Depositional Environment and Hydrocarbon Distribution in the Silurian–Devonian Black Shales of Western Peninsular Malaysia Using Spectroscopic Characterization
Abstract
1. Introduction
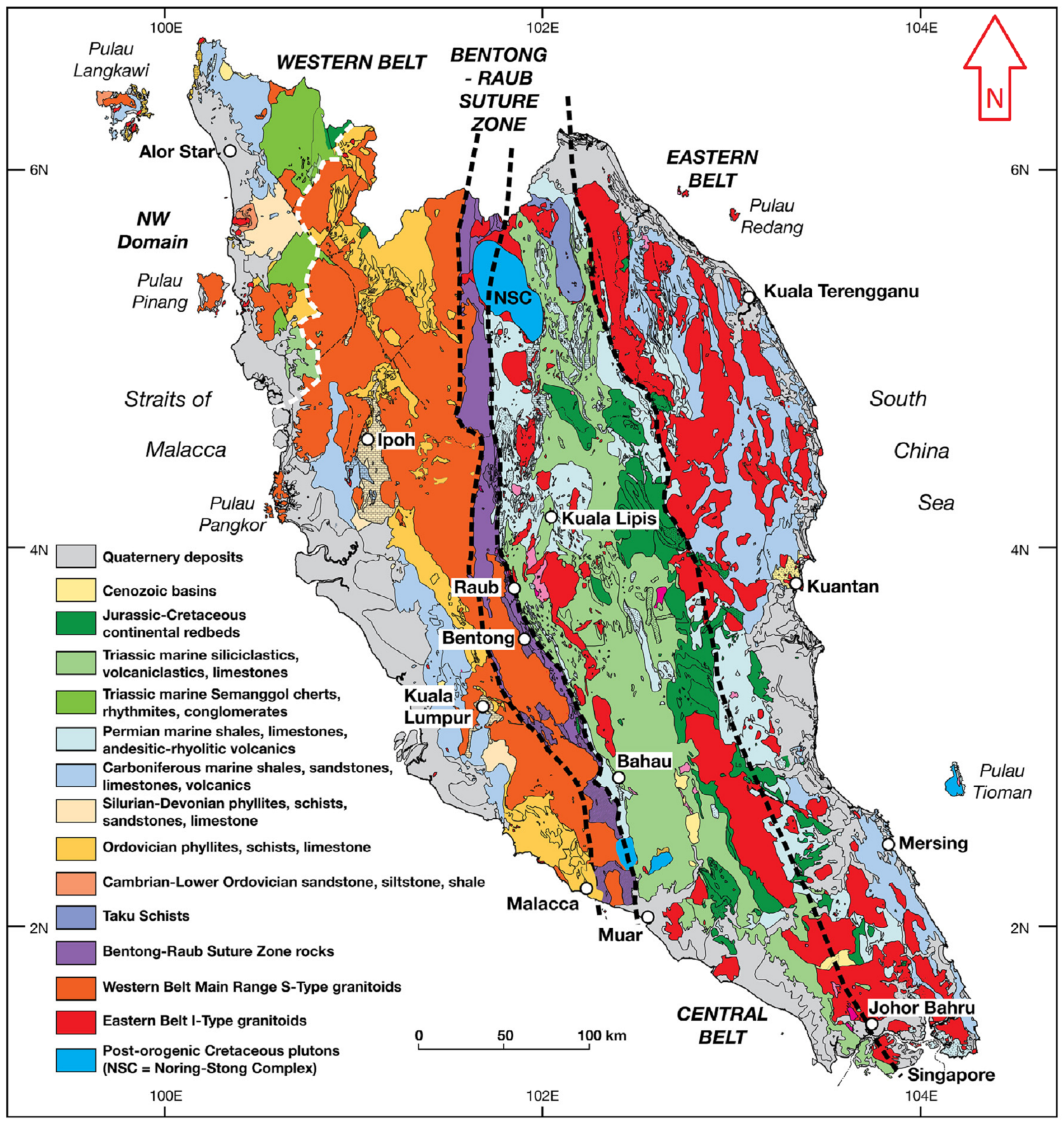
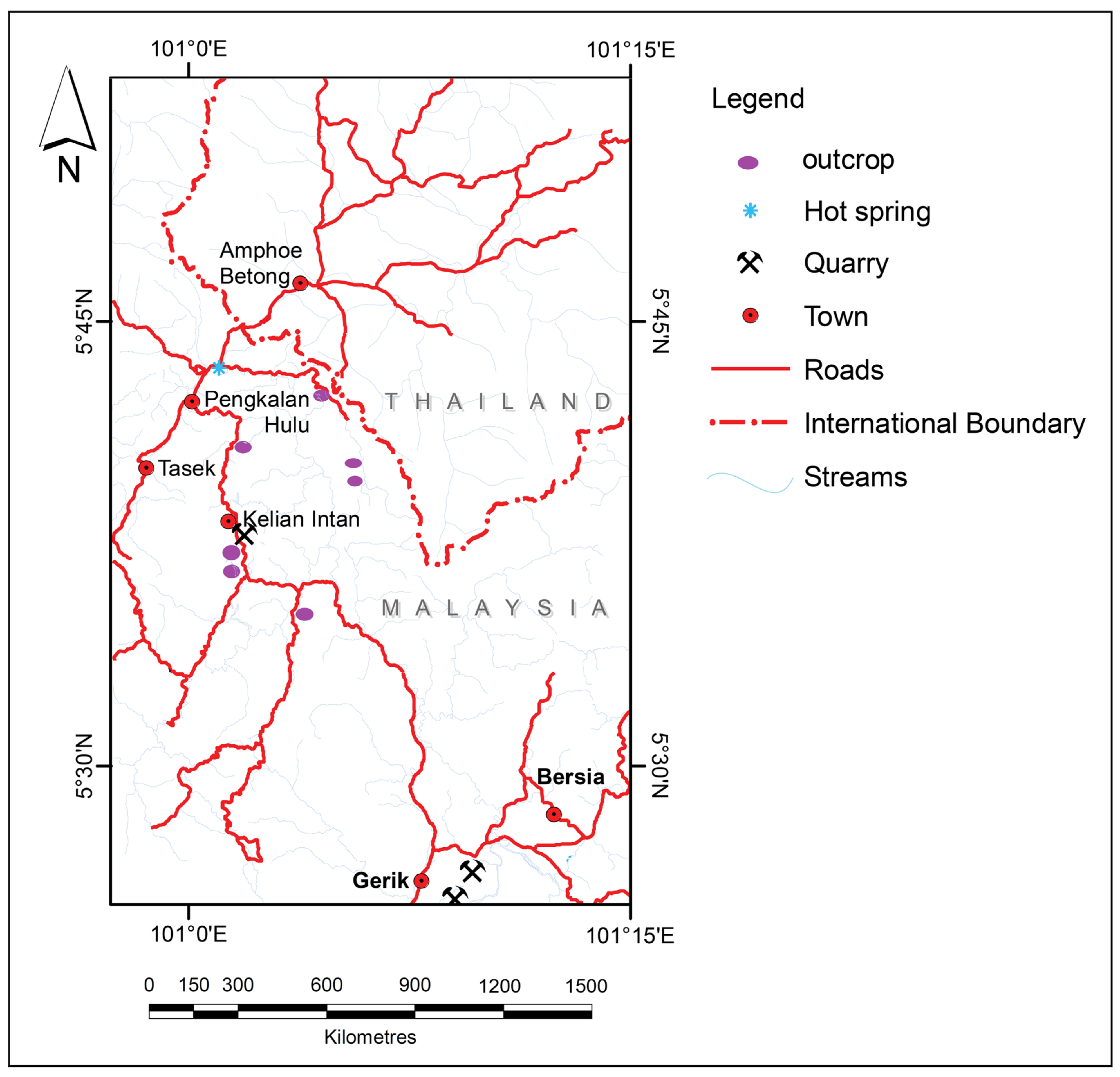
2. Study Area
3. Materials and Methods
3.1. Sampling
3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
3.3. Ultraviolet-Visible Spectroscopy (UV-Vis)
3.4. Total Organic Carbon (TOC)
4. Results
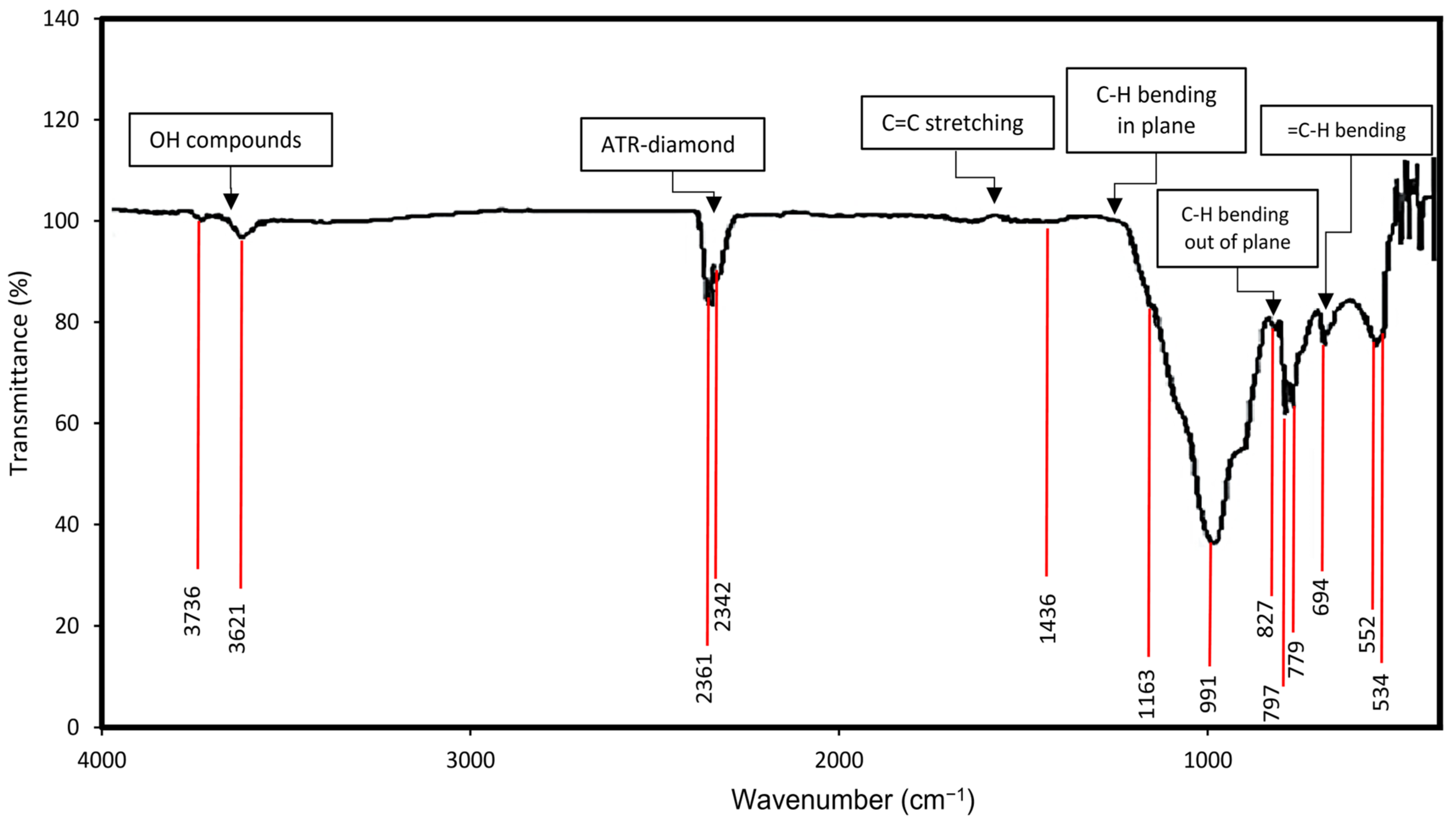
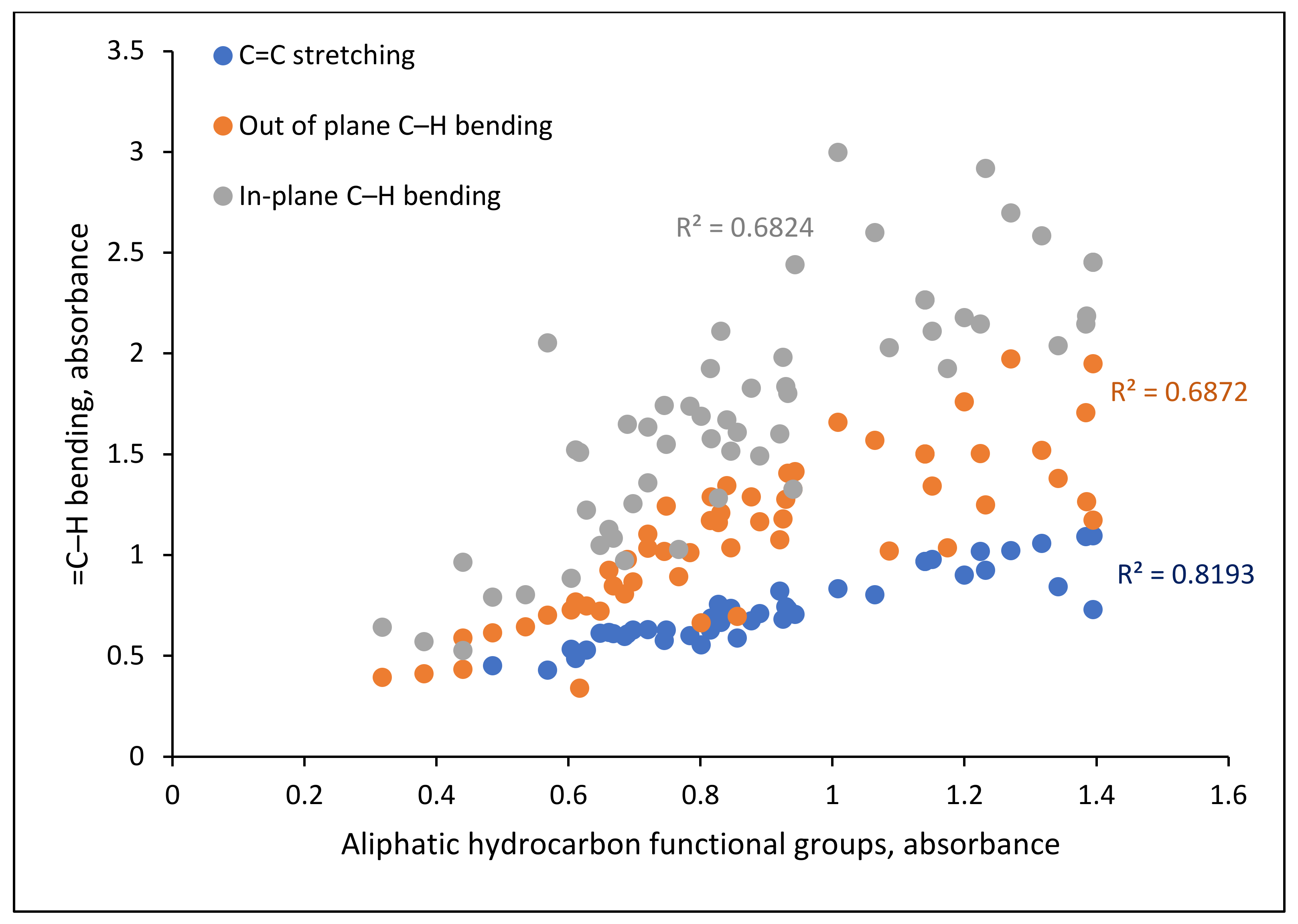
4.1. Hydrocarbon Functional Groups
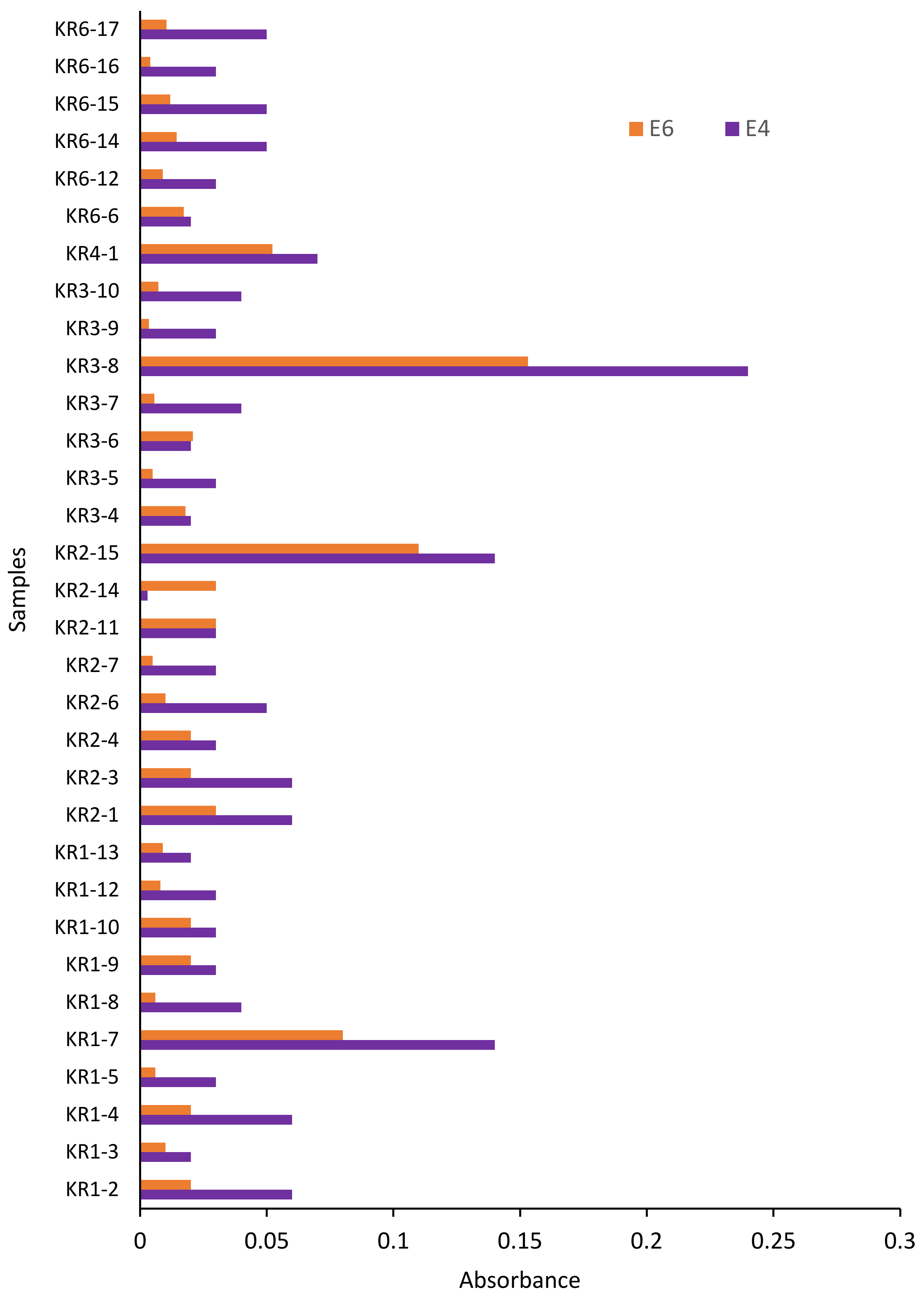
4.2. Distribution of E4 and E6
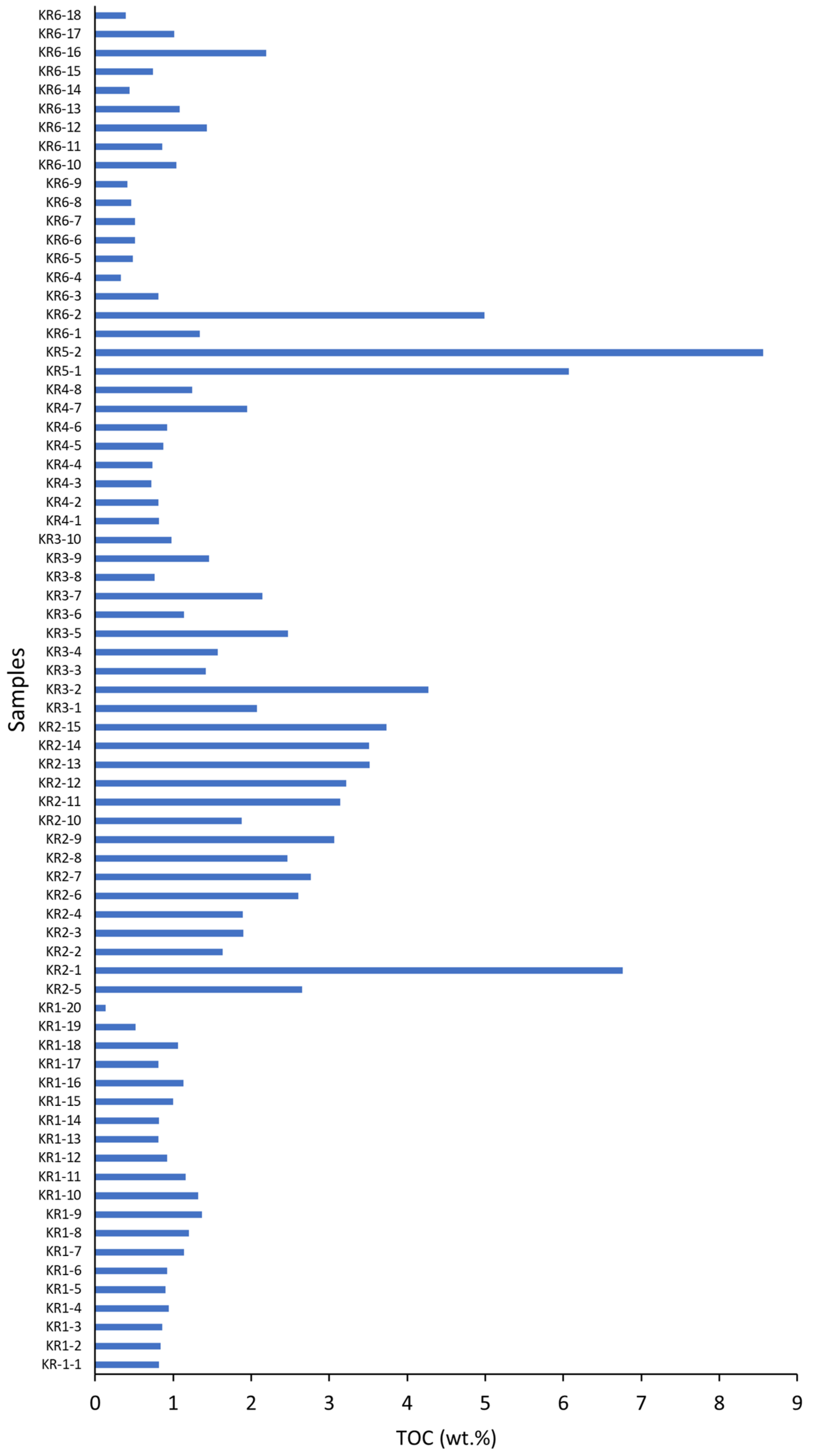
4.3. Total Organic Carbon (TOC)
5. Discussion
5.1. Hydrocarbon Functional Groups Distribution
5.2. Relationship of TOC with Functional Groups
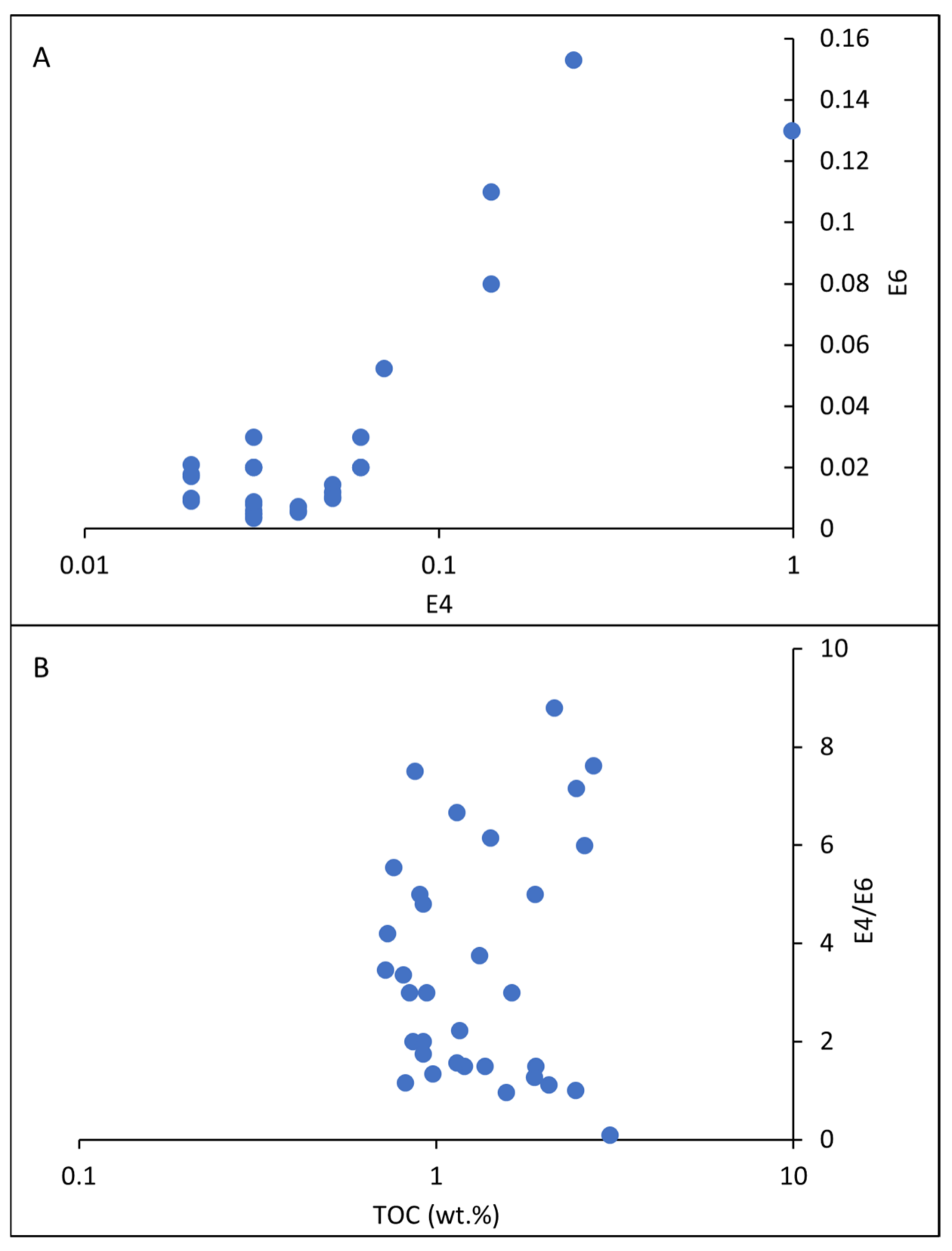
5.3. Hydrocarbon Compound Characterization Using E4/E6
5.4. Depositional Environment of Organic Matter
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Myers, K.J.; Wignall, P.B. Understanding Jurassic organic-rich mudrocks—New concepts using gamma-ray spectrometry and palaeoecology: Examples from the Kimmeridge Clay of Dorset and the Jet Rock of Yorkshire. In Marine Clastic Sedimentology; Leggett, J.K., Zuffa, G.G., Eds.; Springer: Dordrecht, The Netherlands, 1987. [Google Scholar] [CrossRef]
- Owusu, E.B.; Tetteh, G.M.; Asante-Okyere, S.; Tsegab, H. Error correction of vitrinite reflectance in matured black shales: A machine learning approach. Unconv. Resour. 2022, 2, 41–50. [Google Scholar] [CrossRef]
- Tourtelot, H.A. Black shale—Its deposition and diagenesis. Clays Clay Miner. 1979, 27, 313–321. [Google Scholar] [CrossRef]
- Naumann, D.; Meyers, R. Encyclopedia of Analytical Chemistry; John Wiley Sons: Chichester, UK, 2000; pp. 102–131. [Google Scholar]
