The Problem of the Formation of Boehmite and Gibbsite in Bauxite-Bearing Lateritic Profiles
Abstract
:1. Introduction
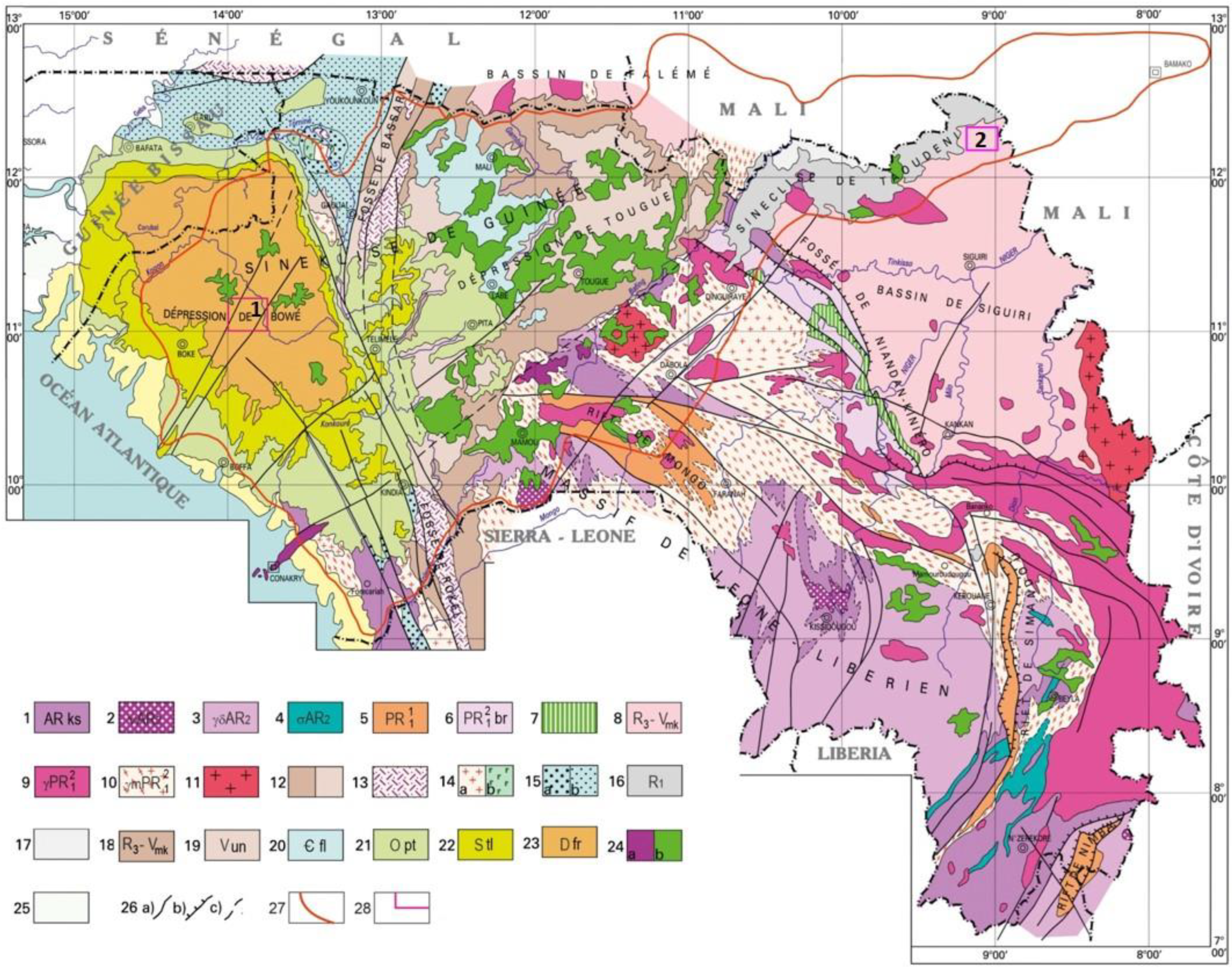
2. Geologic Setting
2.1. Parent Rock Geology
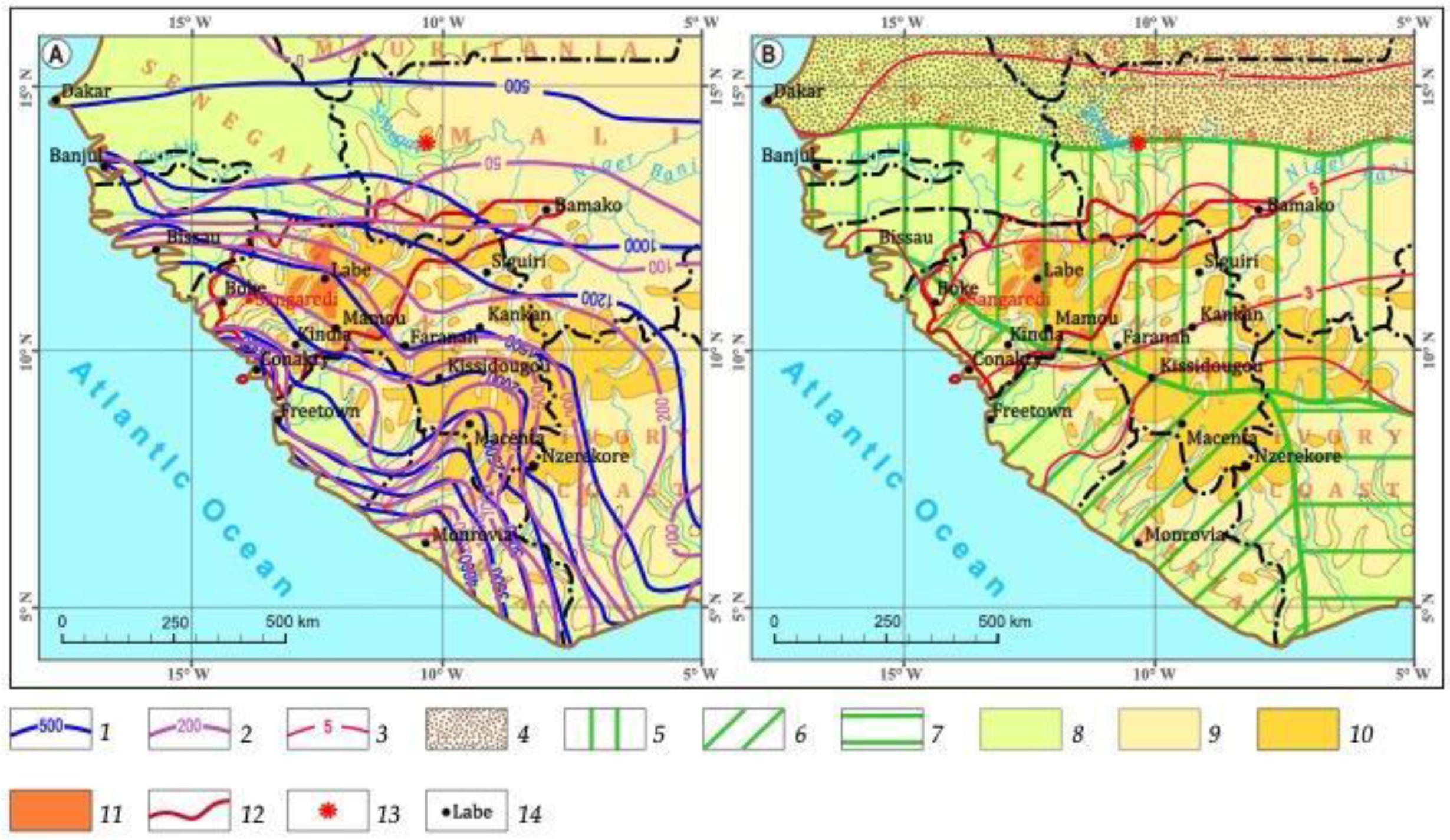
2.2. Climate/Weathering
- The amount of precipitation varies from 3500–4000 mm/year in the southwest to less than 1000 mm/year in the extreme northeast.
- In the direction from the southwest to the northeast, the number of dry months increases from 3–4 to 6–7, and the amount of unevaporated moisture decreases from 600 to 100 mm/year.
