Geochemistry and Mineralogy of Ferromanganese Crusts from the Western Cocos-Nazca Spreading Centre, Pacific
Abstract
:1. Introduction
1.1. Ferromanganese Crust Occurrences in the Cocos-Nazca Ridge
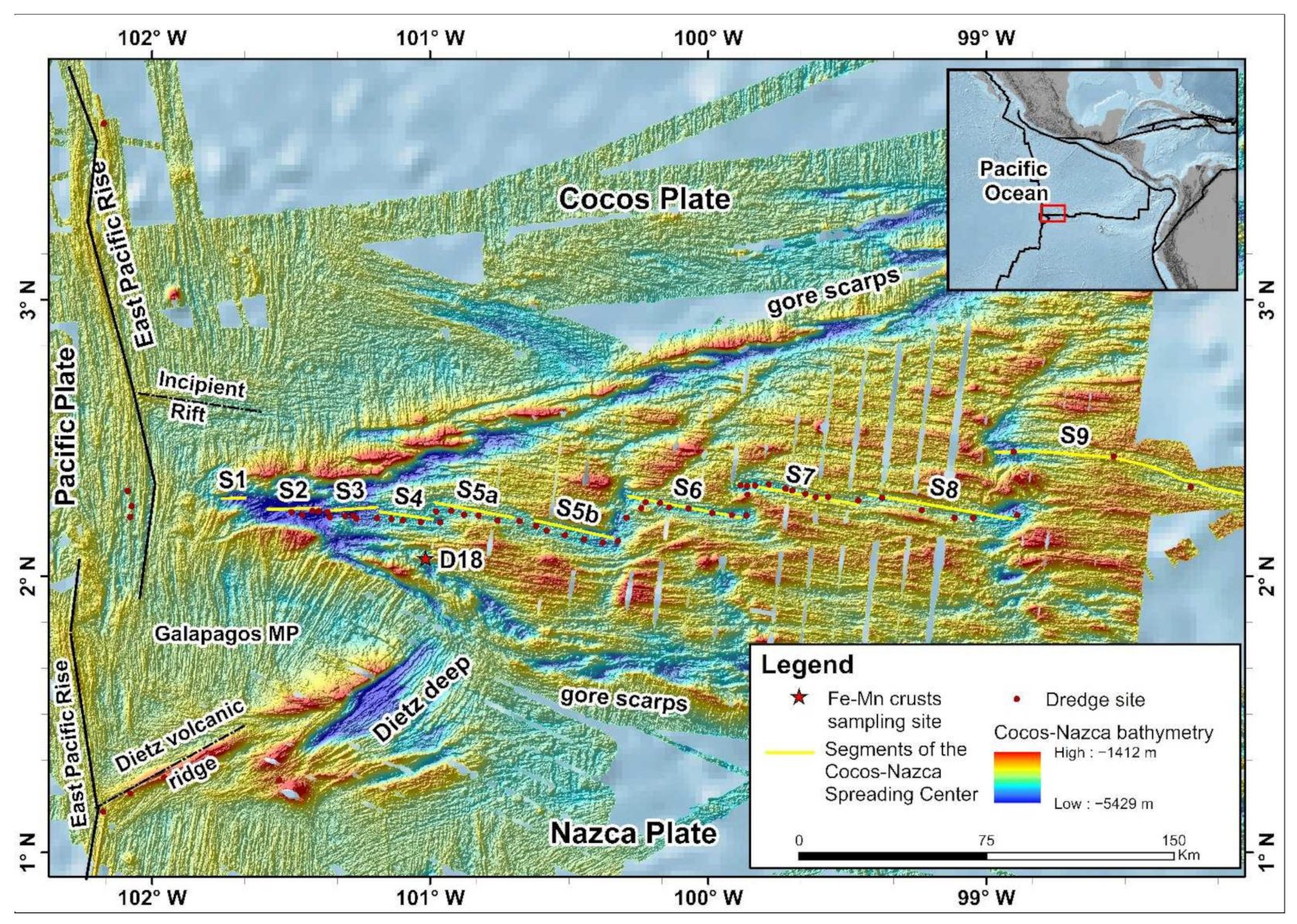
1.2. Study Area
2. Materials and Methods
2.1. Sample Collection and Data Processing
2.2. Methods
2.3. Data Processing, Statistics and Basic Calculations
3. Results
3.1. General Description and Morphology of Crusts
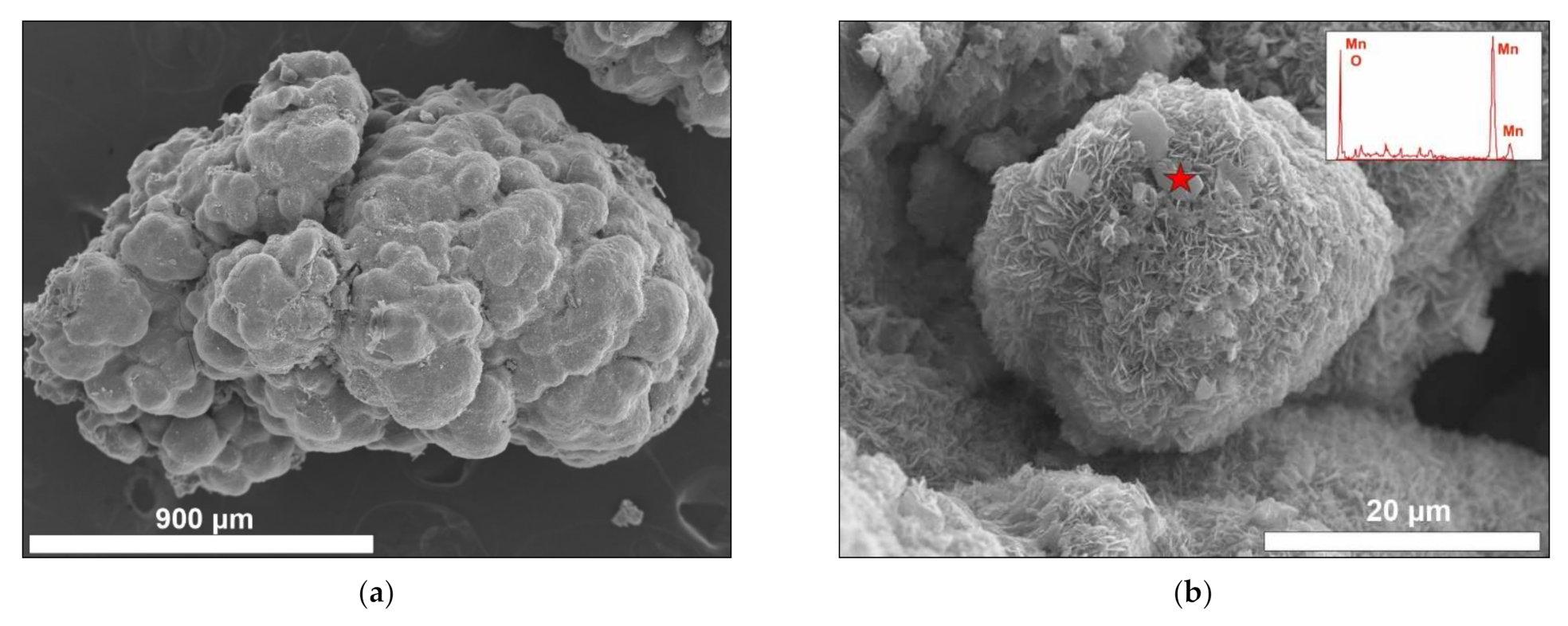
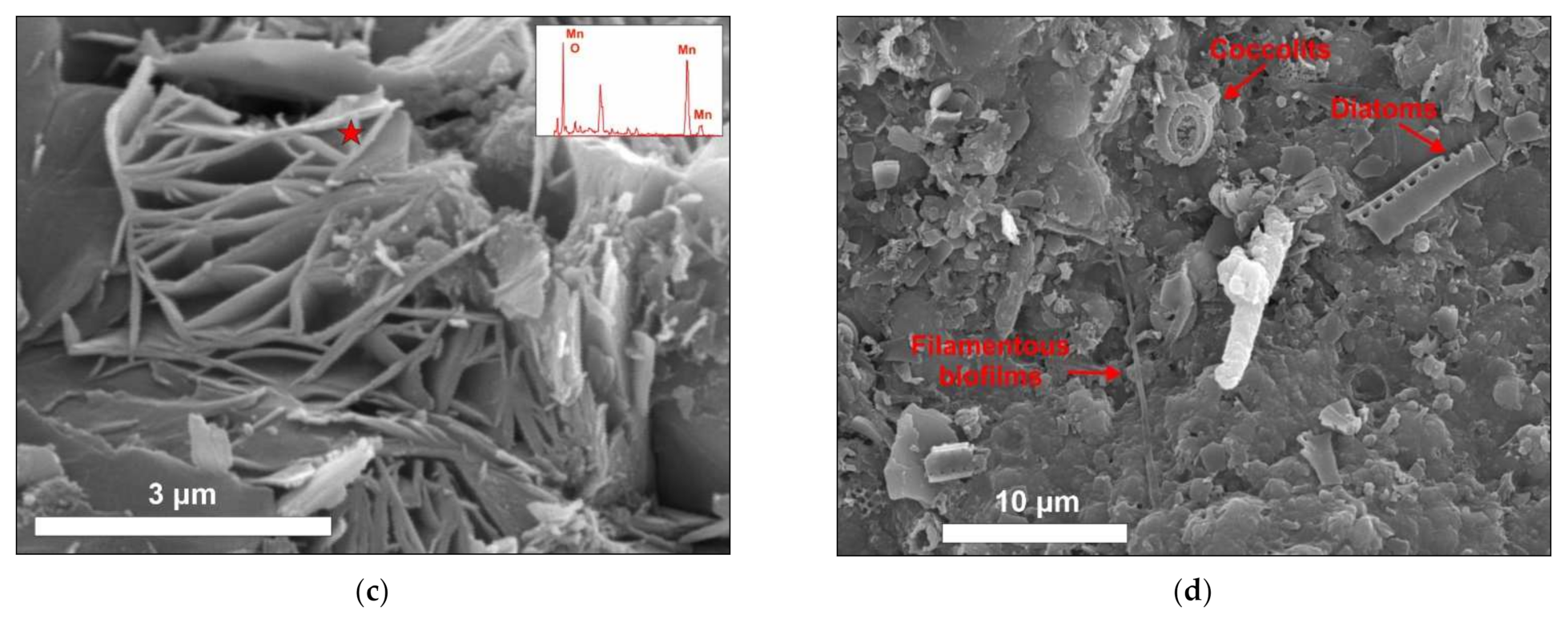
- Non-laminated: Black basaltic substrate, the crust has a smooth botryoidal texture. This crust is fragile and loosely connected to the substrate (Figure 2a–c).
- Layered: dark grey to brownish crust without substrate. These layered crusts show a submetallic luster and low hardness (Figure 2d,e).
- Transitional: crust with botryoidal texture. Basalt substrate covered by a thick laminated dark grey crust, and partially thick black non-laminated crust (Figure 2f).
