A Novel Dissolution and Synchronous Extraction of Rare Earth Elements from Bastnaesite by a Functionalized Ionic Liquid [Hbet][Tf2N]
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instrumentation and Methods
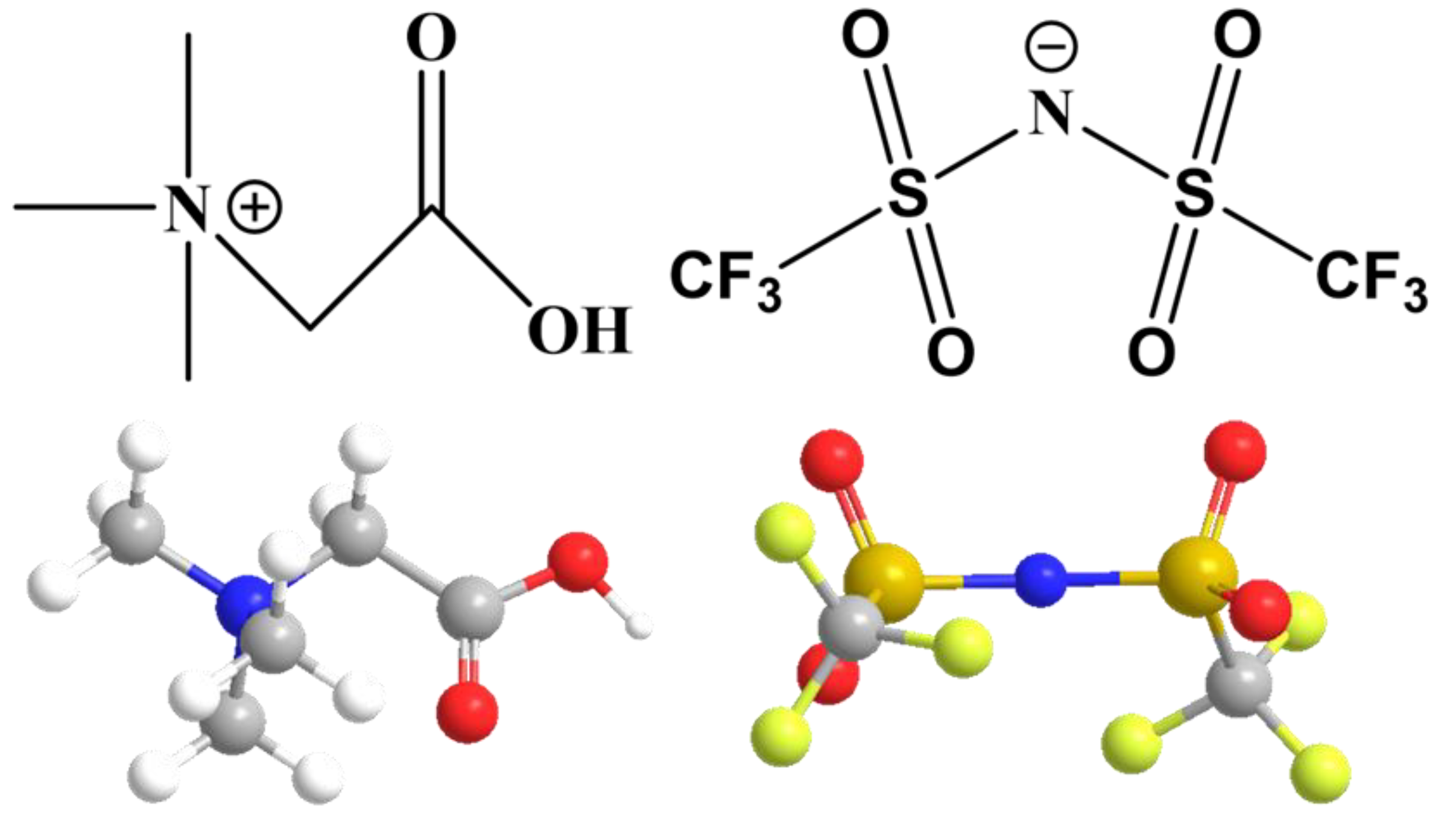
2.3. Synthesis Method of Ionic Liquid
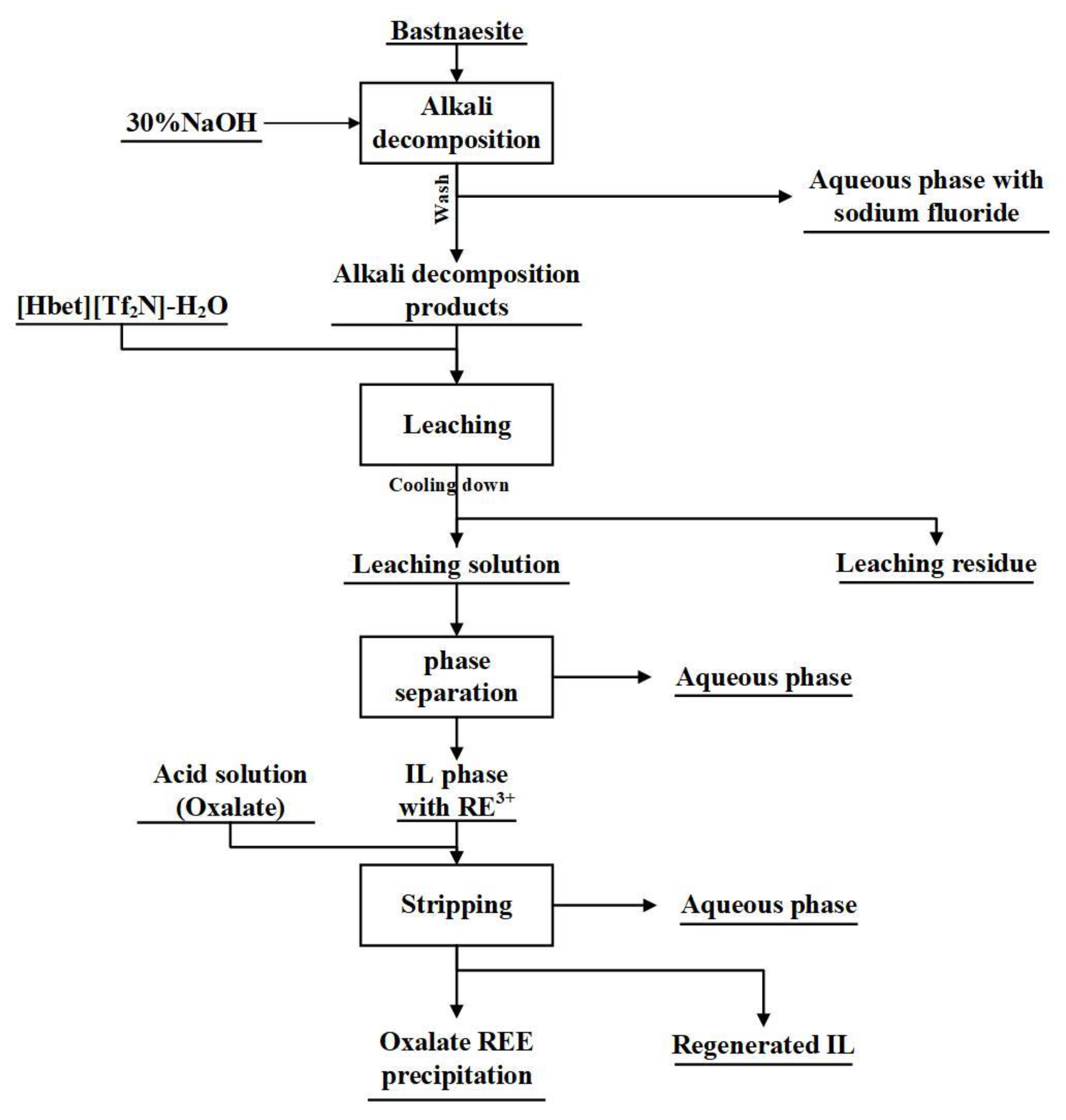
2.4. Experimental Process
2.4.1. Dissolution and Synchronous Extraction of Rare Earth Elements from Bastnaesite
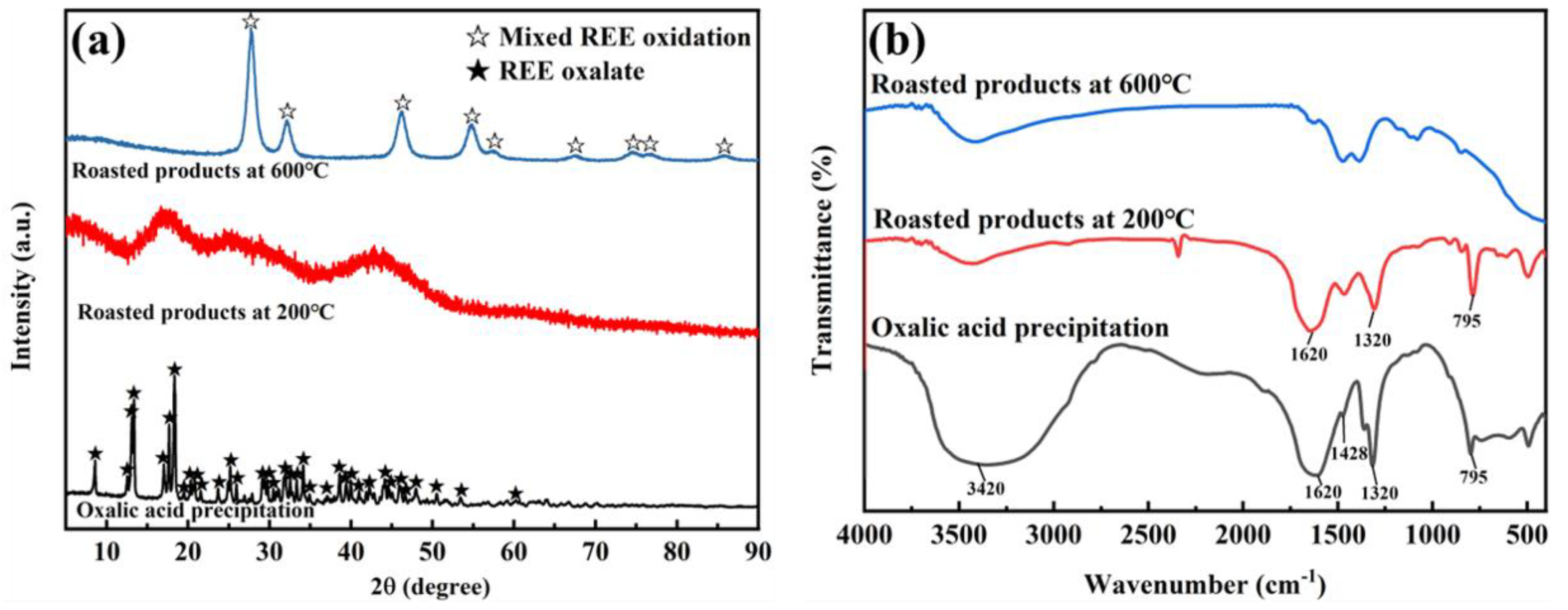
2.4.2. Back Extraction of Rare Earth Elements
3. Results and Discussion
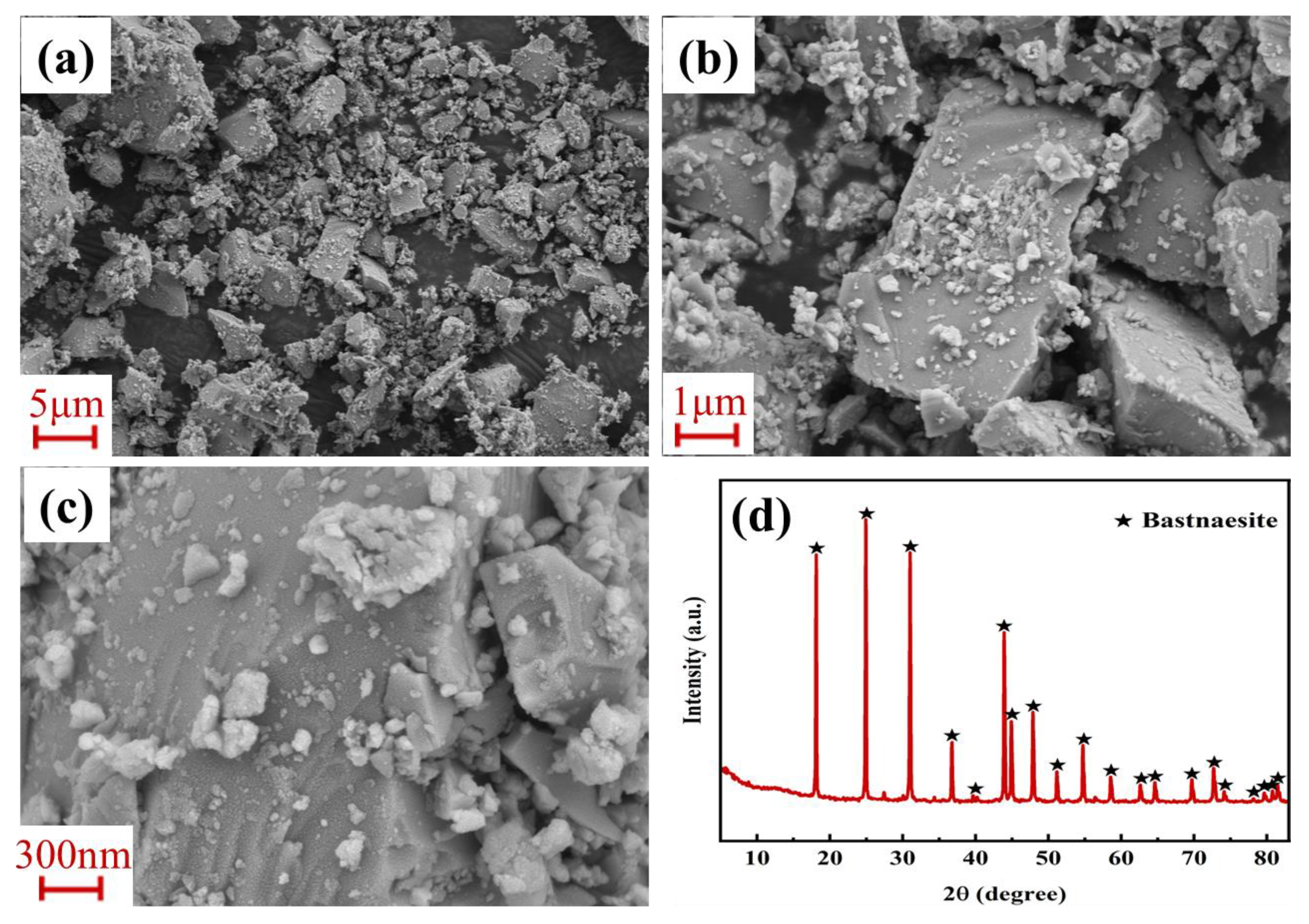
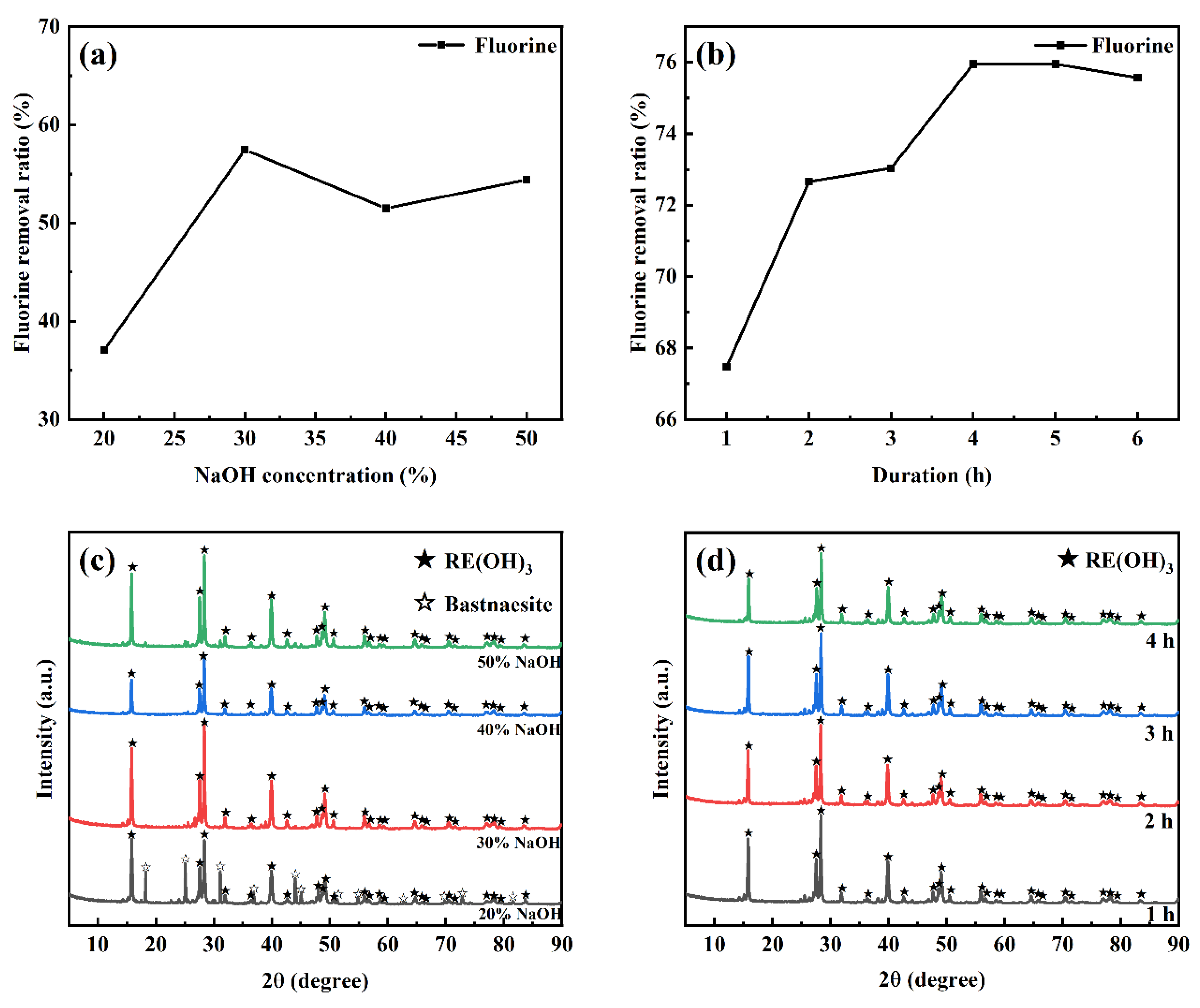
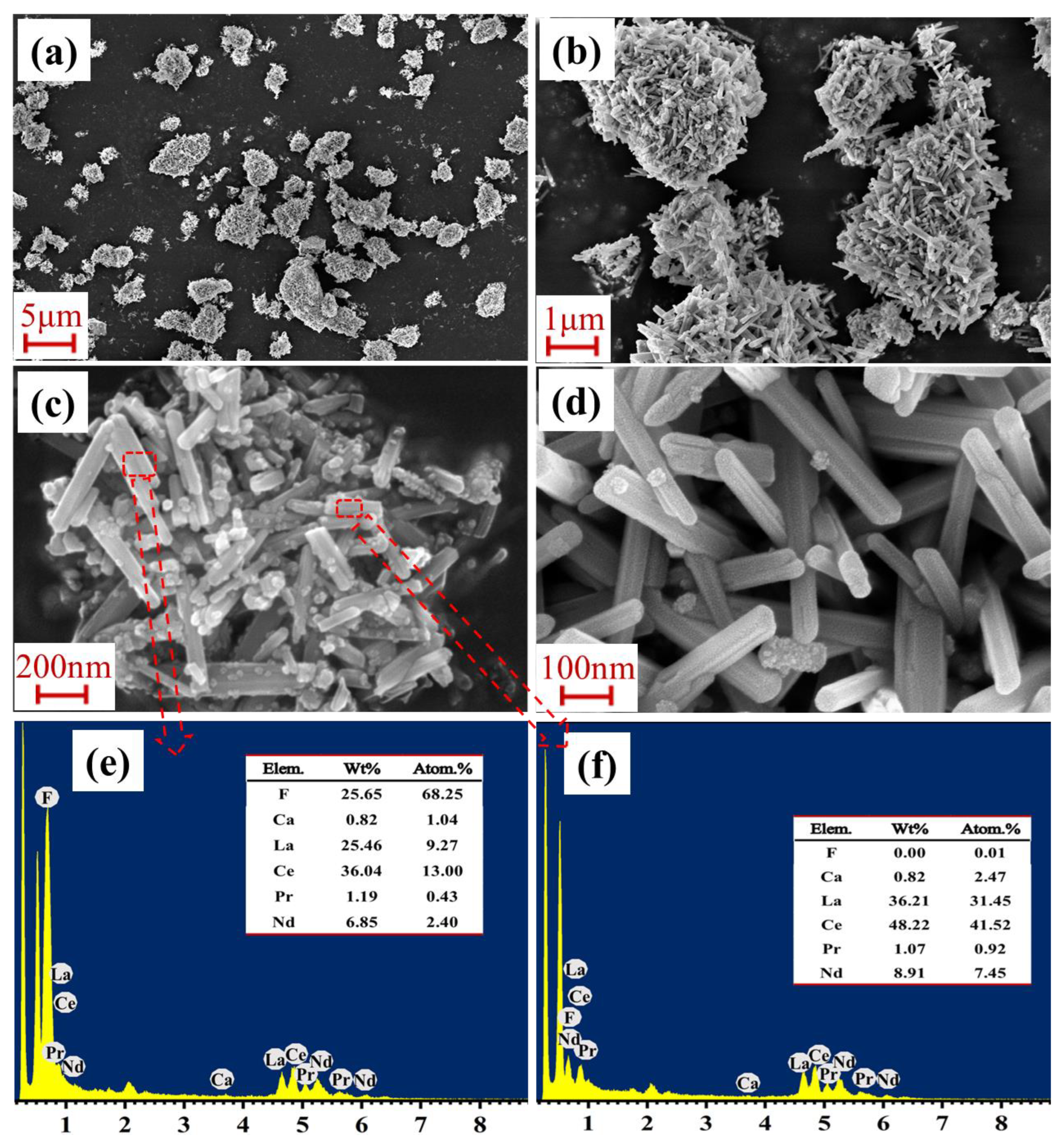
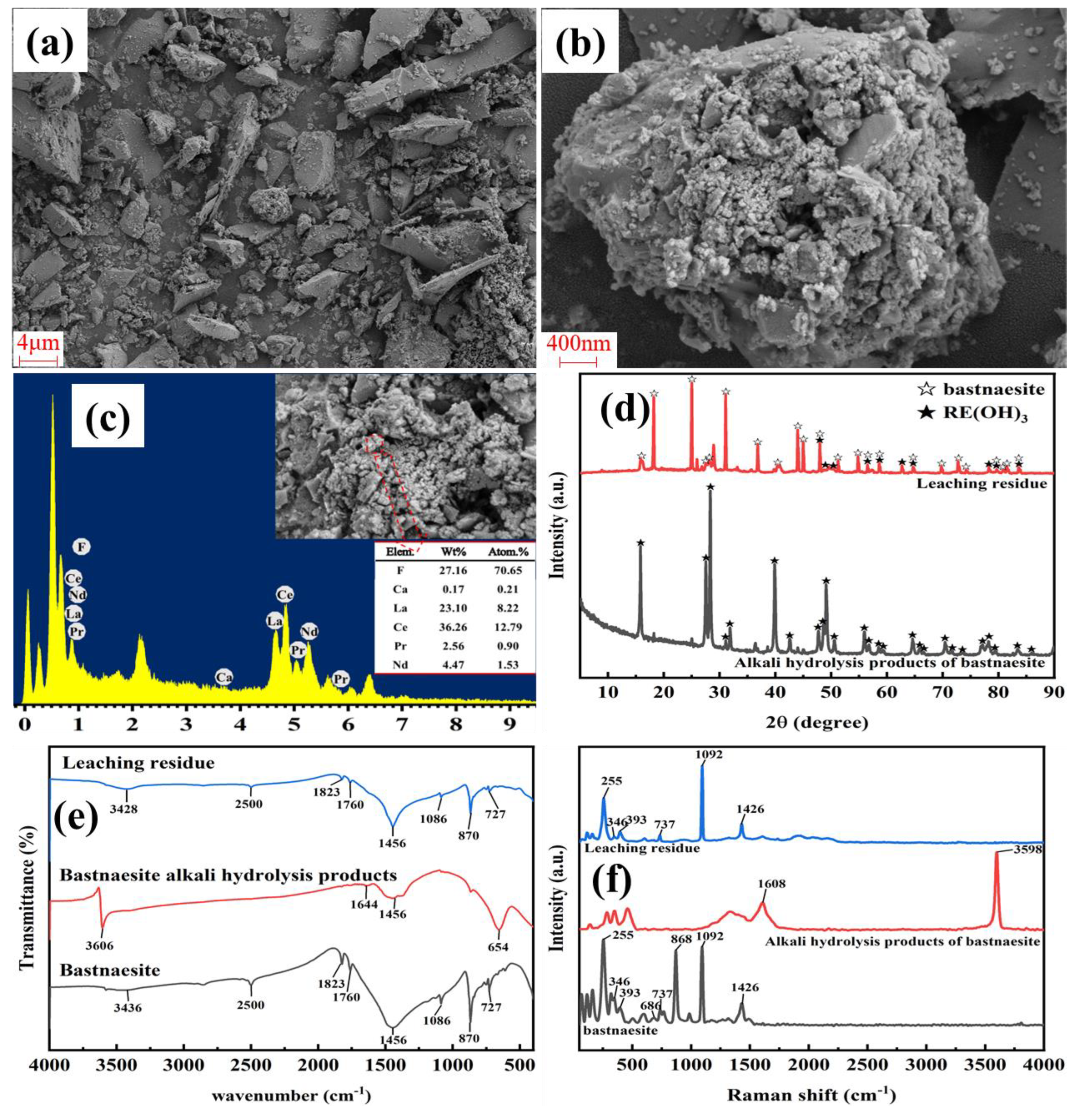
3.1. Alkali Decomposition of Bastnaesite
3.2. Leaching Behavior of REEs from Alkali Decomposition Products
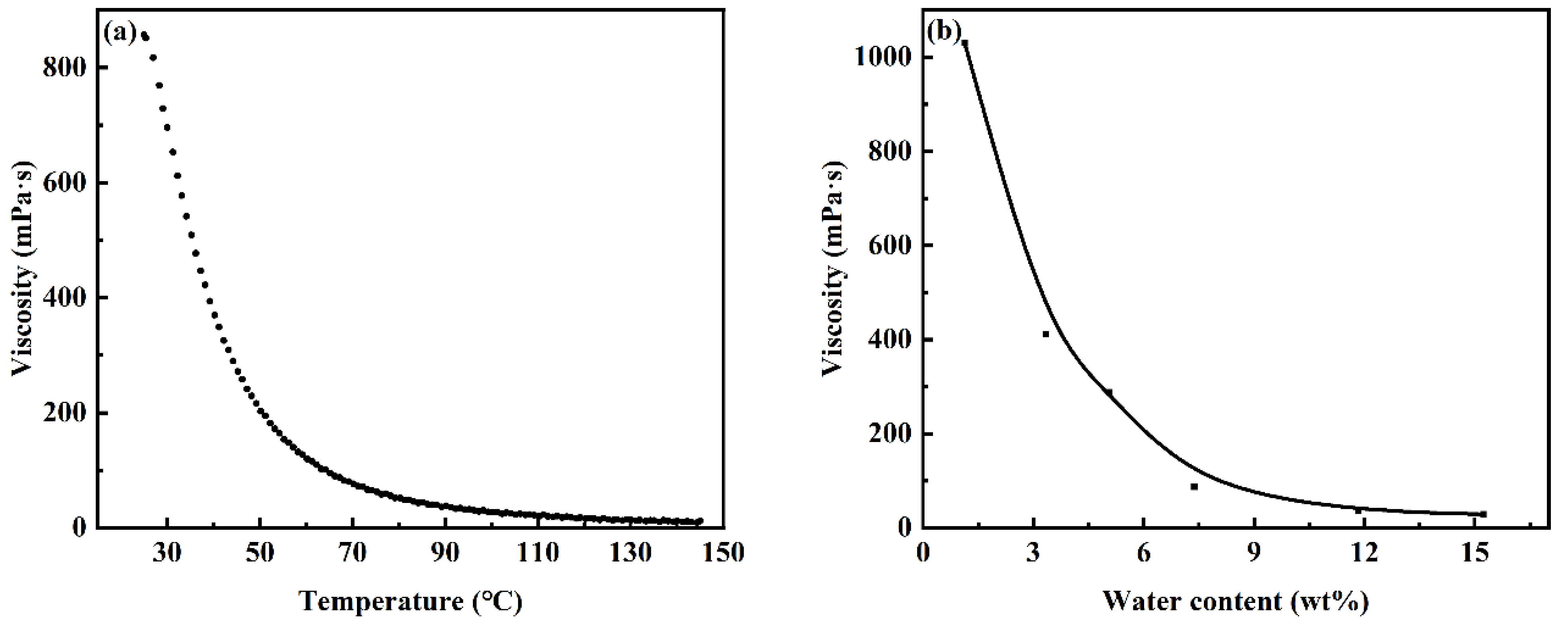
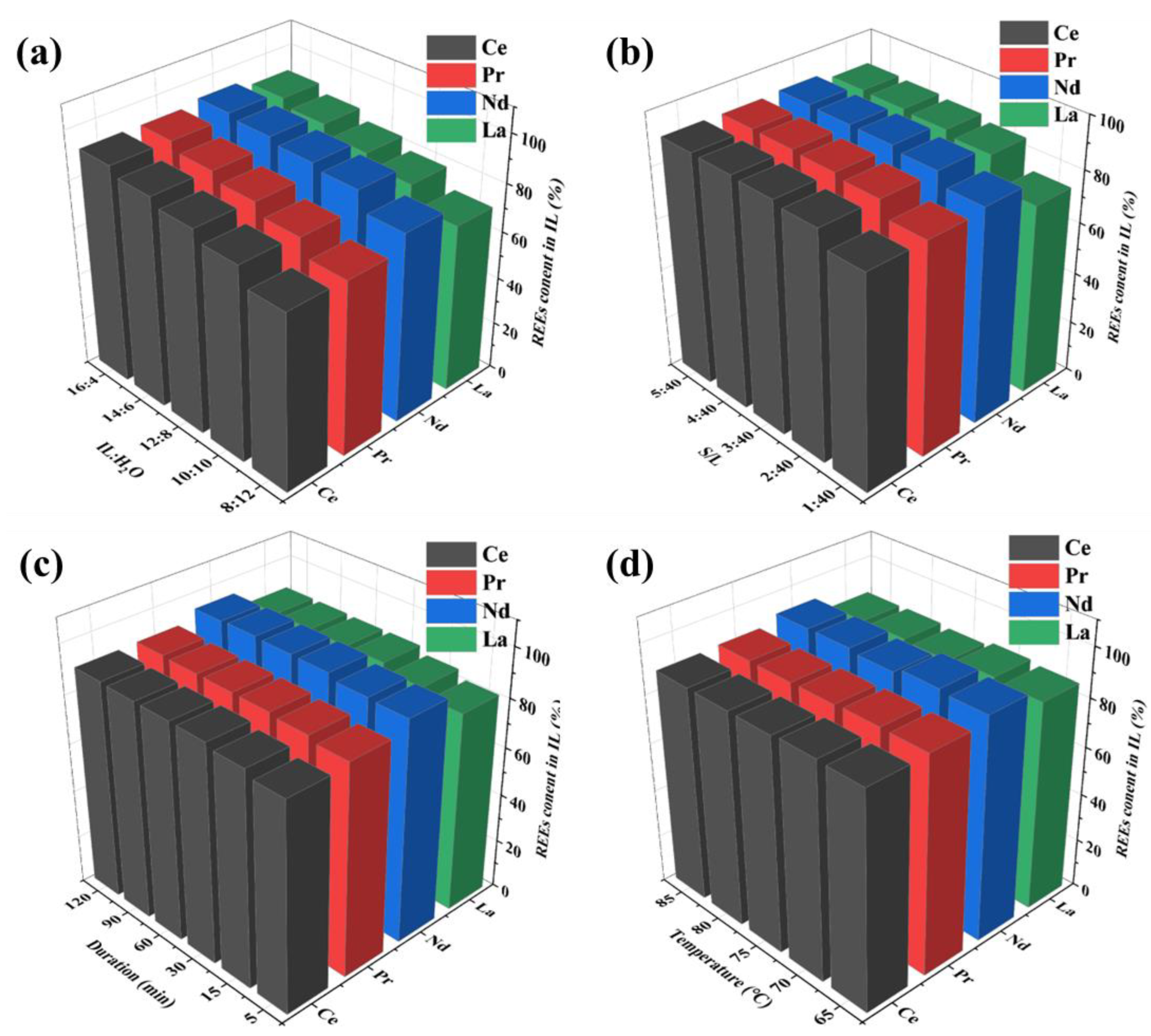
3.3. Extraction of REEs Based on Stratification of [Hbet][Tf2N] and Aqueous Solution
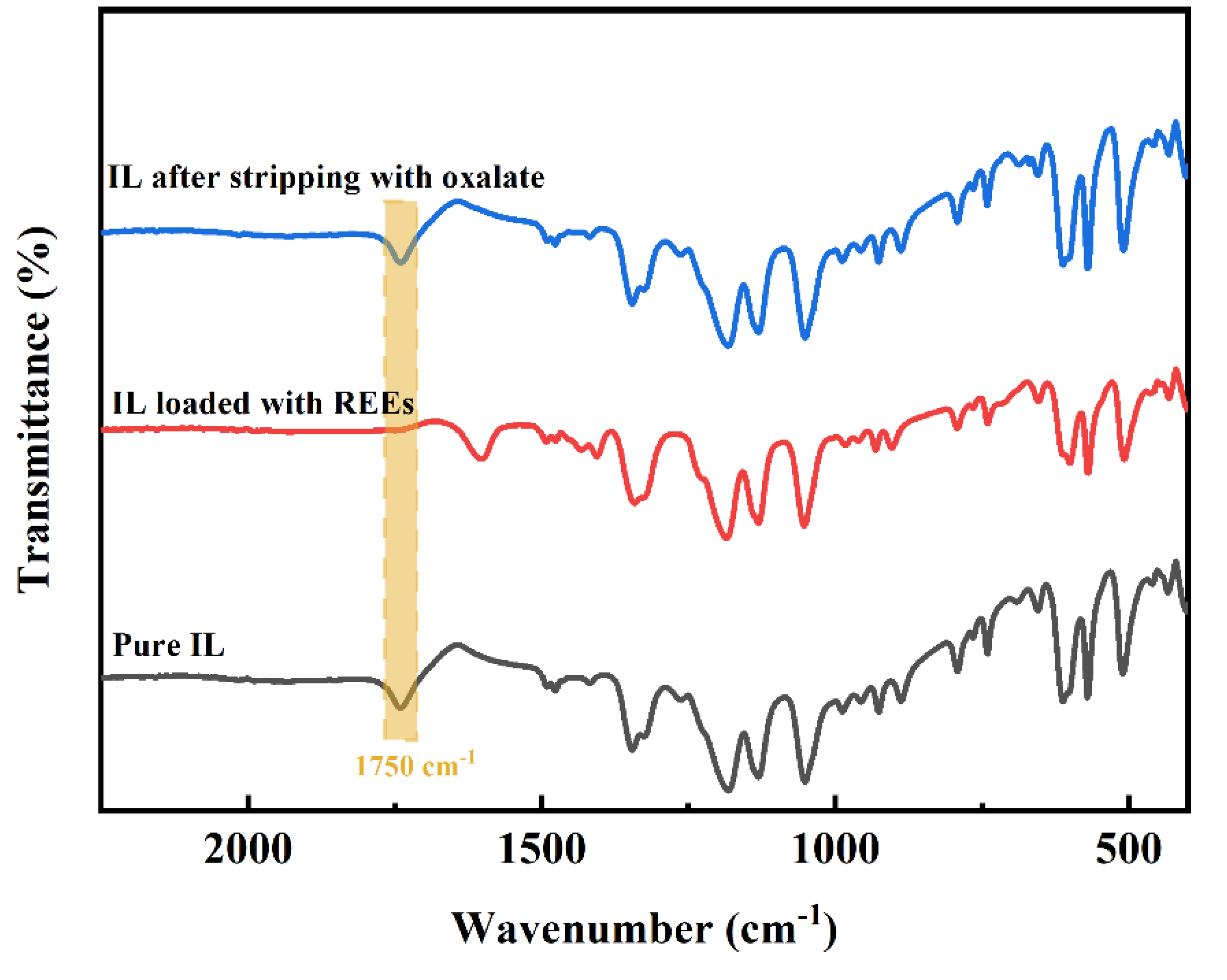
3.4. Back Extraction of [Hbet][Tf2N] Loaded with REEs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jannah, A.N.; Thang, S.G.; Kong, C.; Ilhamsyah, A.B.P.; Chen, S.K.; Abd-Shukor, R. Effects of Yb on the electrical and microstructural properties of (Y1-xYbx)Ba2Cu3O7-delta (x=0-1.0) superconductor. Int. J. Electrochem. Sci. 2021, 16, 150903. [Google Scholar] [CrossRef]
- Junshan, L.; Shengjuan, D.; Longpan, Z.; Shikao, S.; Jiye, W.; Lianshe, F. Preparation, luminescence and potential application of rare earth Sm3+-doped fluorphlogopite phosphors. J. Lumin. 2022, 244, 118685. [Google Scholar]