2.3. Genetic Classification of Bauxites
3. Sampling and Analytical Methods
3.1. Sampling
3.2. Analytical Methods
4. Results
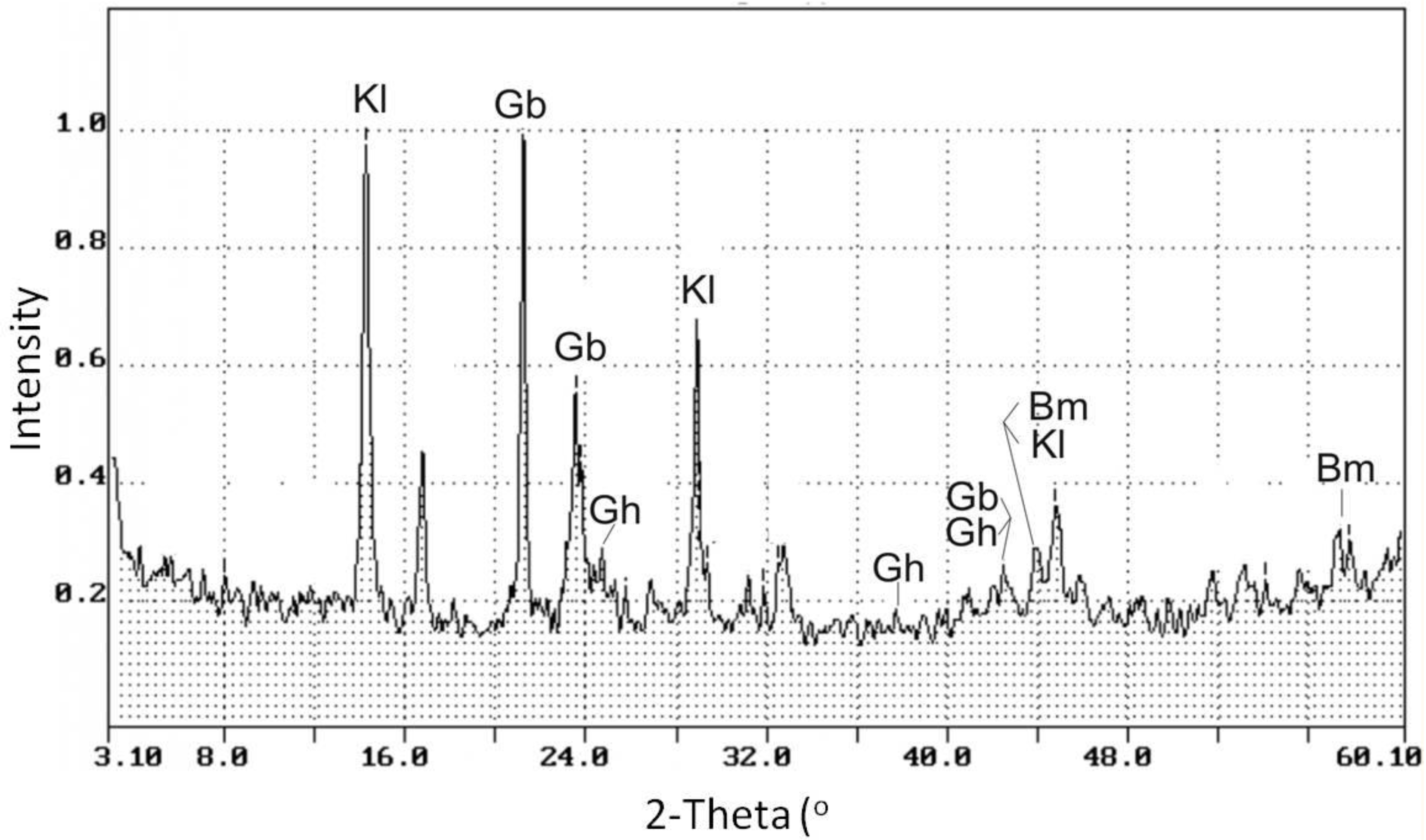
4.1. X-ray Powder Diffraction
4.2. Simultaneous Thermal Analysis
4.3. X-ray Fluorescence
5. Discussion
5.1. Distribution of Boehmite in Bauxite-Bearing Lateritic Profiles Depending on the Genetic Type of Bauxite
5.2. Geomorphologic and Climatic Factors of the Formation of Essentially Boehmite Bauxites
5.3. pH Factor in the Formation of Boehmite
5.4. High Aluminum Concentration as a Factor of Bauxite Formation
5.5. Fire as a Factor of Boehmite Formation
6. Conclusions
- (1)
- Gibbsite is formed in all classical in situ bauxites in the west of the FDM province. The thickness of the laterite covers is no more than 10–15 m. This is the zone of the formation and existence of exclusively gibbsite bauxites.
