Occurrence of SiC and Diamond Polytypes, Chromite and Uranophane in Breccia from Nickel Laterites (New Caledonia): Combined Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Geological Setting
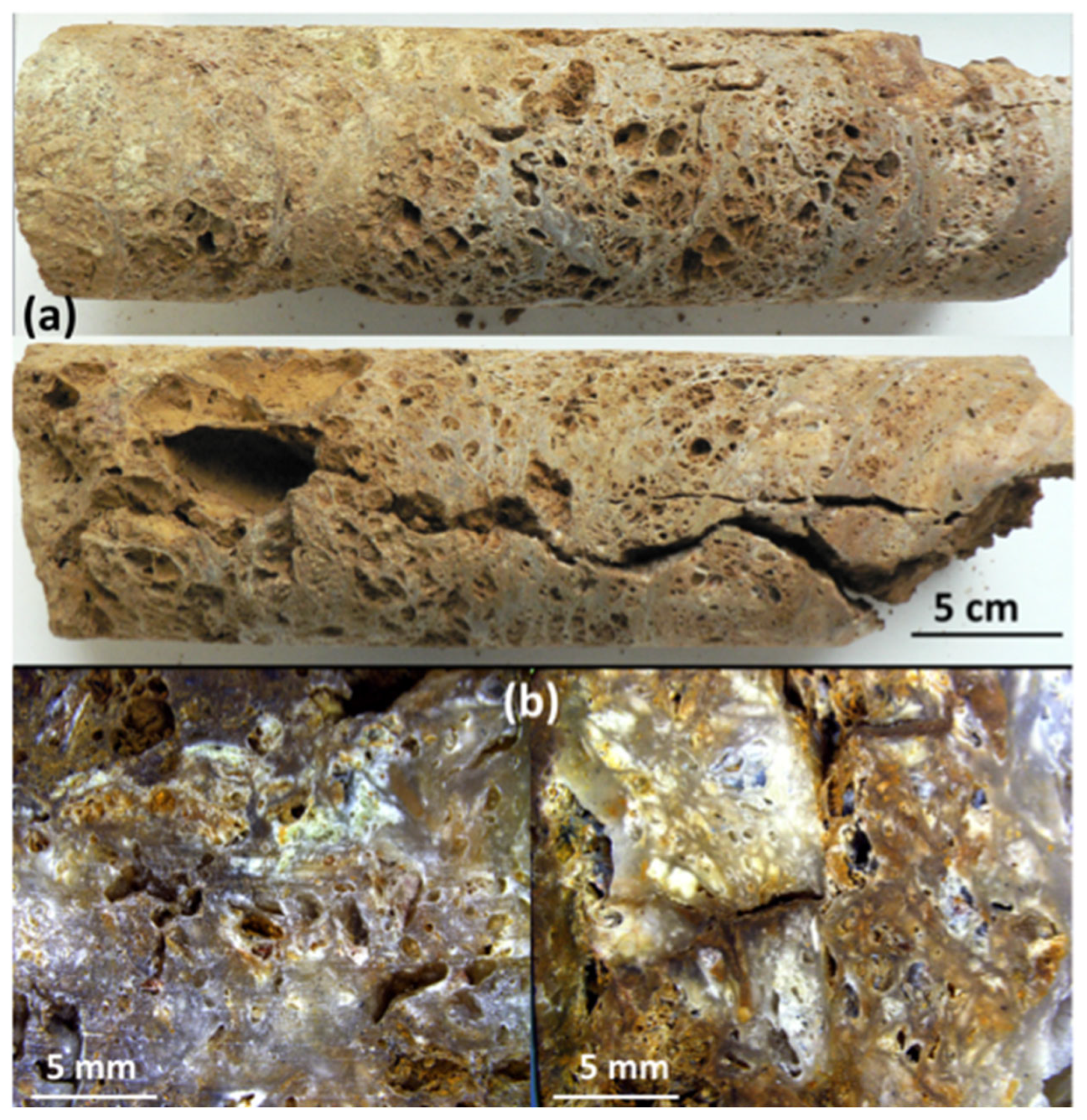
2.3. Sample Description
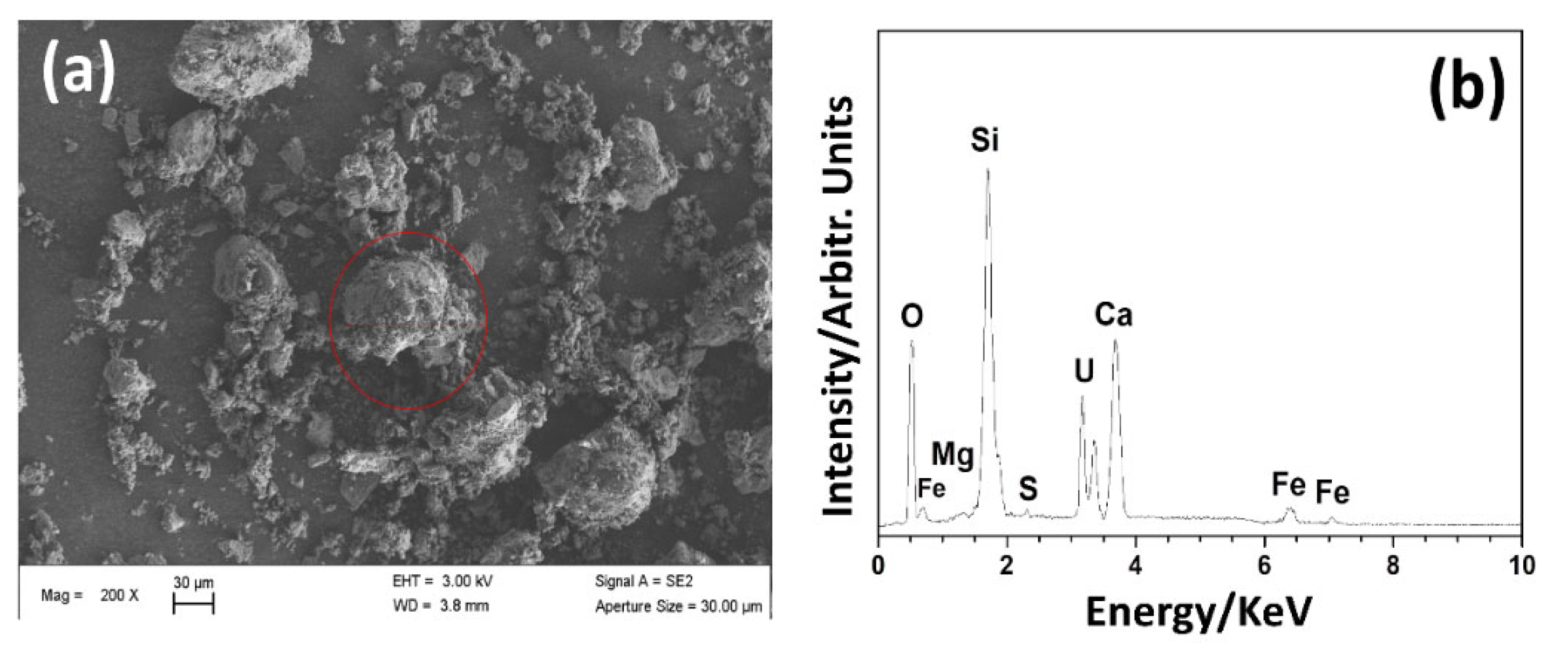
2.4. Analytical Methods
3. Results
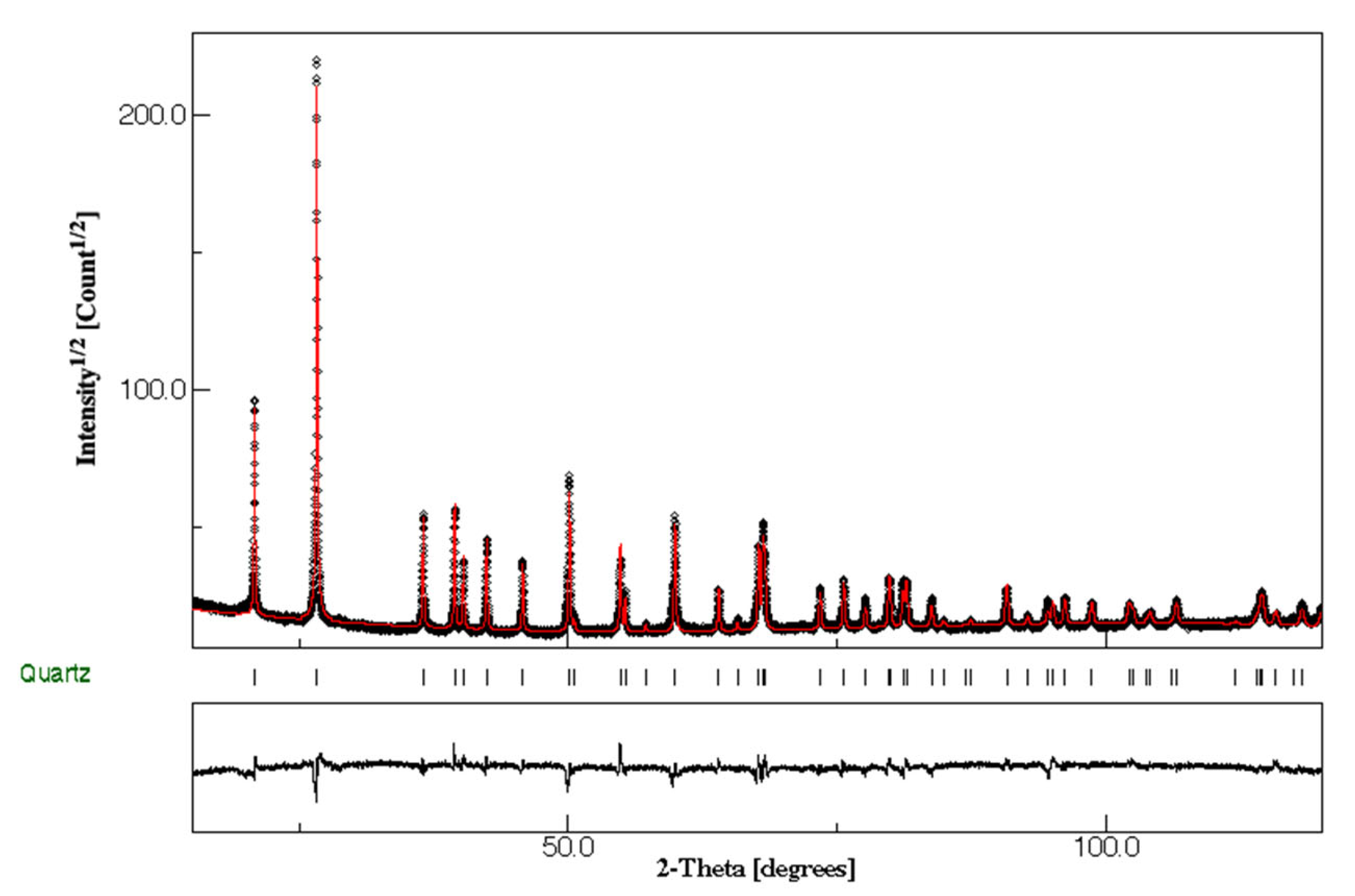
3.1. Major Composition of the Siliceous Breccia
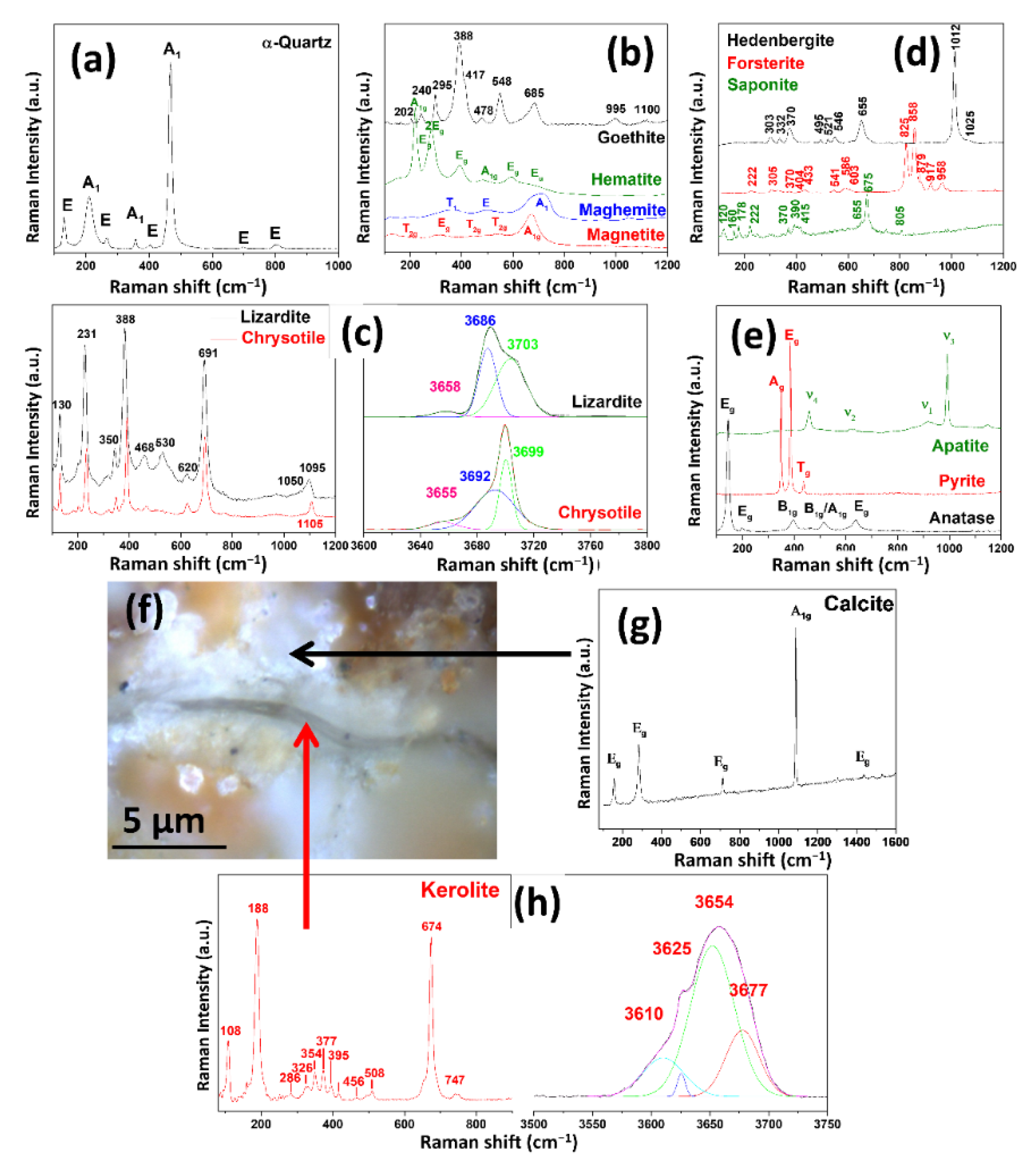
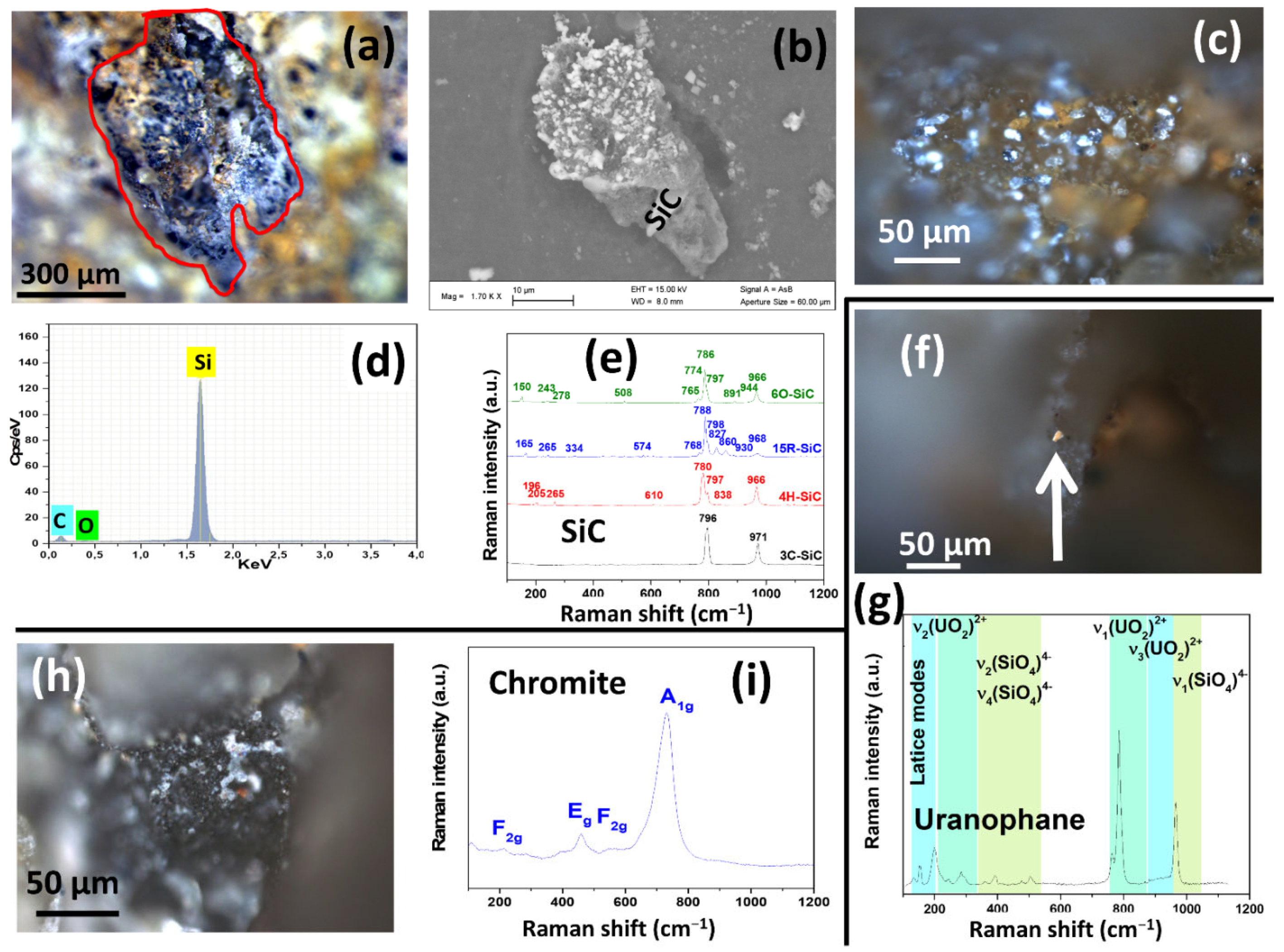
3.2. Pore Fillings in the Siliceous Breccia
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gevorkyan, R.G.; Kaminsky, F.V.; Lunev, V.C.; Osovetsky, V.M.; Nachatryan, N.D. A new occurrence of diamonds in ultramafic rocks in Armenia. Dokl. AN Armen. SSR 1976, 63, 176–181. [Google Scholar]
- Lian, D.; Yang, J.; Dilek, Y.; Wu, W.; Zhang, Z.; Xiong, F.; Liu, F.; Zhou, W. Deep mantle origin and ultra-reducing conditions in podiform chromitite: Diamond, moissanite, and other unusual minerals in podiform chromitites from the Pozanti-Karsanti ophiolite, southern Turkey. Am. Mineral. 2017, 102, 1101–1113. [Google Scholar]
- Moe, K.S.; Yang, J.S.; Johnson, P.; Xu, X.; Wang, W. Spectroscopic analysis of microdiamonds in ophiolitic chromitite and peridotite. Lithosphere 2018, 10, 133–140. [Google Scholar] [CrossRef]
- Pujol-Solà, N.; Proenza, J.A.; Garcia-Casco, A.; González-Jiménez, J.M.; Andreazini, A.; Melgarejo, J.C.; Gervilla, F. An Alternative Scenario on the Origin of Ultra-High Pressure (UHP) and Super-Reduced (SuR) Minerals in Ophiolitic Chromitites: A Case Study from the Mercedita Deposit (Eastern Cuba). Minerals 2018, 8, 433. [Google Scholar] [CrossRef]
- Trumbull, R.B.; Yang, J.S.; Robinson, P.T.; Di Pierro, S.; Vennemann, T.; Wiedenbeck, M. The carbon isotope composition of natural SiC (moissanite) from the Earth’s mantle: New discoveries from ophiolites. Lithos 2009, 113, 612–620. [Google Scholar] [CrossRef]
- Gorshkov, A.I.; Titkov, S.V.; Bao, Y.N.; Ryabchikov, I.D.; Magazina, L.O. Microinclusions in diamonds of octahedral habit from kimberlites of Shandong Province, Eastern China. Geol. Ore Deposit. 2006, 48, 326–334. [Google Scholar] [CrossRef]
- Di Pierro, S.; Gnos, E.; Grobety, B.H.; Armbruster, T.; Bernasconi, S.M.; Ulmer, P.J.S. Rock-forming moissanite (natural alpha-silicon carbide). Am. Mineral. 2003, 88, 1817–1821. [Google Scholar] [CrossRef]
- Bai, W.; Robinson, P.T.; Fang, Q.; Yang, J.; Yan, B.; Zhang, Z.; Hu, X.-F.; Zhou, M.-F.; Malpas, J. The PGE and base-metal alloys in the podiform chromitites of the Luobusa ophiolite, southern Tibet. Can. Mineral. 2000, 38, 585–598. [Google Scholar] [CrossRef]