- Artz, R.R.E.; Chapman, S.J.; Robertson, A.H.J.; Potts, J.M.; Laggoun-Défarge, F.; Gogo, S.; Comont, L.; Disnar, J.-R.; Francez, A.-J. FTIR spectroscopy can be used as a screening tool for organic matter quality in regenerating cutover peatlands. Soil Biol. Biochem. 2008, 40, 515–527. [Google Scholar] [CrossRef]
- Yokota, T.; Scriven, F.; Montgomery, D.S.; Strausz, O.P. Absorption and emission spectra of Athabasca asphaltene in the visible and near ultraviolet regions. Fuel 1986, 65, 1142–1149. [Google Scholar] [CrossRef]
- Evdokimov, I.N.; Losev, A.P. Potential of UV-visible absorption spectroscopy for characterizing crude petroleum oils. Oil Gas Bus. 2007, 1, 1–21. [Google Scholar]
- Eglinton, G. Applications of infrared spectroscopy to organic chemistry. In An Introduction to Spectroscopic Methods for the Identification of Organic Compounds: Nuclear Magnetic Resonance and Infrared Spectroscopy; Pergamon: Oxford, UK, 1970; pp. 123–143. [Google Scholar] [CrossRef]
- Radke, M.; Rullkötter, J.; Vriend, S.P. Distribution of naphthalenes in crude oils from the Java Sea: Source and maturation effects. Geochim. Cosmochim. Acta 1994, 58, 3675–3689. [Google Scholar] [CrossRef]
- Bastow, T.P.; Alexander, R.; Fisher, S.J.; Singh, R.K.; van Aarssen, B.G.K.; Kagi, R.I. Geosynthesis of organic compounds. Part V—Methylation of alkylnaphthalenes. Org. Geochem. 2000, 31, 523–534. [Google Scholar] [CrossRef]
- Evdokimov, I.N.; Eliseev, N.Y.; Akhmetov, B.R. Assembly of asphaltene molecular aggregates as studied by near-UV/visible spectroscopy: II. Concentration dependencies of absorptivities. J. Pet. Sci. Eng. 2003, 37, 145–152. [Google Scholar] [CrossRef]
- Maciel, G.E.; Bartuska, V.J.; Miknis, F.P. Correlation between oil yields of oil shales and 13C nuclear magnetic resonance spectra. Fuel 1978, 57, 505–506. [Google Scholar] [CrossRef]
- Hutchison, C.S.; Tan, D.N.K. Geology of Peninsular Malaysia; The University of Malaya, The Geological Society of Malaysia: Kuala Lumpur, Malaysia, 2009; p. 479. [Google Scholar]
