Use of the Far Infrared Spectroscopy for NaCl and KCl Minerals Characterization—A Case Study of Halides from Kłodawa in Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Geological Setting of Halite and Sylvite Samples
2.2. Samples
2.3. Chemical Analysis of Halides
2.4. XRD Measurements
2.5. IR Spectra Measurements
2.6. Calculations
3. Results
3.1. Chemical Analysis of Halides
3.2. Crystallography Measurements of Selected Halite and Sylvite Samples from Kłodawa Salt Diapirs
3.3. Experimental Spectra of Selected Halite and Sylvite Samples
3.4. Calculated Infrared Spectra for Models of NaCl and KCl Crystals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeljaś, D. Magazyny gazu ziemnego w cechsztynskich formacjach solnych elementem bezpieczenstwa energetycznego Polski. Przegląd Geol. 2020, 68, 824–832. [Google Scholar]
- Cornot-Gandolphe, S. Underground Gas Storage in the World—2018 Status. Cedigaz Insights 2018, 31, 1–17. [Google Scholar]
- Czapowski, G.; Tomassi-Morawiec, H. Stan rozpoznania geologicznego struktur solnych regionu szczecińskiego pod katem oceny mozliwości budowy w ich obrebie kawernowych magazynów i składowisk. Biul. Panstw. Inst. Geol. 2012, 448, 145–156. [Google Scholar]
- Krzywiec, P. Geometria i ewolucja wybranych struktur solnych z obszaru niżu polskiego w świetle danych sejsmicznych. Przegląd Geol. 2009, 57, 812–818. [Google Scholar]
- Kunstman, A.; Poborska-Młynarska, K.; Urbańczyk, K. Zarys Otworowego Ługownictwa Solnego: Aktualne Kierunki Rozwoju; AGH [Akademia Górniczo-Hutnicza]; Uczelniane Wydawnictwa Naukowo-Dydaktyczne: Kraków, Poland, 2002; ISBN 8388408488. [Google Scholar]
- Pierce, W.G.; Rich, E.I. Summary of Rock Salt Deposits in the United States as Possible Storage Sites for Radioactive Waste Materials; US Government Printing Office: Washington, DC, USA,, 1962; Volume 1148.
- Pinho, S.P.; Macedo, E.A. Solubility of NaCl, NaBr, and KCl in water, methanol, ethanol, and their mixed solvents. J. Chem. Eng. Data 2005, 50, 29–32. [Google Scholar] [CrossRef]
- Aquilano, D.; Otálora, F.; Pastero, L.; García-Ruiz, J.M. Three study cases of growth morphology in minerals: Halite, calcite and gypsum. Prog. Cryst. Growth Charact. Mater. 2016, 62, 227–251. [Google Scholar] [CrossRef]
- Okrusch, M.; Frimmel, H.E. Halides. In Mineralogy; Springer: Berlin/Heidelberg, Germany, 2020; pp. 105–109. [Google Scholar]
- Mahadik, P.; Pathak, N.; Sengupta, P. Spectroscopic studies on blue halite. J. Lumin. 2018, 194, 327–333. [Google Scholar] [CrossRef]
- Sonnenfeld, P. The color of rock salt-A review. Sediment. Geol. 1995, 94, 267–276. [Google Scholar] [CrossRef]
- Stoiber, R.E.; Morse, S.A. Isotropic Crystal Identification. In Crystal Identification with the Polarizing Microscope; Springer: Berlin/Heidelberg, Germany, 1994; pp. 76–86. [Google Scholar]
- Zelek, S.M.; Stadnicka, K.M.; Toboła, T.; Natkaniec-Nowak, L. Lattice deformation of blue halite from Zechstein evaporite basin: Kłodawa Salt Mine, Central Poland. Mineral. Petrol. 2014, 108, 619–631. [Google Scholar] [CrossRef][Green Version]
- Mees, F.; Tursina, T.V. Salt minerals in saline soils and salt crusts. In Interpretation of Micromorphological Features of Soils and Regoliths; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 11; pp. 289–321. [Google Scholar]
- Yalçin, Ş.; Mutlu, I.H. Structural characterization of some table salt samples by XRD, ICP, FTIR and XRF techniques. Acta Phys. Pol. A Gen. Phys. 2012, 121, 50. [Google Scholar] [CrossRef]
- Rattanakawin, C.; Lakantha, W.; Kajai, I. Flotation of sylvinite from Thakhek, Lao, PDR. Songklanakarin J. Sci. Technol. 2019, 41, 545–550. [Google Scholar]
- Henry, C.H. Analysis of Raman scattering by F centers. Phys. Rev. 1966, 152, 699. [Google Scholar] [CrossRef]
- Frezzotti, M.L.; Tecce, F.; Casagli, A. Raman spectroscopy for fluid inclusion analysis. J. Geochem. Explor. 2012, 112, 1–20. [Google Scholar]
- Krishnamurthy, N.; Krishnan, R.S. Raman spectra of alkali halides. Z. Phys. 1965, 183, 130–139. [Google Scholar] [CrossRef]
