The Role of Scandium Substitution in Babingtonite Group Minerals
Abstract
1. Introduction
2. Materials and Methods
3. Results
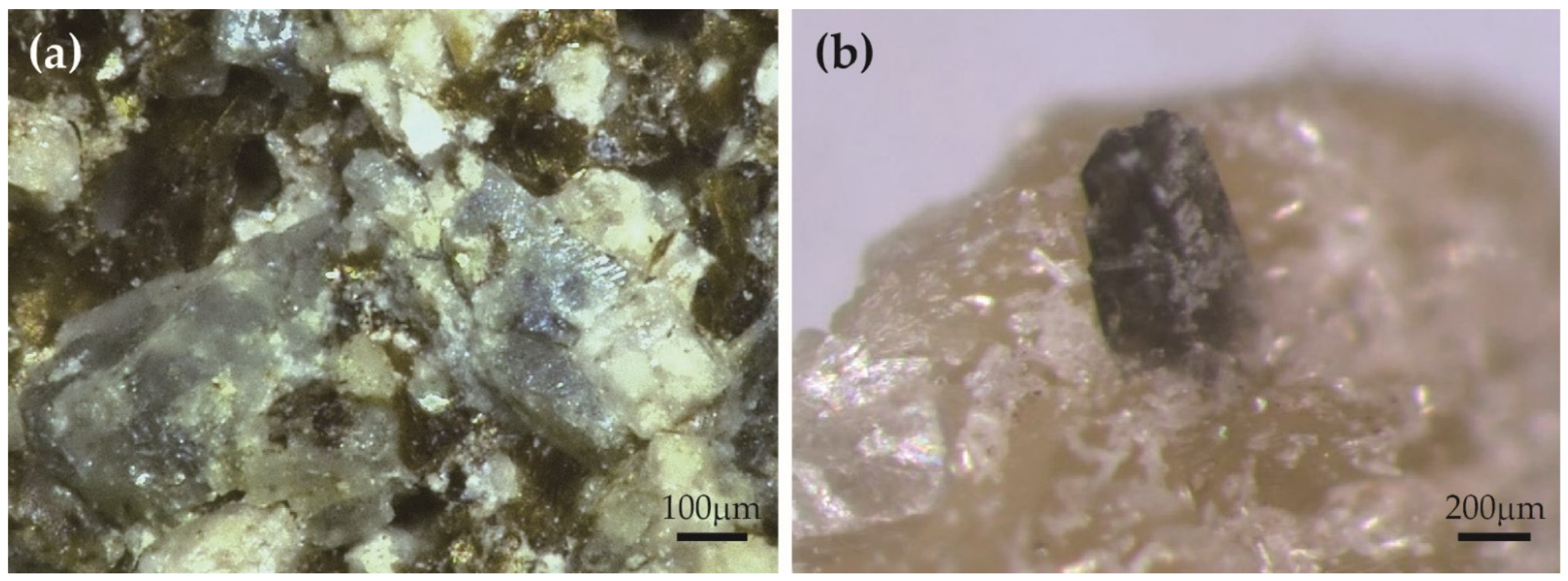
3.1. Chemical Compositions of Sc-Rich Babingtonite
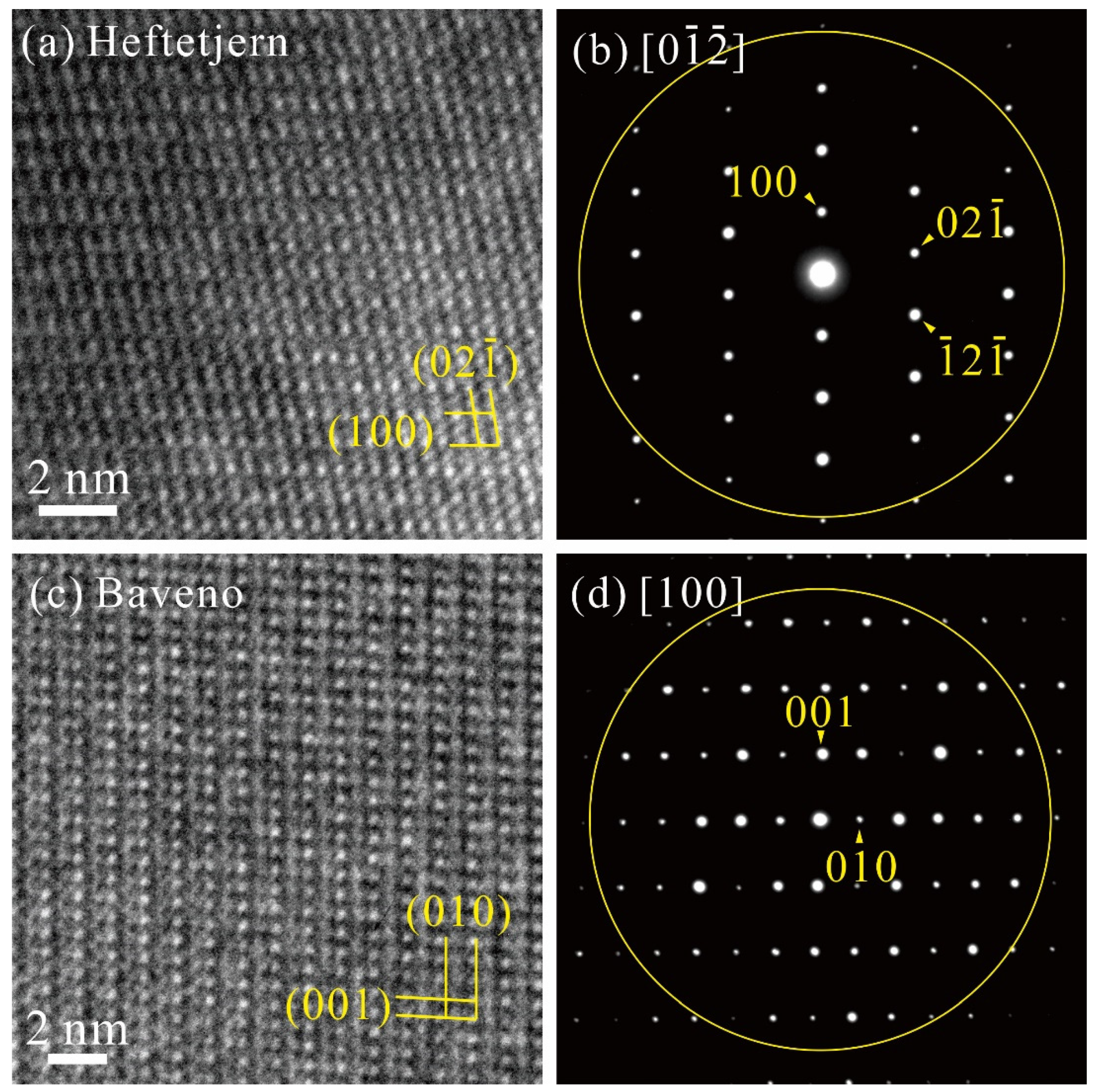
3.2. TEM Observation
3.3. Crystal Structure Solution and Refinements
4. Discussion
4.1. Determination of Cation Distributions at the Octahedral Sites and the Large Polyhedra in the Heftetjern and Baveno Sc-Rich Babingtonite
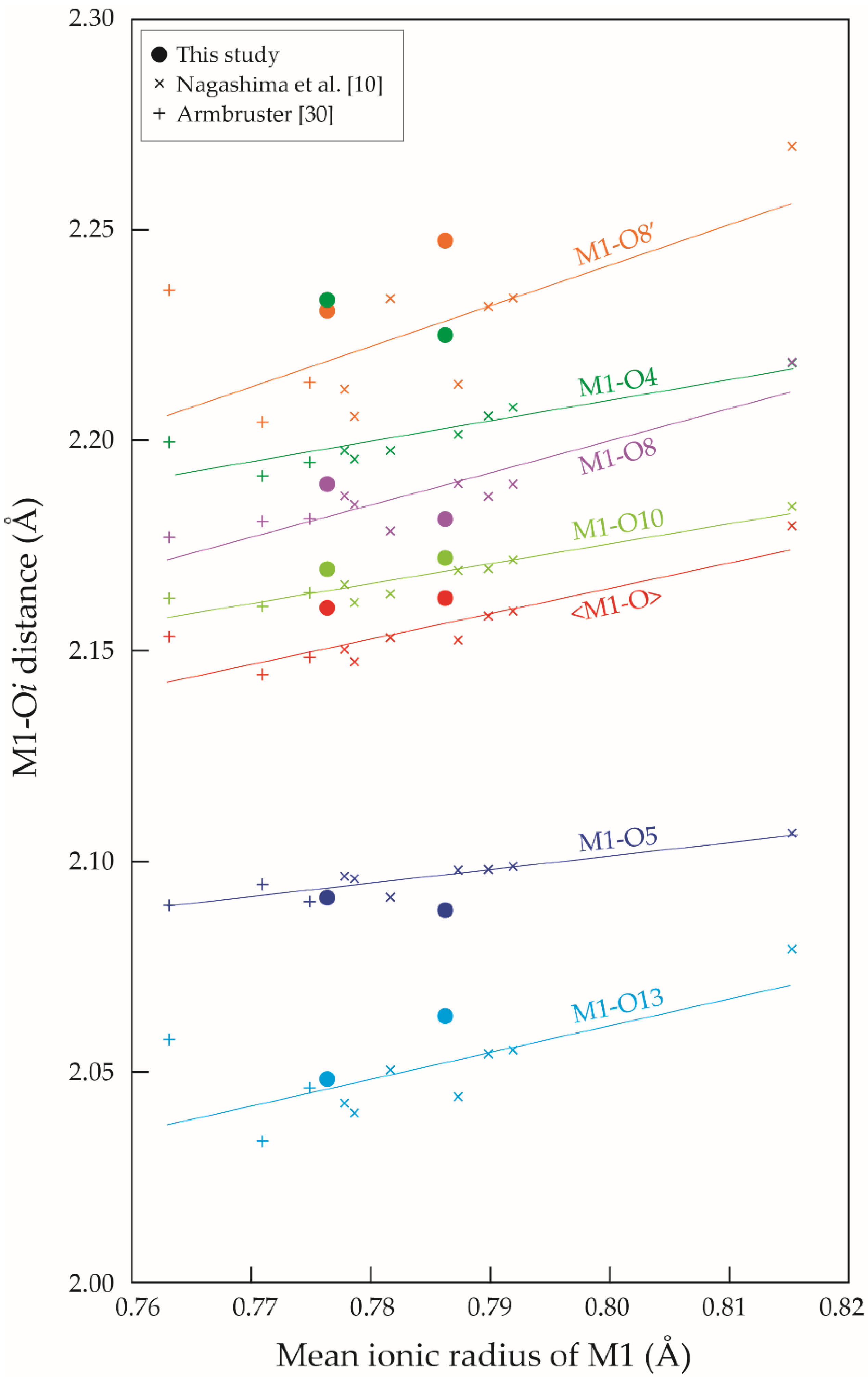
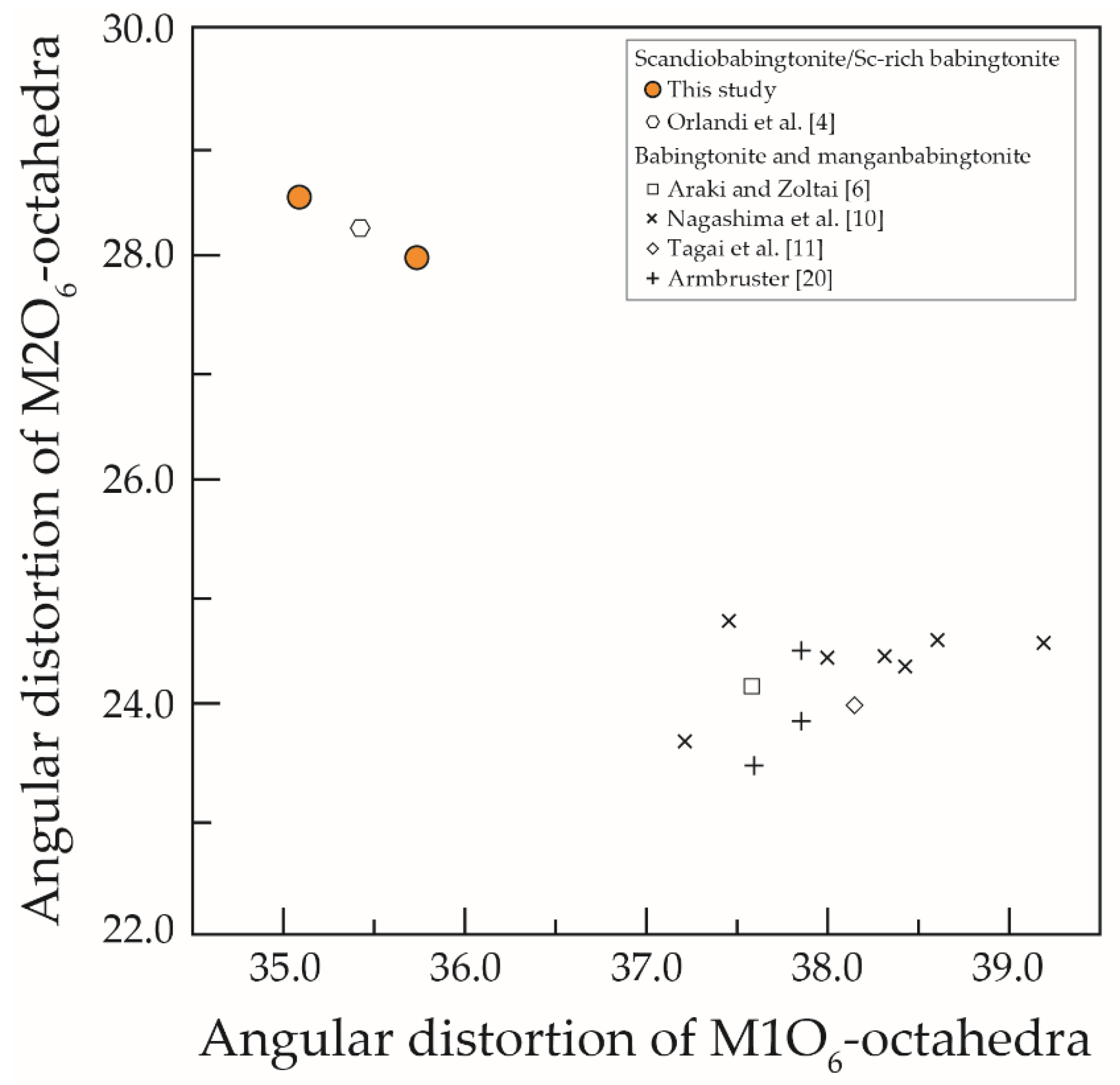
4.2. Cation Distribution and Structural Variation
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wise, W.S.; Moller, W.P. Occurrence of Ca-Fe silicate minerals with zeolites in basalt cavities at Bombay, India. Eur. J. Miner. 1990, 2, 875–883. [Google Scholar] [CrossRef]
