State-of-the-Art Nanoclay Reinforcement in Green Polymeric Nanocomposite: From Design to New Opportunities
Abstract
1. Introduction
2. Nanoclay
3. Polymer/Nanoclay Nanocomposite
| Polymer | Nanoclay | Polymer/Nanoclay Structure | Ref |
|---|---|---|---|
| Polypropylene | Montmorillonite | Exfoliated and intercalated | [51,52] |
| Polypropylene | Kaolinite | Intercalated | [53,54] |
| Polypropylene | Cloisite | Exfoliated | [55,56] |
| Polypropylene | Vinyl clay | Exfoliated | [57] |
| Polypropylene | Sepiolite | Exfoliated or intercalated | [58,59] |
4. Green Polymer/Nanoclay Nanocomposite
4.1. Cellulose/Nanoclay Nanocomposite
4.2. Starch/Nanoclay Nanocomposite
4.3. Poly(Lactic Acid)/Nanoclay Nanocomposite
4.4. Natural Rubber/Nanoclay Nanocomposite
4.5. Silk/Nanoclay Nanocomposite
5. Significance of Green Polymer/Nanoclay Nanocomposite
5.1. Sustainability
5.2. Membranes/Packaging
5.3. Biomedical Relevance
6. Encounters, Future and Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Masini, J.C.; Abate, G. Guidelines to Study the Adsorption of Pesticides onto Clay Minerals Aiming at a Straightforward Evaluation of Their Removal Performance. Minerals 2021, 11, 282. [Google Scholar] [CrossRef]
- Perelomov, L.; Mandzhieva, S.; Minkina, T.; Atroshchenko, Y.; Perelomova, I.; Bauer, T.; Pinsky, D.; Barakhov, A. The synthesis of organoclays based on clay minerals with different structural expansion capacities. Minerals 2021, 11, 707. [Google Scholar] [CrossRef]
- Undabeytia, T.; Shuali, U.; Nir, S.; Rubin, B. Applications of chemically modified clay minerals and clays to water purification and slow release formulations of herbicides. Minerals 2020, 11, 9. [Google Scholar] [CrossRef]
- Jacquet, A.; Geatches, D.L.; Clark, S.J.; Greenwell, H.C. Understanding cationic polymer adsorption on mineral surfaces: Kaolinite in cement aggregates. Minerals 2018, 8, 130. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Yavna, V. Comparative study of the hydrophobicity of organo-montmorillonite modified with cationic, amphoteric and nonionic surfactants. Minerals 2020, 10, 732. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. Introduction to Clay Science: Techniques and Applications: Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–7. [Google Scholar]
- Kumar, J.; Jatoi, A.S.; Mazari, S.A.; Ali, E.Y.; Hossain, N.; Abro, R.; Mubarak, N.M.; Sabzoi, N. Advanced Green Nanocomposite Materials for Wastewater Treatment: Sustainable Nanotechnology for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 297–321. [Google Scholar]
- Alhanish, A.; Ali, G.A.M. Recent Developments in Wastewater Treatment Using Polymer/Clay Nanocompo-sites. In Advances in Nanocomposite Materials for Environmental and Energy Harvesting Applications. Engineering Materials; Shalan, A.E., Hamdy Makhlouf, A.S., Lanceros-Méndez, S., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Tripathi, M.; Singh, B. Synthesis of Green Nanocomposite Material for Engineering Application: Sustainable Nanotechnology for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 135–157. [Google Scholar]
- Shokrani, H.; Shokrani, A.; Jouyandeh, M.; Seidi, F.; Gholami, F.; Kar, S.; Munir, M.T.; Kowalkowska-Zedler, D.; Zarrintaj, P.; Rabiee, N. Green polymer nanocomposites for skin tissue engineering. ACS Appl. Bio Mater. 2022, 5, 2107–2121. [Google Scholar] [CrossRef] [PubMed]
- Barra, A.; Nunes, C.; Ruiz-Hitzky, E.; Ferreira, P. Green Carbon Nanostructures for Functional Composite Materials. Int. J. Mol. Sci. 2022, 23, 1848. [Google Scholar] [CrossRef]
- Thakur, M.; Chandel, M.; Rani, A.; Sharma, A. Introduction to Biorenewable Nanocomposite Materials: Methods of Preparation, Current Developments, and Future Perspectives: Biorenewable Nanocomposite Materials, Vol. 2: Desalination and Wastewater Remediation; ACS Publications: Washington, DC, USA, 2022; pp. 1–24. [Google Scholar]
- Idumah, C.I.; Okonkwo, U.; Obele, C. Recently Emerging Advancements in Montmorillonite Polymeric Nanoarchitectures and Applications. Clean. Mater. 2022, 4, 100071. [Google Scholar] [CrossRef]
- Paul, A.; Augustine, R.; Hasan, A.; Zahid, A.A.; Thomas, S.; Agatemor, C.; Ghosal, K. Halloysite nanotube and chitosan polymer composites: Physicochemical and drug delivery properties. J. Drug Deliv. Sci. Technol. 2022, 72, 103380. [Google Scholar] [CrossRef]
- Palem, R.R.; Rao, K.M.; Shimoga, G.; Saratale, R.G.; Shinde, S.K.; Ghodake, G.S.; Lee, S.-H. Physicochemical characterization, drug release, and biocompatibility evaluation of carboxymethyl cellulose-based hydrogels reinforced with sepiolite nanoclay. Int. J. Biol. Macromol. 2021, 178, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, J.; Mei, C.; Yang, R.; He, W.; Li, X.; Cai, L.; Guo, M.; Li, J.; Xia, C. Thermal and flame-retardant properties of multilayered composites prepared through novel multilayering approach. Environ. Res. 2022, 213, 113724. [Google Scholar] [CrossRef] [PubMed]
- Gamage, A.; Thiviya, P.; Mani, S.; Ponnusamy, P.G.; Manamperi, A.; Evon, P.; Merah, O.; Madhujith, T. Environmental Properties and Applications of Biodegradable Starch-Based Nanocomposites. Polymers 2022, 14, 4578. [Google Scholar] [CrossRef] [PubMed]
- Calesco, M.; Watanuki Filho, A.; de Moura, M.; Aouada, F. Effects of addition of hybrid nanocomposite based on polysaccharide hydrogel and nanoclay on the fresh and hardened properties of cementitious mortars. Cerâmica 2022, 68, 97–107. [Google Scholar] [CrossRef]
- Ramesh, M.; Maniraj, J.; Kumar, S.G. Polysaccharide-Fibrous Clay Bionanocomposites and their Applications. Adv. Appl. Micro Nano Clay Biopolym.-Based Compos. 2022, 125, 1–26. [Google Scholar]
- Mihankhah, P.; Azdast, T.; Mohammadzadeh, H.; Hasanzadeh, R.; Aghaiee, S. Fused filament fabrication of biodegradable polylactic acid reinforced by nanoclay as a potential biomedical material. J. Thermoplast. Compos. Mater. 2021, 2021. [Google Scholar] [CrossRef]
- Oliver-Ortega, H.; Vandemoortele, V.; Bala, A.; Julian, F.; Méndez, J.A.; Espinach, F.X. Nanoclay effect into the biodegradation and processability of poly (lactic acid) nanocomposites for food packaging. Polymers 2021, 13, 2741. [Google Scholar] [CrossRef] [PubMed]
- Chaiwutthinan, P.; Phutfak, N.; Larpkasemsuk, A. Effects of thermoplastic poly (ether-ester) elastomer and bentonite nanoclay on properties of poly (lactic acid). J. Appl. Polym. Sci. 2021, 138, 50443. [Google Scholar] [CrossRef]
- Nair, R.; Bhattacharya, A.; Bhowmik, P.; Kant, R. Effect of surface modification on mechanical properties of filature silk waste and nanoclay filler-based polymer matrix composite. Polym. Polym. Compos. 2021, 29, S696–S706. [Google Scholar] [CrossRef]
- Yadav, R.; Purwar, R.; Bose, U. Studies of rheological, electrical, and optical properties of silk fibroin/Benzalkonium chloride modified MMT clay nanocomposite films. Mater. Today Proc. 2021, 45, 4998–5004. [Google Scholar] [CrossRef]
