Diatomaceous Silica in Environmental Applications: A Case Study from the Lacustrine Deposit of Limnos Island, Aegean Sea, Greece
Abstract
:1. Introduction
2. Materials and Methods
2.1. X-ray Diffraction Analysis (XRD)
2.2. Scanning Electron Microscopy (SEM-EDS)
2.3. X-ray Fluorescence (XRF)
2.4. Physical Parameters
2.4.1. Insulation Block Density
2.4.2. Specific Surface Area
2.4.3. Porosity
3. Results and Discussion
3.1. Mineralogical Composition
3.2. Morphology of the Studied Diatomites
3.3. Chemical Analysis
3.4. Physical Properties
3.4.1. Insulation Block Density and Specific Surface Area
3.4.2. Porosity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Breese, O.Y.R. Diatomite. In Industrial Minerals and Rocks; Carr, D.D., Ed.; Society of Mining, Metallurgy, and Exploration Inc.: Englewood, CO, USA, 1994; pp. 397–411. [Google Scholar]
- Du, Y.; Wang, X.; Wu, J.; Wang, J.; Li, Y.; Dai, H. Mg3Si4O10(OH)2 and MgFe2O4 in situ grown on diatomite: Highly efficient adsorbents for the removal of Cr(VI). Microporous Mesoporous Mater. 2018, 271, 83–91. [Google Scholar] [CrossRef]
- Ilia, K.I.; Stamatakis, G.M.; Perraki, S.T. Mineralogy and technical properties of clayey diatomites from north and central Greece. Cent. Eur. J. Geosci. 2009, 1, 393–403. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Selim, A.Q.; Zayed, A.M.; Komarneni, S.; Mobarak, M.; Seliem, M.K. Enhancing adsorption capacity of Egyptian diatomaceous earth by thermo-chemical purification: Methylene bule uptake. J. Colloid Interface Sci. 2019, 534, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Taoukil, D.; El Meski, Y.; Lahlaouti, M.I.; Djedjig, R.; El Bouardi, A. Effect of the use of diatomite as partial replacement of sand on thermal and mechanical properties of mortars. J. Build. Eng. 2021, 42, 103038. [Google Scholar] [CrossRef]
- Stamatakis, M.; Tsipoura-Vlachou, M. Diatomaceous rocks in Greece. In Proceedings of the 14th International Congress of IMM on Minerals Materials and Industry (Ed IMM), Edinburgh, UK, 2–6 July 1990; pp. 185–192. [Google Scholar]
- Koukouzas, N. Komnina diatomaceous clay deposit overburden lignite and its suitability (W. Macedonia, Northern Greece). Miner. Wealth 1992, 81, 39–52. [Google Scholar]