- Ghomi, M.; Rzepka, E.; Taurel, L. Study of initial stages of F-centre aggregation in NaCl by Raman scattering. Phys. Status Solidi 1979, 92, 447–453. [Google Scholar] [CrossRef]
- Rzepka, E.; Lefrant, S.; Taurel, L. First-order Raman spectra of NaCl containing sodium colloids. Solid State Commun. 1979, 30, 795–796. [Google Scholar] [CrossRef]
- Wesełucha-Birczyńska, A.; Toboła, T.; Natkaniec-Nowak, L. Raman microscopy of inclusions in blue halites. Vib. Spectrosc. 2008, 48, 302–307. [Google Scholar] [CrossRef]
- Osterrothová, K.; Jehlička, J. Investigation of biomolecules trapped in fluid inclusions inside halite crystals by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 83, 288–296. [Google Scholar] [CrossRef]
- Wesełucha-Birczyńska, A.; Zelek, S.; Stadnicka, K. Blue halite colour centre aggregates studied by micro-Raman spectroscopy and X-ray diffraction. Vib. Spectrosc. 2012, 60, 124–128. [Google Scholar] [CrossRef]
- Zelek, S.M.; Wesełucha-Birczyńska, A.; Szklarzewicz, J.; Stadnicka, K.M. Spectroscopic properties of halite from Kłodawa salt mine, central Poland. Mineral. Petrol. 2015, 109, 45–51. [Google Scholar] [CrossRef][Green Version]
- Toboła, T. Raman spectroscopy of organic, solid and fluid inclusions in the Oldest Halite of LGOM area (SW Poland). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 189, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Toboła, T.; Natkaniec-Nowak, L. SEM-EDS investigation of solid state inclusion. In Blue Halite of the Kłodawa Salt; AGH University of Science and Technology Press: Kraków, Poland, 2008; pp. 70–80. [Google Scholar]
- Pironon, J.; Barres, O. Semi-quantitative FT-IR microanalysis limits: Evidence from synthetic hydrocarbon fluid inclusions in sylvite. Geochim. Cosmochim. Acta 1990, 54, 509–518. [Google Scholar] [CrossRef]
- Mougenot, B.; Pouget, M.; Epema, G.F. Remote sensing of salt affected soils. Remote Sens. Rev. 1993, 7, 241–259. [Google Scholar] [CrossRef]
- Howari, F. Spectroscopy of evaporites. Per. Miner. 2002, 71, 191–200. [Google Scholar]
- Hunt, G.R. Spectroscopic properties of rocks and minerals. In Handbook of Physical Properties of Rocks; CRC Press: Boca Raton, FL, USA, 2017; Volume 1, p. 295. [Google Scholar]
- Peters, R.D. Using Spectral Measurements to Differentiate between Aqueous NaCl and Aqueous KCl in Dual-Salt Solutions; University of Saskatchewan: Saskatoon, SK, Canada, 2016. [Google Scholar]
- Peters, R.D.; Noble, S.D. Using near infrared measurements to evaluate NaCl and KCl in water. J. Near Infrared Spectrosc. 2019, 27, 147–155. [Google Scholar] [CrossRef]
- Dadlez, R.; Narkiewicz, M.; Stephenson, R.A.; Visser, M.T.M.; van Wees, J.D. Tectonic evolution of the Mid-Polish Trough: Modelling implications and significance for central European geology. Tectonophysics 1995, 252, 179–195. [Google Scholar] [CrossRef]
- Krzywiec, P. Structural inversion of the Pomeranian and Kuiavian segments of the Mid-Polish Trough--lateral variations in timing and structural style. Geol. Q. 2006, 50, 151–168. [Google Scholar]
- Wachowiak, J.; Pawlikowski, M.; Wilkosz, P. Lithostratigraphy of Zechstein evaporites of the central and north-western parts of the Mogilno Salt Diapir, based on boreholes Z-9 and Z-17. Geol. Geophys. Environ. 2012, 38, 115–151. [Google Scholar] [CrossRef][Green Version]
- Wagner, R. Stratigraphy and evolution of the Zechstein Basin in the Polish Lowland. Pr. Państwowego Inst. Geol. 1994, 156, 3–71. [Google Scholar]
- Wagner, R.; Peryt, T.M. Possibility of sequence stratigraphic subdivision of the Zechstein in the Polish Basin. Geol. Q. 1997, 41, 457–474. [Google Scholar]
- Natkaniec-Nowak, L.; Toboła, T. Występowanie soli niebieskich w Kłodawie. Przegląd Geol. 2003, 51, 435–438. [Google Scholar]
- Rusiniak, P.; Ruszczyńska, A.; Wątor, K.; Bulska, E.; Kmiecik, E. Methodological aspects concerning sampling and determination of total selenium and selenium species in geothermal waters. Bull. Geogr. Phys. Geogr. Ser. 2020, 18, 5–16. [Google Scholar] [CrossRef]
- ISO 11885:2007; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). International Organization for Standarization: Geneva, Switzerland, 2007.