- Wongvasana, B.; Thongnuanchan, B.; Masa, A.; Saito, H.; Sakai, T.; Lopattananon, N. Comparative Structure–Property Relationship between Nanoclay and Cellulose Nanofiber Reinforced Natural Rubber Nanocomposites. Polymers 2022, 14, 3747. [Google Scholar] [CrossRef] [PubMed]
- Miedzianowska, J.; Masłowski, M.; Rybiński, P.; Strzelec, K. Modified nanoclays/straw fillers as functional additives of natural rubber biocomposites. Polymers 2021, 13, 799. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, T.R.; Neeraj, P.; Ramakrishnan, S.; Amarnath, S.; Lorenzetti, D.; Mohamed, P.; Tyres, A. Sepiolite nanoclay: A reinforcing filler in the natural rubber/butadiene rubber (NR/BR) matrix for tire tread compound application. Rubber World 2021, 263, 32–44. [Google Scholar]
- Abulyazied, D.E.; Ene, A. An investigative study on the progress of nanoclay-reinforced polymers: Preparation, properties, and applications: A review. Polymers 2021, 13, 4401. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Daneshvar e Asl, S.; Vossoughi, M.; Simchi, A.; Sadrzadeh, M. Green electrospun membranes based on chitosan/amino-functionalized nanoclay composite fibers for cationic dye removal: Synthesis and kinetic studies. ACS Omega 2021, 6, 10816–10827. [Google Scholar] [CrossRef] [PubMed]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Tullio, S.; Chalcraft, D. Converting natural nanoclay into modified nanoclay augments the toxic effect of natural nanoclay on aquatic invertebrates. Ecotoxic. Environ. Saf. 2020, 197, 110602. [Google Scholar] [CrossRef]
- Galimberti, M. Rubber-Clay Nanocomposites: Science, Technology, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Mousavi, M.; Fini, E.H.; Hung, A.M. Underlying molecular interactions between sodium montmorillonite clay and acidic bitumen. J. Phys. Chem. C 2019, 123, 15513–15522. [Google Scholar] [CrossRef]
- Li, A.; Wang, A.-Q.; Chen, J.-M. Preparation and Properties of Poly (acrylic acid-potassium acrylate)/Attapulgite Superabsorbent Composite. J. Funct. Polym. 2004, 17, 200–206. [Google Scholar]
- Mazloomi, F.; Jalali, M. Effects of vermiculite, nanoclay and zeolite on ammonium transport through saturated sandy loam soil: Column experiments and modeling approaches. Catena 2019, 176, 170–180. [Google Scholar] [CrossRef]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous materials for oil spill cleanup: A review of synthesis and absorbing properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef]
- Srithammaraj, K. Electrical Property-Investigation of Porous Clay Heterostructures Derived from Naturally-Occurring Clay Minerals for Smart Packaging. Ph.D. Thesis, Chulalongkorn University, Bangkok, Thailand, 2017. [Google Scholar]
- Taylor, R.; Smith, T. The engineering geology of clay minerals: Swelling, shrinking and mudrock breakdown. Clay Miner. 1986, 21, 235–260. [Google Scholar] [CrossRef]
- Ma, C.; Eggleton, R.A. Cation exchange capacity of kaolinite. Clays Clay Miner. 1999, 47, 174–180. [Google Scholar]
- Wypych, F.; Bergaya, F.; Schoonheydt, R.A. From Polymers to Clay Polymer Nanocomposites: Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2018; pp. 331–359. [Google Scholar]
- Albdiry, M.; Yousif, B.; Ku, H.; Lau, K. A critical review on the manufacturing processes n relation to the properties of nanoclay/polymer composites. J. Compos. Mater. 2013, 47, 1093–1115. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.B.; Ming, L.Y.; Yong, H.C.; Tahir, M.F.M. Clay-Based Materials in Geopolymer Technology: Cement Based Materials; IntechOpen: London, UK, 2018. [Google Scholar]
- Misaelides, P. Clay Minerals and Zeolites for Radioactive Waste Immobilization and Containment: A Concise Verview: Modified Clay and Zeolite Nanocomposite Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 243–274. [Google Scholar]
- Shunmugasamy, V.C.; Xiang, C.; Gupta, N. Clay/Polymer Nanocomposites: Processing, Properties, and Applications: Hybrid and Hierarchical Composite Materials; Springer: Berlin/Heidelberg, Germany, 2015; pp. 161–200. [Google Scholar]
- Rafiee, R.; Shahzadi, R. Mechanical properties of nanoclay and nanoclay reinforced polymers: A review. Polym. Compos. 2019, 40, 431–445. [Google Scholar] [CrossRef]
- Das, P.; Manna, S.; Behera, A.K.; Shee, M.; Basak, P.; Sharma, A.K. Current synthesis and characterization techniques for clay-based polymer nano-composites and its biomedical applications: A review. Environ. Res. 2022, 212, 113534. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Kwon, Y.R.; Kim, J.S.; Kim, J.-H.; Kim, D.H. Surface-crosslinking in the presence of nanoclay and characteristics of the itaconic acid-based superabsorbent polymer composites. Polym.-Plast. Technol. Mater. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Alias, A.H.; Norizan, M.N.; Sabaruddin, F.A.; Asyraf, M.R.M.; Norrrahim, M.N.F.; Ilyas, A.R.; Kuzmin, A.M.; Rayung, M.; Shazleen, S.S.; Nazrin, A. Hybridization of MMT/lignocellulosic fiber reinforced polymer nanocomposites for structural applications: A review. Coatings 2021, 11, 1355. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A review of the synthesis and applications of polymer–nanoclay composites. Appl. Sci. 2018, 8, 1696. [Google Scholar] [CrossRef]
- Barmouz, M.; Seyfi, J.; Givi, M.K.B.; Hejazi, I.; Davachi, S.M. A novel approach for producing polymer nanocomposites by in-situ dispersion of clay particles via friction stir processing. Mater. Sci. Eng. A 2011, 528, 3003–3006. [Google Scholar] [CrossRef]
- Chinellato, A.; Vidotti, S.; Hu, G.-H.; Pessan, L. Compatibilizing effect of acrylic acid modified polypropylene on the morphology and permeability properties of polypropylene/organoclay nanocomposites. Compos. Sci. Technol. 2010, 70, 458–465. [Google Scholar] [CrossRef]
- Selvakumar, V.; Palanikumar, K.; Palanivelu, K. Studies on mechanical characterization of polypropylene/Na-MMT nanocomposites. J. Miner. Mater. Charact. Eng. 2010, 9, 671. [Google Scholar]
- Tang, W.; Song, L.; Zhang, S.; Li, H.; Sun, J.; Gu, X. Preparation of thiourea-intercalated kaolinite and its influence on thermostability and flammability of polypropylene composite. J. Mater. Sci. 2017, 52, 208–217. [Google Scholar] [CrossRef]
- Tang, W.; Song, L.; Liu, F.; Dessie, W.; Qin, Z.; Zhang, S.; Gu, X. Improving the flame retardancy and thermal stability of polypropylene composites via introducing glycine intercalated kaolinite compounds. Appl. Clay Sci. 2022, 217, 106411. [Google Scholar] [CrossRef]
- Touati, N.; Kaci, M.; Bruzaud, S.; Grohens, Y. The effects of reprocessing cycles on the structure and properties of isotactic polypropylene/cloisite 15A nanocomposites. Polym. Degrad. Stab. 2011, 96, 1064–1073. [Google Scholar] [CrossRef]
- Samal, S.K.; Nayak, S.K.; Mohanty, S. Polypropylene nanocomposites: Effect of organo-modified layered silicates on mechanical, thermal & morphological performance. J. Thermoplas. Compos. Mater. 2008, 21, 243–263. [Google Scholar]
