Multi-Technique Analytical Approach to Quantitative Analysis of Spodumene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Heat Treatment
2.3. X-ray Diffraction (XRD)
2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
3. Results and Discussion
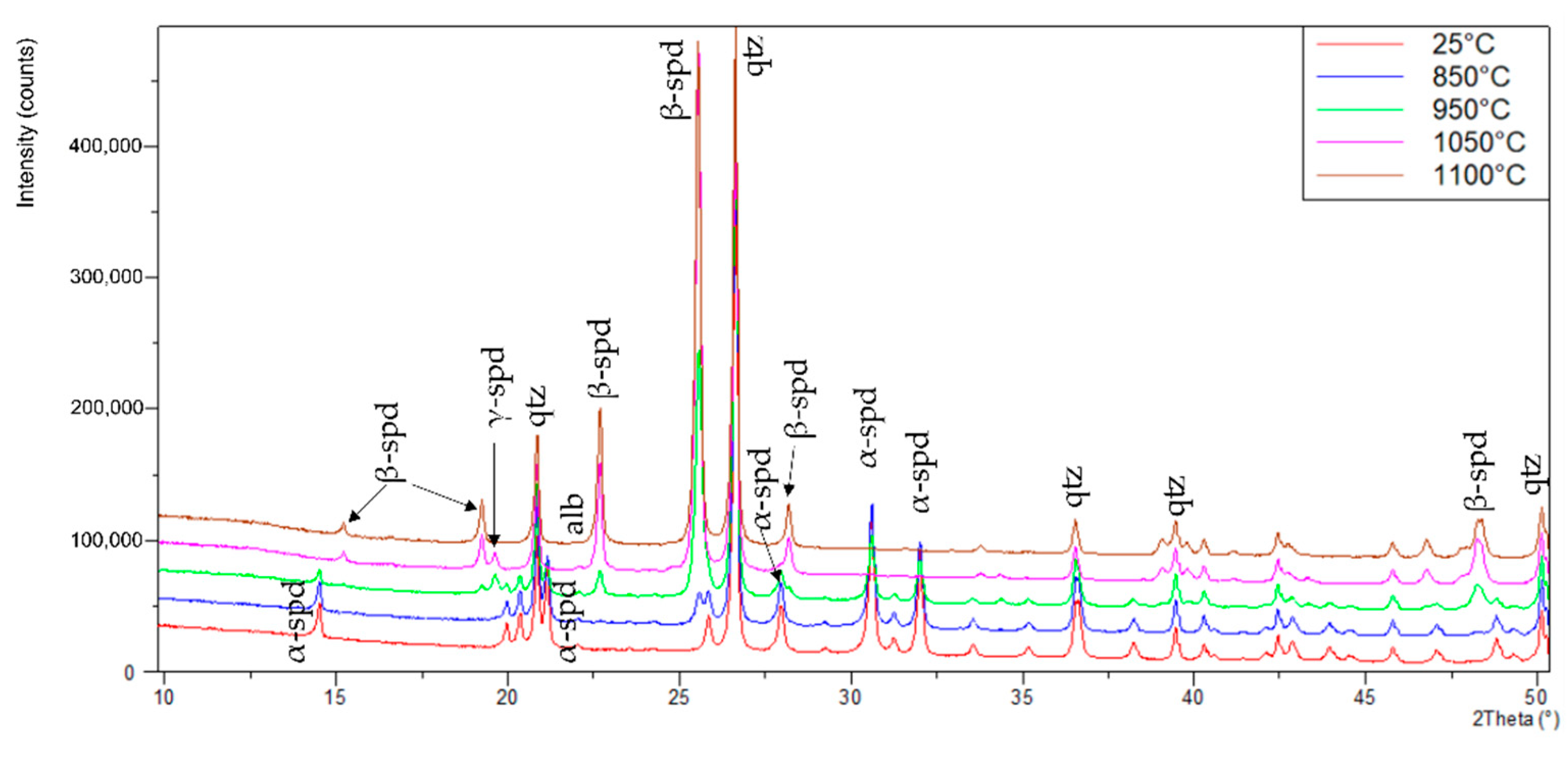
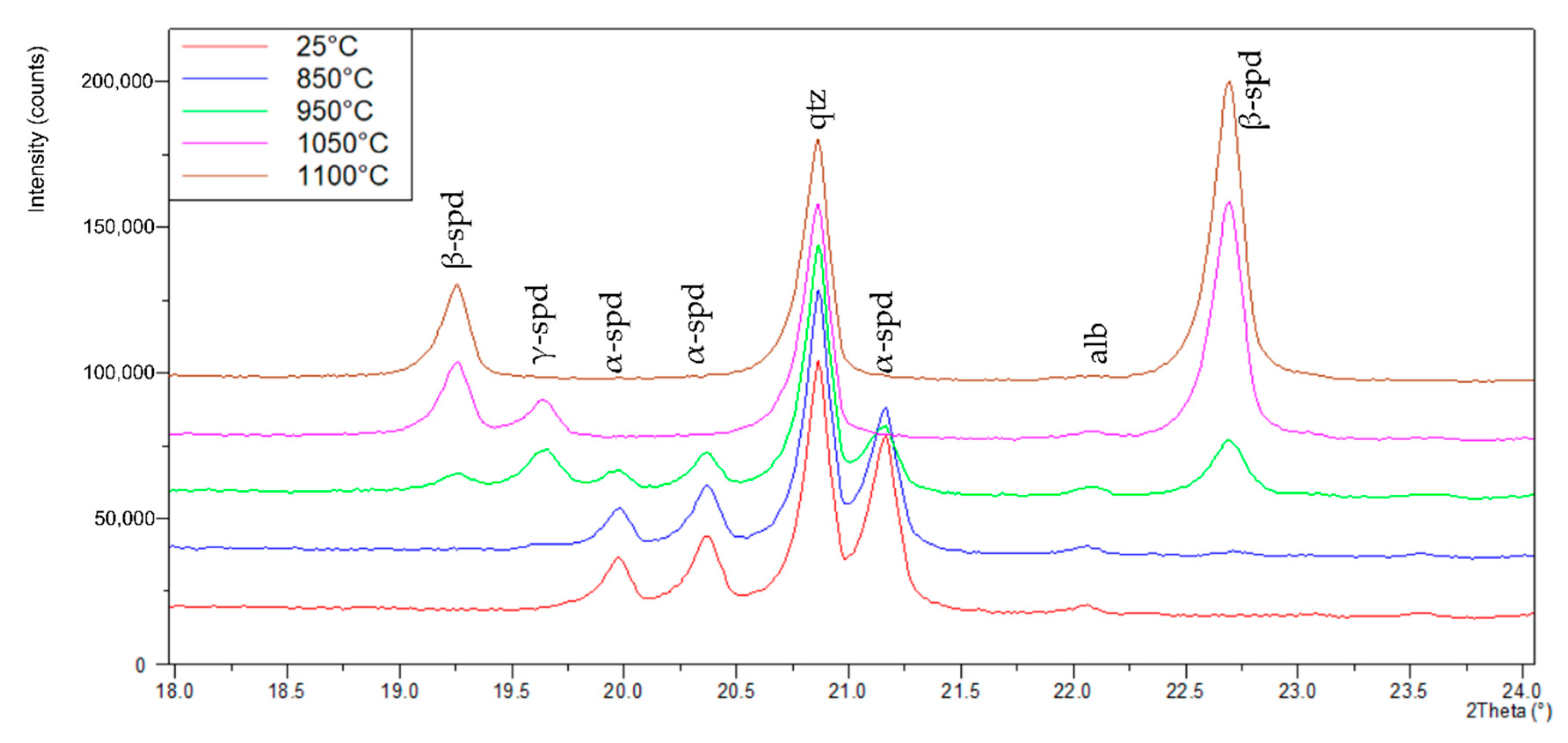
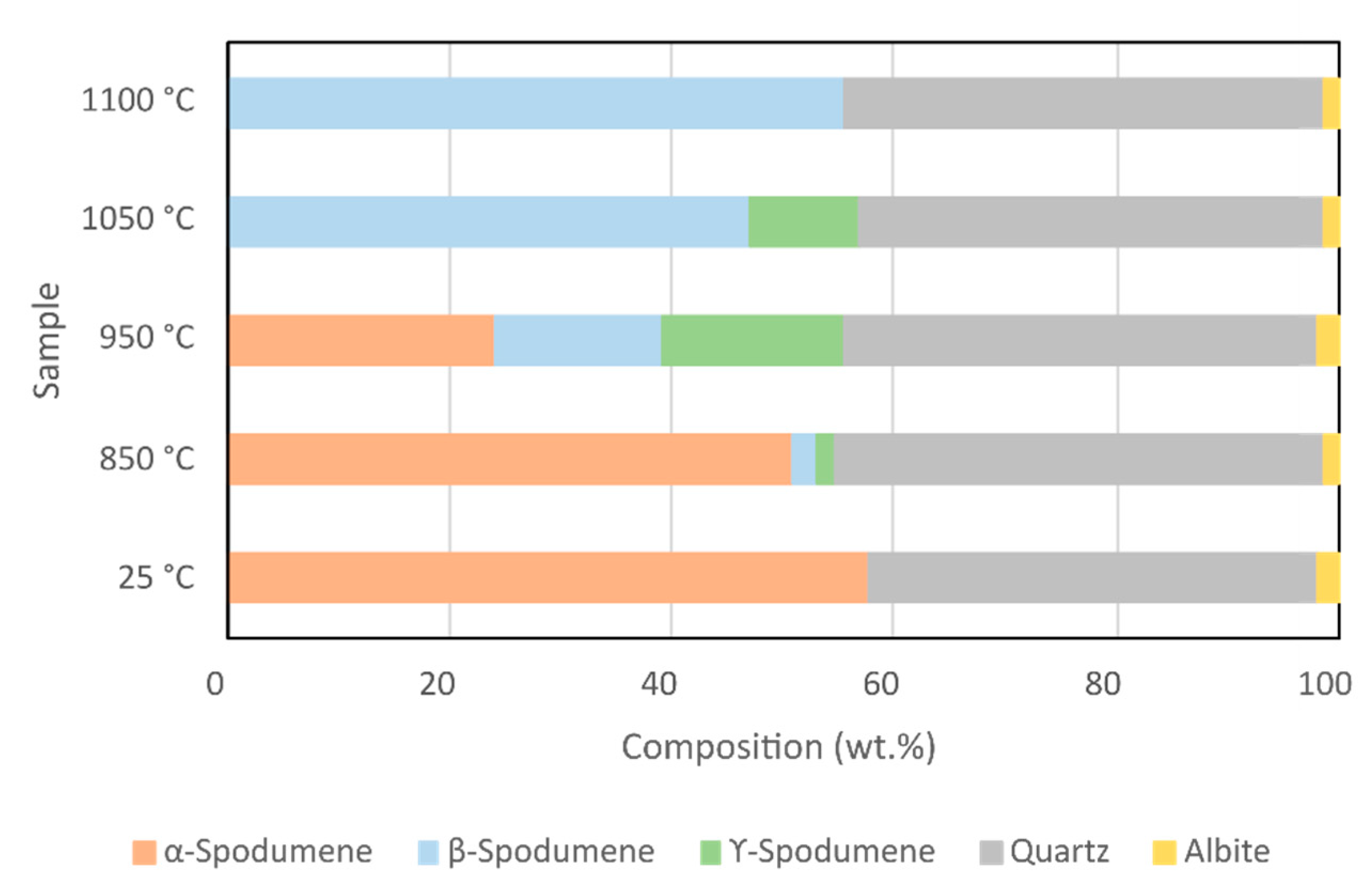
3.1. XRD Analysis of Spodumene