- Xu, S.; Wu, W.; Xiao, W.; Yang, J.; Chen, J.; Ji, S.; Liu, Y. Moissanite in serpentinites from the Dabie Shan Mountains in China. Mineral. Mag. 2008, 72, 899–908. [Google Scholar] [CrossRef]
- Qi, D.; DeYoung, B.J.; Innes, R.W. Structure-Function Analysis of the Coiled-Coil and Leucine-Rich Repeat Domains of the RPS5 Disease Resistance Protein. Plant Physiol. 2012, 158, 1819–1832. [Google Scholar] [CrossRef]
- Bauer, J.; Fiala, J.; Hrichova, R. Natural a-silicon carbide. Am. Mineral. 1963, 48, 620–634. [Google Scholar]
- Schmidt, M.W.; Gao, C.; Golubkova, A.; Rohrbach, A.; Connolly, J.A. Natural moissanite (SiC)—A low temperature mineral formed from highly fractionated ultra-reducing COH-fluids. Prog. Earth Planet. Sci. 2014, 1, 27–30. [Google Scholar] [CrossRef]
- Pearson, D.G.; Davies, G.R.; Nixon, P.H. Geochemical Constraints on the Petrogenesis of Diamond Facies Pyroxenites from the Beni Bousera Peridotite Massif, North Morocco. J. Petrol. 1993, 34, 125–172. [Google Scholar] [CrossRef]
- Davies, G.R.; Nixon, P.H.; Pearson, D.G.; Obata, M. Tectonic implications of graphitized diamonds from the Ronda peridotite massif, southern Spain. Geology 1993, 21, 471–474. [Google Scholar] [CrossRef]
- Pujol-Solà, N.; Garcia-Casco, A.; Proenza, J.A.; González-Jiménez, J.M.; del Campo, A.; Colás, V.; Canals, À.; Sánchez-Navas, A.; Roqué-Rosell, J. Diamond forms during low pressure serpentinisation of oceanic lithosphere. Geochem. Persp. Let. 2020, 15, 19–24. [Google Scholar] [CrossRef]
- Farré-de-Pablo, J.; Proenza, J.A.; González-Jiménez, J.M.; Garcia-Casco, A.; Colás, V.; Roqué-Rossell, J.; Camprubí, A.; Sánchez-Navas, A. A shallow origin for diamonds in ophiolitic chromitites. Geology 2019, 47, 75–78. [Google Scholar] [CrossRef]
- El Mendili, Y.; Orberger, B.; Chateigner, D.; Bardeau, J.-F.; Gascoin, S.; Petit, S.; Perez, O.; Khadraoui, F. Insight into the structural, elastic and electronic properties of a new orthorhombic 6O-SiC polytype. Sci. Rep. 2019, 10, 7562. [Google Scholar] [CrossRef] [PubMed]
- Lovering, T.G. Radioactive Deposits in New Mexico; Geological Survey Bulletin: Reston, VA, USA, 1956; pp. 315–390. [Google Scholar]
- Min, M.; Fang, C.; Fayek, M. Petrography and genetic history of coffinite and uraninite from the Liueryiqi granite-hosted uranium deposit, SE China. Ore Geol. Rev. 2005, 26, 187–197. [Google Scholar] [CrossRef]
- Shukla, M.K.; Sharma, A. A brief review on breccia: It’s contrasting origin and diagnostic signatures. Solid. Earth Sci. 2018, 3, 50–59. [Google Scholar] [CrossRef]
- Leonardos, O.H.; Fernandes, S.M.; Fyfe, W.S.; Powell, M. The mciro-chemistry of uraniferous laterites from Brazil: A natural example of inorganic chromatography. Chem. Geol. 1987, 60, 111–119. [Google Scholar] [CrossRef]
