Valorization of South African Coal Wastes through Dense Medium Separation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of the Head Sample
3.2. Valorization Opportunities
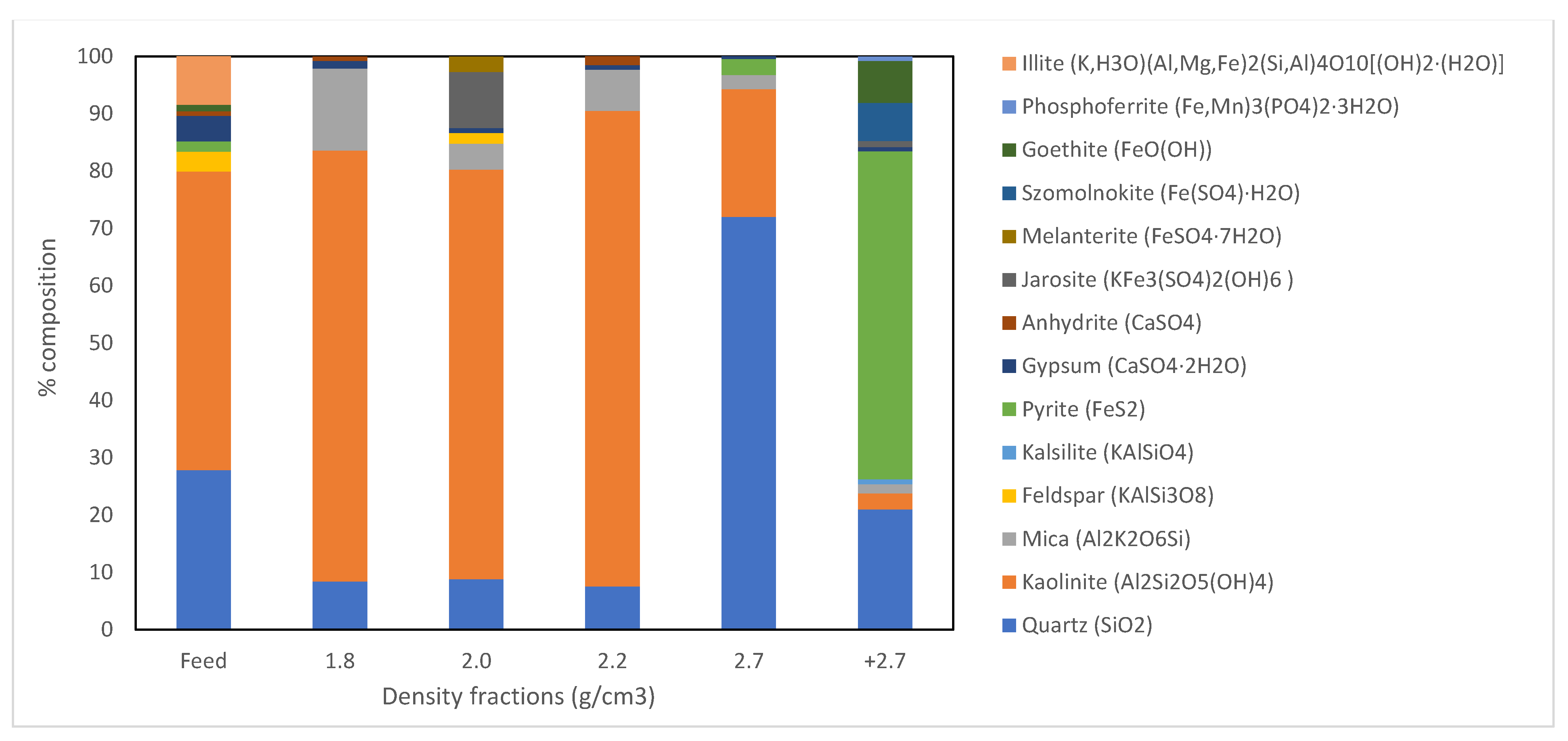
3.2.1. Float Sink Separations
3.2.2. Low Density Fractions
3.2.3. Intermediary Density Fractions
3.2.4. High Density Fraction
3.3. An Integrated Approach to Re-Purposing Coal Mine Waste
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahrami, A.; Ghorbani, Y.; Mirmohammadi, M.; Sheykhi, B.; Kazemi, F. The Beneficiation of Tailing of Coal Preparation Plant by Heavy-Medium Cyclone. Int. J. Coal Sci. Technol. 2018, 5, 374–384. [Google Scholar] [CrossRef]
- Chugh, Y.P.; Behum, P.T. Coal Waste Management Practices in the USA: An Overview. Int. J. Coal Sci. Technol. 2014, 1, 163–176. [Google Scholar] [CrossRef] [Green Version]
- North, B.; Engelbrecht, A.; Oboirien, B. Feasibility Study of Electricity Generation from Discard Coal. J. S. Afr. Inst. Min. Metall. 2015, 115, 573–580. [Google Scholar] [CrossRef]
- Lloyd, P.J. The Potential of Coal Wastes in South Africa. J. S. Afr. Inst. Min. Metall. 2000, 100, 69–72. [Google Scholar]
- Do Amaral Filho, J.R.; Schneider, I.A.H.; de Brum, I.A.S.; Sampaio, C.H.; Miltzarek, G.; Schneider, C. Caracterização de Um Depósito de Rejeitos Para o Gerenciamento Integrado Dos Resíduos de Mineração Na Região Carbonífera de Santa Catarina, Brasil. REM Rev. Esc. Minas 2013, 66, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.T.L.; Broadhurst, J.L.; Opitz, A.; Fundikwa, B.; Stander, H.-M.; Mostert, L.; Amaral Filho, J.; Kotsiopoulos, A. An Industrial Ecology Approach to Sulphide Containing Mineral Wastes to Minimise ARD Formation PART 2: Design for Disposal and Extraction of Products of Value; WRC in Pretoria: Pretoria, South Africa, 2020. [Google Scholar]
- Eberhard, A. Market, Investment, and Policy Challenges for South African Coal. In The Global Coal Market: Supplying the Major Fuel for Emerging Economies; Thurber, M.C., Morse, R.K., Eds.; Cambridge University Press: Cambridge, UK, 2015; pp. 164–203. [Google Scholar]
