Synthesis, Characterization, and Application of Geopolymer/TiO2 Nanoparticles Composite for Efficient Removal of Cu(II) and Cd(II) Ions from Aqueous Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2 Nanoparticles
2.3. Synthesis of Geopolymer and Geopolymer-NanoTiO2 Composites
2.4. Characterization
2.5. Adsorption Experiments
3. Results and Discussion
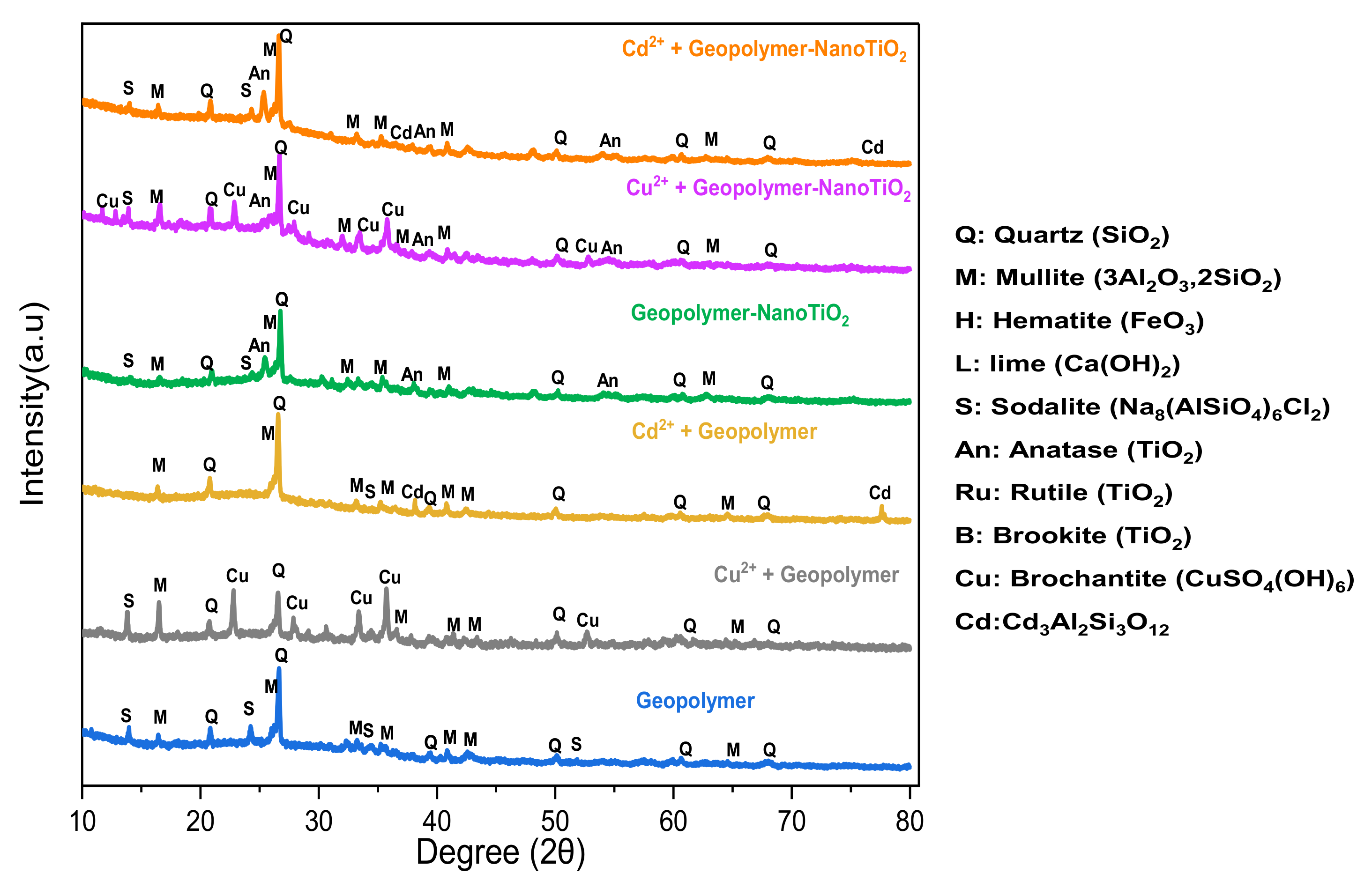
3.1. Sample Characterization
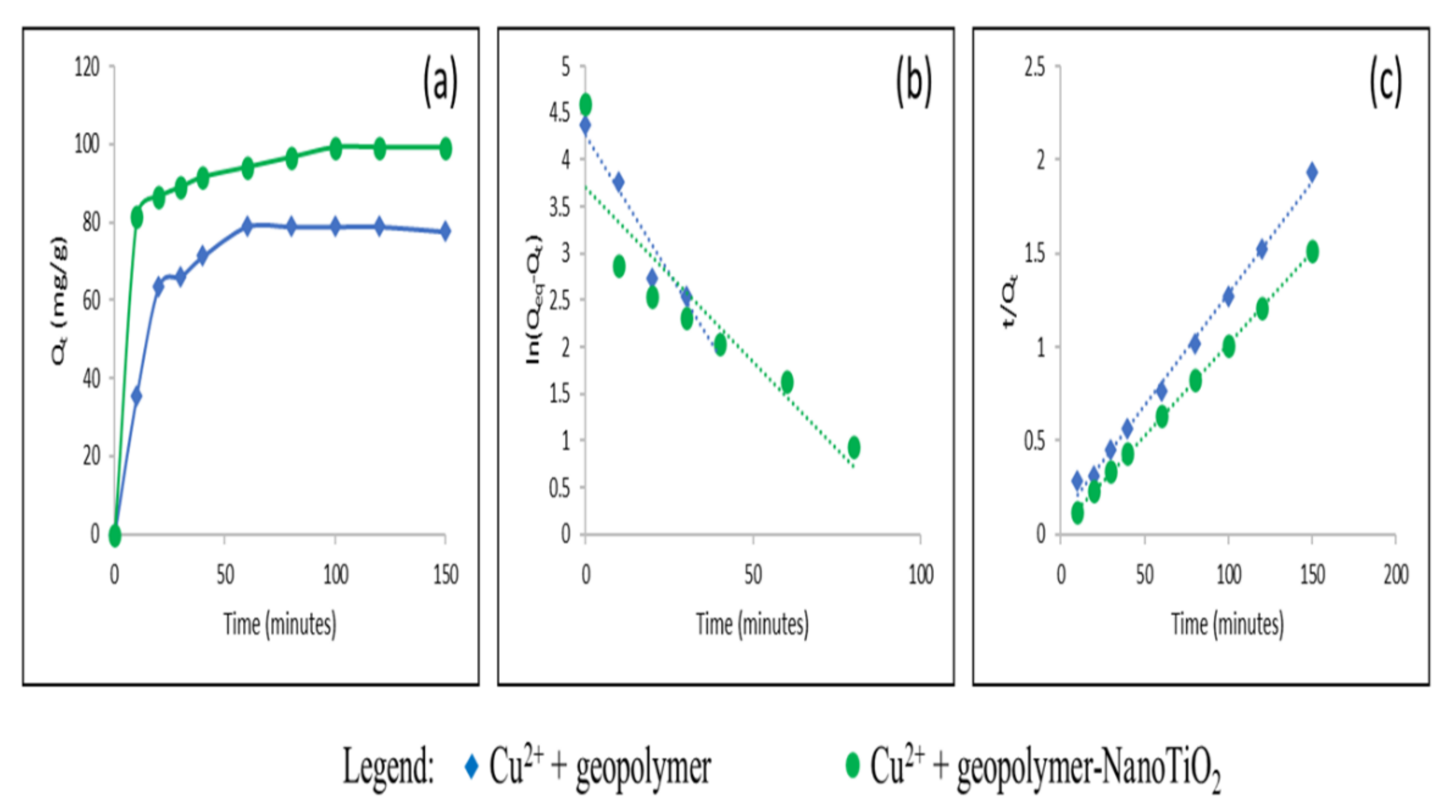
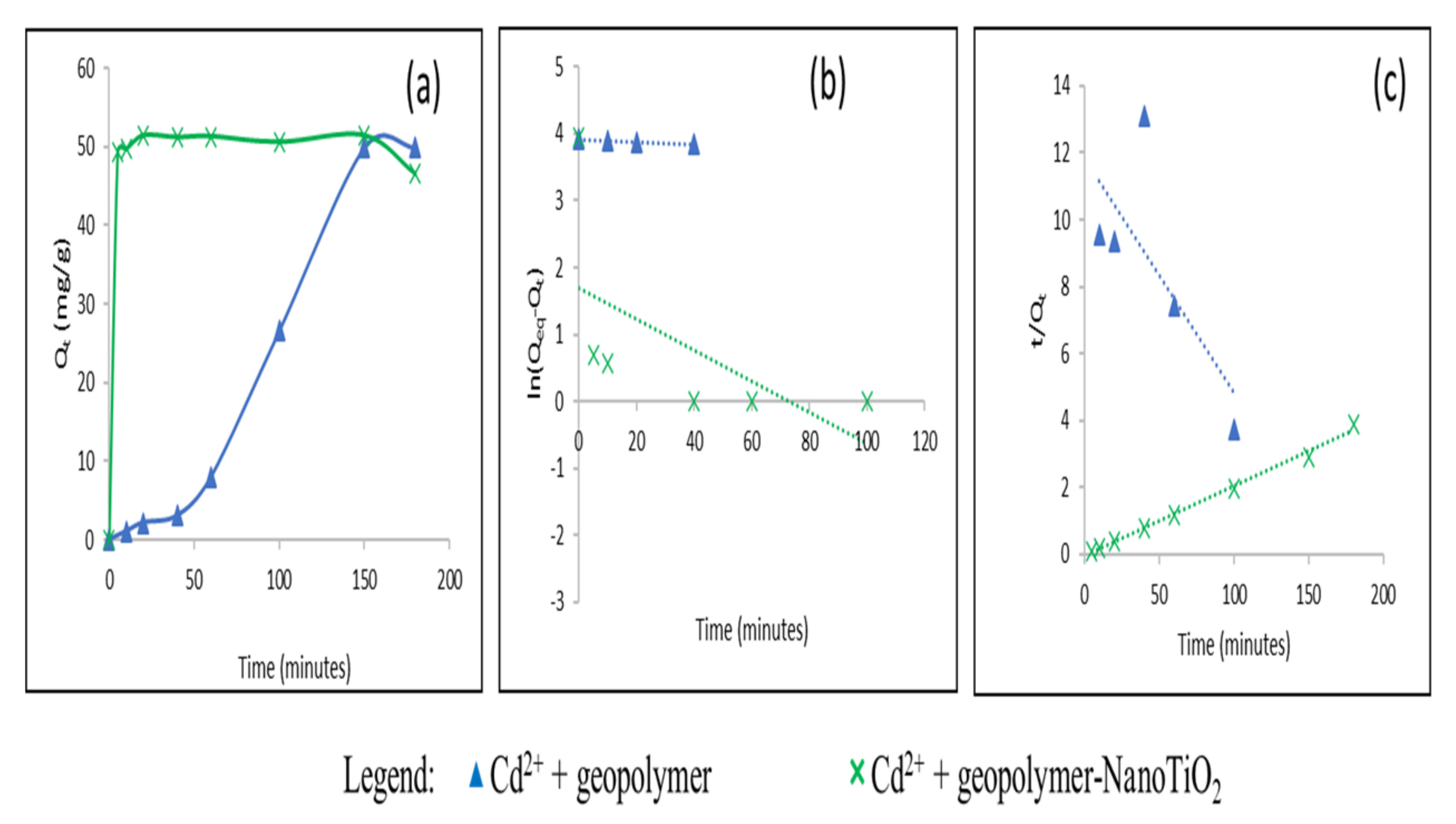
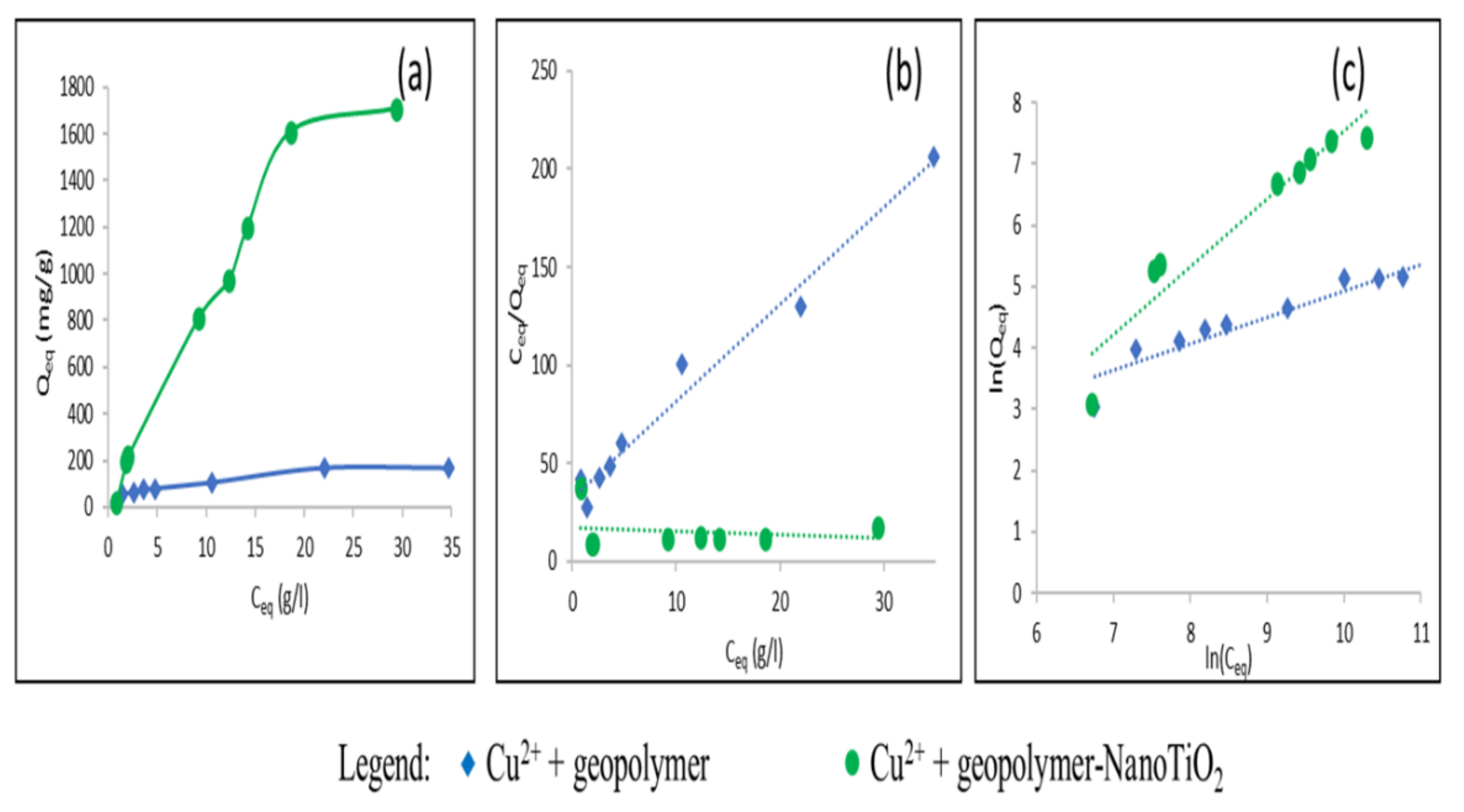
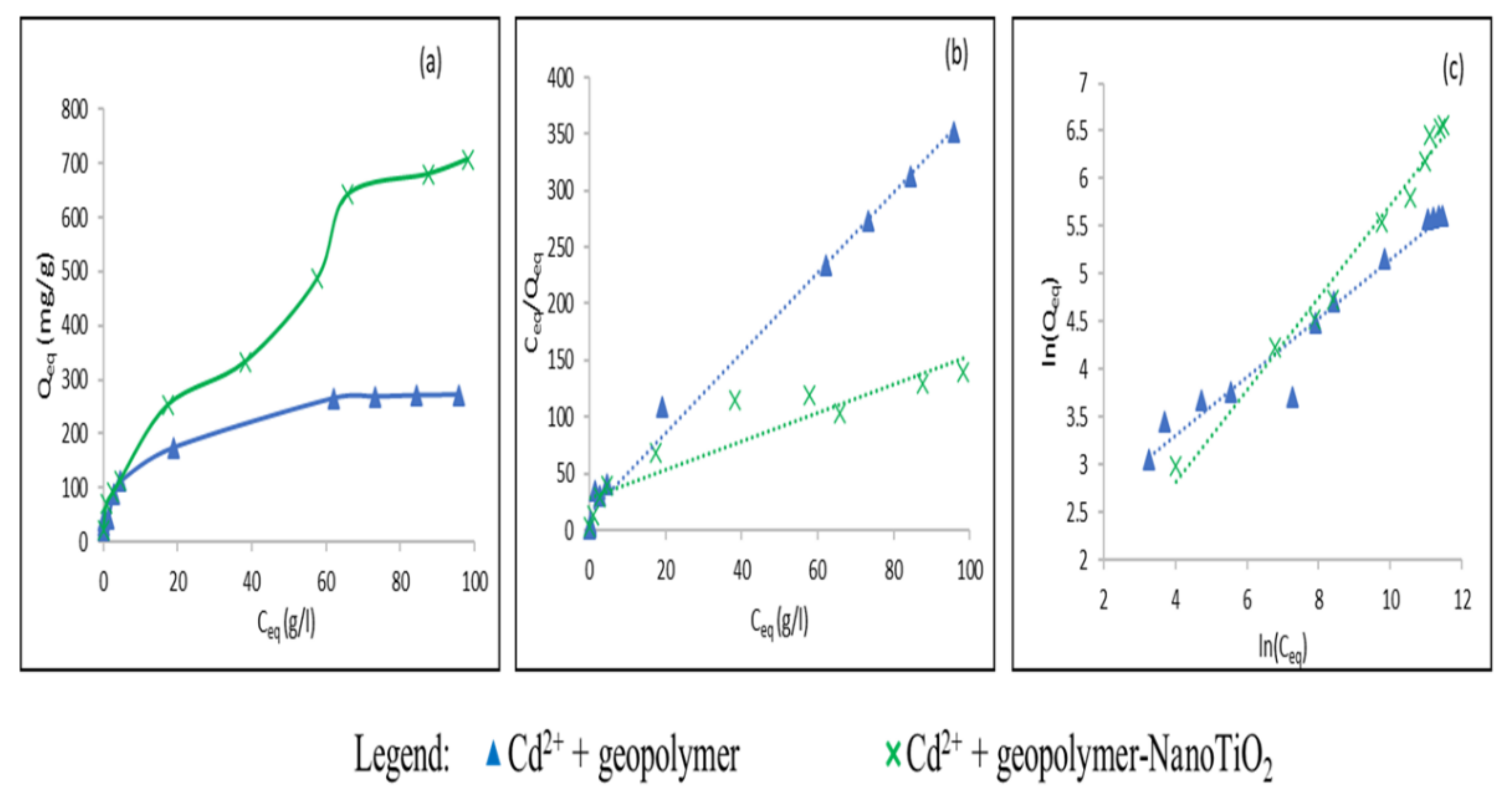
3.2. Study of the Adsorption of Cu2+ and Cd2+ from an Aqueous Solution
3.3. Characterization after Adsorption of Cu2+ and Cd2+ from an Aqueous Solution
3.4. Comparative Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baby, J.; Raj, J.S.; Biby, E.T.; Sankarganesh, P.; Jeevitha, M.V.; Ajisha, S.U.; Rajan, S.S. Toxic Effect of Heavy Metals on Aquatic Environment. Int. J. Biol. Chem. Sci. 2011, 4, 939–952. [Google Scholar] [CrossRef]
- Crini, G. Recent Developments in Polysaccharide-Based Materials Used as Adsorbents in Wastewater Treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Mohammed, A.S.; Kapri, A.; Goel, R. Heavy Metal Pollution: Source, Impact, and Remedies. In Biomanagement of Metal-Contaminated Soils; Springer: Dordrecht, The Netherlands, 2011; Volume 20, pp. 1–28. [Google Scholar] [CrossRef]
- Maleki, A.; Hajizadeh, Z.; Sharifi, V.; Emdadi, Z. A Green, Porous and Eco-Friendly Magnetic Geopolymer Adsorbent for Heavy Metals Removal from Aqueous Solutions. J. Clean. Prod. 2019, 215, 1233–1245. [Google Scholar] [CrossRef]