- Duée, C.; Orberger, B.; Maubec, N.; Laperche, V.; Capar, L.; Bourguignon, A.; Bourrat, X.; Mendili, Y.E.I.; Chateigner, D.; Gascoin, S.; et al. Impact of heterogeneities and surface roughness on pXRF, pIR, XRD and Raman analyses: Challenges for on-line, real-time combined mineralogical and chemical analyses on drill cores and implication for “high speed” Ni-laterite exploration. J. Geochem. Explor. 2019, 198, 1–17. [Google Scholar] [CrossRef]
- El Mendili, Y.; Chateigner, D.; Orberger, B.; Gascoin, S.; Bardeau, J.-F.; Petit, S.; Duee, C.; Guen, M.L.; Pilliere, H. Combined XRF, XRD, SEM-EDS, and Raman Analyses on Serpentinized Harzburgite (Nickel Laterite Mine, New Caledonia): Implications for Exploration and Geometallurgy. ACS Earth Space Chem. 2019, 3, 2237–2249. [Google Scholar] [CrossRef]
- Cassard, D.; Nicolas, A.; Rabinovitch, M.; Moutte, J.; Leblanc, M.; Prinzhofer, A. Structural classification of chromite pods in New Caledonia. Econ. Geol. 1981, 76, 805–881. [Google Scholar] [CrossRef]
- Marchesi, C.; Garrido, C.J.; Godard, M.; Belley, F.; Ferré, E. Migration and accumulation of ultra-depleted subduction-related melts in the Massif du Sud ophiolite (New Caledonia). Chem. Geol. 2009, 266, 171–186. [Google Scholar] [CrossRef]
- Prinzhofer, A.; Allègre, C. Residual peridotite and the mechanism of partial melting. Earth Planet. Sci. Let. 1985, 74, 251–265. [Google Scholar] [CrossRef]
- Moutte, J. Le massif de Tiebaghi, Nouvelle-Calédonie, et ses gites de chromite. Ph.D. Thesis, Ecole Nationale Supérieure des Mines de Paris, Paris, France, 1979. [Google Scholar]
- Bailly, L.; Ambrosi, J.P.; Barbarand, J.; Beauvais, A.; Cluzel, D. Nickel-Typologie des latérites de Nouvelle-Calédonie. Gisements de nickel latéritique, volume II. In Rapport de Recherche: Tome Nickel et Technologie; HAL: Paris, France, 2014; pp. 1–448. [Google Scholar]
- Sevin, B.; Ricordel-Prognon, C.; Quesnel, F.; Cluzel, D.; Lesimple, S.; Maurizot, P. First palaeomagnetic dating of ferricrete in New Caledonia: New insight on the morphogenesis and palaeoweathering of ‘Grande Terre’. Terra Nova 2012, 24, 77–85. [Google Scholar] [CrossRef]
- Mc Rae, E. Nickel Statistics and Information; Annual Publication; USGS: Reston, VA, USA, 2018. [Google Scholar]
- Trescases, J.J. The lateritic nickel-ore deposits. In Soils and Sediments Mineralogy and Geochemistry; Paquet, H., Clauer, N., Eds.; Springer: Berlin, Germany, 1997; pp. 125–138. [Google Scholar]