- Kumar, V.; Singh, A. Polypropylene clay nanocomposites. Rev. Chem. Eng. 2013, 29, 439–448. [Google Scholar] [CrossRef]
- Asensio, M.; Herrero, M.; Núñez, K.; Gallego, R.; Merino, J.C.; Pastor, J.M. In situ polymerization of isotactic polypropylene sepiolite nanocomposites and its copolymers by metallocene catalysis. Eur. Polym. J. 2018, 100, 278–289. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Tripathi, B.P. Polydopamine primed phosphorylated sepiolite-Polypropylene nanocomposite with enhanced thermal, rheological, and flame retardant properties. Polym. Degrad. Stab. 2022, 202, 110005. [Google Scholar] [CrossRef]
- Fakhri, L.A.; Ghanbarzadeh, B.; Dehghannya, J.; Abbasi, F.; Ranjbar, H. Optimization of mechanical and color properties of polystyrene/nanoclay/nano ZnO based nanocomposite packaging sheet using response surface methodology. Food Packag. Shelf Life 2018, 17, 11–24. [Google Scholar] [CrossRef]
- Ahmed, L.; Zhang, B.; Shen, R.; Agnew, R.J.; Park, H.; Cheng, Z.; Mannan, M.S.; Wang, Q. Fire reaction properties of polystyrene-based nanocomposites using nanosilica and nanoclay as additives in cone calorimeter test. J. Therm. Anal. Calor. 2018, 132, 1853–1865. [Google Scholar] [CrossRef]
- Pilevar, Z.; Bahrami, A.; Beikzadeh, S.; Hosseini, H.; Jafari, S.M. Migration of styrene monomer from polystyrene packaging materials into foods: Characterization and safety evaluation. Trends Food Sci. Technol. 2019, 91, 248–261. [Google Scholar] [CrossRef]
- Awad, S.A. Mechanical and thermal characterisations of low-density polyethylene/nanoclay composites. Polym. Polym. Compos. 2021, 29, 1325–1332. [Google Scholar] [CrossRef]
- Karami, S.; Nazockdast, H.; Ahmadi, Z.; Rabolt, J.F.; Noda, I.; Chase, D.B. Microstructure effects on the rheology of nanoclay-filled PHB/LDPE blends. Polym. Compos. 2019, 40, 4125–4134. [Google Scholar] [CrossRef]
- Mansourian, A.; Goahri, A.R.; Khosrowshahi, F.K. Performance evaluation of asphalt binder modified with EVA/HDPE/nanoclay based on linear and non-linear viscoelastic behaviors. Construct. Build. Mater. 2019, 208, 554–563. [Google Scholar] [CrossRef]
- Sánchez-Valdes, S. High-density polyethylene/recycled HDPE/nanoclay composites using an amine-alcohol modified polyethylene as a compatibilizer. Iran. Polym. J. 2021, 30, 297–305. [Google Scholar] [CrossRef]
- Luo, J.; Tian, Q.; Qin, S.; Qi, Y.; Li, J.; Zhang, D. Effect of organoclay content on thermal stability properties of polypropylene/organoclay nanocomposites. J. Thermoplast. Compos. Mater. 2022, 35, 533–554. [Google Scholar] [CrossRef]
- Žiganova, M.; Merijs-Meri, R.; Zicāns, J.; Ivanova, T.; Bochkov, I.; Kalniņš, M.; Błędzki, A.K.; Danilovas, P.P. Characterisation of Nanoclay and Spelt Husk Microfiller-Modified Polypropylene Composites. Polymers 2022, 14, 4332. [Google Scholar] [CrossRef]
- Lu, X. Transparent, Biaxially Oriented Barrier Films from Polypropylene-Clay Nanocomposites. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 2022. [Google Scholar]
- Valério, M.B.; dos Santos, L.E.; de Carvalho, L.J.; Reznik, L.Y.; da Silva, A.L.N. Barrier behavior evaluation of polyamide 12 nanoclay composite to offshore application by electrochemical impedance spectroscopy. J. Appl. Polym. Sci. 2022, 139, 51863. [Google Scholar] [CrossRef]
- Tajuddin, M.; Yusof, N.; Fajrina, N.; Salleh, W.; Ismail, A.; Jaafar, J.; Aziz, F. Tailoring the properties of polyamide thin film membrane with layered double hydroxide nanoclay for enhancement in water separation. Curr. Appl. Phys. 2022, 34, 36–40. [Google Scholar] [CrossRef]
- Paara, T.; Lange, S.; Kristjan, S.; Lõhmus, R.; Krumme, A.; Mändar, H. Improving the Oxygen Barrier of Polyamide Food Packaging by Using Nanoclay. Mater. Sci. 2022, 28, 217–223. [Google Scholar] [CrossRef]
- Bangera, M.K.; Kotian, R.; Natarajan, S.; Somasundaram, J.; Mangalath, D.L. Effects of graphene nanoplatelets and montmorillonite nanoclay reinforcement on dental polymethyl methacrylate. Polym. Compos. 2022, 43, 3626–3638. [Google Scholar] [CrossRef]
- Sengwa, R.; Dhatarwal, P. Toward multifunctionality of PEO/PMMA/MMT hybrid polymer nanocomposites: Promising morphological, nanostructural, thermal, broadband dielectric, and optical properties. J. Phys. Chem. Sol. 2022, 166, 110708. [Google Scholar] [CrossRef]
- Kausar, A.; Bocchetta, P. Poly (methyl methacrylate) Nanocomposite Foams Reinforced with Carbon and Inorganic Nanoparticles—State-of-the-Art. J. Compos. Sci. 2022, 6, 129. [Google Scholar] [CrossRef]
- Hussain, S.; Al-Sarraf, A. Influence of Bioactive and Bio Inert Ceramic Powders on Tribology Properties of PMMA Composite Denture Base. J. Biomimet. Biomater. Biomed. Eng. 2022, 57, 1–8. [Google Scholar] [CrossRef]
- Elakkiya, S.; Arthanareeswaran, G. Hydrophilic nanoclay-polyaniline decorated membrane with improved permeability for separation of endocrine-disrupting chemical and fitness of fouling using model. J. Indus. Eng. Chem. 2022, 110, 234–247. [Google Scholar] [CrossRef]
- Nigil, M.R.; Ramanujam, B.T.S.; Thiruvengadathan, R. Nanoclay-based Polymer Nanocomposites for Electromagnetic Interference Shielding. Adv. Appl. Micro Nano Clay II Synth. Polym. Compos. 2022, 129, 53–78. [Google Scholar]
- Landim, V.P.; Foguel, M.V.; Prado, C.M.; Sotomayor, M.P.; Vieira, I.C.; Silva, B.V.; Dutra, R.F. A Polypyrrole/Nanoclay Hybrid Film for Ultra-Sensitive Cardiac Troponin T Electrochemical Immunosensor. Biosensors 2022, 12, 545. [Google Scholar] [CrossRef] [PubMed]
- Motaung, T.E.; Linganiso, L.Z. Critical review on agrowaste cellulose applications for biopolymers. Int. J. Plast. Technol. 2018, 22, 185–216. [Google Scholar] [CrossRef]
- Gul, S.; Kausar, A.; Muhammad, B.; Jabeen, S. Research progress on properties and applications of polymer/clay nanocomposite. Polym.-Plast. Technol. Mater. 2022, 55, 684–703. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Tabone, M.D.; Cregg, J.J.; Beckman, E.J.; Landis, A.E. Sustainability metrics: Life cycle assessment and green design in polymers. Environ. Sci. Technol. 2010, 44, 8264–8269. [Google Scholar] [CrossRef]
- Wood, C.D.; Cooper, A.I.; DeSimone, J.M. Green synthesis of polymers using supercritical carbon dioxide. Curr. Opin. Sol. Stat. Mater. Sci. 2004, 8, 325–331. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Arabi, M.; Ghaedi, M.; Ostovan, A.; Wang, X.; Li, J.; Chen, L. Dummy molecularly imprinted polymers based on a green synthesis strategy for magnetic solid-phase extraction of acrylamide in food samples. Talanta 2019, 195, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Marti, M.; Molina, L.; Aleman, C.; Armelin, E. Novel epoxy coating based on DMSO as a green solvent, reducing drastically the volatile organic compound content and using conducting polymers as a nontoxic anticorrosive pigment. ACS Sustain. Chem. Eng. 2013, 1, 1609–1618. [Google Scholar] [CrossRef]