- Dermitzakis, M.; Triantafyllou, M.; Stamatakis, M.; Tsaparas, N. Diatomaceous sediments of Gavdos Island, Southern Greece, Stratigraphical and Petrological analysis. In Proceedings of the Interim Colloquium/RCMNS Meditarrenean Geogene Cyclostratigraphy in Marine-Continental Paleo-Environments, Patras, Greece, 27–29 May 1998; Books of Abstracts. p. 22. [Google Scholar]
- Stamatakis, M.; Calvo, J.; Regueiro, M.; Neri, R. Alternating diatomaceous and volcaniclastic deposits in northern Milos Island, Aegean Sea, Greece. In Proceedings of the 15th International Sedimentological Congress, Alicante, Spain, 12–17 April 1998; pp. 738–739. [Google Scholar]
- Chu, H.Q.; Dong, B.Z.; Zhang, Y.L.; Zhou, X.F.; Yu, Z.X. Pollutant removal mechanisms in a bio-diatomite dynamic membrane reactor for micro-polluted surface water purification. Desalination 2012, 293, 38–45. [Google Scholar] [CrossRef]
- Miao, X.L.; Zhu, M.F.; Li, Y.G.; Zhang, Q.H.; Wang, H.Z. Synthesis of dental resins using diatomite and nano sized SiO2 and TiO2. Prog. Nat. Sci. Mater. Int. 2012, 22, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Skubiszewska-Zieba, J.; Charmas, B.; Leboda, R.; Gunko, V.M. Carbon-mineral adsorbents with a diatomaceous earth/perlite matrix modified by carbon deposits. Microporous Mesoporous Mater. 2012, 156, 209–216. [Google Scholar] [CrossRef]
- Cong, P.L.; Chen, S.F.; Chen, H.X. Effects of diatomite on the properties of asphalt binder. Constr. Build. Mater. 2012, 30, 495–499. [Google Scholar] [CrossRef]
- Ergun, A. Effects of the usage of diatomite and waste marble powder as partial replacement of cement on the mechanical properties of concrete. Constr. Build. Mater. 2011, 25, 806–812. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, J.; Sun, D.; Liu, G. Surface complexation modeling calculation of Pb(II) adsorption onto the calcined diatomite. Appl. Surf. Sci. 2015, 359, 48–54. [Google Scholar] [CrossRef]
- Man, J.; Gao, W.; Yan, S.; Liu, G.; Hao, H. Preparation of porous brick from diatomite and sugar filter mud at lower temperature. Constr. Build. Mater. 2017, 156, 1035–1042. [Google Scholar] [CrossRef]
- San, O.; İmaretli, A. Preparation and filtration testing of diatomite filtering layer by acid leaching. Ceram. Int. 2011, 37, 73–78. [Google Scholar] [CrossRef]
- Lyngsie, G.; Katika, K.; Fabricius, I.L.; Hansen, H.C.B.; Borggaard, O.K. Phosphate removal by iron oxide-coated diatomite: Laboratory test of a new method for cleaning drainage water. Chemosphere 2019, 222, 884–890. [Google Scholar] [CrossRef]
