1. Introduction
Acid mine drainage (AMD) is one of the major environmental problems in the mining industry, both due to its serious impacts on the fluvial network and its long-term duration. This process, which begins with the oxidation of sulphides, has been described as a “perpetual pollution machine” [
1,
2]. The weathering reactions of sulphides, such as pyrite, produce acid solutions that are responsible for mobilizing metals, sulphates, and acidity [
3,
4,
5]. The complex chain of biotic and abiotic reactions that involve the oxidative dissolution of sulphides, and processes of precipitation and oxidation reduction resulting in AMD production, have been the subjects of extensive literature reviews [
6,
7,
8,
9].
The Iberian Pyrite Belt is a geological formation that is 230 km long and, on average, 50 km wide. It constitutes one of the largest sulphides deposits in the world [
10] with approximately 1700 MT of reserves. These deposits have been exploited for more than 4000 years (b.p.), with numerous and extensive mining works remaining as evidence of this activity, as well as several million tonnes of ancient slags of different composition [
10]. These sulphides’ massive bodies contain pyrite with associations of sphalerite, galena, and chalcopyrite as well as other minor phases. Therefore, such heavy mining activity throughout history has created a unique scenario regarding contamination by AMD. The waters emerging from inside the mines through tunnels, tailings ponds, mountains of ash, leachate from slag, calcination areas, cementation channels, and runoff from washing sulphur residue spread by the mines all produce pollution once they reach the closest watercourses [
11]. The enrichment or attenuation concentration of different elements is described in Grande et al., 2022 [
12].
The acid drainage from these mines with the drainage network modify the physical-chemical characteristics of the watercourses, increasing the acidity, heavy-metal, and sulphate concentration in waters to extreme levels [
13].
The impact of climate on the AMD polluting process is more than well known, as it affects pyritic materials exposed on the surface. Climatic conditions, and especially precipitation, are the most significant external controlling factors in terms of the degree and type of mining pollution in the area. The study area presents characteristics typical of the semi-arid Mediterranean climate, with annual precipitation of around 630 mm/year; moderate temperatures with average annual values of 17.1 °C, and a temperature range of roughly 50 °C. The precipitation occurs mostly in autumn and winter. Lack of rain in summer and part of spring produces situations of drought because the hydric balance is negative in this region (630 mm rain and 900 mm ETP) [
14].
