Chemical Diversity of Teeth and Bone Fragments from a Newly Discovered Upper Muschelkalk Bone Bed from Silesia, Poland
Abstract
:1. Introduction
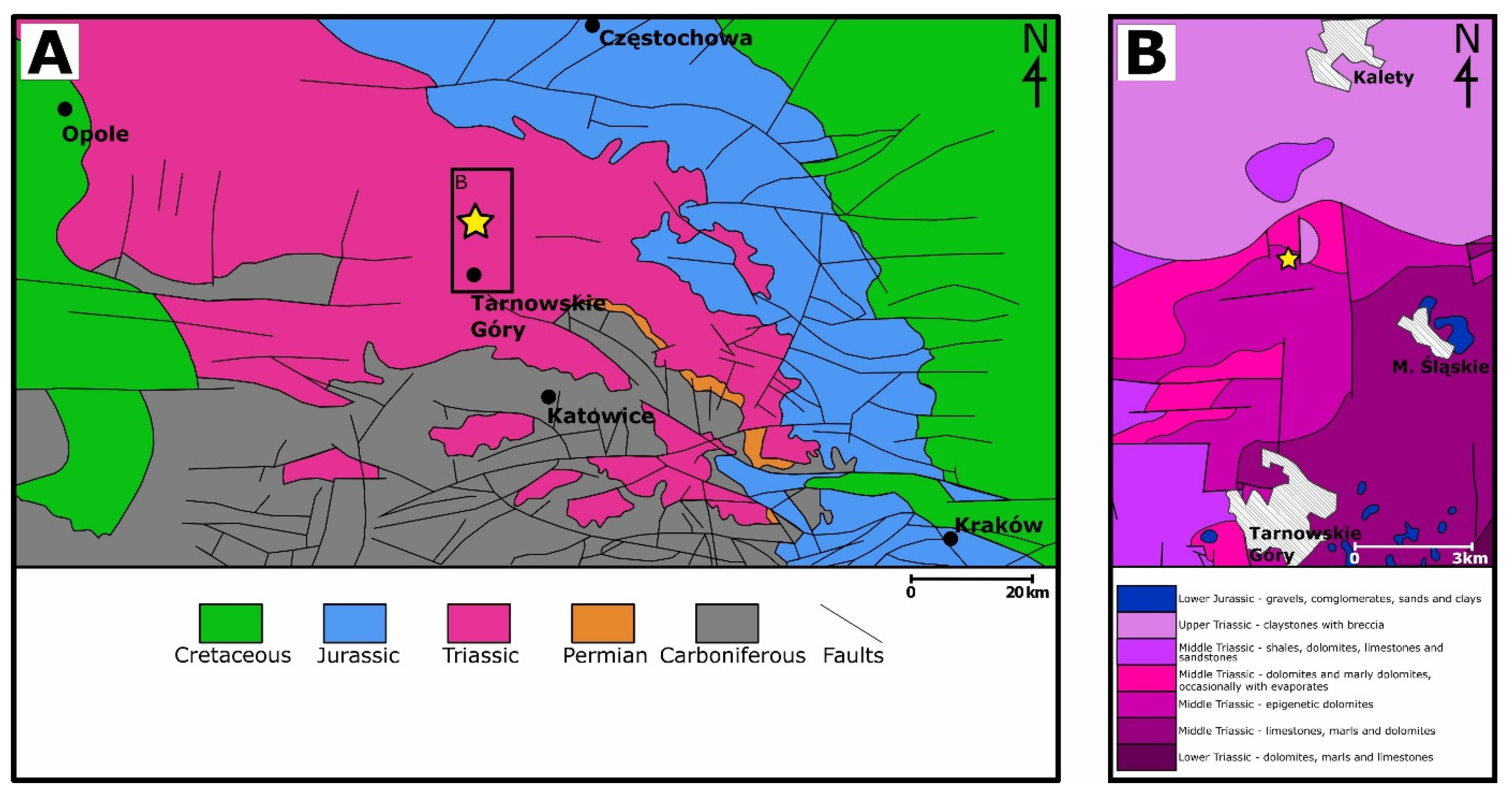
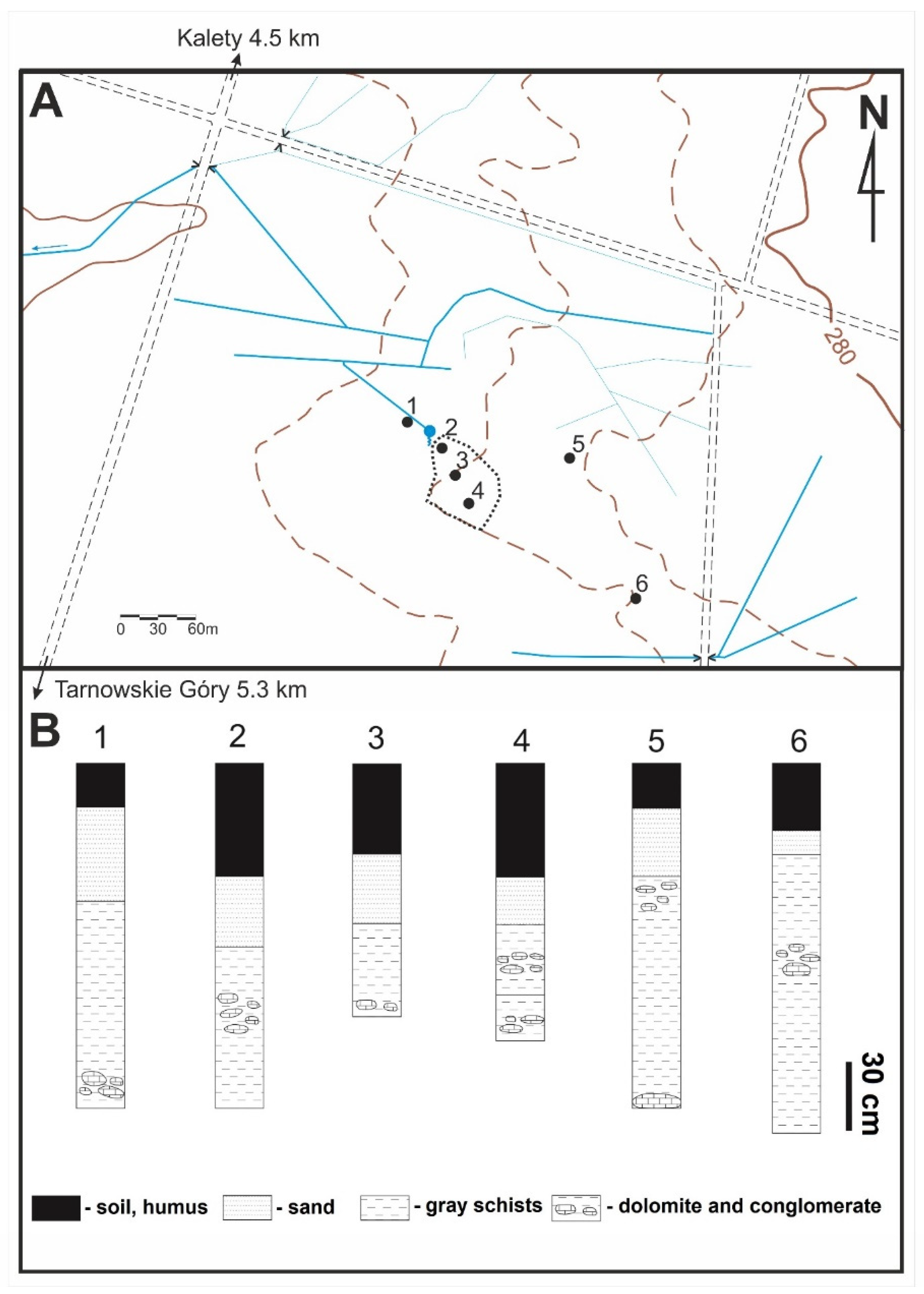
2. Geological Settings
3. Materials and Methods
3.1. Microscope and Electron Probe Micro-Analyses (EPMA)
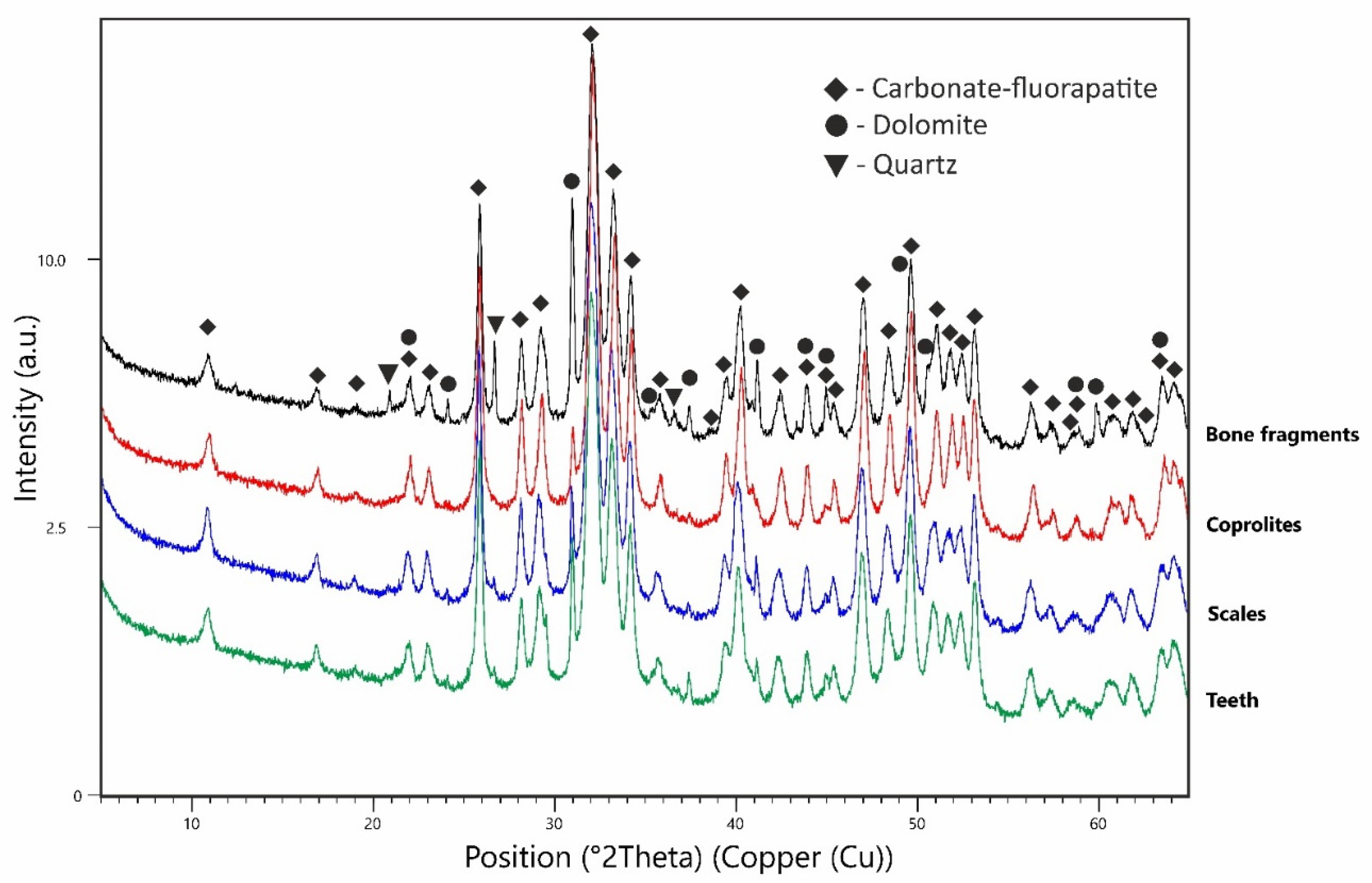
3.2. X-ray Diffraction (XRD)
3.3. Infrared Spectroscopy (IR)
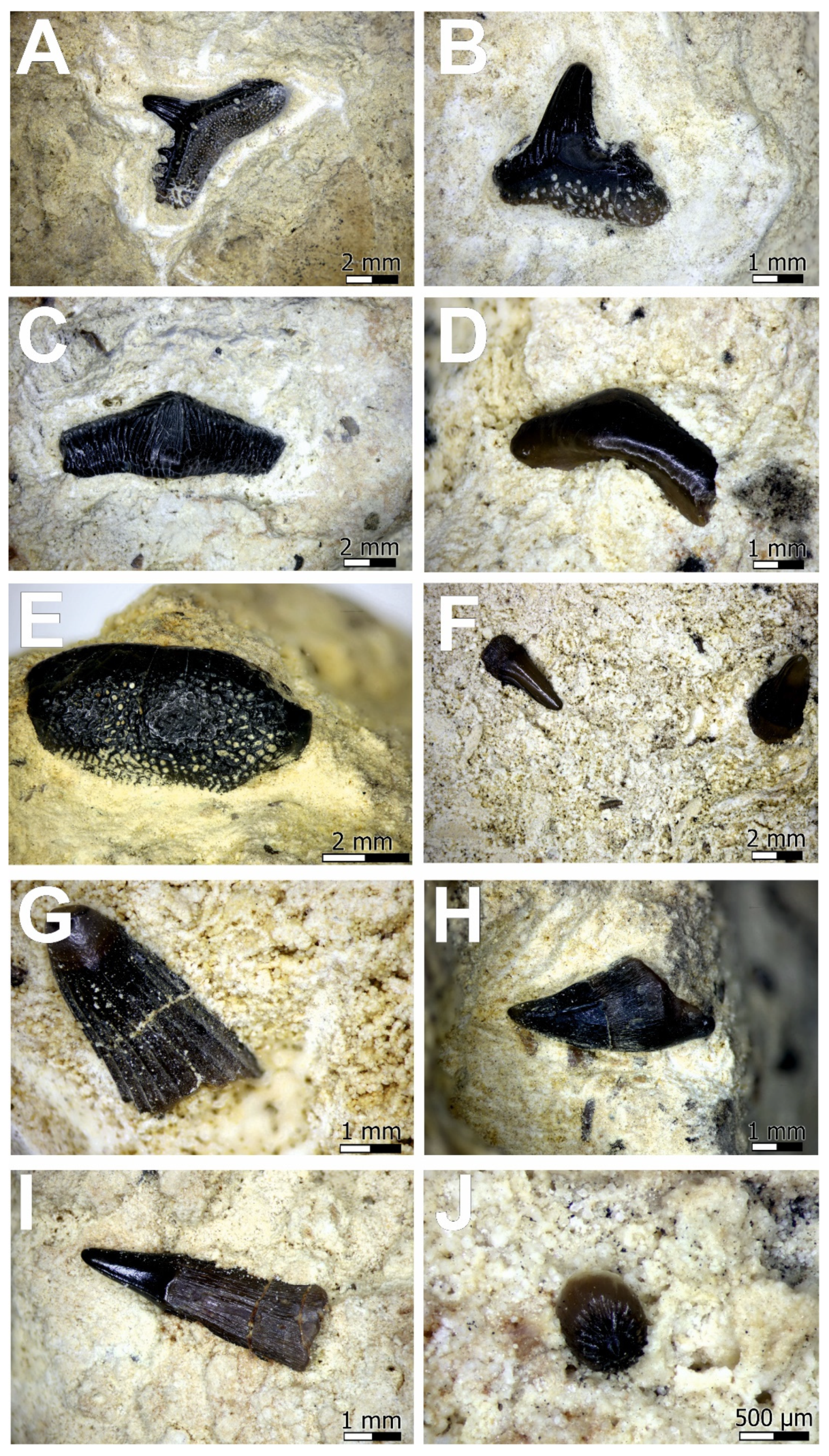
4. Results
5. Discussion
6. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akkus, O.; Adar, F.; Schaffler, M.B. Age-related changes in physiochemical properties of mineral crystals are related to impaired mechanical function of cortical bone. Bone 2004, 34, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Kleeman, C.R. Role of bone in regulation of systemic acid-base balance. Kidney Int. 1991, 39, 9–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keenan, S.W. From bone to fossil: A review of the diagenesis of bioapatite. Am. Min. 2016, 101, 1943–1951. [Google Scholar] [CrossRef]
- Szrek, P.; Niedźwiedzki, G.; Dec, M. Storm origin of bone-bearing beds in the Lower Devonian placoderm sand stone from Podłazie Hill (Holy Cross Mountains, central Poland). Geol. Quat. 2014, 58, 795–806. [Google Scholar]
- Sander, P.M. Early permian depositional environments and pond bonebeds in central archer County, Texas. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1989, 69, 1–21. [Google Scholar] [CrossRef]
- Suarez, C.A.; Morschhauser, E.M.; Suarez, M.B.; You, H.; Li, D.; Dodson, P. Rare earth element geochemistry of bone beds from the Lower Cretaceous Zhonggou Formation of Gansu Province, China. J. Vertebr. Paleontol. 2018, 38, 22–35. [Google Scholar] [CrossRef]
