Nitrogen Assessment in Amended Mining Soils Sown with Coronilla juncea and Piptatherum miliaceum
Abstract
:1. Introduction
2. Materials and Methods
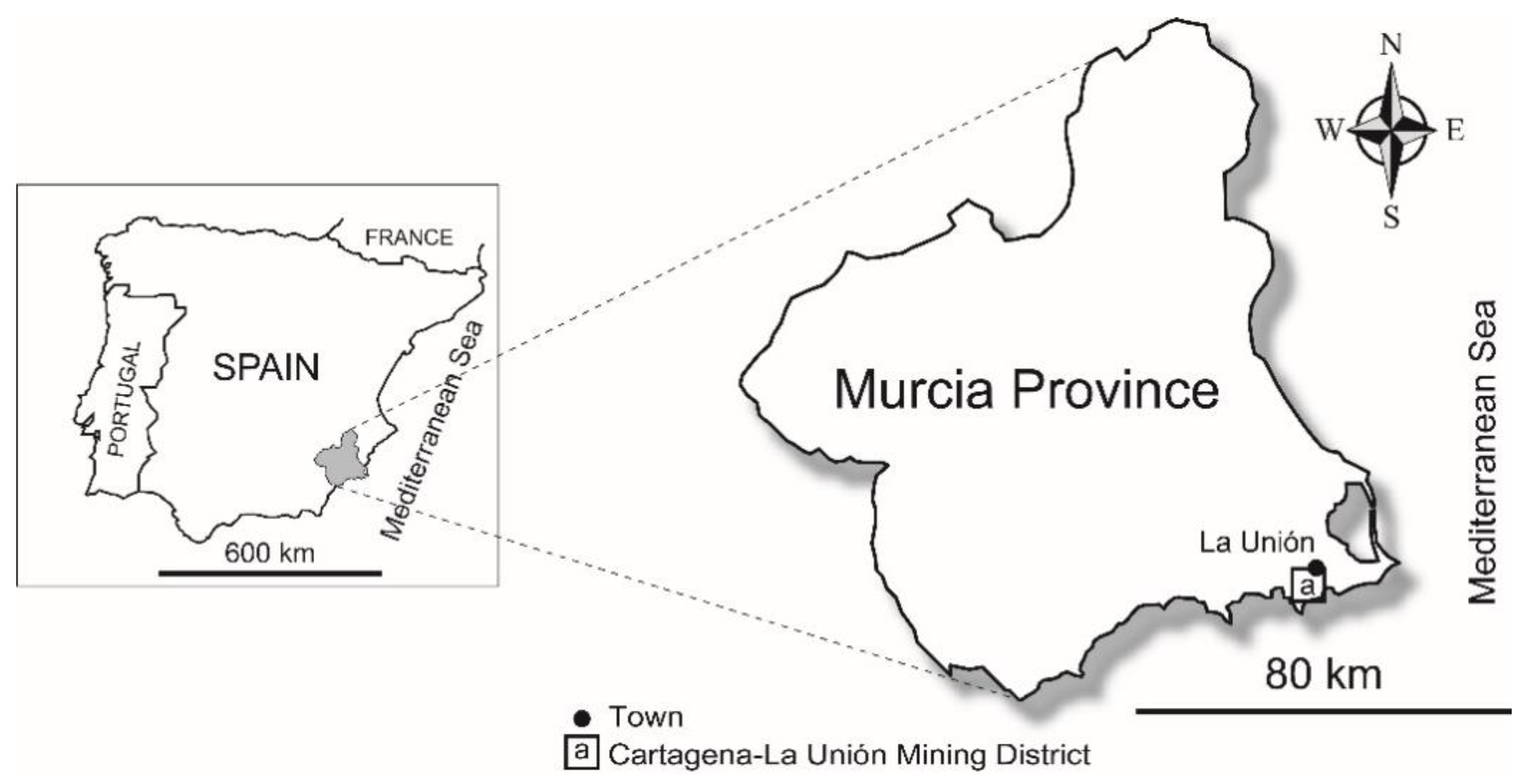
2.1. Study Area
2.2. Field Experiment
- Control (CT);
- Limestone + biochar (L + BC);
- Limestone + compost (L + COM);
- Limestone + biochar + compost (L + BC + COM);
- Limestone + biochar + zeolite (L + BC + Z);
- Limestone + compost + zeolite (L + COM + Z);
- Limestone + biochar + compost + zeolite (L + BC + COM + Z).