- (2)
- The genetic type favorable to the formation of boehmite bauxites is precisely sedimentary-lateritic bauxites. Boehmite formation occurs during the lateritization and resilification of bleached, aqueous continental sediments and of bleached bedrock under these sediments at depths of more than 20–25 m, mainly due to the redistribution (input from above) of aluminum.
- (3)
- Planation causes more deep horizons of sedimentary-lateritic bauxites to become exposed on the surface. In a humid climate, with more intense drainage and the direct influence of organic debris and microorganisms, boehmite becomes unstable. It begins to dissolve and is replaced by gibbsite, which is more stable under these conditions. During gibbsite formation, excess aluminum is redistributed, enriching pore solutions and contributing to their saturation with aluminum during depositions from solutions at any depth.
- (4)
- The concentration of aluminum increases by an order of magnitude or more (up to 1.78 mg/L) under acidic conditions. The concentration of organic acids associated with the vital activity of organisms also increases with depth. At the depth of oxygen-containing groundwater, alumichelate are decomposed, and the supersaturation of solutions and the mass deposition of aluminum oxide occur.
- (5)
- In the northeast part of the province, the current topoclimatic conditions are changing significantly. The rainfall is decreasing by more than two times compared with the Eastern Bamako region, the air humidity is decreasing, and the average annual temperature is increasing. However, the main factor of boehmite formation is apparently the decrease in unevaporated moisture to 100 mm/year. The profile wash is sharply decreasing. The intensity of the effect of the decomposition and alteration products of organic matter and the intensity of bacterial activity on lateritic covers is also expected to decrease.
- (6)
- The combination of these factors ensures the preservation of boehmite bauxites formed by the end of the Late Miocene or during the Pliocene-Early Pleistocene.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bardossy, G.; Aleva, G.J.J. Lateritic Bauxites; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Mehrotra, S.P.; Alex, T.C.; Greifzu, G.; Kumar, R. Mechanical Activation of Gibbsite and Boehmite: New Findings and their Implications. Trans. Indian Inst. Met. 2015, 69, 51–59. [Google Scholar] [CrossRef]
- Mamedov, V.I.; Chausov, A.A.; Okonov, E.A.; Makarova, M.A.; Boeva, N.M. The World’s Largest Fouta Djallon–Mandingo Bauxite Province (West Africa): Part I. Background. J. Geol. Ore Depos 2020, 62, 163–176. [Google Scholar] [CrossRef]
- Mamedov, V.I.; Makarova, M.A.; Boeva, N.M.; Vnuchkov, D.A.; Bortnikov, N.S. The world’s largest bauxite province Futa Jalon—Mandingo (West Africa). Part 2: The influence of the composition of source rocks on the abundance and quality of bauxite. J. Geol. Ore Depos. 2021, 63, 599–624. [Google Scholar] [CrossRef]