- Memon, S.A. Phase change materials integrated in building walls a state-of-the-art review. Renew. Sustain. Energy Rev. 2014, 31, 870–906. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Q.; Zhou, C.; Jin, Q. Enhanced photocatalytic activity of La and N co-doped TiO2/diatomite composite. Powder Technol. 2017, 322, 296–300. [Google Scholar] [CrossRef]
- Losic, D.; Mitchell, J.G.; Voelcker, N.H. Diatomaceous lessons in nanotechnology and advanced materials. Adv. Mater. 2009, 21, 2947–2958. [Google Scholar] [CrossRef]
- Khraishen, M.A.M.; Alg-Houti, M.S. Enhanced dye adsorption by microemulsion-modified calcined diatomite (le-cd). Adsorpt. J. Int. Adsorpt. Soc. 2005, 11, 547–559. [Google Scholar] [CrossRef]
- Mao, X.M.; Tian, X.K.; Yu, C.Y. Capturing and storage of CO2 by micron-nano minerals: Evidence from the nature. Chin. J. Geochem. 2011, 30, 569–575. [Google Scholar] [CrossRef]
- Ediz, N.; Bentli, İ.; Tatar, İ. Improvement in filtration characteristics of diatomite by calcination. Int. J. Miner. Process. 2010, 94, 129–134. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Pan, J.; Cai, J. Separation of diatom valves and girdle bands from coscinodiscus, diatomite by settlin method. J. Mater. Sci. 2010, 45, 5736–5741. [Google Scholar] [CrossRef]
- Innocenti, F.; Manetti, P.; Mazzuoli, R.; Pertusati, P.; Fytical, M.; Kolios, N. Geological map of the Limnos Island (Northern Aegean Sea—Greece); Lithografia Artistica Cartografica: Firenze, Italy, 2010. [Google Scholar]
- Kantiranis, N.; Stergiou, A.; Filippidis, A.; Drakoulis, A. Calculation of the percentage of amorphous material using PXRD patterns. Bull. Geol. Soc. of Greece 2004, 36, 446–453. [Google Scholar] [CrossRef]
- Kantiranis, N.; Sikalidis, K.; Godelitsas, A.; Squires, C.; Papastergios, G.; Filippidis, A. Extra-framework cation release from heulandite-type rich tuffs on exchange with NH4+. J. Environ. Manag. 2011, 92, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Haul, R.; Dümbgen, G. Vereinfachte méthode zur messung von oberflächengrößen durch gasadsorption. Chem. Ing. Tech. 1963, 35, 586–589. [Google Scholar] [CrossRef]
- Wyllie, M.R.J.; Gregory, A.R.; Gardner, G.H.F. An experimental investigation of factors affecting elastic wave velocities in porous media. Geophysics 1958, 23, 421–623. [Google Scholar] [CrossRef]
- Parasnis, S.D. Principles of Applied Geophysics; Chapman & Hall: London, UK, 1997; p. 429. [Google Scholar]