- Zhiwei, W.; Wanjun, T.; Qingliang, Y.; Biao, X.; Guangyong, X. Charge compensating effect of rare earth ions Ln3+ (Ln =Y, La) on the photoluminescence improvement of Sr9MgK(PO4)7:Eu2+ phosphor. J. Lumin. 2022, 244, 118746. [Google Scholar]
- Hou, L.; Ju, J.; Tang, X.; Chen, R.; Yin, W.; Yan, A.; Chen, B.; Du, Y. Sandwiched structure of hot-deformed Nd-Fe-B permanent magnets processed by Nd-Cu eutectic alloys diffusion. J. Rare Earths 2020, 39, 993–997. [Google Scholar] [CrossRef]
- Changsheng, Q.; Hui, W.; Jiangwen, L.; Liuzhang, O.; Min, Z. Tuning hydrogen storage thermodynamic properties of ZrFe2 by partial substitution with rare earth element Y. Int. J. Hydrog. Energy 2021, 46, 18445–18452. [Google Scholar]
- Wenjia, H.; Tao, H.; Bo, J.; Jinhao, Z.; Juan, S.; Rufang, P. Rare-earth, nitrogen-rich, oxygen heterocyclic supramolecular compounds (Nd, Sm, and Eu): Synthesis, structure, and catalysis for ammonium perchlorate. J. Rare Earths 2022, 40, 428–433. [Google Scholar]
- Pikkili, R.; Ch., B.; Rani, D.S.; Jayasankar, C.K. Spectral investigations of Nd3+:Ba(PO3)2+La2O3 glasses for infrared laser gain media applications. Opt. Mater. 2022, 129, 112482. [Google Scholar]
- Kul, M.; Topkaya, Y.; Karakaya, I. Rare earth double sulfates from pre-concentrated bastnasite. Hydrometallurgy 2008, 93, 129–135. [Google Scholar] [CrossRef]
- Lucas, J.; Lucas, P.; Le Mercier, T.; Rollat, A.; Davenport, W. Extracting Rare Earth Elements from Concentrates. In Rare Earths; Elsevier: Amsterdam, The Netherlands, 2015; pp. 47–67. [Google Scholar]