- Cathelineau, M.; Myagkiy, A.; Quesnel, B.; Boiron, M.-C.; Gautier, P.; Boulvais, P.; Ulrich, M.; Truche, L.; Golfier, F.; Drouillet, M. Multistage crack seal vein and hydrothermal Ni enrichment inserpentinized ultramafic rocks (Koniambo massif, New Caledonia). Miner. Depos. 2017, 52, 945–960. [Google Scholar] [CrossRef]
- Dublet, G.; Juillot, F.; Morin, G.; Fritsch, E.; Fandeur, D.; Ona-Nguema, G.; Brown, G.E. Ni speciation in a New Caledonian lateritic regolith: A quantitative. X-ray absorption spectroscopy investigation. Geochim. Cosmochim. Acta 2012, 95, 119–133. [Google Scholar] [CrossRef]
- Manceau, A.; Calas, G.; Decarrau, A. Nickel-bearing clay minerals: I. Optical study of nickel crystal chemistry. Clay Miner. 1985, 20, 367–487. [Google Scholar] [CrossRef]
- Cluzel, D.; Meffre, S.; Maurizot, P.; Crawford, A.J. Earliest Eocene (53 Ma) convergence in the Southwest Pacific: Evidence from pre-obduction dikes in the ophiolite of New Caledonia. Terra Nova 2006, 18, 395–402. [Google Scholar] [CrossRef]
- Jeanpert, J.; Lesimple, S.; Sevin, B.; Maurizot, P.; Robineau, B.; Maréchal, J.-C.; Dewandel, B. Exploration des Galeries Chromical-Massif de Tiébaghi. In Observations Géologiques et Hydrogéologiques à L’intérieur D’un Massif de Péridotites; SGNC/DIMENC: New Caledonia, France, 2015; pp. 1–35. [Google Scholar]
- Lagabrielle, Y.; Chauvet, A.; Ulrich, M.; Guillot, S. Passive obduction and gravity-driven emplacement of large ophiolitic sheets: The New Caledonia ophiolite (SW Pacific) as a case study? Bull. Soc. Géol. Fr. 2013, 6, 545–556. [Google Scholar] [CrossRef]
- Maurizot, P. Formations miocènes de Népoui. In Compilation des Connaissances; BRGM-SGNC: Nouméa, France, 2011; pp. 1–96. [Google Scholar]
- Maurizot, P.; Cluzel, D. Pre-obduction records of Eocene foreland basins in central New Caledonia: An appraisal from surface geology and Cadart-1 borehole data. New Zealand J. Geol. Geophys. 2014, 57, 300–311. [Google Scholar] [CrossRef]
- Camus, H.; Leveneur, D.; Bart, F. Structuration karstique des aquifères dans les massifs ophiolitiques de Nouvelle-Calédonie. In Aquifères de Socle: Le Point Sur Les Concepts et Les Applications Opérationnelles; La Roche-sur-Yon, Pays de la Loire, France, 2015. [Google Scholar]
- Jeanpert, J. Structure et fonctionnement hydrogéologiques des massifs de péridotites d Nouvelle-Calédonie. Ph.D. Thesis, Université de la Réunion, Saint-Denis, France, 2017. [Google Scholar]
- Trittschack, R.; Grobéty, B.; Koch-Müller, M. In situ high-temperature Raman and FTIR spectroscopy of the phase transformation of lizardite. Am. Mineral. 2012, 97, 1965–1976. [Google Scholar] [CrossRef]
