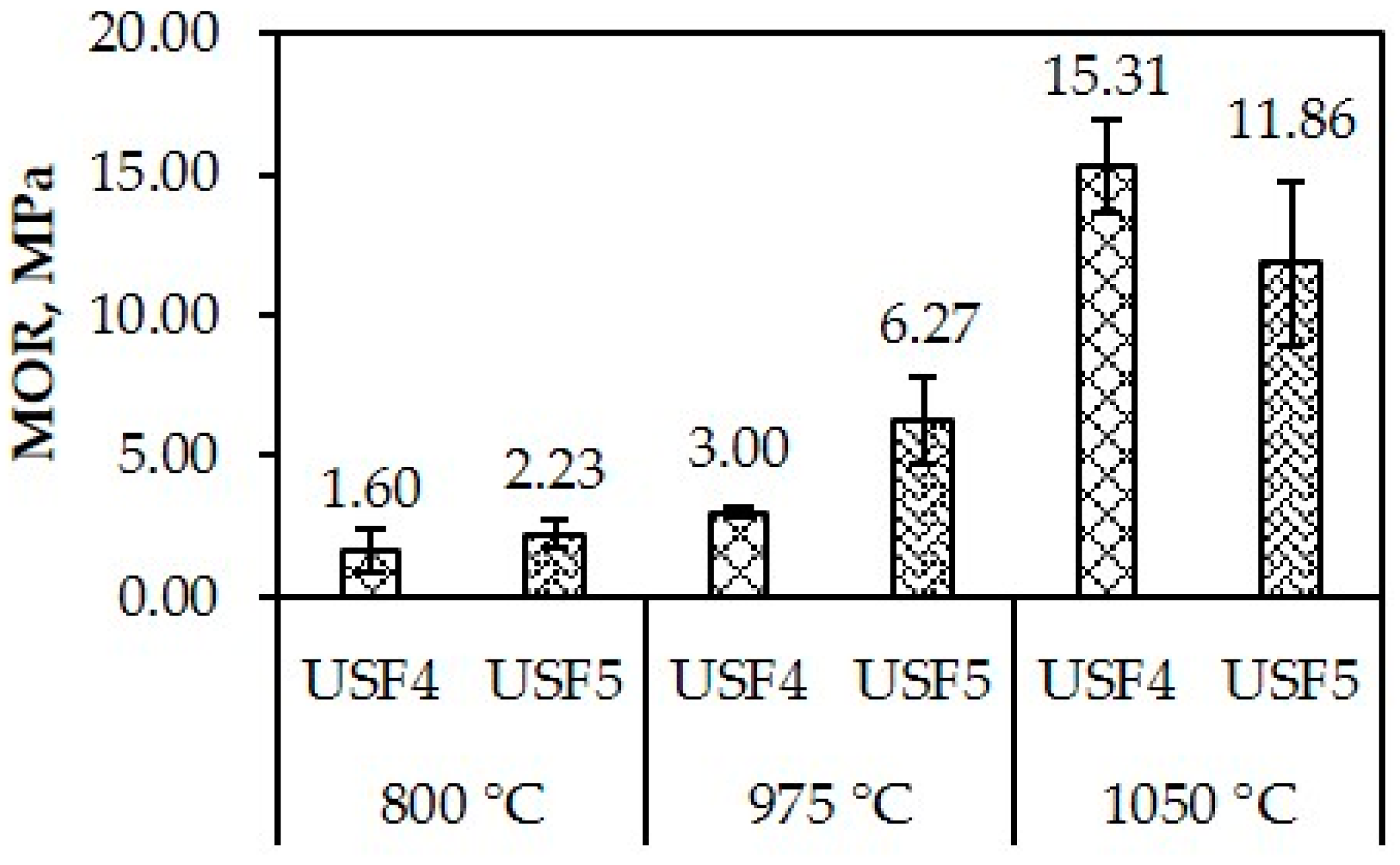
- Tiles were produced from USF4 and USF5 only. Physical properties showed total linear shrinkage, loss on ignition, water absorption and apparent porosity were generally low at low temperatures. Both USF4 and USF5 had initial vitrification at temperatures as early as 975 °C, which compacted the clay matrix at 1050 °C. USF4 and USF5 fired at 1050 °C had the highest MOR.