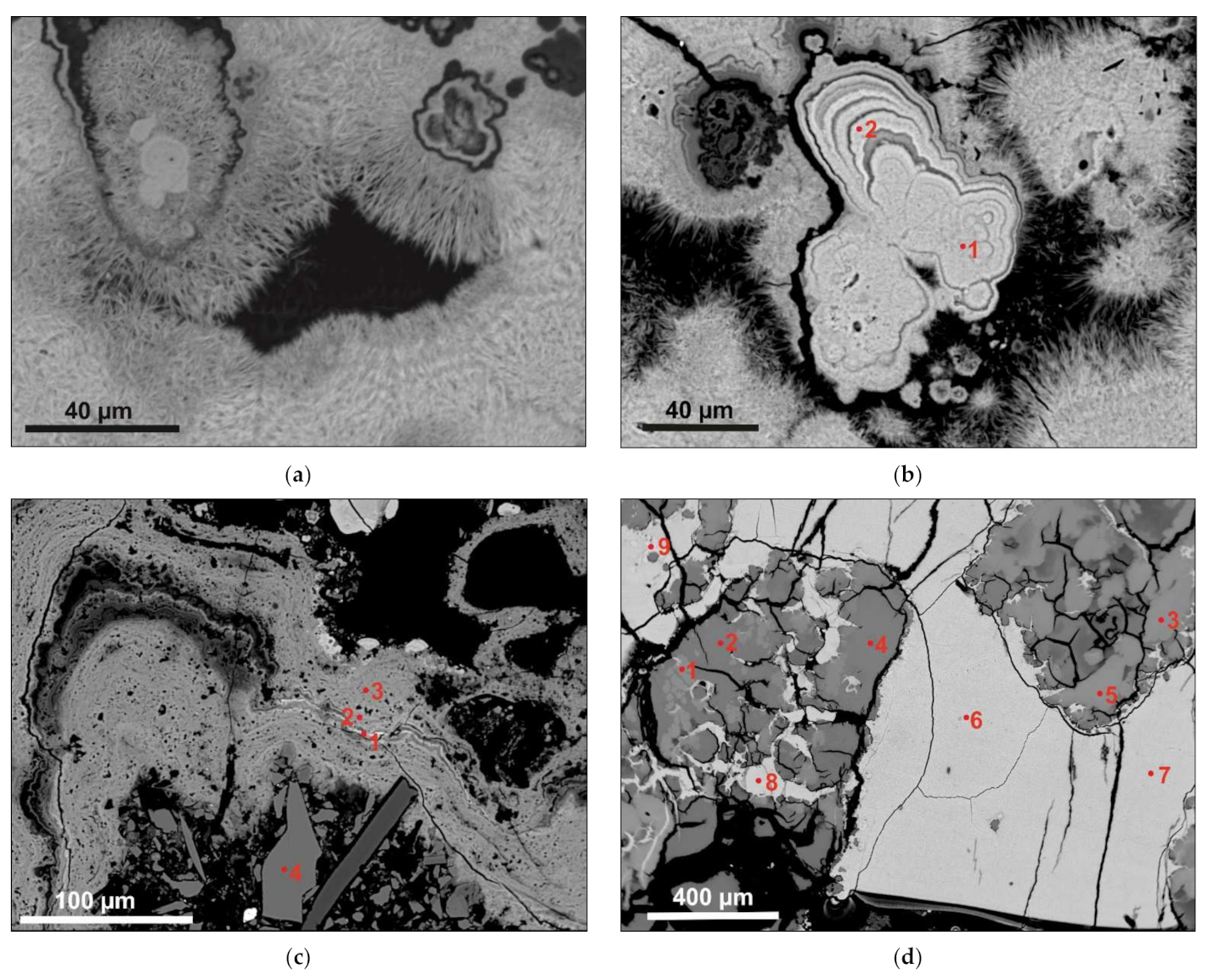
3.2. SEM Textural Description
3.3. Bulk Analysis
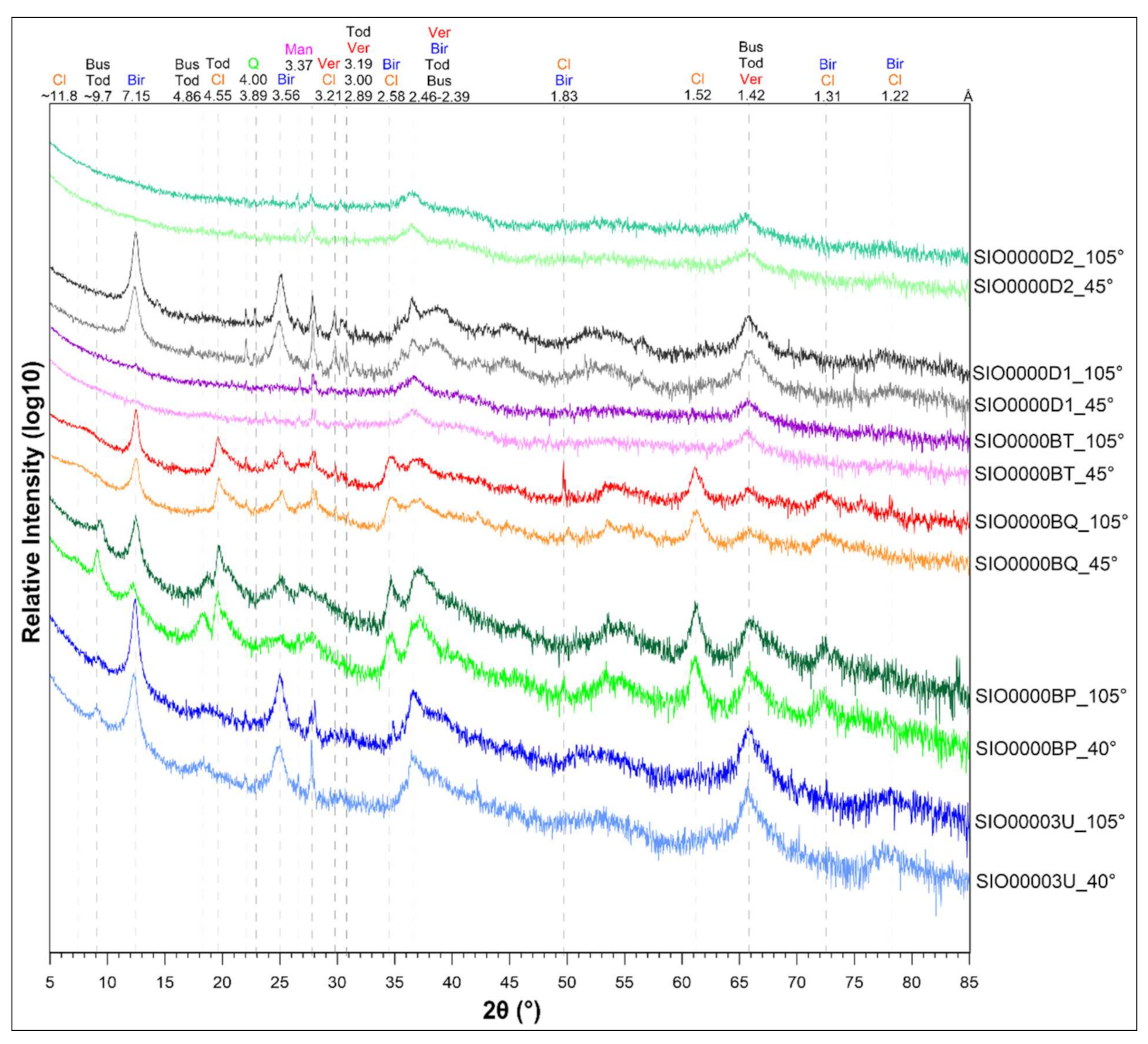
3.3.1. Mineralogy (XRD)
3.3.2. Geochemistry of Major Elements (XRF)
3.3.3. Geochemistry of Minor and Trace Elements (ICP-MS)
3.4. Electron Probe Microanalysis
3.5. Statistical Analysis
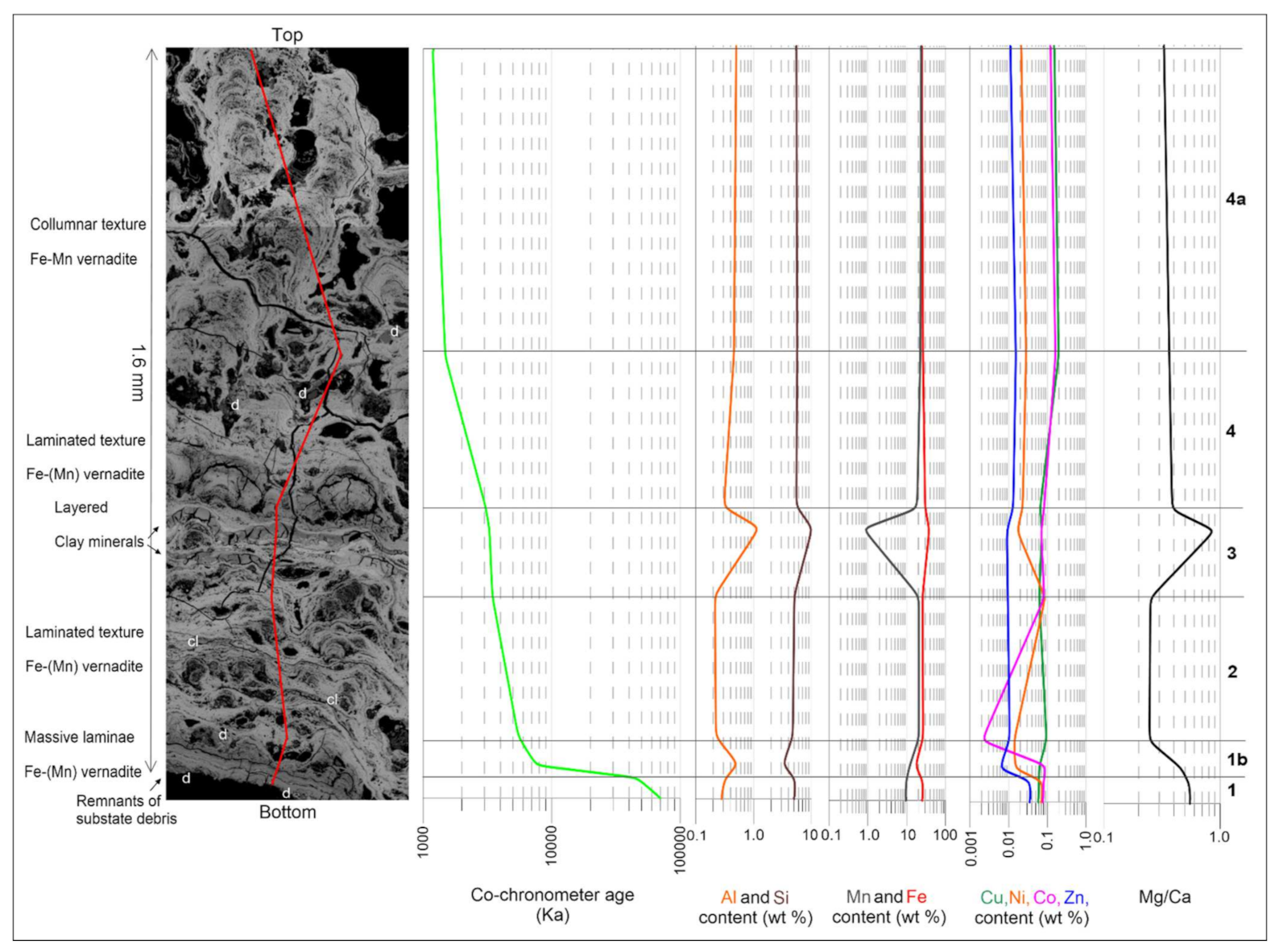
3.6. Growth Rates
4. Discussion
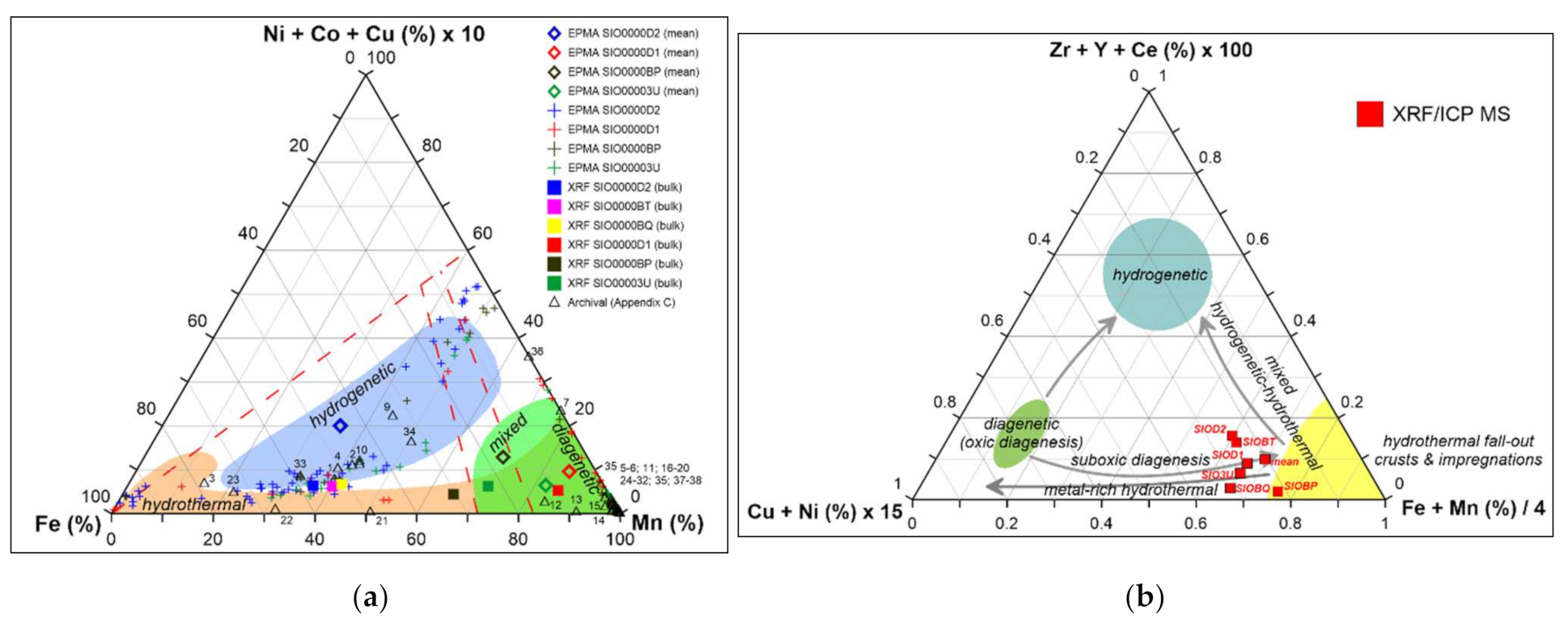