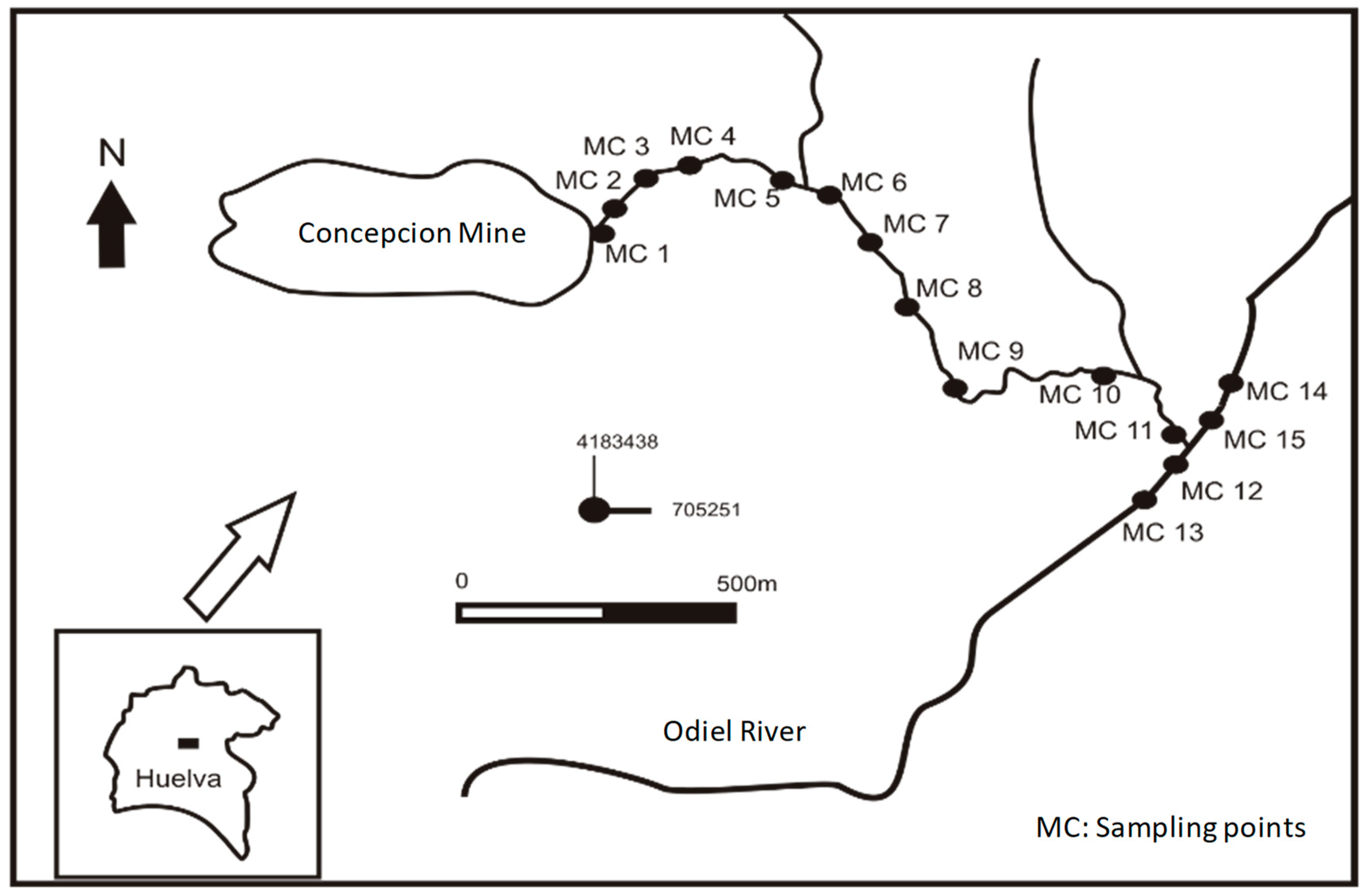
Concepción Mine (
Figure 1) is located in the Iberian Pyrite Belt (FPI), in the central part of the province of Huelva (southwest Spain), more specifically within the stratigraphic record of the Vulcano–Sedimentary Complex. The closest river that runs through the area is the Odiel. It is born in the Sierra de Aracena, north of the province of Huelva, running from north to south until it flows into the Atlantic. This river has a length of 140 km and its waters have an excellent quality in its first 24 km, until the interception with acidic waters from Concepción Mine downstream, i.e., the sampling area of this work. The Concepción Mine is the first exploitation that contributes to the Odiel river pollution. This mine has been closed since the 1990s without complete corrective measures.
The channel under study is approximately 2350 m in length and was born from a channel opened in previous EGMASA restoration works, which intercepts one of the main and oldest underground galleries of the mining exploitation called “Galería del Carmen” [
7], thus allowing the exit of the waters affected by AMD processes inside the open pit lake and the galleries. The acid effluent runs through a concrete channel that also collects the municipality’s rainwater and little waste rock dam. At the end of the concrete channel, the drainage fits naturally through a ravine known as “Barranco de los Diques” until intercepting the Odiel river after a waterfall of about 2 m downstream.
The main objective of this work is focused on establishing the possible reasons for the interdependence between electrical conductivity (EC) and pH, with the load of metals and sulphates, as well as with a set of variables that defines the physico-chemical characteristics of water, such as temperature (T), total dissolved solids (TSD), and redox potential (Eh), transported by a mining channel affected by AMD, through the use of artificial intelligence techniques such as Fuzzy Logic, and also the establishment natural attenuation processes.
In this study, a methodology based on the use of the data mining technique PreFuRGe (Predictive Fuzzy Rules Generator) [
15], is proposed for data treatment. This technique proved to be efficient for modelling the qualitative behaviour of a complex system and can also be applied to establish cause–effect relationships that, in contrast to classical statistical treatments, improve the understanding of the processes involved. PreFuRGe has allowed the discovery of new and relevant information, using datasets drawn for diverse contexts such as, for example: software engineering [
16], systems identification in control, and the modelling of complex environmental systems existing in waters affected by AMD processes [
8,
9,
12,
17].