- Wątor, K.; Kmiecik, E.; Tomaszewska, B. Assessing medicinal qualities of groundwater from the Busko-Zdrój area (Poland) using the probabilistic method. Environ. Earth Sci. 2016, 75, 804. [Google Scholar] [CrossRef]
- Wątor, K.; Kmiecik, E.; Postawa, A.; Rusiniak, P. A probabilistic approach to assesment of the quality of drinking water. Geologos 2019, 25, 249–254. [Google Scholar] [CrossRef]
- Rusiniak, P.; Wątor, K.; Kmiecik, E. Inorganic Chromium Speciation in Geothermal Water of the Podhale Trough (Southern Poland) Used for Recreational Purpose. Energies 2020, 13, 3531. [Google Scholar] [CrossRef]
- ISO 17294:2016-2; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes. International Organization for Standarization: Geneva, Switzerland, 2016.
- ISO 9297:1989; Water Quality—Determination of Chloride—Silver Nitrate Titration with Chromate Indicator (Mohr’s Method). International Organization for Standarization: Geneva, Switzerland, 1989.
- ISO 9963-1:1994; Water Quality—Determination of Alkalinity—Part 1: Determination of Total and Composite Alkalinity. International Organization for Standarization: Geneva, Switzerland, 1994.
- Marciniak, H.; Diduszko, R.; Kozak, M. XRayan, X-ray Phase Analysis Program, Version 4.2.2; Producer “KOMA” Henryk Marciniak: Warszawa, Poland, 2013. [Google Scholar]
- Altomare, A.; Cuocci, C.; Giacovazzo, C.; Moliterni, A.; Rizzi, R.; Corriero, N.; Falcicchio, A. EXPO2013: A kit of tools for phasing crystal structures from powder data. J. Appl. Cryst. 2013, 46, 1231–1235. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian, Gaussian 09, Revision A. 1; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef]
- Ochterski, J.W. Vibrational Analysis in Gaussian, White Papers and Technical Notes, 1999 (Minor Corrections 2018 and 2020). Available online: https://gaussian.com/white/ (accessed on 15 November 2022).
- Burstein, E.; Johnson, F.A.; Loudon, R. A Selection Rules for Second-Order Infrared and Raman Processes in the Rocksalt Structure and Interpretation of the Raman Spectra of NaCl, KBr, and NaI. Phys. Rev. A 1965, 139, A1239. [Google Scholar] [CrossRef]
- Krishnan, R.S. Raman spectra of alkali halides. In Essays in Structural Chemistry; Downs, A.J., Long, D.A., Staveley, L.A.K., Eds.; Springer: Boston, MA, USA, 1971. [Google Scholar]
- Clayman, B.P.; Nolt, I.G.; Sievers, A.J. Far-infrared absorption spectrum of NaI:NaCl. Solid State Commun. 1969, 7, 7–10. [Google Scholar] [CrossRef]
- Morioka, Y.; Nakagawa, I. Hyper-Raman spectra of some cubic crystals. Chem. Phys. Lett. 1985, 122, 150–152. [Google Scholar] [CrossRef]
- Ferraro, J.R. (Ed.) Metal halide vibrations. In Low-Frequency Vibration of Inorganic and Coordination Compounds; Springer: New York, NY, USA, 1971; pp. 111–189. [Google Scholar]
- Li, B.; Michaelides, A.; Scheffler, M. Density functional theory study of flat and stepped NaCl(001). Phys. Rev. B 2007, 76, 075401. [Google Scholar] [CrossRef]
- Jensen, T.L.; Moxnes, J.; Unneberg, E.A. Density Functional Theory Comparison Study of the Surface and Lattice Energy of Sodium Chloride. J. Comput. Theor. Nanosci. 2013, 10, 464–469. [Google Scholar] [CrossRef]
- Hirano, M. Friction at the Atomic Level: Atomistic Approaches in Tribology; John Wiley & Sons: Hoboken, NJ, USA, 2018; ISBN 3527411690. [Google Scholar]
- Available online: https://cccbdb.nist.gov/vibscalejust.asp (accessed on 15 November 2022).