2.3. Sampling and Analytical Methods
2.4. Statistical Analysis
3. Results and Discussion
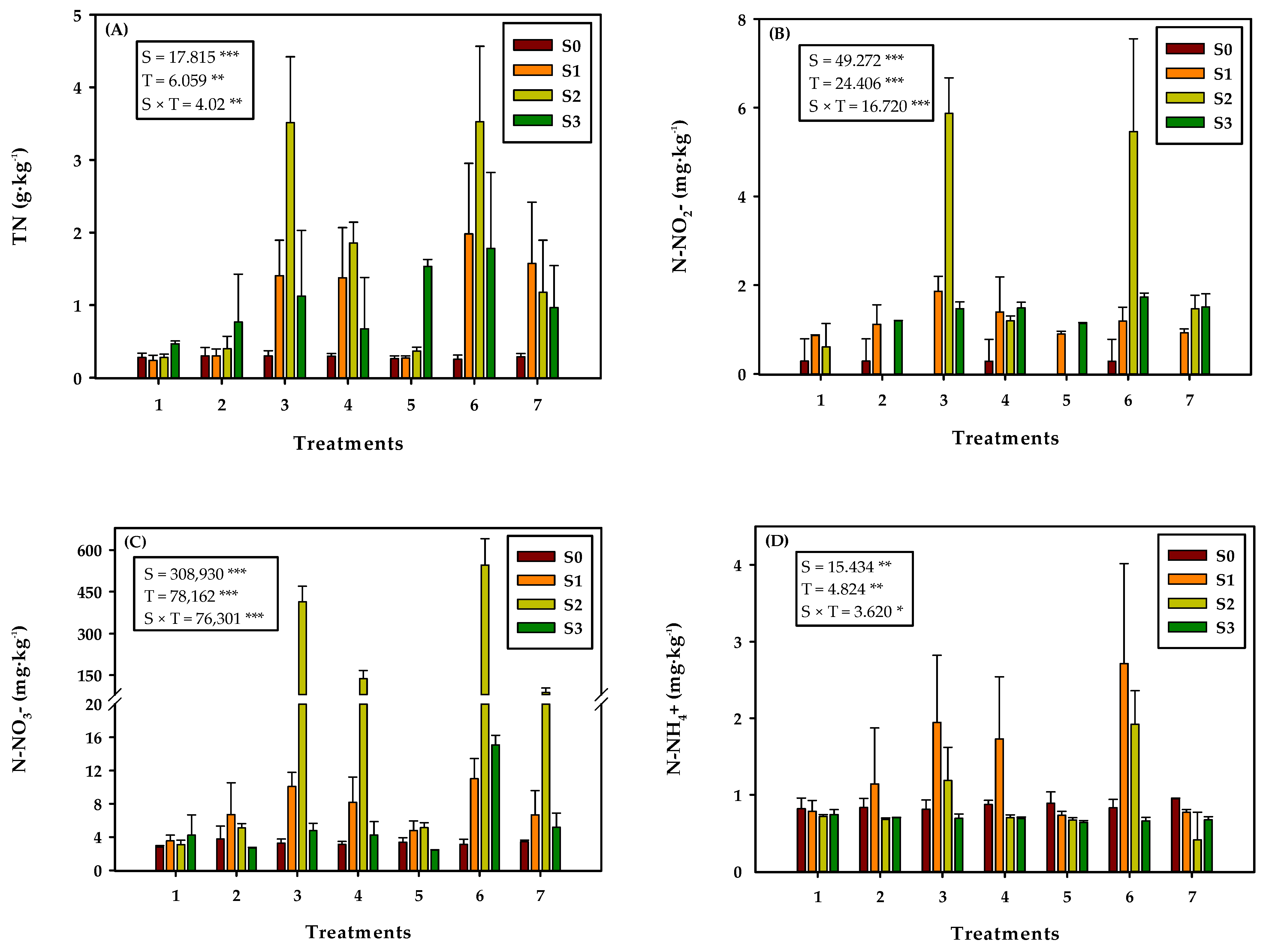
3.1. Effect of the Amendment Application on Soil Nitrogen Content
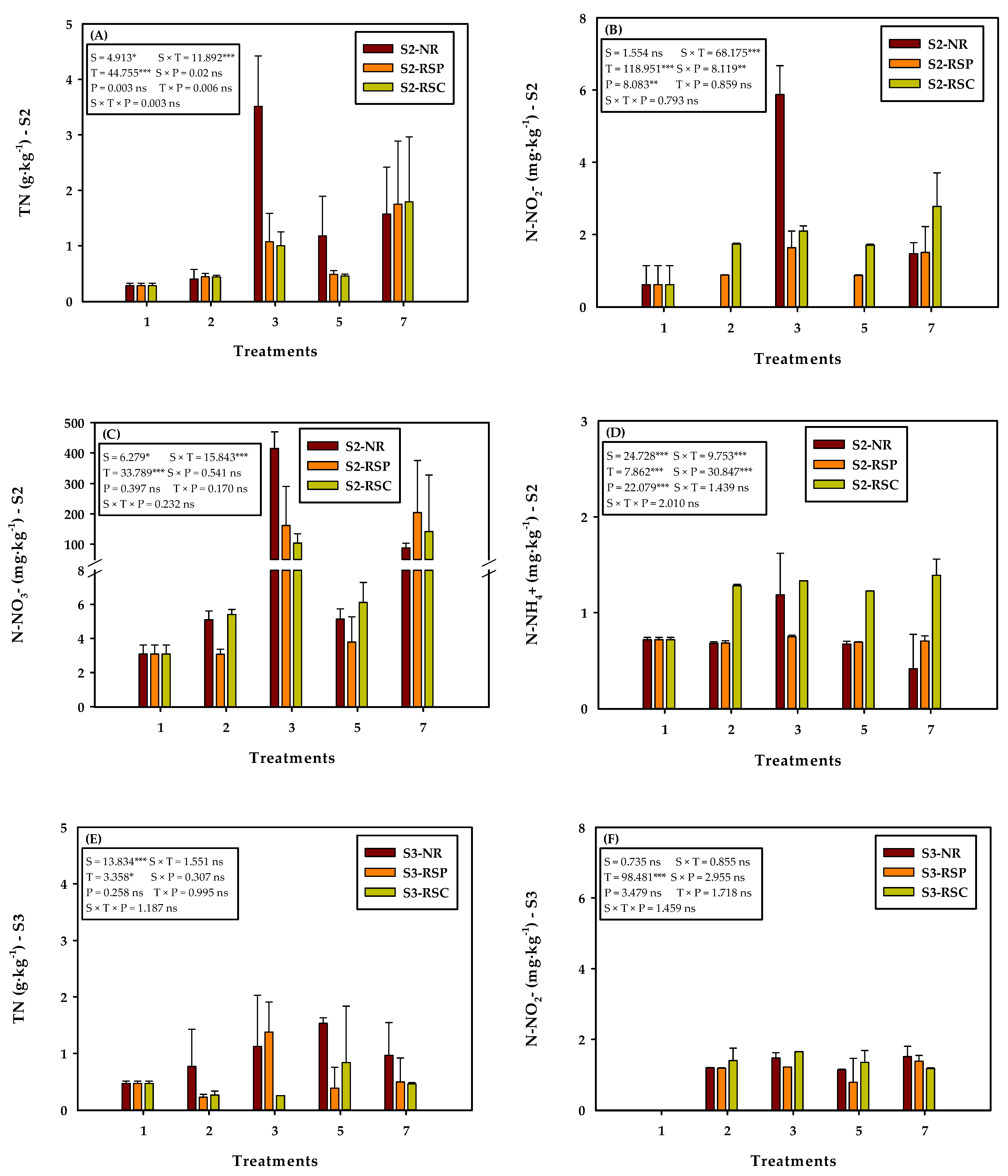
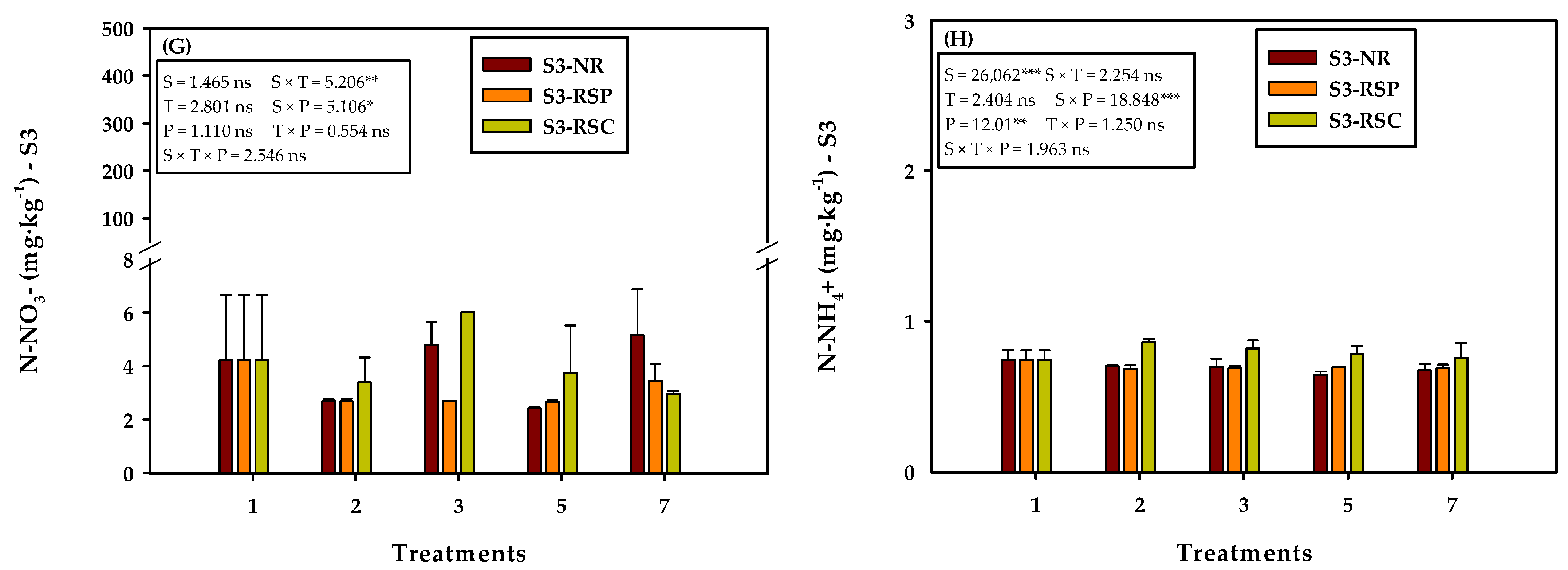
3.2. Effect of the Soil and Plant Type on Soil Nitrogen Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davila, R.B.; Fontes, M.P.F.; Pacheco, A.A.; Ferreira, M. Heavy metals in iron ore tailings and floodplain soils affected by the Samarco dam collapse in Brazil. Sci. Total Environ. 2020, 709, 136151. [Google Scholar] [CrossRef]
- Parraga-Aguado, I.; González-Alcaraz, M.N.; Schulin, R.; Conesa, H.M. El uso potencial de Piptatherum miliaceum para la fitoterapia de relaves mineros en áreas semiáridas: Papel de la fertilidad del suelo y la competencia de las plantas. Rev. Ambient. Ambient. 2015, 158, 74–84. [Google Scholar]
- Kabas, S.; Faz, A.; Acosta, J.A.; Zornoza, R.; Martínez-Martínez, S.; Carmona, D.M.; Bech, J. Efecto de los desechos de mármol y lodo de cerdo sobre el crecimiento de la vegetación nativa y la movilidad de metales pesados en un estanque de relaves de una mina. Rev. De Explor. Geoquímica 2012, 123, 69–76. [Google Scholar]
- Carrillo-González, R. Fitorremediación asistida con enmiendas y fitoestabilización de elementos potencialmente tóxicos. Agro Product. 2017, 10, 4. [Google Scholar]
- Asensio, V.; Covelo, E.F.; Kandeler, E. Soil management of copper mine tailing soils—Sludge amendment and tree vegetation could improve biological soil quality. Sci. Total Environ. 2013, 456, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Barriga, F.; Faz, Á.; Acosta, J.A.; Soriano-Disla, M.; Martínez-Martínez, S.; Zornoza, R. Uso de Piptatherum miliaceum para el fitomando de tecnosoles modificados con biocarbón derivados de relaves piríticos para mejorar la agregación del suelo y reducir la movilidad de los metales (loides). Geoderma 2017, 307, 159–171. [Google Scholar] [CrossRef]
- Radziemska, M.; Bęś, A.; Gusiatin, Z.M.; Cerdà, A.; Jeznach, J.; Mazur, Z.; Brtnický, M. Assisted phytostabilization of soil from a former military area with mineral amendments. Ecotoxicol. Environ. Saf. 2020, 188, 109934. [Google Scholar] [CrossRef]