- Gebretsadik, H.T.; Sum, C.W.; Talib, J.A. Lithostratigraphy of Paleozoic Carbonates in the Kinta Valley, Peninsular Malaysia: Analogue for Paleozoic Successions; ICIPEG 2016; Springer: Singapore, 2017; pp. 559–567. [Google Scholar]
- Shoieb, M.A.; Gebretsadik, H.T.; Rahmani, O.; Ismail, M.S.; Ibad, S.M. Geochemical characteristics of the Silurian-Devonian Kroh black shales, Peninsular Malaysia: An implication for hydrocarbon exploration. J. Geochem. Explor. 2022, 232, 106891. [Google Scholar] [CrossRef]
- Metcalfe, I. Tectonic evolution of the Malay Peninsula. J. Asian Earth Sci. 2013, 76, 195–213. [Google Scholar] [CrossRef]
- Group, M.-T.W. Geology of the Pengkalan Hulu-Betong transect area along the Malaysia-Thailand border. Geol. Pap. 2009, 7, 1–84. [Google Scholar]
- Griffiths, P.R. Fourier transform infrared spectrometry. Science 1983, 222, 297–302. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, C.; Mastalerz, M.; Hu, S.; Gasaway, C.; Tao, X. Applications of micro-fourier transform infrared spectroscopy (FTIR) in the geological sciences—A review. Int. J. Mol. Sci. 2015, 16, 30223–30250. [Google Scholar] [CrossRef]
- McKelvy, M.L.; Britt, T.R.; Davis, B.L.; Gillie, J.K.; Lentz, L.A.; Leugers, A.; Nyquist, R.A.; Putzig, C.L. Infrared spectroscopy. Anal. Chem. 1996, 68, 93–160. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications, Analytical Techniques in the Sciences; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Washburn, K.E.; Birdwell, J.E. Multivariate analysis of ATR-FTIR spectra for assessment of oil shale organic geochemical properties. Org. Geochem. 2013, 63, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.; Senesi, N.; Schnitzer, M. Information provided on humic substances by E4/E6 ratios. Soil Sci. Soc. Am. J. 1977, 41, 352–358. [Google Scholar] [CrossRef]
- Schnitzer, M. Soil organic matter—The next 75 years. Soil Sci. 1991, 151, 41–58. [Google Scholar] [CrossRef]
- Peters, K.E.; Cassa, M.R. Applied source rock geochemistry: Chapter 5: Part II. Essential elements. In The Petroleum System—From Source to Trap; American Association of Petroleum Geologists, AAPG: Tulsa, OK, USA, 1994; Volume 60, pp. 93–120. [Google Scholar]