4.1. Classification and Forming Conditions
4.2. Potential Age
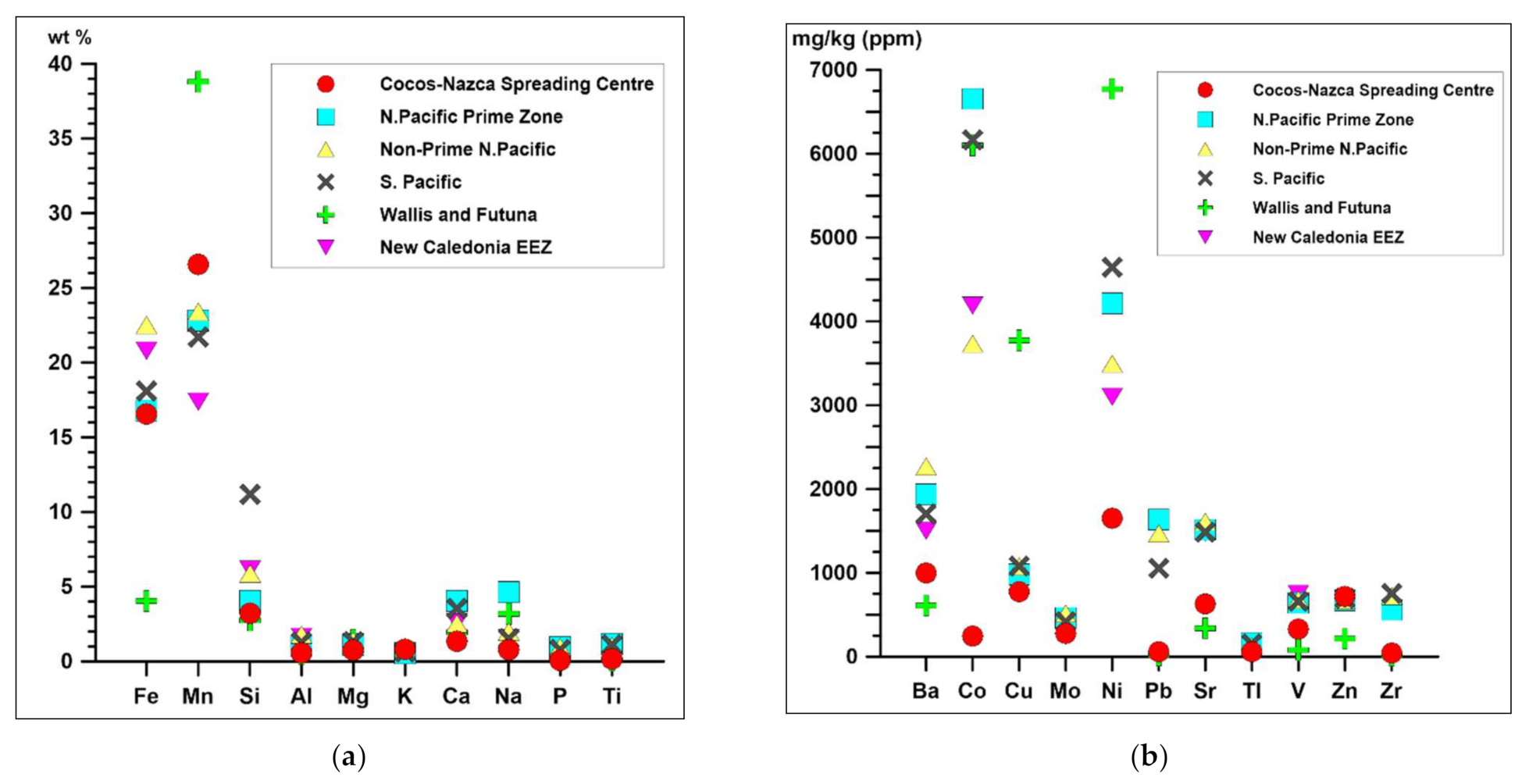
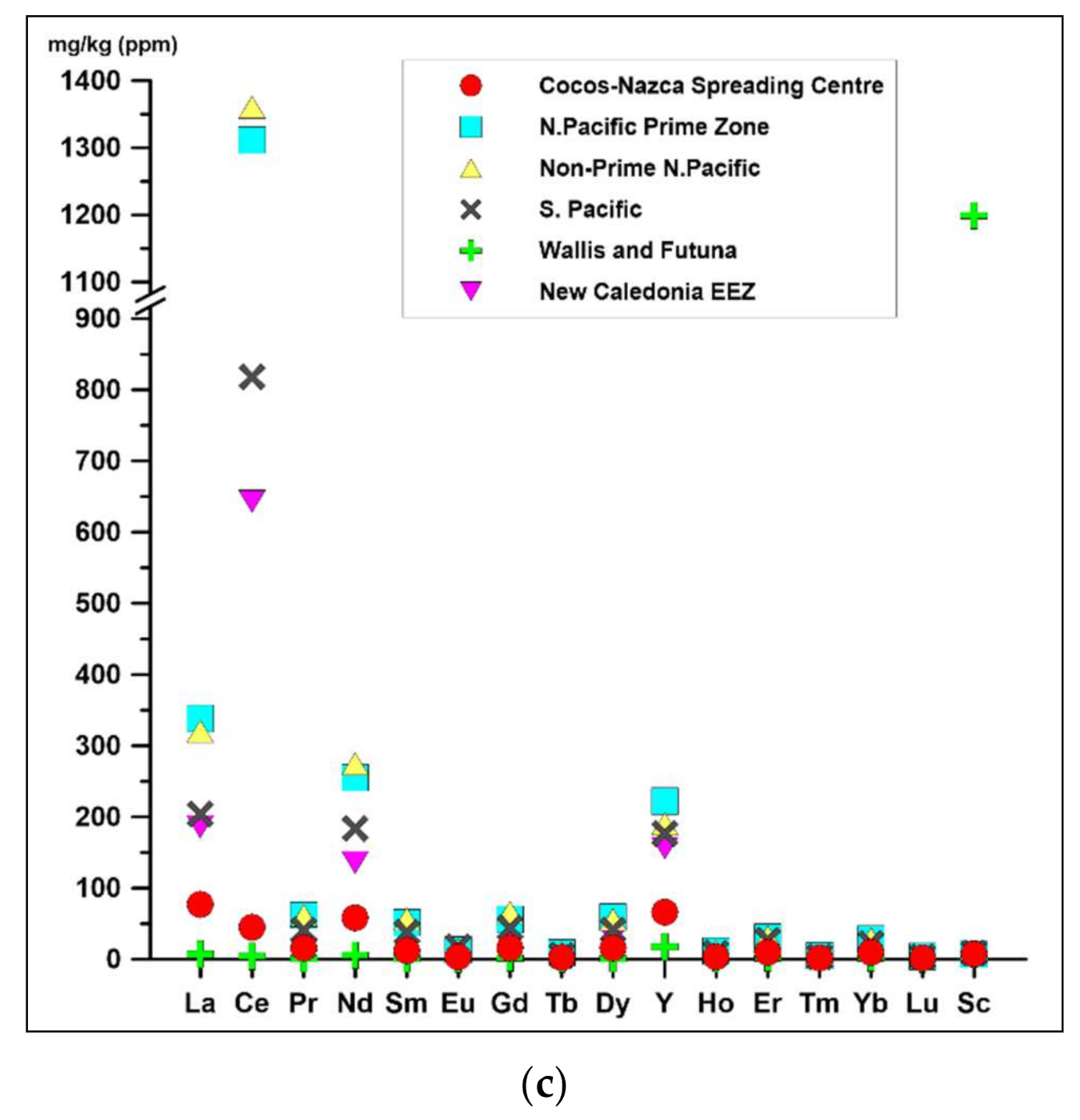
4.3. Economic Potential and Comparison with Other Crusts from Pacific
5. Summary
- Six thin Fe-Mn crusts recovered during dredging from the Cocos-Nazca Spreading Centre in 2018 provide detailed geochemical and mineralogical data to determine their complex and various formation conditions.