- Martínez-Martínez, S.; Zornoza, R.; Gabarrón, M.; Gómez-Garrido, M.; Rosales, R.M.; Muñoz, M.A.; Gómez-López, M.D.; Soriano-Disla, J.M.; Faz, A.; Acosta, J.A. Is aided phytostabilization a suitable technique for the remediation of tailings? Eur. J. Soil Sci. 2019, 70, 862–875. [Google Scholar] [CrossRef]
- Morugan-Coronado, A.; Soriano-Disla, M.; Moreno-Barriga, F.; Linares, C.; Faz, A.; García-Orenes, F.; Zornoza, R. Uso de Piptatherum miliaceum para permitir el establecimiento exitoso de Salvia rosmarinus en tecnosoles desarrollados a partir de relaves piríticos. Quimiosfera 2021, 267, 129281. [Google Scholar]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. El papel del biocarbón y el compost de biocarbón en la mejora de la calidad del suelo y el rendimiento de los cultivos: Una revisión. Ecol. Suelo Apl. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Oldfield, T.L.; Sikirica, N.; Mondini, C.; López, G.; Kuikman, P.J.; Holden, N.M. Biochar, compost y ambient-compost blend como opciones para recuperar nutrientes y secuestrar carbono. Rev. Ambient. Ambient. 2018, 218, 465–476. [Google Scholar]
- Misaelides, P. Aplicación de zeolitas naturales en la remediación ambiental: Una breve revisión. Mater. Microporosos Mesoporosos 2011, 144, 15–18. [Google Scholar] [CrossRef]
- Castaldi, P.; Santoña, L.; Melis, P. Inmovilización de metales pesados por enmiendas químicas en un suelo contaminado e influencia en el crecimiento del lupino blanco. Chemosphere 2005, 60, 365–371. [Google Scholar] [CrossRef]
- Shi, W.Y.; Shao, H.B.; Li, H.; Shao, M.A.; Du, S. Avances en la remediación de suelos peligrosos contaminados con metales pesados mediante zeolita natural. D. Mater. Peligr. 2009, 170, 1–6. [Google Scholar]
- Méndez, M.O.; Maier, R.M. Fitorremediación de relaves mineros en ambientes templados y áridos. Rev. Environ. Sci. Bio/Technol. 2008, 7, 47–59. [Google Scholar]
- Ghosh, D.; Maiti, S.K. Fitorremediación asistida por biocarbón y eliminación de biomasa en suelos mineros contaminados con metales pesados: Una revisión. Rev. Int. Fitorremediación 2021, 23, 559–576. [Google Scholar]