- Liu, J.; Zhang, T.-A.; Dou, Z. Mechanochemical decomposition on (rare earth) bastnaesite concentrate in NaOH solution. Miner. Eng. 2019, 137, 27–33. [Google Scholar] [CrossRef]
- Liu, J.; Dou, Z.; Zhang, T. Kinetic study on bastnaesite concentrate mechanochemical decomposition in NaOH solution. J. Rare Earths 2020, 38, 418–426. [Google Scholar] [CrossRef]
- Guo, W.L.; Xu, Y.H.; Cang, D.Q.; Ma, S.F.; Tian, H.; Meng, Z.J.; Zhang, X.X. Study on alkali liquor roasting and sulphuric acid leaching of bayan obo rare earth concentrate. Metalurgija 2018, 57, 157–161. [Google Scholar]
- Deliang, M.; Meng, W.; Zongyu, F.; Chao, X.; Yanyan, Z.; Xiaowei, H. Behavior of phase transformation of Baotou mixed rare earth concentrate during oxidation roasting. J. Rare Earths 2022, 40, 981–987. [Google Scholar]
- Shi, W. Kinetics on Chlorinating Rare Earth of Weishan Mid-grade Bastnasite in Shandong after Fixed Fluorine Treatment. Chin. J. Process Eng. 2003, 3, 278–282. [Google Scholar]
- Wenzhong, S.H.I.; Guocai, Z.H.U.; Ruan, C.H.I. Kinetics of Chlorinating Rare Earth in Bastnaesite with NH4Cl Chlorinating Method after Fixed Fluorine Treatment. Chin. Rare Earths 2006, 27, 65–69. [Google Scholar]
- Shi, W.; Zhu, G.; Zhang, Z.; Wang, J.; Chi, R. Recovery of RE from Weishan Rare Earth Concentrate with Chlorination Roasting after Fluorine-fixing Treatment. Chin. J. Process Eng. 2002, 2, 523–528. [Google Scholar]