- Hill, V.G.; Zimmerman, K.G. The hydrothermal growth and thermal decomposition of boehmite single crystals. Am. Mineral. 1970, 55, 285–288. [Google Scholar]
- Peng, L.; Xu, X.; Lv, Z.; Song, J.; He, M.; Wang, Q.; Yan, L.; Li, Y.; Li, Z. Thermal and morphological study of Al2O3 nanofibers derived from boehmite precursor. J. Therm. Anal. Calorim. 2011, 110, 749–754. [Google Scholar] [CrossRef]
- Puttewar, S.P.; Bhukte, P.G. Phase transformation of minerals in bauxite. Proc. Int. Semin. Min. Process. Technol. 2006, 5, 443–448. [Google Scholar]
- Bardossy, G. Bauxite Deposits on Carbonate Rocks, Developments in Economic Geology. In Karst Bauxites; Elsevier: Amsterdam, The Netheelands, 1982; Volume 14, p. 441. [Google Scholar]
- Trolard, F.; Tardy, Y. The stabilities of gibbsite, boehmite, aluminous goethites and aluminous hematites in bauxites, ferricretes and laterites as a function of water activity, temperature and particle size. J. Geochim. Cosmochim. Acta 1987, 51, 945–957. [Google Scholar] [CrossRef]
- Tardy, Y.; Nahon, D. Geochemistry of laterites. Stability of Al-goethite, Al-hematite and Fe’+-kaolinite in bauxites and ferricretes: An approach to the mechanism of concretion formation. Am. J. Sci. 1985, 285, 865–903. [Google Scholar] [CrossRef]
- Sinitsyn, V.M. The Climate of the Laterites and Bauxites; Nedra: Leningrad, Russia, 1976; p. 152. [Google Scholar]
- Didier, P.; Nahon, D.; Fritz, B.; Tardy, Y. Activity of water as a geochemical controlling factor in ferricretes. A thermodynamic model in the system: Kaolinite, Fe—Al oxihydroxides. Sci. Geol. Mem. 1984, 71, 35–44. [Google Scholar]
- Balkay, B.; Bardossy, G. Etude des processus elementaires de la lateritisation sur laterites guineennes. Bull. Hung. Geol. Soc. 1967, 97, 91–100. [Google Scholar]
- Karšulin, M. Bauxite Lateritique et Bauxite Karstique. Symp. Bauxites, Oxydes, Hydroxydes D’aluminium; Académie Yougoslave des Sciences et des Arts: Zagreb, Yugoslavia, 1964; Volume 1, pp. 7–29. [Google Scholar]
- Ball, P.J.; Gilkes, R.J. The Mt. Saddleback bauxite deposit, south Western Australia. Chem. Geol. 1987, 60, 215–225. [Google Scholar] [CrossRef]
- De Lapparent, J. Les hydroxydes d’aluminium des argiles bauxitiques de l’Ayrshire (Écosse). J. Bull. Minéralogie 1935, 58, 246–267. [Google Scholar] [CrossRef]
- Bronevoy, V.A.; Kopeikin, V.A.; Tenyakov, V.A. Equilibrium conditions of the gibbsite—kaolinite system and some problems of laterite and bauxite formation. In New Data on the Geology of Bauxite; VIMS: Moscow, Russia, 1975; Volume 3, pp. 16–30. [Google Scholar]
- Keller, W.D. Observation on the origin of Missouri high-alumina clays. In Problems of Clay and Laterite Genesis; AIME: New York, NY, USA, 1952; pp. 115–135. [Google Scholar]
- Beneslavsky, S.I. The Mineralogy of Bauxites; Gosgeoltechizdat: Moscow, Russia, 1963; p. 170. [Google Scholar]
