HAp/β-TCP Biphasic Ceramics Obtained by the Pechini Method: An Antibacterial Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
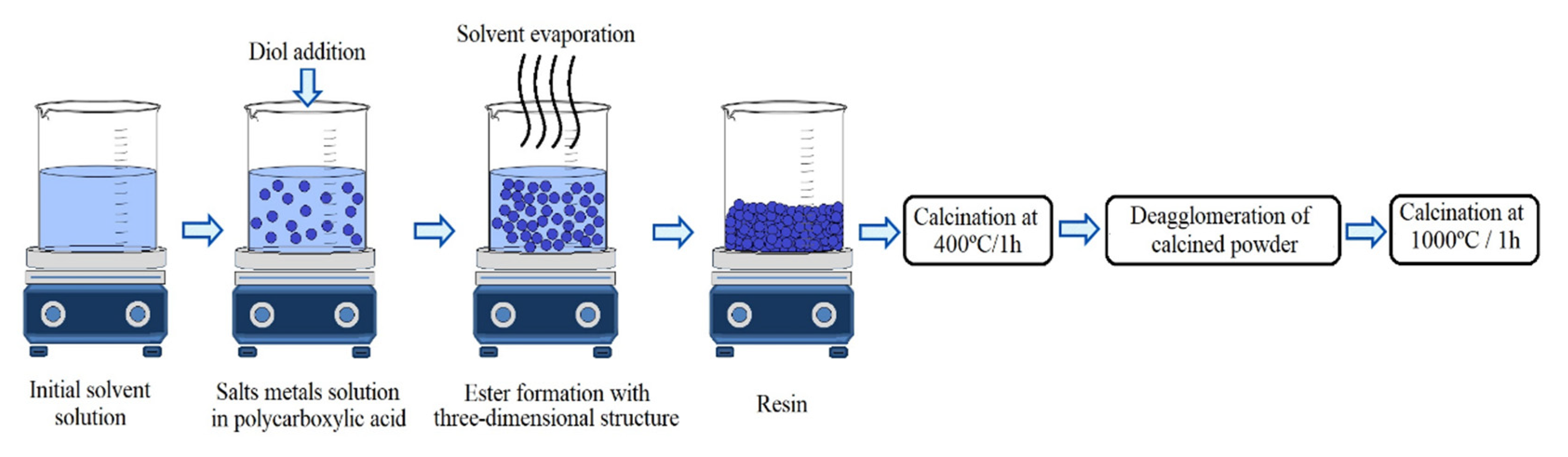
2.2. Synthesis of Bioceramics by the Pechini Method
2.3. Characterization
2.4. Biological Tests
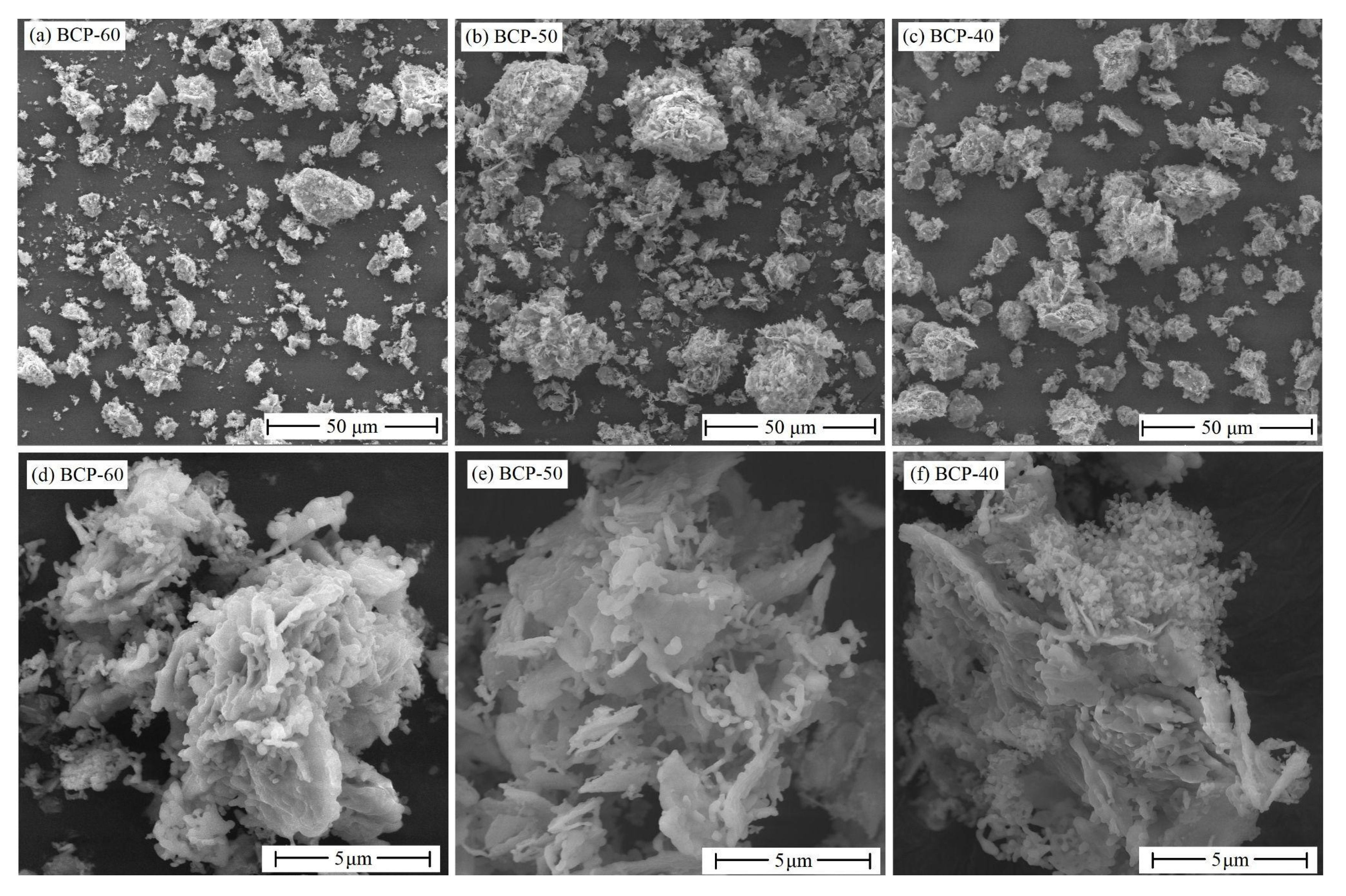
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munerato, M.S.; Biguetti, C.C.; Silva, R.B.P.; Silva, A.C.R.; Bacelar, A.C.Z.; Couto, M.C.R.; Duarte, M.A.H.; Santiago-Junior, J.F.; Bossini, P.S.; Matsumoto, M.A. Using different physicochemical biomaterials for oral and maxillofacial reconstruction, and inflammatory response and macrophage polarization. Mater. Sci. Eng. C 2020, 107, 110229. [Google Scholar] [CrossRef] [PubMed]
- Nihouannen, D.L.; Daculsi, G.; Saffarzadeh, A.; Gauthier, O.; Delplace, S.; Pilet, P.; Layrolle, P.; Araújo, D.S.; Diniz, V.C.S.; Dantas, J.; et al. Avaliação da fotoluminescência do TiO2 sintetizado pelo método Pechini. Cerâmica 2017, 63, 367. [Google Scholar]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.-D.; Smeets, R. Current trends and future perspectives of bone substitute materials–From space holders to innovative biomaterials. J. Cranio-Maxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Ablikim, Z.; Chen, T.; Cai, Q.; Xia, J.; Jiang, D.; Wang, S. The preparation and characterization of HA/β-TCP biphasic ceramics from fish bones. Ceram. Int. 2017, 43, 12213–12220. [Google Scholar] [CrossRef]