- Darmayanti, L.; Kadja, G.T.M.; Notodarmojo, S.; Damanhuri, E.; Mukti, R.R. Structural Alteration within Fly Ash-Based Geopolymers Governing the Adsorption of Cu2+ from Aqueous Environment: Effect of Alkali Activation. J. Hazard. Mater. 2019, 377, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Ratnarathorn, N.; Chailapakul, O.; Henry, C.S.; Dungchai, W. Simple Silver Nanoparticle Colorimetric Sensing for Copper by Paper-Based Devices. Talanta 2012, 99, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Cui, X.; Liao, C.; Li, Z. Facile Fabrication of Green Geopolymer/Alginate Hybrid Spheres for Efficient Removal of Cu(II) in Water: Batch and Column Studies. Chem. Eng. J. 2017, 311, 126–134. [Google Scholar] [CrossRef]
- Yu, Z.; Dang, Q.; Liu, C.; Cha, D.; Zhang, H.; Zhu, W.; Zhang, Q.; Fan, B. Preparation and Characterization of Poly(Maleic Acid)-Grafted Cross-Linked Chitosan Microspheres for Cd(II) Adsorption. Carbohydr. Polym. 2017, 172, 28–39. [Google Scholar] [CrossRef]
- Meng, J.; Cui, J.; Yu, J.; Huang, W.; Wang, P.; Wang, K.; Liu, M.; Song, C.; Chen, P. Preparation of Green Chelating Fibers and Adsorption Properties for Cd(II) in Aqueous Solution. J. Mater. Sci. 2017, 53, 2277–2289. [Google Scholar] [CrossRef]
- Wang, X.S.; Miao, H.H.; He, W.; Shen, H.L. Competitive Adsorption of Pb(II), Cu(II), and Cd(II) Ions on Wheat-Residue Derived Black Carbon. J. Chem. Eng. Data 2011, 56, 444–449. [Google Scholar] [CrossRef]
- Lan, T.; Li, P.; Rehman, F.U.; Li, X.; Yang, W.; Guo, S. Efficient Adsorption of Cd2+ from Aqueous Solution Using Metakaolin Geopolymers. Environ. Sci. Pollut. Res. 2019, 26, 33555–33567. [Google Scholar] [CrossRef]