- Goretskiy, Y.K. Regularities of Bauxite Deposits Location; VIMS: Moscow, Russia, 1960; Volume 5, p. 257. [Google Scholar]
- Bronevoy, V.A.; Kim, Y.I.; Kulikova, G.V. Features of Mineral Formation During the Formation of Bauxites in the Western Part of the Liberian Shield. In Proceedings of the SNIIGIMS, Novosibirsk, Russia, 1971; pp. 164–167. [Google Scholar]
- Gastuche, M.; Herbillon, A. Etude des gels d’alumine: Cristallisation en milieu desionise. Bull. Soc. Chem. 1962, 7, 1404–1412. [Google Scholar]
- Souza—Santos, P.; Vallejo-Freire, A.; Souza-Santos, H.L. Electron microscope studies on the ageing of amorphous colloidal aluminum hydroxide. Colloid Polym. Sci. 1953, 133, 101–107. [Google Scholar]
- Pedro, G.; Berrier, J.; Tessier, D. Recherches experimental sur l’alteration des argiles dioctaedriques de type kaolinite et illite. Bull. Groupe Français Argiles 1970, 22, 29–50. [Google Scholar] [CrossRef]
- Harrassowitz, H.L. Material und Versuch erdgeschichtlicher Auswertung. Fortschr. Geol. Palaont. 1926, 4, 253–566. [Google Scholar]
- Panasyuk, G.P.; Kozerozhets, I.V.; Voroshilov, I.L.; Belan, V.N.; Semenov, E.A.; Luchkov, I.V. The thermodynamic properties and role of water contained in dispersed oxides in precursor-boehmite conversion, based on the example of aluminum hydroxide and oxide under hydrothermal conditions in different environments. Russ. J. Phys. Chem. 2015, 89, 592–597. [Google Scholar] [CrossRef]
- Panasyuk, G.P.; Belan, V.N.; Voroshilov, I.L.; Kozerozhets, I.V. The hydrargillite→boehmite transformation. Inorg. Mater. 2010, 46, 747–753. [Google Scholar] [CrossRef]
- Karimov, T.M.; Mukhammadiev, A.D.; Gilmutdinov, I.I.; Gilmutdinov, I.M.; Kuznetsova, I.V.; Sabirzyanov, A.N. Experimental study of the process of obtaining aluminum hydroxide (boehmite) by hydrothermal synthesis. Bull. Technol. Univ. 2018, 21, 4290–4293. [Google Scholar]
- Makarova, M.A.; Mamedov, V.I.; Alekhin, Y.V.; Shipilova, E.S. The Unique Role of Pore Water in Lateritic Bauxite Formation, Republic of Guinea. J. Dokl. Earth Sci. 2019, 489, 1297–1300. [Google Scholar] [CrossRef]
- Akaemov, S.T.; Pastukhova, M.V.; Tenyakov, V.A.; Yasamanov, N.A. Time and conditions for the formation of bauxites in the laterite covers of the Earth’s equatorial zone. In Problems of Bauxite Genesis; Nauka: Moscow, Russia, 1975; pp. 55–77. [Google Scholar]
- Boeva, N.M.; Slukin, A.D.; Shipilova, E.S.; Makarova, M.A.; Balashov, F.V.; Zhegallo, E.A.; Zaytseva, L.V.; Bortnikov, N.S. Features of morphology and composition of supergenic minerals of rare and rare earth elements in lateritized bauxites of the Chadobets uplift (Siberian platform). Dokl. Earth Sci. 2021, 500, 720–727. [Google Scholar] [CrossRef]
- Chesworth, W. The stability of gibbsite and boehmite at the surface of the earth. Clays Clay Miner. 1972, 20, 369–374. [Google Scholar] [CrossRef]
- Peryea, F.J.; Kittrick, J.A. Relative solubilities of corundum, gibbsite, boehmite and diaspor at standart state conditions. Clays Clay Miner. 1988, 36, 391–396. [Google Scholar] [CrossRef]