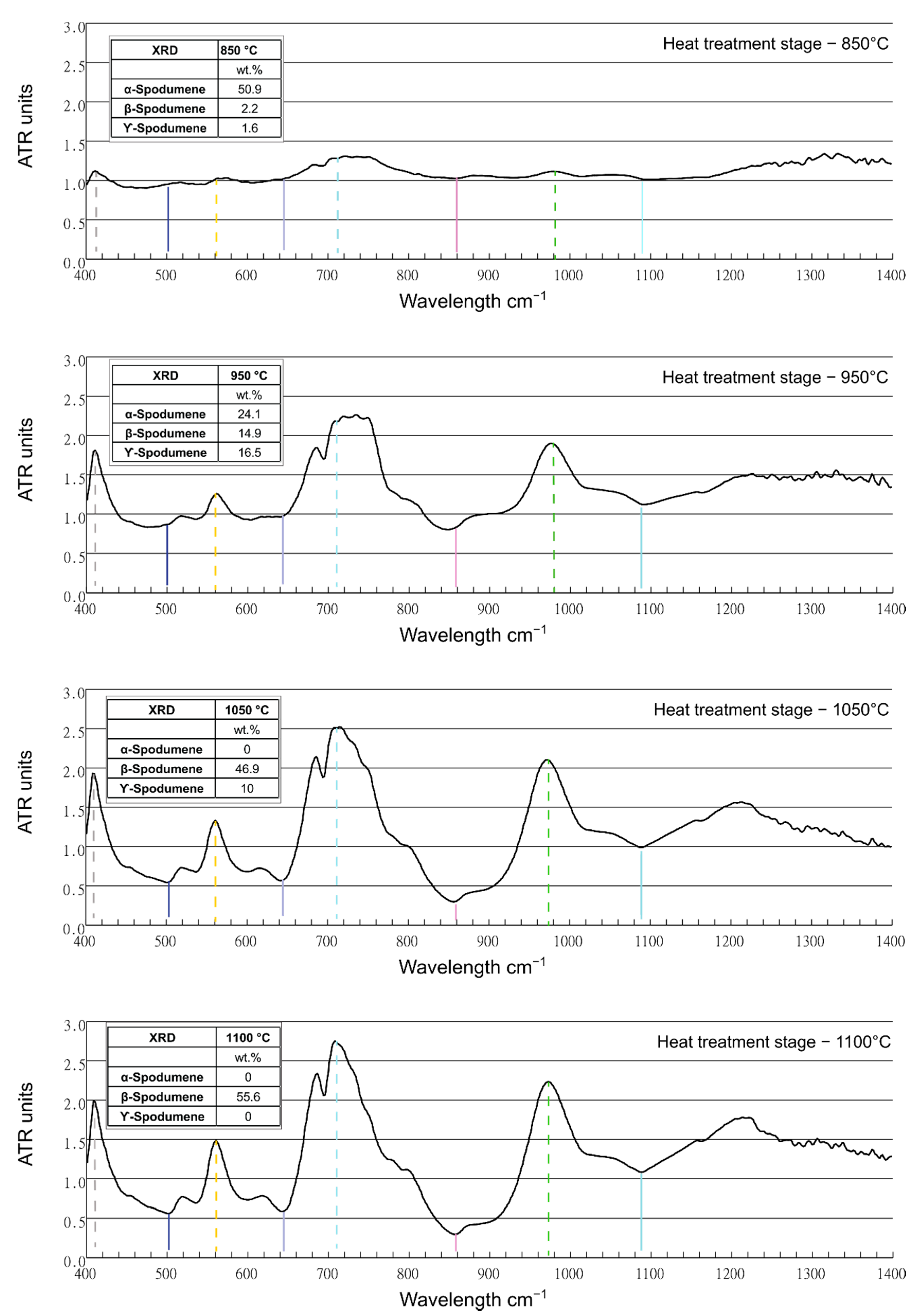
3.2. FTIR Analysis of Spodumene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Summaries, M.C. Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2021; p. 200. [Google Scholar]
- Projection Total Lithium Demand Globally 2030. Available online: https://www.statista.com/statistics/452025/projected-total-demand-for-lithium-globally (accessed on 15 December 2021).