- Jeffrey, L.S. Characterization of the Coal Resources of South Africa. J. S. Afr. Inst. Min. Metall. 2005, 105, 95–102. [Google Scholar]
- Kazadi Mbamba, C.; Harrison, S.T.L.; Franzidis, J.; Broadhurst, J.L. Mitigating Acid Rock Drainage Risks While Recovering Low-Sulfur Coal from Ultrafine Colliery Wastes Using Froth Flotation. Miner. Eng. 2012, 29, 13–21. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Machado, C.M.; Duarte, G.W.; Peterson, M. Beneficiation of Pyrite from Coal Mining. J. Clean Prod. 2016, 139, 821–827. [Google Scholar] [CrossRef]
- Weiler, J.; Schneider, I.A.H. Pyrite Utilization in the Carboniferous Region of Santa Catarina, Brazil-Potentials, Challenges, and Environmental Advantages. REM Int. Eng. J. 2019, 72, 515–522. [Google Scholar] [CrossRef]
- Taha, Y.; Elghali, A.; Derhy, M.; Amrani, M.; Hakkou, R.; Benzaazoua, M. Towards an Integrated Approach for Zero Coal Mine Waste Storage: Solutions Based on Materials Circularity and Sustainable Resource Governance. Miner. Processing Extr. Metall. Rev. 2022, 1–14. [Google Scholar] [CrossRef]
- Patricio Ferreira, L.; Müller, T.G.; Cargnin, M.; de Oliveira, C.M.; Peterson, M. Valorization of Waste from Coal Mining Pyrite Beneficiation. J. Environ. Chem. Eng. 2021, 9, 105759. [Google Scholar] [CrossRef]
- Amar, H.; Benzaazoua, M.; Edahbi, M.; Villeneuve, M. Reprocessing Feasibility of Polymetallic Waste Rock for Cleaner and Sustainable Mining. J. Geochem. Explor. 2020, 220, 106683. [Google Scholar] [CrossRef]
- Amar, H.; Benzaazoua, M.; Elghali, A.; Bussière, B.; Duclos, M. Upstream Environmental Desulphurisation and Valorisation of Waste Rocks as a Sustainable AMD Management Approach. J. Geochem. Explor. 2020, 215, 106555. [Google Scholar] [CrossRef]
- Department of Mineral Resources. Review of the Sulphur Industry in the Republic of South Africa; Report R101/2013; Arcadia: Pretoria, South Africa, 2012.