- Hossner, L.R.; Hons, F.M. Reclamation of mine tailings. In Soil Restoration; Lal, R., Stewart, B.A., Eds.; Advances in Soil Science; Springer: New York, NY, USA, 1992; Volume 17, pp. 311–350. [Google Scholar]
- Fagorzi, C.; Checcucci, A.; DiCenzo, G.C.; Debiec-Andrzejewska, K.; Dziewit, L.; Pini, F.; Mengoni, A. Aprovechamiento de los rizobios para mejorar la fitorremediación de metales pesados por las legumbres. Genes 2018, 9, 542. [Google Scholar] [CrossRef] [Green Version]
- Chapin, F.S., III; Shaver, G.R.; Kedrowski, R.A. Controles ambientales sobre las fracciones de carbono, nitrógeno y fósforo en Eriophorum vaginatum en la tundra de matas de Alaska. La Rev. Ecol. 1986, 74, 167–195. [Google Scholar]
- Robles, A.B.; Allegretti, L.I.; Passera, C.B. Coronilla juncea es un arbusto forrajero nutritivo y útil en la rehabilitación de tierras agrícolas marginales mediterráneas abandonadas. Rev. Entornos Áridos 2002, 50, 381–392. [Google Scholar]
- Martínez-Martínez, S.; Acosta, J.A.; Cano, A.F.; Carmona, D.M.; Zornoza, R.; Cerda, C. Assessment of the lead and zinc contents in natural soils and tailing ponds from the Cartagena-La Unión mining district, SE Spain. J. Geochem. Explor. 2013, 124, 166–175. [Google Scholar] [CrossRef]
- Toro, F.D.J.C.; Benítez, L.M.L.; Herrera, M.I.Á. La zeolita en la mitigación ambiental. Rev. Lasallista Investig. 2006, 3, 30–34. [Google Scholar]
- Doula, M.K.; Kavvadias, V.A.; Elaiopoulos, K. Zeolitas en procesos de remediación de suelos. Man. Zeolitas Nat. 2012, 519–568. [Google Scholar]
- Querol, X.; Alastuey, A.; Moreno, N.; Alvarez-Ayuso, E.; García-Sánchez, A.; Cama, J.; Simón, M. Immobilization of heavy metals in polluted soils by the addition of zeolitic material synthesized from coal fly ash. Chemosphere 2006, 62, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Uso de zeolita para neutralizar el níquel en un ambiente de suelo. Monit. Y Evaluación Ambient. 2018, 190, 1–13. [Google Scholar]
- Schad, P. Tecnosoles en la Base Mundial de Referencia para los Recursos del Suelo: Historia y definiciones. Cienc. Suelo Nutr. Veg. 2018, 64, 138–144. [Google Scholar]
- Peech, M. Actividad de Iones de Hidrógeno. In Métodos de Análisis de Suelos: Parte 2 Propiedades Químicas y Microbiológicas; American Society of Agronomy: New York, NY, USA, 1965; Volume 9, pp. 914–926. [Google Scholar]