- Nawang, R.; Hussein, M.Z.; Matori, K.A.; Abdullah, C.A.C.; Hashim, M. Physicochemical properties of hydroxyapatite/montmorillonite nanocomposite prepared by powder sintering. Results Phys. 2019, 15, 102540. [Google Scholar] [CrossRef]
- Omori, Y.; Okada, M.; Takeda, S.; Matsumoto, N. Fabrication of dispersible calcium phosphate nanocrystals via a modified Pechini method under non-stoichiometric conditions. Mater. Sci. Eng. C 2014, 42, 562–568. [Google Scholar] [CrossRef]
- Jiang, T.; Li, Z.; Xiao, P.; Cheng, Y.; Liu, Z.; Yu, S. Synthesis and growth mechanism of calcium phosphate layer on SiC-coated carbon/carbon composites with surface pre-oxidation. Appl. Surf. Sci. 2019, 495, 143427. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Botelho, M.G.; Dorozhkin, S.V. Biphasic calcium phosphates bioceramics (HA/TCP): Concept, physicochemical properties and the impact of standardization of study protocols in biomaterials research. Mater. Sci. Eng. C 2017, 71, 1293–1312. [Google Scholar] [CrossRef]
- Aghayan, M.A.; Rodríguez, M.A. Influence of fuels and combustion aids on solution combustion synthesis of bi-phasic calcium phosphates (BCP). Mater. Sci. Eng. C 2012, 32, 2464–2468. [Google Scholar] [CrossRef]
- Ruiz-Aguilar, C.; Olivares-Pinto, U.; Aguilar-Reyes, E.A.; López-Juárez, R.; Alfonso, I. Characterization of -tricalcium phosphate powders synthesized by sol–gel and mechanosynthesis. Boletín De La Soc. Española De Cerámica Y Vidr. 2018, 57, 213–220. [Google Scholar] [CrossRef]