- Do Amaral Filho, J.R.; Weiler, J.; Broadhurst, J.L.; Schneider, I.A.H. The Use of Static and Humidity Cell Tests to Assess the Effectiveness of Coal Waste Desulfurization on Acid Rock Drainage Risk. Mine Water Environ. 2017, 36, 429–435. [Google Scholar] [CrossRef]
- Argane, R.; Benzaazoua, M.; Bouamrane, A.; Hakkou, R. Cement Hydration and Durability of Low Sulfide Tailings-Based Renders: A Case Study in Moroccan Constructions. Miner. Eng. 2015, 76, 97–108. [Google Scholar] [CrossRef]
- Dos Santos, C.R.; do Filho, J.R.A.; Tubino, R.M.C.; Schneider, I.A.H. Use of Coal Waste as Fine Aggregates in Concrete Paving Blocks. Geomaterials 2013, 3, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Firpo, B.A.; Amaral Filho, J.R.D.; Schneider, I.A.H. A Brief Procedure to Fabricate Soils from Coal Mine Wastes Based on Mineral Processing, Agricultural, and Environmental Concepts. Miner. Eng. 2015, 76, 81–86. [Google Scholar] [CrossRef]
- Amaral Filho, J.R.; Firpo, B.A.; Broadhurst, J.L.; Harrison, S.T.L. On the Feasibility of South African Coal Waste for Production of ‘FabSoil’, a Technosol. Miner. Eng. 2020, 146, 106059. [Google Scholar] [CrossRef]
- Weiler, J.; Firpo, B.A.; Schneider, I.A.H. Technosol as an Integrated Management Tool for Turning Urban and Coal Mining Waste into a Resource. Miner. Eng. 2020, 147, 106179. [Google Scholar] [CrossRef]
- Runkel, M.; Sturm, P. Pyrite Roasting, an Alternative to Sulphur Burning. J. S. Afr. Inst. Min. Metall. 2009, 109, 491–496. [Google Scholar]
- Lopes, F.A. Producao Hidrometalurgica de Oxidos Magneticos a Partir de Concentrado de Pirita Proveniente de Rejeitos Da Mineracao de Carvao; UFRGS: Porto Alegre, Brazil, 2017. [Google Scholar]
- Vigânico, E.M. Protótipo Em Escala Piloto Para Produção de Sulfato Ferroso a Partir de Concentrado de Pirita Da Mineração de Carvão. Ph.D. Thesis, Federal University of Rio Grande Sul, Porto Alegre, Brazil, 2014. [Google Scholar]
- Weiler, J.; do Amaral Filho, J.R.; Schneider, I.A.H. Processamento de Rejeito de Carvão Visando a Redução de Custos No Tratamento Da Drenagem Ácida de Minas—Estudo de Caso Na Região Carbonífera de Santa Catarina. Eng. Sanit. Ambient. 2016, 21, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.T.L.H.; Opitz, A.K.B.; Jera, M.K.; Mostert, L.D.; Kotsiopoulos, A. An Industrial Ecology Approach to Sulphide Containing Mineral Wastes to Minimise ARD Formation: Characterising Potential for ARD, Design for Disposal and Extraction of Products with Value; Report in Preparation; Arcadia: Pretoria, South Africa, 2013. [Google Scholar]
- Cebeci, Y.; Ulusoy, U.; Sönmez, I. Determination of Optimum Washing Conditions for a Lignite Coal Based on Ash and Sulfur Content. Fuel 2014, 123, 52–58. [Google Scholar] [CrossRef]