- Both USF4 and USF5 passed the CNS Type III water absorption requirement for floor tiles and ISO standard 13006 type AIII for water absorption for the three firing temperatures. Both USF4 and USF5 fired at 1050 °C passed the modulus of rupture for ISO standard 13006 type AIII and AIIb-2. At 1050 °C, only USF4 passed the PNS MOR requirement for wall tile, the ICCTAS MOR requirement for wall tile and ISO standard 130006 MOR requirement for type AIIa-2.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, I.; Kanazawa, Y.; Sato, N.; Galtchandmani, P.; Jha, M.K.; Tabelin, C.B.; Jeon, S.; Ito, M.; Hiroyoshi, N. Beneficiation of Low-Grade Rare Earth Ore from Khalzan Buregtei Deposit (Mongolia) by Magnetic Separation. Minerals 2021, 11, 1432. [Google Scholar] [CrossRef]
- Hannah Ritchie, M.R. CO2 and Greenhouse Gas Emissions. 2017. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 21 March 2022).
- Tabelin, C.B.; Park, I.; Phengsaart, T.; Sanghee, J.; Villacorte-Tabelin, M.; Alonzo, D.; Yoo, K.; Ito, M.; Hiroyoshi, N. Copper and Critical Metals Production from Porphyry Ores and E-Wastes: A Review of Resource Availability, Processing/Recycling Challenges, Socio-Environmental Aspects, and Sustainability Issues. Resour. Conserv. Recycl. 2021, 170, 105610. [Google Scholar] [CrossRef]
- Hund, K.; La Porta, D.; Fabregas, T.P.; Laing, T.; Drexhage, J. Minerals for Climate Action: The Mineral Intensity of the Clean Energy Transition. World Bank. 2020. Available online: https://pubdocs.worldbank.org/en/961711588875536384/Minerals-for-Climate-Action-The-Mineral-Intensity-of-the-Clean-Energy-Transition.pdf (accessed on 21 March 2022).
- Balanay, R.M.; Halog, A. Promoting life cycle thinking for sustainability in the mining sector of the Philippines. Int. J. Life Cycle Assess. 2016, 22, 1864–1874. [Google Scholar] [CrossRef]
- Mineral Commodity Summaries; USGS Unnumbered Series; US Geological Survey: Reston, VA, USA, 2021. Available online: https://pubs.usgs.gov/periodicals/mcs2021/mcs2021.pdf (accessed on 21 March 2022).
- Aquino, K.A.; Arcilla, C.A.; Schardt, C.; Tupaz, C.A.J. Mineralogical and Geochemical Characterization of the Sta. Cruz Nickel Laterite Deposit, Zambales, Philippines. Minerals 2022, 12, 305. [Google Scholar] [CrossRef]
- Tupaz, C.A.J.; Watanabe, Y.; Sanematsu, K.; Echigo, T.; Arcilla, C.; Ferrer, C. Ni-Co Mineralization in the Intex Laterite Deposit, Mindoro, Philippines. Minerals 2020, 10, 579. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S.; Canbulat, I. Towards a Low-Carbon Society: A Review of Lithium Resource Availability, Challenges and Innovations in Mining, Extraction and Recycling, and Future Perspectives. Miner. Eng. 2020, 163, 106743. [Google Scholar] [CrossRef]
- Mines and Geoscience Bureau. Mineral Industry at a Glance. Mineral Statistics. 2020. Available online: https://region10.mgb.gov.ph/wp-content/uploads/2021/06/Mineral-Industry-at-a-Glance-2020.pdf (accessed on 21 March 2022).
- Senoro, D.B.; Bonifacio, P.B.; Mascareñas, D.R.; Tabelin, C.B.; Ney, F.P.; Lamac, M.R.L.; Tan, F.J. Spatial distribution of agricultural yields with elevated metal concentration of the island exposed to acid mine drainage. J. Degraded Min. Lands Manag. 2020, 8, 2551–2558. [Google Scholar] [CrossRef]
- Tomiyama, S.; Igarashi, T.; Baltazar, C.; Tangviroon, T.P.; Ii, H. Acid Mine Drainage Sources and Hydrogeochemistry at the Yatani Mine, Yamagata, Japan: A Geochemical and Isotopic Study. J. Contam. Hydrol. 2019, 225, 103502. [Google Scholar] [CrossRef]
- Delina, R.E.; Arcilla, C.; Otake, T.; Garcia, J.J.; Tan, M.; Ito, A. Chromium occurrence in a nickel laterite profile and its implications to surrounding surface waters. Chem. Geol. 2020, 558, 119863. [Google Scholar] [CrossRef]
- Promentilla, M.A.B.; Beltran, A.B.; Orbecido, A.H.; Bernardo-Arugay, I.; Resabal, V.J.; Villacorte-Tabelin, M.; Dalona, I.M.; Opiso, E.; Alloro, R.; Alonzo, D. Systems Approach toward a Greener Eco-Efficient Mineral Extraction and Sustainable Land Use Management in the Philippines. Chem. Eng. Trans. 2021, 88, 1171–1176. [Google Scholar]
- Nickel, E.H.; Nichols, M.C. Mineral Database; MDI Minerals Data: Livermore, CA, USA, 2007. [Google Scholar]
- Brindley, G.; Brown, G. Crystal Structures of Clay Minerals and Their X-Ray Identification; The Mineralogical Society of Great Britain and Ireland: London, UK, 1982; Volume 5. [Google Scholar]
- Spears, D.A.B. Velde Introduction to Clay Minerals. Chemistry, Origins, Uses and Environmental Significance. Chapman & Hall, London, 1992. Vii+ 198 Pp. Price£ 14.95 Isbn: 0.412. 37030.1. Clay Miner. 1993, 28, 161–162. [Google Scholar]
- ASTM D4318-10; Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. ASTM International: West Conshohocken, PA, USA, 2010.