- Hemingway, B.S.; Robie, R.A.; Apps, J.A. Revised values for thermodynamic properties of boehmite, AlO (OH), and related species and phases in the system Al-H-O. Am. Mineral. 1991, 76, 445–457. [Google Scholar]





| Sampling Interval | Count of Samples | SiO2, wt % | Al2O3, wt % | Al2O3mono, wt % | Fe2O3, wt % | TiO2, wt % | LOI, wt % |
|---|---|---|---|---|---|---|---|
| Bauxites after sedimentary clays (geliform and oolitic) | |||||||
| 0–5 m | 173 | 1.06 | 57.74 | 1.47 | 7.75 | 2.69 | 30.01 |
| 5–10 m | 186 | 0.87 | 59.93 | 3.87 | 5.30 | 2.67 | 30.48 |
| 10–20 m | 176 | 1.11 | 63.87 | 16.72 | 2.51 | 4.40 | 27.37 |
| 20–30 m | 81 | 0.80 | 63.49 | 17.67 | 2.89 | 5.13 | 26.94 |
| over 30 m | 11 | 1.23 | 61.07 | 4.72 | 3.37 | 3.99 | 29.60 |
| Psammite bauxites | |||||||
| 0–5 m | 229 | 0.75 | 58.62 | 3.31 | 5.91 | 4.23 | 29.73 |
| 5–10 m | 208 | 0.76 | 60.65 | 5.64 | 4.16 | 3.85 | 29.84 |
| 10–20 m | 258 | 0.89 | 62.75 | 11.16 | 2.89 | 3.69 | 29.03 |
| 20–30 m | 106 | 0.96 | 60.70 | 11.12 | 4.04 | 5.52 | 28.04 |
| over 30 m | 19 | 1.72 | 57.67 | 12.06 | 8.22 | 6.47 | 25.17 |
| Gravelstone bauxites | |||||||
| 0–5 m | 239 | 0.83 | 59.27 | 3.06 | 5.89 | 3.23 | 30.03 |
| 5–10 m | 207 | 0.87 | 61.00 | 3.12 | 3.25 | 3.03 | 31.10 |
| 10–20 m | 216 | 0.87 | 62.42 | 7.06 | 2.49 | 3.16 | 30.31 |
| 20–30 m | 79 | 0.74 | 62.14 | 14.11 | 4.31 | 4.64 | 27.43 |
| over 30 m | 12 | 2.71 | 56.18 | 6.28 | 10.34 | 4.67 | 25.35 |
| Conglomerate bauxites | |||||||
| 0–5 m | 39 | 0.81 | 62.62 | 8.10 | 3.10 | 2.80 | 29.92 |
| 5–10 m | 73 | 0.60 | 63.49 | 10.53 | 2.03 | 3.37 | 29.76 |
| 10–20 m | 283 | 0.68 | 64.11 | 14.17 | 2.25 | 3.50 | 28.71 |
| 20–30 m | 194 | 0.68 | 64.90 | 18.25 | 2.65 | 3.48 | 27.54 |
| over 30 m | 27 | 1.60 | 64.43 | 16.40 | 3.34 | 3.11 | 26.77 |
| Intensity of Lateritization | After Sedimentary Clays | After Psammite Sediments | After Gravel Sediments | After Gravel and Gravel-Pebble Sediments | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Value | Count of Samples | Content | Count of Samples | Content | Count of Samples | Content | Count of Samples | Content | |||||||||||||
| SiO2 | Al2O3 | Al2O3mono | Fe2O3 | SiO2 | Al2O3 | Al2O3mono | Fe2O3 | SiO2 | Al2O3 | Al2O3mono | Fe2O3 | SiO2 | Al2O3 | Al2O3mono | Fe2O3 | ||||||
| SiO2 < 2 | wt % | 69 | 1 | 58 | 1 | 8 | 168 | 1 | 59 | 4 | 5 | 208 | 1 | 60 | 3 | 4 | 48 | 1 | 63 | 10 | 2 |
| kg/m3 | 20 | 1062 | 27 | 143 | 16 | 1169 | 79 | 108 | 14 | 1080 | 56 | 78 | 12 | 1229 | 196 | 43 | |||||
| input/output, kg/m3 | –37 | 108 | –250 | 62 | –41 | 164 | –116 | 1 | –46 | 86 | –145 | –16 | –54 | 111 | –145 | –39 | |||||
| 2 < SiO2 < 5 | wt % | 7 | 4 | 59 | 17 | 5 | 16 | 3 | 58 | 11 | 6 | 18 | 4 | 59 | 12 | 6 | 11 | 4 | 61 | 19 | 5 |
| kg/m3 | 61 | 1014 | 294 | 86 | 57 | 1005 | 195 | 107 | 61 | 994 | 200 | 94 | 67 | 1117 | 341 | 82 | |||||