- Toboła, T.; Natkaniec-Nowak, L.; Szybist, A.; Misiek, G.; Janiów, S. Blue salts in Kłodawa salt mine. Miner. Resour. Manag. IGSMiE PAN 2007, 23, 117–132. [Google Scholar]
- Zelek, S. Structural and Physical-Chemical Characteristics of Blue Halite from Kłodawa Salt Mine and Embedded Solid Inclusions. Ph.D. Thesis, Jagiellonian University, Kraków, Poland, 2012. [Google Scholar]
- Toboła, T.; Cyran, K.; Rembiś, M. Microhardness analysis of halite from different salt-bearing formations. Geol. Q. 2019, 63, 771–785. [Google Scholar] [CrossRef]
- Hunsche, U.; Hampel, A. Rock salt—the mechanical properties of the host rock material for a radioactive waste repository. Eng. Geol. 1999, 52, 271–291. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, C.; Gao, H.; Yang, X.; Xu, S.; Zhao, Y. Experiments on mechanical properties of salt rocks under cyclingloading. J. Rock Mech. Geotech. Eng. 2012, 4, 54–61. [Google Scholar] [CrossRef]
- Carter, N.L.; Hansen, F.D. Creep of rock salt. Tectonophysics 1983, 92, 275–333. [Google Scholar] [CrossRef]



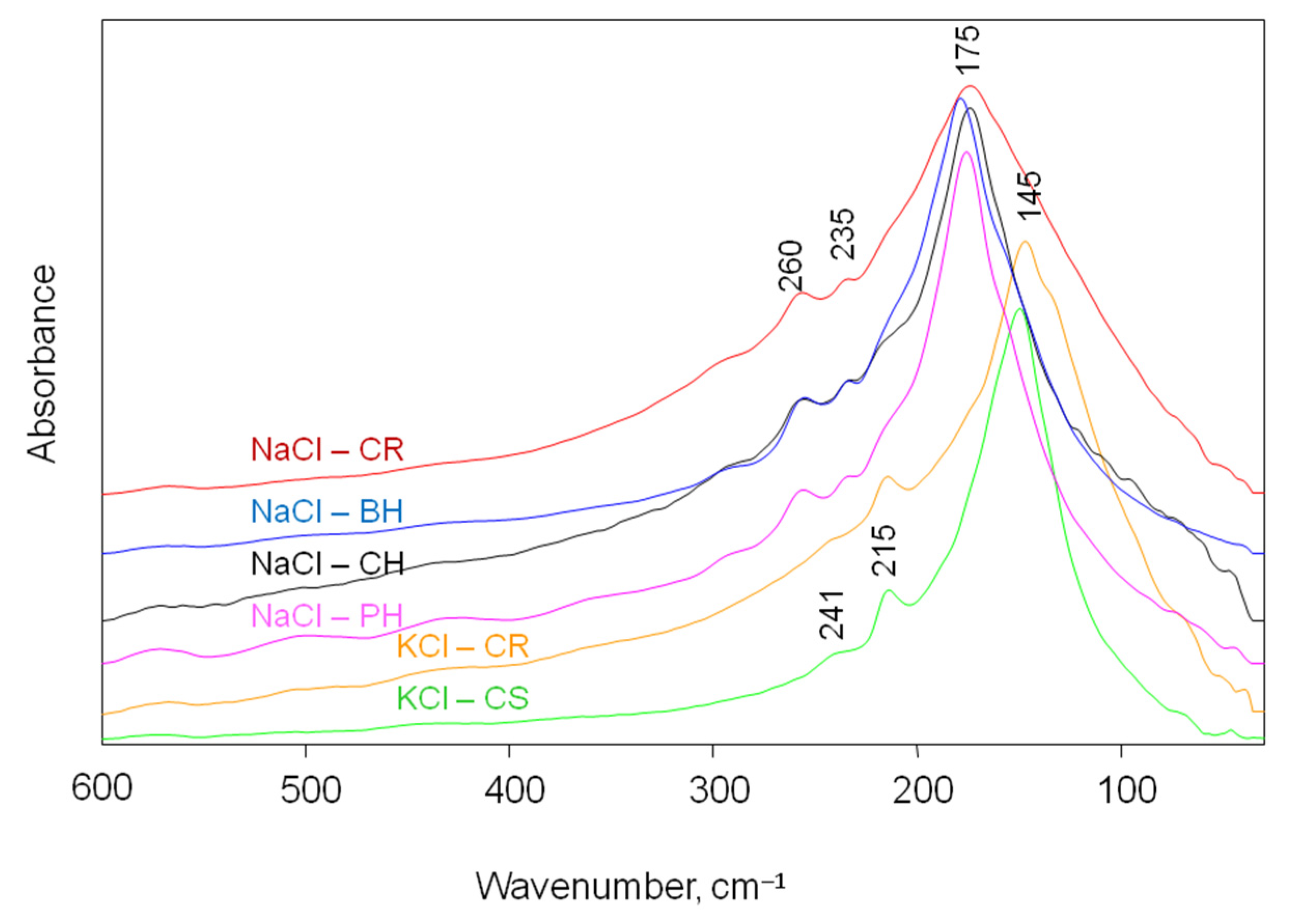
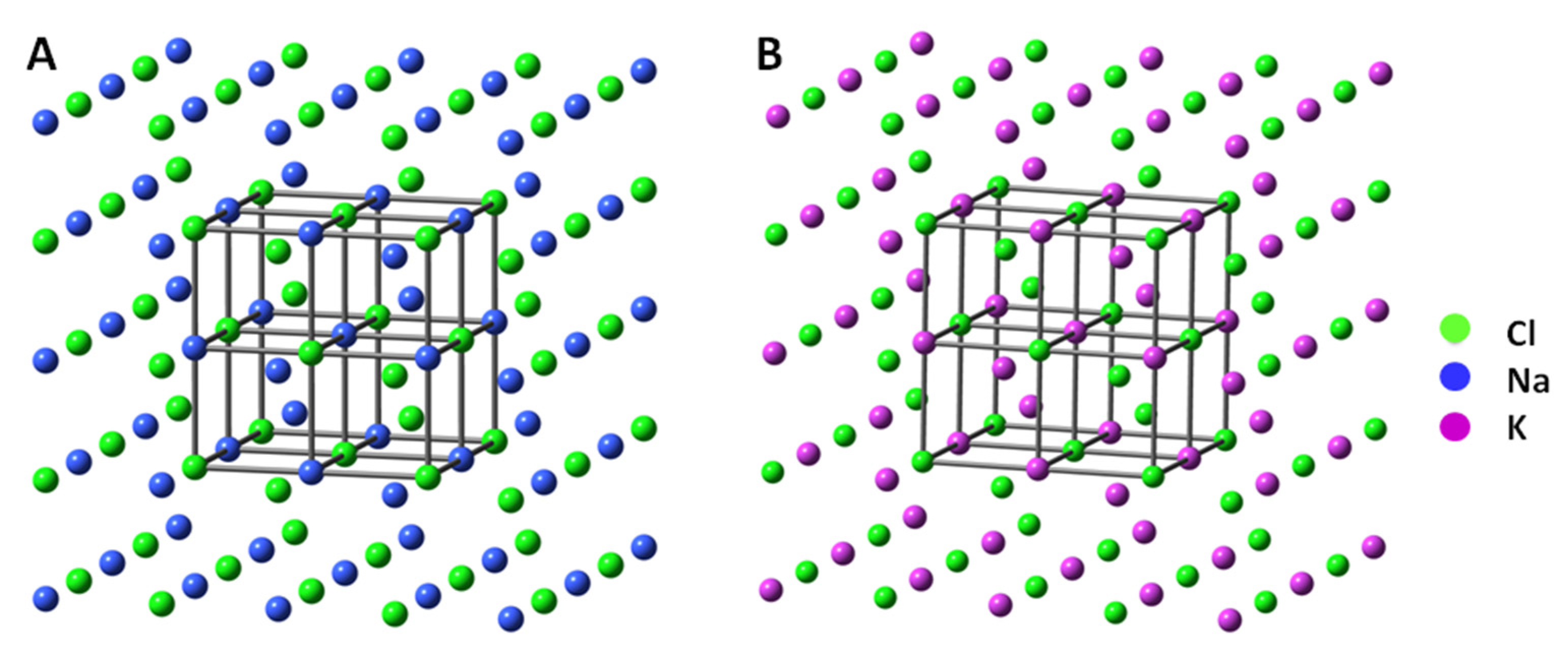

| Structural and Spectroscopic Characteristic of Halites and Sylvite | |
|---|---|
| Experiments | Theoretical Calculations |
|
|
|
|
|
|
| |
| Name of Sample | Component [% wt.] | ||||||
|---|---|---|---|---|---|---|---|