- Cobertera, E. Edafología Aplicada. Suelos, Producción Agraria, Planificación territorial e Impactos Ambientales; Gráficas Rógar, S.A.: Madrid, Spain, 1993; 326p. [Google Scholar]
- Ramírez-García, J.; Almendros, P.; Quemada, M. Ground cover and leaf area index relationship in a grass, legume and crucifer crop. Plant Soil Environ. 2012, 58, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-García, J.; Gabriel, J.L.; Alonso-Ayuso, M.; Quemada, M. Quantitative characterization of five cover crop species. Rev. Cienc. Agrícolas 2015, 153, 1174–1185. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Monedero, M.A.; Roig, A.; Paredes, C.; Bernal, M.P. Transformación de nitrógeno durante el compostaje de residuos orgánicos por el sistema de Rutgers y sus efectos sobre el pH, EC y madurez de las mezclas de compostaje. Tecnol. Biorecursos 2001, 78, 301–308. [Google Scholar]
- Zubillaga, M.S.; Branzini, A.; Lavado, R.S. Problemas de fitotoxicidad en compost. Pilquen-Sección Agron. 2008, 9, 1. [Google Scholar]
- Barbaro, L.; Karlanian, M.; Rizzo, P.; Riera, N. Caracterización de diferentes compost para su uso como componente de sustratos. Chil. J. Agric. Anim. Sci. 2019, 35, 126–136. [Google Scholar] [CrossRef]
- Montero, R.G.; Medina, P.P.; Rastrero, M.R.; Herráiz, M.J.S.; Guirado, M.; Torres, R.M.G. Biochar y sus aplicaciones potenciales en el suelo. Técnica Ind. 2021, 328, 44–53. [Google Scholar]
- Flores, A. Suelos Salinos y Sódicos; Instituto Superior de Ciencias Agropecuarias de la Habana, Facultad de Agronomía, Departamento de Suelos y Riego: La Habana, Cuba, 1991; 32p. [Google Scholar]
- Ramirez, L.R.; José, G.L.D.; Rosales, J.G.; Edgar, R.C.; Hernández, J.D. Validación del modelo LEACHM para predecir la salinidad en un suelo del valle de Quibor, con cultivo de cebolla bajo riego localizado. Rev. For. Latinoam. 2005, 38, 97–119. [Google Scholar]