2. Materials and Methods
In order to evaluate the physico-chemical characteristics of the acid channel and its receptor medium, water sampling was carried out at the end of April, coinciding with the end of the rainy season, from the birth of the mining channel (MC1) to its mouth in the Odiel river (MC11), as well as four other points in the same river, before (MC14 and MC15) and after (MC12 and MC13) being intercepted by the studied channel (
Figure 2).
The determination of pH, electrical conductivity (EC), total dissolved solids (TDS), temperature (T), and redox potential (Eh) was carried out in situ by using a portable multi-parameter equipment of the Crison brand, performing 3 consecutive measures to avoid reading errors. Additionally, the sulphates concentration was also determined using a photometer from the Macherey-Nagel commercial brand, model FP-11, based on the turbidimetric measurement method, and a Sulfate Test Kit from the HANNA Instruments brand.
A sample of water was taken after field measurements at each defined point in 100 mL sterile polyethylene cans for the determination of heavy metals and metalloids.
In the field, the water samples were filtered with 0.45 micron Cellulose Nitrate filters (Sartorius 11406-47-ACN), then nitric acid was added to achieve a pH < 2 to avoid precipitation of metals during transport to the laboratory. Samples were carried out in a portable refrigerator at 4 °C.
In the laboratory, the concentration of metals and metalloids was determined, using an Inductively Coupled Plasma Mass Spectrometer (ICP-MS) of the Agilent 7700 trademark and an Inductively Coupled Plasma Optical Emission Spectrophotometer (ICP-OES) of the Jobin Yvon Ultima 2 brand, belonging to CIDERTA (University of Huelva).
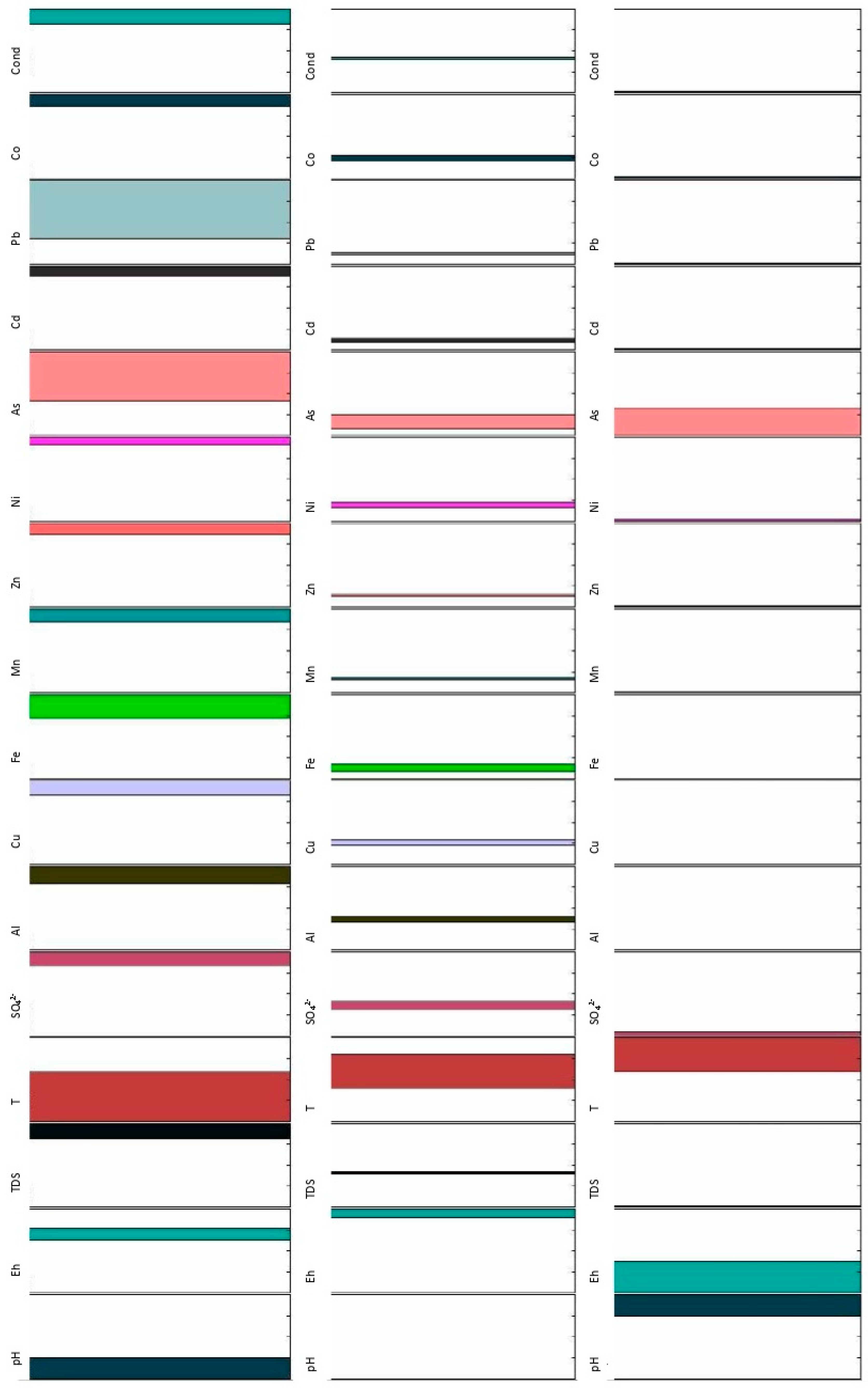
The data mass obtained was treated using graphical and statistical analyses. Spatial evolution and Cluster Analysis were studied with the software package Statgraphics Centurion XVI. Additionally, Fuzzy Logic techniques (
Figure 3) were applied using the PreFuRGe tool [
8,
9,
12,
15,
16,
17]. This tool allows the interpretation of the complexity of AMD affected waters.
3. Results
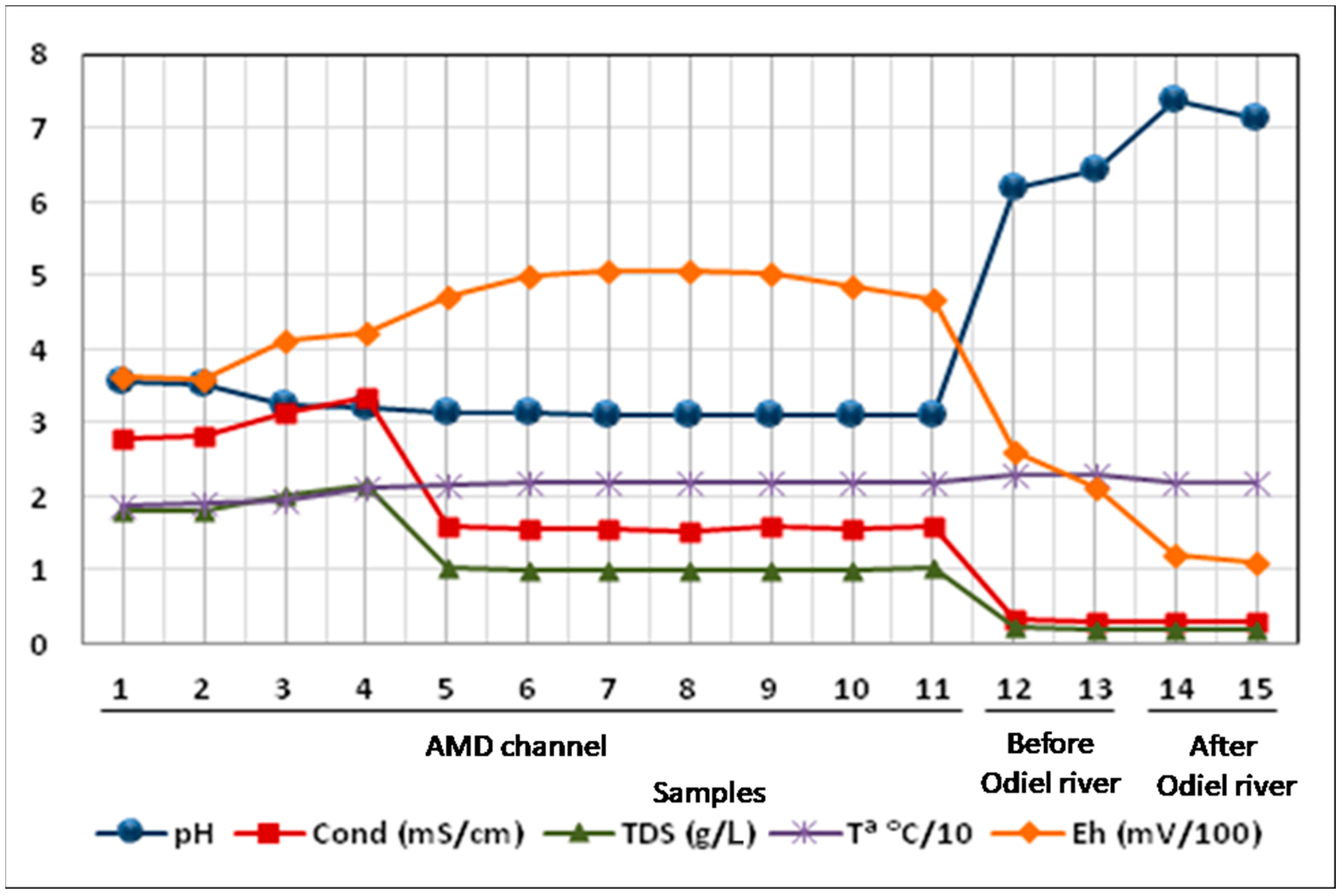
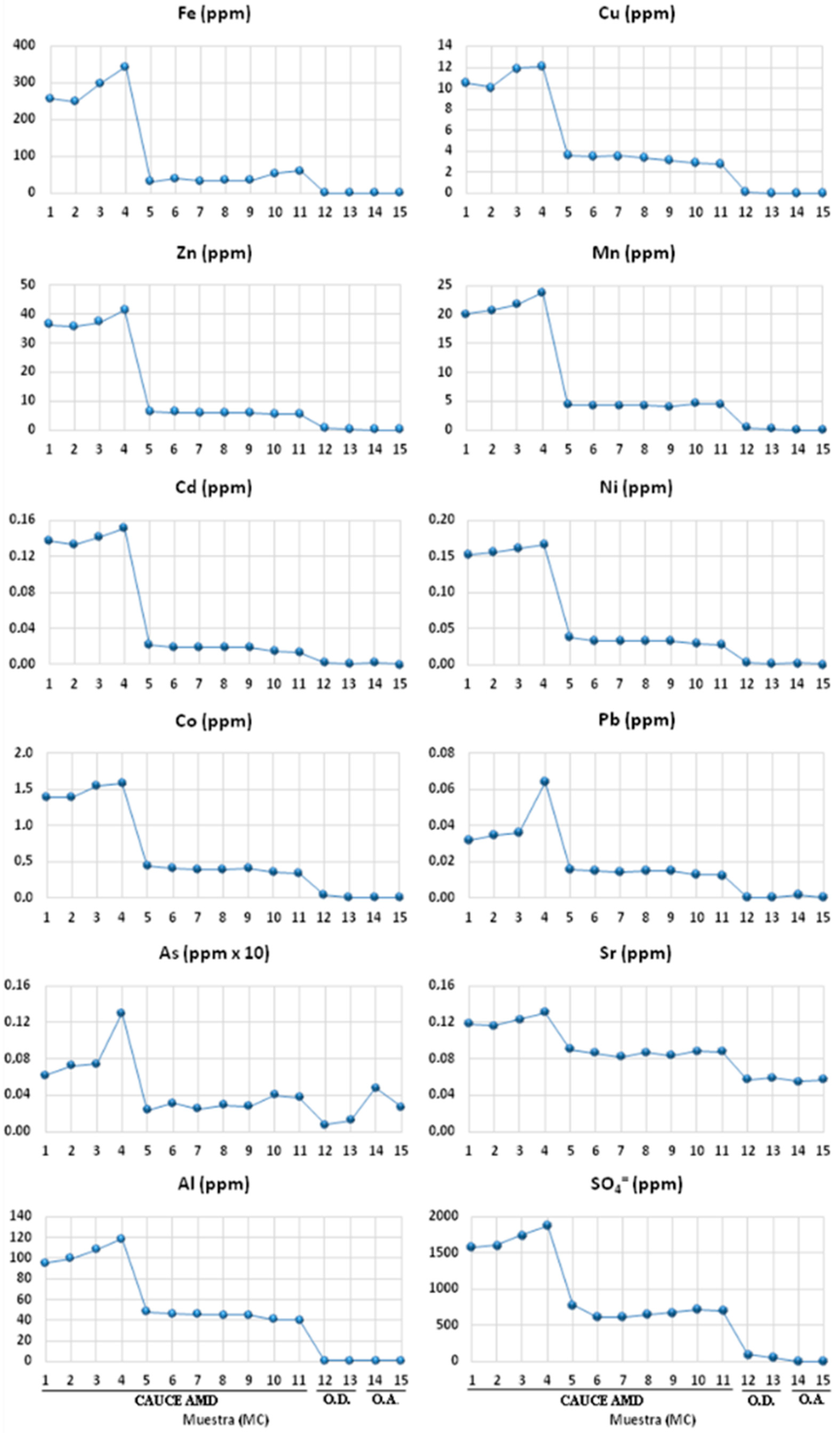
Figure 4 and
Figure 5 represent the spatial evolution of the in situ measured parameters and laboratory determinations during and after the sampling campaign, respectively. In the mining effluent (MC1 to MC 11), the electrical conductivity and the total dissolved solids show the same trend, presenting a moderate increase in their values from the source of the channel (MC1) to MC4, where they reach their maximum values. From this point on, the values of both parameters drop sharply (MC5) and stabilize along the channel even before intercepting its receiving medium (MC11). Once the channel intersects with Odiel river (samples MC12 and MC13) these parameters undergo another sharp drop until they reach their minimum values.
Unlike what happened with the previous parameters, within the acid channel, the pH shows its highest value at point MC1, decreasing moderately to point MC4 where, from this point on, the tendency is to decrease smoothly to point MC11. When it intercepts the Odiel River (MC12), the pH values rise sharply again. The Eh presents a totally opposite behaviour, increasing its values from the source of the effluent until reaching its maximum at point MC8, from which it decreases smoothly to point MC11. When the effluent intercepts Odiel (MC12), the Eh decreases its value, showing its minimum at point MC15 upstream of the confluence with Odiel.
Regarding the temperature, it shows its minimum value at the beginning of the acid effluent (MC1) and gradually increases until the point MC5, from which it stabilizes its values along the channel until it intercepts with its receptor medium.
It is observed, in a generalized way, that the concentrations of all the elements analysed within the studied channel present a moderate and progressive increase from point MC1 to MC4, where they reach their maximum values. From point MC4 to MC5, concentrations show a sharp decrease, and from the latter to the mouth (MC11), concentrations tend to decrease slightly except for Fe, Mn, As, and Sr, which increase their values from the point MC10. The same happens with SO42 = from point MC8. Once the acid effluent intercepts with the Odiel river (MC12 and MC13), the concentrations register minimum values.
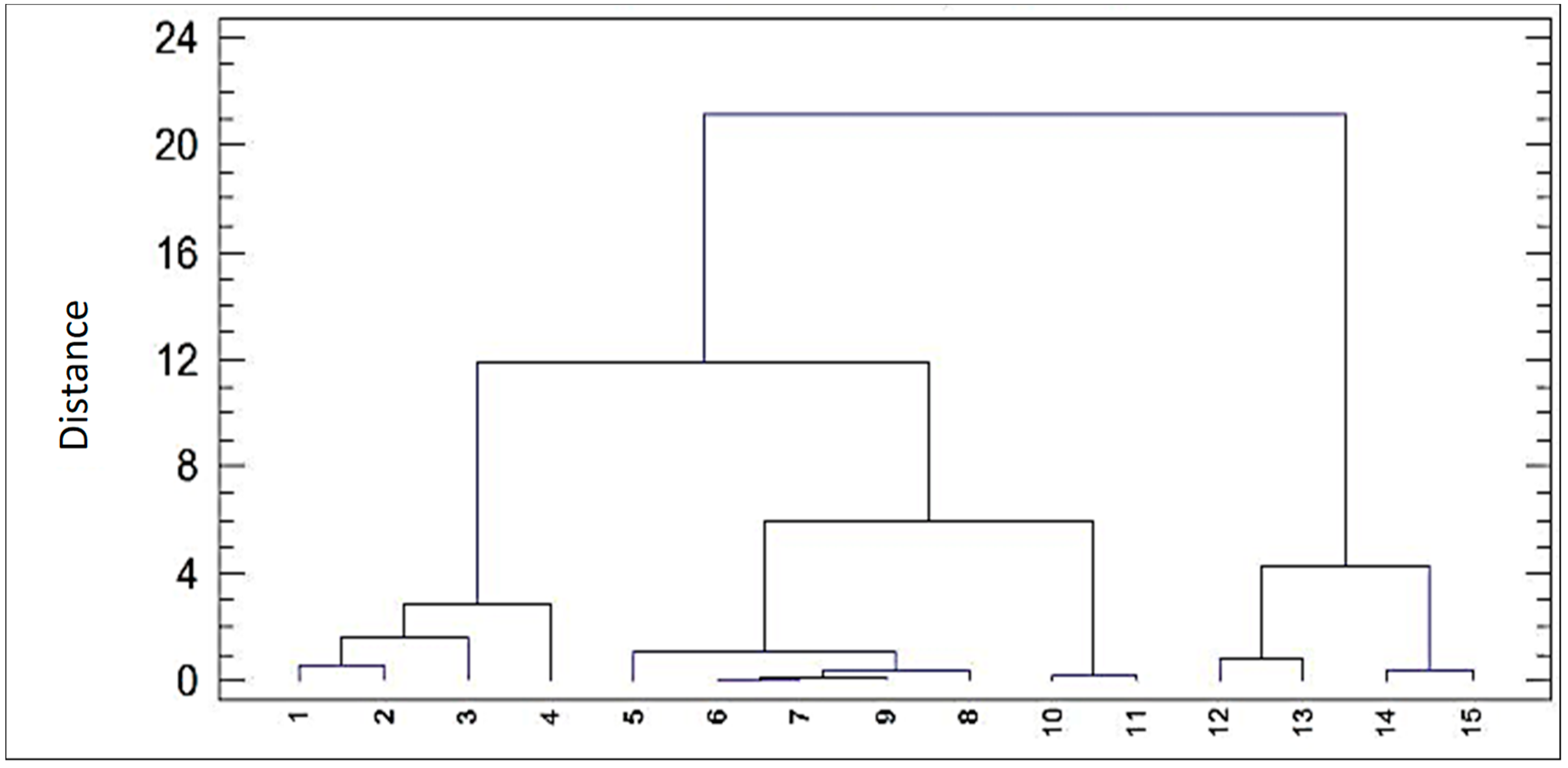
Figure 6 shows the dendrogram of observations made from the studied points. Remember that the software input is the set of the considered physico-chemical variables, while the system output that appears in the cluster is each one of the observation points affected by the totality of the variables’ values with the information each one transports. Two main groups were defined, the first formed by points MC1 to MC11, taken in the affected mining channel, and a second group formed by points MC12 to MC15, taken in the Odiel river. In the first group, there are two subclusters: subcluster 1, formed by samples MC1 to MC4, which have a high affinity, and another subgroup formed by samples MC5 to MC11, the latter being subdivided into two other subgroups, formed by samples MC5 to MC9 and by MC10 and MC11. Finally, within the group of samples taken in the Odiel river (subcluster 2), they can be subdivided into the waters contaminated by the studied channel, formed by points MC12 and MC13, and a second subgroup formed by samples MC14 and MC15 corresponding to the samples taken before the confluence with Odiel. Noteworthily, clusters present a very good Pearson proximity and are not more than a “graphic representation” of the correlation matrix, showing the good fit of the measures.
The data mass obtained was treated using Fuzzy Logic techniques by PreFuRGe tool [
10,
11].
Figure 7 and
Figure 8 show the fuzzy rules obtained for the variables analysed in the water samples taken at the different sampling points defined in the Concepción mine, taking as consequence the conductivity (
Figure 7) and the pH (
Figure 8), respectively. The application of Fuzzy Logic techniques to this data mass allows us to extend the proposed hydrochemical characterization models in the same watercourse, but using classical statistics.
Figure 7 shows how conductivity maintains, as expected, an inverse relationship with pH. As the conductivity increases its values, the pH decreases. Quite the opposite occurs with the analysed metallic charge and sulphates, which increase or decrease their concentrations in the same way that conductivity does. It should be noted that As presents a similar behaviour to the one described for sulphates and metals, presenting a universe of discourse which is always somehow larger than for the other parameters.