- Cheng, Q.; Huang, Q.; Khan, S.; Liu, Y.; Liao, Z.; Li, G.; Ok, Y.S. Adsorption of Cd by Peanut Husks and Peanut Husk Biochar from Aqueous Solutions. Ecol. Eng. 2016, 87, 240–245. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Kim, A.G. Fly Ash Characterization by SEM–EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Li, L.; Wang, S.; Zhu, Z. Geopolymeric Adsorbents from Fly Ash for Dye Removal from Aqueous Solution. J. Colloid Interface Sci. 2006, 300, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous Photocatalytic Degradation of Organic Contaminants over Titanium Dioxide: A Review of Fundamentals, Progress and Problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Cho, H.; Oh, D.; Kim, K. A Study on Removal Characteristics of Heavy Metals from Aqueous Solution by Fly Ash. J. Hazard. Mater. 2005, 127, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Aboulayt, A.; Jaafri, R.; Samouh, H.; Cherki El Idrissi, A.; Roziere, E.; Moussa, R.; Loukili, A. Stability of a New Geopolymer Grout: Rheological and Mechanical Performances of Metakaolin-Fly Ash Binary Mixtures. Constr. Build. Mater. 2018, 181, 420–436. [Google Scholar] [CrossRef]
- Kai, M.F.; Dai, J.G. Understanding Geopolymer Binder-Aggregate Interfacial Characteristics at Molecular Level. Cem. Concr. Res. 2021, 149, 106582. [Google Scholar] [CrossRef]
- Sumesh, M.; Alengaram, U.J.; Jumaat, M.Z.; Mo, K.H.; Alnahhal, M.F. Incorporation of Nano-Materials in Cement Composite and Geopolymer Based Paste and Mortar—A Review. Constr. Build. Mater. 2017, 148, 62–84. [Google Scholar] [CrossRef]