- Rodella, C.B.; Nunes, L.A.O.; Saeki, M.J.; Padilha, P.M.; Florentino, A.O. Caracterização textural e estrutural de V2O5/TiO2 obtidos via sol-gel: Comparação entre secagem convencional e supercrítica. Química Nova 2002, 25, 209–213. [Google Scholar] [CrossRef]
- Agrawal, K.; Singh, G.; Puri, D.; Prakash, S. Synthesis and Characterization of Hydroxyapatite Powder by Sol-Gel Method for Biomedical Application. J. Miner. Mater. Charact. Eng. 2011, 10, 727–734. [Google Scholar] [CrossRef]
- Arokiasamy, P.; Abdullah, M.M.A.B.; Rahim, S.Z.A.; Luhar, S.; Sandu, A.V.; Jamil, N.H.; Nabiałek, M. Synthesis methods of hydroxyapatite from natural sources: A review. Ceram. Int. 2022, 48, 14959–14979. [Google Scholar] [CrossRef]
- Galea, L.; Bohner, M.; Thuering, J.; Doebelin, N.; Ring, T.A.; Aneziris, C.G.; Graule, T. Growth kinetics of hexagonal sub-micrometric β-tricalcium phosphate particles in ethylene glycol. Acta Biomater. 2014, 10, 3922–3930. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.M.; Mahapatra, C.; Kim, H.W.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.; Biswas, C.K.; Paul, S. A comprehensive review on the preparation and application of calcium hydroxyapatite: A special focus on atomic doping methods for bone tissue engineering. Ceram. Int. 2021, 47, 28122–28144. [Google Scholar] [CrossRef]