| input/output, kg/m3 | –49 | 121 | 196 | 14 | –56 | 22 | –2 | –7 | –64 | –10 | –10 | –58 | –80 | 28 | –11 | –12 | |||||
| 5 < SiO2 <10 | wt % | 10 | 7 | 55 | 6 | 4 | 14 | 6 | 56 | 11 | 7 | 23 | 7 | 57 | 15 | 6 | 12 | 8 | 59 | 19 | 5 |
| kg/m3 | 109 | 892 | 98 | 72 | 113 | 983 | 197 | 115 | 124 | 1004 | 259 | 101 | 147 | 1089 | 352 | 94 | |||||
| input/output, kg/m3 | –102 | 83 | –25 | 36 | –77 | 137 | –29 | 37 | –70 | 38 | –1 | 19 | –73 | 169 | 110 | 16 | |||||
| 10 < SiO2 < 15 | wt % | 6 | 14 | 54 | 8 | 2 | 4 | 12 | 54 | 14 | 5 | 5 | 11 | 56 | 15 | 5 | 3 | 13 | 54 | 14 | 5 |
| kg/m3 | 211 | 809 | 122 | 35 | 190 | 846 | 225 | 78 | 194 | 966 | 260 | 82 | 220 | 920 | 242 | 78 | |||||
| input/output, kg/m3 | –47 | 5 | 5 | –20 | –83 | 41 | 135 | 24 | –78 | 115 | 58 | 3 | –99 | –8 | 51 | 22 | |||||
| 15 < SiO2 < 20 | wt % | 9 | 17 | 52 | 8 | 4 | 11 | 17 | 51 | 6 | 3 | 5 | 17 | 53 | 13 | 5 | 3 | 18 | 52 | 11 | 3 |
| kg/m3 | 257 | 804 | 117 | 55 | 272 | 805 | 90 | 54 | 272 | 851 | 202 | 79 | 319 | 928 | 191 | 56 | |||||
| input/output, kg/m3 | –373 | 245 | 113 | 31 | –351 | 215 | 57 | –3 | –376 | 299 | 199 | 0 | –377 | 330 | 188 | 5 | |||||
| Sediments least altered by lateritization | wt % | 1 | 44 | 39 | 0 | 2 | 1 | 41 | 39 | 2 | 4 | 1 | 42 | 36 | 0 | 5 | 1 | 44 | 37 | 0 | 3 |
| kg/m3 | 631 | 559 | 4 | 24 | 624 | 590 | 33 | 56 | 648 | 552 | 3 | 79 | 697 | 598 | 3 | 52 | |||||
| Region | Count of Samples | Content, wt % | ||||
|---|---|---|---|---|---|---|
| Variation | SiO2 | Al2O3 | Al2O3Mono | Fe2O3 | ||
| Western Guinea | 22 | from | 0.5 | 46.7 | 4.1 | 8.4 |
| to | 1.5 | 59.5 | 16.3 | 28.3 | ||
| average | 1.2 | 52 | 8.2 | 17.1 | ||
| Mali (West Bamako, Falesa, Kenyeba) | 9 | from | 0.5 | 54.36 | 15.1 | 1.96 |
| to | 4.47 | 69.31 | 50.7 | 18.28 | ||
| average | 1.17 | 63.45 | 27.86 | 5.82 | ||
| Mali (East Bamako, Bafoulabe) | 11 | from | 0.43 | 63.29 | 24.1 | 2.0 |
| to | 1.07 | 70.7 | 62.0 | 6.05 | ||
| average | 0.73 | 67.46 | 45.82 | 3.25 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamedov, V.; Boeva, N.; Makarova, M.; Shipilova, E.; Melnikov, P. The Problem of the Formation of Boehmite and Gibbsite in Bauxite-Bearing Lateritic Profiles. Minerals 2022, 12, 389. https://doi.org/10.3390/min12030389
Mamedov V, Boeva N, Makarova M, Shipilova E, Melnikov P. The Problem of the Formation of Boehmite and Gibbsite in Bauxite-Bearing Lateritic Profiles. Minerals. 2022; 12(3):389. https://doi.org/10.3390/min12030389
Chicago/Turabian StyleMamedov, Vladimir, Natalia Boeva, Marina Makarova, Elena Shipilova, and Philimon Melnikov. 2022. "The Problem of the Formation of Boehmite and Gibbsite in Bauxite-Bearing Lateritic Profiles" Minerals 12, no. 3: 389. https://doi.org/10.3390/min12030389
APA StyleMamedov, V., Boeva, N., Makarova, M., Shipilova, E., & Melnikov, P. (2022). The Problem of the Formation of Boehmite and Gibbsite in Bauxite-Bearing Lateritic Profiles. Minerals, 12(3), 389. https://doi.org/10.3390/min12030389