- Glendinning, S.; Jones, C.J.; Lamont-Black, J. The Use of Electrokinetic Geosynthetics to Improve Soft Soils. In Ground Improvement Case Histories; Elsevier: Amsterdam, The Netherlands, 2015; pp. 403–452. [Google Scholar]
- Torres, P.; Manjate, R.S.; Fernandes, H.R.; Olhero, S.M.; Ferreira, J.M.F. Incorporation of River Silt in Ceramic Tiles and Bricks. Ind. Ceram. 2009, 29, 1–8. [Google Scholar]
- Ma, B.; Li, Y.; Liu, G.; Liang, D. Preparation and properties of Al2O3–MgAl2O4 ceramic foams. Ceram. Int. 2015, 41, 3237–3244. [Google Scholar] [CrossRef]
- Fischer, J.; Stawarczyk, B.; Hämmerle, C.H.F. Flexural Strength of Veneering Ceramics for Zirconia. J. Dent. 2008, 36, 316–321. [Google Scholar] [CrossRef]
- Ryan, W.; Radford, C. Whitewares Production, Testing, and Quality Control: Including Materials, Body Formulations, and Manufacturing Processes; Pergamon: London, UK, 1987; pp. 200–203. ISSN 0073-9022. [Google Scholar]
- Canillo, S.; Cortez, C.; Bernardo-Arugay, I. Design and Production of Buchner Funnel Using Maria Cristina Clay by Slip Casting Method. In Proceedings of the METCON 2017, Batac, Philippines, 26 November 2017. [Google Scholar]
- Bernardo, I.C.; Diamante, J.C.; Lanticse-Diaz, L.J. Characterization of Philippine Clay. In Proceedings of the AUNSEED-Net METCON 2011, Olongapo City, Philippines, 27–28 October 2011. [Google Scholar]
- Longos, A.; Tigue, A.; Dollente, I.; Malenab, R.; Bernardo-Arugay, I.; Hinode, H.; Kurniawan, W.; Promentilla, M. Optimization of the Mix Formulation of Geopolymer Using Nickel-Laterite Mine Waste and Coal Fly Ash. Minerals 2020, 10, 1144. [Google Scholar] [CrossRef]
- Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B.; Fukushima, T.; Park, I.; Jeon, S.; Igarashi, T.; Sato, T.; Nyambe, I. Detoxification of Lead-Bearing Zinc Plant Leach Residues from Kabwe, Zambia by Coupled Extraction-Cementation Method. J. Environ. Chem. Eng. 2020, 8, 104197. [Google Scholar] [CrossRef]
- Opiso, E.M.; Tabelin, C.B.; Maestre, C.V.; Aseniero, J.P.J.; Park, I.; Villacorte-Tabelin, M. Synthesis and characterization of coal fly ash and palm oil fuel ash modified artisanal and small-scale gold mine (ASGM) tailings based geopolymer using sugar mill lime sludge as Ca-based activator. Heliyon 2021, 7, e06654. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Silwamba, M.; Paglinawan, F.C.; Mondejar, A.J.S.; Duc, H.G.; Resabal, V.J.; Opiso, E.M.; Igarashi, T.; Tomiyama, S.; Ito, M.; et al. Solid-phase partitioning and release-retention mechanisms of copper, lead, zinc and arsenic in soils impacted by artisanal and small-scale gold mining (ASGM) activities. Chemosphere 2020, 260, 127574. [Google Scholar] [CrossRef] [PubMed]
- Rizov, B. Phase Transformations from Goethite to Hematite and Thermal Decomposition in Various Nickeliferous Laterite Ores. J. Univ. Chem. Technol. Metall. 2012, 47, 207–210. [Google Scholar]
- Lv, X.; Bai, C.; He, S.; Huang, Q. Mineral Change of Philippine and Indonesia Nickel Lateritic Ore during Sintering and Mineralogy of Their Sinter. ISIJ Int. 2010, 50, 380–385. [Google Scholar] [CrossRef]
- Huang, Q.; Lv, X. Phases transformation of nickel lateritic ore during dehydration. J. Min. Met. Sect. B Met. 2011, 47, 45–51. [Google Scholar] [CrossRef]
- Khanlari, G.R.; Namazi, A.; Abdi Lor, Y. Assessment of Engineering Properties of Clay Soils as Brick and Tiles Materials in Hamedan, West of Iran. In Proceedings of the 10th International Congress for Applied Mineralogy (ICAM); Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Budhu, M. Soil Mechanics and Foundations; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- ASTM D2487-11; Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM International: West Conshohocken, PA, USA, 2011.