- Peña, J.; Vallet-Regı, M. Hydroxyapatite, tricalcium phosphate and biphasic materials prepared by a liquid mix technique. J. Eur. Ceram. Soc. 2003, 23, 1687–1696. [Google Scholar] [CrossRef]
- Scherrer, P. Nachrichten von der Gesellschaft der Wissenschaften zu Gottingen. Math.-Phys. Kl. 1918, 2, 98–100. [Google Scholar]
- Zheng, L.Y.; Zhu, J.F. Study on Antimicrobial Activity of Chitosan with Different Molecular Weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Roopalakshmi, S.; Ravishankar, R.; Belaldavar, S.; Prasad, R.G.S.V.; Phani, A.R. Investigation of Structural and Morphological Characteristic of Hydroxyapatite Synthesized by Sol-Gel Process. Mater. Today Proc. 2017, 4, 12026–12031. [Google Scholar] [CrossRef]
- Stötzel, C.; Müller, E.A.; Reinert, E.; Niederdraenk, E.; Barralet, J.E.; Gbureck, U. Ion adsorption behavior of hydroxyapatite with different crystallinities. Colloids Surf. B Biointerfaces 2009, 74, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Sun, R.; Wei, Z.; Zhao, H.; Li, F., II. Size-dependent defluoridation properties of synthetic hydroxyapatite. J. Fluor Chem. 2009, 130, 550–556. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Patai, P.; Futalan, C.M.; Utara, S.; Khemthong, P.; Kamonwannasit, S. Structural characterization of cerium-doped hydroxyapatite nanoparticles synthesized by an ultrasonic-assisted sol-gel technique. Results Phys. 2018, 10, 956–963. [Google Scholar]