- Kantiranis, N.; Christaras, B.; Filippidis, A.; Tsirambides, A. The usage of ultrasonic techniques at calcination studies. In Proceedings of the International Meeting “Aggregate 2001—Environment and Economy”, Helsinki, Finland, 6–10 August 2001; pp. 389–394. [Google Scholar]
- Kantiranis, N.; Filippidis, A.; Tsirambides, A.; Christaras, B. Thermal decomposition study of crystalline limestone using P-wave velocity. Constr. Build. Mater. 2005, 19, 359–365. [Google Scholar] [CrossRef]
- Stamatakis, M.G.; Hein, J.R.; Magganas, A.C. Geochemistry and diagenesis of Miocene lacustrine siliceous sedimentary and pyroclastic rocks, Mitilinii basin, Samos Island, Greece. Sediment. Geol. 1989, 64, 65–78. [Google Scholar] [CrossRef]
- Minoura, K.; Susaki, T.; Horiuchi, K. Lithification of biogenic siliceous sediments: Evidence from Neogene diatomaceous sequences of northeast Japan. Sediment. Geol. 1996, 107, 45–59. [Google Scholar] [CrossRef]
- Reid, S.; Mc Intyre, J.L. Monterey Formation porcelanite reservoirs of the Elk Hills field, Kern County, California. Am. Assoc. Pet. Geol. Bull. 2001, 85, 169–189. [Google Scholar]
- Harben, P.W. Diatomite. In The Industrial Minerals Handybook IV; Industrial Minerals Informations: Surrey, UK, 1992; pp. 118–122. [Google Scholar]
- Stefanou, E.; Kantiranis, N.; Chatzicharalambous, K.; Stamatakis, M.; Georgiadis, G. Diatomaceous silica in environmental applications. A case study from the lacustrine deposit of Limnos Island, Aegean Sea Greece. In Proceedings of the Coastal Landscapes, Mining Activities & Preservation of Cultural Heritage, Milos Island, Greece, 17–20 September 2014. [Google Scholar]
- Chatterjee, K.K. Uses of Industrial Minerals, Rocks and Freshwater; Nova Science Publishers Inc.: New York, NY, USA, 2009; p. 584. [Google Scholar]
- Stamatakis, M.G.; Fragoulis, D.; Antonopoulou, S.; Stamatakis, G. The opaline silica rich sedimentary rocks of Milos Island, Greece and their behavior as pozzolanas in the manufacture of cement. Adv. Cem. Res. 2010, 22, 171–183. [Google Scholar] [CrossRef]
- Chen, F.; Miao, Y.; Ma, L.; Zhan, F.; Wang, W.; Chen, N.; Xie, Q. Optimization of pore structure of a clayey diatomite. Part. Sci. Technol. 2019, 38, 522–528. [Google Scholar] [CrossRef]