- De Souza, K.F.; Sampaio, C.H.; Kussler, J.A.T. Washability Curves for the Lower Coal Seams in Candiota Mine—Brazil. Fuel Process. Technol. 2012, 96, 140–149. [Google Scholar] [CrossRef]
- Stewart, W.A.; Miller, S.D.; Smart, R. Advances in Acid Rock Drainage (ARD) Characterisation of Mine Wastes. In Proceedings of the 7th International Conference on Acid Rock Drainage 2006, ICARD—Also Serves as the 23rd Annual Meetings of the American Society of Mining and Reclamation, St. Louis, MO, USA, 26–30 March 2006; Volume 3, pp. 2098–2119. [Google Scholar]
- Miller, S. ACARP Project C15034: Development of ARD Assessment for Coal Process Wastes; Environmental Geochemistry International Pty Ltd.: Balmain, Australia, 2008. [Google Scholar]
- Schumann, R.; Stewart, W.; Miller, S.; Kawashima, N.; Li, J.; Smart, R. Acid-Base Accounting Assessment of Mine Wastes Using the Chromium Reducible Sulfur Method. Sci. Total Environ. 2012, 424, 289–296. [Google Scholar] [CrossRef]
- Grobler, J.D.; Sandenbergh, R.F.; Pistorius, P.C. The Stability of Ferrosilicon Dense Medium Suspensions. J. S. Afr. Inst. Min. Metall. 2002, 102, 83–86. [Google Scholar]
- Kotsiopoulos, A.; Harrison, S.T.L. Co-Disposal of Benign Desulfurised Tailings with Sulfidic Waste Rock to Mitigate ARD Generation: Influence of Flow and Contact Surface. Miner. Eng. 2018, 116, 62–71. [Google Scholar] [CrossRef]
- Taha, Y.; Benzaazoua, M.; Hakkou, R.; Mansori, M. Coal Mine Wastes Recycling for Coal Recovery and Eco-Friendly Bricks Production. Miner. Eng. 2017, 107, 123–138. [Google Scholar] [CrossRef]
- Mjonono, D.; Harrison, S.T.L.; Kotsiopoulos, A. Supplementing Structural Integrity of Waste Rock Piles through Improved Packing Protocols to Aid Acid Rock Drainage Prevention Strategies. Miner. Eng. 2019, 135, 13–20. [Google Scholar] [CrossRef]
- Weiler, J.; Amaral Filho, J.R.; Schneider, I.A. Processamento de Rejeitos de Carvão e Redução Do Impacto Ambiental. Augmdomus 2014, 6, 80–94. [Google Scholar]
- Falcon, R.; Ham, A.J. Characteristics of Southern African Coals. J. S. Afr. Inst. Min. Metall. 1988, 88, 145–161. [Google Scholar]
- McCarthy, T.S. The Impact of Acid Mine Drainage in South Africa. S. Afr. J. Sci. 2011, 107, 1–7. [Google Scholar] [CrossRef]
- Oliveira, M.L.S.; Ward, C.R.; Izquierdo, M.; Sampaio, C.H.; De Brum, I.A.S.; Kautzmann, R.M.; Sabedot, S.; Querol, X.; Silva, L.F.O. Chemical Composition and Minerals in Pyrite Ash of an Abandoned Sulphuric Acid Production Plant. Sci. Total Environ. 2012, 430, 34–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akinwekomi, V.; Maree, J.P.; Masindi, V.; Zvinowanda, C.; Osman, M.S.; Foteinis, S.; Mpenyana-Monyatsi, L.; Chatzisymeon, E. Beneficiation of Acid Mine Drainage (AMD): A Viable Option for the Synthesis of Goethite, Hematite, Magnetite, and Gypsum–Gearing towards a Circular Economy Concept. Miner. Eng. 2020, 148, 106204. [Google Scholar] [CrossRef]