- Li, H.; Eksteen, J.; Kuang, G. Recovery of Lithium from Mineral Resources: State-of-the-Art and Perspectives—A Review. Hydrometallurgy 2019, 189, 105129. [Google Scholar] [CrossRef]
- Li, C.-T.; Peacor, D.R. The Crystal Structure of LiAlSi206-II (“P Spodumene”). Z. Für Krist. 1968, 126, 46–65. [Google Scholar] [CrossRef]
- Dessemond, C.; Soucy, G.; Harvey, J.-P.; Ouzilleau, P. Phase Transitions in the α–γ–β Spodumene Thermodynamic System and Impact of γ-Spodumene on the Efficiency of Lithium Extraction by Acid Leaching. Minerals 2020, 10, 519. [Google Scholar] [CrossRef]
- Peltosaari, O.; Tanskanen, P.; Heikkinen, E.-P.; Fabritius, T. A→γ→β-Phase Transformation of Spodumene with Hybrid Microwave and Conventional Furnaces. Miner. Eng. 2015, 82, 54–60. [Google Scholar] [CrossRef]
- Dessemond, C.; Lajoie-Leroux, F.; Soucy, G.; Laroche, N.; Magnan, J.-F. Spodumene: The Lithium Market, Resources and Processes. Minerals 2019, 9, 334. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.L.; Mann, J.P.; Montoya, A.; Haynes, B.S. In Situ Synchrotron XRD Analysis of the Kinetics of Spodumene Phase Transitions. Phys. Chem. Chem. Phys. 2018, 20, 10753–10761. [Google Scholar] [CrossRef]
- Barbosa, L.I.; Valente, G.; Orosco, R.P.; González, J.A. Lithium Extraction from β-Spodumene through Chlorination with Chlorine Gas. Miner. Eng. 2014, 56, 29–34. [Google Scholar] [CrossRef]
- Fosu, A.Y.; Kanari, N.; Bartier, D.; Hodge, H.; Vaughan, J.; Chagnes, A. Physico-Chemical Characteristics of Spodumene Concentrate and Its Thermal Transformations. Materials 2021, 14, 7423. [Google Scholar] [CrossRef]
- Kuang, G.; Chen, Z.B.; Guo, H.; Li, M.H. Lithium Extraction Mechanism from α-Spodumene by Fluorine Chemical Method. AMR 2012, 524–527, 2011–2016. [Google Scholar] [CrossRef]
- Xia, L.; Wen, G.; Song, L.; Wang, X. Sol–Gel Synthesis and Crystallization Behaviour of β-Spodumene. J. Sol-Gel Sci. Technol. 2009, 52, 134–139. [Google Scholar] [CrossRef]
- Shu, K.; Xu, L.; Wu, H.; Tang, Z.; Luo, L.; Yang, J.; Xu, Y.; Feng, B. Selective Flotation Separation of Spodumene from Feldspar Using Sodium Alginate as an Organic Depressant. Sep. Purif. Technol. 2020, 248, 117122. [Google Scholar] [CrossRef]
- Jarvis, I.; Jarvis, K.E. Inductively Coupled Plasma-Atomic Emission Spectrometry in Exploration Geochemistry. J. Geochem. Explor. 1992, 44, 139–200. [Google Scholar] [CrossRef]
- Post, J.E.; Bish, D.L. Rietveld Refinement of Crystal Structures Using Powder X-Ray Diffraction Data. Mineral. Soc. Am. 1989, 20, 277–308. [Google Scholar]
- Kaufhold, S.; Hein, M.; Dohrmann, R.; Ufer, K. Quantification of the Mineralogical Composition of Clays Using FTIR Spectroscopy. Vib. Spectrosc. 2012, 59, 29–39. [Google Scholar] [CrossRef]
- Abdullah, A.A.; Oskierski, H.C.; Altarawneh, M.; Senanayake, G.; Lumpkin, G.; Dlugogorski, B.Z. Phase Transformation Mechanism of Spodumene during Its Calcination. Miner. Eng. 2019, 140, 105883. [Google Scholar] [CrossRef]