- Guillou-Frottier, L.; Beauvais, A.; Bailly, L.; Wyns, R.; Augé, T.; Audion, A.S. Transient hydrothermal corrugations within mineralized ultramafic laterites. In Proceedings of the SGA Biannual conference, Nancy, France, 24–27 August 2015. [Google Scholar]
- El Mendili, Y.; Vaitkus, A.; Merkys, A.; Gražulis, S.; Chateigner, D.; Mathevet, F.; Gascoin, S.; Petit, S.; Bardeau, J.-F.; Zanatta, M.; et al. Raman Open Database: First interconnected Raman-XRD open-access resource for material identification. J. Appl. Cryst. 2019, 52, 618–625. [Google Scholar] [CrossRef]
- Le Page, Y.; Donnay, G. Refinement of the Crystal Structure of Low-Quartz. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1976, 32, 2456–2459. [Google Scholar] [CrossRef]
- Neeway, J.; Abdelouas, A.; Ribet, S.; David, K.; El Mendili, Y.; Grambow, B.; Schumacher, S. Effect of Callovo-Oxfordian clay rock on the dissolution rate of the SON68 simulated nuclear waste glass. J. Nucl. Mater. 2015, 459, 291–300. [Google Scholar] [CrossRef]
- Čermáková, Z.; Hradil, D.; Bezdička, P.; Hradilová, J. New data on “kerolite–pimelite” series and the colouring agent of Szklary chrysoprase, Poland. Phys. Chem. Miner. 2017, 44, 193–202. [Google Scholar] [CrossRef]
- Cheung, R. Silicon Carbide Microelectromechanical Systems for Harsh Environments; Imperial College Press: London, UK, 2006. [Google Scholar]
- Lu, Y.P.; He, D.W.; Zhu, J.; Yang, X.D. First-principles study of pressure-induced phase transition in silicon carbide. Phys. B. 2008, 403, 3543–3546. [Google Scholar] [CrossRef]
- Nakashima, S.; Harima, H. Raman Investigation of SiC Polytypes. Phys. Status Solidi 1997, 162, 39–64. [Google Scholar] [CrossRef]
- Frondel, C.; Marvin, U.B. Lonsdaleite, a hexagonal polymorph of diamond. Nature 1967, 214, 587–589. [Google Scholar] [CrossRef]
- Spear, K.E.; Phelps, A.W.; White, W.B. Diamond polytypes and their vibrational spectra. J. Mater. Res. 1990, 5, 2277–2285. [Google Scholar] [CrossRef]
- Kraus, D.; Ravasio, A.; Gauthier, M.; Gericke, D.O.; Vorberger, J.; Frydrych, S.; Helfrich, J.; Fletcher, L.B.; Schaumann, G.; Nagler, B.; et al. Nanosecond formation of diamond and lonsdaleite by shock compression of graphite. Nat. Commun. 2016, 7, 10970. [Google Scholar] [CrossRef]
- Smith, D.C.; Godard, G. UV and VIS Raman spectra of natural lonsdaleites: Towards a recognised standard. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 428–435. [Google Scholar] [CrossRef]
- Nishitani-Gamo, M.; Sakaguchi, I.; Loh, K.P.; Kanda, H.; Ando, T. Confocal Raman spectroscopic observation on hexagonal diamond formation from dissolved carbon in nickel under chemical vapor deposition conditions. Appl. Phys. Lett. 1998, 73, 765–767. [Google Scholar] [CrossRef]