- Iordanskii, A.; Kamaev, P.; Olkhov, A.; Wasserman, A. Water transport phenomena in greenand petrochemicalpolymers. Differences and similarities. Desalination 1999, 126, 139–145. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, M.; Luo, J.; Li, X.; Gao, Q.; Li, J. A novel water-based process produces eco-friendly bio-adhesive made from green cross-linked soybean soluble polysaccharide and soy protein. Carbohydr. Polym. 2017, 169, 417–425. [Google Scholar] [CrossRef]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017, 12, 2957. [Google Scholar] [CrossRef] [PubMed]
- Green, J.J.; Elisseeff, J.H. Mimicking biological functionality with polymers for biomedical applications. Nature 2016, 540, 386–394. [Google Scholar] [CrossRef]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer–Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Sevastyanova, O.; Qin, W.; Kadla, J. Effect of nanofillers as reinforcement agents for lignin composite fibers. J. Appl. Polym. Sci. 2010, 117, 2877–2881. [Google Scholar] [CrossRef]
- Patanair, B.; Saiter-Fourcin, A.; Thomas, S.; Thomas, M.G.; Parathukkamparambil Pundarikashan, P.; Gopalan Nair, K.; Kumar, V.K.; Maria, H.J.; Delpouve, N. Promoting interfacial interactions with the addition of lignin in poly (lactic acid) hybrid nanocomposites. Polymers 2021, 13, 272. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Chen, N.; Zhang, Q.; Wang, K.; Fu, Q.; Zhang, X. Preparation and properties of chitosan nanocomposites with nanofillers of different dimensions. Polym. Degrad. Stab. 2009, 94, 124–131. [Google Scholar] [CrossRef]
- Fauzi, B.; Nawawi, M.G.M.; Fauzi, R.; Mamauod, S.N.L. Physicochemical characteristics of sago starch-chitosan nanofillers film. BioResources 2019, 14, 8324–8330. [Google Scholar]
- Grossman, A.; Vermerris, W. Lignin-based polymers and nanomaterials. Curr. Opin. Biotechnol. 2019, 56, 112–120. [Google Scholar] [CrossRef]
- Lancefield, C.S.; Westwood, N.J. The synthesis and analysis of advanced lignin model polymers. Green Chem. 2015, 17, 4980–4990. [Google Scholar] [CrossRef]
- Jayrajsinh, S.; Shankar, G.; Agrawal, Y.K.; Bakre, L. Montmorillonite nanoclay as a multifaceted drug-delivery carrier: A review. J. Drug Deliv. Sci. Technol. 2017, 39, 200–209. [Google Scholar] [CrossRef]
- Nazir, M.S.; Kassim, M.H.M.; Mohapatra, L.; Gilani, M.A.; Raza, M.R.; Majeed, K. Characteristic Properties of Nanoclays and Characterization of Nanoparticulates and Nanocomposites: Nanoclay Reinforced Polymer Composites; Springer: Berlin/Heidelberg, Germany, 2016; pp. 35–55. [Google Scholar]
- Hosseini, S.M.S.; Mirzaei, M. Assessment of the colloidal montmorillonite dispersion as a low-cost and eco-friendly nanofluid for improving thermal performance of plate heat exchanger. SN Appl. Sci. 2020, 2, 1719. [Google Scholar] [CrossRef]
- Penchah, H.R.; Ghaemi, A.; Godarziani, H. Eco-friendly CO2 adsorbent by impregnation of diethanolamine in nanoclay montmorillonite. Environ. Sci. Pollut. Res. 2021, 28, 55754–55770. [Google Scholar] [CrossRef] [PubMed]
- Ngwabebhoh, F.A.; Erdem, A.; Yildiz, U. Synergistic removal of Cu (II) and nitrazine yellow dye using an eco-friendly chitosan-montmorillonite hydrogel: Optimization by response surface methodology. J. Appl. Polym. Sci. 2016, 133, 29. [Google Scholar] [CrossRef]
- Bordes, P.; Pollet, E.; Avérous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M. Synthesis and properties of biodegradable poly (vinyl alcohol)/organo-nanoclay bionanocomposites. J. Polym. Environ. 2012, 20, 732–740. [Google Scholar] [CrossRef]
- Kim, Y. TEMPO-Oxidized Nanofibrillated Cellulose Film (NFC) Incorporating Graphene Oxide (GO) Nanofillers; Virginia Tech: Blacksburg, VA, USA, 2017. [Google Scholar]
- Wu, Y.-Y.; Zhang, J.; Liu, C.; Zheng, Z.; Lambert, P. Effect of graphene oxide nanosheets on physical properties of ultra-high-performance concrete with high volume supplementary cementitious materials. Materials 2020, 13, 1929. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, J.; Kang, H.; Zhang, L. Design, preparation and properties of bio-based elastomer composites aiming at engineering applications. Compos. Sci. Technol. 2016, 133, 136–156. [Google Scholar] [CrossRef]
- Alves, L.; Ferraz, E.; Gamelas, J. Composites of nanofibrillated cellulose with clay minerals: A review. Adv. Coll. Interf. Sci. 2019, 272, 101994. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Agarwal, S.; Kumar, A.; Kumar, V.; Prajapati, S.K.; Kumar, T.; Singhal, N. Waste Clothes to Microcrystalline Cellulose: An Experimental Investigation. J. Polym. Environ. 2022, 1–15. [Google Scholar] [CrossRef]
- Olusanya, J.O.; Mohan, T.; Kanny, K. Effect of cellulose-nanoparticles (CNPs) and nanoclay (NC) reinforced starch based biocomposite films on thermal and mechanical properties. Res. Sq. 2022, 1–24. [Google Scholar] [CrossRef]
- Chakkour, M.; Ould Moussa, M.; Khay, I.; Balli, M.; Ben Zineb, T. Towards widespread properties of cellulosic fibers composites: A comprehensive review. J. Reinf. Plast. Compos. 2022. [Google Scholar] [CrossRef]
- Barbi, S.; Taurino, C.; La China, S.; Anguluri, K.; Gullo, M.; Montorsi, M. Mechanical and structural properties of environmental green composites based on functionalized bacterial cellulose. Cellulose 2021, 28, 1431–1442. [Google Scholar] [CrossRef]
- Ming, S.; Chen, G.; He, J.; Kuang, Y.; Liu, Y.; Tao, R.; Ning, H.; Zhu, P.; Liu, Y.; Fang, Z. Highly transparent and self-extinguishing nanofibrillated cellulose-monolayer clay nanoplatelet hybrid films. Langmuir 2017, 33, 8455–8462. [Google Scholar] [CrossRef]
- Ferfera-Harrar, H.; Dairi, N. Green nanocomposite films based on cellulose acetate and biopolymer-modified nanoclays: Studies on morphology and properties. Iran. Polym. J. 2014, 23, 917–931. [Google Scholar] [CrossRef]
- Duan, J.; Gong, S.; Gao, Y.; Xie, X.; Jiang, L.; Cheng, Q. Bioinspired ternary artificial nacre nanocomposites based on reduced graphene oxide and nanofibrillar cellulose. ACS Appl. Mater. Interf. 2016, 8, 10545–10550. [Google Scholar] [CrossRef]
- Kadumudi, F.B.; Trifol, J.; Jahanshahi, M.; Zsurzsan, T.-G.; Mehrali, M.; Zeqiraj, E.; Shaki, H.; Alehosseini, M.; Gundlach, C.; Li, Q. Flexible and green electronics manufactured by origami folding of nanosilicate-reinforced cellulose paper. ACS Appl. Mater. Interf. 2020, 12, 48027–48039. [Google Scholar] [CrossRef] [PubMed]
- Mary, S.K.; Koshy, R.R.; Arunima, R.; Thomas, S.; Pothen, L.A. A review of recent advances in starch-based materials: Bionanocomposites, pH sensitive films, aerogels and carbon dots. Carbohydr. Polym. Technol. Appl. 2022, 3, 100190. [Google Scholar] [CrossRef]
- Zhiguang, C.; Junrong, H.; Huayin, P.; Keipper, W. The Effects of Temperature on Starch Molecular Conformation and Hydrogen Bonding. Starch-Stärke 2022, 74, 2100288. [Google Scholar] [CrossRef]