- Stamatakis, M.G.; Fragoulis, D.; Csirik, G.; Bedelean, I.; Pedersen, S. The influence of biogenic micro-silica-rich rocks on the properties of blended cements. Cem. Concr. Compos. 2003, 25, 177–184. [Google Scholar] [CrossRef]
- Yilmaz, B.; Ediz, N. The use of raw and calcined diatomite in cement production. Cem. Concr. Compos. 2008, 30, 202–211. [Google Scholar] [CrossRef]
- Koukouzas, N. Mineralogy and geochemistry of diatomite associated with lignite seams in the Komnina Lignite Basin, Ptolemais, Northern Greece. Int. J. Coal Geol. 2007, 71, 276–286. [Google Scholar] [CrossRef]
- Fragoulis, D.; Stamatakis, M.G.; Chaniotakis, E.; Columbus, G. Characterization of lightweight aggregates produced with clayey diatomite rocks originating from Greece. Mater. Charact. 2004, 53, 307–316. [Google Scholar] [CrossRef]
- Lee, W.E.; Souza, G.P.; McConville, C.J.; Tarvornpanich, T.; Iqbal, Y. Mullite formation in clays and clay-derived vitreous ceramics. J. Eur. Ceram. Soc. 2008, 28, 465–471. [Google Scholar] [CrossRef]
- Iqbal, Y.; Lee, W.E. Microstructural evolution in triaxial porcelain. J. Am. Ceram. Soc. 2000, 83, 3121–3127. [Google Scholar] [CrossRef]
- Santana, L.N.L.; Gomes, J.; Neves, G.A.; Lira, H.L.; Menezes, R.R.; Segadaes, A.M. Mullite formation from bentonites containing kaolinite: Effect of composition and synthesis parameters. Appl. Clay Sci. 2014, 87, 28–33. [Google Scholar] [CrossRef]
- Yang, K.H.; Wu, J.H.; His, C.S.; Lu, H.Y. Morphologically textured mullite in sintered tape-cast kaolin. J. Am. Ceram. Soc. 2011, 94, 938–944. [Google Scholar] [CrossRef]
- Vogiatzis, D. Usage of Fly Ash and Natural Zeolite in Making Lightweight Mortar. Master’s Thesis, Aristotle University, Thessaloniki, Greece, 2009. [Google Scholar]
- Pimraksa, K.; Chindaprasirt, P. Lightweight bricks made of diatomaceous eart, lime and gypsum. Ceram. Int. 2009, 35, 471–478. [Google Scholar] [CrossRef]
- Degirmenci, N.; Yilmaz, A. Use of diatomite as partial replacement for Portland cement in cement mortars. Construct. Build. Mater. 2009, 23, 284–288. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Performance of novel thermal energy storage engineered cementitious composites incorporating a paraffin/diatomite composite phase change material. Appl. Energy 2014, 121, 114–122. [Google Scholar] [CrossRef]