- Zornoza, R.; Moreno-Barriga, F.; Acosta, J.A.; Muñoz, M.A.; Faz, A. Estabilidad, disponibilidad de nutrientes e hidrofobicidad de biochares derivados de estiércol, residuos de cultivos y desechos sólidos municipales para su uso como enmiendas del suelo. Chemosphere 2016, 144, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Escalante Rebolledo, A.; Pérez López, G.; Hidalgo Moreno, C.; López Collado, J.; Campo Alves, J.; Valtierra Pacheco, E.; Etchevers Barra, J.D. Biocarbón (biochar) I: Naturaleza, historia, fabricación y uso en el suelo. Terra Latinoam. 2016, 34, 367–382. [Google Scholar]
- Espejel-Ayala, F.; Solís-López, M.; Schouwenaars, R.; Ramírez-Zamora, R.M. Síntesis de zeolita P utilizando jales de cobre. Rev. Mex. Ing. Química 2015, 14, 205–212. [Google Scholar]
- Zornoza, R.; Faz, Á.; Carmona, D.M.; Acosta, J.A.; Martínez-Martínez, S.; de Vreng, A. Mineralización de carbono, actividad microbiana y dinámica de metales en estanques de relaves modificados con purín de cerdo y residuos de mármol. Chemosphere 2013, 90, 2606–2613. [Google Scholar] [CrossRef]
- Valero, C.; Yazmin, T. Desnitrificación del Suelo Bajo dos Tratamientos de Riego para Estimar el Rendimiento y Emisión de Metano en Arroz; Repositorio institucional de la Universidad Nacional Agraria La Molina: Lima, Peru, 2020. [Google Scholar]
- Cabrera, O.G.; Vermoesen, A.; Van Cleemput, O.; Cabriales, J.P. Efecto del tipo de suelo, humedad y fuente de nitrógeno en las emisiones de N2 y N2O. Terra Latinoam. 2000, 18, 1–9. [Google Scholar]
- Hayatsu, M.; Tago, K.; Saito, M. Diversos actores en el ciclo del nitrógeno: Diversidad y funciones de los microorganismos implicados en la nitrificación y desnitrificación. Cienc. Suelo Nutr. Veg. 2008, 54, 33–45. [Google Scholar]
- Salisbury, F.B.; Ross, C.W. Fisiología Vegetal; Grupo Editorial Iberoamérica, México, D.F.: Lima, Peru, 1994. [Google Scholar]
- Lee, C.; Chon, H.; Jung, M. Heavy metal contamination in the vicinity of the Daduk Au–Ag–Pb–Zn mine in Korea. Appl. Geochem. 2001, 16, 1377–1386. [Google Scholar] [CrossRef]
- Galán, A.; Romero, E. Contaminación de Suelos Por Metales Pesados. Macla Revista de la Sociedad Española de Mineralogía 10. 2008, pp. 48–60. Available online: http://www.ehu.eus/sem/macla_pdf/macla10/Macla10_48.pdf (accessed on 28 August 2021).
- Jordanova, D.; Rao, S.; Kotsev, T.; Jordanova, N. Industrial contamination of alluvial soils near Fe–Pb mining site revealed by magnetic and geochemical studies. Geoderma 2013, 192, 237–248. [Google Scholar] [CrossRef]