- Burns, G.R.; Dyar, M.D. Crystal chemistry and Mössbauer spectra of babingtonite. Am. Miner. 1991, 76, 892–899. [Google Scholar]
- Vinogradova, R.A.; Sychkoba, V.A.; Kabalov, Y.K. Manganiferous babingtonite from the Rudnyi Kaskad deposit, Eastern Sayan. Dokl. Acad. Nauk. SSSR 1966, 169, 434–437. [Google Scholar]
- Orlandi, P.; Pasero, M.; Vezzalini, G. Scandiobabingtonite, a new mineral from the Baveno pegmatite, Piedmont, Italy. Am. Miner. 1998, 83, 1330–1334. [Google Scholar] [CrossRef]
- Raade, G.; Erambert, M. An intergrowth of scandiobabingtonite and cascandite from the Heftetjern granite pegmatite, Norway. N. Jb. Miner. Mh. 1999, 1999, 545–550. [Google Scholar]
- Araki, T.; Zoltai, T. Crystal structure of babingtonite. Z. Kristallogr. 1972, 135, 355–375. [Google Scholar] [CrossRef]
- Amthauer, G. 57Fe Mössbauer study of babingtonite. Am. Miner. 1980, 65, 157–162. [Google Scholar]
- Amthauer, G.; Rossman, G.R. Mixed valence of iron in minerals with cation clusters. Phys. Chem. Miner. 1984, 11, 37–51. [Google Scholar] [CrossRef]
- Akasaka, M.; Kimura, T.; Nagashima, M. Rietveld and 57Fe Mössbauer study of babingtonite from Shimane Peninsula, Japan. J. Miner. Pet. Sci. 2013, 108, 121–130. [Google Scholar] [CrossRef]
- Nagashima, M.; Mitani, K.; Akasaka, M. Structural variation of babingtonite depending on cation distribution at the octahedral sites. Miner. Pet. 2014, 108, 287–301. [Google Scholar] [CrossRef]
- Tagai, T.; Joswig, W.; Fuess, H. Neutron diffraction study of babingtonite at 80 K. Miner. J. 1990, 15, 8–18. [Google Scholar] [CrossRef][Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Kristiansen, R. A unique assemblage of scandium-bearing minerals from the Heftetjern-pegmatite, Tørdal, south Norway. In Proceedings of the Kongsberg Mineral Symposium, Norsk Bergverksmuseum Skirift, Kongsberg, Norway, 23 May 2009; Volume 41, pp. 75–104. [Google Scholar]
- Gramaccioli, C.A.; Campostrini, I.; Orlandi, P. Scandium minerals in the miaroles of granite at Baveno, Italy. Eur. J. Miner. 2004, 16, 951–956. [Google Scholar] [CrossRef]
- Bruker SMART and SAINT-Plus; Versions 6.01; Bruker AXS Inc.: Madison, WI, USA, 1999.