- Shahbaz, A.; Hussain, N.; Basra, M.A.R.; Bilal, M. Polysaccharides-Based Nano-Hybrid Biomaterial Platforms for Tissue Engineering, Drug Delivery, and Food Packaging Applications. Starch-Stärke 2022, 74, 2200023. [Google Scholar] [CrossRef]
- Madhumitha, G.; Fowsiya, J.; Mohana Roopan, S.; Thakur, V.K. Recent advances in starch–clay nanocomposites. Int. J. Polym. Anal. Charact. 2018, 23, 331–345. [Google Scholar] [CrossRef]
- Paluch, M.; Ostrowska, J.; Tyński, P.; Sadurski, W.; Konkol, M. Structural and Thermal Properties of Starch Plasticized with Glycerol/Urea Mixture. J. Polym. Environ. 2022, 30, 728–740. [Google Scholar] [CrossRef]
- Ben, Z.Y.; Samsudin, H.; Yhaya, M.F. Glycerol: Its properties, polymer synthesis, and applications in starch based films. Eur. Polym. J. 2022, 175, 111377. [Google Scholar] [CrossRef]
- Mansour, G.; Zoumaki, M.; Marinopoulou, A.; Tzetzis, D.; Prevezanos, M.; Raphaelides, S.N. Characterization and properties of non-granular thermoplastic starch—Clay biocomposite films. Carbohydr. Polym. 2020, 245, 116629. [Google Scholar] [CrossRef]
- Pandey, J.K.; Singh, R.P. Green nanocomposites from renewable resources: Effect of plasticizer on the structure and material properties of clay-filled starch. Starch-Stärke 2005, 57, 8–15. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Entezami, A.A. Physicochemical properties of starch–CMC–nanoclay biodegradable films. Int. J. Biol. Macromol. 2010, 46, 1–5. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, J.; Zhang, R.; Kong, R.; Lu, W.; Wang, X. Characterization and application of the microencapsulated carvacrol/sodium alginate films as food packaging materials. Int. J. Biol. Macromol. 2019, 141, 259–267. [Google Scholar] [CrossRef]
- Hasany, M.; Talebian, S.; Sadat, S.; Ranjbar, N.; Mehrali, M.; Wallace, G.G.; Mehrali, M. Synthesis, properties, and biomedical applications of alginate methacrylate (ALMA)-based hydrogels: Current advances and challenges. Appl. Mater. Today 2021, 24, 101150. [Google Scholar] [CrossRef]
- Wei, J.; Wang, B.; Li, Z.; Wu, Z.; Zhang, M.; Sheng, N.; Liang, Q.; Wang, H.; Chen, S. A 3D-printable TEMPO-oxidized bacterial cellulose/alginate hydrogel with enhanced stability via nanoclay incorporation. Carbohydr. Polym. 2020, 238, 116207. [Google Scholar] [CrossRef]
- Shahbazi, M.; Jäger, H.; Ahmadi, S.J.; Lacroix, M. Electron beam crosslinking of alginate/nanoclay ink to improve functional properties of 3D printed hydrogel for removing heavy metal ions. Carbohydr. Polym. 2020, 240, 116211. [Google Scholar] [CrossRef]
- Zhou, Z.; Cai, K.; Shen, J.; Cai, L.; Dai, B.; Wang, Z.; Ma, P.; Liu, J.; Shen, X. Fabrication and biological assessment of halloysite-doped micro/nano structures on titanium surface. Ceram. Int. 2022. [Google Scholar] [CrossRef]
- Karolina Pierchala, M.; Kadumudi, F.B.; Mehrali, M.; Zsurzsan, T.G.; Kempen, P.J.; Serdeczny, M.P.; Spangenberg, J.; Andresen, T.L.; Dolatshahi-Pirouz, A. Soft electronic materials with combinatorial properties generated via mussel-inspired chemistry and halloysite nanotube reinforcement. ACS Nano 2021, 15, 9531–9549. [Google Scholar] [CrossRef]
- Makaremi, M.; Pasbakhsh, P.; Cavallaro, G.; Lazzara, G.; Aw, Y.K.; Lee, S.M.; Milioto, S. Effect of morphology and size of halloysite nanotubes on functional pectin bionanocomposites for food packaging applications. ACS Appl. Mater. Interf. 2017, 9, 17476–17488. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Lazzara, G. Polysaccharides/Halloysite nanotubes for smart bionanocomposite materials. Carbohydr. Polym. 2020, 245, 116502. [Google Scholar] [CrossRef] [PubMed]
- Parvathy, S.; Hema, S.; Sajith, M.; Sulthan, R.; Sreelekshmi, C.; Sambhudevan, S.; Shankar, B. A Review on Barrier Properties of Nanocellulose and Polylactic acid Composites. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1258, 012017. [Google Scholar] [CrossRef]
- Shao, L.; Xi, Y.; Weng, Y. Recent Advances in PLA-Based Antibacterial Food Packaging and Its Applications. Molecules 2022, 27, 5953. [Google Scholar] [CrossRef] [PubMed]
- Darie, R.N.; Pâslaru, E.; Sdrobis, A.; Pricope, G.M.; Hitruc, G.E.; Poiată, A.; Baklavaridis, A.; Vasile, C. Effect of nanoclay hydrophilicity on the poly (lactic acid)/clay nanocomposites properties. Indus. Eng. Chem. Res. 2014, 53, 7877–7890. [Google Scholar] [CrossRef]
- Grigora, M.-E.; Terzopoulou, Z.; Tsongas, K.; Bikiaris, D.N.; Tzetzis, D. Physicochemical Characterization and Finite Element Analysis-Assisted Mechanical Behavior of Polylactic Acid-Montmorillonite 3D Printed Nanocomposites. Nanomaterials 2022, 12, 2641. [Google Scholar] [CrossRef]
- Bai, J.; Goodridge, R.D.; Hague, R.J.; Okamoto, M. Processing and characterization of a polylactic acid/nanoclay composite for laser sintering. Polym. Compos. 2017, 38, 2570–2576. [Google Scholar] [CrossRef]
- Eng, C.C.; Ibrahim, N.A.; Zainuddin, N.; Ariffin, H.; Yunus, W.M.; Wan, Z.; Then, Y.Y.; Teh, C.C. Enhancement of mechanical and thermal properties of polylactic acid/polycaprolactone blends by hydrophilic nanoclay. Indian J. Mater. Sci. 2013, 2013, 816503. [Google Scholar] [CrossRef]
- Irandoost, M.; Pezeshki-Modaress, M.; Javanbakht, V. Removal of lead from aqueous solution with nanofibrous nanocomposite of polycaprolactone adsorbent modified by nanoclay and nanozeolite. J. Water Proc. Eng. 2019, 32, 100981. [Google Scholar] [CrossRef]
- Sri, H.; Ganapathy, D.; Sivaswamy, V.; Veeraiyan, D.N.; Venugopalan, S. Osteogenic Effectiveness of 3d Printed Polycaprolactone Scaffold Implanted with Metallic Bone Regenerative Compounds—A Systematic Review. J. Pharmaceut. Negat. Res. 2022, 13, 514–523. [Google Scholar]
- Kazemzadeh, G.; Jirofti, N.; Kazemi Mehrjerdi, H.; Rajabioun, M.; Alamdaran, S.A.; Mohebbi-Kalhori, D.; Mirbagheri, M.S.; Taheri, R. A review on developments of in-vitro and in-vivo evaluation of hybrid PCL-based natural polymers nanofibers scaffolds for vascular tissue engineering. J. Indus. Text. 2022, 52, 15280837221128314. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Yazdanpanah, Z.; Soltani, Y.A.; Sardroud, H.A.; Nasirtabrizi, M.H.; Chen, X. Curcumin-loaded electrospun polycaprolactone/montmorillonite nanocomposite: Wound dressing application with anti-bacterial and low cell toxicity properties. J. Biomater. Sci. Polym. Ed. 2020, 31, 169–187. [Google Scholar] [CrossRef]
- Stodolak-Zych, E.; Kurpanik, R.; Dzierzkowska, E.; Gajek, M.; Zych, Ł.; Gryń, K.; Rapacz-Kmita, A. Effects of Montmorillonite and Gentamicin Addition on the Properties of Electrospun Polycaprolactone Fibers. Materials 2021, 14, 6905. [Google Scholar] [CrossRef]