- Dow, W.G.; Pearson, D.B. Organic matter in Gulf Coast sediments. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 4 May 1975; pp. 85–94. [Google Scholar] [CrossRef]
- Wiberley, S.E.; Gonzalez, R.D. Infrared spectra of polynuclear aromatic compounds in the CH stretching and out-of-plane bending regions. Appl. Spectrosc. 1961, 15, 174–177. [Google Scholar] [CrossRef]
- Ibad, S.M.; Padmanabhan, E. Exploring the relationship between hydrocarbons with total carbon and organic carbon in black shale from Perak, Malaysia. Pet. Coal 2017, 59, 933–943. [Google Scholar]
- Yang, G.-P. Polycyclic aromatic hydrocarbons in the sediments of the South China Sea. Environ. Pollut. 2000, 108, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Helgeson, H.C.; Richard, L.; McKenzie, W.F.; Norton, D.L.; Schmitt, A. A chemical and thermodynamic model of oil generation in hydrocarbon source rocks. Geochim. Cosmochim. Acta 2009, 73, 594–695. [Google Scholar] [CrossRef]
- Tissot, B.P.; Welte, D.H. Petroleum Formation and Occurrence; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar] [CrossRef]
- Tan, J.; Horsfield, B.; Mahlstedt, N.; Zhang, J.; Boreham, C.J.; Hippler, D.; van Graas, G.; Tocher, B.A. Natural gas potential of Neoproterozoic and lower Palaeozoic marine shales in the Upper Yangtze Platform, South China: Geological and organic geochemical characterization. Int. Geol. Rev. 2015, 57, 305–326. [Google Scholar] [CrossRef]
- Ross, D.J.K.; Bustin, R.M. Characterizing the shale gas resource potential of Devonian–Mississippian strata in the Western Canada sedimentary basin: Application of an integrated formation evaluation. Am. Assoc. Pet. Geol. Bull. 2008, 92, 87–125. [Google Scholar] [CrossRef]
- Jarvie, D.M.; Hill, R.J.; Ruble, T.E.; Pollastro, R.M. Unconventional shale-gas systems: The Mississippian Barnett Shale of north-central Texas as one model for thermogenic shale-gas assessment. Am. Assoc. Pet. Geol. Bull. 2007, 91, 475–499. [Google Scholar] [CrossRef]