- He, J.; Li, Y.; Xue, X.; Ru, H.; Huang, X.; Yang, H. Leaching of fluorine and rare earths from bastnaesite calcined with aluminum hydroxide and the recovery of fluorine as cryolite. RSC Adv. 2017, 7, 14053–14059. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.; Wei, Y.; Li, Y.; He, J. Separation of Fluoride/Cerium by Aluminum Salt Coordination from Bastnasite Sulfuric Acid Leaching Solution. J. Chin. Soc. Rare Earths 2013, 31, 393–398. [Google Scholar]
- He, J.G.; Li, Y.; Xue, X.X.; Ru, H.Q.; Huang, X.W.; Yang, H. Separation of fluorine/cerium from fluorine-bearing rare earth sulfate solution by selective adsorption using hydrous zirconium oxide. RSC Adv. 2016, 6, 43814–43822. [Google Scholar] [CrossRef]
- Mihkel, K. Ionic Liquids in Chemical Analysis. Crit. Rev. Anal. Chem. 2005, 35, 177–192. [Google Scholar]
- Watanabe, H.; Doi, H.; Saito, S.; Matsugami, M.; Fujii, K.; Kanzaki, R.; Kameda, Y.; Umebayashi, Y. Hydrogen bond in imidazolium based protic and aprotic ionic liquids. J. Mol. Liq. 2015, 217, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Davis, H.; James, J. Task-Specific Ionic Liquids. Chem. Lett. 2004, 33, 1072–1077. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, S.; Wang, D.; Yao, X. Hydrogen bonds in imidazolium ionic liquids. J. Phys. Chem. A 2006, 110, 9775–9782. [Google Scholar] [CrossRef]
- Cocalia, V.A.; Holbrey, J.D.; Gutowski, K.E.; Bridges, N.J.; Rogers, R.D. Separations of metal ions using ionic liquids: The challenges of multiple mechanisms. Tsinghua Sci. Technol. 2006, 11, 188–193. [Google Scholar] [CrossRef]