- Bokobza, L. Natural rubber nanocomposites: A review. Nanomaterials 2018, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Morera, J.; Verdejo, R.; López-Manchado, M.A.; Santana, M.H. Sustainable mobility: The route of tires through the circular economy model. Waste Manag. 2021, 126, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Choi, K.; Nam, J.-D.; Choi, H.J. Magnetorheological elastomers: Fabrication, characteristics, and applications. Materials 2020, 13, 4597. [Google Scholar] [CrossRef]
- Keereerak, A.; Sukkhata, N.; Lehman, N.; Nakaramontri, Y.; Sengloyluan, K.; Johns, J.; Kalkornsurapranee, E. Development and Characterization of Unmodified and Modified Natural Rubber Composites Filled with Modified Clay. Polymers 2022, 14, 3515. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.J.; Egodage, S.M.; Walpalage, S. Enhancement of mechanical properties of natural rubber–clay nanocomposites through incorporation of silanated organoclay into natural rubber latex. e-Polymers 2020, 20, 144–153. [Google Scholar] [CrossRef]
- Siririttikrai, N.; Thanawan, S.; Suchiva, K.; Amornsakchai, T. Comparative study of natural rubber/clay nanocomposites prepared from fresh or concentrated latex. Polym. Test. 2017, 63, 244–250. [Google Scholar] [CrossRef]
- George, S.C.; Rajan, R.; Aprem, A.S.; Thomas, S.; Kim, S.S. The fabrication and properties of natural rubber-clay nanocomposites. Polym. Test. 2016, 51, 165–173. [Google Scholar] [CrossRef]
- Sookyung, U.; Nakason, C.; Venneman, N.; Thaijaroend, W. Influence concentration of modifying agent on properties of natural rubber/organoclay nanocomposites. Polym. Test. 2016, 54, 223–232. [Google Scholar] [CrossRef]
- Kapoor, S.; Kundu, S.C. Silk protein-based hydrogels: Promising advanced materials for biomedical applications. Acta Biomater. 2016, 31, 17–32. [Google Scholar] [CrossRef]
- Purwar, R. Bionanocomposites Based on Silk Proteins and Nanoclay: Nanotechnology in Textiles; Jenny Stanford Publishing: Singapore, 2020; pp. 179–204. [Google Scholar]
- Devi, R.R.; Mandal, M.; Maji, T.K. Physical properties of simul (red-silk cotton) wood (Bombax ceiba L.) chemically modified with styrene acrylonitrile co-polymer and nanoclay. Holzforschung 2012, 66, 365–371. [Google Scholar] [CrossRef]
- Doblhofer, E.; Schmid, J.; Rieß, M.; Daab, M.; Suntinger, M.; Habel, C.; Bargel, H.; Hugenschmidt, C.; Rosenfeldt, S.; Breu, J. Structural insights into water-based spider silk protein–nanoclay composites with excellent gas and water vapor barrier properties. ACS Appl. Mater. Interf. 2016, 8, 25535–25543. [Google Scholar] [CrossRef]
- Chen, P.; Xie, F.; McNally, T. Understanding the effects of montmorillonite and sepiolite on the properties of solution-cast chitosan and chitosan/silk peptide composite films. Polym. Int. 2021, 70, 527–535. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Zhang, A.; Ling, C.; Sheng, R.; Li, X.; Yao, Q.; Chen, J. Enzymatically crosslinked silk-nanosilicate reinforced hydrogel with dual-lineage bioactivity for osteochondral tissue engineering. Mater. Sci. Eng. C 2021, 127, 112215. [Google Scholar] [CrossRef]
- Kadumudi, F.B.; Jahanshahi, M.; Mehrali, M.; Zsurzsan, T.G.; Taebnia, N.; Hasany, M.; Mohanty, S.; Knott, A.; Godau, B.; Akbari, M.; et al. A Protein-Based, Water-Insoluble, and Bendable Polymer with Ionic Conductivity: A Roadmap for Flexible and Green Electronics. Adv. Sci. 2019, 6, 1801241. [Google Scholar] [CrossRef]
- Jawaid, M.; Chee, S.S.; Asim, M.; Saba, N.; Kalia, S. Sustainable kenaf/bamboo fibers/clay hybrid nanocomposites: Properties, environmental aspects and applications. J. Clean. Product. 2022, 330, 129938. [Google Scholar] [CrossRef]
- Spencer, P.; Spares, R.; Sweeney, J.; Coates, P. Modelling the large strain solid phase deformation behaviour of polymer nanoclay composites. Mech. Time-Depen. Mater. 2008, 12, 313–327. [Google Scholar] [CrossRef]
- Shakrani, S.A.; Ayob, A.; Rahim, M.A.A. A review of nanoclay applications in the pervious concrete pavement. AIP Conf. Proc. 2017, 1885, 020049. [Google Scholar]
- Shakrani, S.A.; Ayob, A.; Rahim, M.A.A. Applications of waste material in the pervious concrete pavement: A review. AIP Conf. Proc. 2017, 1885, 020048. [Google Scholar]
- Elango, K.; Gopi, R.; Saravanakumar, R.; Rajeshkumar, V.; Vivek, D.; Raman, S.V. Properties of pervious concrete—A state of the art review. Mater. Today Proc. 2021, 45, 2422–2425. [Google Scholar] [CrossRef]
- Mansi, A.; Sor, N.H.; Hilal, N.; Qaidi, S.M. The impact of nano clay on normal and high-performance concrete characteristics: A review. IOP Conf. Ser. Earth Environ. Sci. 2022, 961, 012085. [Google Scholar] [CrossRef]
- Ramadhansyah, P.; Mohd Ibrahim, M.Y.; Hainin, M.R.; Warid, M.N.M.; Ibrahim, M.H. Porous concrete pavement containing nano-silica: Pre-review. Adv. Mater. Res. 2014, 911, 454–458. [Google Scholar] [CrossRef]
- Biswas, B.; Warr, L.N.; Hilder, E.F.; Goswami, N.; Rahman, M.M.; Churchman, J.G.; Vasilev, K.; Pan, G.; Naidu, R. Biocompatible functionalisation of nanoclays for improved environmental remediation. Chem. Soc. Rev. 2019, 48, 3740–3770. [Google Scholar] [CrossRef]
- Soleimani, M.; Amini, N. Remediation of Environmental Pollutants Using Nanoclays: Nanoscience and Plant–Soil Systems; Springer: Berlin/Heidelberg, Germany, 2017; pp. 279–289. [Google Scholar]
- Lu, T.; Gou, H.; Rao, H.; Zhao, G. Recent progress in nanoclay-based Pickering emulsion and applications. J. Environ. Chem. Eng. 2021, 9, 105941. [Google Scholar] [CrossRef]
- Li, W.; Qamar, S.A.; Qamar, M.; Basharat, A.; Bilal, M.; Iqbal, H.M. Carrageenan-based nano-hybrid materials for the mitigation of hazardous environmental pollutants. Int. J. Biol. Macromol. 2021, 190, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Kataki, M.S.; Kakoti, B.B.; Deka, K.; Rajkumari, A. Nanotechnology Applications in Natural Nanoclays Production and Application for Better Sustainability. Sustain. Nanotechnol. Strateg. Prod. Appl. 2022, 159–171. [Google Scholar] [CrossRef]
- Iravani, R.; An, C.; Adamian, Y.; Mohammadi, M. A Review on the Use of Nanoclay Adsorbents in Environmental Pollution Control. Water Air Soil Pollut. 2022, 233, 109. [Google Scholar] [CrossRef]
- Floody, M.C.; Theng, B.; Reyes, P.; Mora, M. Natural nanoclays: Applications and future trends—A Chilean perspective. Clay Miner. 2009, 44, 161–176. [Google Scholar] [CrossRef]
- Vasilev, I.Y.; Ananev, V.; Sultanova, Y.M.; Kolpakova, V. The Influence of the Composition of Polyethylene, Starch, and Monoglyceride Biodegradable Compositions on Their Physicomechanical Properties and Structure. Polym. Sci. Ser. D 2022, 15, 122–127. [Google Scholar] [CrossRef]
- Pan, Y.; Farmahini-Farahani, M.; OHearn, P.; Xiao, H.; Ocampo, H. An overview of bio-based polymers for packaging materials. J. Bioresour. Bioprod. 2016, 1, 106–113. [Google Scholar]
- Emblem, A. Plastics Properties for Packaging Materials: Packaging Technology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 287–309. [Google Scholar]