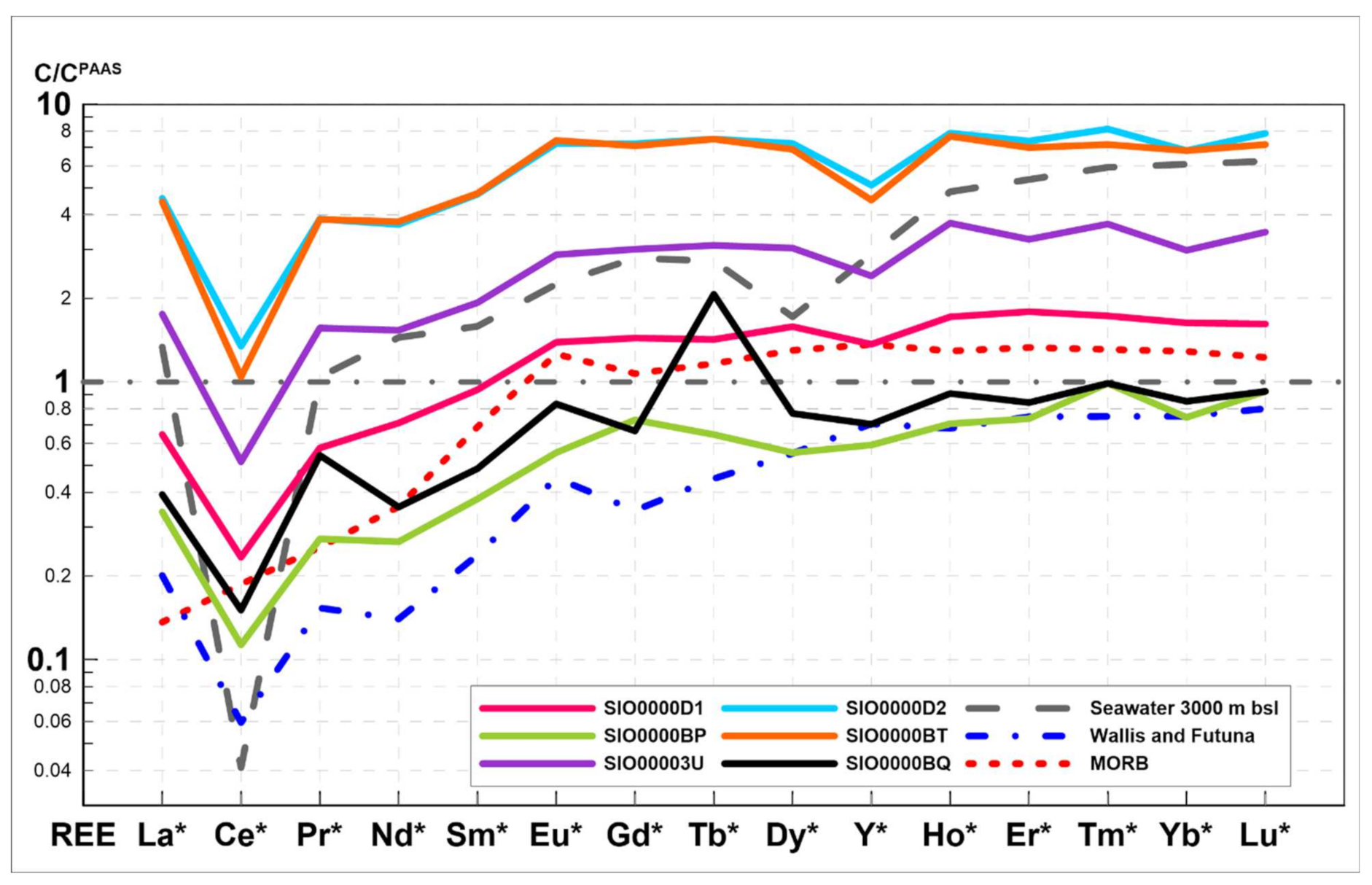
- Based on bulk chemical composition, Mn (mean 26.6 ± 5.9%) and Fe (mean 16.5 ± 4.1%) dominate. The sum of mean concentrations for three prospective metals (∑Cu + Ni + Co) is low, at 0.27 wt %. The total content of ΣREY is also low, with a range from 81 to 741 mg/kg (mean 339 mg/kg). The studied crusts were characterized by visible negative Ce anomalies and positive Eu anomalies, which are typical for hydrothermal deposits, hydrothermally altered deposits or stratabound deposits. Analyzed samples also show strong depletion in Zr and Ti that indicate low influences of external components (e.g., terrestrial debris).
- The dominant mineral phases in studied crusts consist mostly of birnessite, todorokite-buserite, and Mn-(Fe) vernadite. Traces of other minerals were found such as diagenetic manganates (asbolane), Fe-oxides and oxyhydroxides or hydrothermally associated and pure Mn-oxyhydroxides (manganite). The elevated concentrations of ∑Cu + Ni + Co (>1.5 wt %) are associated predominantly with birnessite. Todorokite-buserite and Fe-(Mn) vernadite are generally metal-depleted, with respect to Ni, Cu and Co (usually 0.2–0.6 wt % in total).
- According to the geochemical and mineralogical data, analyzed crusts originated hydrothermally and transformed due to some interaction with seawater, acquiring mixed hydrogenetic and diagenetic signatures. The complicity of analyzed crusts is surprisingly high considering the short length of dredging. Analyzed petrological features show that relatively young seafloor areas forming conditions may provide a complicated image of ferromanganese crusts, potentially affected with strong local spatiotemporal variability of geologic and/or oceanographic features near spreading centers.
- The growth rates of C-NSC crusts range from 0.10 to 1.94 mm/kyr, with mean 0.66 mm/kyr, which can be classified as rapid growth characteristics typical for hydrothermal Mn-oxide deposits. The calculated growth rates of crust thickness, assuming lack of any hiatuses, indicate maximum age of studied crusts at approximately 86 ka (Late Pleistocene).
- Low crusts thickness and low strategic metals concentrations (e.g., Co, Ni, Cu, REY) suggest that, at the time of writing, the C-NSC encrustations need to be considered as poor and economically non-prospective.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hein, J.R.; Spinardi, F.; Okamoto, N.; Mizell, K.; Thorburn, D.; Tawake, A. Critical metals in manganese nodules from the Cook Islands EEZ, abundances and distributions. Ore Geol. Rev. 2015, 68, 97–116. [Google Scholar] [CrossRef]
- Machida, S.; Fujinaga, K.; Ishii, T.; Nakamura, K.; Hirano, N.; Kato, Y. Geology and geochemistry of ferromanganese nodules in the Japanese Exclusive Economic Zone around Minamitorishima Island. Geochem. J. 2016, 50, 539–555. [Google Scholar] [CrossRef] [Green Version]
- González, F.J.; Somoza, L.; Hein, J.R.; Medialdea, T.; León, R.; Urgorri, V.; Reyes, J.; Martín-Rubí, J.A. Phosphorites, Co-rich Mn nodules, and Fe-Mn crusts from Galicia Bank, NE Atlantic: Reflections of Cenozoic tectonics and paleoceanography. Geochem. Geophys. Geosyst. 2016, 17, 346–374. [Google Scholar] [CrossRef] [Green Version]
- Conrad, T.A.; Hein, J.R.; Paytan, A.; Clague, D.A. Formation of Fe-Mn crusts within a continental margin environment. Ore Geol. Rev. 2017, 87, 25–40. [Google Scholar] [CrossRef]
- Konstantinova, N.; Hein, J.R.; Gartman, A.; Mizell, K.; Barrulas, P.; Cherkashov, G.; Mikhailik, P.; Khanchuk, A. Mineral Phase-Element Associations Based on Sequential Leaching of Ferromanganese Crusts, Amerasia Basin Arctic Ocean. Minerals 2018, 8, 460. [Google Scholar] [CrossRef] [Green Version]
- Zawadzki, D.; Maciąg, Ł.; Kotliński, R.A.; Kozub-Budzyń, G.A.; Piestrzyński, A.; Wróbel, A. Geochemistry of cobalt-rich ferromanganese crusts from the Perth Abyssal Plain (E Indian Ocean). Ore Geol. Rev. 2018, 101, 520–531. [Google Scholar] [CrossRef]
- Maciąg, Ł.; Zawadzki, D.; Kotliński, R.A.; Kozub-Budzyń, G.A.; Piestrzyński, A.; Wróbel, A. Mineralogy of Cobalt-Rich Ferromanganese Crusts from the Perth Abyssal Plain (E Indian Ocean). Minerals 2019, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Marino, E.; González, F.J.; Lunar, R.; Reyes, J.; Medialdea, T.; Castillo-Carrión, M.; Bellido, E.; Somoza, L. High-Resolution Analysis of Critical Minerals and Elements in Fe–Mn Crusts from the Canary Island Seamount Province (Atlantic Ocean). Minerals 2018, 8, 285. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Sun, X.; Guan, Y.; Xiao, Z.; Liu, Y.; Liao, J.; Guo, Z. Distribution of Rare Earth Elements plus Yttrium among Major Mineral Phases of Marine Fe–Mn Crusts from the South China Sea and Western Pacific Ocean: A Comparative Study. Minerals 2019, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Marino, E.; González, F.J.; Kuhn, T.; Madureira, P.; Wegorzewski, A.V.; Mirao, J.; Medialdea, T.; Oeser, M.; Miguel, C.; Reyes, J.; et al. Hydrogenetic, Diagenetic and Hydrothermal Processes Forming Ferromanganese Crusts in the Canary Island Seamounts and Their Influence in the Metal Recovery Rate with Hydrometallurgical Methods. Minerals 2019, 9, 439. [Google Scholar] [CrossRef] [Green Version]
- Koschinsky, A.; Hein, J.R.; Kraemer, D.; Foster, A.L.; Kuhn, T.; Halbach, P. Platinum enrichment and phase associations in marine ferromanganese crusts and nodules based on a multi-method approach. Chem. Geol. 2020, 539, 119426. [Google Scholar] [CrossRef]
- Nishi, K.; Usui, A.; Nakasato, Y.; Yasuda, H. Formation age of the dual structure and environmental change recorded in hydrogenetic ferromanganese crusts from Northwest and Central Pacific seamounts. Ore Geol. Rev. 2017, 87, 62–70. [Google Scholar] [CrossRef]
- Usui, A.; Nishi, K.; Sato, H.; Nakasato, Y.; Thornton, B.; Kashiwabara, T.; Tokumaru, A.; Sakaguchi, A.; Yamaoka, K.; Kato, S.; et al. Continuous growth of hydrogenetic ferromanganese crusts since 17 Myr ago on Takuyo-Daigo Seamount, NW Pacific, at water depths of 800–5500 m. Ore Geol. Rev. 2017, 87, 71–87. [Google Scholar] [CrossRef] [Green Version]
- Halbach, P.; Jahn, A.; Cherkashov, G. Marine Co-rich ferromanganese crust deposits: Description and formation, occurrences and distribution, estimated world-wide resources. In Deep-Sea Mining; Sharma, R., Ed.; Springer: Cham, Switzerland, 2017; pp. 65–141. [Google Scholar] [CrossRef]
- Yeo, I.; Dobson, K.; Josso, P.; Pearce, R.; Howarth, S.; Lusty, P.A.J.; Le Bas, T.; Murton, B. Assessment of the mineral resource potential of Atlantic ferromanganese crusts based on their growth history, microstructure, and texture. Minerals 2018, 8, 327. [Google Scholar] [CrossRef] [Green Version]
- Bonatti, E.; Kraemer, T.; Rydell, H. Classification and genesis of submarine iron-manganese deposits. In Ferromanganese Deposits on the Ocean Floor, 1st ed.; Horn, D.R., Ed.; IDOE Publ: Washington, DC, USA, 1972; pp. 149–161. [Google Scholar]
- Koschinsky, A.; Hein, J.R. Uptake of elements from seawater by ferromanganese crusts: Solid-phase associations and seawater speciation. Mar. Geol. 2003, 198, 331–351. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A. Deep-ocean ferromanganese crusts and nodules. In The Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 12, pp. 273–291. [Google Scholar] [CrossRef]
- Hein, J.R.; Conrad, T.A.; Staudigel, H. Seamount mineral deposits. A source of rare metals for high-technology industries. Oceanography 2010, 23, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Hein, J.R. Cobalt-Rich Ferromanganese Crusts: Global Distribution, Composition, Origin and research Activities. In: Polymetallic Massive Sulphides and Cobalt-Rich Ferromanganese Crusts: Status and Prospects. International Seabed Authority. Tech. Study 2000, 2, 37–77. [Google Scholar]
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Johnson, K.S.; Coale, K.H.; Berelson, W.M.; Gordon, R.M. On the Formation of the Manganese Maximum in the Oxygen Minimum. Geochim. Cosmochim. Acta. 1996, 60, 1291–1299. [Google Scholar] [CrossRef]
- Dubinin, A.V. Geochemistry of Rare Earth Elements in the Ocean. Lithol. Miner. Resour. 2004, 39, 289–307. (In Russian) [Google Scholar] [CrossRef]
- Asavin, A.M.; Kubrakova, I.V.; Mel’nikov, M.E.; Tyutyunnik, O.A.; Chesalova, E.I. Geochemical Zoning in Ferromanganese Crusts of Ita-MaiTai Guyot. Geochemistry Int. 2010, 48, 423–445. [Google Scholar] [CrossRef]
- Fitzgerald, C.E.; Gillis, K.M. Hydrothermal manganese oxide deposits from Baby Bare seamount in the Northeast Pacific. Ocean. Mar. Geol. 2006, 225, 145–156. [Google Scholar] [CrossRef]
- Klitgord, K.D.; Mudie, J.D. The Galapagos spreading center: A near-bottom geophysical survey. Geophys. J. R. Astron. Soc. 1974, 38, 563–588. [Google Scholar] [CrossRef] [Green Version]
- Hekinian, R.; Rosendahl, B.R.; Cronan, D.S.; Dmitriev, Y.; Fodor, R.V.; Goll, R.M.; Hoffert, M.; Humphris, S.E.; Mattey, D.P.; Matland, J.; et al. Hydrothermal deposits and associated basement rocks from the Galapagos Spreading Center. Oceanolog. Acta 1978, 1, 473–482. [Google Scholar]
- Corliss, J.B.; Lyle, M.; Dymond, J.; Crane, K. The chemistry of hydrothermal mounds near the Galapagos Rift. Earth Planet. Sci. Lett. 1978, 40, 12–24. [Google Scholar] [CrossRef]
- Green, K.E.; Von Herzen, R.P.; Williams, D.L. The Galapagos Spreading Center at 86°W: A detailed geothermal field study. J. Geophys. Res. 1981, 86, 979–986. [Google Scholar] [CrossRef]
- Clague, D.A.; Frey, F.A.; Thompson, G.; Rindge, S. Minor and trace element geochemistry of volcanic rocks dredged from the Galapagos Spreading Center: Role of crystal fractionation and mantle heterogeneity. J. Geophys. Res. 1981, 86, 9469–9482. [Google Scholar] [CrossRef]