- Cimdina, L.B.; Borodajenko, N. Research of Calcium Phosphates Using FourierTransform Infrared Spectroscopy, Infrared Spectroscopy-Materials Science, Engineering and Technology; Intech: Shanghai, China, 2012; ISBN 978-953-51-0537-4. [Google Scholar]
- Rameshbabu, N.; Kumar, T.S.S.; Prabhakar, T.G.; Sastry, V.S.; Murty, K.V.G.K.; Rao, K.P. Antibacterial nanosized silver substituted hydroxyapatite: Synthesis and characterization. J. Biomed. Mater. Res. Part A 2007, 3, 80. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Kadir, M.R.A.; Malek, N.A.N.N.; Mahmood, N.H.; Murali, M.R.; Kamarul, T. Rapid microwave-assisted synthesis and characterization of nanosized silver-doped hydroxyapatite with antibacterial properties. Mater. Lett. 2012, 89, 118–122. [Google Scholar] [CrossRef]
- Piccirillo, C.; Pullar, R.C.; Tobaldi, D.M.; Castro, P.M.L.; Pintado, M.M.E. Hydroxyapatite and chloroapatite derived from sardine by-products. Ceram. Int. 2014, 40, 13231–13240. [Google Scholar] [CrossRef]
- MashreghI, A.; Davoudi, F. The effect of ethylene glycol/citric acid molar ratio in the initial precursor of TiO2 nanoparticle paste synthesized by a polymerizable complex method on the photovoltaic properties of dye-sensitized solar cells. Mater. Sci. Semicond. Processing 2015, 30, 618–624. [Google Scholar] [CrossRef]