- Sheldrick, G.M. SADABS, Program for Area Detector Adsorption Correction; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Matsumoto, T.; Yamano, Y.; Sato, T.; Ferrara, J.D.; White, F.J.; Meyer, M. “What is this?” a structure analysis tool for rapid and automated solution of small molecule structures. J. Chem. Crystallogr. 2021, 51, 438–450. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Franks, F. (Ed.) Water: A Comprehensive Treatise. Vol. 2, Water in Crystalline Hydrates Aqueous Solutions of Simple Nonelectrolytes; Plenum Press: New York, NY, USA, 1973; p. 648. [Google Scholar]
- Nespolo, M.; Ferraris, G.; Ohashi, H. Charge distribution as a tool to investigate structural details: Meaning and application to pyroxenes. Acta Crystallogr. Sect. B Struct. Sci. 1999, B55, 902–916. [Google Scholar] [CrossRef]
- Nespolo, M.; Ferraris, G.; Ivaldi, G.; Hoppe, R. Charge distribution as a tool to investigate structural details. II. Extension to hydrogen bonds, distorted and hetero–ligand polyhedra. Acta Crystallogr. Sect. B Struct. Sci. 2001, B57, 652–664. [Google Scholar] [CrossRef]
- Nespolo, M. Charge distribution as a tool to investigate structural details. IV. A new route to heteroligand polyhedra. Acta Crystallogr. Sect. B 2016, B72, 51–66. [Google Scholar] [CrossRef]
- Hoppe R, Effective coordination numbers (ECoN) and mean fictive ionic radii (MEFIR). Z. Kristallogr. 1979, 150, 23–52. [CrossRef]
- Baur, H. The geometry of polyhedral distortions. Predictive relationships for the phosphate group. Acta Crystallogr. Sect. B 1974, B30, 1195–1215. [Google Scholar] [CrossRef]
- Robinson, K.; Gibbs, G.V.; Ribbe, P.H. Quadratic elongation: A quantitative measure of distortion in coordination polyhedral. Science 1971, 172, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Bosi, F.; Hatert, F.; Hålenius, U.; Pasero, M.; Miyawaki, R.; Mills, S. On the application of the IMA-CNMNC dominant-valency rule to complex mineral compositions. Miner. Mag. 2019, 83, 627–632. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database. Acta Crystallogr. Sect. B 1985, B41, 244–247. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. Sect. B Struct. Sci. 1991, B47, 192–197. [Google Scholar] [CrossRef]
- Armbruster, T. Cation distribution in Mg, Mn-bearing babingtonite from Arvigo, Val Calanca Grisons, Switzerland. Schweiz. Miner. Petrog. Mitt. 2000, 80, 279–284. [Google Scholar]
- Kosoi, A.L. The structure of babingtonite. Sov. Phys. Crystallogr. 1976, 20, 446–451. [Google Scholar]
- Nagashima, M.; Armbruster, T. Saneroite: Chemical and structural variations of manganese pyroxenoids with hydrogen bonding in the silicate chain. Eur. J. Miner. 2010, 22, 393–402. [Google Scholar] [CrossRef]

| Locality | Heftetjern, Norway | Baveno, Italy | ||
|---|---|---|---|---|
| Ave. | Std. | Ave. | Std. | |
| n = 7 | n = 20 | |||
| SiO2 | 53.75 | 0.40 | 52.59 | 0.30 |
| TiO2 | 0.02 | 0.03 | 0.04 | 0.03 |
| Al2O3 | 0.04 | 0.04 | 0.26 | 0.24 |
| Cr2O3 *1 | 0.01 | 0.01 | 0.00 | 0.01 |
| V2O3 *1 | 0.01 | 0.01 | 0.01 | 0.01 |
| FeO *1 | 8.63 | 0.87 | 11.13 | 2.01 |
| Sc2O3 *1 | 13.78 | 1.35 | 8.44 | 2.16 |
| MnO *1 | 2.73 | 0.30 | 4.41 | 0.74 |
| MgO | 0.24 | 0.03 | 0.07 | 0.02 |
| CaO | 18.32 | 0.74 | 17.83 | 0.45 |
| Na2O | 0.76 | 0.51 | 0.91 | 0.26 |