- Goryainov, S.V.; Likhacheva, A.Y.; Rashchenko, S.V.; Shubin, A.S.; Afanas’ev, V.P.; Pokhilenko, N.P. Raman identification of lonsdaleite in Popigai impactites. J. Raman Spectrosc. 2014, 45, 305–313. [Google Scholar] [CrossRef]
- Wu, B.R. Structural and vibrational properties of the 6H diamond: First-principles study. Diam. Relat. Mater. 2007, 16, 21–28. [Google Scholar] [CrossRef]
- Denisov, V.N.; Mavrin, B.N.; Serebryanaya, N.R.; Dubitsky, G.A.; Aksenenkov, V.V.; Kirichenko, A.N.; Kuzmin, N.V.; Kulnitskiy, B.A.; Perezhogin, I.A.; Blank, V.D. First-principles, UV Raman, X-ray diffraction and TEM study of the structure and lattice dynamics of the diamond–lonsdaleite system. Diam. Relat. Mater. 2011, 20, 951–953. [Google Scholar] [CrossRef]
- Dovesi, R.; Orlando, R.; Erba, A.; Zicovich-Wilson, C.M.; Civalleri, B.; Casassa, S.; Maschio, L.; Ferrabone, M.; De La Pierre, M.; D’Arco, P.; et al. CRYSTAL14: A program for the ab initio investigation of crystalline solids. Int. J. Quantum Chem. 2014, 114, 1287–1317. [Google Scholar] [CrossRef]
- Reddy, B.J.; Frost, R.L. Spectroscopic characterization of chromite from the Moa-Baracoa Ophiolitic Massif, Cuba. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2005, 61, 1721–1728. [Google Scholar] [CrossRef][Green Version]
- Driscoll, R.J.P.; Wolverson, D.; Mitchels, J.M.; Skelton, J.M.; Parker, S.C.; Molinari, M.; Khan, I.; Geeson, D.; Allen, G.C. Raman spectroscopic study of uranyl minerals from Cornwall, UK. RSC. Adv. 2014, 4, 59137–59149. [Google Scholar] [CrossRef]
- Bonales, L.J.; Menor-Salván, C.; Cobos, J. Study of the alteration products of a natural uraninite by Raman spectroscopy. J. Nucl. Mater. 2015, 462, 296–303. [Google Scholar] [CrossRef]
- Frost, R.L.; Cejka, J.; Weier, M.L.; Martens, W. Molecular structure of the uranyl silicates—A Raman spectroscopic study. J. Raman Spectrosc. 2006, 37, 538–551. [Google Scholar] [CrossRef]
- Alexander, E.B.; Coleman, R.G.; Keeler-Wolfe, T.; Harrison, S.P. Serpentine Geoecology of Western North America: Geology, Soils and Vegetation; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Frost, B.R.; Beard, J. On silica activity and serpentinization. J. Petrol. 2007, 48, 1351–1368. [Google Scholar] [CrossRef]
- Fyfe, W.S. Heats of chemical reactions and submarine heat production. Geophys. J. Roy. Astron. Soc. 1974, 37, 213–215. [Google Scholar] [CrossRef]
- Monin, C.; Chavagnac, V.; Boulart, C.; Ménez, B.; Gérard, M.; Gérard, E.; Quemeneur, M.; Erauso, G.; Postec, A.; Guentas-Dombrowski, L.; et al. The low temperature hyperalkaline hydrothermal system of the Prony Bay (New Caledonia). Biogeosci. Discuss. 2014, 11, 6221–6267. [Google Scholar]