- Honnorez, J.; von Herzen, R.P.; Barrett, T.J.; Becker, K.; Bender, M.L.; Borella, P.E.; Hubberten, H.W.; Jones, S.C.; Karato, S.; Laverne, C.; et al. Hydrothermal mounds and young ocean crust of the Galapagos: Preliminary Deep Sea Drilling results, Leg 70. Geo. Soc. Am. Bull. 1981, 92, 457–472. [Google Scholar] [CrossRef]
- Barrett, T.J.; Friedrichsen, H.; Fleet, A.J. Elemental and Stable Isotopic Composition of Some Metalliferous and Pelagic Sediments from the Galapagos Mounds Area. Deep Sea Drilling Project Leg 1983, 70, 315–323. [Google Scholar] [CrossRef]
- Lonsdale, P. Deep-tow observations at the mounds abyssal hydrothermal field, Galapagos Rift. Earth Planet. Sci. Lett. 1977, 36, 92–110. [Google Scholar] [CrossRef]
- Borella, P.E. Sediment lithostratigraphy of the Galapagos hydrothermal mounds. DSDP Rep. Publ. 1983, LXX, 183–195. [Google Scholar] [CrossRef]
- Natland, J.; Rosendahl, B.; Hekinian, R.; Dmitriev, Y.; Fodor, R.; Goll, R.; Hoffert, M.; Humphris, S.; Mattey, D.; Petersen, N.; et al. Galapagos hydrothermal mounds: Stratigraphy and chemistry revealed by deep sea drilling. Science 1979, 204, 613–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varentsov, I.M.; Sakharov, B.A.; Drits, V.A.; Tsipursky, S.I.; Choporov, D.Y.; Aleksandrova, V.A. Hydrothermal Deposits of the Galapagos Rift Zone, Leg 70: Mineralogy and Geochemistry of Major Components. Initial. Rep. Deep. Sea Drill. Proj. 1983, 70, 235–268. [Google Scholar] [CrossRef]
- Lalou, C.; Brichet, E.; Leclaire, H.; Duplessy, J.D. Uranium Series Disequilibrium and Isotope Stratigraphy in Hydrothermal Mound Samples from DSDP Sites 506-509, Leg 70, and Site 424, Leg 54: An Attempt at Chronology. In Initial Reports of the Deep Sea Drilling Project covering Leg 70 of the Cruises of the Drilling Vessel Glomar Challenger, Balboa, Panama to Callao, Peru, November–December; U.S. Govt. Printing Office: Washington, DC, USA, 1979; pp. 303–314. [Google Scholar] [CrossRef]
- Moore, W.S.; Vogt, P.R. Hydrothermal manganese crusts from two sites near the Galapagos spreading axis. Earth Planet. Sci. Lett. 1976, 29, 349–356. [Google Scholar] [CrossRef]
- Herzig, P.M.; Becker, K.P.; Stoffers, P.; Backer, H.; Blum, N. Hydrothermal silica chimney fields in the Galapagos spreading center at 86° W. Earth Planet. Sci. Lett. 1988, 89, 261–272. [Google Scholar] [CrossRef]
- Nagender Nath, B. Rare Earth Element Geochemistry of the Sediments, Ferromanganese Nodules and Crusts from the Indian Ocean. Ph.D. Thesis, GOA University, Goa, India, 1993. [Google Scholar]
- Smith, D.K.; Schouten, H.; Parnell-Turner, R.; Klein, E.M.; Cann, J.R.; Dunham, C.; Alodia, G.; Blasco, I.; Wernette, B.W.; Zawadzki, D.; et al. The Evolution of Seafloor Spreading Behind the Tip of the Westward Propagating Cocos-Nazca Spreading Center. Geochem. Geophys. Geosyst. 2020, 21, e2020GC008957. [Google Scholar] [CrossRef]
- Smith, D.K.; Schouten, H. Opening of Hess Deep Rift at the Galapagos Triple Junction. Geophys. Res. Lett. 2018, 45, 3942–3950. [Google Scholar] [CrossRef] [Green Version]
- DeMets, C.; Gordon, R.G.; Argus, D.F.; Stein, S. Effect of recent revisions to the geomagnetic reversal time scale on estimates of current plate motions. Geophys. Res. Lett. 1994, 21, 2191–2194. [Google Scholar] [CrossRef]
- Lonsdale, P. Regional shape and tectonics of the equatorial East Pacific Rise. Mar. Geophys. Res. 1977, 3, 295–315. [Google Scholar] [CrossRef]
- Smith, D.K.; Schouten, H.; Montési, L.; Zhu, W. The recent history of the Galapagos triple junction preserved on the Pacific plate. Earth Planet. Sci. Lett. 2013, 371–372, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.K.; Klein, E.M.; Schouten, H.; Parnell-Turner, R.; Cann, J.R.; Dunham, C.; Alodia, G.; Blasco, I.; Wernette, B.W.; Zawadzki, D.; et al. Transition from rifting to seafloor spreading behind the tip of the westward propagating Cocos-Nazca spreading center. In Proceedings of the American Geophysical Union Fall Abstracts, Washington, DC, USA, 10–14 December 2018. [Google Scholar]
- Schilling, J.G.; Kingsley, R.H.; Devine, J.D. Galapagos hot spot spreading center system 1. Spatial petrological and geochemical variations (83° W–101° W). J. Geophys. Res. 1982, 87, 5593–5610. [Google Scholar] [CrossRef]
- Puchelt, H.; Emmermann, R. Petrogenetic implications of tholeiitic basalt glasses from the East Pacific Rise and the Galápagos Spreading Center. Chem. Geol. 1983, 38, 39–56. [Google Scholar] [CrossRef]
- Hekinian, R.; Bideau, D.; Francheteau, J.; Cheminee, J.L.; Armijo, R.; Lonsdale, P.; Blum, N. Petrology of the East Pacific Rise crust and upper mantle exposed in Hess Deep (eastern equatorial Pacific). J. Geophys. Res. 1993, 98, 8069–8094. [Google Scholar] [CrossRef]
- Francheteau, J.; Armijo, R.; Cheminee, J.L.; Hekinian, R.; Lonsdale, P.; Blum, N. 1 Ma East Pacific Rise oceanic crust and uppermost mantle exposed by rifting in Hess Deep (equatorial Pacific Ocean). Earth Planet. Sci. Lett. 1990, 101, 281–295. [Google Scholar] [CrossRef]
- Mével, C.; Gillis, K.M. Introduction and principal results. In Proceedings of the Ocean Drilling Program, Initial Reports; Gillis, K., Mével, C., Allan, J., Eds.; Ocean Drilling Program: College Station, TX, USA, 1993; Volume 147. [Google Scholar] [CrossRef]
- Karson, J.A.; Klein, E.M.; Hurst, S.D.; Lee, C.D.; Rivizzigno, P.A.; Curewitz, D.; Morris, A.R.; Party, H.D.S. Structure of uppermost fast spreading oceanic crust exposed at the Hess Deep Rift: Implications for subaxial processes at the East Pacific Rise. Geochem. Geophys. Geosyst. 2002, 3. [Google Scholar] [CrossRef]
- Stewart, M.A.; Klein, E.M.; Karson, J.A. The geochemistry of dikes and lavas from the north wall of the Hess Deep Rift: Insights into the four-dimensional character of crustal construction at fast-spreading mid-ocean ridges. J. Geophys. Res. 2002, 107, EPM 4-1–EPM 4-24. [Google Scholar] [CrossRef]
- Hey, R. Tectonic evolution of the Cocos-Nazca spreading center. Geo. Soc. Am. Bull. 1977, 88, 1404–1420. [Google Scholar] [CrossRef]
- Lonsdale, P. Structural patterns of the Galapagos Microplate and evolution of the Galapagos triple junction. J. Geophys. Res. 1988, 93, 13551–13574. [Google Scholar] [CrossRef]
- Schouten, H.; Smith, D.K.; Montési, L.; Zhu, W.; Klein, E.M. Unstable Northern Rifts of the Galapagos Triple Junction. Geology 2008, 36, 330–342. [Google Scholar] [CrossRef]
- Lonsdale, P.; Blum, N.; Puchelt, H. The RRR triple junction at the southern end of the Pacific-Cocos East Pacific Rise. Earth Planet. Sci. Lett. 1992, 109, 73–85. [Google Scholar] [CrossRef]
- Beaulieu, S.E.; Baker, E.T.; German, C.R.; Maffei, A. An authoritative global database for active submarine hydrothermal vent fields. Geochem. Geophys. Geosys. 2013, 14, 4892–4905. [Google Scholar] [CrossRef] [Green Version]
- Baker, E.T.; Haymon, R.M.; Resing, J.A.; White, S.M.; Walker, S.L.; Macdonald, K.C.; Nakamura, K. High-resolution surveys along the hot spot–affected Galápagos Spreading Center: 1. Distribution of hydrothermal activity. Geochem. Geophys. Geosyst. 2008, 9, Q09003. [Google Scholar] [CrossRef]
- Klein, E.M.; Smith, D.K.; Cann, J.R.; Asfhar, S.; Alodia, G.; Blasco, I.; Curry, S.; Dunham, C.; Latypova, E.; Zawadzki, D. The Transition from Rifting to Seafloor Spreading at the Western Tip of the Cocos-Nazca Rift, R/V Sally Ride Leg 1806 Manzanillo, Mexico to San Diego, CA, USA, 20 April–26 May 2018; Cruise Report Investigation of the Cocos-Nazca Spreading Center; National Science Foundation: Alexandria, VA, USA, 2018. [Google Scholar]
- Brouwer, P. Theory of XRF: Getting Acquainted with the Principles; PANalytical: Almela, The Netherlands, 2010. [Google Scholar]
- Sindern, S. Analysis of Rare Earth Elements in Rock and Mineral Samples by ICP-MS and LA-ICP-MS. Phys. Sci. Rev. 2017, 2, 1–14. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific Publication: Carlton, Australia, 1985; p. 312. [Google Scholar]
- Bau, M.; Schmidt, K.; Koschinsky, A.; Hein, J.R.; Kuhn, T.; Usui, A. Discriminating between different genetic types of marine ferromanganese crusts and nodules based on rare earth elements and yttrium. Chem. Geol. 2014, 381, 1–9. [Google Scholar] [CrossRef]
- Dubinin, A.V.; Sval’nov, V.N. Geochemistry of Rare Earth Elements in Micro and Macronodules from the Pacific Bioproductive Zone. Lithol. Miner. Resour. 2000, 35, 25–39. [Google Scholar] [CrossRef]
- Grazulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.T.; Quiros, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An open-access collection of crystal structures. J. Appl. Cryst. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Cui, H.; Qiu, G.; Feng, X.; Tan, W.; Liu, F. Birnessites with different average manganese oxidation states synthesized, characterized, and transformed to todorokite at atmospheric pressure. Clays Clay Miner. 2009, 57, 715–724. [Google Scholar] [CrossRef]
- Golden, D.C.; Chen, C.C.; Dixon, J.B. Transformation of Birnessite to Buserite, Todorokite, and Manganite under Mild Hydrothermal Treatment. Clays Clay Miner. 1987, 35, 271–280. [Google Scholar] [CrossRef]
- Wegorzewski, A.V.; Kuhn, T.; Dohrmann, R.; Wirth, R.; Grangeon, S. Mineralogical characterization of individual growth structures of Mn-nodules with different Ni+Cu content from the central Pacific Ocean. Am. Mineral. 2015, 100, 2497–2508. [Google Scholar] [CrossRef]
- Lee, S.; Xu, H. XRD and TEM Studies on Nanophase Manganese Oxides in Freshwater Ferromanganese Nodules from Green Bay, Lake Michigan. Clays Clay Miner. 2016, 64, 523–536. [Google Scholar] [CrossRef]
- The Mineralogy Database. Available online: Webmineral.com (accessed on 2 February 2022).