| NiO | 0.02 | 0.02 | 0.02 | 0.03 |
| ZnO | 0.02 | 0.02 | 0.01 | 0.02 |
| SnO2 | 0.00 | 0.00 | 1.42 | 0.22 |
| Li2O *2 | - | 0.03 | 0.01 | |
| 98.38 | 97.17 | |||
| ΣCations = 9 | ||||
| Si | 5.00 | 0.03 | 4.98 | 0.02 |
| Ti | 0.00 | 0.00 | 0.00 | 0.00 |
| Al | 0.00 | 0.00 | 0.03 | 0.03 |
| Cr3+ | 0.00 | 0.00 | 0.00 | 0.00 |
| V3+ | 0.00 | 0.00 | 0.00 | 0.00 |
| Fe | 0.67 | 0.07 | 0.88 | 0.16 |
| Sc3+ | 1.12 | 0.10 | 0.70 | 0.18 |
| Mn2+ | 0.21 | 0.02 | 0.36 | 0.06 |
| Mg | 0.03 | 0.00 | 0.01 | 0.00 |
| Ca | 1.83 | 0.08 | 1.81 | 0.05 |
| Na | 0.14 | 0.09 | 0.17 | 0.05 |
| Ni | 0.00 | 0.00 | 0.00 | 0.00 |
| Zn | 0.00 | 0.00 | 0.00 | 0.00 |
| Sn | 0.00 | 0.00 | 0.05 | 0.01 |
| Li | - | 0.01 | 0.00 | |
| Total | 9.00 | 9.00 | ||
| Recalculated values from stoichiometry (total cations = 9 per 14.5 oxygens) and charge balance | ||||
| Fe2+ | 0.65 | 0.49 | ||
| Fe3+ | 0.02 | 0.39 | ||
| Locality | Heftetjern, Norway | Baveno, Italy | |
|---|---|---|---|
| Space group | |||
| Crystal size (mm) | 0.11 × 0.08 × 0.07 | 0.08 × 0.06 × 0.045 | |
| Cell parameters | a (Å) | 7.5272(1) | 7.5199(2) |
| b (Å) | 11.7175(1) | 11.7145(3) | |
| c (Å) | 6.7613(1) | 6.7408(2) | |
| α (°) | 91.710(1) | 91.756(2) | |
| β (°) | 93.637(1) | 93.786(2) | |
| γ(°) | 104.522(1) | 104.549(2) | |
| V (Å3) | 575.49(2) | 572.83(3) | |
| Dcalc (g/cm3) | 3.22 | 3.26 | |
| Radiation | MoKα (λ = 0.71073 Å) | ||
| Monochromator | Graphite | VariMax optics | |
| Diffractometer | Bruker APEXII CCD | RIGAKU HyPix-6000HE | |
| Scan type | φ–ω scan [15] | ω scan | |
| Absorption correction | SADABS [16] | CrysAlisPro [17] | |
| Absorption coefficient μ (mm−1) | 3.23 | 3.38 | |
| Corrected reflections | 17,219 | 15,995 | |
| Unique reflections | 5505 | 3498 | |
| Criterion for observed reflections | |||
| Rint (%) | 3.11 | 3.35 | |
| Rs (%) | 4.01 | 2.42 | |
| θmax(°) | 36.3 | 30.5 | |
| Index limit | −12 ≤ h ≤ 12, −19 ≤ k ≤ 19, −11 ≤ l ≤ 11 | −10 ≤ h ≤ 10, −16 ≤ k ≤ 16, −9 ≤ l ≤ 9 | |
| Refinement on F2 using | SHELXL-97 [18] | ||
| R1 (%) | 3.29 | 3.65 | |
| wR2 (%) | 8.04 | 9.56 | |
| S | 1.06 | 1.09 | |
| No. of parameters | 223 | 223 | |
| Weighting scheme * | w = 1/[s2(Fo2) + (0.0341P)2 + 0.19P] | w = 1/[s2(Fo2) + (0.0476P)2 + 1.06P] | |
| ∆rmax (e Å−3) | 1.00 (0.65 Å from O3) | 1.19 (1.85 Å from H1) | |
| ∆rmin (e Å−3) | −0.99 (0.44 Å from A2) | −0.88 (0.78 Å from M2) | |
| Site | Heftetjern, Norway | Baveno, Italy | Site | Heftetjern, Norway | Baveno, Italy | ||
|---|---|---|---|---|---|---|---|
| A1 | x | 0.78285(6) | 0.78537(8) | O4 | x | 0.32403(18) | 0.3231(3) |
| y | 0.94184(3) | 0.94247(5) | y | 0.34392(11) | 0.34236(18) | ||
| z | 0.14510(6) | 0.14439(9) | z | 0.2511(2) | 0.2489(3) | ||
| Ueq | 0.01193(8) | 0.01691(13) | Ueq | 0.0093(2) | 0.0159(4) | ||
| Occ. | Ca1.00 | Ca1.00 | O5 | x | 0.54658(18) | 0.5476(3) | |
| A2 | x | 0.23511(5) | 0.23498(8) | y | 0.62362(11) | 0.62281(18) | |
| y | 0.52449(3) | 0.52256(6) | z | 0.3605(2) | 0.3620(3) | ||
| z | 0.29664(6) | 0.29944(10) | Ueq | 0.0104(2) | 0.0170(4) | ||
| Ueq | 0.01020(13) | 0.0167(2) | O6 | x | 0.67996(18) | 0.6797(3) | |
| Occ. | Ca0.879(5)Na0.121 | Ca0.819(8)Na0.181 | y | 0.37438(11) | 0.37379(18) | ||
| M1 | x | 0.59133(4) | 0.59151(6) | z | 0.37721(19) | 0.3767(3) | |