- Maiti, M.; Sarkar, M.; Maiti, S.; Malik, M.A.; Xu, S. Modification of Geopolymer with Size Controlled TiO2 Nanoparticle for Enhanced Durability and Catalytic Dye Degradation under UV Light. J. Clean. Prod. 2020, 255, 120183. [Google Scholar] [CrossRef]
- Sivasakthi, M.; Jeyalakshmi, R.; Rajamane, N.P. Investigation of Microstructure and Thermomechanical Properties of Nano-TiO2 Admixed Geopolymer for Thermal Resistance Applications. J. Mater. Eng. Perform. 2021, 30, 3642–3653. [Google Scholar] [CrossRef]
- Jézéquel, H.; Chu, K. Removal of Arsenate from Aqueous Solution by Adsorption onto Titanium Dioxide Nanoparticles. J. Environ. Sci. Health Part A 2007, 41, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.C.; Fu, S.H.; Tseng, J.J.; Chu, H.; Ko, T.H. Study on Photocatalytic Degradation of Gaseous Dichloromethane Using Pure and Iron Ion-Doped TiO2 Prepared by the Sol–Gel Method. Chemosphere 2007, 66, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Hema, M.; Arasi, A.Y.; Tamilselvi, P.; Anbarasan, R. Titania Nanoparticles Synthesized by Sol-Gel Technique. Chem Sci Trans 2013, 2, 239–245. [Google Scholar] [CrossRef]
- Sindhunata; Van Deventer, J.S.J.; Lukey, G.C.; Xu, H. Effect of Curing Temperature and Silicate Concentration on Fly-Ash-Based Geopolymerization. Ind. Eng. Chem. Res. 2006, 45, 3559–3568. [Google Scholar] [CrossRef]
- Khatib, K.; Kerroumi, H.; El Azhari, M. Synthesis, Characterization and Optimization of New Adsorbent Materials Based on Industrial Discharges for the Decontamination of Liquid Effluents. Mater. Today Proc. 2020, 22, 120–125. [Google Scholar] [CrossRef]