- Manheim, F.T.; Lane-Bostwick, C.M. Cobalt in ferromanganese crusts as a monitor of hydrothermal discharge on the Pacific sea floor. Nature 1988, 335, 59–62. [Google Scholar] [CrossRef]
- Skowronek, A.; Maciąg, Ł.; Zawadzki, D.; Strzelecka, A.; Baláž, P.; Mianowicz, K.; Abramowski, T.; Konečný, P.; Krawcewicz, A. Chemostratigraphic and Textural Indicators of Nucleation and Growth of Polymetallic Nodules from the Clarion–Clipperton Fracture Zone (IOM Claim Area). Minerals 2021, 11, 868. [Google Scholar] [CrossRef]
- De Baar, H.J.; Brewer, P.G.; Bacon, M.P. Anomalies in rare earth distributions in seawater: Gd and Tb. Geochim. Cosmochim. Acta 1985, 49, 1961–1969. [Google Scholar] [CrossRef]
- Josso, P.; Pelleter, E.; Pourret, O.; Fouquet, Y.; Etoubleau, J.; Cheron, S.; Bollinger, C. A new discrimination scheme for oceanic ferromanganese deposits using high field strength and rare earth elements. Ore Geol. Rev. 2017, 87, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Gale, A.; Dalton, C.A.; Langmuir, C.H.; Su, Y.; Schilling, J.G. The mean composition of ocean ridge basalts. Geochem. Geophys. Geosyst. 2013, 14, 489–518. [Google Scholar] [CrossRef] [Green Version]
- Puteanus, D.; Halbach, P. Correlation of Co concentration and growth rate—A method for age determination of ferromanganese crusts. Chem. Geol. 1988, 69, 73–85. [Google Scholar] [CrossRef]
- German, C.R.; Colley, S.; Palmer, M.R.; Khripounoff, A.; Klinkhammer, G.P. Hydrothermal plume-particle fluxes at 13°N on the East Pacific Rise. Deep-Sea Res. I Oceanogr. Res. Pap. 2002, 49, 1921–1940. [Google Scholar] [CrossRef]
- Bonatti, E.; Zerbi, M.; Kay, R.; Rydell, H. Metalliferous deposits from the Apennine ophiolites: Mesozoic equivalent of modern deposits from oceanic spreading center. Geol. Soc. Am. Bull. 1976, 87, 83–94. [Google Scholar] [CrossRef]
- Bau, M. Rare-earth element mobility during hydrothermal and metamorphic fluid– rock interaction and the significance of the oxidation state of europium. Chem. Geol. 1991, 93, 219–230. [Google Scholar] [CrossRef]
- Dubinin, A.V.; Sval’nov, V.N. Geochemistry of the Manganese Ore Process in the Ocean: Evidence from Rare Earth Elements. Lithol. Miner. Resour. 2003, 38, 91–100. [Google Scholar] [CrossRef]
- Ohta, A.; Ishii, S.; Sakakibara, M.; Mizuno, A.; Kawabe, I. Systematic correlation of the Ce anomaly with the Co/(Ni + Cu) ratio and Y fractionation from Ho in distinct types of Pacific deep-sea nodules. Geochem. J. 1999, 33, 399–417. [Google Scholar] [CrossRef]
- Shatrov, V.A.; Sirotin, V.I.; Voitsekhovsky, G.V.; Belyavtseva, E.E. Rare earth elements as indicators of the tectonic activity of the basement. Dokl. Earth Sci. 2008, 423, 672–673. [Google Scholar] [CrossRef]
- Nagender Nath, B.; Balaram, V.; Sudhakar, M.; Plüger, W.L. Rare earth element geochemistry of ferromanganese deposits from the Indian Ocean. Mar. Chem. 1992, 38, 185–208. [Google Scholar] [CrossRef]
- Yuan, L.; Sun, L.; Fortin, D.; Wang, Y.; Yin, X. Microscale Characterization and Trace Element Distribution in Bacteriogenic Ferromanganese Coatings on Sand Grains from an Intertidal Zone of the East China Sea. PLoS ONE 2015, 10, e0119080. [Google Scholar] [CrossRef] [Green Version]
- Toth, J.R. Deposition of submarine crusts rich in manganese and iron. Geo. Soc. Am. Bull. 1980, 91, 44–54. [Google Scholar] [CrossRef]
- Nagender Nath, B.; Plüger, W.L.; Roelandts, I. Geochemical constraints on the hydrothermal origin of ferromanganese encrustations from the Rodriguez Triple Junction, Indian Ocean. Geol. Soc. Lond. Spec. Publ. 1997, 119, 199–211. [Google Scholar] [CrossRef]
- Corliss, J.B.; Dymond, J. Nazca plate metalliferous sediments. I. Elemental distribution patterns in surface sediments (abs). EOS 1975, 56, 445. [Google Scholar]
- Atkins, A.L.; Shaw, S.; Peacock, C.L. Nucleation and growth of todorokite from birnessite: Implications for trace-metal cycling in marine sediments. Geochim. Cosmochim. Acta 2014, 144, 109–125. [Google Scholar] [CrossRef] [Green Version]
- Nath, B.N.; Roelandts, I.; Sudhakar, M.; Plüger, W.; Balaram, V. Cerium anomaly variations in ferromanganese nodules and crusts from the Indian Ocean. Mar. Geol. 1994, 120, 385–400. [Google Scholar] [CrossRef]
- Choi, J.H.; Hariya, Y. Geochemistry and depositional environment of Mn oxide deposits in the Tokora Belt, Northeastern Hokkaido, Japan. Econ. Geol. 1992, 87, 12–24. [Google Scholar] [CrossRef]
- Prakash, S.L.; Ray, D.; Nagender Nath, B.; Satyanarayanan, M.; Kamesh Raju, K.A.; Kurian, J.P.; Kumar, D.; Srinivas Rao, A. Anomalous phase association of REE in ferromanganese crusts from Indian mid-oceanic ridges: Evidence for large scale dispersion of hydrothermal iron. Chem. Geol. 2020, 549, 119679. [Google Scholar] [CrossRef]
- Liao, L.; Xu, X.W.; Jiang, X.W.; Wang, C.S.; Zhang, D.S.; Ni, J.Y.; Wu, M. Microbial diversity in deep-sea sediment from the cobalt-rich crust deposit region in the Pacific Ocean. FEMS Microbiol. Ecol. 2011, 78, 565–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, S.; Hirai, M.; Ohkuma, M.; Suzuki, K. Microbial metabolisms in an abyssal ferromanganese crust from the Takuyo-Daigo Seamount as revealed by metagenomics. PLoS ONE 2019, 14, e0224888. [Google Scholar] [CrossRef] [PubMed]
- Crerar, D.A.; Namson, J.; Chyi, M.S.; Williams, I.; Feigenson, M.D. Manganiferrous cherts of the Franciscian assemblage: General geology, ancient and modern analogues, and implications for hydrothermal convection at oceanic spreading centres. Econ. Geol. 1982, 77, 519–540. [Google Scholar] [CrossRef]
- Nicholson, K. Genetic types of manganese oxide deposits near Inverness, Scotland. A Devonian sublacustrine hot-spring deposit. Miner. Deposita 1992, 25, 126–131. [Google Scholar]
- Hein, J.R.; Koschinsky, A.; Halbach, P.; Manheim, F.T.; Bau, M.; Kang, J.K.; Lubick, N. Iron and manganese oxide mineralization in the Pacific. In Manganese Mineralization: Geochemistry and Mineralogy of Terrestrial and Marine Deposits; Nicholson, K., Hein, J.R., Bühn, B., Dasgupta, S., Eds.; Geological Society, Special Publications: London, UK, 1997; Volume 119, pp. 123–138. [Google Scholar]
- Hein, J.R.; Petersen, S. The Geology of Cobalt-rich Ferromanganese Crusts. In Deep Sea Minerals: Cobalt-Rich Ferromanganese Crusts, A Physical, Biological, Environmental, and Technical Review; Baker, E., Beaudoin, Y., Eds.; Secretariat of the Pacific Community; GRID-Arendal: Arendal, Norway, 2013; Volume 1. [Google Scholar]
- Benjamin, J.; Rovere, A.; Fontana, A.; Furlani, S.; Vacchi, M.; Inglis, R.H.; Galili, E.; Antonioli, F.; Sivan, D.; Miko, S.; et al. Late Quaternary sea-level changes and early human societies in the central and eastern Mediterranean Basin: An interdisciplinary review. Quat. Int. 2017, 449, 29–57. [Google Scholar] [CrossRef] [Green Version]
- Lea, D.W.; Pak, D.K.; Spero, H.J. Climate Impact of Late Quaternary Equatorial Pacific Sea Surface Temperature Variations. Science 2000, 289, 1719–1724. [Google Scholar] [CrossRef] [Green Version]
- Theyer, F.; Mayer, L.; Barron, J.; Thomas, E. The equatorial Pacific High-Productivity Belt: Elements for a Synthesis of Deep Sea Drilling Project Leg 85 Results. Initial Reports DSDP 1985, 85. [Google Scholar] [CrossRef]
- Staszak, P.; Collot, J.; Josso, P.; Pelleter, E.; Etienne, S.; Patriat, M.; Cheron, S.; Boissier, A.; Guyomard, Y. Origin and Composition of Ferromanganese Deposits of New Caledonia Exclusive Economic Zone. Minerals 2022, 12, 255. [Google Scholar] [CrossRef]
- Baturin, G.N. Geochemistry of Hydrothermal Ferromanganese Crusts of the Sea of Japan. In Doklady Earth Sciences; Springer Nature BV: Dordrecht, The Netherlands, 2012; Volume 445, pp. 862–867. [Google Scholar]
- Mikhailik, P.E.; Mikhailik, V.; Blokhin, M.G.; Zarubina, N.V. Sources of gallium in ferromanganese crusts from the Sea of Japan. Russ. Geol. Geophys. 2015, 56, 1148–1153. [Google Scholar] [CrossRef]
- Lui-Heung, C.; Hein, J.R. Lithium contents and isotopic compositions of ferromanganese deposits from the global ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 1147–1162. [Google Scholar] [CrossRef]
- International Seabed Authority. Available online: http://www.isa.org.jm/ (accessed on 6 June 2021).