- Du, X.; Song, X.; Zhang, M.; Lu, Y.; Lu, Y.; Chen, P.; Liu, Z.; Yang, S. Shale gas potential of the Lower Permian Gufeng Formation in the western area of the Lower Yangtze Platform, China. Mar. Pet. Geol. 2015, 67, 526–543. [Google Scholar] [CrossRef]
- Egbobawaye, I.E. Petroleum Source-Rock Evaluation and Hydrocarbon Potential in Montney Formation Unconventional Reservoir, Northeastern British Columbia, Canada, Natural Resources. Nat. Resour. 2017, 8, 716. [Google Scholar] [CrossRef]
- Ibad, S.M.; Padmanabhan, E. Methane sorption capacities and geochemical characterization of Paleozoic shale Formations from Western Peninsula Malaysia: Implication of shale gas potential. Int. J. Coal Geol. 2020, 224, 103480. [Google Scholar] [CrossRef]
- Abou El-Anwar, E.; Salman, S.; Mousa, D.; Aita, S.; Makled, W.; Gentzis, T. Organic Petrographic and Geochemical Evaluation of the Black Shale of the Duwi Formation, El Sebaiya, Nile Valley, Egypt. Minerals 2021, 11, 1416. [Google Scholar] [CrossRef]
- Li, B.; Feng, C.; Li, X.; Chen, Y.; Niu, J.; Shen, Z. Spatial distribution and source apportionment of PAHs in surficial sediments of the Yangtze Estuary, China. Mar. Pollut. Bull. 2012, 64, 636–643. [Google Scholar] [CrossRef]
- Wu, F.; Xu, L.; Sun, Y.; Liao, H.; Zhao, X.; Guo, J. Exploring the relationship between polycyclic aromatic hydrocarbons and sedimentary organic carbon in three Chinese lakes. J. Soils Sediments 2012, 12, 774–783. [Google Scholar] [CrossRef]
- González-Vila, F.J.; Amblès, A.; del Rıo, J.C.; Grasset, L. Characterisation and differentiation of kerogens by pyrolytic and chemical degradation techniques. J. Anal. Appl. Pyrolysis 2001, 58, 315–328. [Google Scholar] [CrossRef]
- Cunha, T.J.F.; Novotny, E.H.; Madari, B.E.; Martin-Neto, L.; Rezende, M.O.d.O.; Canelas, L.P.; Benites, V.d.M. Spectroscopy characterization of humic acids isolated from Amazonian Dark Earth Soils (terra preta de Índio). In Amazonian Dark Earths: Wim Sombroek’s Vision; Springer: Berlin/Heidelberg, Germany, 2009; pp. 363–372. [Google Scholar] [CrossRef]