| Na+ | K+ | Mg2+ | Zn2+ | Cu2+ | Cl− | Br− | |
| SYLVITE | 0.356 | 51.196 | <0.011 | 0.003 | 0.002 | 48.242 | 0.201 |
| WHITE HALITE | 38.567 | 0.169 | 0.010 | 0.004 | 0.004 | 61.247 | <0.011 |
| PURPLE HALITE | 38.265 | 0.662 | <0.011 | 0.003 | 0.002 | 61.068 | <0.011 |
| BLUE HALITE | 39.006 | 0.391 | <0.011 | 0.004 | 0.002 | 60.575 | 0.022 |
| Name of Sample | Crystal Systems | Symmetry Space Group | Unit Cell Parameters | Volume | |
|---|---|---|---|---|---|
| a = b = c [Å] | α = β = γ [°] | V [Å3] | |||
| COLORLESS SYLVITE | isometric | Fm-3m | 6.2905(1) | 90.000 | 248.920(5) |
| COLORLESS HALITE | isometric | Fm-3m | 5.6418(1) | 90.000 | 179.579(7) |
| PURPLE HALITE | isometric | Fm-3m | 5.6375(1) | 90.000 | 179.171(5) |
| BLUE HALITE | isometric | Fm-3m | 5.6397(1) | 90.000 | 179.379(6) |
| Halide | Restriction for Geometry Optimiztion | Bond Distance [Å] (Na-Cl, K-Cl) | Angle [°] (Na-Cl-Na, Cl-Na-Cl, K-Cl-K, Cl-K-Cl) | ||||
|---|---|---|---|---|---|---|---|
| Mean Value | Maximum, Minimum | Standard Deviation | Mean Value | Maximum, Minimum | Standard Deviation | ||
| NaCl | None | 2.786 | 2.875, 2.653 | 0.053 | 89.96 | 99.90, 83.71 | 2.72 |
| All angles 90° | 2.784 | 2.790, 2.778 | 0.006 | 90.00 | 90.00, 90.00 | 0.00 | |
| KCl | None | 3.167 | 3.229, 3.052 | 0.042 | 90.00 | 95.75, 86.62 | 1.50 |