- Guo, R.; Huang, J.; Chen, X.; Luo, Q.; Luo, L.; Xiong, Y.; Zhang, S. Pechini sol-gel synthesis of La2CaB8O16:Eu3+ red phosphor and its photoluminescence spectral properties. J. Lumin. 2019, 206, 15–20. [Google Scholar] [CrossRef]
- Bakar, S.N.A.; Talib, I.A.; Osman, N. The effect of different molar ratio citric acid and ethylene glycol to metal cation on the ceramics powder of Y3+ doped BaZrO3. Solid State Sci. Technol. 2010, 18, 6936–6944. [Google Scholar]
- Jouannaux, J.; Haeussler, A.; Drobek, M.; Ayral, A.; Abanades, S.; Julbe, A. Lanthanum manganite perovskite ceramic powders for CO2 splitting: Influence of Pechini synthesis parameters on sinterability and reactivity. Ceram. Int. 2019, 45, 15636–15648. [Google Scholar] [CrossRef]
- Karampas, I.A.; Kontoyannis, C.G. Characterization of calcium phosphates mixtures. Vib. Spectrosc. 2013, 64, 126–133. [Google Scholar] [CrossRef]
- Tõnsuaadu, K.; Gross, K.A.; Pluduma, L.; Veiderma, M. A review on the thermal stability of calcium apatites. A review on the thermal stability of calcium apatites. J. Therm. Anal. Calorim. 2012, 110, 647–659. [Google Scholar]
- Schnieders, J.; Gbureck, U.; Vorndran, E.; Schossig, M.; Kissel, T. The effect of porosity on drug release kinetics from vancomycin microsphere/calcium phosphate cement composites. J. Biomed. Mater. Res. 2011, 99, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Mukai, M.; Uchida, K.; Sugo, K.; Nakasu, M.; Nakajima, T.; Takata, K.; Takaso, M.; Urabe, K. Long-term antibacterial activity of vancomycin from calcium phosphate cement in vivo. Bio-Med. Mater. Eng. 2022, 33, 41–50. [Google Scholar] [CrossRef]