| IGSNID 1 | Crust Thickness (mm) | Textural Type | EPMA | ICP-MS | SEM | XRF | XRD 2 |
|---|---|---|---|---|---|---|---|
| SIO0000D2 | 1–6 | 1 | x | x | - | x | x |
| SIO0000BT | 1–4 | 1 | - | x | x | x | x |
| SIO0000BQ | 3–10 | 1 | - | x | x | x | x |
| SIO0000D1 | 20 | 2 | x | x | - | x | x |
| SIO0000BP | 30 | 2 | x | x | x | x | x |
| SIO00003U | 1–10 | 3 | x | x | x | x | x |
| Major | Minor | |
|---|---|---|
| SIO0000D2 | Vernadite | - |
| SIO0000BT | Vernadite | Buserite, Manganite |
| SIO0000BQ | Birnessite, Vernadite | Buserite, Clays |
| SIO0000D1 | Birnessite, Vernadite | - |
| SIO0000BP | Todorokite, Buserite, Vernadite | Birnessite, Fe-ox., Clays, Manganite |
| SIO00003U | Birnessite, Vernadite | Todorokite, Buserite |
| MDL * | SIO0000D2 | SIO0000BT | SIO0000BQ | SIO0000D1 | SIO0000BP | SIO00003U | Mean | ||
|---|---|---|---|---|---|---|---|---|---|
| Fe | wt % | 0.01 | 26.86 | 23.88 | 18.31 | 4.96 | 13.49 | 11.75 | 16.54 |
| Mn | 0.01 | 17.11 | 18.01 | 15.00 | 44.13 | 28.70 | 36.48 | 26.57 | |
| Si | 0.01 | 2.72 | 2.91 | 6.30 | 1.30 | 4.39 | 1.70 | 3.22 | |
| Al | 0.01 | 0.68 | 0.65 | 0.68 | 0.68 | 0.20 | 0.59 | 0.58 | |
| Mg | 0.01 | 0.42 | 0.41 | 0.90 | 1.15 | 0.89 | 0.75 | 0.75 | |
| K | 0.01 | 0.32 | 0.36 | 1.32 | 0.97 | 1.20 | 0.64 | 0.80 | |
| Ca | 0.01 | 1.67 | 1.60 | 0.91 | 1.65 | 0.75 | 1.47 | 1.34 | |
| Na | 0.01 | 0.55 | 0.62 | 0.79 | 1.02 | 0.81 | 0.97 | 0.79 | |
| P | 0.001 | 0.144 | 0.104 | 0.015 | 0.031 | 0.009 | 0.075 | 0.063 | |
| Ti | 0.01 | 0.26 | 0.23 | 0.09 | 0.11 | 0.03 | 0.14 | 0.14 | |
| Element | MDL ** | SIO0000D2 | SIO0000BT | SIO0000BQ | SIO0000D1 | SIO0000BP | SIO00003U | Mean | |
| As | mg/kg (ppm) | 0.2 | 188.0 | 125.0 | 18.0 | 37.0 | 21.0 | 78.0 | 78.0 |
| Ba | 1 | 1160 | 1222 | 397 | 645 | 1639 | 920 | 997 | |
| Be | 1 | 4 | 3 | 1 | <1 | <1 | <1 | 2 | |
| Bi | 0.04 | 1.95 | 1.47 | 0.27 | 0.43 | 0.15 | 0.79 | 0.84 | |
| Cd | 0.02 | 2.80 | 3.80 | 7.40 | 19.60 | 27.10 | 20.00 | 13.45 | |
| Co | 0.2 | 449.0 | 373.0 | 78.0 | 298.7 | 72.0 | 202.0 | 245.5 | |
| Cr | 1 | 14 | 18 | 43 | 58 | 6 | 14 | 26 | |
| Cs | 0.1 | <0.1 | <0.1 | 1.1 | <0.1 | 0.6 | <0.1 | 0.9 | |
| Cu | 0.1 | 860.0 | 902.0 | 758.0 | 644.4 | 569.0 | 899.0 | 772.1 | |
| Ga | 0.02 | 3.74 | 4.31 | 10.36 | 10.03 | 11.79 | 14.09 | 9.05 | |
| Hf | 0.02 | 0.08 | <0.02 | 1.14 | 2.07 | 0.52 | <0.02 | 0.95 | |
| In | 0.01 | 0.16 | 0.19 | 0.06 | 0.09 | 0.01 | 0.08 | 0.10 | |
| Li | 0.1 | 6.0 | 22.0 | 209.0 | 738.0 | 277.0 | 514.0 | 294.3 | |
| Mo | 0.05 | 198.00 | 145.00 | 159.00 | 432.50 | 284.00 | 436.00 | 275.75 | |
| Nb | 0.04 | 0.92 | 1.94 | 1.64 | 3.16 | 0.97 | 5.50 | 2.36 | |
| Ni | 0.1 | 1683.0 | 1487.0 | 1563.0 | 1769.7 | 1288.0 | 2118.0 | 1651.5 | |
| Pb | 0.02 | 147.00 | 122.00 | 16.00 | 20.02 | 8.00 | 52.00 | 60.84 | |
| Rb | 0.1 | 3.0 | 3.0 | 43.0 | 5.3 | 32.0 | 5.0 | 15.2 | |
| Re | 0.002 | <0.002 | 0.002 | <0.002 | <0.002 | <0.002 | <0.002 | - | |
| Sb | 0.02 | 21.00 | 19.00 | 38.00 | 59.43 | 69.00 | 96.00 | 50.41 | |
| Se | 0.3 | 2.2 | 1.6 | 0.5 | 0.3 | 0.3 | 1.3 | 1.0 | |
| Sn | 0.1 | 2.7 | 2.3 | 0.8 | 0.8 | 0.2 | 1.7 | 1.4 | |
| Sr | 1 | 1146 | 1055 | 205 | 445 | 328 | 599 | 630 | |
| Ta | 0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | - | |
| Te | 0.05 | 3.92 | 3.83 | 0.33 | 0.83 | 0.49 | 1.67 | 1.85 | |
| Th | 0.1 | 4.9 | 4.3 | 0.8 | 0.9 | 0.4 | 2.6 | 2.3 | |
| Tl | 0.05 | 35.00 | 19.00 | 57.00 | 71.88 | 84.00 | 94.00 | 60.15 | |
| U | 0.1 | 8.9 | 6.6 | 1.7 | 3.6 | 3.4 | 5.7 | 5.0 | |
| W | 0.1 | 11.8 | 13.9 | 4.2 | 9.9 | 6.1 | 18.4 | 10.7 | |
| V | 1 | 549 | 532 | 113 | 261 | 138 | 375 | 328 | |
| Zn | 0.2 | 574.0 | 512.0 | 619.0 | 960.5 | 711.0 | 933.0 | 718.3 | |
| Zr | 0.2 | 5.9 | 8.2 | 82.0 | 117.4 | 38.1 | 16.1 | 44.6 | |
| La | 0.1 | 175.0 | 170.0 | 15.0 | 24.7 | 13.0 | 67.0 | 77.5 | |
| Ce | 0.02 | 107.00 | 83.00 | 12.00 | 18.58 | 9.00 | 41.00 | 45.10 | |
| Pr | 0.1 | 34.1 | 34.0 | 4.8 | 5.1 | 2.4 | 13.8 | 15.7 | |
| Nd | 0.1 | 125.0 | 128.0 | 12.0 | 24.1 | 9.0 | 52.0 | 58.4 | |
| Sm | 0.1 | 26.3 | 26.5 | 2.7 | 5.2 | 2.1 | 10.7 | 12.3 | |
| Eu | 0.1 | 7.8 | 8.0 | 0.9 | 1.5 | 0.6 | 3.1 | 3.7 | |
| Gd | 0.1 | 33.6 | 33.0 | 3.1 | 6.7 | 3.4 | 14.0 | 15.6 | |
| Tb | 0.1 | 5.8 | 5.8 | 1.6 | 1.1 | 0.5 | 2.4 | 2.9 | |
| Dy | 0.1 | 33.8 | 32.2 | 3.6 | 7.4 | 2.6 | 14.2 | 15.6 | |
| Y | 0.1 | 138.0 | 122.0 | 19.0 | 36.9 | 16.0 | 65.0 | 66.2 | |
| Ho | 0.1 | 7.8 | 7.6 | 0.9 | 1.7 | 0.7 | 3.7 | 3.7 | |
| Er | 0.1 | 21.0 | 19.9 | 2.4 | 5.1 | 2.1 | 9.3 | 10.0 | |
| Tm | 0.1 | 3.3 | 2.9 | 0.4 | 0.7 | 0.4 | 1.5 | 1.5 | |
| Yb | 0.1 | 19.2 | 19.2 | 2.4 | 4.6 | 2.1 | 8.4 | 9.3 | |
| Lu | 0.1 | 3.4 | 3.1 | 0.4 | 0.7 | 0.4 | 1.5 | 1.6 | |
| Sc | 0.1 | 11.6 | 10.6 | 7.7 | 8.8 | 1.6 | 5.3 | 7.6 | |
| ΣREY | - | 741.10 | 695.20 | 81.20 | 144.08 | 64.30 | 307.60 | 338.91 | |
| ΣLREE | - | 475.20 | 449.50 | 47.40 | 79.18 | 36.10 | 187.60 | 212.50 | |
| ΣHREE | - | 265.90 | 245.70 | 33.80 | 64.90 | 28.20 | 120.00 | 126.42 | |
| LREE/HREE | - | 0.53 | 0.57 | 0.51 | 0.37 | 0.34 | 0.48 | 0.47 | |
| CeSN/CeSN * | - | 0.32 | 0.25 | 0.32 | 0.38 | 0.37 | 0.31 | 0.33 | |
| LaSN/LuSN * | - | 0.58 | 0.62 | 0.43 | 0.40 | 0.37 | 0.51 | 0.48 | |
| Euan | - | 1.21 | 1.25 | 1.45 | 1.17 | 1.00 | 1.16 | 1.21 | |
| YSN/HoSN | - | 0.65 | 0.59 | 0.77 | 0.80 | 0.84 | 0.64 | 0.72 | |
| ∑(Ni,Cu,Co) | mg/kg (ppm) | - | 2992 | 2762 | 2399 | 2713 | 1929 | 3219 | 2669 |
| Fe/Mn | - | 1.57 | 1.33 | 1.22 | 0.11 | 0.47 | 0.32 | 0.84 | |
| Mn/Fe | - | 0.64 | 0.75 | 0.81 | 8.90 | 2.13 | 3.10 | 2.72 | |
| Co/Zn | - | 0.78 | 0.73 | 0.13 | 0.31 | 0.10 | 0.22 | 0.34 | |
| Si/Al | - | 4.02 | 4.46 | 9.29 | 1.91 | 22.41 | 2.88 | 7.49 | |
| Th/U | - | 0.55 | 0.65 | 0.47 | 0.25 | 0.12 | 0.46 | 0.46 |
| Si | Al | Cl | Ba | Ca | Fe | Mn | Ti | P | Pb | Cu | Co | K | Sr | Zn | Tl | Ni | S | Na | Mg | As | Rest * | Expl. var. (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Si | |||||||||||||||||||||||
| Al | 0.63 | ||||||||||||||||||||||
| Cl | 0.11 | 0.05 | |||||||||||||||||||||
| Ba | −0.14 | −0.13 | −0.06 | ||||||||||||||||||||
| Ca | −0.25 | −0.16 | 0.52 | 0.10 | |||||||||||||||||||
| Fe | 0.77 | 0.62 | 0.33 | −0.07 | 0.17 | ||||||||||||||||||
| Mn | −0.87 | −0.68 | −0.27 | 0.13 | −0.02 | −0.96 | |||||||||||||||||
| Ti | 0.46 | 0.46 | 0.42 | 0.19 | 0.45 | 0.77 | −0.70 | ||||||||||||||||
| P | 0.41 | 0.36 | 0.47 | 0.01 | 0.57 | 0.85 | −0.73 | 0.81 | |||||||||||||||
| Pb | 0.25 | 0.11 | 0.05 | 0.08 | 0.12 | 0.34 | −0.32 | 0.27 | 0.31 | ||||||||||||||
| Cu | −0.13 | 0.29 | −0.19 | −0.04 | 0.12 | −0.06 | 0.04 | −0.01 | 0.05 | −0.01 | |||||||||||||
| Co | 0.32 | 0.29 | 0.47 | 0.00 | 0.35 | 0.54 | −0.49 | 0.59 | 0.57 | 0.15 | −0.04 | ||||||||||||
| K | 0.11 | −0.29 | −0.37 | −0.15 | −0.59 | −0.46 | 0.34 | −0.64 | −0.70 | −0.14 | −0.27 | −0.42 | |||||||||||
| Sr | 0.22 | 0.24 | 0.08 | 0.09 | 0.07 | 0.30 | −0.27 | 0.40 | 0.31 | 0.16 | 0.12 | 0.20 | −0.23 | ||||||||||