- Abbott, A.P.; McKenzie, K.J. Application of ionic liquids to the electrodeposition of metals. Phys. Chem. Chem. Phys. 2006, 8, 4265–4279. [Google Scholar] [CrossRef]
- Paiva, A.P.; Nogueira, C.A. Ionic Liquids in the Extraction and Recycling of Critical Metals from Urban Mines. Waste Biomass Valorization 2020, 12, 1725–1747. [Google Scholar] [CrossRef]
- Kim, B.-K.; Lee, E.J.; Kang, Y.; Lee, J.-J. Application of ionic liquids for metal dissolution and extraction. J. Ind. Eng. Chem. 2018, 61, 388–397. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Olea, F.; Sepúlveda, R.; Castillo, J.; Cabezas, R.; Merlet, G.; Romero, J. Possibilities and challenges for ionic liquids in hydrometallurgy. Sep. Purif. Technol. 2020, 251, 117289. [Google Scholar] [CrossRef]
- Potdar, M.K.; Mohile, S.S.; Salunkhe, M.M. Coumarin syntheses via Pechmann condensation in Lewis acidic chloroaluminate ionic liquid. Tetrahedron Lett. 2001, 42, 9285–9287. [Google Scholar] [CrossRef]
- Hsiu, S.I.; Huang, J.F.; Sun, I.W.; Yuan, C.H.; Shiea, J. Lewis acidity dependency of the electrochemical window of zinc chloride-1-ethyl-3-methylimidazolium chloride ionic liquids. Electrochim. Acta 2002, 47, 4367–4372. [Google Scholar] [CrossRef]
- Yang, H.; Gu, Y.; Deng, Y.; Shi, F. Electrochemical activation of carbon dioxide in ionic liquid: Synthesis of cyclic carbonates at mild reaction conditions. Chem. Commun. 2002, 3, 274–275. [Google Scholar] [CrossRef]