- Ulrich, M.; Picard, C.; Guillot, S.; Chauvel, C.; Cluzel, D.; Meffre, S. Multiple melting stages and refertilization as indicators for ridge to subduction formation: The New Caledonia ophiolite. Lithos 2010, 115, 223–236. [Google Scholar] [CrossRef]
- Chamberlain, J.A.; McLeod, C.R.; Traill, R.J.; Lachance, G.R. Native metals in the Muskox Intrusion. Can. J. Earth Sci. 1965, 2, 188–215. [Google Scholar] [CrossRef]
- Yamamoto, S.; Komiya, T.; Hirose, K.; Maruyama, S. Coesite and clinopyroxene exsolution lamellae in chromitites: In-situ ultrahigh-pressure evidence from podiform chromitites in the Luobusa ophiolite, southern Tibet. Lithos 2009, 109, 314–322. [Google Scholar] [CrossRef]
- Cartigny, P. Stable Isotopes and the Origin of Diamond. Elements 2005, 1, 79–84. [Google Scholar] [CrossRef]
- Bouilhol, P.; Debret, B.; Inglis, E.C.; Warembourg, M.; Grocolas, T.; Rigaudier, T.; Villeneuve, J.; Burton, K.W. Decoupling of inorganic and organic carbon during slab mantle devolatilisation. Nature. Comm. 2022, 13, 308. [Google Scholar] [CrossRef]
- McCollom, T.M.; Bach, W. Thermodynamic constraints on hydrogen generation during serpentinization of ultramafic rocks. Geochim. Cosmochim. Acta 2009, 73, 856–875. [Google Scholar] [CrossRef]
- Stagno, V.; Frost, D.J. Carbon speciation in the asthenosphere: Experimental measurements of the redox conditions at which carbonate-bearing melts coexist with graphite or diamond in peridotite assemblages. Earth Planet. Sci. Lett. 2010, 300, 72–84. [Google Scholar] [CrossRef]
- Bundy, T.P.; Kasper, J.S. Hexagonal Diamond-A New Form of Carbon. Chem. Phys. 1967, 46, 3437–3446. [Google Scholar] [CrossRef]
- Daulton, T.L.; Amari, S.; Scott, A.C.; Hardiman, M.; Pinter, N.; Anderson, R.S. Comprehensive Analysis of Nanodiamond Evidence Relating to the Younger Drays Impact Hypothesis. J. Quat. Sci. 2017, 32, 7–34. [Google Scholar] [CrossRef]
- Cayron, C.; Hertog, M.D.; Latu-Romain, L.; Mouchet, C.; Secouard, C.; Rouviere, J.-L.; Rouviere, E.; Simonato, J.-P. Odd electron diffraction patterns in silicon nanowires and silicon thin films explained by microtwins and nanotwins. J. Appl. Cryst. 2008, 42, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.O.; Gurney, J.J. Proceedings of the Fourth International Kimberlite Conference, Kimberlites and Related Rocks; Their Mantle/Crust Setting, Diamonds and Diamond Exploration; Geological Society of Australia Special Publication 14; Blackwell Scientic: Cambridge, UK, 1989; pp. 1029–1041. [Google Scholar]
- Mudd, G.M. Compilation of Uranium Production History and Uranium Deposit Data Across Australia; SEA-US Inc.: Melbourne, Australia, 2006; pp. 1–46. [Google Scholar]