For extreme-high conductivity values, the Eh maintains high values, while for low-extreme conductivity values, the Eh takes values ranging from extreme-low to medium. When the conductivity takes extreme-high values, all metal load and sulphates show extreme-low values or zero.
If we take the pH as a consequence (
Figure 8) we can see how, at low pH values, the metallic and sulphates load present values that go from medium to extreme-high, except for lead, which remains between low to medium values. As the pH increases its value, the metallic charge and the sulphates decrease their values, with the exception of arsenic, which maintains extreme low values when the pH takes extreme-high values, taking into account that the discourse universe of pH reaches up to 7, in this case.
4. Discussion
Throughout the studied effluent, all the parameters, with the exception of pH, show an increasing trend in the first 200 m, from its source (MC1) to point MC4. This trend is justified by the greater oxidizing capacity of the channel waters due to contact with the atmosphere as they move away from the place of origin. As the medium is enriched with oxygen, Fe
2+ oxidizes to Fe
3+, releasing hydrogen ions to the medium, causing a decrease in pH and an increase in the concentrations of sulphates, total dissolved solids, and, consequently, electrical conductivity [
12]. Between points MC4 and MC5, the channel is led by a concreted channel about 500 m long, which collects the waters of the acid channel and the town’s rainwater. This channel is fully upholstered with other products, the result of the iron oxy-hydroxides precipitation.
Therefore, in the first section of the AMD channel, iron oxidation and hydrolysis phenomena occur, while in the second section, with the mix of water from different sources, dilution and precipitation phenomena occur. By precipitating iron hydroxides, natural attenuation phenomena would be taking place, causing a large part of the analysed pollutants to precipitate as well.
The corresponding section from point MC5, where the channel runs naturally throughout the orography of the place to point MC11, just before intercepting the Odiel river, shows more stable values of all of the physico-chemical parameters analysed. It can be observed that, for the majority of parameters, the general tendency is to gently decrease their concentrations, except for the parameter TDS, CE, SO42−, Fe, Mn, As, and Sr, which show a slight increase from the MC10 point onwards. This can be explained by the confluence of a small drainage, which intercepts the main acid channel and provides it with waters with different hydrochemical characteristics.
Once the studied channel intercepts clean waters from the Odiel river (points MC14 and MC15), there is a sudden increase in the pH values, reaching conditions close to neutrality, which causes a precipitation phenomenon.
Likewise, it can be seen that values of all analysed parameters in the Odiel river section affected by the contaminated channel (points MC12 and MC13) tend to be quite close to those measured in its clean waters before being intercepted (points MC14 and MC15). This fact is ratified in the Fuzzy Logic graphs, where it is seen that when the conductivity takes extreme values under the metallic and sulphate load, it is practically non-existent because the Odiel river is clean.
According to Grande et al., 2010 [
17], the presence of total dissolved As is closely related to temperature and precipitation, and therefore to pH, which is corroborated by what is observed in the Fuzzy Logic graphs (
Figure 8). Thus, very high total As values are only compatible with extremely low pH levels and vice versa.
5. Conclusions
The oxidation of metal sulphides in AMD media is a complex process, which includes reactions such as oxidation, reduction, hydrolysis, and precipitation. The surface exposure of acidic waters from inside of the mine undergo the aforementioned processes in a short time and space, continuing the generation of acidity and sulphates outside the point of emergence, thanks to the residual particulate matter deposited in its channel.
It is evident that the waters of the Odiel river, once it has been intercepted by the mining effluent, has sufficient neutralizing capacity to buffer the new physico-chemical conditions that it brings. The fact that pH maintains an inverse relationship with conductivity and dissolved solids may be due to the sulphides oxidation processes that generate, on the one hand, sulphates (which increase with the conductivity), and at the same time, hydrogen emissions (acidifying water).
The present work concluded the existence of natural attenuation processes for the mining channel, despite of the entrances of other drainages in the AMD channel with different hydrochemical characteristics imposing modifications on it. This indicates that these media have a high vulnerability to external stimuli.
In this context, the PreFuRGe computer tool used in this study acquires a high dimension effectiveness for the qualitative diagnosis of the contamination, improves work considerably, and makes knowledge of the processes involved easier. It can also be applied to establish cause–effect relationships in contrast to classical statistical treatments. The application of Fuzzy Logic to characterize AMD improves the previously proposed models using classical statistics.