Cu Dynamics in the Rhizosphere of Native Tropical Species: Assessing the Potential for Phytostabilization in Mining-Impacted Soils
Abstract
:1. Introduction
2. Materials and Methods
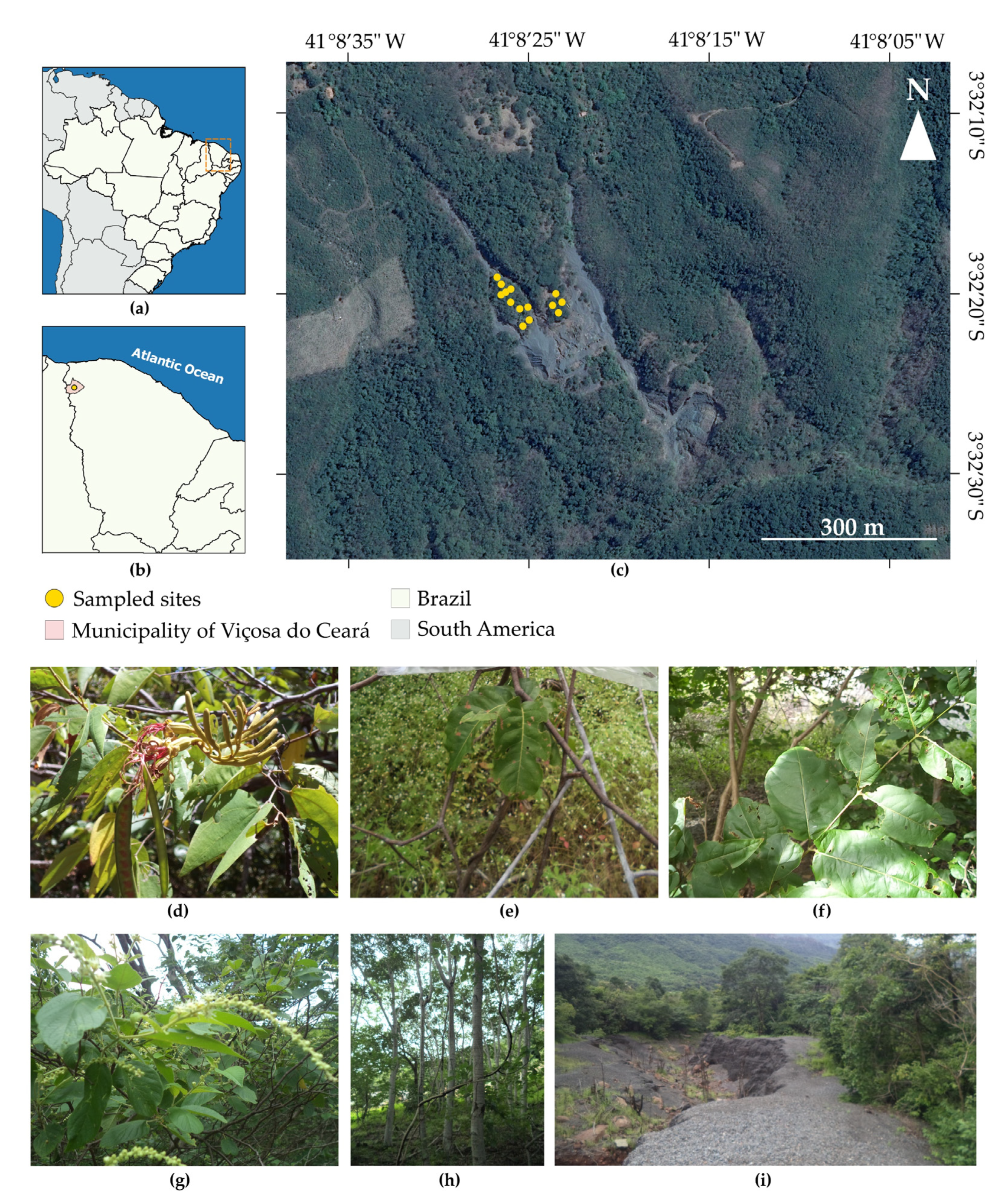
2.1. Site Description
2.2. Plant and Soil Sampling
2.3. Chemical Analyses: pH, Organic Matter Content, and Cu Solid-Phase Fractionation
- Exchangeable or soluble (CuEX)–extracted using a MgCl2 1 mol L−1 solution at a pH 7.0.
- Associated with carbonates (CuCAR)–extracted with a 1 mol L−1 NaOAC solution at a pH 5.0.
- Associated with organic matter (CuOM) extracted with 6% NaOCl at a pH 8.0;
- Associated with amorphous iron oxides (CuAM), extracted with an oxalic acid solution (0.2 mol L−1) + ammonium oxalate (0.2 mol L−1) at a pH 3.0;
- mol L oxides (CuOX), extracted with sodium bicarbonate (0.25 mol L−1) + sodium bicarbonate (0.11 mol L−1) + 3 g sodium dithionite;
- Associated with sulfides (CuS), extracted with a 4 mol L−1 HNO3 solution;
- Residual Cu (CuRES) was extracted by triacid digestion (HCl + HNO3 and HF) during microwave-assisted digestion.
2.4. Plant Tissues Digestion and Factors Calculation
2.5. Determination of Cu Concentrations and Quality Procedures
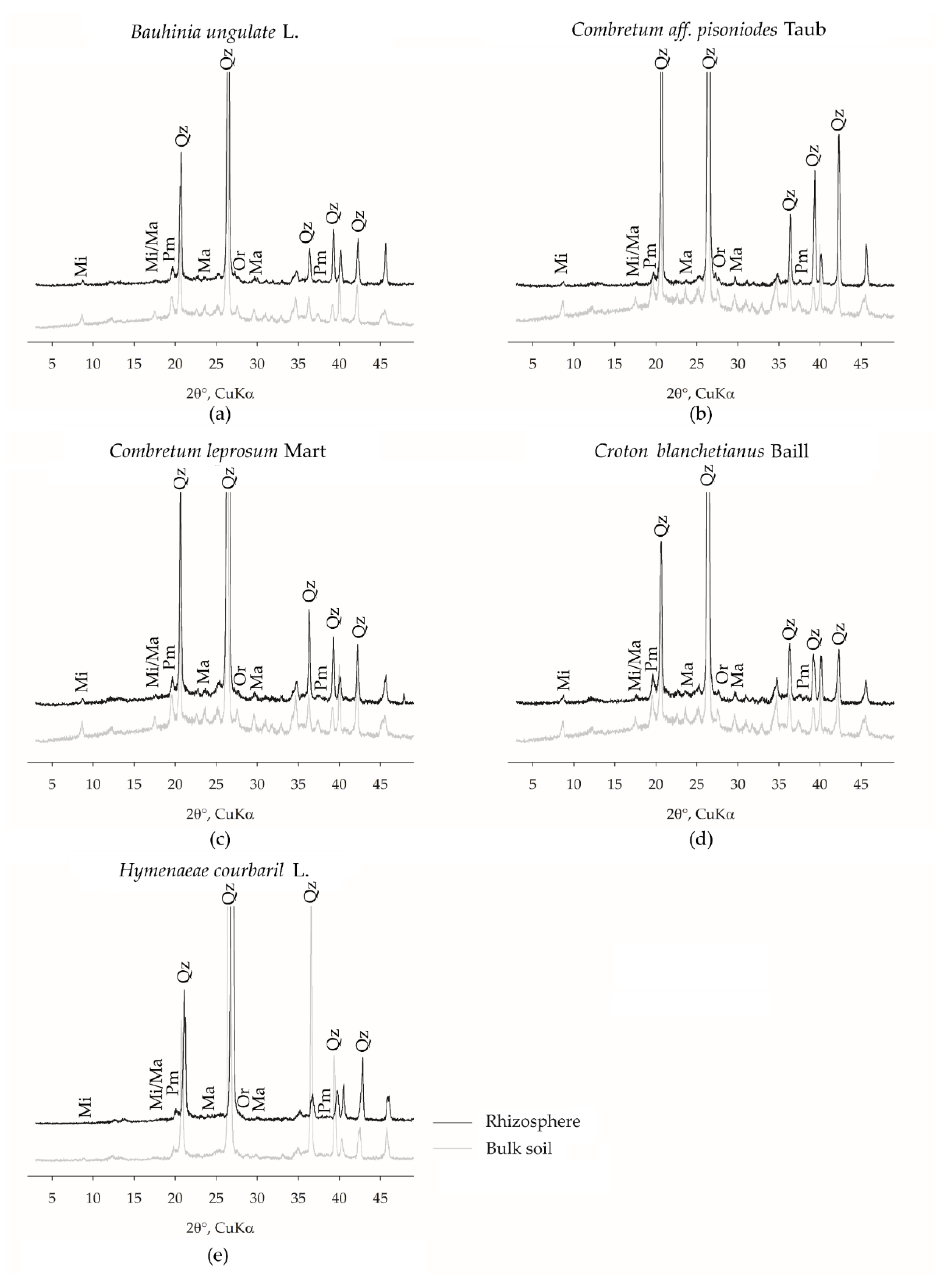
2.6. Mineralogical Characterization
2.7. Statistical Analysis
3. Results and Discussions
3.1. Plant Species Effects on Cooper Geochemistry in the Bulk and Rhizospheric Soils
3.2. Cooper Content in Plants Tissues and their Potential to Phytoremediation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akala, V.A.; Lal, R. Soil Organic Carbon Pools and Sequestration Rates in Reclaimed Minesoils in Ohio. J. Environ. Qual. 2001, 30, 2098–2104. [Google Scholar] [CrossRef]
- Barrutia, O.; Artetxe, U.; Hernández, A.; Olano, J.M.; García-Plazaola, J.I.; Garbisu, C.; Becerril, J.M. Native Plant Communities in an Abandoned Pb-Zn Mining Area of Northern Spain: Implications for Phytoremediation and Germplasm Preservation. Int. J. Phytoremediation 2011, 13, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Asensio, V.; Covelo, E.F.; Kandeler, E. Soil management of copper mine tailing soils—Sludge amendment and tree vegetation could improve biological soil quality. Sci. Total Environ. 2013, 456–457, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Perlatti, F.; Ferreira, T.O.; Romero, R.E.; Costa, M.C.G.; Otero, X.L. Copper accumulation and changes in soil physical-chemical properties promoted by native plants in an abandoned mine site in northeastern Brazil: Implications for restoration of mine sites. Ecol. Eng. 2015, 82, 103–111. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, Z.; Niu, S.; Li, X.; Wang, Y.; Bai, Z. Reclamation promotes the succession of the soil and vegetation in opencast coal mine: A case study from Robinia pseudoacacia reclaimed forests, Pingshuo mine, China. CATENA 2018, 165, 72–79. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Nieto, J.M.; Sarmiento, A.M.; Olías, M.; Canovas, C.R.; Riba, I.; Kalman, J.; Delvalls, T.A. Acid mine drainage pollution in the Tinto and Odiel rivers (Iberian Pyrite Belt, SW Spain) and bioavailability of the transported metals to the Huelva Estuary. Environ. Int. 2007, 33, 445–455. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Palutoglu, M.; Akgul, B.; Suyarko, V.; Yakovenko, M.; Kryuchenko, N.; Sasmaz, A. Phytoremediation of Cadmium by Native Plants Grown on Mining Soil. Bull. Environ. Contam. Toxicol. 2018, 100, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.J.M.; McGrath, S.P.; Reeves, R.D.; Smith, J.A.C. Metal Hyperaccumulator Plants: A Review of the Ecology and Physiology of a Biological Resource for Phytoremediation of Metal-Polluted Soils. In Phytoremediation of Contaminated Soil and Water; CRC-Press: Boca Raton, FL, USA, 2000; p. 23. ISBN 9780367803148. [Google Scholar]
- Gleba, D.; Borisjuk, N.V.; Borisjuk, L.G.; Kneer, R.; Poulev, A.; Skarzhinskaya, M.; Dushenkov, S.; Logendra, S.; Gleba, Y.Y.; Raskin, I. Use of plant roots for phytoremediation and molecular farming. Proc. Natl. Acad. Sci. USA 1999, 96, 5973–5977. [Google Scholar] [CrossRef] [Green Version]
- Baker, A.J.M. Accumulators and excluders -strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Barceló, J.; Poschenrieder, C. Phytoremediation: Principles and perspectives. Contrib. Sci. 2003, 2, 333–344. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Garbisu, C.; Alkorta, I. Phytoextraction: A cost-effective plant-based technology for the removal of metals from the environment. Bioresour. Technol. 2001, 77, 229–236. [Google Scholar] [CrossRef]
- Bolan, N.S.; Park, J.H.; Robinson, B.; Naidu, R.; Huh, K.Y. Phytostabilization: A Green Approach to Contaminant Containment. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 145–204. ISBN 9780123855381. [Google Scholar]
- Pereira, B.F.F.; de Abreu, C.A.; Herpin, U.; de Abreu, M.F.; Berton, R.S. Phytoremediation of lead by jack beans on a Rhodic Hapludox amended with EDTA. Sci. Agric. 2010, 67, 308–318. [Google Scholar] [CrossRef] [Green Version]
- de Paiva, H.N.; de Carvalho, J.G.; Siqueira, J.O. Effect of the cadmium application on nutrients content in cedro (cedrela fissilis vell.) Seedlings. Ciência Florest. 2001, 11, 153. [Google Scholar] [CrossRef] [Green Version]
- Mench, M.; Lepp, N.; Bert, V.; Schwitzguébel, J.-P.; Gawronski, S.W.; Schröder, P.; Vangronsveld, J. Successes and limitations of phytotechnologies at field scale: Outcomes, assessment and outlook from COST Action 859. J. Soils Sediments 2010, 10, 1039–1070. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—A review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Bidone, E.; Cesar, R.; Santos, M.C.; Sierpe, R.; Silva-Filho, E.V.; Kutter, V.; Dias da Silva, L.I.; Castilhos, Z. Mass balance of arsenic fluxes in rivers impacted by gold mining activities in Paracatu (Minas Gerais State, Brazil). Environ. Sci. Pollut. Res. 2018, 25, 9085–9100. [Google Scholar] [CrossRef] [PubMed]
- Adler Miserendino, R.; Guimarães, J.R.D.; Schudel, G.; Ghosh, S.; Godoy, J.M.; Silbergeld, E.K.; Lees, P.S.J.; Bergquist, B.A. Mercury Pollution in Amapá, Brazil: Mercury Amalgamation in Artisanal and Small-Scale Gold Mining or Land-Cover and Land-Use Changes? ACS Earth Space Chem. 2018, 2, 441–450. [Google Scholar] [CrossRef]
- Ericsson, M.; Löf, O. Mining’s contribution to national economies between 1996 and 2016. Miner. Econ. 2019, 32, 223–250. [Google Scholar] [CrossRef] [Green Version]
- Afonso, T.F.; Demarco, C.F.; Pieniz, S.; Camargo, F.A.O.; Quadro, M.S.; Andreazza, R. Potential of Solanum viarum Dunal in use for phytoremediation of heavy metals to mining areas, southern Brazil. Environ. Sci. Pollut. Res. 2019, 26, 24132–24142. [Google Scholar] [CrossRef]
- Asensio, V.; Flórido, F.G.; Ruiz, F.; Perlatti, F.; Otero, X.L.; Ferreira, T.O. Screening of native tropical trees for phytoremediation in copper-polluted soils. Int. J. Phytoremediation 2018, 20, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Sparovek, G.; De Jong Van Lier, Q.; Dourado Neto, D. Computer assisted Koeppen climate classification: A case study for Brazil. Int. J. Climatol. 2007, 27, 257–266. [Google Scholar] [CrossRef]
- Perlatti, F.; Martins, E.P.; de Oliveira, D.P.; Ruiz, F.; Asensio, V.; Rezende, C.F.; Otero, X.L.; Ferreira, T.O. Copper release from waste rocks in an abandoned mine (NE, Brazil) and its impacts on ecosystem environmental quality. Chemosphere 2021, 262, 127843. [Google Scholar] [CrossRef] [PubMed]
- Perlatti, F.; Otero, X.L.; Macias, F.; Ferreira, T.O. Geochemical speciation and dynamic of copper in tropical semi-arid soils exposed to metal-bearing mine wastes. Sci. Total Environ. 2014, 500–501, 91–102. [Google Scholar] [CrossRef]
- Perlatti, F.; Ferreira, T.O.; da Costa Roberto, F.A.; Romero, R.E.; Sartor, L.R.; Otero, X.L. Trace metal/metalloid concentrations in waste rock, soils and spontaneous plants in the surroundings of an abandoned mine in semi-arid NE-Brazil. Environ. Earth Sci. 2015, 74, 5427–5441. [Google Scholar] [CrossRef]
- Hinsinger, P. How Do Plant Roots Acquire Mineral Nutrients? Chemical Processes Involved in the Rhizosphere. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 1998; Volume 64, pp. 225–265. [Google Scholar]
- Otero, X.L.; Álvarez, E.; Fernández-Sanjurjo, M.J.; Macías, F. Micronutrients and toxic trace metals in the bulk and rhizospheric soil of the spontaneous vegetation at an abandoned copper mine in Galicia (NW Spain). J. Geochemical Explor. 2012, 112, 84–92. [Google Scholar] [CrossRef]
- Silveira, M.L.; Alleoni, L.R.F.; O’Connor, G.A.; Chang, A.C. Heavy metal sequential extraction methods—A modification for tropical soils. Chemosphere 2006, 64, 1929–1938. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 3540312102. [Google Scholar]
- Gimeno-García, E.; Andreu, V.; Boluda, R. Distribution of heavy metals in rice farming soils. Arch. Environ. Contam. Toxicol. 1995, 29, 476–483. [Google Scholar] [CrossRef]
- Siregar, A.; Kleber, M.; Mikutta, R.; Jahn, R. Sodium hypochlorite oxidation reduces soil organic matter concentrations without affecting inorganic soil constituents. Eur. J. Soil Sci. 2005, 56, 481–490. [Google Scholar] [CrossRef]
- Zasoski, R.J.; Burau, R.G. A rapid nitric-perchloric acid digestion method for multi-element tissue analysis. Commun. Soil Sci. Plant Anal. 1977, 8, 425–436. [Google Scholar] [CrossRef]
- Sekabira, K.; Oryem-Origa, H.; Mutumba, G.; Kakudidi, E.; Basamba, T.A. Heavy metal phytoremediation by Commelina benghalensis (L) and Cynodon dactylon (L) growing in urban stream sediments. Int. J. Plant Physiol. Biochem. 2011, 3, 133–142. [Google Scholar]
- Sas, L.; Rengel, Z.; Tang, C. Excess cation uptake, and extrusion of protons and organic acid anions by Lupinus albus under phosphorus deficiency. Plant Sci. 2001, 160, 1191–1198. [Google Scholar] [CrossRef]
- Kabas, S.; Saavedra-Mella, F.; Huynh, T.; Kopittke, P.M.; Carter, S.; Huang, L. Metal uptake and organic acid exudation of native Acacia species in mine tailings. Aust. J. Bot. 2017, 65, 357. [Google Scholar] [CrossRef]
- Zeng, F.; Chen, S.; Miao, Y.; Wu, F.; Zhang, G. Changes of organic acid exudation and rhizosphere pH in rice plants under chromium stress. Environ. Pollut. 2008, 155, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Masto, R.E.; Ram, L.C.; Selvi, V.A.; Srivastava, N.K.; Tripathi, R.C.; George, J. Rhizosphere soil microbial index of tree species in a coal mining ecosystem. Soil Biol. Biochem. 2009, 41, 1824–1832. [Google Scholar] [CrossRef]
- Liljeroth, E.; Kuikman, P.; Van Veen, J.A. Carbon translocation to the rhizosphere of maize and wheat and influence on the turnover of native soil organic matter at different soil nitrogen levels. Plant Soil 1994, 161, 233–240. [Google Scholar] [CrossRef]
- Kent, A.D.; Triplett, E.W. Microbial Communities and Their Interactions in Soil and Rhizosphere Ecosystems. Annu. Rev. Microbiol. 2002, 56, 211–236. [Google Scholar] [CrossRef] [PubMed]
- Hinsinger, P.; Plassard, C.; Tang, C.; Jaillard, B. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil 2003, 248, 43–59. [Google Scholar] [CrossRef]
- USDA Soil Survey Manual Agriculture. Handbook 18. USDA Nat. Resour. Conserv. Serv. 2017, 18, 483. [Google Scholar]
- Brunetto, G.; Miotto, A.; Ceretta, C.A.; Schmitt, D.E.; Heinzen, J.; de Moraes, M.P.; Canton, L.; Tiecher, T.L.; Comin, J.J.; Girotto, E. Mobility of copper and zinc fractions in fungicide-amended vineyard sandy soils. Arch. Agron. Soil Sci. 2014, 60, 609–624. [Google Scholar] [CrossRef]
- Meers, E.; Tack, F.M.G.; Van Slycken, S.; Ruttens, A.; Du Laing, G.; Vangronsveld, J.; Verloo, M.G. Chemically Assisted Phytoextraction: A Review of Potential Soil Amendments for Increasing Plant Uptake of Heavy Metals. Int. J. Phytoremediation 2008, 10, 390–414. [Google Scholar] [CrossRef]
- Karlsson, T.; Persson, P.; Skyllberg, U. Complexation of Copper(II) in Organic Soils and in Dissolved Organic Matter—EXAFS Evidence for Chelate Ring Structures. Environ. Sci. Technol. 2006, 40, 2623–2628. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, G.; Guidi, G.; Lubrano, L. Organic matter as an influencing factor on copper and cadmium adsorption by soils. Water Air Soil Pollut. 1978, 9, 263–269. [Google Scholar] [CrossRef]
- Martínez, C.E.; McBride, M.B. Dissolved and Labile Concentrations of Cd, Cu, Pb, and Zn in Aged Ferrihydrite−Organic Matter Systems. Environ. Sci. Technol. 1999, 33, 745–750. [Google Scholar] [CrossRef]
- Gao, P.M.Y. Efficiency of organic ligands in adsorptive dissolution and photoreductive dissolution of hematite. Int. J. Environ. Sci. Technol. 2016, 13, 1195–1206. [Google Scholar] [CrossRef] [Green Version]
- Fritsch, E.; Allard, T.; Benedetti, M.F.; Bardy, M.; do Nascimento, N.R.; Li, Y.; Calas, G. Organic complexation and translocation of ferric iron in podzols of the Negro River watershed. Separation of secondary Fe species from Al species. Geochim. Cosmochim. Acta 2009, 73, 1813–1825. [Google Scholar] [CrossRef]
- Kraemer, S.M. Iron oxide dissolution and solubility in the presence of siderophores. Aquat. Sci.-Res. Bound. 2004, 66, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.R.; Owens, G.; Naidu, R. Effect of Root-Induced Chemical Changes on Dynamics and Plant Uptake of Heavy Metals in Rhizosphere Soils. Pedosphere 2010, 20, 494–504. [Google Scholar] [CrossRef]
- Bravin, M.N.; Garnier, C.; Lenoble, V.; Gérard, F.; Dudal, Y.; Hinsinger, P. Root-induced changes in pH and dissolved organic matter binding capacity affect copper dynamic speciation in the rhizosphere. Geochim. Cosmochim. Acta 2012, 84, 256–268. [Google Scholar] [CrossRef]
- Turpault, M.-P.; Righi, D.; Utérano, C. Clay minerals: Precise markers of the spatial and temporal variability of the biogeochemical soil environment. Geoderma 2008, 147, 108–115. [Google Scholar] [CrossRef]
- April, R.; Keller, D. Mineralogy of the rhizosphere in forest soils of the eastern United States. Biogeochemistry 1990, 9, 1–18. [Google Scholar] [CrossRef]
- Graham, R.C.; Diallo, M.M.; Lund, L.J. Soils and Mineral Weathering on Phyllite Colluvium and Serpentinite in Northwestern California. Soil Sci. Soc. Am. J. 1990, 54, 1682–1690. [Google Scholar] [CrossRef]
- Marie-Pierre, T.; Claude, N.; Christophe, C. Rhizosphere impact on the dissolution of test minerals in a forest ecosystem. Geoderma 2009, 153, 147–154. [Google Scholar] [CrossRef]
- Paz, A.; Gagen, E.J.; Levett, A.; Zhao, Y.; Kopittke, P.M.; Southam, G. Biogeochemical cycling of iron oxides in the rhizosphere of plants grown on ferruginous duricrust (canga). Sci. Total Environ. 2020, 713, 136637. [Google Scholar] [CrossRef]
- Lazo, D.E.; Dyer, L.G.; Alorro, R.D. Silicate, phosphate and carbonate mineral dissolution behaviour in the presence of organic acids: A review. Miner. Eng. 2017, 100, 115–123. [Google Scholar] [CrossRef]
- Gobran, G.R.; Turpault, M.-P.; Courchesne, F. Contribution of rhizospheric processes to mineral weathering in forest soils. In Biogeochemistry of Trace Elements in the Rhizosphere; Elsevier: Amsterdam, The Netherlands, 2005; pp. 3–28. [Google Scholar]
- van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Thornton, I.; Webb, J.S. Trace Elements in Soils and Plants. In Food Chains and Human Nutrition; Springer: Dordrecht, The Netherlands, 1980; pp. 273–315. [Google Scholar]
- Cluis, C. Junk-greedy greens: Phytoremediation as a new option for soil decontamination. BioTeach J. 2004, 2, 61–67. [Google Scholar]
- Chen, J.; Shafi, M.; Li, S.; Wang, Y.; Wu, J.; Ye, Z.; Peng, D.; Yan, W.; Liu, D. Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of Moso bamboo (Phyllostachys pubescens). Sci. Rep. 2015, 5, 13554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radziemska, M.; Vaverková, M.; Baryła, A. Phytostabilization—Management Strategy for Stabilizing Trace Elements in Contaminated Soils. Int. J. Environ. Res. Public Health 2017, 14, 958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhargava, A.; Carmona, F.F.; Bhargava, M.; Srivastava, S. Approaches for enhanced phytoextraction of heavy metals. J. Environ. Manag. 2012, 105, 103–120. [Google Scholar] [CrossRef]
- Vassilev, A.; Schwitzguebél, J.-P.; Thewys, T.; van der Lelie, D.; Vangronsveld, J. The Use of Plants for Remediation of Metal-Contaminated Soils. Sci. World J. 2004, 4, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Rivelli, A.R.; De Maria, S.; Puschenreiter, M.; Gherbin, P. Accumulation of Cadmium, Zinc, and Copper by Helianthus Annuus L.: Impact on Plant Growth and Uptake of Nutritional Elements. Int. J. Phytoremediation 2012, 14, 320–334. [Google Scholar] [CrossRef]

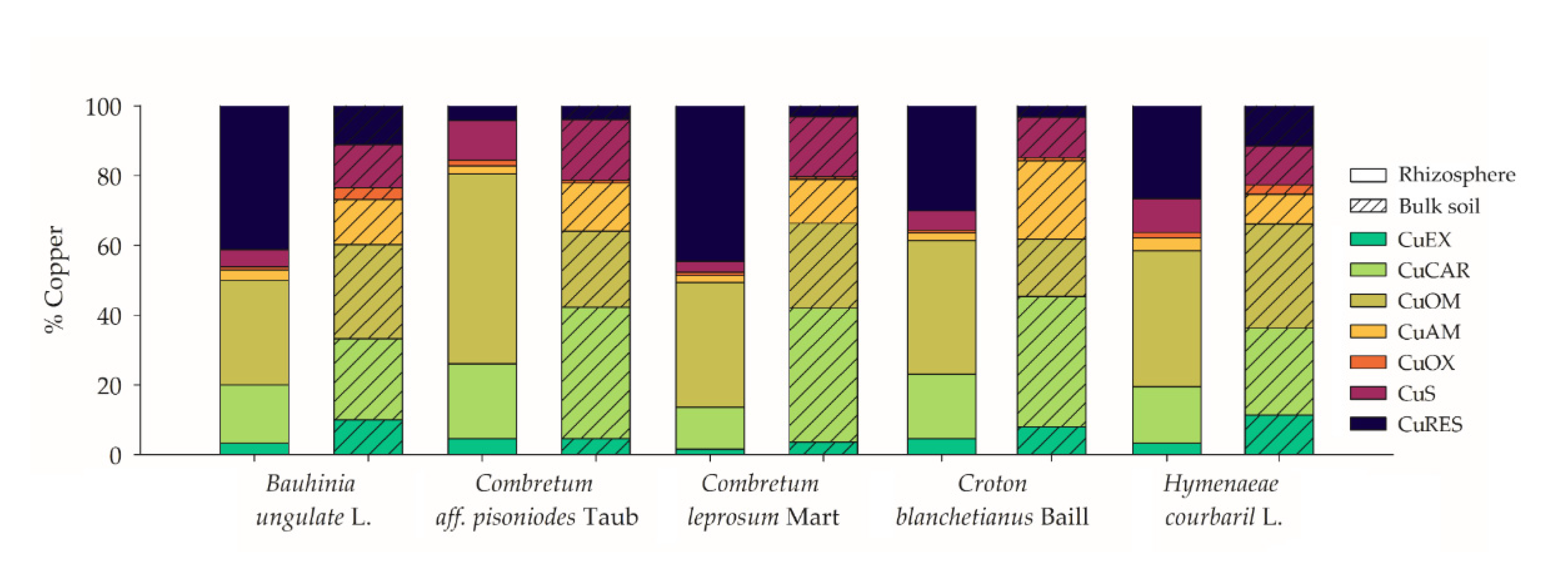
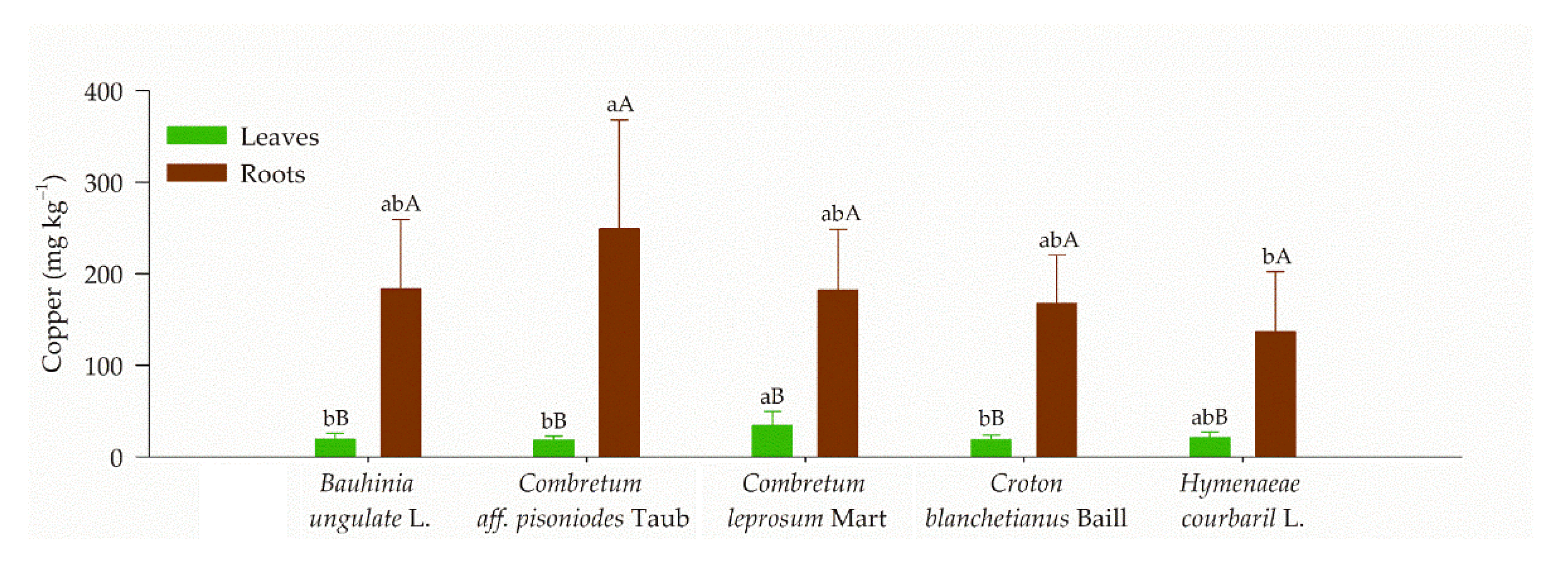
| Plant Specie | Total Cu Content (mg kg−1) | Pseudo Total Cu * (mg kg−1) | BCF Leaves | BCF Roots | TF | |
|---|---|---|---|---|---|---|
| Leaves | Roots | |||||
| Bauhinia ungulate L. | 19 | 183 | 642 | 0.030 | 0.285 | 0.104 |
| Combretum aff. pisoniodes Taub | 18 | 249 | 401 | 0.045 | 0.621 | 0.072 |
| Combretum leprosum Mart | 34 | 182 | 877 | 0.039 | 0.208 | 0.187 |
| Croton blanchetianus Bail | 19 | 168 | 868 | 0.021 | 0.193 | 0.113 |
| Hymenaeae courbaril L. | 21 | 136 | 355 | 0.059 | 0.384 | 0.154 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, D.P.; Queiroz, H.M.; Perlatti, F.; Ferreira, A.D.; Asensio, V.; Nóbrega, G.N.; Otero, X.L.; Ferreira, T.O. Cu Dynamics in the Rhizosphere of Native Tropical Species: Assessing the Potential for Phytostabilization in Mining-Impacted Soils. Minerals 2022, 12, 130. https://doi.org/10.3390/min12020130
de Oliveira DP, Queiroz HM, Perlatti F, Ferreira AD, Asensio V, Nóbrega GN, Otero XL, Ferreira TO. Cu Dynamics in the Rhizosphere of Native Tropical Species: Assessing the Potential for Phytostabilization in Mining-Impacted Soils. Minerals. 2022; 12(2):130. https://doi.org/10.3390/min12020130
Chicago/Turabian Stylede Oliveira, Daniel Pontes, Hermano Melo Queiroz, Fabio Perlatti, Amanda Duim Ferreira, Verónica Asensio, Gabriel Nuto Nóbrega, Xosé Luis Otero, and Tiago Osório Ferreira. 2022. "Cu Dynamics in the Rhizosphere of Native Tropical Species: Assessing the Potential for Phytostabilization in Mining-Impacted Soils" Minerals 12, no. 2: 130. https://doi.org/10.3390/min12020130
APA Stylede Oliveira, D. P., Queiroz, H. M., Perlatti, F., Ferreira, A. D., Asensio, V., Nóbrega, G. N., Otero, X. L., & Ferreira, T. O. (2022). Cu Dynamics in the Rhizosphere of Native Tropical Species: Assessing the Potential for Phytostabilization in Mining-Impacted Soils. Minerals, 12(2), 130. https://doi.org/10.3390/min12020130