- Calvert, C.S.; Palkowsky, D.A.; Pevear, D.R. A Combined X-Ray Powder Diffraction and Chemical Method for the Quantitative Mineral Analysis of Geologic Samples. In Quantitative Mineral Analysis of Clays; Clay Minerals Society Workshop Lectures: Chantilly, VA, USA, 1989; ISBN 978-1-881208-21-1. [Google Scholar]
- Bish, D.L.; Post, J.E. Quantitative Mineralogical Analysis Using the Rietveld Full-Pattern Fitting Method. Am. Mineral. 1993, 78, 932–940. [Google Scholar]
- Lemière, B.; Uvarova, Y.A. New Developments in Field-Portable Geochemical Techniques and on-Site Technologies and Their Place in Mineral Exploration. Geochem. Explor. Environ. Anal. 2020, 20, 205–216. [Google Scholar] [CrossRef]
- Mathia, E.; Ratcliffe, K.; Wright, M. Brittleness Index—A Parameter to Embrace or Avoid? In Proceedings of the 4th Unconventional Resources Technology Conference; American Association of Petroleum Geologists, San Antonio, TX, USA, 1–3 August 2016. [Google Scholar]
- Montgomery, P.; Ratcliffe, K.; Bell, C.R.; Locklair, R. System and Method for Determining Stratigraphic Location and Areal Extent of Total Organic Carbon Using an Integrated Stratigraphic Approach 2019. U.S. Patent No. 10,365,261, 30 July 2019. [Google Scholar]
- Dehaine, Q.; Tijsseling, L.T.; Rollinson, G.K.; Buxton, M.W.N.; Glass, H.J. Geometallurgical Characterisation with Portable FTIR: Application to Sediment-Hosted Cu-Co Ores. Minerals 2022, 12, 15. [Google Scholar] [CrossRef]
- Murthy, M.K.; Kirby, E.M. Infrared Study of Compounds and Solid Solutions in the System Lithia-Alumina-Silica. J. Am. Ceram. Soc. 1962, 45, 324–329. [Google Scholar] [CrossRef]
- Yan, W.; Liu, D.; Tan, D.; Yuan, P.; Chen, M. FTIR Spectroscopy Study of the Structure Changes of Palygorskite under Heating. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1052–1057. [Google Scholar] [CrossRef]
- Nayak, P.S.; Singh, B.K. Instrumental Characterization of Clay by XRF, XRD and FTIR. Bull. Mater. Sci. 2007, 30, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Database of Raman Spectroscopy, X-ray Diffraction and Chemistry of Minerals. Available online: https://rruff.info/ (accessed on 20 December 2021).
- Moore, R.L.; Haynes, B.S.; Montoya, A. Effect of the Local Atomic Ordering on the Stability of β-Spodumene. Inorg. Chem. 2016, 55, 6426–6434. [Google Scholar] [CrossRef] [PubMed]
- Ncube, T.; Oskierski, H.; Senanayake, G.; Dlugogorski, B.Z. Two-Step Reaction Mechanism of Roasting Spodumene with Potassium Sulfate. Inorg. Chem. 2021, 60, 3620–3625. [Google Scholar] [CrossRef] [PubMed]



| Al2O3 | SiO2 | TiO2 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | Sc | Y | S | Li | LOI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | % | % | % | ppm | ppm | ppm | ppm | % | |
| ICP-OES | 16.9 | 78.3 | 0.02 | 0.21 | 0.15 | 0.29 | 0.35 | 0.36 | 0.16 | 0.04 | 8.09 | 24.5 | 272 | 22,485 | n.d. |
| XRF | 16.9 | 76.6 | <0.01 | 0.08 | 0.17 | 0.11 | 0.07 | 0.34 | 0.12 | 0.04 | n.d. | n.d. | 19.1 | n.d. | 0.46 |