- Varma, P.R.H.; Fernandez, F.B. Bioceramics in Tissue Engineering: Retrospect and Prospects. In Biomaterials in Tissue Engineering and Regenerative Medicine; Springer: Singapore, 2021; pp. 61–87. [Google Scholar]
- Erdem, U.; Dogan, M.; Metin, A.U.; Baglar, S.; Turkoz, M.B.; Turk, M.; Nezir., S. Hydroxyapatite-based nanoparticles as a coating material for the dentine surface: An antibacterial and toxicological effect. Ceram. Int. 2020, 46, 270–280. [Google Scholar] [CrossRef]
- Stanić, V.; Dimitrijević, S.; Antić-Stanković, J.; Mitrić, M.; Jokić, B.; Plećaš, I.B.; Raičević, S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Oliveira, C.; Oliveira, A.L.M.; Chantelle, L.; Cavalcanti, G.R.S.; Landers, R.; Medina-Carrasco, S.; Orta, M.D.M.; Filho, E.C.S.; Jaber, M.; Fonseca, M.G. Functionalization of the hydroxyapatite surface with ZnO for alizarin immobilization. Appl. Surf. Sci. 2022, 593, 153412. [Google Scholar] [CrossRef]
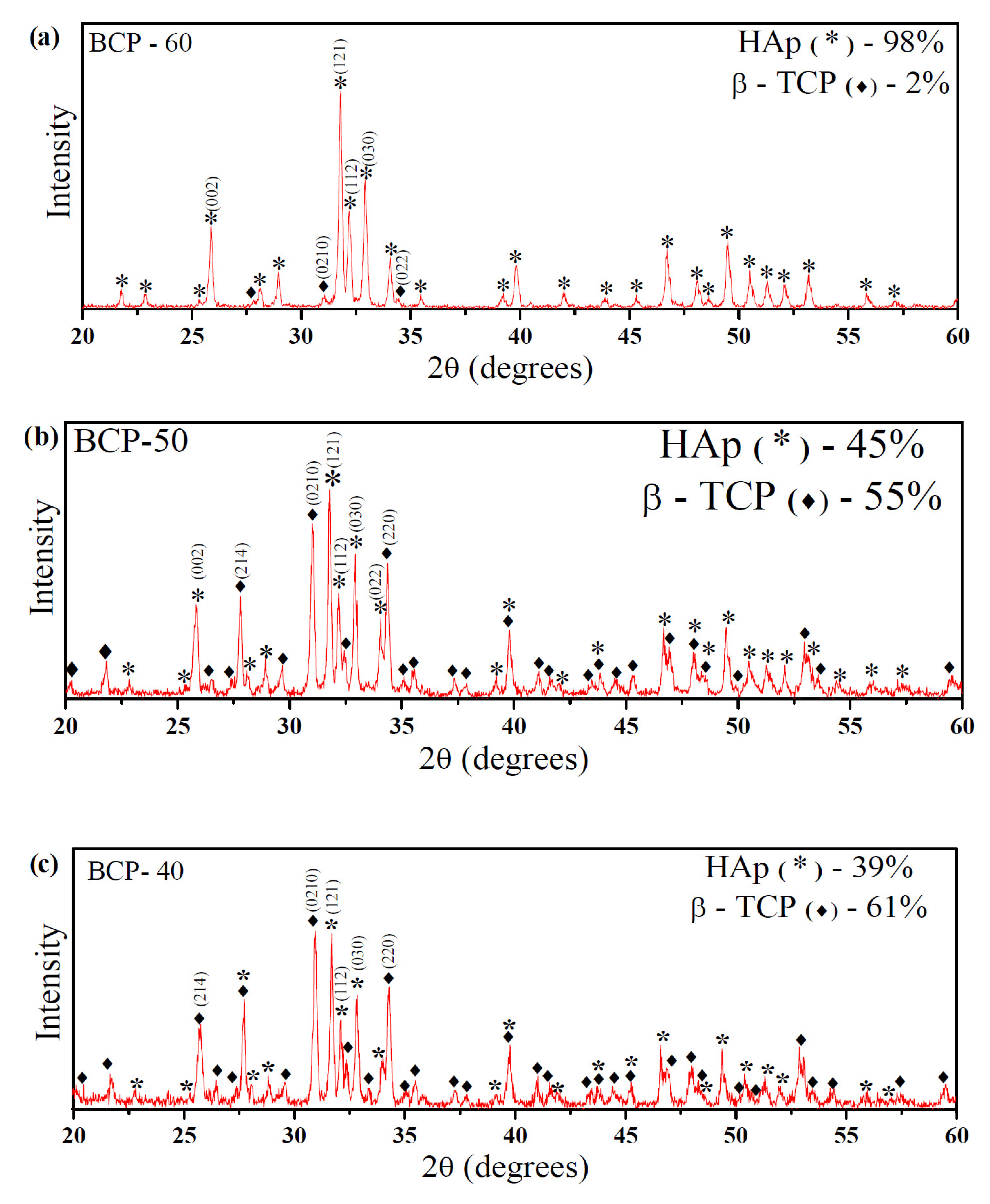
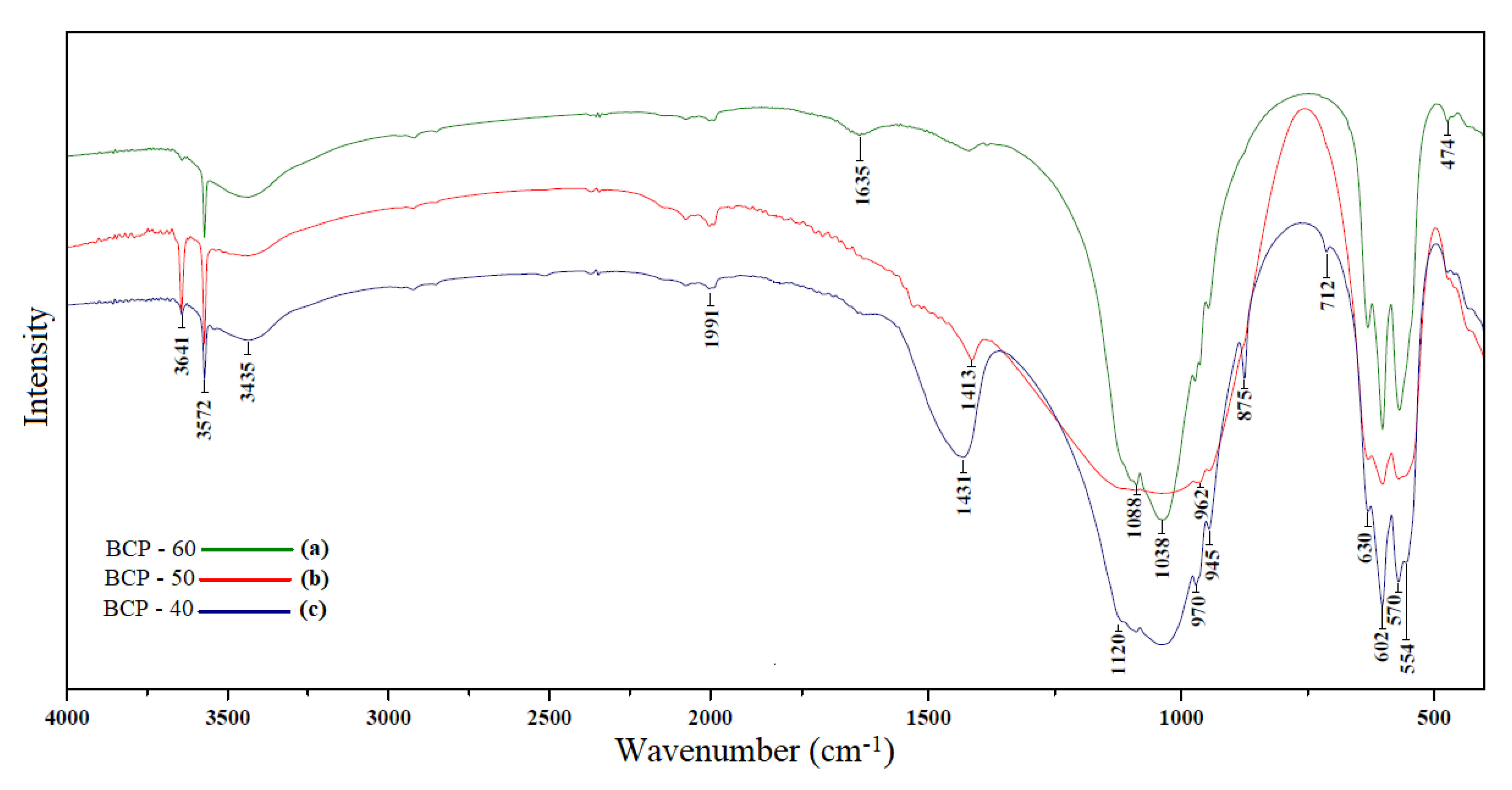
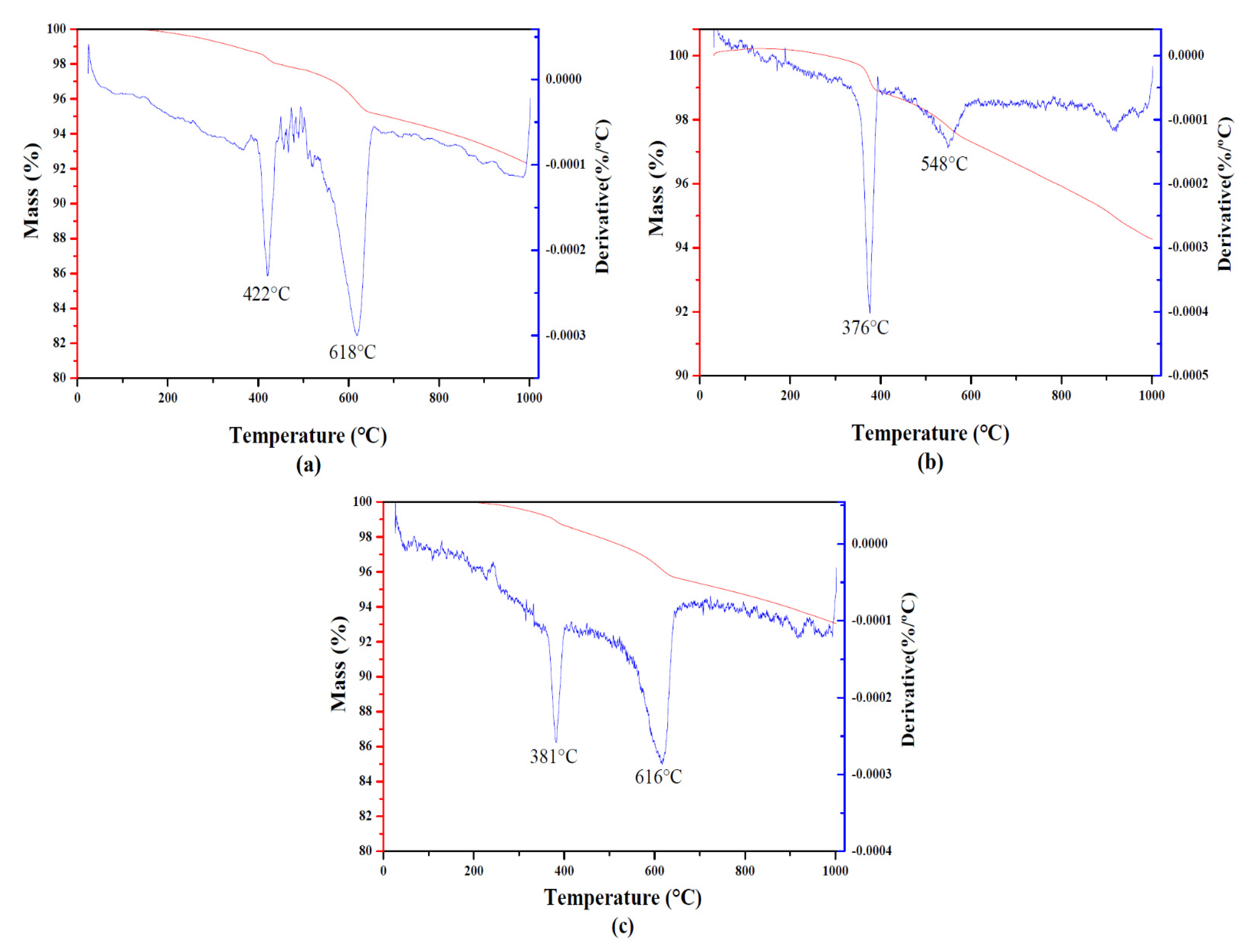

| Quantification of the Phases (%) | HAp Crystallite Size (nm) | Degree of Crystallinity (%) | ||
|---|---|---|---|---|
| Sample | HAp | β-TCP | ||
| BCP-60 | 98 | 02 | 63.1 | 94.8 |
| BCP-50 | 45 | 55 | 61.1 | 88.1 |
| BCP-40 | 39 | 61 | 60.6 | 82.3 |