| y | 0.64458(2) | 0.64463(4) | Ueq | 0.0093(2) | 0.0160(4) | ||
| z | 0.05998(4) | 0.06114(6) | O7 | x | 0.96595(18) | 0.9668(3) | |
| Ueq | 0.00717(8) | 0.01336(15) | y | 0.38837(11) | 0.38725(18) | ||
| Occ. | Fe0.912(1) | Fe0.914(2) | z | 0.1589(2) | 0.1589(3) | ||
| M2 | x | 0.04577(4) | 0.04582(6) | Ueq | 0.0098(2) | 0.0167(4) | |
| y | 0.23481(3) | 0.23560(4) | O8 | x | 0.67605(18) | 0.6765(3) | |
| z | 0.18689(5) | 0.18698(6) | y | 0.47579(12) | 0.47449(18) | ||
| Ueq | 0.00735(8) | 0.01383(14) | z | 0.0325(2) | 0.0310(3) | ||
| Occ. | Sc1.056(2) | Sc1.219(3) | Ueq | 0.0096(2) | 0.0166(4) | ||
| Si1 | x | 0.29210(7) | 0.29043(11) | O9 | x | 0.92492(18) | 0.9255(3) |
| y | 0.05364(4) | 0.05399(7) | y | 0.57242(11) | 0.57179(17) | ||
| z | 0.34008(7) | 0.34089(11) | z | 0.3369(2) | 0.3364(3) | ||
| Ueq | 0.00759(10) | 0.01390(16) | Ueq | 0.0091(2) | 0.0155(4) | ||
| Si2 | x | 0.46362(7) | 0.46313(10) | O10 | x | 0.86916(18) | 0.8700(3) |
| y | 0.31596(4) | 0.31550(7) | y | 0.75391(11) | 0.75439(18) | ||
| z | 0.42663(7) | 0.42583(11) | z | 0.12336(19) | 0.1243(3) | ||
| Ueq | 0.00672(9) | 0.01318(16) | Ueq | 0.0091(2) | 0.0166(4) | ||
| Si3 | x | 0.80612(7) | 0.80656(10) | O11 | x | 0.01987(19) | 0.0191(3) |
| y | 0.44861(4) | 0.44743(7) | y | 0.21715(12) | 0.21830(18) | ||
| z | 0.21360(7) | 0.21295(11) | z | 0.4867(2) | 0.4845(3) | ||
| Ueq | 0.00640(9) | 0.01269(15) | Ueq | 0.0116(3) | 0.0187(4) | ||
| Si4 | x | 0.98787(7) | 0.98887(10) | O12 | x | 0.20278(18) | 0.2041(3) |
| y | 0.71562(4) | 0.71504(7) | y | 0.73870(11) | 0.73891(18) | ||
| z | 0.30648(7) | 0.30714(11) | z | 0.2453(2) | 0.2462(3) | ||
| Ueq | 0.00662(9) | 0.01298(16) | Ueq | 0.0097(2) | 0.0163(4) | ||
| Si5 | x | 0.33021(7) | 0.32945(10) | O13 | x | 0.51046(18) | 0.5112(3) |
| y | 0.83865(4) | 0.83870(7) | y | 0.79903(12) | 0.80065(18) | ||
| z | 0.10790(8) | 0.10746(12) | z | 0.0580(2) | 0.0577(3) | ||
| Ueq | 0.00726(9) | 0.01359(16) | Ueq | 0.0108(2) | 0.0173(4) | ||
| O1 | x | 0.2007(2) | 0.2003(3) | O14 | x | 0.79688(18) | 0.7992(3) |
| y | 0.99084(12) | 0.99011(19) | y | 0.13677(11) | 0.13810(18) | ||
| z | 0.5332(2) | 0.5344(3) | z | 0.0780(2) | 0.0791(3) | ||
| Ueq | 0.0134(3) | 0.0205(4) | Ueq | 0.0101(2) | 0.0174(4) | ||
| O2 | x | 0.13377(18) | 0.1319(3) | O15 | x | 0.40070(18) | 0.3977(3) |
| y | 0.07586(12) | 0.07670(19) | y | 0.96704(11) | 0.96769(18) | ||
| z | 0.1828(2) | 0.1836(3) | z | 0.2338(2) | 0.2318(3) | ||
| Ueq | 0.0098(2) | 0.0170(4) | Ueq | 0.0115(3) | 0.0194(4) | ||
| O3 | x | 0.43477(18) | 0.4344(3) | H1 | x | 0.124(4) | 0.124(5) |
| y | 0.17383(11) | 0.17352(17) | y | 0.9097(10) | 0.9095(16) | ||
| z | 0.4293(2) | 0.4309(3) | z | 0.521(5) | 0.507(7) | ||
| Ueq | 0.0106(2) | 0.0173(4) | Uiso | 0.05 | 0.05 |
| Heftetjern, Norway | Baveno, Italy | Heftetjern, Norway | Baveno, Italy | ||||
|---|---|---|---|---|---|---|---|
| A1- | O1 | 2.277(1) | 2.268(2) | A2- | O8 | 2.364(1) | 2.365(2) |
| O13 | 2.328(2) | 2.327(2) | O5 | 2.347(1) | 2.353(2) | ||
| O14 | 2.320(1) | 2.325(2) | O7 | 2.360(1) | 2.351(2) | ||
| O2 | 2.359(1) | 2.355(2) | O4 | 2.391(1) | 2.388(2) | ||
| O10 | 2.450(1) | 2.444(2) | O6 | 2.442(1) | 2.431(2) | ||
| O2′ | 2.705(1) | 2.675(2) | O9 | 2.563(1) | 2.560(2) | ||