- Sastry, K.V.S.G.K.; Sahitya, P.; Ravitheja, A. Influence of Nano TiO2 on Strength and Durability Properties of Geopolymer Concrete. Mater. Today Proc. 2021, 45, 1017–1025. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J. Effect of the Alkali Metal Activator on the Properties of Fly Ash-Based Geopolymers. Ind. Eng. Chem. Res. 1999, 38, 3932–3941. [Google Scholar] [CrossRef]
- Nath, S.K.; Maitra, S.; Mukherjee, S.; Kumar, S. Microstructural and Morphological Evolution of Fly Ash Based Geopolymers. Constr. Build. Mater. 2016, 111, 758–765. [Google Scholar] [CrossRef]
- Yang, L.Y.; Jia, Z.J.; Zhang, Y.M.; Dai, J.G. Effects of Nano-TiO2 on Strength, Shrinkage and Microstructure of Alkali Activated Slag Pastes. Cem. Concr. Compos. 2015, 57, 1–7. [Google Scholar] [CrossRef]
- Karanac, M.; Đolić, M.; Veličković, Z.; Kapidžić, A.; Ivanovski, V.; Mitrić, M.; Marinković, A. Efficient Multistep Arsenate Removal onto Magnetite Modified Fly Ash. J. Environ. Manage. 2018, 224, 263–276. [Google Scholar] [CrossRef]
- Vijayalakshmi, R.; Rajendran, V. Synthesis and Characterization of Nano-Tio2 via Different Methods. Arch. Appl. Sci. Res. 2012, 4, 1183–1190. [Google Scholar]
- Nikolić, V.; Komljenović, M.; Marjanović, N.; Baščarević, Z.; Petrović, R. Lead Immobilization by Geopolymers Based on Mechanically Activated Fly Ash. Ceram. Int. 2014, 40, 8479–8488. [Google Scholar] [CrossRef]
- Khataee, R.; Heydari, V.; Moradkhannejhad, L.; Safarpour, M.; Joo, S.W. Self-Cleaning and Mechanical Properties of Modified White Cement with Nanostructured TiO2. J. Nanosci. Nanotechnol. 2013, 13, 5109–5114. [Google Scholar] [CrossRef]
- Zailan, S.N.; Mahmed, N.; Abdullah, M.M.A.B.; Rahim, S.Z.A.; Halin, D.S.C.; Sandu, A.V.; Vizureanu, P.; Yahya, Z. Potential Applications of Geopolymer Cement-Based Composite as Self-Cleaning Coating: A Review. Coatings 2022, 12, 133. [Google Scholar] [CrossRef]