| ICP-MS | Be | V | Cr | Co | Ni | Cu | Zn | Ga | Rb | Sr | Y | Zr | Nb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | |
| 7.32 | 3.56 | 54.7 | 0.69 | 32.5 | 7.71 | 13.4 | 77.4 | 22.9 | 12.9 | 24.8 | 355 | 10.3 | |
| Mo | Sn | Cs | Ba | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | |
| ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | |
| 6.78 | 367 | 20.8 | 91.4 | 1.12 | 1.98 | 0.25 | 0.86 | 0.19 | 0.04 | 0.18 | 0.03 | 0.16 | |
| Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Tl | Pb | Th | U | ||
| ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ||
| 0.03 | 0.06 | 0.01 | 0.07 | 0.01 | 8.32 | 5.81 | 1.35 | 0.09 | 3.06 | 0.40 | 0.44 |
| XRD | 25 °C | 850 °C | 950 °C | 1050 °C | 1100 °C |
|---|---|---|---|---|---|
| wt.% | wt.% | wt.% | wt.% | wt.% | |
| α-Spodumene | 57.8 | 50.9 | 24.1 | 0.0 | 0.0 |
| β-Spodumene | 0.0 | 2.2 | 14.9 | 46.9 | 55.6 |
| γ-Spodumene | 0.0 | 1.6 | 16.5 | 10.0 | 0.0 |
| Quartz | 40.4 | 43.9 | 42.7 | 41.7 | 43.1 |
| Albite | 1.8 | 1.4 | 1.8 | 1.4 | 1.3 |
| Wavenumber cm−1 | 25 °C | 850 °C | 950 °C | 1050 °C | 1100 °C | Mineral Phase * |
|---|---|---|---|---|---|---|
| 425–450 | - | - | (sh) | (sh) | (sh) | (γ, β)-Spd |
| 450 | 0.234 | 0.215 | 0.214 | 0.173 | 0.185 | Spd, Qtz |
| 518 | (sh) | (sh) | (sh) | 0.073 | 0.077 | Spd, Qtz |
| 555 | - | - | - | (sh) | (sh) | β-Spd |
| 593 | 0.08 | 0.079 | 0.07 | (sh) | (sh) | Spd |
| 641 | 0.066 | 0.067 | 0.063 | - | - | (α, γ)-Spd |
| 695 | 0.033 | 0.039 | 0.05 | 0.06 | 0.07 | Qtz |
| 779 | 0.09 | 0.1 | 0.11 | 0.1 | 0.11 | Qtz |
| 797 | 0.09 | 0.11 | 0.11 | 0.1 | 0.1 | Qtz |
| 860 | 0.99 | 0.098 | (sh) | - | - | α, γ-Spd |
| 919 | 0.141 | 0.146 | (sh) | - | - | α, γ-Spd |
| 970–1020 (1011) | (sh) | (sh) (0.293) | (sh) (0.316) | β-Spd | ||
| 1060 | 0.246 | 0.263 | 0.312 | 0.280 | 0.304 | Qtz |
| 1072 | (sh) | (sh) | - | - | - | α-Spd |
| 1085 | (sh) | (sh) | (sh) | - | - | Qtz |
| 1163 | 0.10 | 0.10 | 0.12 | 0.13 | 0.14 | Qtz |
| α-Spodumene | 57.8 | 50.9 | 24.1 | 0 | 0 | |
| β-Spodumene | 0 | 2.2 | 14.9 | 46.9 | 55.6 | |
| γ-Spodumene | 0 | 1.6 | 16.5 | 10 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardisco, L.; Hannula, P.-M.; Pearce, T.J.; Morgan, L. Multi-Technique Analytical Approach to Quantitative Analysis of Spodumene. Minerals 2022, 12, 175. https://doi.org/10.3390/min12020175
Sardisco L, Hannula P-M, Pearce TJ, Morgan L. Multi-Technique Analytical Approach to Quantitative Analysis of Spodumene. Minerals. 2022; 12(2):175. https://doi.org/10.3390/min12020175
Chicago/Turabian StyleSardisco, Lorenza, Pyry-Mikko Hannula, Tim J. Pearce, and Luke Morgan. 2022. "Multi-Technique Analytical Approach to Quantitative Analysis of Spodumene" Minerals 12, no. 2: 175. https://doi.org/10.3390/min12020175
APA StyleSardisco, L., Hannula, P.-M., Pearce, T. J., & Morgan, L. (2022). Multi-Technique Analytical Approach to Quantitative Analysis of Spodumene. Minerals, 12(2), 175. https://doi.org/10.3390/min12020175