- Akter, T.; Hossain, M.S. Application of plant fibers in environmental friendly composites for developed properties: A review. Clean. Mater. 2021, 2, 100032. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, R.; Shaqib, M.; Sarkar, A.; Chauhan, K.D. Biodegradable Polymers as Packaging Materials: Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 245–259. [Google Scholar]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Borowski, G.; Szczypiński, M.; Klepka, T.; Thakur, V.K. Recent progress in biodegradable polymers and nanocomposite-based packaging materials for sustainable environment. Int. J. Polym. Anal. Charact. 2018, 23, 383–395. [Google Scholar] [CrossRef]
- Goel, P.; Arora, R.; Haleem, R.; Shukla, S.K. Advances in Bio-degradable Polymer Composites-Based Packaging Material. Chem. Afr. 2022, 1–21. [Google Scholar] [CrossRef]
- Najeeb, J.; Naeem, S. Biodegradable Food Packaging Materials: Handbook of Biodegradable Materials; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–29. [Google Scholar]
- Kumar, M.; Panjagari, N.R.; Kanade, P.P.; Singh, A.K.; Badola, R.; Ganguly, S.; Behare, P.V.; Sharma, R.; Alam, T. Sodium caseinate-starch-modified montmorillonite based biodegradable film: Laboratory food extruder assisted exfoliation and characterization. Food Packag. Shelf Life 2018, 15, 17–27. [Google Scholar] [CrossRef]
- Garusinghe, U.M.; Varanasi, S.; Raghuwanshi, V.S.; Garnier, G.; Batchelor, W. Nanocellulose-montmorillonite composites of low water vapour permeability. Coll. Surf. A Physicochem. Eng. Asp. 2018, 540, 233–241. [Google Scholar] [CrossRef]
- Shori, S.; Chen, X.; Peralta, M.; Gao, H.; zur Loye, H.C.; Ploehn, H.J. Effect of interfacial pretreatment on the properties of montmorillonite/poly (vinyl alcohol) nanocomposites. J. Appl. Polym. Sci. 2015, 132, 18. [Google Scholar] [CrossRef]
- David, P.P., Jr. Review on the preparation, structure and property relation of clay-based polymer nanocomposites. KIMIKA 2017, 28, 44–56. [Google Scholar]
- Aulin, C.; Salazar-Alvarez, G.; Lindström, T. High strength, flexible and transparent nanofibrillated cellulose–nanoclay biohybrid films with tunable oxygen and water vapor permeability. Nanoscale 2012, 4, 6622–6628. [Google Scholar] [CrossRef] [PubMed]
- Farmahini-Farahani, M.; Bedane, A.H.; Pan, Y.; Xiao, H.; Eic, M.; Chibante, F. Cellulose/nanoclay composite films with high water vapor resistance and mechanical strength. Cellulose 2015, 22, 3941–3953. [Google Scholar] [CrossRef]
- de Souza, A.G.; Dos Santos, N.M.A.; da Silva Torin, R.F.; dos Santos Rosa, D. Synergic antimicrobial properties of Carvacrol essential oil and montmorillonite in biodegradable starch films. Int. J. Biol. Macromol. 2020, 164, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Cross, L.M.; Peak, C.W.; Gold, K.; Carrow, J.K.; Brokesh, A.; Singh, K.A. 2D nanoclay for biomedical applications: Regenerative medicine, therapeutic delivery, and additive manufacturing. Adv. Mater. 2019, 31, 1900332. [Google Scholar] [CrossRef]
- Peña-Parás, L.; Sánchez-Fernández, J.A.; Vidaltamayo, R. Nanoclays for biomedical applications. In Handbook of Ecomaterials; Springer: Cham, Switzerland, 2018; Volume 5, pp. 3453–3471. [Google Scholar]
- Gebeshuber, I.C. Biomimetic Nanotechnology Vol. 2. Biomimetics 2022, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Nilforoushzadeh, M.A.; Amirkhani, M.A.; Seirafianpour, F.; Pakbaz, Y.; Zare, S.; Goodarzi, A. Regenerative Medicine in Dermatology. J. Skin Stem Cell 2022, 9, e124222. [Google Scholar] [CrossRef]
- Jafari, A.; Ajji, Z.; Mousavi, A.; Naghieh, S.; Bencherif, S.A.; Savoji, H. Latest Advances in 3D Bioprinting of Cardiac Tissues. Adv. Mater. Technol. 2022, 7, 2101636. [Google Scholar] [CrossRef]
- Nouri, M.; Mokhtari, J.; Rostamloo, M. Electrospun poly (ɛ-caprolactone)/nanoclay nanofibrous mats for tissue engineering. Fibers Polym. 2013, 14, 957–964. [Google Scholar] [CrossRef]
- Balavigneswaran, C.K.; Muthuvijayan, V. Nanohybrid-Reinforced Gelatin-Ureidopyrimidinone-Based Self-healing Injectable Hydrogels for Tissue Engineering Applications. ACS Appl. Bio Mater. 2021, 4, 5362–5377. [Google Scholar] [CrossRef]
- Molchanov, V.S.; Efremova, M.A.; Kiseleva, T.Y.; Philippova, O.G.E.E. Injectable ultra soft hydrogel with natural nanoclay. Нанoсистемы физика химия математика 2019, 10, 76–85. [Google Scholar] [CrossRef]
- Mattausch, H. Properties and Applications of Nanoclay Composites: Polymer Nanoclay Composites; Elsevier: Amsterdam, The Netherlands, 2015; pp. 127–155. [Google Scholar]
- Ferrández-Rives, M.; Beltrán-Osuna, Á.A.; Gómez-Tejedor, J.A.; Gomez Ribelles, J.L. Electrospun PVA/bentonite nanocomposites mats for drug delivery. Materials 2017, 10, 1448. [Google Scholar] [CrossRef]
- Hsu, W.-H.; Chang, H.-M.; Lee, Y.-L.; Prasannan, A.; Hu, C.-C.; Wang, J.-S.; Lai, J.-Y.; Yang, J.M.; Jebaranjitham, N.; Tsai, H.-C. Biodegradable polymer-nanoclay composites as intestinal sleeve implants installed in digestive tract for obesity and type 2 diabetes treatment. Mater. Sci. Eng. C 2020, 110, 110676. [Google Scholar] [CrossRef] [PubMed]
- Dana, K.; Sarkar, M. Organophilic Nature of Nanoclay: Clay Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 117–138. [Google Scholar]
- Villalba-Rodríguez, A.M.; Martínez-González, S.; Sosa-Hernández, J.E.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M. Nanoclay/Polymer-Based Hydrogels and Enzyme-Loaded Nanostructures for Wound Healing Applications. Gels 2021, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Rezanejad Gatabi, Z.; Heshmati, N.; Mirhoseini, M.; Dabbaghianamiri, M. The Application of Clay-Based Nanocomposite Hydrogels in Wound Healing. Arab. J. Sci. Eng. 2022, 1–14. [Google Scholar] [CrossRef]
- Bibi, S.; Mir, S.; Rehman, W.; Menaa, F.; Gul, A.; Alaryani, F.S.S.; Alqahtani, A.M.; Haq, S.; Abdellatif, M.H. Synthesis and In Vitro/Ex Vivo Characterizations of Ceftriazone-Loaded Sodium Alginate/Poly (Vinyl Alcohol) Clay Reinforced Nanocomposites: Possible Applications in Wound Healing. Materials 2022, 15, 3885. [Google Scholar] [CrossRef] [PubMed]
- Asthana, N.; Pal, K.; Aljabali, A.A.; Tambuwala, M.M.; de Souza, F.G.; Pandey, K. Polyvinyl alcohol (PVA) mixed green–clay and aloe vera based polymeric membrane optimization: Peel-off mask formulation for skin care cosmeceuticals in green nanotechnology. J. Mol. Struct. 2021, 1229, 129592. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Abdallah, H.M.; Mohamed, N.A.; Mohamed, R.R. Synthesis, characterization and application of biodegradable crosslinked carboxymethyl chitosan/poly (vinyl alcohol) clay nanocomposites. Mater. Sci. Eng. C 2015, 56, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Barage, S.; Lakkakula, J.; Sharma, A.; Roy, A.; Alghamdi, S.; Almehmadi, M.; Hossain, M.; Allahyani, M.; Abdulaziz, O. Nanomaterial in Food Packaging: A Comprehensive Review. J. Nanomater. 2022, 2022, 6053922. [Google Scholar] [CrossRef]