- Román, P.; Martínez, M.; Pantoja, A. Manual de Compostaje del Agricultor. Santiago de Chile (Chile). 2013. Available online: http://www.fao.org/docrep/019/i3388s/i3388s.pdf (accessed on 16 November 2021).
- Díaz, L.; Laguna, H.; Gutiérrez, Y.; Melo, A.; Vega, A. Tratamiento de suelos mineros mediante compostaje con Biochar, estiércol ovino y residuos orgánicos domiciliarios. Rev. Medio Ambiente Min. 2020, 5, 11–18. [Google Scholar]
- Rodríguez Valdivia, M. Evaluacion de la Capacidad de Adsorción de Nh4+ y Metales Pesados Pb2+, Cd2+, Cu2+, Zn2+ y Mn2+ Empleando Zeolitas Naturales y Sintéticas; UNAS: Arequipa, Peru, 2017. [Google Scholar]
- Bochet, E.; García-Palacios, P.; Peco, B.; Tormo, J.; García-Fayos, P. Procesos Ecológicos y Restauración de la Cubierta Vegetal. In Restauración Ecológica de Áreas Afectadas por Infraestructuras de Transporte: Bases Científicas para Soluciones Técnicas; Fundación Biodiversidad: Madrid, Spain, 2011; pp. 101–141. [Google Scholar]
- Vázquez, M.; Poschenrieder, C.; Barcelo, J. Pulvinus structure and leaf abscission in cadmium-treated bean plants (Phaseolus vulgaris). Can. J. Bot. 1989, 67, 2756–2764. [Google Scholar] [CrossRef]
- Barceló, J.; Poschenrieder, C. Respuestas de las plantas a la contaminación por metales pesados. Suelo Planta 1992, 2, 345–361. [Google Scholar]
- Vilagrosa, A.; Caturla, R.N.; Hernández, N.; Cortina, J.; Bellot, J.; Vallejo, V.R. Reforestación en ambiente semiárido del sureste peninsular. Resultados de las investigaciones desarrolladas para optimizar la supervivencia y el crecimiento de especies autóctonas. In Montes para la sociedad del nuevo milenio. III Congreso Forestal Español. Junta Andal. Cons. Medio Ambiente 2001, 4, 5. [Google Scholar]
- García-Gil, J.C.; Plaza, C.; Senesi, N.; Brunetti, G.; Polo, A.A. Efectos de la enmienda de lodos de depuradora sobre los ácidos húmicos y las propiedades microbiológicas de un suelo mediterráneo semiárido. Biol. Fertil. Suelos 2004, 39, 320–328. [Google Scholar]
- Rincón, L.E.C.; Gutiérrez, F.A.A. Dinámica del ciclo del nitrógeno y fósforo en suelos. Rev. Colomb. de Biotecnol. 2012, 14, 285–295. [Google Scholar]