- Nockemann, P.; Thijs, B.; Pittois, S.; Thoen, J.; Glorieux, C.; Hecke, K.V.; Meervelt, L.V.; Kirchner, B.; Binnemans, K. Task-Specific Ionic Liquid for Solubilizing Metal Oxides. J. Phys. Chem. B 2006, 110, 20978–20992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaeffer, N.; Grimes, S.; Cheeseman, C. Interactions between trivalent rare earth oxides and mixed [Hbet][Tf2N]:H2O systems in the development of a one-step process for the separation of light from heavy rare earth elements. Inorg. Chim. Acta 2016, 439, 55–60. [Google Scholar] [CrossRef]
- Richter, J.; Ruck, M. Dissolution of metal oxides in task-specific ionic liquid. RSC Adv 2019, 9, 29699–29710. [Google Scholar] [CrossRef] [Green Version]
- Nockemann, P.; Thijs, B.; Parac-Vogt, T.N.; Hecke, K.V.; Meervelt, L.V.; Tinant, B.; Hartenbach, I.; Schleid, T.; Ngan, V.T.; Nguyen, M.T.; et al. Carboxyl-Functionalized Task-Specific Ionic Liquids for Solubilizing Metal Oxides. Inorg. Chem. 2008, 47, 9987–9999. [Google Scholar] [CrossRef]
- Hoogerstraete, T.V.; Onghena, B.; Binnemans, K. Homogeneous Liquid-Liquid Extraction of Metal Ions with a Functionalized Ionic Liquid. J. Phys. Chem. Lett. 2013, 4, 1659–1663. [Google Scholar] [CrossRef] [PubMed]
- Davris, P.; Balomenos, E.; Panias, D.; Paspaliaris, I. Selective leaching of rare earth elements from bauxite residue (red mud), using a functionalized hydrophobic ionic liquid. Hydrometallurgy 2016, 164, 125–135. [Google Scholar] [CrossRef]
- Taggart, R.K.; Hower, J.C.; Hsu-Kim, H. Effects of roasting additives and leaching parameters on the extraction of rare earth elements from coal fly ash. Int. J. Coal Geol. 2018, 196, 106–114. [Google Scholar] [CrossRef]