- Ono, Y.; Amano, Y.; Aikawa, M.; Machida, M. Zur Theorie Der Sogenannten Adsorption Geloster Stoffe. K. Sven. Vetenskapsakademiens. Handl. 1898, 24, 1–39. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of Dye from Aqueous Solution by Peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, K.; Yaseen, M.; Tong, Z.; Cui, X. Synthesis of Highly Efficient Porous Inorganic Polymer Microspheres for the Adsorptive Removal of Pb2+from Wastewater. J. Clean. Prod. 2018, 193, 351–362. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.S.; Al Zboon, K.; Al-Makhadmeh, L.; Hararah, M.; Mahasneh, M. Fly Ash Based Geopolymer for Heavy Metal Removal: A Case Study on Copper Removal. J. Environ. Chem. Eng. 2015, 3, 1669–1677. [Google Scholar] [CrossRef]
- Onutai, S.; Kobayashi, T.; Thavorniti, P.; Jiemsirilers, S. The Adsorption of Cadmium Ions on Fly Ash Based Geopolymer Particles. Key Eng. Mater. 2018, 766, 65–70. [Google Scholar] [CrossRef]
- Balamurugan, S.; Balu, A.; Usharani, K.; Suganya, M.; Anitha, S.; Prabha, D.L.; Ilangovan, S. Synthesis of CdO Nanopowders by a Simple Soft Chemical Method and Evaluation of Their Antimicrobial Activities. Pac. Sci. Rev. A Nat. Sci. Eng. 2016, 18, 228–232. [Google Scholar] [CrossRef]
- Pandey, S.; Tiwari, S. Facile Approach to Synthesize Chitosan Based Composite—Characterization and Cadmium(II) Ion Adsorption Studies. Carbohydr. Polym. 2015, 134, 646–656. [Google Scholar] [CrossRef]