- Strachan, M.G.; Alexander, R.; Kagi, R.I. Trimethyl naphthalenes in crude oils and sediments: Effects of source and maturity. Geochim. Cosmochim. Acta 1988, 52, 1255–1264. [Google Scholar] [CrossRef]
- Volkman, J.K.; Alexander, R.; Kagi, R.I.; Rowland, S.J.; Sheppard, P.N. Biodegradation of aromatic hydrocarbons in crude oils from the Barrow Sub-basin of Western Australia. Org. Geochem. 1984, 6, 619–632. [Google Scholar] [CrossRef]
- Wang, X.; Cai, T.; Wen, W.; Ai, J.; Ai, J.; Zhang, Z.; Zhu, L.; George, S.C. Surfactin for enhanced removal of aromatic hydrocarbons during biodegradation of crude oil. Fuel 2020, 267, 117272. [Google Scholar] [CrossRef]
- Cheng, X.; Hou, D.; Mao, R.; Xu, C. Severe biodegradation of polycyclic aromatic hydrocarbons in reservoired crude oils from the Miaoxi Depression, Bohai Bay Basin. Fuel 2018, 211, 859–867. [Google Scholar] [CrossRef]
- Asahina, K.; Suzuki, N. Alkyl naphthalenes and tetralins as indicators of source and source rock lithology–Pyrolysis of a cadinane-type sesquiterpene in the presence and absence of montmorillonite. J. Pet. Sci. Eng. 2016, 145, 657–667. [Google Scholar] [CrossRef]
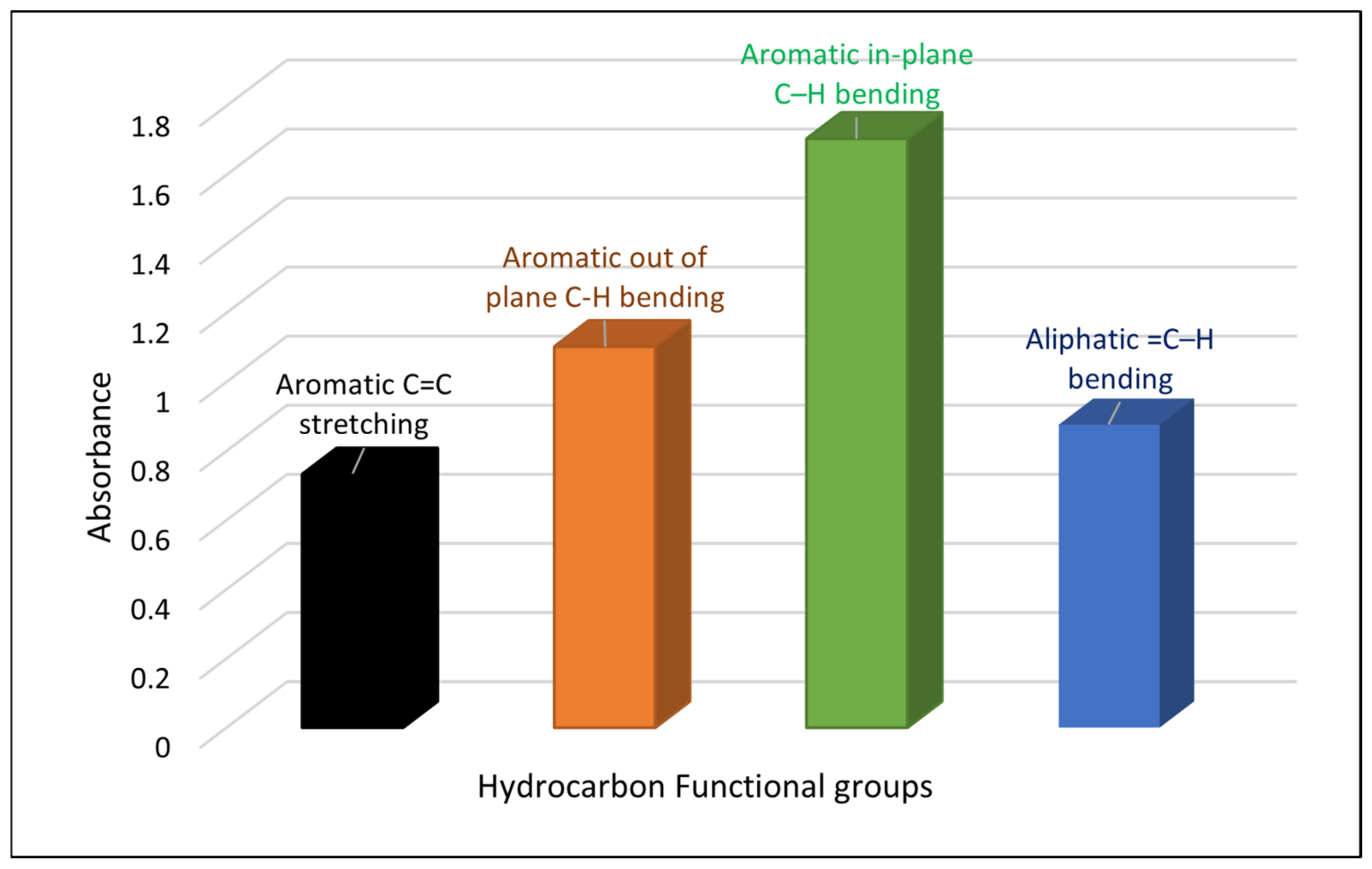

| Samples | Aromatic Hydrocarbon | Aliphatic Hydrocarbon 700–600 C–H Bending Absorbance | ||
|---|---|---|---|---|
| 1600–1430 C=C Stretching Absorbance | 900–690 Out of Plane C-H Bending Absorbance | 1275–1000 In-Plane C–H Bending Absorbance | ||
| KR1-2 | 0.903 | 1.761 | 2.18 | 1.2 |
| KR1-3 | N.A | 1.105 | 1.361 | 0.72 |
| KR1-4 | 0.806 | 1.57 | 2.602 | 1.064 |
| KR1-5 | 0.708 | 1.416 | 2.443 | 0.943 |
| KR1-6 | 0.734 | 1.408 | 1.804 | 0.932 |
| KR1-7 | 0.745 | 1.279 | 1.838 | 0.929 |
| KR1-8 | 0.712 | 1.167 | 1.494 | 0.89 |
| KR1-9 | 0.531 | 0.749 | 1.224 | 0.627 |
| KR1-10 | 0.97 | 1.503 | 2.267 | 1.14 |
| KR1-11 | 0.845 | 1.381 | 2.04 | 1.342 |
| KR1-12 | 0.63 | 1.173 | 1.928 | 0.815 |
| KR2-2 | 0.534 | 0.73 | 0.887 | 0.604 |
| KR2-3 | 1.019 | 1.505 | 2.148 | 1.224 |
| KR2-4 | 0.737 | 1.038 | 1.518 | 0.846 |
| KR2-6 | 0.981 | 1.344 | 2.113 | 1.151 |
| KR2-8 | 0.599 | 0.809 | 0.974 | 0.685 |
| KR2-9 | 0.823 | 1.077 | 1.602 | 0.92 |
| KR2-12 | 0.63 | 0.868 | 1.257 | 0.698 |
| KR2-14 | 0.615 | 0.724 | 1.049 | 0.648 |
| KR3-1 | N.A | 0.436 | 0.529 | 0.44 |
| KR3-3 | 0.453 | 0.617 | 0.793 | 0.485 |
| KR3-4 | 0.619 | 0.927 | 1.129 | 0.661 |
| KR3-5 | N.A | 0.413 | 0.573 | 0.381 |
| KR3-6 | 0.757 | 1.163 | 1.284 | 0.827 |
| KR3-7 | 0.613 | 0.849 | 1.086 | 0.668 |
| KR3-8 | N.A | 0.895 | 1.03 | 0.767 |
| KR3-10 | N.A | 0.646 | 0.806 | 0.535 |
| KR3-13 | N.A | 1.328 | 1.328 | 0.94 |
| KR4-1 | 0.834 | 1.661 | 3 | 1.008 |
| KR4-2 | 0.63 | 1.244 | 1.552 | 0.748 |
| KR4-3 | N.A | 0.591 | 0.967 | 0.44 |
| KR4-4 | 1.023 | 1.974 | 2.698 | 1.27 |
| KR4-5 | 0.633 | 1.036 | 1.636 | 0.72 |
| KR4-6 | 0.689 | 1.29 | 1.578 | 0.816 |
| KR4-7 | 0.61 | 0.98 | 1.651 | 0.689 |
| KR4-8 | 0.707 | 1.346 | 1.673 | 0.84 |
| KR5-1 | N.A | 0.395 | 0.643 | 0.318 |
| KR5-2 | 0.676 | 1.29 | 1.829 | 0.877 |
| KR6-1 | 0.927 | 1.251 | 2.92 | 1.232 |
| KR6-2 | N.A | 1.021 | 2.031 | 1.086 |
| KR6-3 | 0.602 | 1.014 | 1.74 | 0.784 |
| KR6-4 | 0.579 | 1.019 | 1.744 | 0.745 |
| KR6-5 | 0.667 | 1.211 | 2.113 | 0.831 |
| KR6-6 | 0.431 | 0.703 | 2.055 | 0.568 |
| KR6-8 | 0.488 | 0.769 | 1.524 | 0.611 |
| KR6-9 | 0.683 | 1.181 | 1.982 | 0.925 |
| KR6-10 | 0.97 | 1.503 | 2.267 | 1.14 |
| KR6-11 | 1.059 | 1.521 | 2.585 | 1.317 |
| KR6-12 | 0.732 | 1.175 | 2.455 | 1.395 |
| KR6-13 | N.A | 0.341 | 1.511 | 0.617 |
| KR6-14 | 0.591 | 0.698 | 1.61 | 0.856 |
| KR6-15 | 0.556 | 0.665 | 1.69 | 0.801 |
| KR6-18 | 1.094 | 1.709 | 2.148 | 1.384 |
| KR6-19 | N.A | 1.267 | 2.187 | 1.385 |
| KR6-20 | N.A | 1.038 | 1.928 | 1.174 |
| KR7-1 | 1.098 | 1.95 | 2.455 | 1.395 |
| Formation | Age | TOC (wt.%) | Average TOC (wt.%) | Reference |
|---|---|---|---|---|
| Longmaxi, China | Lower Silurian | 0.44–4 | 2.32 | [33] |
| Niutitang, China | Lower Cambrian | 0.39–10.2 | 5.26 | [33] |
| Muskwa, Besa & Fort Simpson, Canada | Devonian– Mississippian | 0.18–4.72 | 1.39 | [34] |
| Barnett shale, USA | Mississippian | 2.62–11.47 | 4.66 | [35] |
| Gufeng, China | Lower Permian | 0.04–22.1 | 3.4 | [36] |
| Montney, Canada | Lower Triassic | 0.03–8.2 | 2.64 | [37] |
| Baling and Bendang Riang, Malaysia | Silurian–Devonian | 0.73–24.6 | 6.71 | [38] |
| Kubang Pasu, Malaysia | Lower Permian | 1.01–19.65 | 5.74 | [38] |
| Duwi Formation, El Sebaiya, Egypt | Late Campanian–early Maastrichtian | 0.21–2.77 | 1.4 | [39] |
| Kroh shale, Malaysia | Silurian–Devonian | 0.13–8.56 | 1.71 | Current study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoieb, M.A.; Gebretsadik, H.T.; Ibad, S.M.; Rahmani, O. Depositional Environment and Hydrocarbon Distribution in the Silurian–Devonian Black Shales of Western Peninsular Malaysia Using Spectroscopic Characterization. Minerals 2022, 12, 1501. https://doi.org/10.3390/min12121501
Shoieb MA, Gebretsadik HT, Ibad SM, Rahmani O. Depositional Environment and Hydrocarbon Distribution in the Silurian–Devonian Black Shales of Western Peninsular Malaysia Using Spectroscopic Characterization. Minerals. 2022; 12(12):1501. https://doi.org/10.3390/min12121501
Chicago/Turabian StyleShoieb, Monera Adam, Haylay Tsegab Gebretsadik, Syed Muhammad Ibad, and Omeid Rahmani. 2022. "Depositional Environment and Hydrocarbon Distribution in the Silurian–Devonian Black Shales of Western Peninsular Malaysia Using Spectroscopic Characterization" Minerals 12, no. 12: 1501. https://doi.org/10.3390/min12121501
APA StyleShoieb, M. A., Gebretsadik, H. T., Ibad, S. M., & Rahmani, O. (2022). Depositional Environment and Hydrocarbon Distribution in the Silurian–Devonian Black Shales of Western Peninsular Malaysia Using Spectroscopic Characterization. Minerals, 12(12), 1501. https://doi.org/10.3390/min12121501