| Zn | −0.24 | −0.04 | −0.21 | −0.12 | 0.15 | −0.20 | 0.21 | −0.13 | −0.04 | −0.11 | 0.53 | −0.11 | −0.07 | 0.13 | |||||||||
| Tl | −0.14 | −0.12 | −0.15 | −0.02 | −0.12 | −0.18 | 0.19 | −0.16 | −0.16 | −0.12 | 0.03 | −0.22 | 0.17 | −0.03 | 0.25 | ||||||||
| Ni | −0.17 | 0.24 | −0.17 | −0.05 | 0.13 | −0.11 | 0.09 | −0.04 | 0.02 | −0.02 | 0.94 | −0.05 | −0.26 | 0.13 | 0.55 | 0.02 | |||||||
| S | 0.03 | 0.03 | 0.56 | 0.05 | 0.73 | 0.34 | −0.23 | 0.47 | 0.63 | 0.18 | 0.06 | 0.40 | −0.51 | 0.18 | 0.06 | −0.17 | 0.13 | ||||||
| Na | −0.53 | −0.47 | −0.32 | −0.15 | −0.18 | −0.72 | 0.72 | −0.62 | −0.66 | −0.31 | −0.09 | −0.48 | 0.35 | −0.29 | 0.09 | 0.10 | −0.07 | −0.33 | |||||
| Mg | −0.50 | −0.34 | −0.34 | −0.05 | −0.28 | −0.77 | 0.73 | −0.67 | −0.70 | −0.25 | 0.26 | −0.45 | 0.54 | −0.17 | 0.18 | 0.21 | 0.32 | −0.33 | 0.40 | ||||
| As | −0.31 | −0.13 | −0.29 | −0.01 | −0.16 | −0.49 | 0.46 | −0.41 | −0.45 | −0.09 | 0.26 | −0.30 | 0.34 | −0.12 | 0.13 | 0.10 | 0.31 | −0.25 | 0.22 | 0.69 | |||
| Rest * | −0.44 | −0.27 | −0.06 | 0.00 | −0.06 | −0.28 | 0.19 | −0.19 | −0.20 | −0.08 | −0.10 | −0.11 | −0.19 | −0.18 | −0.05 | −0.01 | −0.10 | −0.17 | 0.04 | −0.07 | −0.11 | ||
| FA | |||||||||||||||||||||||
| Factor 1 | 0.86 | 0.77 | 0.15 | −0.13 | −0.09 | 0.93 | −0.97 | 0.67 | 0.66 | 0.28 | 0.03 | 0.46 | −0.31 | 0.34 | −0.13 | −0.13 | −0.03 | 0.12 | −0.68 | −0.68 | −0.46 | −0.23 | 33.7 |
| Factor 2 | −0.18 | 0.31 | −0.23 | −0.07 | 0.12 | −0.09 | 0.08 | −0.02 | 0.03 | −0.03 | 0.95 | −0.05 | −0.31 | 0.16 | 0.62 | −0.002 | 0.95 | 0.07 | −0.08 | 0.30 | 0.35 | −0.05 | 13.6 |
| Factor 3 | −0.14 | −0.12 | 0.73 | 0.01 | 0.90 | 0.27 | −0.11 | 0.51 | 0.65 | 0.07 | −0.0004 | 0.51 | −0.60 | 0.11 | 0.06 | −0.15 | 0.04 | 0.85 | −0.23 | −0.33 | −0.26 | −0.12 | 11.8 |
| Factor 4 | 0.33 | 0.08 | 0.05 | −0.10 | −0.07 | 0.01 | 0.004 | −0.06 | −0.08 | 0.09 | 0.01 | 0.008 | 0.50 | 0.13 | −0.02 | 0.06 | 0.05 | 0.08 | −0.004 | 0.39 | 0.44 | −0.84 | 6.0 |
| Factor 5 | −0.05 | −0.07 | −0.13 | 0.84 | 0.11 | 0.06 | 0.001 | 0.31 | 0.14 | 0.46 | 0.001 | 0.02 | −0.19 | 0.38 | −0.13 | −0.03 | 0.0004 | 0.08 | −0.30 | −0.05 | 0.08 | −0.03 | 5.6 |
| Factor 6 | 0.05 | 0.13 | 0.13 | 0.004 | −0.02 | 0.02 | −0.07 | −0.01 | −0.03 | 0.14 | −0.0003 | 0.18 | −0.04 | −0.27 | −0.51 | −0.81 | 0.01 | 0.01 | −0.08 | 0.03 | 0.20 | 0.11 | 5.0 |
| Other factors | (below 5% of explained variance) | Sum = 75.7% | |||||||||||||||||||||
| Co-Chronometer Data | |||||||
|---|---|---|---|---|---|---|---|
| Id | SIO0000D2 | SIO0000BT | SIO0000BQ | SIO0000D1 | SIO0000BP | SIO00003U | Mean |
| Co (mg/kg) | 449 | 373 | 78 | 299 | 72 | 202 | 246 |
| Co (wt %) | 0.0449 | 0.0373 | 0.0078 | 0.02987 | 0.0072 | 0.0202 | 0.02455 |
| Fe | 26.86 | 23.88 | 18.31 | 4.96 | 13.49 | 11.74 | 16.54 |
| Mn | 17.11 | 18.01 | 15.00 | 44.13 | 28.70 | 36.48 | 26.57 |
| Fe + Mn | 43.97 | 41.89 | 33.31 | 49.09 | 42.19 | 48.23 | 43.11 |
| Con | 0.05 | 0.04 | 0.01 | 0.03 | 0.01 | 0.02 | 0.03 |
| GR (mm/kyr) * | 0.10 | 0.12 | 1.14 | 0.23 | 1.94 | 0.43 | 0.66 |
| GR (mm/kyr) ** | 0.11 | - | - | - | - | - | - |
| Maximum thickness (mm) | 6 | 4 | 10 | 20 | 30 | 10 | 13 |
| Maximal predicted age (ka) * | 61 (69 **) | 33 | 9 | 86 | 15 | 23 | 38 |
| Mean age (±ka) | 38.0 ± 25.3 | ||||||
| Sample ID | Classification | Dominating Mineralogy 1 | Geochemistry | GR 2: |
|---|---|---|---|---|
| SIO0000D2 (textural type 1) | hydrogenetic > hydrothermal 3 hydrogenetic-detrital 4 hydrogenetic→ hydrothermal 5 | Fe-(Mn) vernadite > Mn-(Fe) vernadite >> birnessite ≅ asbolane ≅ Fe-oxyhydroxides columnar growth and biomorphic textures | Fe > Mn (Mn/Fe = 0.64) Ca-Na-Mg-K Si > Al Ni-Cu-Co; Co/Zn = 0.78 ∑REY ***,6 | * |
| SIO0000BT (textural type 1) | hydrogenetic ≅ hydrothermal 3 hydrogenetic-detrital 4 hydrogenetic → hydrothermal 5 | Fe-(Mn) vernadite >> todorokite-buserite filamentous structures of potential microbial growth; biomorphic textures | Fe > Mn (Mn/Fe = 0.75) Ca-Na-Mg-K Si > Al Ni-Cu-(Co); Co/Zn = 0.73 ∑REY ***,6 | * |
| SIO0000BQ (textural type 1) | hydrogenetic ≅ hydrothermal 3 hydrothermal 4 hydrothermal 5 | todorokite-buserite > Fe-(Mn) vernadite ≅ Na-birnessite ≅ other Fe-(Mn) bioclasts and filamentous biofilms mixed with Mn-Fe oxyhydroxides potentially transformed todorokite to buserite and birnessite | Fe > Mn (Mn/Fe = 0.81) K-Ca-Mg-Na Si >> Al Ni-Cu; Co/Zn = 0.13 ∑REY *,6 | *** |
| SIO0000D1 (textural type 2) | diagenetic >> hydrogenetic ≅ hydrothermal 3 hydrogenetic-detrital → detrital-diagenetic 4 hydrothermal 5 | birnessite >> Fe-(Mn) vernadite radial and globular micro aggregates; biomorphic textures non-transformed birnessite | Mn >> Fe (Mn/Fe = 8.9) Ca-Mg-Na-K Si > Al Ni-Cu-(Co); Co/Zn = 0.31 ∑REY **,6 | ** |
| SIO0000BP (textural type 2) | mixed: diagenetic > hydrogenetic ≅ hydrothermal 3 hydrothermal 4 hydrothermal 5 | todorokite-buserite >> Fe-(Mn) vernadite ≅ Ni-(Cu) asbolane > birnessite micro-crystals of with clay-like tabular or rosette-type aggregates intercalation and agglomerates of phyllosilicates partially transformed todorokite to buserite and birnessite | Mn >> Fe (Mn/Fe = 2.13) K-Mg-Na-Ca Si >> Al Ni-Cu; Co/Zn = 0.10 ∑REY *,6 | *** |
| SIO00003U (textural type 3) | diagenetic >> hydrogenetic > hydrothermal 3 hydrogenetic-detrital → detrital diagenetic 4 hydrothermal 5 | birnessite >> Fe-(Mn) vernadite > todorokite/buserite radial and globular micro aggregates partially transformed todorokite to buserite and birnessite | Mn >> Fe (Mn/Fe = 3.1) Ca-Na-Mg-K Si > Al Ni-Cu-(Co); Co/Zn = 0.22 ∑REY **,6 | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawadzki, D.; Maciąg, Ł.; Blasco, I.; González, F.J.; Wernette, B.; Marino, E.; Kozub-Budzyń, G.A.; Piestrzyński, A.; Wróbel, R.J.; McCartney, K. Geochemistry and Mineralogy of Ferromanganese Crusts from the Western Cocos-Nazca Spreading Centre, Pacific. Minerals 2022, 12, 538. https://doi.org/10.3390/min12050538
Zawadzki D, Maciąg Ł, Blasco I, González FJ, Wernette B, Marino E, Kozub-Budzyń GA, Piestrzyński A, Wróbel RJ, McCartney K. Geochemistry and Mineralogy of Ferromanganese Crusts from the Western Cocos-Nazca Spreading Centre, Pacific. Minerals. 2022; 12(5):538. https://doi.org/10.3390/min12050538
Chicago/Turabian StyleZawadzki, Dominik, Łukasz Maciąg, Iker Blasco, Francisco Javier González, Benjamin Wernette, Egidio Marino, Gabriela A. Kozub-Budzyń, Adam Piestrzyński, Rafał J. Wróbel, and Kevin McCartney. 2022. "Geochemistry and Mineralogy of Ferromanganese Crusts from the Western Cocos-Nazca Spreading Centre, Pacific" Minerals 12, no. 5: 538. https://doi.org/10.3390/min12050538
APA StyleZawadzki, D., Maciąg, Ł., Blasco, I., González, F. J., Wernette, B., Marino, E., Kozub-Budzyń, G. A., Piestrzyński, A., Wróbel, R. J., & McCartney, K. (2022). Geochemistry and Mineralogy of Ferromanganese Crusts from the Western Cocos-Nazca Spreading Centre, Pacific. Minerals, 12(5), 538. https://doi.org/10.3390/min12050538