- Ge, Y.; Cui, X.; Kong, Y.; Li, Z.; He, Y.; Zhou, Q. Porous Geopolymeric Spheres for Removal of Cu(II) from Aqueous Solution: Synthesis and Evaluation. J. Hazard. Mater. 2015, 283, 244–251. [Google Scholar] [CrossRef]
- Yu, Z.; Song, W.; Li, J.; Li, Q. Improved Simultaneous Adsorption of Cu(II) and Cr(VI) of Organic Modified Metakaolin-Based Geopolymer. Arab. J. Chem. 2020, 13, 4811–4823. [Google Scholar] [CrossRef]
- Taamneh, Y.; Sharadqah, S. The Removal of Heavy Metals from Aqueous Solution Using Natural Jordanian Zeolite. Appl. Water Sci. 2016, 7, 2021–2028. [Google Scholar] [CrossRef]

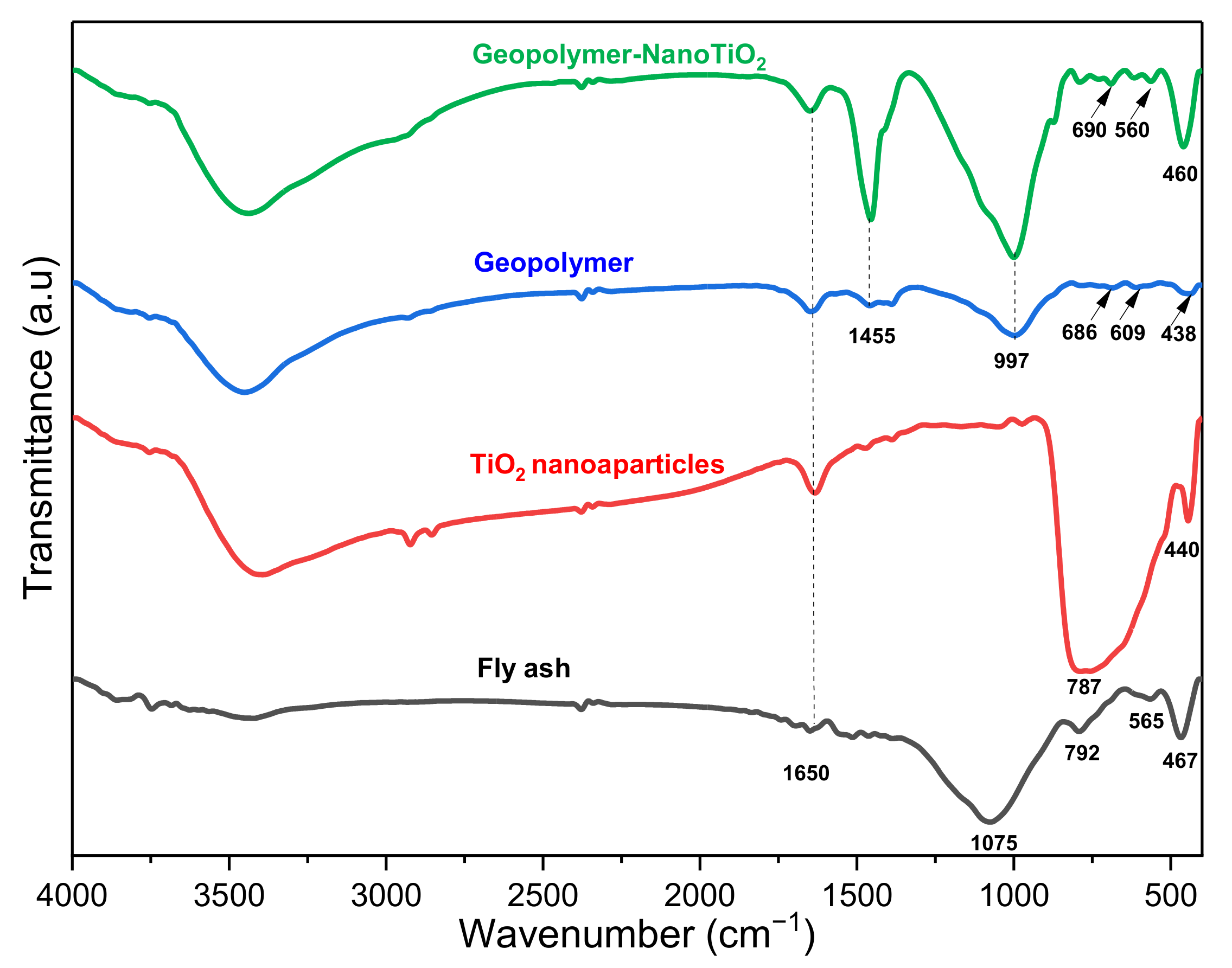
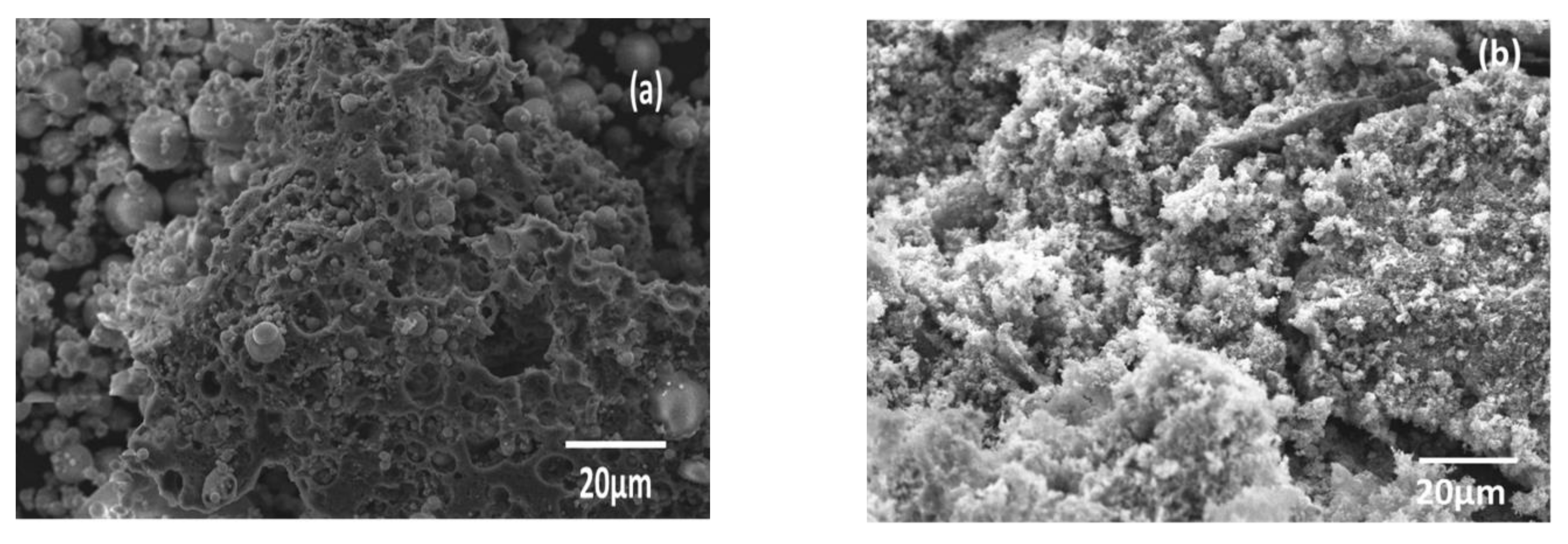
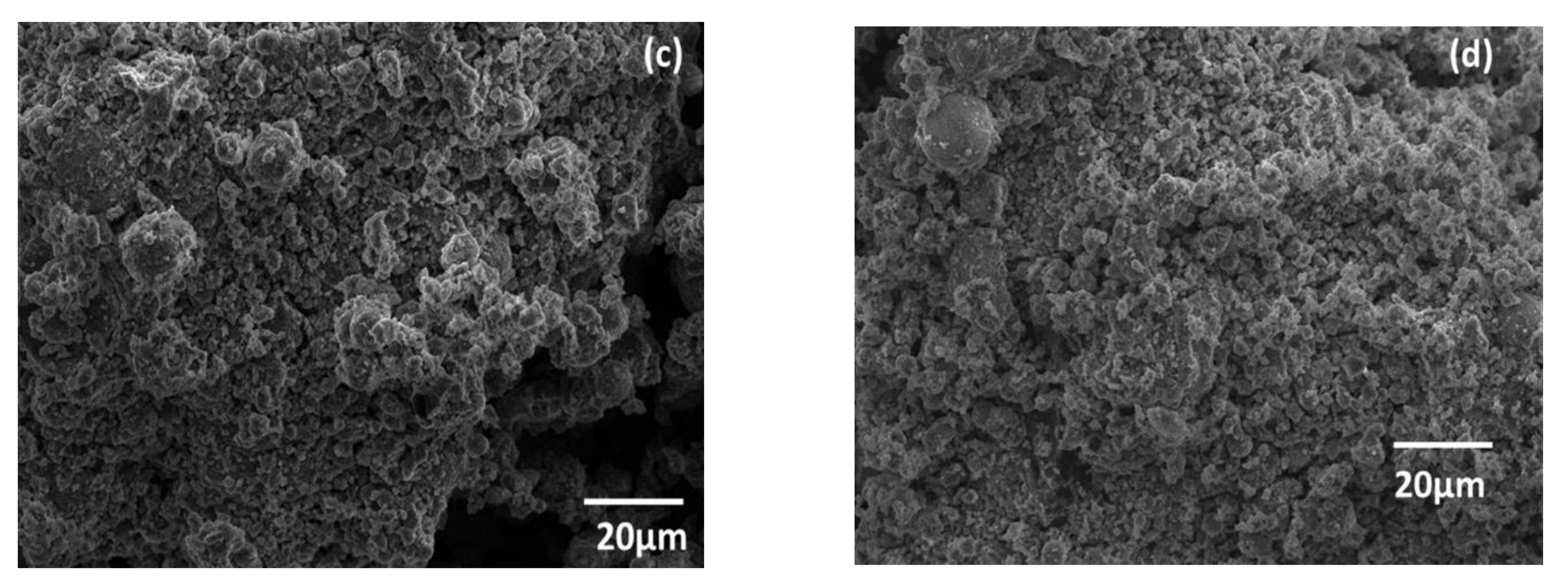
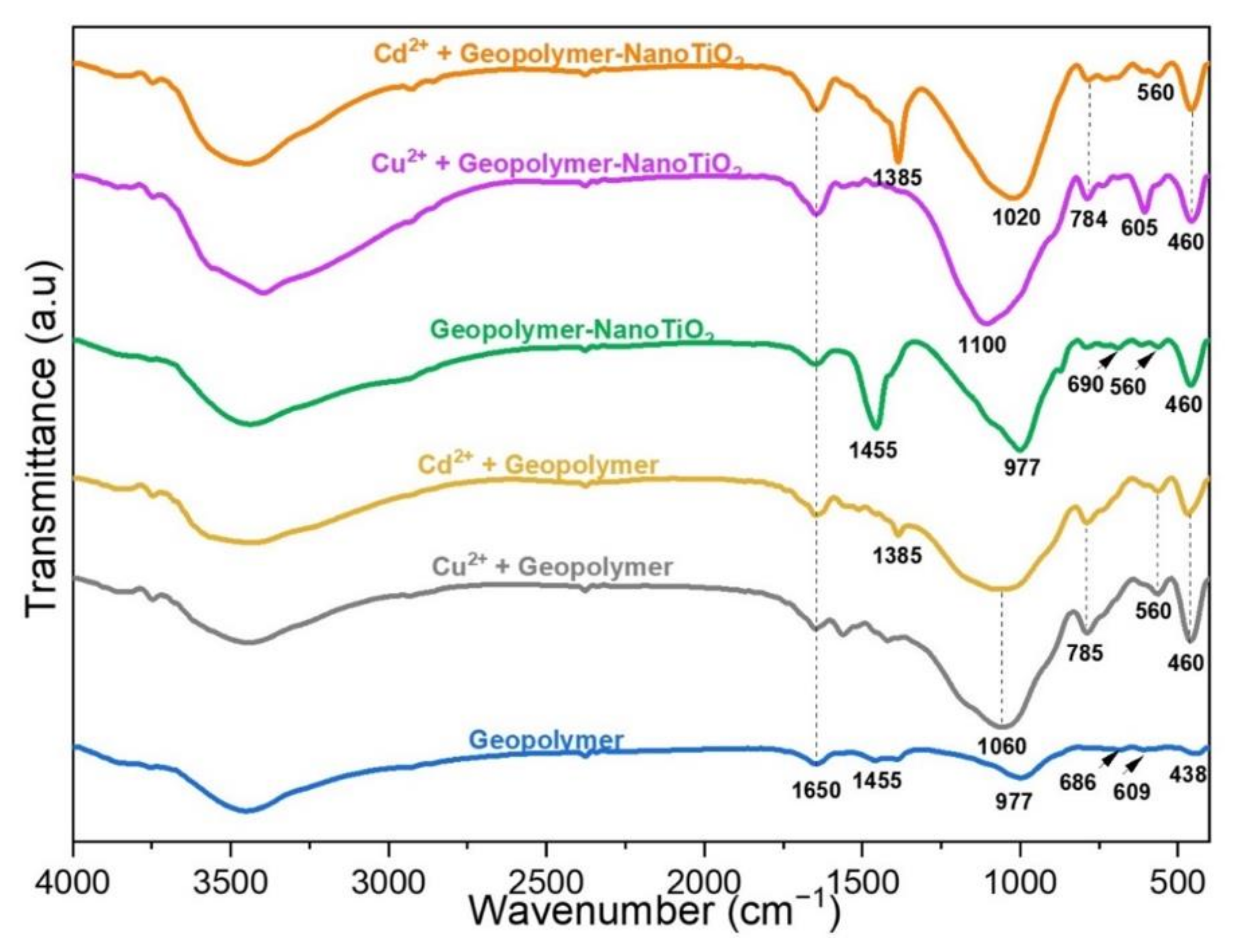
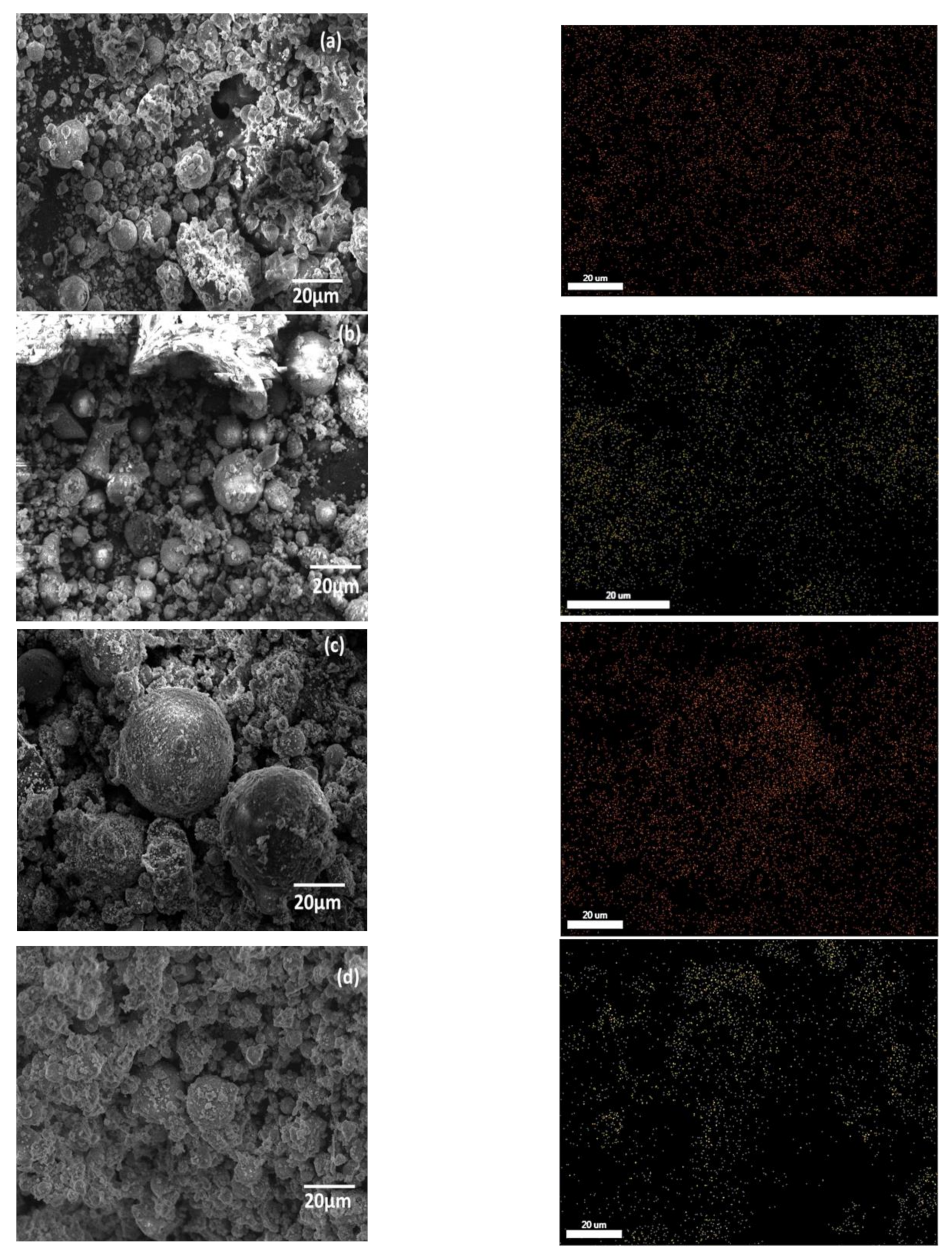
| Component | SiO2 | Al2O3 | Fe2O3 | CaO | K2O | TiO2 | MgO | Na2O | P2O5 | MnO | Loss Amount |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 33.83 | 13.12 | 5.58 | 4.27 | 2.18 | 0.95 | 0.93 | 0.83 | 0.45 | 0.06 | 37.76 |
| Adsorbate | Adsorbents | Qeq (mg/g) Experimental | Pseudo First Order | Pseudo Second Order | ||||
|---|---|---|---|---|---|---|---|---|
| Qeq (mg/g) | K1 (min−1) | R2 | Qeq (mg/g) | K2 (g.mg−1. min−1) | R2 | |||
| Cu2+ | Geopolymer | 78.87 | 37.62 | 0.0244 | 0.86 | 84.03 | 1.5.10−3 | 0.99 |
| Geopolymer-NanoTiO2 | 99.23 | 40.28 | 0.0372 | 0.83 | 102 | 2.62 | 0.99 | |
| Cd2+ | Geopolymer | 49.9 | 56.68 | 7.5.10−3 | 0.81 | 16.72 | 2.8.10−4 | 0.68 |
| Geopolymer-NanoTiO2 | 51.43 | 1.79 | 0.031 | 0.009 | 48.07 | 0.01 | 0.99 | |
| Adsorbate | Adsorbents | Qmax (mg/g) Experimental | Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|---|---|---|
| Qmax (mg/g) | KL (L/mg) | RL | R2 | n | KF (mg/g)(L/mg)1/n | R2 | |||