- Balmforth, N.J.; Provenzale, A. Geomorphological Fluid Mechanics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2001; Volume 582. [Google Scholar]
- Kreirzti, L.K.; Benamara, L.; Boudjenane, N.-E. Valorization of dredging sediments of dam BOUHNIFIA in ceramic. J. Aust. Ceram. Soc. 2019, 55, 1081–1089. [Google Scholar] [CrossRef]
- Grim, R.E. Applied Clay Mineralogy; Mcgraw Hill Book Coy. Inc.: New York, NY, USA, 1962; pp. 37–43. [Google Scholar]
- Baccour, H.; Medhioub, M.; Jamoussi, F.; Mhiri, T. Densification Behaviour of a Red Firing Tunisian Triassic Clay. Am. J. Appl. Sci. 2008, 5, 263–269. [Google Scholar] [CrossRef][Green Version]
- Worrall, W.E. Ceramic Raw Materials, 2nd ed.; Institute of Ceramics Textbook Series; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Department of Trade and Industry. Philippine National Standard. Available online: http://www.puntofocal.gov.ar/notific_otros_miembros/phl60_t.pdf (accessed on 21 March 2022).
- Lin, D.-F.; Wang, W.-J.; Chen, C.-W.; Lin, K.-L. Applying Mixture of Municipal Incinerator Bottom Ash and Sewage Sludge Ash for Ceramic Tile Manufacturing. Materials 2021, 14, 3863. [Google Scholar] [CrossRef] [PubMed]
- Indian Council of Ceramic Tiles and Sanitaryware—ICCTAS Magazine. 2019. Available online: http://www.icctas.com/pdf/ICTAS%20NOV%20-19.pdf (accessed on 21 March 2022).



| Mass % | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | NiO | Cr2O3 | MnO | TiO2 | Na2O | K2O | SrO | SO3 | P2O5 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMW | 24.34 | 9.20 | 46.26 | 15.10 | 0.71 | 1.47 | 2.14 | 0.72 | 0.05 | — | |||||
| San Nicholas clay [25] | 53.7 | 16.9 | 12.9 | 4.72 | 1.79 | — | — | 0.12 | 1.09 | 1.8 | 0.35 | — | 0.09 | 6.61 | |
| Ma. Cristina Red Clay [24] | 29 | 18 | 46.33 | — | 1.38 | 0.06 | 0.13 | 0.62 | 3.13 | — | 0.08 | 0.05 | 0.6 | — | — |
| Feldspar | 60.65 | 12.59 | 0.31 | 5.16 | 21.12 | 0.02 | 0.15 | — | — | — | — |
| Mineral ID | Chemical Formula |
|---|---|
| Goethite | Fe3+O(OH) |
| Lizardite | Mg3Si2O5(OH)4 |
| Spinel | MgAl2O4 |
| Quartz | SiO2 |
| Magnetite | Fe3O4 |
| Maghemite | γ-Fe2O3 |
| Hematite | Fe2O3 |
| Montmorillonite | (Na, Ca)0.3 (Al, Mg)2 Si4 O10 (OH)2 • n H2O |
| Andesine | (Na, Ca) (Si, Al)4 O8 |
| Labradorite | (Ca, Na)(Si, Al)4 O8 |
| Anorthite | CaAl2Si2O8 |
| Code | Type of Soil | PI | LL | Position (Basis: “A” Line) |
|---|---|---|---|---|
| CL-ML | Silty clay | 4–7 | On or above | |
| ML | Silt | <50 | Below | |
| MH | Elastic Silt | ≥50 | Below | |
| OL | Organic clay | ≥4 | <50 | On or above |
| Organic silt | <4 | Below | ||
| OH | Organic clay | ≥50 | On or above | |
| Organic silt | Below |
| Formulation | Empirical Formula |
|---|---|