- Costa, J.A.C.; Martinelli, A.E.; Donascimento, R.M.; Mendes, A.M. Microstructural design and thermal characterization of composite diatomite-vermiculite paraffin-based form-stable PCM for cementitious mortars. Construct. Build. Mater. 2010, 232, 117–167. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Li, C.; Monteiro, P.J. Green concrete containing diatomaceous earth and limestone: Workability, mechanical properties, and life-cycle assessment. J. Clean. Prod. 2019, 223, 662–679. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, X. Effect of diatomite on thermal insulation properties of straw fiber cement-based composites. IOP Conf. Ser. Earth Environ. Sci. 2019, 295, 032047. [Google Scholar] [CrossRef]
- Saridemir, M.; Çelikten, S.; Yıldırım, A. Mechanical and microstructural properties of calcined diatomite powder modified high strength mortars at ambient and high temperatures. Adv. Powder Technol. 2020, 31, 3004–3017. [Google Scholar] [CrossRef]
- Hamdi, B.; Hamdi, S. Thermal properties of Algerian diatomite, study of the possibility to its use in the thermal insulation. In Proceedings of the International Congress on Energy Efficiency and Energy Related Materials, (ENEFM2013), Antalya, Turkey, 9–12 October 2013; Springer Proceedings in Physics. Volume 155, pp. 27–32. [Google Scholar]
- Fragoulis, D.; Stamatakis, M.G.; Chaniotakis, E.; Columbus, G. The Utilization of Clayey Diatomite in the Production of Lightweight Aggregates and Concrete. Tile Brick Int. 2003, 19, 392–397. [Google Scholar]
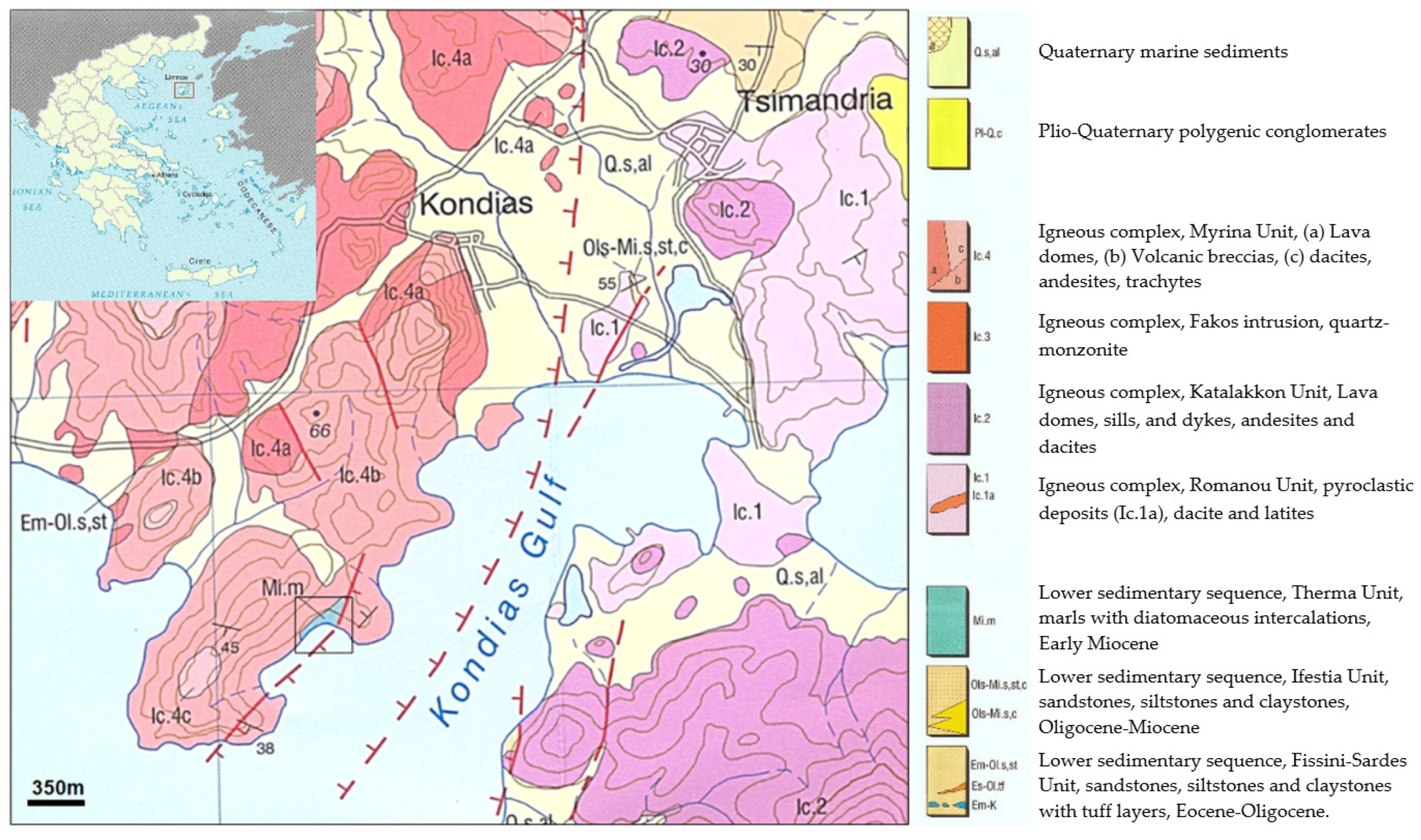
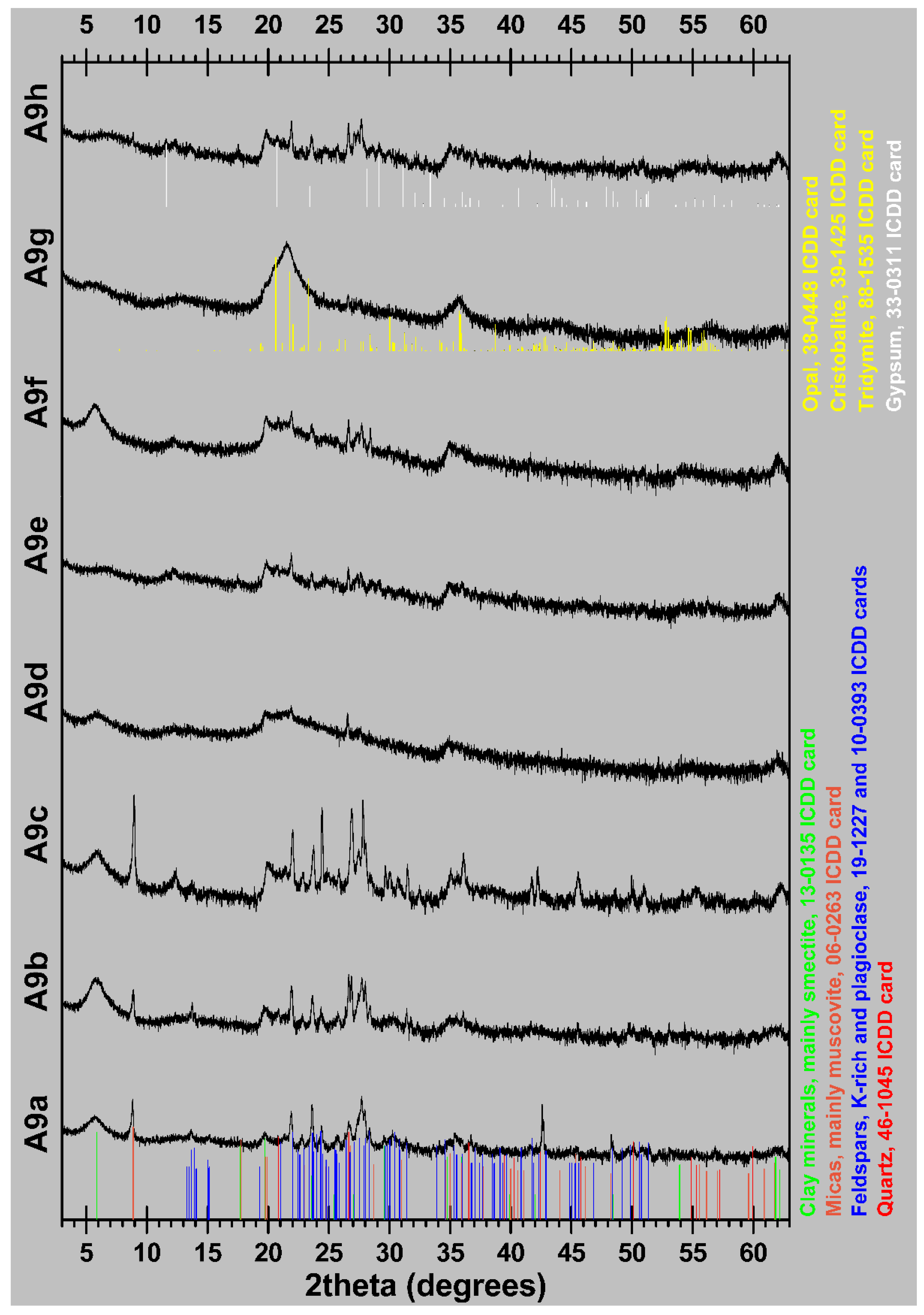


| Sample | Diatomite Type | CM | M | Qz | Fsp | Opal (A + CT) | Gp | VG |
|---|---|---|---|---|---|---|---|---|
| A9a | marl | 24 | 45 | 4 | 20 | - | - | 7 |
| A9b | marl | 44 | 19 | 14 | 15 | - | - | 8 |
| A9c | marl | 19 | 60 | - | 21 | - | - | - |
| A9d | porcelanite | 25 | - | 6 | 3 | 57 | - | 9 |
| A9e | clayey/marly diatomite | 18 | 3 | 8 | 12 | 40 | 11 | 8 |
| A9f | clayey/marly diatomite | 45 | - | 7 | 5 | 38 | - | - |
| A9g | porcelanite | 4 | - | 2 | 2 | 85 | - | 7 |
| A9h | clayey/marly diatomite | 12 | 8 | 12 | 30 | 22 | 16 | - |
| Sample | Diatomite Type | SiO2 | TiO2 | Al2O3 | Fe2O3t | MnO | MgO | CaO | Na2O | K2O | P2O5 | L.O.I. | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A9a | marl | 56.43 | 0.83 | 17.87 | 4.45 | 0.02 | 1.38 | 3.45 | 3.74 | 2.62 | 0.41 | 8.56 | 99.76 |
| A9b | marl | 56.86 | 0.76 | 16.90 | 6.19 | 0.02 | 1.60 | 2.47 | 3.05 | 2.19 | 0.21 | 9.54 | 99.79 |
| A9c | marl | 53.45 | 1.24 | 21.26 | 4.22 | 0.02 | 1.47 | 1.24 | 1.72 | 2.73 | 0.17 | 12.12 | 99.64 |
| A9d | porcelanite | 77.49 | 0.33 | 7.58 | 1.52 | 0.02 | 0.51 | 0.39 | 0.39 | 0.47 | 0.03 | 11.43 | 100.16 |
| A9e | porcelanite | 58.12 | 0.63 | 15.62 | 4.08 | 0.01 | 0.81 | 0.67 | 0.58 | 0.98 | 0.11 | 18.52 | 100.13 |
| A9f | moler | 67.83 | 0.53 | 11.59 | 1.91 | 0.01 | 0.80 | 0.68 | 0.62 | 0.88 | 0.04 | 14.96 | 99.85 |
| A9g | porcelanite | 81.60 | 0.17 | 4.50 | 1.17 | 0.01 | 0.44 | 0.43 | 0.25 | 0.32 | 0.02 | 11.15 | 100.06 |
| A9h | moler | 49.76 | 0.86 | 19.36 | 6.53 | 0.01 | 1.20 | 1.01 | 1.11 | 1.91 | 0.17 | 18.17 | 100.09 |
| Sample | A (Sample A9f) Moler-Type | Β (Sample A9g) Porcelanite-Type | ||
|---|---|---|---|---|
| (g/cm3) | (Lb/ft3) | (g/cm3) | (Lb/ft3) | |
| Insulation block density (dried at 105 °C) | 0.831 | 51.8 | 0.819 | 51.1 |
| Insulation block density (calcined at 1100 °C) | 0.753 | 47.0 | 0.757 | 47.3 |
| (% v/v) | (% v/v) | |||
| Porosity (dried at 105 °C) | 26.1 | 28.6 | ||
| Porosity (calcined at 1100 °C) | 15.7 | 16.0 | ||
| (m2/g) | (m2/g) | |||
| Specific surface area (dried at 105 °C) | 77 | 132 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanou, E.; Kantiranis, N.; Chatzicharalambous, K.; Mytiglaki, C.; Stamatakis, M.; Georgiadis, G. Diatomaceous Silica in Environmental Applications: A Case Study from the Lacustrine Deposit of Limnos Island, Aegean Sea, Greece. Minerals 2022, 12, 523. https://doi.org/10.3390/min12050523
Stefanou E, Kantiranis N, Chatzicharalambous K, Mytiglaki C, Stamatakis M, Georgiadis G. Diatomaceous Silica in Environmental Applications: A Case Study from the Lacustrine Deposit of Limnos Island, Aegean Sea, Greece. Minerals. 2022; 12(5):523. https://doi.org/10.3390/min12050523
Chicago/Turabian StyleStefanou, Evangelos, Nikolaos Kantiranis, Konstantinos Chatzicharalambous, Christina Mytiglaki, Michael Stamatakis, and George Georgiadis. 2022. "Diatomaceous Silica in Environmental Applications: A Case Study from the Lacustrine Deposit of Limnos Island, Aegean Sea, Greece" Minerals 12, no. 5: 523. https://doi.org/10.3390/min12050523
APA StyleStefanou, E., Kantiranis, N., Chatzicharalambous, K., Mytiglaki, C., Stamatakis, M., & Georgiadis, G. (2022). Diatomaceous Silica in Environmental Applications: A Case Study from the Lacustrine Deposit of Limnos Island, Aegean Sea, Greece. Minerals, 12(5), 523. https://doi.org/10.3390/min12050523