| Cu2+ | Geopolymer | 172.83 | 188.67 | 1.7.10−4 | 0.02 < RL < 0.52 | 0.99 | 2.32 | 1.86 | 0.89 |
| Geopolymer-NanoTiO2 | 1708.2 | 5000 | 1.17.10−5 | 0.25 < RL < 0.94 | 0.033 | 0.9 | 0.029 | 0.91 | |
| Cd2+ | Geopolymer | 271.66 | 285.71 | 2.3.10−4 | 0.006 < RL < 0.77 | 0.99 | 3.25 | 8.03 | 0.95 |
| Geopolymer-NanoTiO2 | 706.9 | 833.33 | 3.78.10−5 | 0.07 < RL < 0.95 | 0.84 | 2.03 | 2.18 | 0.97 | |
| Fly Ash | Nano TiO2 | Geopolymer | Cu2++ Geopolymer | Cd2++ Geopolymer | Geopolymer-NanoTiO2 | Cu2++ Geopolymer-NanoTiO2 | Cd2++ Geopolymer-NanoTiO2 | |
|---|---|---|---|---|---|---|---|---|
| Element | Weight% | Weight% | Weight% | Weight% | Weight% | Weight% | Weight% | Weight% |
| C | 61.48 | 3.07 | 2.31 | 14.5 | 24.54 | 12.59 | 22.42 | 22.78 |
| O | 19.29 | 39.59 | 36.72 | 38.74 | 43.32 | 39.24 | 36.43 | 39.40 |
| Fe | 0.08 | - | 0.51 | 0.05 | 2.38 | 0.00 | 3.08 | 3.33 |
| Na | 0.19 | - | 16.10 | - | 0.84 | 13.84 | - | 2.04 |
| Mg | 0.39 | - | 0.84 | 0.11 | 0.55 | 0.11 | 0.77 | 0.72 |
| Al | 4.87 | - | 7.16 | 5.43 | 8.22 | 6.30 | 6.22 | 7.29 |
| Si | 10.28 | - | 19.40 | 9.87 | 16.42 | 17.20 | 9.81 | 12.13 |
| S | 0.61 | - | - | 4.53 | - | - | 1.5 | - |
| K | 0.98 | - | 6.31 | 0.89 | 1.09 | 1.30 | 0.96 | 1.02 |
| Ca | 1.84 | - | 10.64 | 1.13 | 1.03 | 3.83 | 1.13 | 2.83 |
| N | - | 0.08 | - | 0.38 | - | - | - | - |
| Br | - | - | - | - | - | - | - | |
| Ti | - | 57.26 | - | - | - | 5.60 | 3.71 | 3.05 |
| Cu | - | - | - | 24.36 | - | - | 13.97 | - |
| Cd | - | - | - | - | 1.61 | - | - | 5.41 |
| Adsorbent | Adsorbates | pH | Time (min) | Temperature °C | Q(mg/g) | Ref. |
|---|---|---|---|---|---|---|
| Porous geopolymer | