Abstract
The synthesis and characterization of a new aluminophosphate, Na2Al2O(PO4)2·0.12H2O obtained as single crystals, is reported. Centrosymmetric tetramers built from AlO5 polyhedra sharing edges and vertices, represent the distinguished feature of the compound. These tetrameric units of AlO5 bipyramids are cross-linked by PO4 tetrahedra to form two-periodic slabs alternating with Na+ ions and a small amount of H2O molecules. The Na2Al2O(PO4)2·0.12H2O with an original crystal architecture is chemically and structurally related to the mineral tinsleyite, KAl2(PO4)2(OH)·2H2O. Similar clusters of Al-centered polyhedra are essential building blocks of both monoclinic structures. The main difference between them consists of the type of the Al coordination by O atoms: in tinsleyite, the clusters are designed from AlO4(OH)2 and AlO4(OH)(H2O) octahedra. In both cases, alkali Na or K atoms significantly distinct in size, act as structure regulating agents, determining the character of the developing crystal architecture. The flexibility of aluminophosphate constructions allows them to self-organize around structure-forming Na+ or K+ ions into anionic layers in Na2Al2O(PO4)2·0.12H2O or a framework (tinsleyite). The synthesis of sodium aluminophosphate under mild hydrothermal conditions and the topological resemblance of its structure with that of the mineral tinsleyite suggest a high probability of a mineral equivalent of the Na2Al2O(PO4)2·0.12H2O in nature.
1. Introduction
Most rocks are formed by oxosalts, minerals containing complex anions, including phosphate anions, crystallized under the conditions of a lithophilic geochemical system. The latter is characterized by an excess of oxygen in it, both in the bound forms, and in the free forms of O3, O2−, O2, etc. Rocks, as a rule, contain different types of water—from hydroxyl and crystallization in minerals to capillary, etc. It is with the active participation of water in rocks that various processes of their transformations occur. Statistical data on the chemical composition of natural oxosalts show that most of them are typical metals with amphoteric properties. These are, first of all, iron and aluminum, as well as manganese, copper, beryllium, zirconium, and some others. In high-alkaline medium- and low-temperature natural systems (late pegmatites and hydrothermalites), complexes of these elements often play the role of anion formers, creating, together with acid groups, anionic structures of a mixed crystal chemical nature, for example, aluminophosphate. Under these conditions, zeolite-like macro- and microporous minerals are quite common, which have the properties of sorbents, molecular sieves, ion exchangers, etc. Simulation of such environments in the laboratory and subsequent detailed study of synthetic analogs of minerals allows, on the one hand, to trace the regular relationships between the physicochemical parameters of the crystallization system and the specific features of the crystal structures of the forming phases (structural typomorphism), and, on the other hand, to obtain new compounds with promising properties.
The mineral tinsleyite, KAl2(PO4)2(OH)·2H2O is a member of the leucophosphite group, with the general formula AB2(PO4)2(OH)·2H2O (A = NH4, K; B = Al, Fe). This mineral group apart from leucophosphite, KFe2[PO4]2(OH)·2H2O [1] and tinsleyite, also includes sphenscidite, NH4Fe2(PO4)2(OH)·2H2O [2] and ammoniotinsleyite, (NH4)2Al2(PO4)2(OH)2·2H2O [3]. The above-mentioned species are known to occur in two different paragenetic associations: late products of the hydrothermal alteration of primary phosphates in granitic pegmatites, or biominerals. Tinsleyite was first described from the Tip Top granitic pegmatite, in South Dakota. It is found in pods of highly altered triphylite in the intermediate zone of the pegmatite in association with leucophosphite, on which it commonly occurs as a morphologically continuous overgrowth [4]. Its crystal structure was established and refined by Dick [5], who used a synthetic crystal, obtained by the reaction of gibbsite with a potassium phosphate solution of pH = 7 at 423 K. Two steps in the thermal loss of water, at 341 and 471 K, were observed. A potassium-rich variant of tinsleyite was synthesized under hydrothermal conditions at 553 K from the water solution of KH2PO4 and Al(OH)3 [6]. Compared to the mineral tinsleyite, the new variant with the crystal chemical formula |K1.5(H2O)0.5|[Al2(OH){(OH)0.5(H2O)0.5}(PO4)2] differs not only in the quantity of K+ cations in the framework channels, but also in the amount of H2O and in the way it is distributed in the structure. It was suggested that the capacity of the minerals of the leucophosphite group to accommodate the K+ (or NH4+) ions is coupled with the H2O content in the framework interstices, which is, therefore, variable. As it happens, the synthetic NH4,Al end member of ammoniotinsleyite called AlPO4˗15 has been obtained and investigated [7,8] 25 years before the mineral was discovered. This material was studied by means of a charge-density analysis [9] and first-principal calculations [10]. The same compound was obtained later and described in [11]. The crystal structure of leucophosphite, KFe2[PO4]2(OH)·2H2O was refined by Dick and Zeiske [12] using a synthetic crystal. They found hydrogen atom positions by Rietveld refinement based on powder neutron-scattering data. The structure of the NH4,Fe end-member sphenscidite, (NH4)Fe2[PO4]2(OH)·2H2O, was refined by Yakubovich and Dadashov [13] with crystals grown at 423 K from a hydrogel containing an organic compound, urea or carbamide CO(NH2)2, which is found in the urine of mammalia, birds, and some reptiles. In the presence of water, the urea gives off ammonia that enters the forming crystal structure. This is a usual way in which sphenscidite crystallizes in nature [2]. As part of our experimental study of synthetic analogues of minerals [14], we report here the new aluminophosphate, Na2Al2O(PO4)2·0.12H2O, with original crystal architecture chemically and structurally related to tinsleyite and might be considered as its hypothetical alteration product of Na metasomatism.
2. Materials and Methods
2.1. Hydrothermal Synthesis and Crystallization
Single crystals of the new compound were synthesized under hydrothermal conditions. Chemical compounds of analytical grade were taken in the mass ratio NaCl:Al(OH)3 = 2:3, which corresponds to 1g (17 mmol) NaCl and 1.5 g (19 mmol) Al(OH)3. This starting mixture was melted in a 10% solution of H3PO4 (5.6 mL), sealed in a poly(tetrafluoroethylene) (PTFE)-lined stainless steel pressure vessel of 7 mL in volume (fill factor 80%). It was kept in a furnace at a temperature of 553 K and a pressure of 7 MPa for 10 days, followed by slow cooling to room temperature. The reaction products were colorless crystals with an irregular shape up to 0.3 mm long (Figure 1). They were washed with water, dried and subjected to a SEM-EDX analysis and to single-crystal X-ray diffraction. The SEM-EDX analysis was carried out on a Jeol SEM (JSM-6480LV) Oxford X-MaxN equipped with an energy-dispersive diffraction spectrometer (Laboratory of Local Methods for Studying Materials, Department of Petrology, Faculty of Geology, M. V. Lomonosov Moscow State University). The measurements were performed at 20 kV and 0.7 nA using the sample covered by a carbon film with a thickness of about 25 nm. The crystals were stable under these conditions. X-ray spectral semiquantitative analysis of unpolished samples revealed Na, P, Al, and O atoms in their composition with the P:Al:Na ratio close to 1:1:1, which is consistent with the data of our X-ray diffraction structural study.
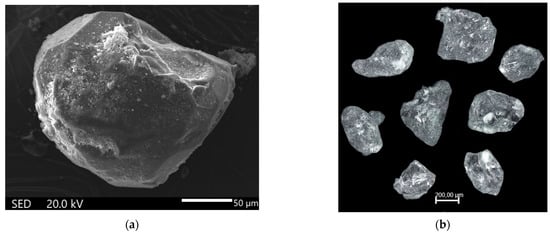
Figure 1.
SEM image (a), showing the sample morphology, and photograph (b) of the crystals.
2.2. X-ray Diffraction and Crystal Structure Determination
X-ray diffraction data were collected from the crystal of 0.05 × 0.15 × 0.26 mm in size at T = 150 K on an Oxford Diffraction Gemini single crystal diffractometer equipped with a CCD detector; Mo Kα radiation (λ = 0.71073 Å). The dataset was corrected for background, Lorentz and polarization effects, and absorption [15]. All calculations were performed within the WinGX program system [16]. The monoclinic crystal structure was solved by direct methods in the space group P21/c and refined in anisotropic approximation with the SHELX programs [17,18] using the F2 data to residual R = 0.0221 [for 2063 reflections with I > 2σ(I)], S = 1.177. In Table 1, we report the crystallographic characteristics of the new aluminophosphate and the experimental conditions of data collection and refinement. Table S1 presents the final results of the atom positions and equivalent isotropic displacement parameters. Characteristic distances are given in Table 2. A bond-valence calculation (Table 3) was performed using the algorithm and parameters given by Brown and Altermatt [19]. Data from Table 3 clearly confirm the assignment of O and the absence of OH ligands. CDS 2167937 contains the supplementary crystallographic data for this paper. The data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, accessed on 21 April 2022, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK, fax: +44 1223 336033.

Table 1.
Na2Al2O(PO4)2·0.12H2O: crystal data and details of X-ray diffraction and structure refinement.

Table 2.
Na2Al2O(PO4)2·0.12H2O. Characteristic distances, Å.

Table 3.
Na2Al2O(PO4)2·0.12H2O. Bond valance data *.
3. Results
3.1. Interatomic Distances and Crystal Structure Description
The basic structural elements of the title compound are shown in Figure 2a. The Al3+ ions in two symmetrically independent positions are surrounded by O atoms, forming trigonal bipyramids. The Al1-centered polyhedron has three close Al1–O distances that vary from 1.774(1) to 1.831(1) Å, and two longer distances to apical O atoms of 1.880 (1) and 1.898(1) Å. The distortion of the Al2-centered five-vertex polyhedron is different: all Al2–O distances lie in the interval 1.816(1)–1.844(1) Å. The pattern of distortion of the AlO5 polyhedra is consistent with the bond-valence calculation (Table 3). The asymmetric unit of the structure includes two P sites in tetrahedral coordination. In the P1 tetrahedron, there are two pairs of close P1–O bond lengths, one of 1.529(1) Å and another of about 1.54 Å. Similarly, two close P2–O distances are of 1.505(1) and 1.521(1) Å and two longer ones of about 1.55 Å characterize the P2O4 polyhedron. The site of Na1 is split into two positions at 0.46 Å statistically populated by Na atoms for 93 and 7% with six Na1–O distances ranging from 2.190(1) to 3.033(3) Å, and six Na1′–O distances from 2.18(2) to 3.09(3) Å. In Na2-centered eight-vertex polyhedron the Na2–O distances lie between 2.309(1) and 3.086(1) Å.

Figure 2.
(a) The main structural units of the Na2Al2O(PO4)2·0.12H2O. Displacement ellipsoids are drawn at the 90% probability level. Symmetry operations: (*) – x, 1 – y, 1 – z; (**) x, 1½ – y, ½+z; (’)—x, – ½ + y, 1½ – z; (”) 1 – x, –0.5 + y, 1½ – z. (b) The tetramer of AlO5 bipyramids sharing edges and vertices, and adjacent PO4 tetrahedra.
Centrosymmetric tetramers built from two Al1O5 polyhedra sharing an edge and two additional Al2O5 polyhedra attached by corner linkage at each side of this common edge represent the distinguished feature of the title compound (Figure 2b). These tetrameric units of four AlO5 bipyramids are cross-linked by PO4 tetrahedra to form two-periodic slabs parallel to the yz plane. The Na+ ions and a small amount of H2O molecules at the symmetry centers occupy positions between the anionic slabs of the [Al2O(PO4)2]2 composition (Figure 3) with eight-membered windows built from alternating sharing vertices P- and Al-centered polyhedra, and open in the {101} direction (Figure 4).
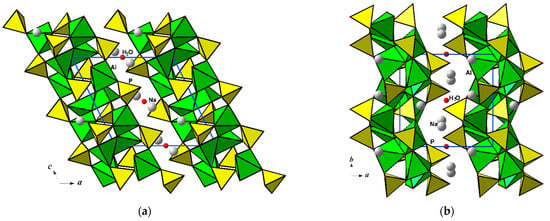
Figure 3.
The Na2Al2O(PO4)2·0.12H2O crystal structure displayed in xz (a) and xy (b) projections.
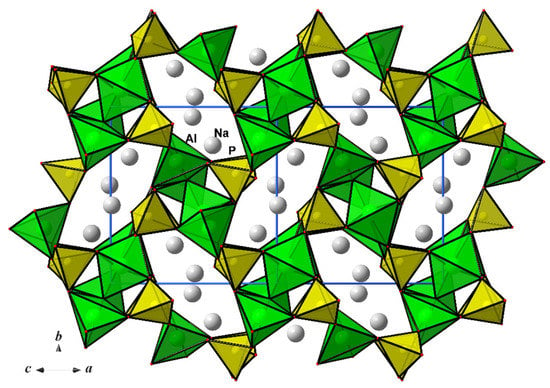
Figure 4.
The Na2Al2O(PO4)2·0.12H2O crystal structure viewed along the {101} direction.
3.2. Crystal Chemical Regularities in the Family of Aluminum Phosphates/Aluminophosphates
Natural phases, formed within the Al–P–O–(H) compositions, are generally supergene minerals of sedimentary rocks; they also occur in weathering zones and late hydrothermal formations. Although these minerals contain water in a different form, they often have rather dense crystal structures formed by cationic layers (augelite, Al2(OH)3[PO4]) or columns (senegalite, Al2(OH)3[PO4]·H2O) of either edge-sharing AlO6 octahedra and AlO5 polyhedra, or columns of aluminum octahedra shared faces and vertices (trolleite, Al4(OH)3[PO4]3). Phosphate tetrahedra unite two- or one-periodic cationic fragments designed by Al-centered polyhedra into framework structures. The water-rich representatives in this mineral group are characterized by a microporous architecture with voids and channels containing H2O molecules (wavellite, Al3(OH)3[PO4]2·5H2O, kingite, Al3(OH)3[PO4]2· 9H2O, etc.). In the crystal structures of the minerals characterized by the ratio Al:P = 1 (variscite and metavariscite, AlPO4 · 2H2O), all vertices of AlO6 octahedra are shared with phosphate tetrahedra [20]. In the crystal structure of mineral berlinite, AlPO4 (an indicator of high-temperature conditions of phase formation [21]) with the ratio Al:P = 1, the framework of which represents a superstructure based on quartz, Al atoms are in the tetrahedral coordination.
Incorporation of Na into the composition of aluminum phosphates is usually connected with metasomatic reactions. In nature, two minerals are known in the Na-Al-P-O-H system—wwardite and brazilianite. Both structures are based on mixed anionic frameworks of Al octahedra and P tetrahedra, but the topology of the frameworks differs significantly. Brazilianite, NaAl3(PO4)2(OH)4 is considered to form in granitic pegmatites as a product of Na-metasomatic alteration of primary pegmatite minerals of the montebrasite-amblygonite series. The crystal structure of brazilianite is designed from columns of AlO4(OH)2 and AlO3(OH)3 octahedra sharing edges; these columns are linked by PO4 tetrahedra in a framework with cavities populated by Na atoms [22,23]. The mineral wardite, NaAl3(PO4)2(OH)4·2H2O of hydrothermal origin usually occurs in P-rich zones of granite pegmatites. In its tetragonal crystal structure, Al-centered octahedra sharing OH vertices are aligned in intercrossing chains along the {100} and {010} directions. These chains form cellular layers parallel to the ab plane with Na atoms occupying positions near their centers. The sheets of AlO2(OH)4, AlO3(OH)2(H2O) and NaO6(H2O)2 polyhedra sharing edges and vertices are linked along the c axis via phosphate tetrahedra and hydrogen bonds [24,25].
Natural potassium and aluminum phosphates are mainly weathering products of clay minerals under the action of phosphate-containing solutions of guano or anthropogenic fertilizers. Potassium aluminophosphate taranakite, K3Al5(HPO4)6(PO4)2·18H2O mostly occurs in humid caves where phosphate-rich excrements of birds, penguins, or bats react with clay minerals. It is also known as a reaction product of soil clays with phosphate-containing fertilizers [26]. In the rhombohedral structure of taranakite, six [K3Al5(HPO4)6(PO4)2(H2O)12] layers alternate along {001} with layers of H2O molecules. Non-exchangeable K atoms are trapped within the layers. Hydrogen bonds in taranakite act within the rigid layers, within the water interlayers, and between the layers and interlayers [27]. The product of taranakite dehydration, francoanellite, occurs at the contact of “terra rossa” with bat guano in the karst cave. The mineral crystal structure is built of the same “taranakite” [K3Al5(HPO4)6(PO4)2(H2O)12] layers connected by hydrogen bonds. Hydrogen bonds in francoanellite are formed within the rigid layers and between them. It has been shown that single crystals of synthetic francoanellite could be obtained by topochemical dehydration of taranakite crystals with loosing of every second water interlayer, when a first-order staging product of the deintercalation of water from taranakite was formed [28].
Known to date, several structural topologies of aluminophosphates are derived from the assemblage of PO4 tetrahedra and a number of types of Al-centered polyhedra (tetrahedra, octahedra, trigonal bipyramids, and tetragonal pyramids) in the framework. Five-vertex polyhedra formed by O atoms in the Al surrounding are not as common as usual AlO4 tetrahedra or AlO6 octahedra. There are compounds, for instance Na6Al3P5O20, in which the microporous framework is built from mutually AlO6 and AlO4 polyhedra, and PO4 tetrahedra having vertex-bridging contacts [29]. Obtained by a high-temperature molten salt method, the Ba3Al2P4O16 phase with an Al/P ratio of 1:2, is characterized by unique [Al2P4O16]∞ chains constructed from PO4, AlO4, and AlO5 groups [30]. Two complex aluminophosphates with an Al:P ratio equal to 5:7, namely, [Al5(OH)(PO4)3(PO3OH)4][NH3(CH2)2NH3]2[2H2O] and [Al5(PO4)5(PO3OH)2][NH3(CH2)3NH3]2[H2O] are layered materials with aluminum atoms in three different coordination states, AlO4, AlO5, and AlO6, and the interlayer space contains the amines and water molecules [31].
The title compound Na2Al2O(PO4)2·0.12H2O represents an example of the layered sodium aluminophosphate built from PO4 and AlO5 polyhedra. Its monoclinic crystal structure is very similar to that of mineral tinsleyite, KAl2(PO4)2(OH)·2H2O with K atoms in the framework channels. The main difference between the two structures consists of the type of Al coordination by O atoms. As shown above, clusters of AlO5 bipyramids sharing edges and vertices are essential building blocks of the Na2Al2O(PO4)2·0.12H2O. Similar clusters, but designed from AlO4(OH)2 and AlO4(OH)(H2O) octahedra, form the tinsleyite crystal structure (Figure 5). The topologies of both Al/P substructures within the bc layers are identical; the amount and distribution of alkali Na+ or K+ ions and the H2O molecules are different. Significantly distinct sizes of Na+ and K+ ionic radii define here not only a type of Al coordination by O atoms in order to arrange the necessary environment around one or another alkali metal. They also specify a periodicity of the aluminophosphate anionic construction: layers alternating along the {100} direction with Na atoms in the Na2Al2O(PO4)2·0.12H2O or a framework with channels populated by a two times lower quantity of K atoms in the case of tinsleyite (Table 4). The synthesis of sodium aluminophosphate under mild hydrothermal conditions and the topological resemblance of its structure with that of the mineral tinsleyite and a number of its structural analogues, suggest a high probability of the existence of a mineral equivalent of the Na2Al2O(PO4)2·0.12H2O in nature.
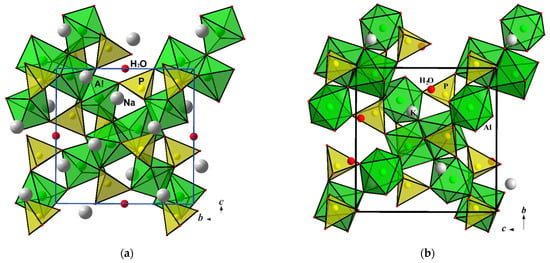
Figure 5.
Crystal structures of Na2Al2O(PO4)2·0.12H2O (a) and tinsleyite, KAl2(OH)(H2O)(PO4)2·H2O (b) projected along the a axis.
A certain structural resemblance between hydrothermally obtained sodium aluminophosphate hydroxide Na2Al3(OH)2(PO4)3 and the mineral minyulite, KAl2F(H2O)4(PO4)2 has been noticed earlier [32]. Minyulite was first synthesized by Haseman et al. [33], as well as a number of other hydrous phosphates, including tinsleyite, by the treatment of clays with phosphate anions at pH ranges appropriate for soil environments and at a temperature less than 95 °C. A synthetic hydroxide analogue of minyulite, namely, KAl2(OH)(H2O)4(PO4)2 could also be obtained by the reaction of gibbsite with a potassium phosphate solution of pH = 5.5 at 333 K [34].


Table 4.
Structurally related sodium and potassium microporous aluminophosphates.
Table 4.
Structurally related sodium and potassium microporous aluminophosphates.
| Compound/Mineral | Unit Cell Parameters, Å, β, o, V, Å3 | Space Group, Z, ρ, g/cm3 | Clusters of Al-Centered Polyhedra | Anionic Structural Fragment | Reference |
|---|---|---|---|---|---|
| Tinsleyite, synthetic KAl2(OH)(H2O)(PO4)2·H2O | a = 9.499(2) | P21/n | Al4(OH)2(H2O)2O16 | Framework | [5] |
| b = 9.503(2) | 4 | ||||
| c = 9.535(2) | 2.66 | ||||
| β = 103.26(3) | |||||
| V = 837.8(2) | |||||
| Na2Al2O(PO4)2·0.12H2O * | a = 9.9927(3) | P21/c | Al4O18 | Layers | This work |
| b = 8.8811(2) | 4 | ||||
| c = 9.7005(3) | 2.63 | ||||
| β = 116.155(4) | |||||
| V = 772.73(4) | |||||
| Minyulite, KAl2F(H2O)4(PO4)2 | a = 9.337(5) | Pba2 | Al2F(H2O)4O6 | Layers | [35] |
| b = 9.740(5) | 2 | ||||
| c = 5.522(3) | 2.47 | ||||
| V = 502.2(5) | |||||
| Na2Al3(OH)2(PO4)3 | a = 8.475(2) | P212121 | Al3(OH)2O12 | Framework | [32] |
| b = 8.471(2) | 4 | ||||
| c = 14.319(3) | 2.88 | ||||
| V = 1028.0(4) |
* Geometric characteristics correspond to the temperature of 150 K.
Crystal structures of these Na- and K-bearing pseudo tetragonal compounds look similar in the ab projection. The elementary blocks of the anionic layers of the mixed type in the minyulite structure are dimers consisting of two Al octahedra sharing F vertices (or OH vertices in the structure of its synthetic variant). These pairs of octahedra are connected in layers via PO4 tetrahedra. Voids of the octagonal cross-section in layers parallel to the {001} plane contain K+ ions. In the direction of the c axis, the aluminophosphate layers are linked by hydrogen bonds [35]. Blocks of sharing vertices Al-centered polyhedra crosslinked by PO4 tetrahedra can be distinguished in the structure of Na2Al3(OH)2(PO4)3; however, in this case, two AlO4(OH) polyhedra and one AlO4(OH)2 octahedron form an elementary cluster of a horseshoe appearance (Figure 6). The Al-centered five-vertex polyhedra are moved up and down along {001} from the Al-centered octahedron. As a consequence, the Al3(OH)2O12 trimers with the AlO4(OH)2 octahedron at their centers occur disposed at two levels along the z axis (Figure 6b); thus increasing the unit cell c parameter to 14.319 Å, as compared with c = 5.522 Å of minyulite having the layered structure (Figure 6c). In the framework structure of sodium aluminophosphate, the clusters are linked via phosphate tetrahedra not only in the ab plane (as in the minyulite structure), but also in the {001} direction. Each Al-centered polyhedron overlaps along the z axis with its counterpart at a distance of about 4.9 Å, and the trimeric clusters look like dimers in the xy projection (Figure 6a).
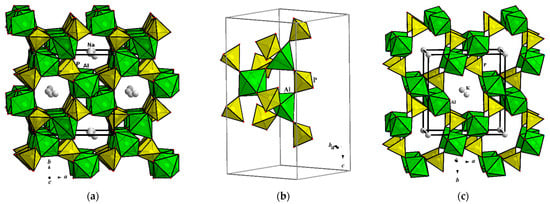
Figure 6.
Crystal structures of Na2Al3(OH)2(PO4)3 (a) and minyulite (c) projected along the c axis of their pseudo tetragonal unit cells; (b) fragment of the Na2Al3(OH)2(PO4)3 structure showing the horseshoe-like cluster built from the AlO4(OH)2 octahedron and two AlO4(OH) five-vertex polyhedra.
The absence, at ambient conditions, of isostructural chemically similar but Na- or K-based compounds is well-known and is mainly due to the difference in ionic radii of Na+ and K+. The abovementioned examples demonstrate the crystal chemical variations between Na- and K-representatives of aluminophosphates, the structures of which include clusters of Al-centered polyhedra interconnected in the framework through oxygen-bridging contacts of PO4 tetrahedra. In both cases, alkali Na or K atoms work as structure regulating agents, determining the character of the developing crystal structure, namely, the types of Al-centered polyhedra and the structure periodicity (layer or framework). The flexibility of aluminophosphate constructions, which include clusters of Al-centered octahedra and/or five-vertex polyhedra connected by phosphate groups, allows them to self-organize around structure-forming Na+ or K+ ions into anionic layers or frameworks. The implementation of such structures, also in nature in the form of minerals, is largely determined by the participation of water, in one form or another, which stabilizes the crystallizing phases due to the forming hydrogen bonds.
4. Conclusions
Various structural topologies of aluminophosphates are derived from the grouping of PO4 tetrahedra and a number of types of Al-centered polyhedra (tetrahedra, octahedra, trigonal bipyramids, and tetragonal pyramids) in the framework. A novel representative Na2Al2O(PO4)2·0.12H2O within this family was obtained as single crystals in middle-temperature hydrothermal conditions. Its original layered crystal structure was established by low-temperature X-ray diffraction. A mineralogically probable phase is discussed as a sodium alternative of tinsleyite, KAl2(OH)(H2O)(PO4)2·H2O, a member of the leucophosphite mineral group, which also includes sphenscidite and ammoniotinsleyite. These minerals are known to occur in two different paragenetic associations: as late products of the hydrothermal alteration of primary phosphates in granitic pegmatites, or as biominerals.
The crystal structures Na2Al2O(PO4)2·0.12H2O and tinsleyite contain similar fragments: clusters of four Al-centered polyhedra sharing edges and vertices, surrounded by PO4 tetrahedra. In contrast to tinsleyite, where tetramers are built from AlO4(OH)2 and AlO4(OH)(H2O) octahedra, analogous clusters of AlO5 bipyramids are the main structural units of sodium aluminophosphate. We showed that alkali Na or K atoms, which have strongly distinct ionic radii, act as structure regulators, determining the character of the developing crystal structure, namely the types of Al-centered polyhedra and the structure periodicity. The flexibility of aluminophosphate constructions, which include clusters of Al-centered octahedra or five-vertex polyhedra connected by phosphate groups, allows them to self-organize around structure-forming Na+ or K+ ions into anionic layers or frameworks. We assume that the synthesis of sodium aluminophosphate under mild hydrothermal conditions and the topological resemblance of its structure with that of the mineral tinsleyite and a number of its structural analogues, suggest a high probability of the existence of a mineral equivalent of the Na2Al2O(PO4)2·0.12H2O in nature.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min12050542/s1, Table S1: Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) for Na2Al2O(PO4)2·0.12H2O.
Author Contributions
Conceptualization, O.Y.; validation, O.Y.; investigation, O.Y., G.K., P.V., S.S., A.V. and O.D.; writing—original draft preparation, O.Y.; writing—review and editing, G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by state budget research project AAAA-A16-116033010121-7, N 6-5.
Data Availability Statement
Not applicable.
Acknowledgments
We thank V.O. Yapaskurt for the microprobe analysis of the crystals.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moore, P.B. Octahedral tetramer in the crystal structure of leucophosphite, K2[Fe3+4(OH)2(H2O)2(PO4)4]·2H2O. Am. Miner. 1972, 57, 397–410. [Google Scholar]
- Wilson, M.J.; Bain, D.C. Sphenscidite, a new phosphate mineral from Elephant Island, British Antarctic Territory. Miner. Mag. 1986, 50, 291–293. [Google Scholar] [CrossRef] [Green Version]
- Chukanov, N.V.; Möhn, G.; Pekov, I.V.; Zubkova, N.V.; Ksenofontov, D.A.; Belakovskiy, D.I.; Vozchikova, S.A.; Britvin, S.N.; Desor, J. Ammoniotinsleyite, (NH4)Al2(PO4)2(OH)·2H2O, a new mineral species from guano deposit at Pabellón de Pica, Iquique Province, Chile. Miner. Mag. 2020, 84, 705–711. [Google Scholar] [CrossRef]
- Dunn, P.J.; Rouse, R.C.; Campbell, T.J.; Roberts, W.L. Tinsleyite, the aluminum analogue of leucophosphite, from the Tip Top pegmatite in South Dakota. Am. Miner. 1984, 69, 374–376. [Google Scholar]
- Dick, S. Ueber die Struktur von synthetischem Tinsleyit K(Al2(PO4)2(OH)(H2O))·(H2O). Z. Nat. Teil B. Anorg. Chem. Organ. Chem. 1999, 54, 1385–1390. [Google Scholar]
- Yakubovich, O.V.; Massa, W.; Dimitrova, O.V. A novel potassium-rich variant of tinsleyite, |K1.5(H2O)0.5 |[Al2(OH){(OH)0.5(H2O)0.5}(PO4)2]. Can. Miner. 2012, 50, 559–569. [Google Scholar] [CrossRef]
- Parise, J.B. Preparation and structure of the aluminium ammonium phosphate dihydrate Al2(NH4)(OH)(PO4)2·2H2O: A tunnel structure with ammonium ions in the channels. Acta Crystallogr. 1984, C40, 1641–1643. [Google Scholar]
- Pluth, J.J.; Smith, J.V.; Bennett, J.M.; Cohen, J.P. Structure of NH4Al2(OH)(H2O)(PO4)2·H2O, the ammonium aluminum analog of GaPO4·2H2O and leucophosphite. Acta Crystallogr. 1984, C40, 2008–2011. [Google Scholar]
- Aubert, E.; Porcher, F.; Souhassou, M.; Lecomte, C. Characterization of intra-framework and guest/host interactions in the AlPO4–15 molecular sieve by charge-density analysis. Acta Crystallogr. 2003, B59, 687–700. [Google Scholar] [CrossRef] [Green Version]
- Byrne, P.J.; Warren, J.E.; Morris, R.E.; Ashbrook, S.E. Structure and NMR assignment in AlPO4–15: A combined study by diffraction, MAS NMR and first-principles calculations. Solid State Sci. 2009, 11, 1001–1006. [Google Scholar] [CrossRef]
- Vaughan, D.E.W.; Yennawar, H.; Perrotta, A. Synthesis and structure of a 3D aluminophosphate (PSU-3). Micropor. Mesopor. Mater. 2012, 153, 18–23. [Google Scholar] [CrossRef]
- Dick, S.; Zeiske, T. Leucophosphite K[Fe2(PO4)2(OH)(H2O)]·H2O: Hydrogen bonding and structural relationships. J. Solid State Chem. 1997, 133, 508–515. [Google Scholar] [CrossRef]
- Yakubovich, O.V.; Dadashov, M.S. Synthesis and crystal structure of a ammonium analogue of the leucophosphite, NH4{Fe2[PO4]2(OH)(H2O)}·H2O. Sov. Phys. Crystallogr. 1992, 37, 757–760. [Google Scholar]
- Yakubovich, O.V.; Khasanova, N.R.; Antipov, E.V. Mineral-inspired materials: Synthetic phosphate analogues for battery applications. Minerals 2020, 10, 524. [Google Scholar] [CrossRef]