- Catlos, E.J.; Mark, D.F.; Suarez, S.; Brookfield, M.E.; Miller, C.G.; Schmitt, A.K.; Gallagher, V.; Kelly, A. Late Silurian zircon U–Pb ages from the Ludlow and Downtonbone beds, Welsh Basin, UK. J. Geol. Soc. London 2021, 178, jgs2020-107. [Google Scholar] [CrossRef]
- Niedźwiedzki, R.; Krzykawski, T.; Salamon, M. New position of “bone beds” from the Upper Silesian Triassic, preliminary results. In Proceedings of the Śląskie Dinozaury (nie tylko) z Krasiejowa, Kraków, Poland, 9 December 2015. [Google Scholar]
- Chrząstek, A.; Niedźwiedzki, R. Kręgowce retu i dolnego wapienia muszlowego na Śląsku. Pr. Geol. Mineral. U. Wr. 1998, 64, 69–81. [Google Scholar]
- Liszkowski, J. A bone bed from the “Wallen-Beds” of the lower Muschelkalk (Lowermost Anisian) at Wolica near Kielce (Holly Cross Mountains). Prz. Geol. 1973, 21, 644–648. [Google Scholar]
- Sulej, T.; Niedźwiedzki, G.; Niedźwiedzki, R.; Surmik, D.; Stachacz, M. New vertebrate assemblage from the marginal-marine and land Lower Keuper strata (Ladinian, Middle Triassic) of Miedary, Silesia, SW Poland. Prz. Geol. 2011, 59, 426–430. [Google Scholar]
- Pawlak, W.; Rozwalak, P.; Sulej, T. Triassic fish faunas from Miedary (Upper Silesia, Poland) and their implications for understanding paleosalinity. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2022, 590, 110–860. [Google Scholar] [CrossRef]
- Kotlicki, S. Geological Map of Poland without Quaternary Deposits (1:200,000); Polish Geological Institute: Warsw, Poland, 1977. [Google Scholar]
- Szulc, J. Middle Triassic evolution of the northern Peri-Tethys area as influenced by early opening of the Tethys Ocean. Ann. Soc. Geol. Pol. 2000, 70, 1–48. [Google Scholar]
- Nawrocki, J.; Szulc, J. The Middle Triassic magnetostratigraphy from the Peri- Tethys basin in Poland. Earth Planet. Sci. Lett. 2000, 182, 77–92. [Google Scholar] [CrossRef]
- Wyczółkowski, J. Objaśnienia do Szczegółowej Mapy Geologicznej Polski; Arkusz Kalety (M34-50B); Wydawnictwa Geologiczne: Warsaw, Poland, 1968; p. 86. [Google Scholar]
- Dadlez, R.; Marek, S.; Pokorski, J. (Eds.) Geological Map of Poland without Cenozoic Deposits (1:1,000,000); Polish Geological Institute: Warsw, Poland, 2000. [Google Scholar]
- Roemer, F.; Geologie von Oberschlesien; Eine Erläuterung zu der im Auftrage des Königl. Preuss. In Handels-Ministeriums von dem Verfasser Bearbeiteten Geologischen Karte von Oberschlesien in 12 Sektionen; Nischkowsky: Breslau, Poland, 1870; 587p. [Google Scholar]
- Siewniak-Witruk, A. Konodonty wapienia muszlowego rejonu Kalet. Geologia 1978, 243, 119–133. [Google Scholar]
- Odrzywolska-Bieńkowa, E. Skamieniałości warstw boruszowickich rejonu Bibiela-Kalety. Przeg. Geol. 1962, 10, 217–218. [Google Scholar]
- Senkowiczowa, H. Trias północno-wschodniego obrzeżenia Górnośląskiego Zagłębia Węglowego. Biul. Państwowego Inst. Geol. 1998, 378, 5–66. [Google Scholar]
- Assmann, P. Revision der Fauna der Wirbellosen der oberschlesischen Trias. Abh. Königlich Preuss. Geol. Landesanst. 1937, 170, 1–134. [Google Scholar]
- Matysik, M.; Surmik, D. Depositional conditions of vertebrate remains within the Lower Muschelkalk (Anisian) peritidal carbonates of the “Stare Gliny” quarry near Olkusz (Kraków-Silesia region, southern Poland). Przeg. Geol. 2016, 64, 495–503. [Google Scholar]
- Liszkowski, J. Die Selachierfauna des Muschelkalks in Polen: Zusammensetzung, Stratigraphie und Paläoökologie. In Muschelkalk, Schöntaler Symposium; Hagdom, H., Seilacher, A., Eds.; Goldschneck Verlag: Stuttgart, Germany, 1993; pp. 175–185. [Google Scholar]
- Dzik, J.; Sulej, T.; Kaim, A.; Niedźwiedzki, R. Późnotriasowe cmentarzysko kręgowców lądowych w Krasiejowie na Śląsku Opolskim. Przeg. Geol. 2000, 3, 226–235. [Google Scholar]
- Dzik, J.; Sulej, T. An early late Triassic long-necked reptile with a bony pectoral shield and gracile appendages. Acta Palaeontol. Pol. 2016, 61, 805–823. [Google Scholar]
- Skawiński, T.; Ziegler, M.; Czepiński, Ł.; Szermański, M.; Tałanda, M.; Surmik, D.; Niedźwiedzki, G. A re-evaluation of the historical ‘dinosaur’ remains from the Middle-Upper Triassic of Poland. Hist. Biol. 2017, 29, 442–472. [Google Scholar] [CrossRef]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore Suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef] [Green Version]
- Schuffert, J.D.; Kastner, M.; Emanuele, G.; Jahnke, R.A. Carbonate-ion substitution in francolite: A new equation. Geochim. Cosmochim. Acta 1990, 54, 2323–2328. [Google Scholar] [CrossRef]
- Trueman, C.; Tuross, N. Trace Elements in Recent and Fossil Bone Apatite. Rev. Mineral. Geochem. 2002, 48, 489–521. [Google Scholar] [CrossRef]
- Kohn, M.J.; Schoeninger, M.J.; Barker, W.W. Altered states: Effects of diagenesis on fossil tooth chemistry. Geochim. Cosmochim. Acta 1999, 63, 2737–2747. [Google Scholar] [CrossRef]
- Perdikatsis, B. X-ray powder diffraction study of francolite by the Rietveld method. Mat. Sci. Forum 1991, 79, 809–814. [Google Scholar]
- LeGeros, R.Z.; Suga, S. Crystallographic nature of fluoride in enameloids of fish. Calcif. Tissue Int. 1980, 32, 169–174. [Google Scholar] [CrossRef]
- Ryanskaya, A.; Kiseleva, D.; Shilovsky, O.; Shagalov, E. XRD study of the Permian fossil bone tissue. Powder Diffr. 2019, 34, S14–S17. [Google Scholar] [CrossRef]
- Nemliher, J.G.; Baturin, G.N.; Kallaste, T.E.; Murdmaa, I.O. Transformation of Hydroxyapatite of Bone Phosphate from the Ocean Bottom during Fossilization. Lithol. Miner. Resour. 2004, 39, 468–479. [Google Scholar] [CrossRef]
- Kallaste, T.; Nemliher, J. Apatite varieties in extant and fossil vertebrate mineralized tissues. J. Appl. Cryst. 2005, 38, 587–594. [Google Scholar] [CrossRef]
- Brudevold, F.; Söremark, R. Chemistry of the mineral phase of enamel. In Structural and Chemical Organization of Teeth; Miles, A.E.W., Ed.; Academic Press: New York, NY, USA, 1967; Volume 2, pp. 247–277. [Google Scholar]
- Trautz, O.R. Crystalline Organization of Dental Mineral. In Structural and Chemical Organization of Teeth (I); Miles, A.E.W., Ed.; Academic Press: New York, NY, USA, 1967; pp. 165–200. [Google Scholar]
- Rowles, S.L. Chemistry of the mineral phase of dentine. In Structural and Chemical Organization of Teeth; Miles, A.E.W., Ed.; Academic Press: New York, NY, USA, 1967; Volume 2, pp. 201–246. [Google Scholar]

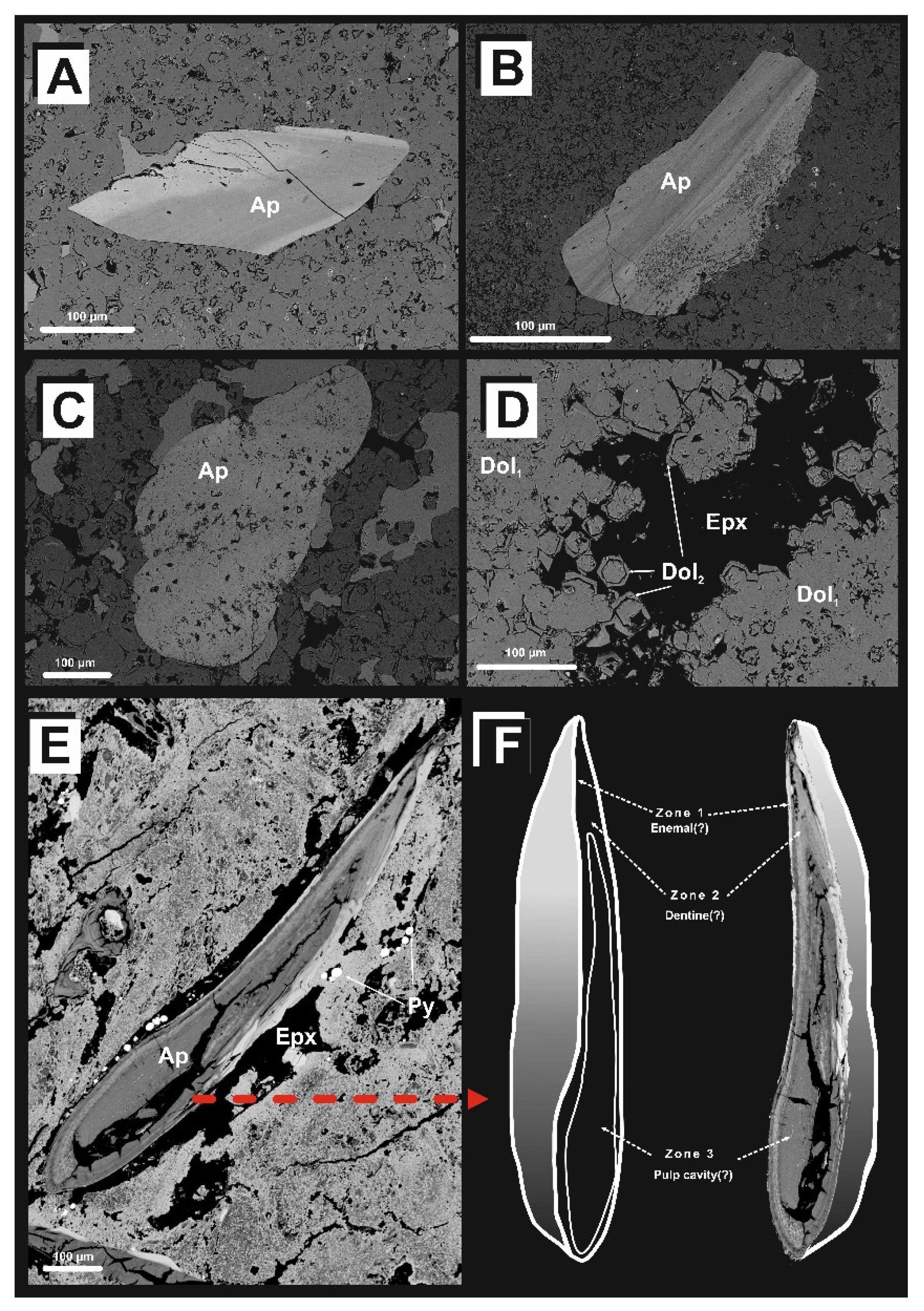
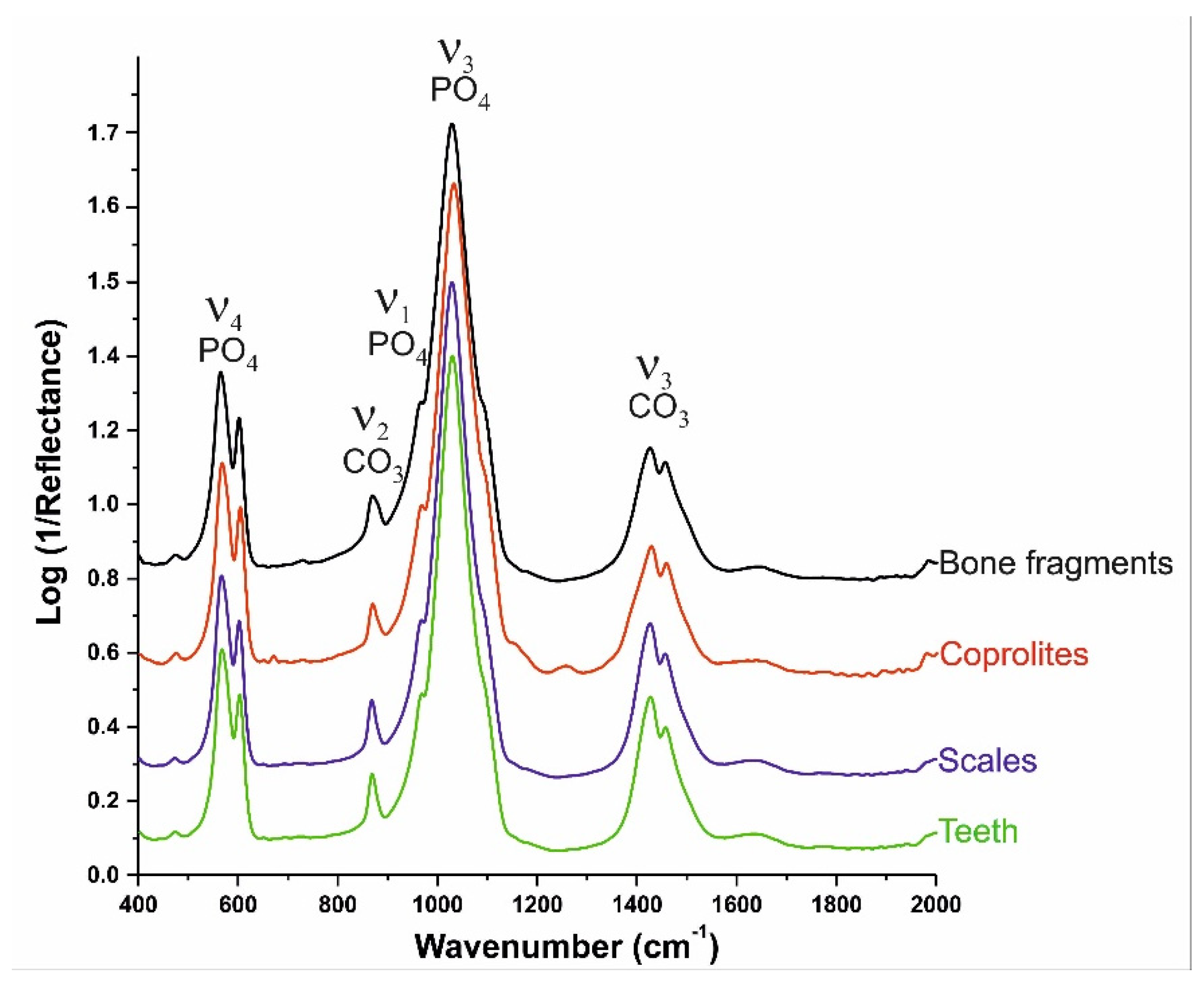
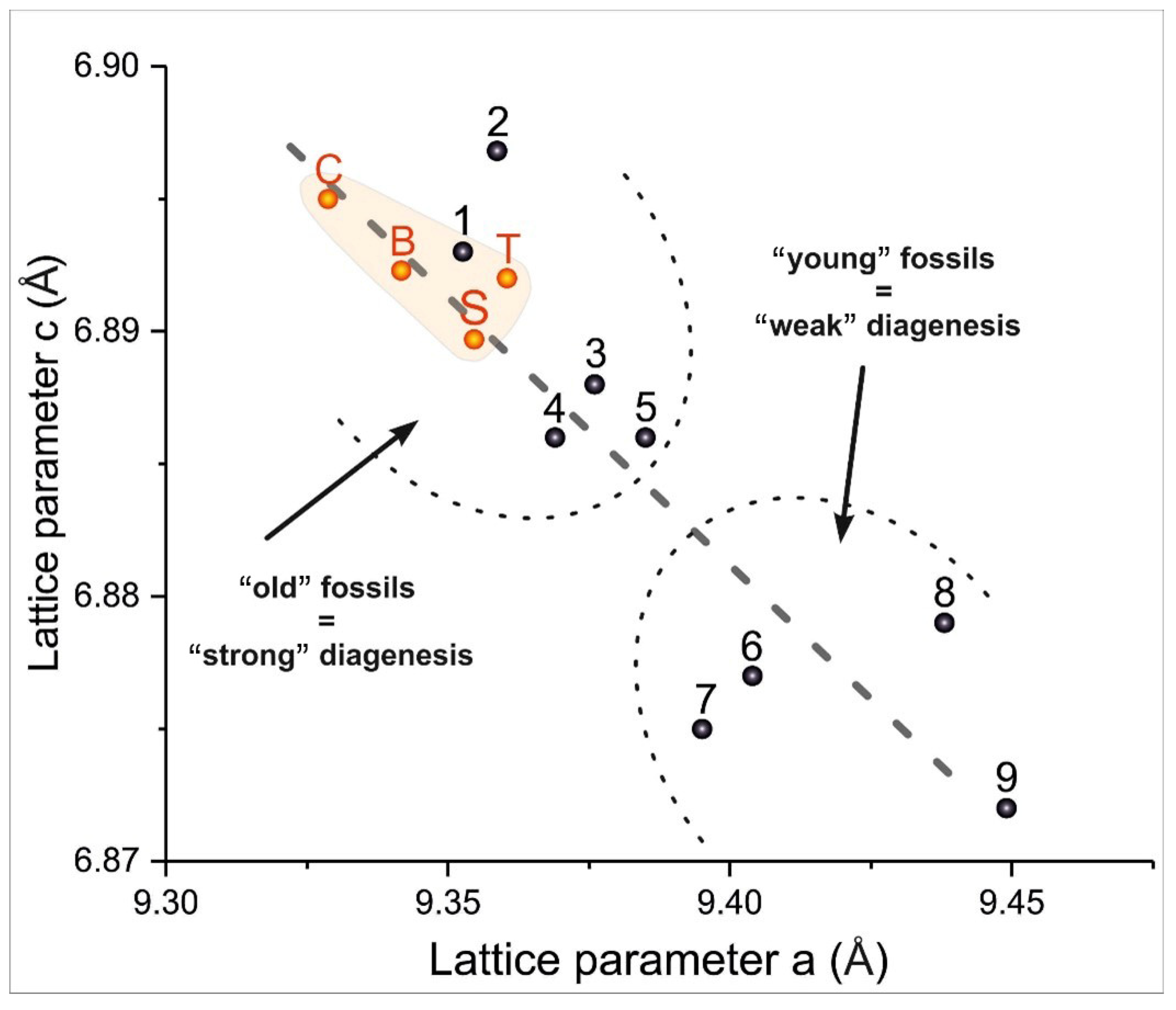
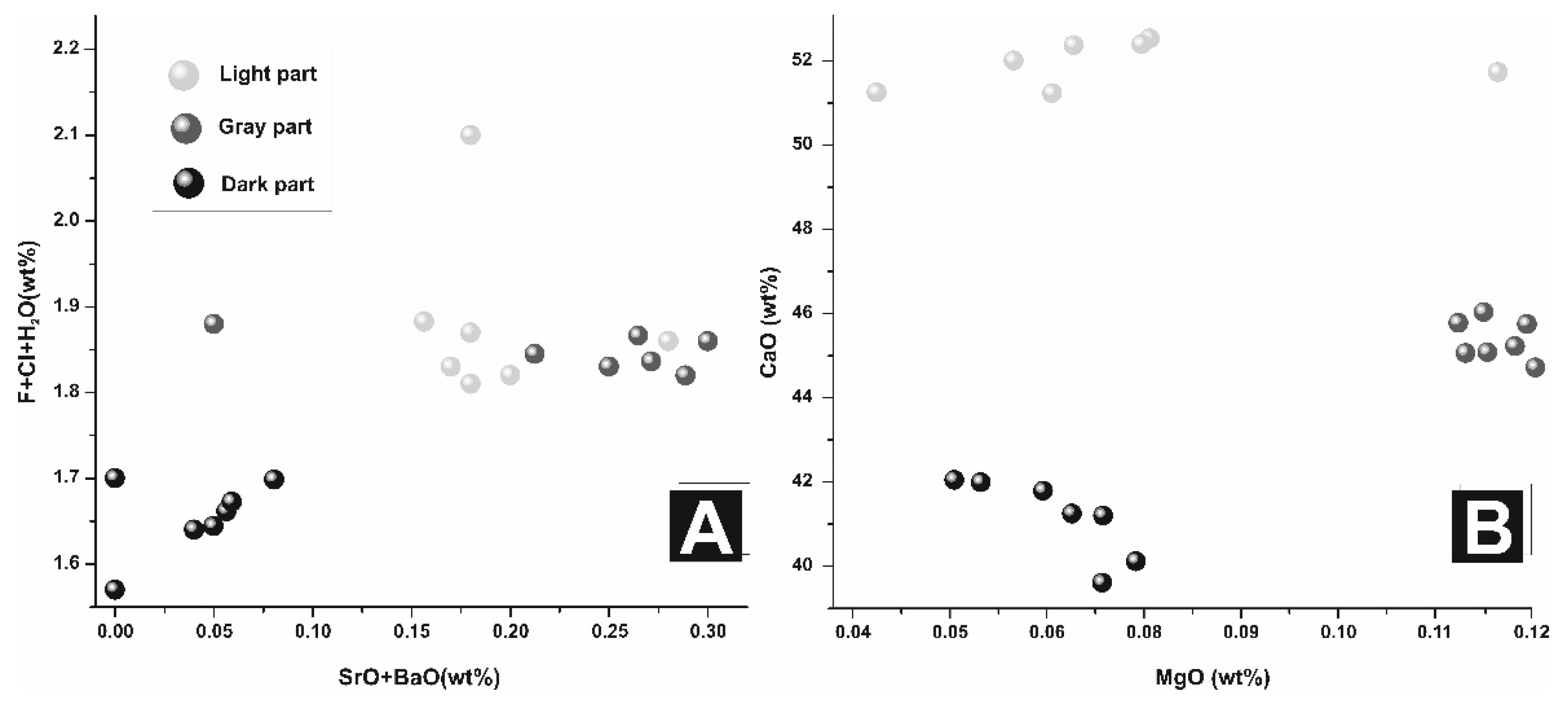
| Sample | Long Bones | Coprolites | Scales | Teeth |
|---|---|---|---|---|
| Lattice parameters | ||||
| a/Å | 9.3417 (4) | 9.3287 (4) | 9.3546 (5) | 9.3604 (5) |
| c/Å | 6.8923 (3) | 6.8950 (2) | 6.8897 (3) | 6.8920 (3) |
| alpha/° | 90 | 90 | 90 | 90 |
| beta/° | 90 | 90 | 90 | 90 |
| gamma/° | 120 | 120 | 120 | 120 |
| V/106 pm3 | 520.895 | 519.643 | 522.133 | 522.958 |
| V ESD/106 pm3 | 0.029 | 0.028 | 0.038 | 0.037 |
| Agreement Indices of Rietveld fit | ||||
| R (expected)/% | 5.301 | 5.262 | 5.289 | 5.139 |
| R (profile)/% | 7.583 | 6.614 | 7.402 | 6.496 |
| R (weighted profile)/% | 10.082 | 8.723 | 10.248 | 8.509 |
| GOF | 1.902 | 1.658 | 1.937 | 1.656 |
| Compound/Sample | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| lBSE#1 | lBSE#2 | lBSE#3 | lBSE#4 | lBSE#5 | gBSE#1 | gBSE#2 | gBSE#3 | dBSE#1 | dBSE#2 | dBSE#3 | dBSE#3 | |
| SO3 (wt%) | 1.07 | 0.96 | 0.97 | 0.92 | 0.68 | 0.87 | 1.35 | 1.12 | 1.17 | 1.21 | 1.36 | 1.19 |
| P2O5 | 35.43 | 35.18 | 34.82 | 34.54 | 36.53 | 32.38 | 32.01 | 32.37 | 29.08 | 28.66 | 28.14 | 27.98 |
| CO2 * | 7.54 | 8.60 | 9.82 | 10.08 | 7.67 | 16.68 | 18.25 | 16.98 | 25.50 | 27.50 | 28.75 | 27.32 |
| MgO | 0.08 | 0.06 | 0.04 | 0.07 | 0.12 | 0.12 | 0.12 | 0.11 | 0.05 | 0.08 | 0.07 | 0.07 |
| CaO | 53.04 | 52.53 | 51.51 | 51.63 | 51.69 | 46.40 | 45.21 | 46.15 | 41.86 | 39.85 | 39.11 | 40.72 |
| MnO | 0.06 | b.d.l. | b.d.l. | 0.04 | 0.06 | 0.04 | b.d.l. | 0.10 | b.d.l. | 0.07 | 0.04 | b.d.l. |
| FeO | 0.31 | 0.23 | 0.25 | 0.28 | 0.44 | 0.60 | 0.34 | 0.55 | 0.32 | 0.60 | 0.29 | 0.45 |
| SrO | 0.15 | 0.17 | 0.12 | 0.12 | 0.26 | 0.20 | 0.12 | 0.18 | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| BaO | 0.04 | b.d.l. | 0.06 | 0.09 | 0.02 | 0.10 | 0.13 | b.d.l. | 0.04 | b.d.l. | 0.06 | b.d.l. |
| Na2O | 0.42 | 0.42 | 0.62 | 0.42 | 0.68 | 0.76 | 0.65 | 0.70 | 0.33 | 0.47 | 0.50 | 0.55 |
| H2O * | 0.64 | 0.71 | 0.72 | 0.60 | 0.86 | 0.75 | 0.63 | 0.60 | 0.56 | 0.54 | 0.46 | 0.50 |
| F | 1.17 | 1.07 | 1.03 | 1.18 | 0.89 | 0.81 | 1.00 | 1.08 | 0.83 | 0.71 | 0.85 | 0.80 |
| Cl | 0.05 | 0.05 | 0.06 | 0.05 | 0.10 | 0.30 | 0.21 | 0.20 | 0.25 | 0.32 | 0.36 | 0.39 |
| F | −0.85 | −0.78 | −0.75 | −0.86 | −0.65 | −0.66 | −0.57 | −0.60 | −0.59 | −0.64 | −0.62 | −0.58 |
| Cl | −0.01 | −0.01 | −0.02 | −0.01 | −0.03 | −0.07 | −0.09 | −0.11 | −0.13 | −0.14 | −0.11 | −0.11 |
| TOTAL | 92.46 | 91.40 | 90.18 | 89.92 | 92.33 | 83.32 | 81.75 | 83.02 | 74.50 | 72.50 | 71.25 | 72.68 |
| S(apfu) | 0.14 | 0.13 | 0.13 | 0.12 | 0.68 | 0.13 | 0.20 | 0.13 | 0.19 | 0.20 | 0.23 | 0.20 |
| P | 5.22 | 5.24 | 5.26 | 5.23 | 5.24 | 5.29 | 5.28 | 5.29 | 5.27 | 5.33 | 5.31 | 5.22 |
| C | 0.78 | 0.76 | 0.74 | 0.77 | 0.76 | 0.71 | 0.72 | 0.71 | 0.73 | 0.67 | 0.69 | 0.78 |
| Mg | 0.02 | 0.02 | 0.01 | 0.02 | 0.12 | 0.03 | 0.03 | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 |
| Ca | 9.89 | 9.90 | 9.84 | 9.90 | 51.69 | 9.59 | 9.44 | 9.59 | 9.60 | 9.37 | 9.33 | 9.61 |
| Mn | 0.06 | |||||||||||
| Fe | 0.05 | 0.03 | 0.04 | 0.04 | 0.44 | 0.10 | 0.06 | 0.10 | 0.06 | 0.11 | 0.05 | 0.08 |
| Sr | 0.01 | 0.01 | 0.01 | 0.01 | 0.26 | 0.01 | 0.01 | 0.01 | ||||
| Ba | 0.02 | |||||||||||
| Na | 0.07 | 0.07 | 0.11 | 0.07 | 0.68 | 0.14 | 0.12 | 0.14 | 0.07 | 0.10 | 0.11 | 0.12 |
| OH | 0.75 | 0.83 | 0.86 | 0.71 | 0.86 | 0.96 | 0.82 | 0.96 | 0.80 | 0.79 | 0.69 | 0.74 |
| F | 0.65 | 0.60 | 0.58 | 0.67 | 0.89 | 0.49 | 0.61 | 0.49 | 0.56 | 0.49 | 0.60 | 0.56 |
| Cl | 0.01 | 0.01 | 0.02 | 0.01 | 0.10 | 0.10 | 0.07 | 0.10 | 0.09 | 0.12 | 0.14 | 0.15 |
| SrO + BaO | 0.18 | 0.17 | 0.18 | 0.20 | 0.28 | 0.30 | 0.25 | 0.18 | 0.04 | 0.00 | 0.06 | 0.00 |
| F + Cl + H2O | 1.87 | 1.83 | 1.81 | 1.82 | 1.86 | 1.86 | 1.83 | 1.88 | 1.64 | 1.57 | 1.67 | 1.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzykawski, T.; Szopa, K.; Niedźwiedzki, R.; Setkiewicz, K.; Czaja, M. Chemical Diversity of Teeth and Bone Fragments from a Newly Discovered Upper Muschelkalk Bone Bed from Silesia, Poland. Minerals 2022, 12, 469. https://doi.org/10.3390/min12040469
Krzykawski T, Szopa K, Niedźwiedzki R, Setkiewicz K, Czaja M. Chemical Diversity of Teeth and Bone Fragments from a Newly Discovered Upper Muschelkalk Bone Bed from Silesia, Poland. Minerals. 2022; 12(4):469. https://doi.org/10.3390/min12040469
Chicago/Turabian StyleKrzykawski, Tomasz, Krzysztof Szopa, Robert Niedźwiedzki, Krzysztof Setkiewicz, and Maria Czaja. 2022. "Chemical Diversity of Teeth and Bone Fragments from a Newly Discovered Upper Muschelkalk Bone Bed from Silesia, Poland" Minerals 12, no. 4: 469. https://doi.org/10.3390/min12040469
APA StyleKrzykawski, T., Szopa, K., Niedźwiedzki, R., Setkiewicz, K., & Czaja, M. (2022). Chemical Diversity of Teeth and Bone Fragments from a Newly Discovered Upper Muschelkalk Bone Bed from Silesia, Poland. Minerals, 12(4), 469. https://doi.org/10.3390/min12040469