- Finch, R.; Ewing, R. The corrosion of uraninite under oxidizing conditions. J. Nucl. Mater. 1992, 190, 133–156. [Google Scholar] [CrossRef]
- Pérez, I.; Casas, I.; Martín, M.; Bruno, J. The thermodynamics and kinetics of uranophane dissolution in bicarbonate test solutions. Geochim. Cosmochim. Acta 2000, 64, 603–608. [Google Scholar] [CrossRef]
- Shvareva, T.Y.; Mazeina, L.; Gorman-Lewis, D.; Burns, P.C.; Szymanowski, J.E.; Fein, J.B.; Navrotsky, A. Thermodynamic characterization of boltwoodite and uranophane: Enthalpy of formation and aqueous solubility. Geochim. Cosmochim. Acta 2011, 75, 5269–5282. [Google Scholar] [CrossRef]
- Skinner, H.C.W.; Jahren, A.H. Biomineralization. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 8, pp. 1–69. [Google Scholar]
- Ayers, J.C.; Watson, E.B. Solubility of Apatite, Monazite, Zircon, and Rutile in Supercritical Aqueous Fluids with Implications for Subduction Zone Geochemistry. Philos. Trans. Phys. Sci. Eng. 1991, 335, 365–375. [Google Scholar]
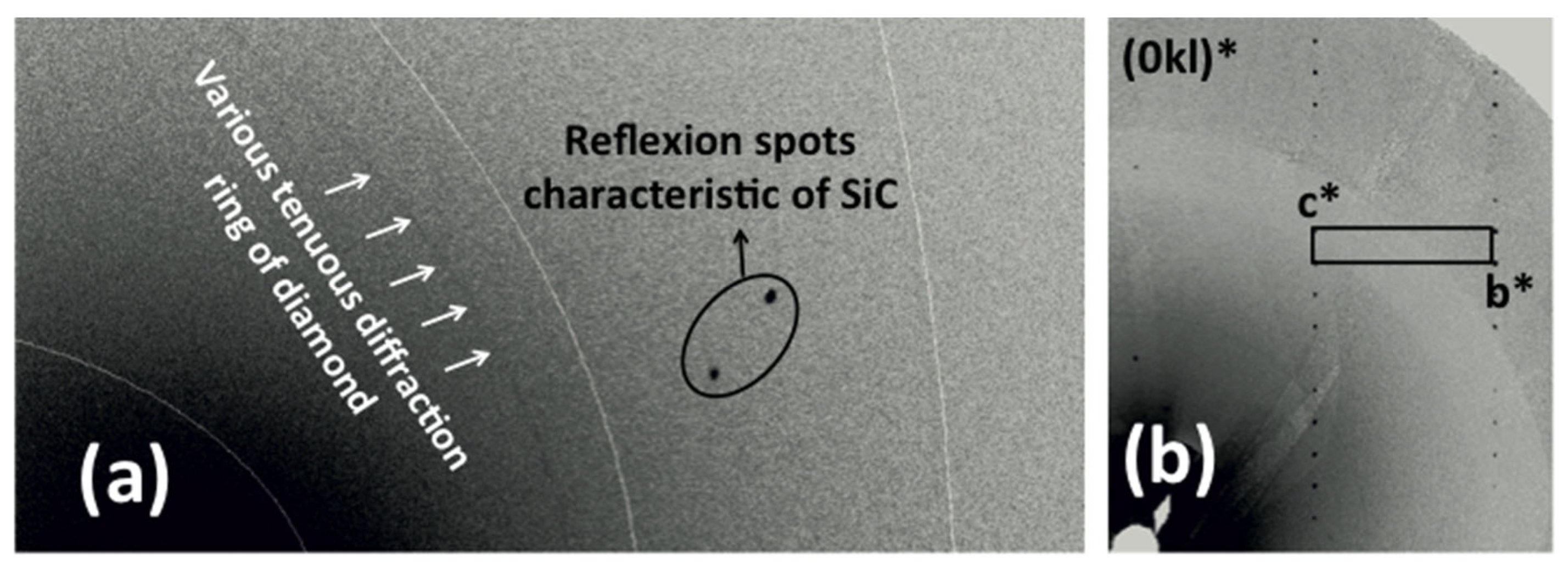
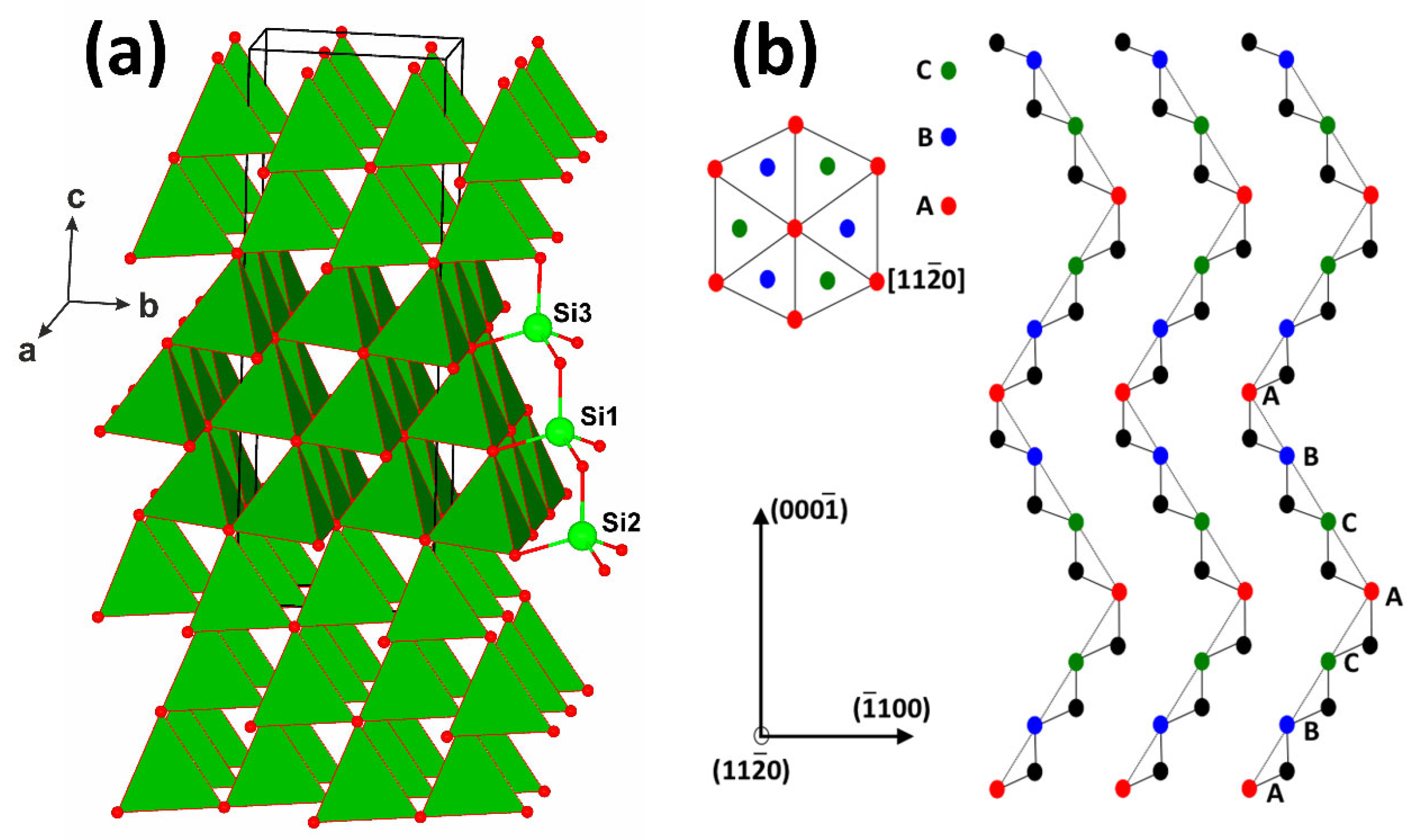
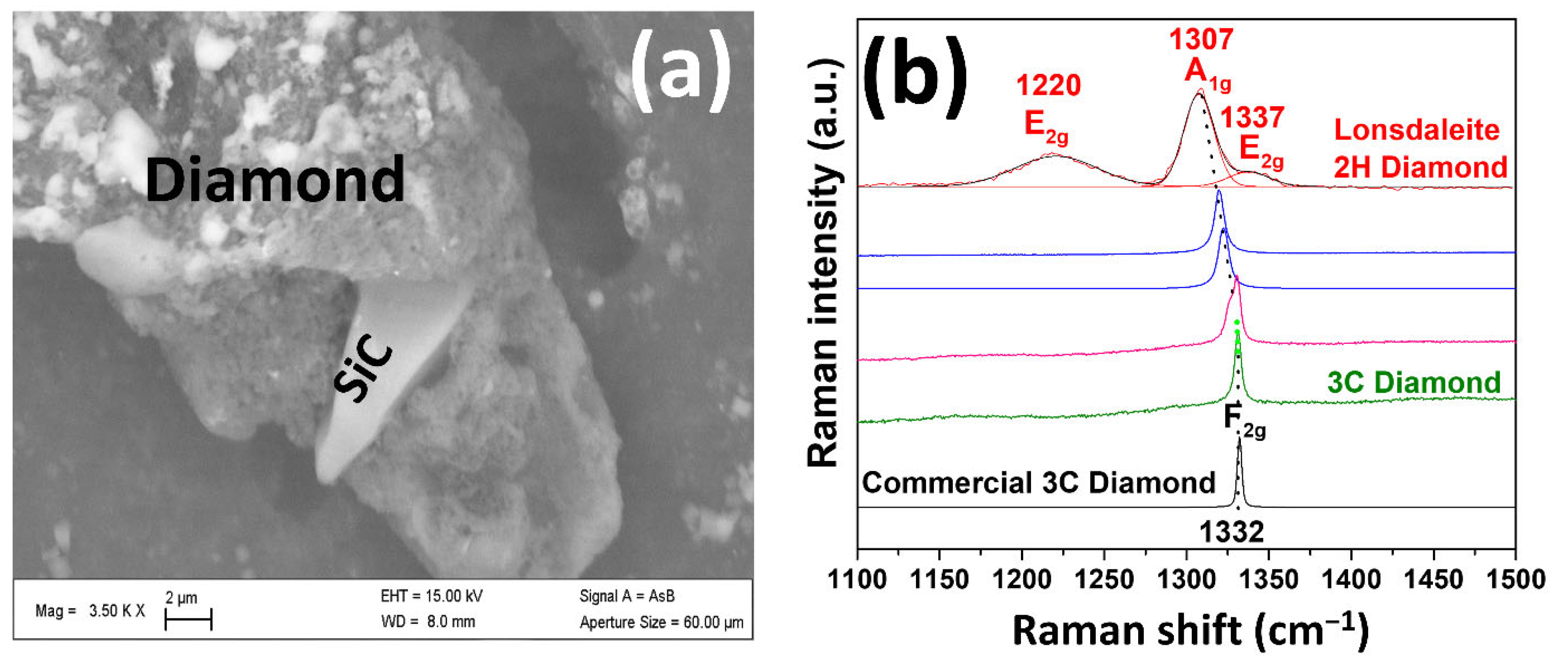
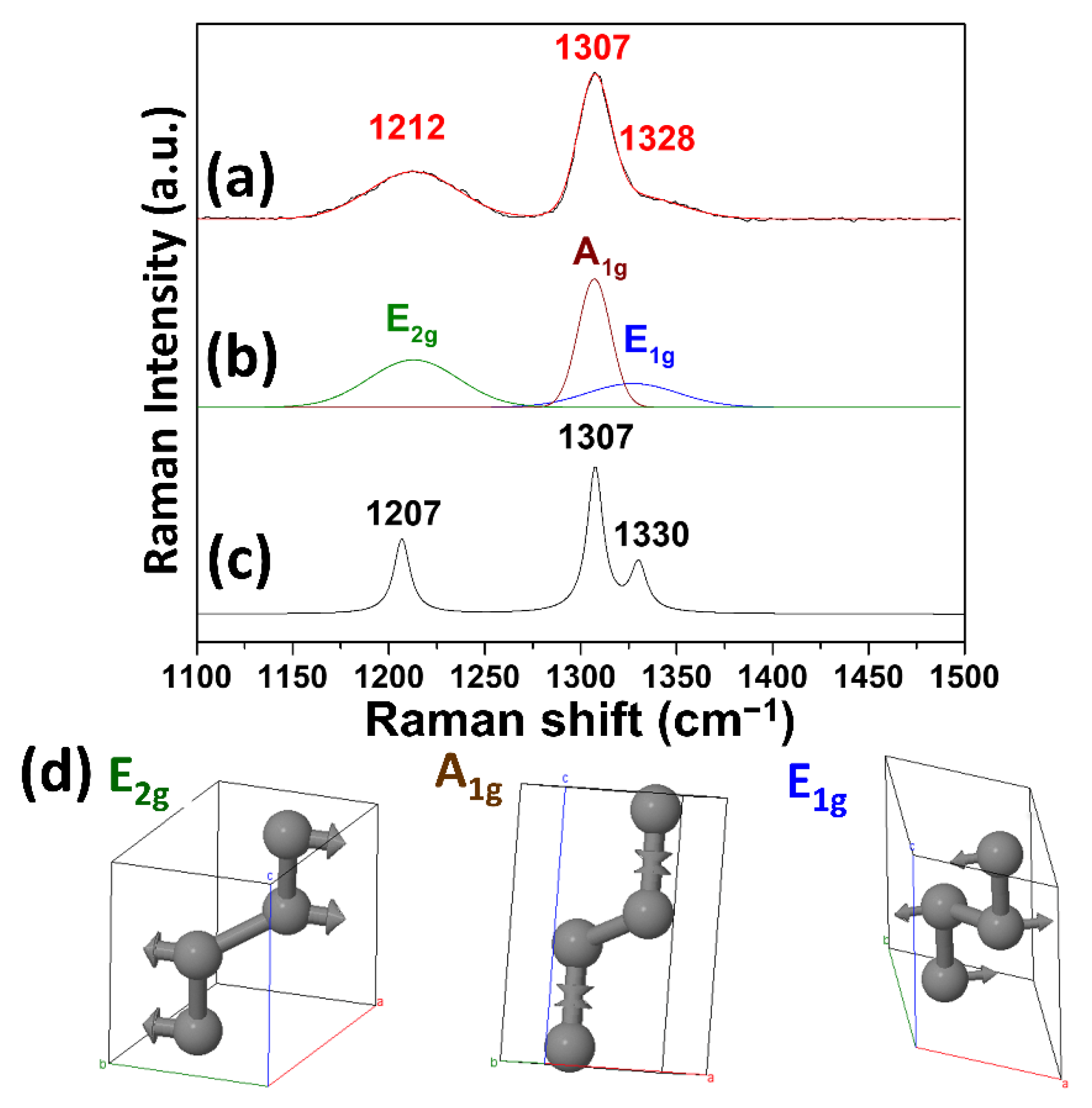
| Element | Composition (wt.%) |
|---|---|
| O | 42.7 ± 1.5 |
| Si | 25.3 ± 0.8 |
| Mg | 21.7 ± 0.8 |
| Fe | 4.9 ± 0.4 |
| Ni | 0.6 ± 0.2 |
| Al | 1.1 ± 0.1 |
| Ca | 1.0 ± 0.1 |
| Cr | 1.2 ± 0.1 |
| Na | 1.25 ± 0.2 |
| K | 0.20 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Mendili, Y.; Orberger, B.; Chateigner, D.; Bardeau, J.-F.; Gascoin, S.; Petit, S.; Perez, O. Occurrence of SiC and Diamond Polytypes, Chromite and Uranophane in Breccia from Nickel Laterites (New Caledonia): Combined Analyses. Minerals 2022, 12, 196. https://doi.org/10.3390/min12020196
El Mendili Y, Orberger B, Chateigner D, Bardeau J-F, Gascoin S, Petit S, Perez O. Occurrence of SiC and Diamond Polytypes, Chromite and Uranophane in Breccia from Nickel Laterites (New Caledonia): Combined Analyses. Minerals. 2022; 12(2):196. https://doi.org/10.3390/min12020196
Chicago/Turabian StyleEl Mendili, Yassine, Beate Orberger, Daniel Chateigner, Jean-François Bardeau, Stéphanie Gascoin, Sébastien Petit, and Olivier Perez. 2022. "Occurrence of SiC and Diamond Polytypes, Chromite and Uranophane in Breccia from Nickel Laterites (New Caledonia): Combined Analyses" Minerals 12, no. 2: 196. https://doi.org/10.3390/min12020196
APA StyleEl Mendili, Y., Orberger, B., Chateigner, D., Bardeau, J.-F., Gascoin, S., Petit, S., & Perez, O. (2022). Occurrence of SiC and Diamond Polytypes, Chromite and Uranophane in Breccia from Nickel Laterites (New Caledonia): Combined Analyses. Minerals, 12(2), 196. https://doi.org/10.3390/min12020196