- Villetti, P.I.C. Produção de Coagulante Férrico a Partir Da Lixiviação de Concen—Trado de Pirita Da Mineração de Carvão via Cristalização/Solubilização de Sulfato Ferroso: Estudo Comparativo Entre Rejeitos de Duas Jazidas. Master’s Thesis, Federal University of Rio Grande do Sul—UFRGS, Porto Alegre, Brazil, 2017. [Google Scholar]
- Vigânico, E.M.; Colling, A.V.; Silva, R.D.A.; Schneider, I.A.H. Biohydrometallurgical/UV Production of Ferrous Sulphate Heptahydrate Crystals from Pyrite Present in Coal Tailings. Miner. Eng. 2011, 24, 1146–1148. [Google Scholar] [CrossRef]
- Menezes, J.C.C.C.; Colling, A.V.; Silva, R.A.S.; dos Santos, R.H.; Scheneider, I.A.H. Ferric Sulphate Coagulant Obtained by Leaching from Coal Tailings. Mine Water Environ. 2017, 36, 457–460. [Google Scholar] [CrossRef]
- De Silva, R.A.; Secco, M.P.; Lermen, R.T.; Schneider, I.A.H.; Hidalgo, G.E.N.; Sampaio, C.H. Optimizing the Selective Precipitation of Iron to Produce Yellow Pigment from Acid Mine Drainage. Miner. Eng. 2019, 135, 111–117. [Google Scholar] [CrossRef]
- Tambwe, O.; Kotsiopoulos, A.; Harrison, S.T.L. Desulphurising High Sulphur Coal Discards Using an Accelerated Heap Leach Approach. Hydrometallurgy 2020, 197, 105472. [Google Scholar] [CrossRef]
- Menezes, J.C.C.C.; Colling, A.V.; Silva, R.A.S.; Scheneider, I.A.H. Effect of Pyrite Concentration on the Quality of Ferric Sulfate Coagulants Obtained by Leaching from Coal Tailings. Miner. Metall. Processing 2016, 33, 77–81. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Amaral Filho, J. Aproveitamento de Rejeitos de Carvão em Santa Catarina. Ph.D. Thesis, UFRGS, Porto Alegre, Brazil, 2014. [Google Scholar]
- Machado De Oliveira, C.; Gesser Müller, T.; Patricio Ferreira, L.; Prado Cechinel, M.A.; Peterson, M.; Raupp-Pereira, F. Valorization of Iron Pyrite from Coal Mining in Southern Brazil. J. Environ. Chem. Eng. 2019, 7, 102931. [Google Scholar] [CrossRef]
- Akdogan, G.; Bradshaw, S.; Dorfling, C.; Bergmann, C.; Ghosh, T.; Campbell, Q. Characterization of Rare Earth Elements by XRT Sorting Products of a South African Coal Seam. Int. J. Coal Prep. Util. 2022, 42, 1071–1087. [Google Scholar] [CrossRef]
| ROM Coal | Thermal Coal | |
|---|---|---|
| Sulfur (%) | 0.5–15 | 0.7–1 |
| Calorific Value (MJ/kg) | 16–21 | 19–27 |
| Ash (%) | 20–40 | 20–30 |
| Mineral Phase | Chemical Formula | (%) |
|---|---|---|
| Quartz | SiO2 | 27.9 |
| Kaolinite | Al4Si4O10(OH)2·H2O | 52.1 |
| Gypsum | CaSO4·2H2O | 4.4 |
| Illite | (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2,(H2O)] | 8.5 |