- Dupont, D.; Binnemans, K. Recycling of rare earths from NdFeB magnets using a combined leaching/extraction system based on the acidity and thermomorphism of the ionic liquid [Hbet][Tf2N]. Green Chem. 2015, 17, 2150–2163. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Duan, Z.; Bai, N.; Wang, H.; Cao, Y.; Song, X.; Peng, W.; Zhu, X. Highly selective dissolution and synchronous extraction of zinc from zinc-cobalt slag by an ionic liquid [Hbet][Tf2N]–H2O system: A novel method for separating zinc and cobalt. J. Clean. Prod. 2021, 315, 128301. [Google Scholar] [CrossRef]
- Dupont, D.; Binnemans, K. Rare-earth recycling using a functionalized ionic liquid for the selective dissolution and revalorization of Y2O3:Eu3+ from lamp phosphor waste. Green Chem. 2015, 17, 856–868. [Google Scholar] [CrossRef] [Green Version]
- Deferm, C.; Luyten, J.; Oosterhof, H.; Fransaer, J.; Binnemans, K. Purification of crude In(OH)3 using the functionalized ionic liquid betainium bis(trifluoromethylsulfonyl)imide. Green Chem. 2018, 20, 412–424. [Google Scholar] [CrossRef]
- Onghena, B.; Binnemans, K. Recovery of Scandium (III) from Aqueous Solutions by Solvent Extraction with the Functionalized Ionic Liquid Betainium Bis(trifluoromethylsulfonyl)imide. Ind. Eng. Chem. Res. 2015, 54, 1887–1898. [Google Scholar] [CrossRef]
- Davris, P.; Balomenos, E.; Panias, D.; Paspaliaris, I. Developing New Process for Selective Extraction of Rare Earth Elements from Bauxite Residue Based on Functionalized Ionic Liquids. In TMS 2018: Light Metals; Springer: Cham, Switzerland, 2018; pp. 149–156. [Google Scholar]
- Fan, F.-L.; Qin, Z.; Cao, S.-W.; Tan, C.-M.; Huang, Q.-G.; Chen, D.-S.; Wang, J.-R.; Yin, X.-J.; Xu, C.; Feng, X.-G. Highly Efficient and Selective Dissolution Separation of Fission Products by an Ionic Liquid [Hbet][Tf2N]: A New Approach to Spent Nuclear Fuel Recycling. Inorg. Chem. 2019, 58, 603–609. [Google Scholar] [CrossRef] [PubMed]



| Elements | Ce | La | Nd | Pr | Ca | P | F |
|---|---|---|---|---|---|---|---|
| Wt% | 19.98 | 15.95 | 7.41 | 4.79 | 0.37 | 0.02 | 7.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wang, D.; Duan, Z.; Liu, J.; Cao, Y.; Peng, W. A Novel Dissolution and Synchronous Extraction of Rare Earth Elements from Bastnaesite by a Functionalized Ionic Liquid [Hbet][Tf2N]. Minerals 2022, 12, 1592. https://doi.org/10.3390/min12121592
Huang Y, Wang D, Duan Z, Liu J, Cao Y, Peng W. A Novel Dissolution and Synchronous Extraction of Rare Earth Elements from Bastnaesite by a Functionalized Ionic Liquid [Hbet][Tf2N]. Minerals. 2022; 12(12):1592. https://doi.org/10.3390/min12121592
Chicago/Turabian StyleHuang, Yukun, Dasong Wang, Zhuo Duan, Jiang Liu, Yijun Cao, and Weijun Peng. 2022. "A Novel Dissolution and Synchronous Extraction of Rare Earth Elements from Bastnaesite by a Functionalized Ionic Liquid [Hbet][Tf2N]" Minerals 12, no. 12: 1592. https://doi.org/10.3390/min12121592
APA StyleHuang, Y., Wang, D., Duan, Z., Liu, J., Cao, Y., & Peng, W. (2022). A Novel Dissolution and Synchronous Extraction of Rare Earth Elements from Bastnaesite by a Functionalized Ionic Liquid [Hbet][Tf2N]. Minerals, 12(12), 1592. https://doi.org/10.3390/min12121592