| Mean (VI) | 2.407 | 2.399 | O12 | 2.613(1) | 2.636(2) | ||
| O15 | 3.059(1) | 3.096(2) | O9′ | 2.957(1) | 2.931(2) | ||
| O15′ | 3.173(1) | 3.141(3) | Mean | 2.505 | 2.502 | ||
| Mean (VIII) | 2.584 | 2.579 | |||||
| Si1- | O3 | 1.615(1) | 1.613(2) | ||||
| M1- | O13 | 2.049(1) | 2.064(2) | O2 | 1.622(1) | 1.620(2) | |
| O5 | 2.092(1) | 2.089(2) | O1 | 1.624(2) | 1.626(2) | ||
| O10 | 2.170(1) | 2.172(2) | O15 | 1.631(1) | 1.630(2) | ||
| O8 | 2.190(1) | 2.182(2) | Mean | 1.623 | 1.622 | ||
| O4 | 2.234(1) | 2.225(2) | |||||
| O8′ | 2.231(1) | 2.248(2) | Si2- | O5 | 1.600(1) | 1.599(2) | |
| Mean | 2.161 | 2.163 | O3 | 1.626(1) | 1.624(2) | ||
| VM1 | 13.25 | 13.29 | O4 | 1.630(2) | 1.629(2) | ||
| DI | 0.028 | 0.027 | O6 | 1.660(1) | 1.660(2) | ||
| <λoct> | 1.012 | 1.012 | Mean | 1.629 | 1.628 | ||
| σq(oct)2 | 35.08 | 35.73 | |||||
| Si3- | O8 | 1.608(1) | 1.607(2) | ||||
| M2- | O14 | 2.017(1) | 1.995(2) | O7 | 1.597(1) | 1.597(2) | |
| O7 | 2.045(1) | 2.020(2) | O6 | 1.631(1) | 1.630(2) | ||
| O11 | 2.060(1) | 2.040(2) | O9 | 1.668(1) | 1.670(2) | ||
| O2 | 2.128(1) | 2.119(2) | Mean | 1.626 | 1.626 | ||
| O4 | 2.172(1) | 2.152(2) | |||||
| O10 | 2.230(1) | 2.230(2) | Si4- | O11 | 1.596(1) | 1.598(2) | |
| Mean | 2.109 | 2.092 | O10 | 1.621(2) | 1.621(2) | ||
| VM2 | 12.35 | 12.07 | O9 | 1.648(1) | 1.647(2) | ||
| DI | 0.032 | 0.035 | O12 | 1.653(1) | 1.653(2) | ||
| <λoct> | 1.010 | 1.01 | Mean | 1.630 | 1.630 | ||
| σq(oct)2 | 28.51 | 27.98 | |||||
| Si5- | O13 | 1.592(1) | 1.593(2) | ||||
| O1…O11 | 2.576(2) | 2.575(3) | O14 | 1.608(1) | 1.607(2) | ||
| O15 | 1.654(1) | 1.651(2) | |||||
| O12 | 1.664(1) | 1.661(2) | |||||
| Mean | 1.630 | 1.628 | |||||
| Locality | Site | Chemical Analysis | Site-Scattering | Determined Site Occupancies *2 | Calculated No.e− | δobs-calc | Observed Bond Distance (Å) | Estimated Bond Distance (Å) | δobs-calc |
|---|---|---|---|---|---|---|---|---|---|
| (epfu) *1 | (epfu) | (epfu) | (epfu) | (Å) | |||||
| Heftetjern | M1 | 45.86 | 23.71(3) | Sc3+0.42Fe2+0.37Mn2+0.21 | 23.70 | 0.01 | 2.161 | 2.139 | 0.022 |
| M2 | 22.17(4) | Sc3+0.68Fe2+0.27Mg0.03Fe3+0.02 | 22.18 | −0.01 | 2.109 | 2.113 | −0.004 | ||
| 45.88 | |||||||||
| Baveno | M1 | 48.65 | 23.76(5) | Sc3+0.43Mn2+0.36Fe2+0.21 | 23.49 | 0.27 | 2.163 | 2.146 | 0.017 |
| M2 | 25.60(6) | Fe3+0.36Fe2+0.30Sc3+0.26Sn4+0.05Al0.03 | 25.51 | 0.09 | 2.092 | 2.074 | 0.018 | ||
| 49.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagashima, M.; Nishio-Hamane, D.; Matsumoto, T.; Fukuda, C. The Role of Scandium Substitution in Babingtonite Group Minerals. Minerals 2022, 12, 333. https://doi.org/10.3390/min12030333
Nagashima M, Nishio-Hamane D, Matsumoto T, Fukuda C. The Role of Scandium Substitution in Babingtonite Group Minerals. Minerals. 2022; 12(3):333. https://doi.org/10.3390/min12030333
Chicago/Turabian StyleNagashima, Mariko, Daisuke Nishio-Hamane, Takashi Matsumoto, and Chihiro Fukuda. 2022. "The Role of Scandium Substitution in Babingtonite Group Minerals" Minerals 12, no. 3: 333. https://doi.org/10.3390/min12030333
APA StyleNagashima, M., Nishio-Hamane, D., Matsumoto, T., & Fukuda, C. (2022). The Role of Scandium Substitution in Babingtonite Group Minerals. Minerals, 12(3), 333. https://doi.org/10.3390/min12030333