| Sample | Staphylococcus aureus (% of Inhibition) | Escherichia coli (% of Inhibition) |
|---|---|---|
| BCP-60 | 35.34 | 38.06 |
| BCP-50 | 43.10 | 73.39 |
| BCP-40 | 30.17 | 25.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, G.K.; Farias, J.R.S.; Lima, I.S.; Nascimento, A.M.; Neves, G.A.; Menezes, R.; Osajima, J.A.; Braga, A.; Silva-Filho, E.C. HAp/β-TCP Biphasic Ceramics Obtained by the Pechini Method: An Antibacterial Approach. Minerals 2022, 12, 1482. https://doi.org/10.3390/min12121482
Carvalho GK, Farias JRS, Lima IS, Nascimento AM, Neves GA, Menezes R, Osajima JA, Braga A, Silva-Filho EC. HAp/β-TCP Biphasic Ceramics Obtained by the Pechini Method: An Antibacterial Approach. Minerals. 2022; 12(12):1482. https://doi.org/10.3390/min12121482
Chicago/Turabian StyleCarvalho, Geysivana K., José R. S. Farias, Idglan S. Lima, Ariane M. Nascimento, Gelmires A. Neves, Romualdo Menezes, Josy A. Osajima, Aluska Braga, and Edson C. Silva-Filho. 2022. "HAp/β-TCP Biphasic Ceramics Obtained by the Pechini Method: An Antibacterial Approach" Minerals 12, no. 12: 1482. https://doi.org/10.3390/min12121482
APA StyleCarvalho, G. K., Farias, J. R. S., Lima, I. S., Nascimento, A. M., Neves, G. A., Menezes, R., Osajima, J. A., Braga, A., & Silva-Filho, E. C. (2022). HAp/β-TCP Biphasic Ceramics Obtained by the Pechini Method: An Antibacterial Approach. Minerals, 12(12), 1482. https://doi.org/10.3390/min12121482