| K-feldspar | KAlSi3O8 | 3.5 |
| Pyrite | FeS2 | 1.8 |
| Anhydrite | CaSO4 | 0.8 |
| Magnetite | Fe2O4 | 1.1 |
| Sieve Aperture (mm) | Retained Mass (%) | Cum. Mass (%) |
|---|---|---|
| 31.5 | 20.8 | 20.8 |
| 22.5 | 4.5 | 25.3 |
| 16.0 | 10.2 | 35.5 |
| 11.2 | 15.8 | 51.3 |
| 8.0 | 6.2 | 57.5 |
| 5.6 | 21.5 | 79.0 |
| 4.5 | 7.0 | 86.0 |
| 2.0 | 14.0 | 100.0 |
| Sample Name | Total S (%) | MPA | ANC kg H2SO4/t | NAPP | ARD Classification |
|---|---|---|---|---|---|
| Bulk sample (feed) | 1.6 | 49.9 | 3.9 | 45.9 | PAF |
| Density Fraction (g/cm) | Mass (%) | Proximate Analysis (%) | Ultimate Analysis (%) | CV (MJ/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ash | FC | Moisture | VM | C | H | N | S | |||
| 1.8 | 58.5 | 19.6 | 53.2 | 2.7 | 24.5 | 60.5 | 3.3 | 1.6 | 0.5 | 24.6 |
| 2.0 | 3.5 | 49.4 | 25.3 | 1.8 | 23.5 | 42.0 | 2.7 | 1.2 | 0.4 | 13.1 |
| 2.2 | 7.9 | 59.1 | 18.4 | 2.2 | 20.3 | 35.0 | 2.4 | 1.0 | 2.8 | 9.4 |
| 2.7 | 28.1 | 80.4 | 1.4 | 1.6 | 16.6 | 12.7 | 1.6 | 0.5 | 1.5 | 1.2 |
| >2.7 | 2.0 | 60.6 | 11.2 | 1.7 | 26.5 | 11.4 | 0.9 | 0.4 | 34.7 | 8.8 |
| Density Fraction (g/cm3) | Sulfur Forms (%) | ||
|---|---|---|---|
| Organic S | Sulfate S | Sulfide S | |
| 1.8 | 0.01 | 0.09 | 0.40 |
| 2.0 | 0.01 | 0.08 | 0.30 |
| 2.2 | 0.01 | 0.56 | 2.07 |
| 2.7 | 0.01 | 0.20 | 1.40 |
| >2.7 | 0.01 | 0.70 | 33.30 |
| Density Fraction (g/cm3) | Total S (%) | MPA | ANC kg H2SO4/t | NAPP | ARD Classification |
|---|---|---|---|---|---|
| 1.8 | 0.5 | 15.3 | 17.8 | −3.0 | NAF |
| 2.0 | 0.4 | 12.2 | 0 | 12.2 | PAF |
| 2.2 | 2.8 | 85.7 | 13.1 | 98.8 | PAF |
| 2.7 | 1.5 | 45.9 | 4.4 | 50.3 | PAF |
| >2.7 | 34.7 | 1061.8 | 0 | 1061.8 | AF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral Filho, J.R.d.; Gcayiya, M.; Kotsiopoulos, A.; Broadhurst, J.L.; Power, D.; Harrison, S.T.L. Valorization of South African Coal Wastes through Dense Medium Separation. Minerals 2022, 12, 1519. https://doi.org/10.3390/min12121519
Amaral Filho JRd, Gcayiya M, Kotsiopoulos A, Broadhurst JL, Power D, Harrison STL. Valorization of South African Coal Wastes through Dense Medium Separation. Minerals. 2022; 12(12):1519. https://doi.org/10.3390/min12121519
Chicago/Turabian StyleAmaral Filho, Juarez R. do, Msimelelo Gcayiya, Athanasios Kotsiopoulos, Jennifer L. Broadhurst, David Power, and Susan T. L. Harrison. 2022. "Valorization of South African Coal Wastes through Dense Medium Separation" Minerals 12, no. 12: 1519. https://doi.org/10.3390/min12121519
APA StyleAmaral Filho, J. R. d., Gcayiya, M., Kotsiopoulos, A., Broadhurst, J. L., Power, D., & Harrison, S. T. L. (2022). Valorization of South African Coal Wastes through Dense Medium Separation. Minerals, 12(12), 1519. https://doi.org/10.3390/min12121519