- Agilent. CrysAlis PRO; Agilent Technologies Ltd.: Yarnton, UK, 2014. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. 1985, 41, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Strunz, H.; Nickel, E.H. Strunz Mineralogical Tables; E. Schweizerbart’sche: Stuttgart, Germany, 2001. [Google Scholar]
- Onac, P.B.; Effenberger, H.S. Re-examination of berlinite (AlPO4) from the Cioclovina Cave, Romania. Amer. Miner. 2007, 92, 1998–2005. [Google Scholar] [CrossRef]
- Gatehouse, B.M.; Miskin, B.K. The crystal structure of brazilianite, NaAI3(PO4)2(OH)4. Acta Cryst. 1974, B30, 1311–1317. [Google Scholar] [CrossRef]
- Gatta, G.D.; Vignola, P.; Meven, M.; Rinaldi, R. Neutron diffraction in gemology: Single-crystal diffraction study of brazilianite, NaAl3(PO4)2(OH)4. Amer. Miner. 2013, 98, 1624–1630. [Google Scholar] [CrossRef] [Green Version]
- Fanfani, L.; Nunzi, A.; Zanazzi, P.P. The crystal structure of wardite. Miner. Mag. 1970, 37, 598–605. [Google Scholar] [CrossRef]
- Gatta, G.D.; Guastoni, A.; Fabelo, O.; Fernandez-Diaz, M.T. A single-crystal neutron diffraction study of wardite, NaAl3(PO4)2(OH)4·2H2O. Phys. Chem. Miner. 2019, 46, 427–435. [Google Scholar] [CrossRef]
- Lindsay, W.; Vlek, P.P.; Chien, S.; Dixon, J.B. Minerals in Soil Environments; Dixon, J.B., Weed, S.B., Eds.; SSSA Book Series No. I: Madison, WI, USA, 1989; p. 1089. [Google Scholar]
- Dick, S.; Goẞner, U.; Weiẞ, A.; Robl, C.; Groẞmann, G.; Ohms, G.; Zeiske, T. Taranakite—The mineral with the longest crystallographic axis. Inorg. Chim. Acta. 1998, 269, 47–57. [Google Scholar] [CrossRef]
- Dick, S.; Zeiske, T. Francoanellit K3A15HPO4)6(PO4)2·12H2O: Struktur und Synthese durch topochemische Entwässerung von Taranakit. Z. Nat. 1998, 53, 711–719. [Google Scholar]
- Yakubovich, O.V.; Kiriukhina, G.V.; Volkov, A.S.; Dimitrova, O.V.; Borovikova, E.Y. Novel aluminophosphate Na6[Al3P5O20] with the original microporous crystal structure established in the study of a pseudomerohedric microtwin. Acta Cryst. 2021, 77, 232–240. [Google Scholar] [CrossRef]
- Duan, M.; Kong, B.; Li, R. Molten salt synthesis of an open-frame aluminum phosphate Ba3Al2P4O16 with a rare-pyramidal AlO5 group. Inorg. Chem. 2020, 59, 12978–12982. [Google Scholar] [CrossRef] [PubMed]
- Bouchevreau, B.; Charlotte Martineau, C.; Mellot-Draznieks, C.; Dutour, J.; Tuel, A.; Suchomel, M.R.; Trébosc, J.; Lafon, O.; Amoureux, J.-P.; Taulelle, F. High-resolution structural characterization of two layered aluminophosphates by synchrotron powder diffraction and NMR crystallographies. Chem. Mater. 2013, 25, 2227–2242. [Google Scholar] [CrossRef]
- Yakubovich, O.V.; Dimitrova, O.V.; Urusuv, V.S. Crystal structure of a new microporous Na2{Al3(OH)2[PO4]3} aluminum phosphate. Dokl. Phys. 2003, 48, 209–215. [Google Scholar] [CrossRef]
- Haseman, J.F.; Lehr, J.R.; Smith, J.P. Mineralogical character of some iron and aluminum phosphates containing potassium and ammonium. Soil Sci. Soc. Am. Proc. 1950, 15, 76–84. [Google Scholar]
- Dick, S.; Großmann, G.; Ohms, G.; Zeiske, T. Aluminiumphosphate mit nichtzentrosymmetrischen Schicht- und Raumnetzstrukturen aus topologisch verwandten Motiven: 1. KA12(PO4)2(OH)·4H2O. Z. Nat. 1997, 52, 1439–1446. [Google Scholar]
- Kampf, A.R. Minyulite: Its atomic arrangement. Amer. Miner. 1977, 62, 256–262. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).