| All angles 90° | 3.164 | 3.176, 3.152 | 0.012 | 90.00 | 90.00, 90.00 | 0.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chruszcz-Lipska, K.; Zelek-Pogudz, S.; Solecka, U.; Solecki, M.L.; Szostak, E.; Zborowski, K.K.; Zając, M. Use of the Far Infrared Spectroscopy for NaCl and KCl Minerals Characterization—A Case Study of Halides from Kłodawa in Poland. Minerals 2022, 12, 1561. https://doi.org/10.3390/min12121561
Chruszcz-Lipska K, Zelek-Pogudz S, Solecka U, Solecki ML, Szostak E, Zborowski KK, Zając M. Use of the Far Infrared Spectroscopy for NaCl and KCl Minerals Characterization—A Case Study of Halides from Kłodawa in Poland. Minerals. 2022; 12(12):1561. https://doi.org/10.3390/min12121561
Chicago/Turabian StyleChruszcz-Lipska, Katarzyna, Sylwia Zelek-Pogudz, Urszula Solecka, Marek Leszek Solecki, Elżbieta Szostak, Krzysztof Kazimierz Zborowski, and Michał Zając. 2022. "Use of the Far Infrared Spectroscopy for NaCl and KCl Minerals Characterization—A Case Study of Halides from Kłodawa in Poland" Minerals 12, no. 12: 1561. https://doi.org/10.3390/min12121561
APA StyleChruszcz-Lipska, K., Zelek-Pogudz, S., Solecka, U., Solecki, M. L., Szostak, E., Zborowski, K. K., & Zając, M. (2022). Use of the Far Infrared Spectroscopy for NaCl and KCl Minerals Characterization—A Case Study of Halides from Kłodawa in Poland. Minerals, 12(12), 1561. https://doi.org/10.3390/min12121561