- Klotz, M.G.; Stain, L.Y. Nitrifier genomics and evolution of the nitrogen cycle. FEMS Microbiol. Lett. 2008, 278, 146–156. [Google Scholar] [CrossRef]
- Noguez-Inesta, A.; López-Sánchez, A.S.; Carrillo-González, R.; González-Chávez, M.C.A. Uso de leguminosas (Fabaceae) en fitorremediación. Agroproductividad 2017, 10, 4. [Google Scholar]
- Paredes Campos, P.D.S.; Rodríguez Rojas, J.J. Especies Vegetales en la Fitorremediación de Suelos Contaminados por Metales Pesados. Bachelor’s Thesis, Universidad César Vallejo, Trujillo, Peru, 2020. Available online: Repositorio.ucv.edu.pe (accessed on 14 March 2021).

| pH | EC (mS·cm−1) | TOC (%) | TN (mg·kg−1) | N-NO2− (mg·kg−1) | N-NO3− (mg·kg−1) | N-NH4+ (mg·kg−1) | |
|---|---|---|---|---|---|---|---|
| Biochar | 11.1 | 1.5 | 71.4 | 760 | 0.89 | 12.5 | 0.7 |
| Compost | 9.5 | 12.1 | 17.7 | 1910 | 10.3 | 107.3 | 26.6 |
| Zeolite | 7.0 | 0.14 | * | * | * | * | * |
| Limestone | 8.7 | 0.04 | * | * | * | * | * |
| Treatment | T-Control | T-1 | T-2 | T-3 | T-4 | T-5 | T-6 |
|---|---|---|---|---|---|---|---|
| % survival | 0.0 | 5.9 | 5.9 | 0.0 | 15.7 | 0.0 | 11.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrá, J.C.; Gabarrón, M.; Faz, Á.; Zornoza, R.; Acosta, J.A.; Martínez-Martínez, S. Nitrogen Assessment in Amended Mining Soils Sown with Coronilla juncea and Piptatherum miliaceum. Minerals 2022, 12, 433. https://doi.org/10.3390/min12040433
Beltrá JC, Gabarrón M, Faz Á, Zornoza R, Acosta JA, Martínez-Martínez S. Nitrogen Assessment in Amended Mining Soils Sown with Coronilla juncea and Piptatherum miliaceum. Minerals. 2022; 12(4):433. https://doi.org/10.3390/min12040433
Chicago/Turabian StyleBeltrá, Juan Carlos, María Gabarrón, Ángel Faz, Raúl Zornoza, José A. Acosta, and Silvia Martínez-Martínez. 2022. "Nitrogen Assessment in Amended Mining Soils Sown with Coronilla juncea and Piptatherum miliaceum" Minerals 12, no. 4: 433. https://doi.org/10.3390/min12040433
APA StyleBeltrá, J. C., Gabarrón, M., Faz, Á., Zornoza, R., Acosta, J. A., & Martínez-Martínez, S. (2022). Nitrogen Assessment in Amended Mining Soils Sown with Coronilla juncea and Piptatherum miliaceum. Minerals, 12(4), 433. https://doi.org/10.3390/min12040433