- Moradihamedani, P. Recent development in polymer/montmorillonite clay mixed matrix membranes for gas separation: A short review. Polym. Bull. 2022, 1–25. [Google Scholar] [CrossRef]
- Chen, B.; Evans, J.R.; Greenwell, H.C.; Boulet, P.; Coveney, P.V.; Bowden, A.A.; Whiting, A. A critical appraisal of polymer–clay nanocomposites. Chem. Soc. Rev. 2008, 37, 568–594. [Google Scholar] [CrossRef]
- LeBaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate nanocomposites: An overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar] [CrossRef]
- Nourizadeh, H.; Bakhshayesh, A. Nanoclay-Based Products Across Global Markets. In StatNano Applied and Industrial Series: Nanoclay-Based Products Across Global Markets: Applications and Properties; StatNano Publications: Lund, Sweden, 2020; pp. 3–33. [Google Scholar]
- Gilman, J.W. Flammability and thermal stability studies of polymer layered-silicate (clay) nanocomposites. Appl. Clay Sci. 1999, 15, 31–49. [Google Scholar] [CrossRef]
- Beall, G.W.; Powell, C.E. Fundamentals of Polymer-Clay Nanocomposites; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Kawasumi, M. The discovery of polymer-clay hybrids. J. Polym. Sci. A Polym. Chem. 2004, 42, 819–824. [Google Scholar] [CrossRef]
- Lee, S.-R.; Park, H.-M.; Lim, H.; Kang, T.; Li, X.; Cho, W.-J.; Ha, C.-S. Microstructure, tensile properties, and biodegradability of aliphatic polyester/clay nanocomposites. Polymer 2002, 43, 2495–2500. [Google Scholar] [CrossRef]
- Avella, M.; De Vlieger, J.J.; Errico, M.E.; Fischer, S.; Vacca, P.; Volpe, M.G. Biodegradable starch/clay nanocomposite films for food packaging applications. Food Chem. 2005, 93, 467–474. [Google Scholar] [CrossRef]
- Aysa, N.H.; Shalan, A.E. Green Nanocomposites: Magical Solution for Environmental Pollution Problems: Advances in Nanocomposite Materials for Environmental and Energy Harvesting Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 389–417. [Google Scholar]
- Niroumand, H.; Zain, M.; Alhosseini, S.N. The influence of nano-clays on compressive strength of earth bricks as sustainable materials. Proced-Soc. Behav. Sci. 2013, 89, 862–865. [Google Scholar] [CrossRef]
- Khan, S.H. Green Nanotechnology for the Environment and Sustainable Development: Green Materials for Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2020; pp. 13–46. [Google Scholar]
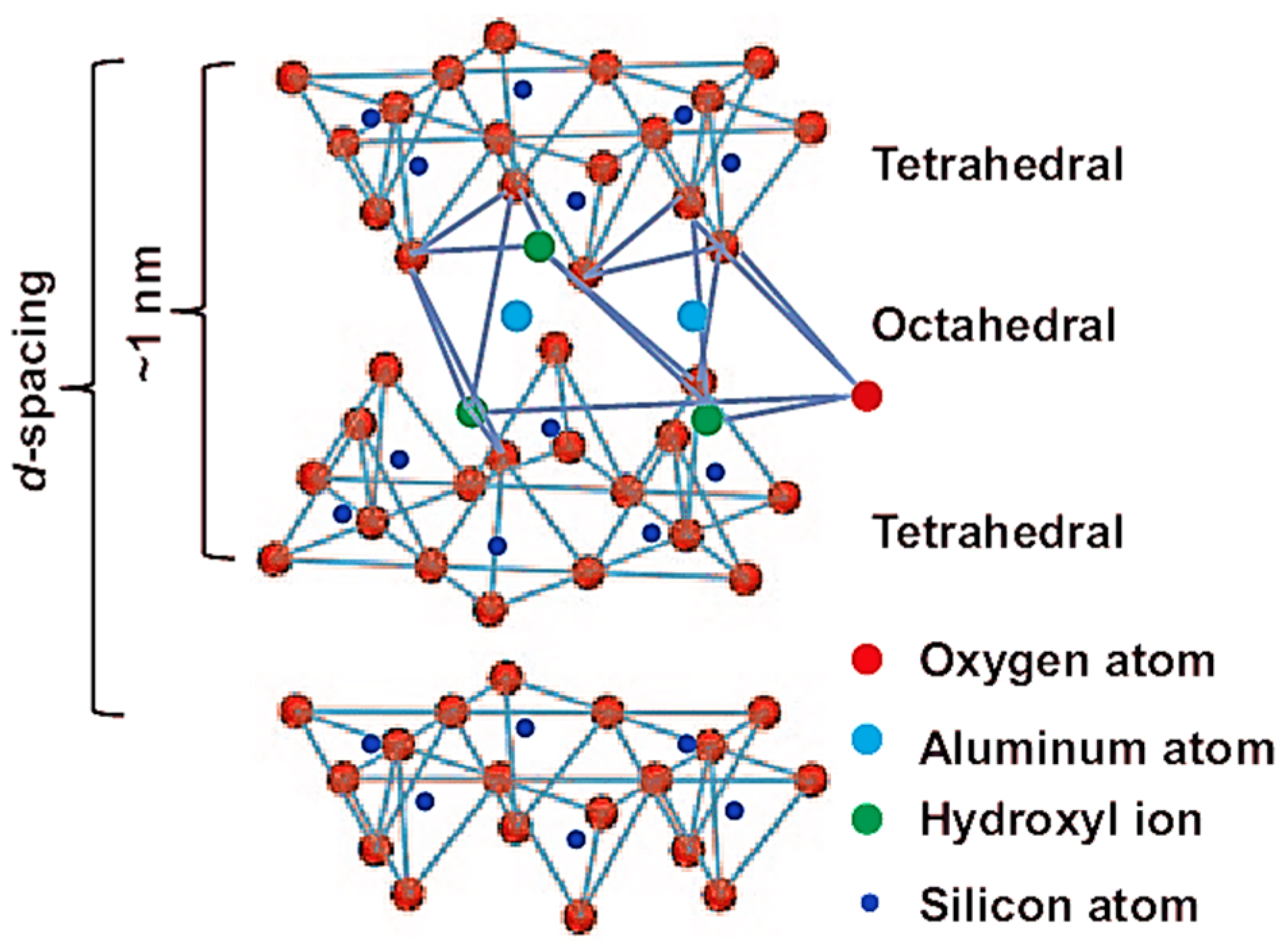
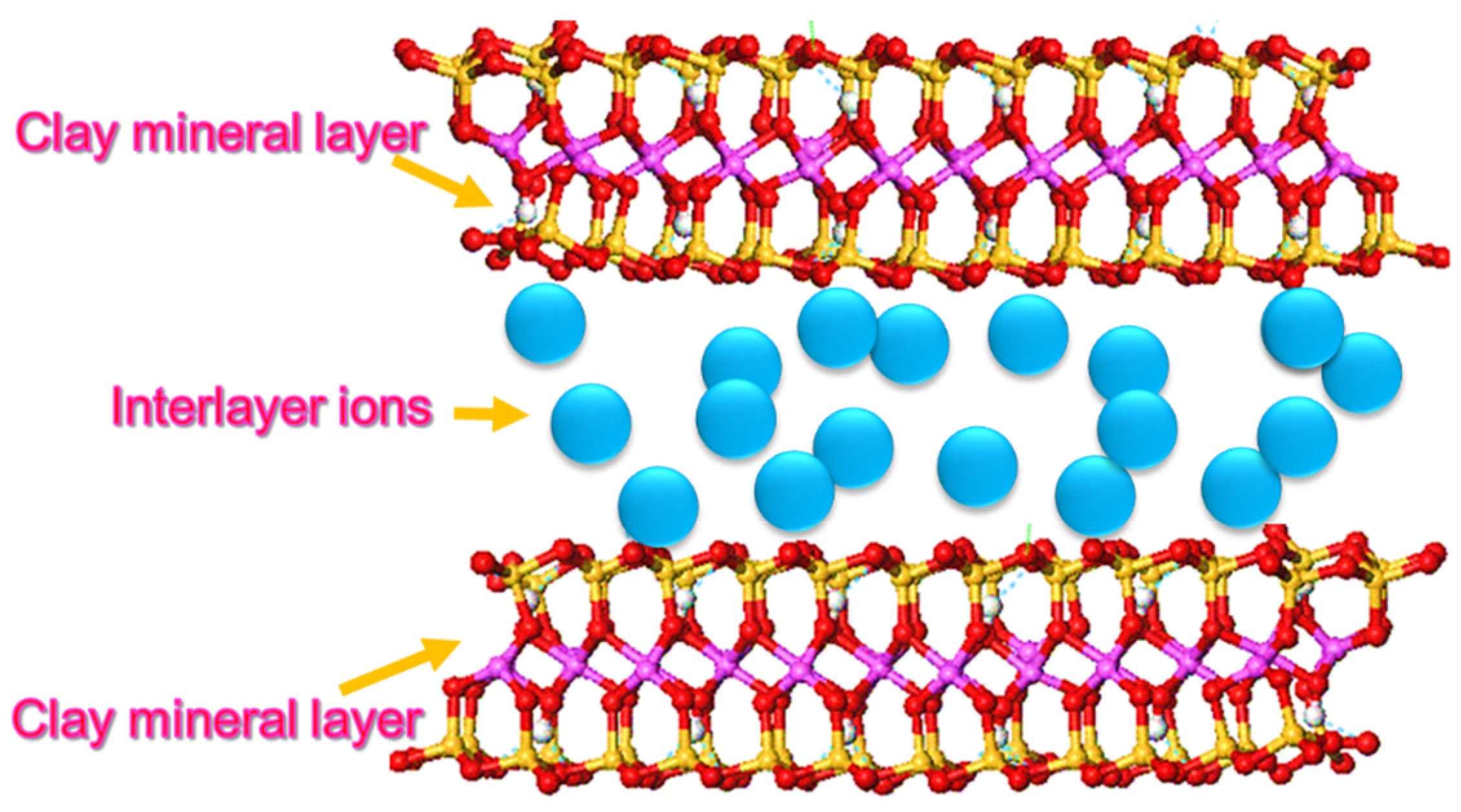
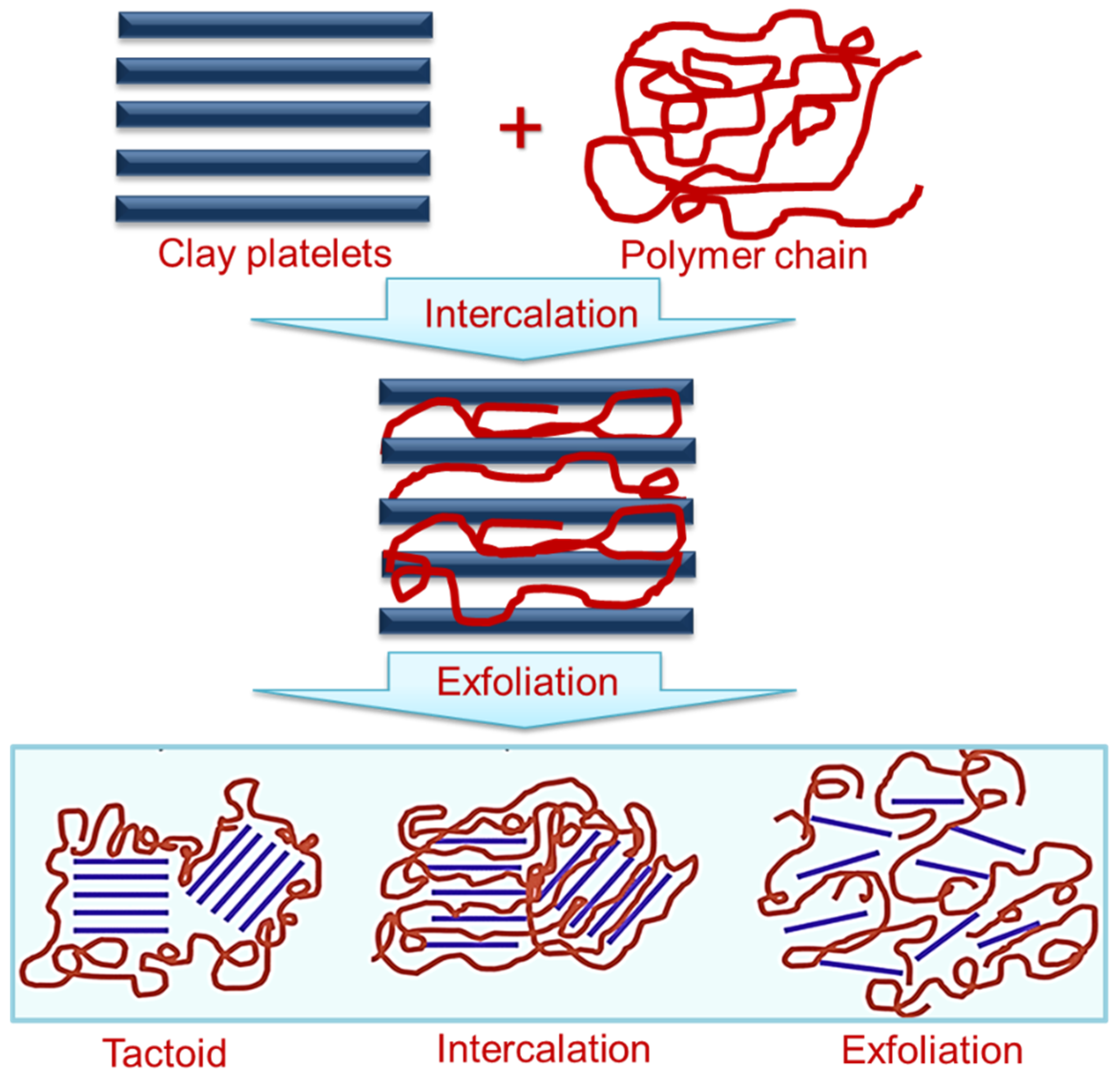
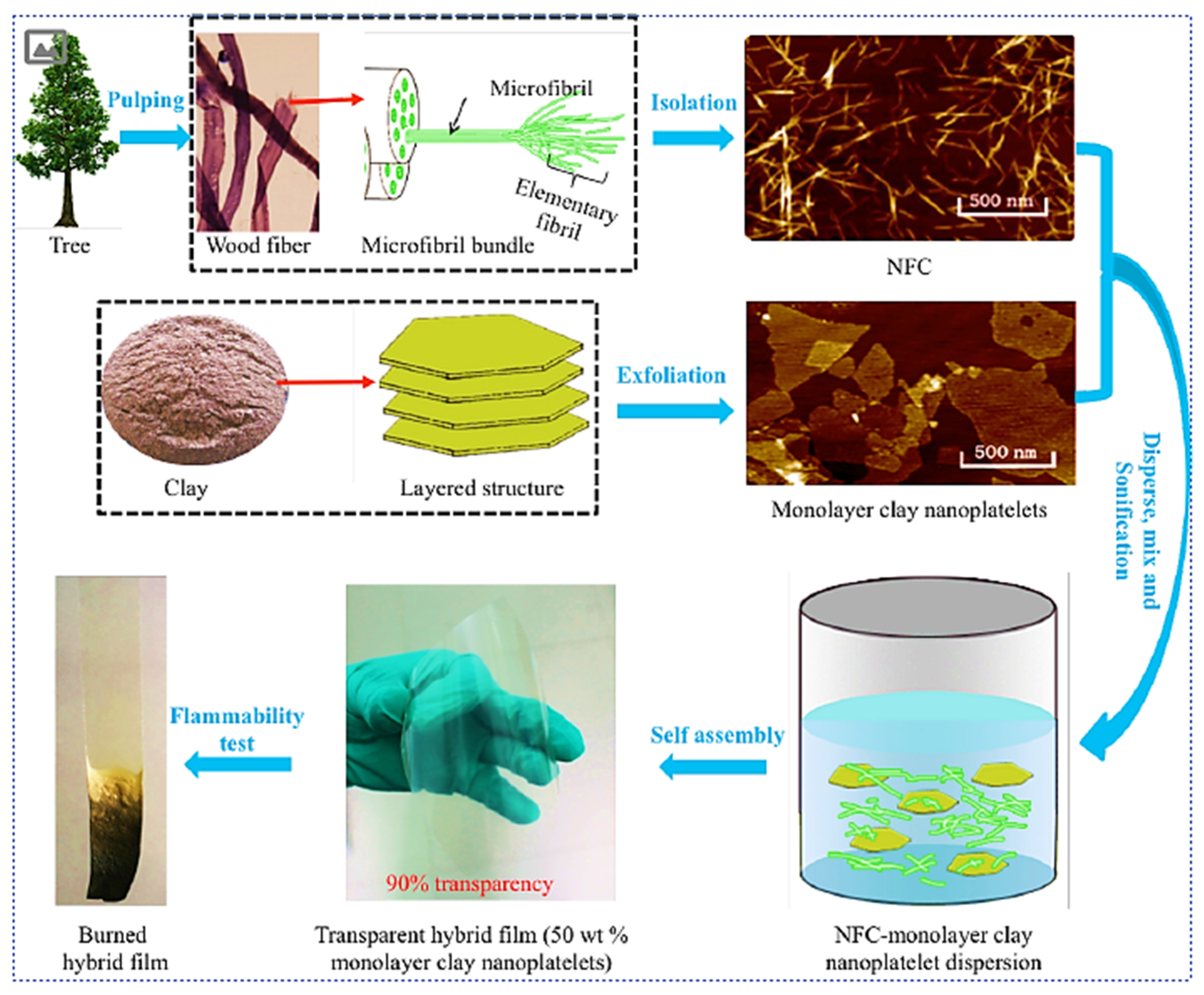
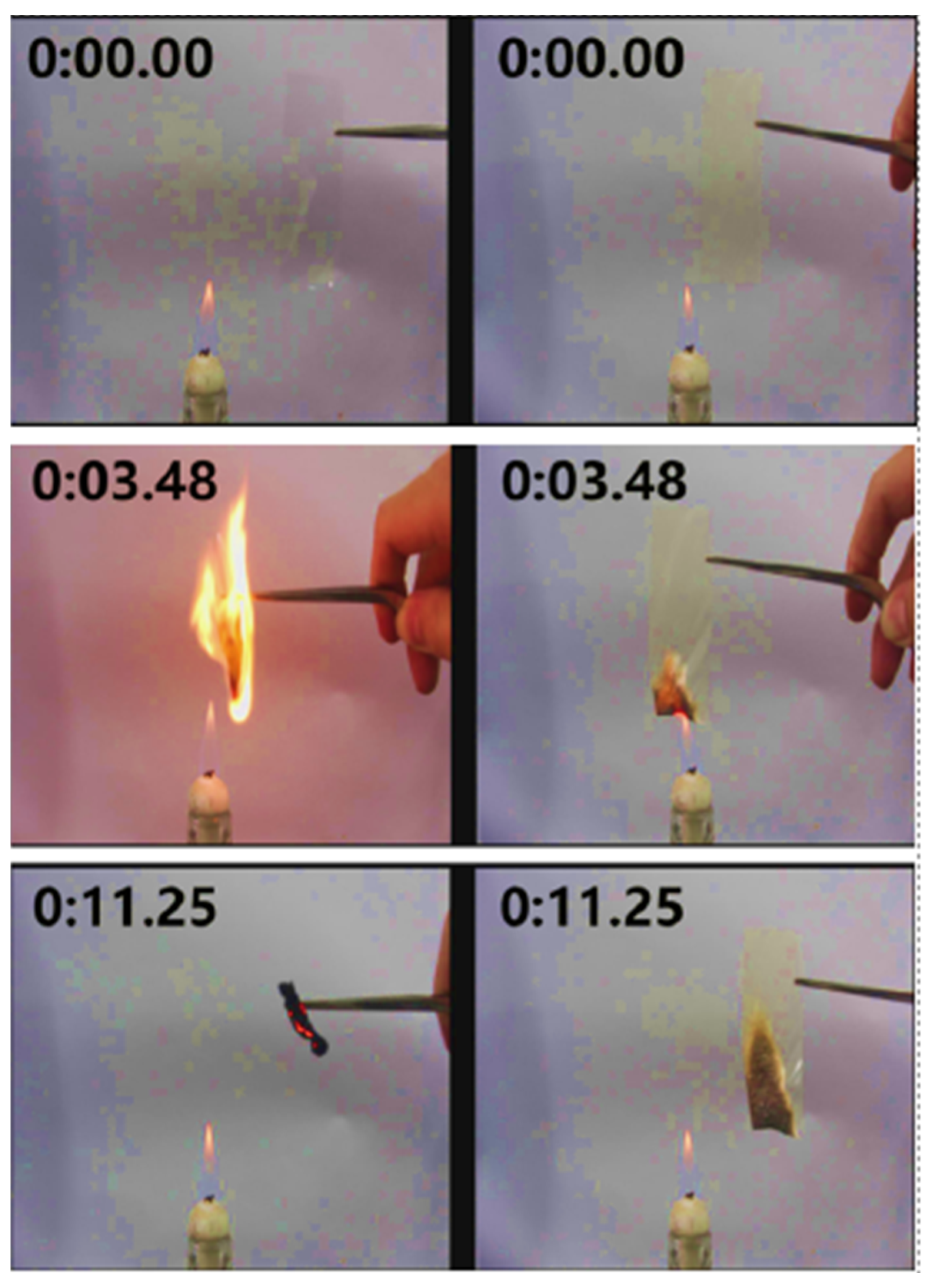
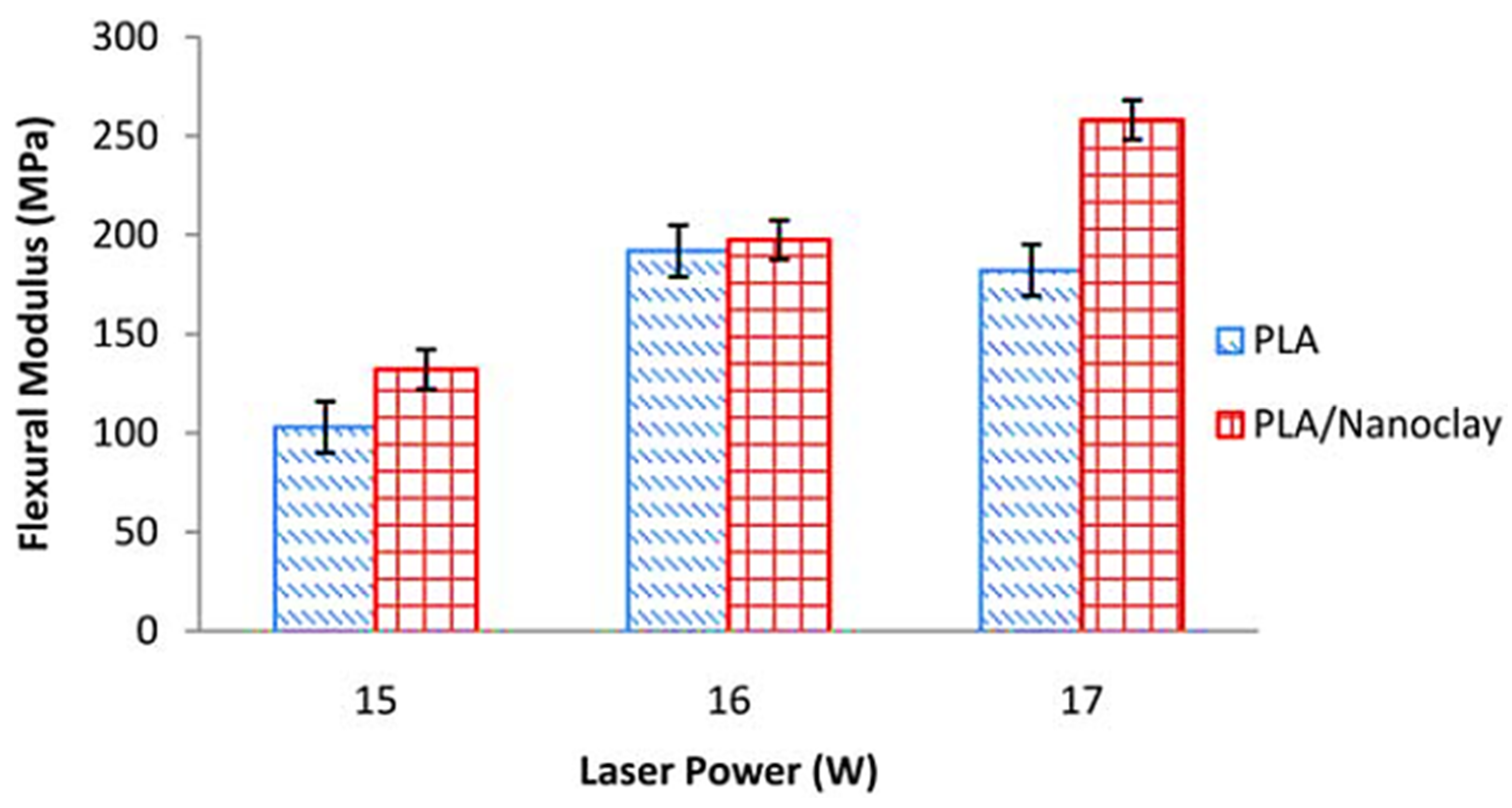

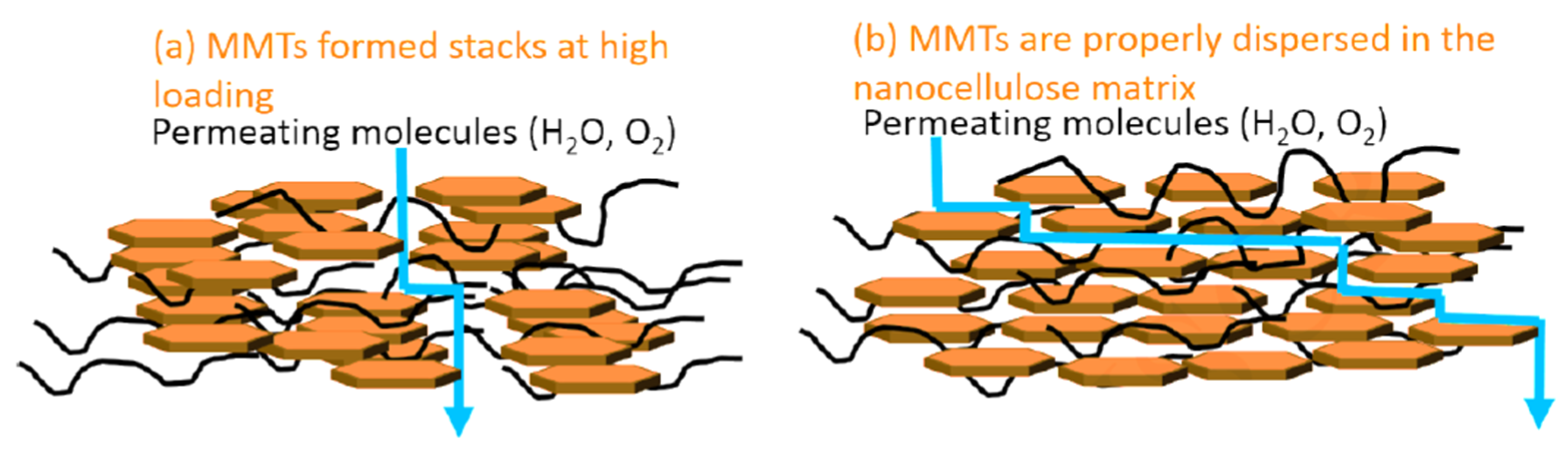
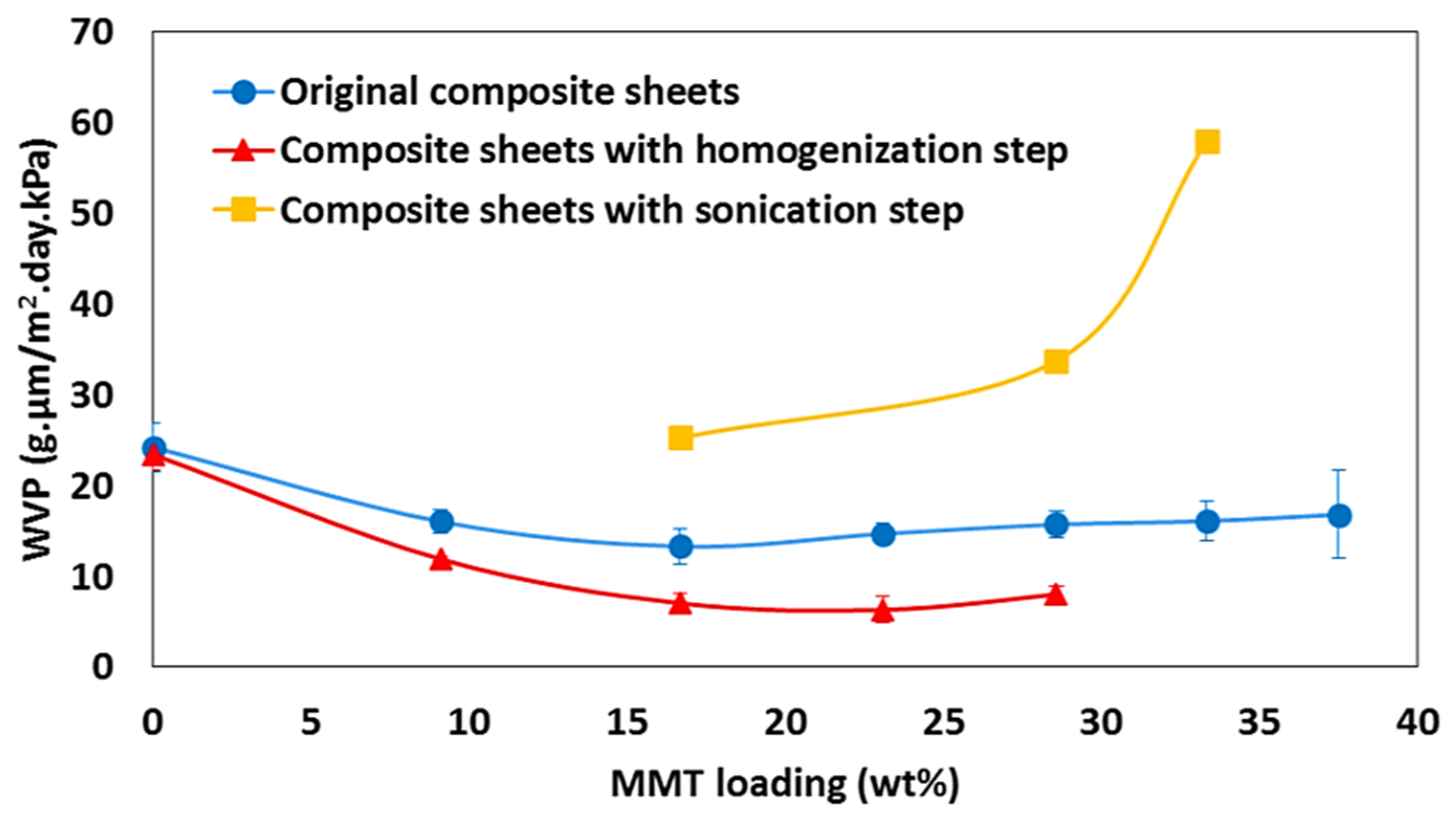
| Properties | Nanoclay | Alumina Silicate |
|---|---|---|
| Structure | Platelets or layered | Particles |
| Cation exchange capability | High due to layered structure | No cation exchange ability |
| Plastic behavior | Upon wetting | No plasticity |
| Swelling behavior | High on wetting | No swelling |
| Permeability | Low | High |
| Catalytic abilities | High | Low |
| Dimensional stability | Low | High |
| Dielectric/thermal insulation | Low | High |
| Resistance to thermal impacts | Low | High |
| Chemical stability | Low | High |
| Heat resistant | Low | 1300 °C |
| Application in metal plating | No | Yes |
| Matrix | Nanoclay | Properties | Ref |
|---|---|---|---|
| Polystyrene | Montmorillonite; Bentonite |
| [60,61,62] |
| Polyethylene (low and high density) | Montmorillonite | [63,64,65,66] | |
| Polypropylene | Montmorillonite | [67,68,69] | |
| Polyamide | Montmorillonite | [70,71,72] | |
| Poly(methyl methacrylate) | Montmorillonite; halloysite nanoclay | [73,74,75,76] | |
| Polyaniline | Montmorillonite; kaolinite; bentonite | [77,78] | |
| Polypyrrole | Montmorillonite | [79] |
| Montmorillonite | Cloisite | Halloysite Nanotube | Application of Nanoclays with Green Polymers |
|---|---|---|---|
| Thermal stability | Heat resistance | Thermal stability | Montmorillonite and halloysite nanoclay in cellulose and starch matrices for electrical, magnetic, and optical devices |
| Mechanical strength | Mechanical stability | Strength properties | Montmorillonite and halloysite nanoclay based green nanomaterials for drug delivery/tissue engineering |
| Gas permeability | Dimensional stability | Flame retardance | Cellulose and starch with montmorillonite for antimicrobials |
| Barrier properties | Rheological properties | Anticorrosion coatings | Cloisite in natural rubber for civil structures |
| Wastewater treatment | Thickener in lubrication oils | Wastewater treatment | Montmorillonite and halloysite nanoclay in cellulose and starch for packaging |
| Polymer | Nanofiller | Processing | Property/Application | Ref |
|---|---|---|---|---|
| Wood | Montmorillonite | Solution/melt method | Sustainable construction materials | [206] |
| Cellulose, starch, poly(lactic acid) | Montmorillonite | Solution/melt method | Packaging | [180,181,182] |
| Nanocellulose | Montmorillonite 16.7–23.1 wt.% | Solution method | Low water vapor permeability 6.3–13.3 g·μm/m2·day·kPa; packaging | [183] |
| Nanocellulose nanofiber | Vermiculite nanoclay | Solution method | Oxygen permeability 0.07 cm3μm·m−2d−1kPa−1 at 50% relative humidity; packaging | [186] |
| Cellulose | Montmorillonite | Solution method | Low water vapor transmission rate 43 g/m2/day; packaging | [187] |
| Starch | Montmorillonite | Solution method | Antimicrobial effect against E. coli bacterial strain | [188] |
| Poly(ɛ-caprolactone) | Montmorillonite | Electrospinning | Tissue engineering; better cell adhesion and bioactivity | [194] |
| Poly-(DL-lactic acid) | Laponite | Solution method | Drug delivery; Type-2 diabetes curation | [199] |
| Poly(vinyl alcohol) | Montmorillonite | Solution method | Wound healing; penicillin drug to wounds | [203] |
| Poly(vinyl alcohol) | Montmorillonite | Solution method | Wound healing; high microbiological stability | [204] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kausar, A.; Ahmad, I.; Maaza, M.; Eisa, M.H. State-of-the-Art Nanoclay Reinforcement in Green Polymeric Nanocomposite: From Design to New Opportunities. Minerals 2022, 12, 1495. https://doi.org/10.3390/min12121495
Kausar A, Ahmad I, Maaza M, Eisa MH. State-of-the-Art Nanoclay Reinforcement in Green Polymeric Nanocomposite: From Design to New Opportunities. Minerals. 2022; 12(12):1495. https://doi.org/10.3390/min12121495
Chicago/Turabian StyleKausar, Ayesha, Ishaq Ahmad, Malik Maaza, and M.H. Eisa. 2022. "State-of-the-Art Nanoclay Reinforcement in Green Polymeric Nanocomposite: From Design to New Opportunities" Minerals 12, no. 12: 1495. https://doi.org/10.3390/min12121495
APA StyleKausar, A., Ahmad, I., Maaza, M., & Eisa, M. H. (2022). State-of-the-Art Nanoclay Reinforcement in Green Polymeric Nanocomposite: From Design to New Opportunities. Minerals, 12(12), 1495. https://doi.org/10.3390/min12121495