| USF1 | 4.11 MgO• 0.18 CaO• Al2O3• 4.55 SiO2• 3.17 Fe2O3 |
| USF2 | 4.03 MgO• 0.26 CaO• Al2O3• 4.65 SiO2• 3.08 Fe2O3 |
| USF3 | 3.40 MgO• 0.85 CaO• Al2O3• 5.39 SiO2• 2.44 Fe2O3 |
| USF4 | 2.77 MgO• 1.44 CaO• Al2O3• 6.14 SiO2• 1.79 Fe2O3 |
| USF5 | 1.97 MgO• 2.18 CaO• Al2O3• 7.08 SiO2• 0.98 Fe2O3 |
| Formulation | Temperature | Judgement Criteria | ||
|---|---|---|---|---|
| NMW Tile | ||||
| Modulus of Rupture, MPa | Water Absorption, % | Remarks | ||
| USF4 | 800 °C | 2.23 ± 0.51 | 35.04 ± 0.32 |   |
| 975 °C | 3.00 ± 0.18 | 35.27 ± 0.08 |   | |
| 1050 °C | 15.31± 1.66 | 21.61 ± 0.34 |        | |
| USF5 | 800 °C | 1.60 ± 0.77 | 37.15 ± 0.71 |   |
| 975 °C | 6.27 ± 1.52 | 27.81± 0.48 |   | |
| 1050 °C | 11.86 ± 2.92 | 23.60 ± 0.99 |     | |
 —Water absorption passed for CNS Type III floor tile.
—Water absorption passed for CNS Type III floor tile.  —Modulus of Rupture passed for ICCTAS wall tile.
—Modulus of Rupture passed for ICCTAS wall tile.  —Modulus of Rupture passed for PNS wall tile.
—Modulus of Rupture passed for PNS wall tile.  —Water absoprtion passed for ISO Standard 13006 AIII.
—Water absoprtion passed for ISO Standard 13006 AIII.  —Modulus of Rupture passed for ISO Standard 13006 AIII.
—Modulus of Rupture passed for ISO Standard 13006 AIII.  —Modulus of Rupture passed for ISO Standard 13006 AIIa-2.
—Modulus of Rupture passed for ISO Standard 13006 AIIa-2.  —Modulus of Rupture passed for ISO Standard 13006 AIIb-2.
—Modulus of Rupture passed for ISO Standard 13006 AIIb-2. Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardo-Arugay, I.C.; Echavez, F.J.A.; Aquiatan, R.H.L.; Tabelin, C.B.; Virtudazo, R.V.R.; Resabal, V.J.T. Development of Ceramic Tiles from Philippine Nickel Laterite Mine Waste by Ceramic Casting Method. Minerals 2022, 12, 579. https://doi.org/10.3390/min12050579
Bernardo-Arugay IC, Echavez FJA, Aquiatan RHL, Tabelin CB, Virtudazo RVR, Resabal VJT. Development of Ceramic Tiles from Philippine Nickel Laterite Mine Waste by Ceramic Casting Method. Minerals. 2022; 12(5):579. https://doi.org/10.3390/min12050579
Chicago/Turabian StyleBernardo-Arugay, Ivyleen C., Fel Jane A. Echavez, Rae Homer L. Aquiatan, Carlito B. Tabelin, Raymond V. Rivera Virtudazo, and Vannie Joy T. Resabal. 2022. "Development of Ceramic Tiles from Philippine Nickel Laterite Mine Waste by Ceramic Casting Method" Minerals 12, no. 5: 579. https://doi.org/10.3390/min12050579
APA StyleBernardo-Arugay, I. C., Echavez, F. J. A., Aquiatan, R. H. L., Tabelin, C. B., Virtudazo, R. V. R., & Resabal, V. J. T. (2022). Development of Ceramic Tiles from Philippine Nickel Laterite Mine Waste by Ceramic Casting Method. Minerals, 12(5), 579. https://doi.org/10.3390/min12050579








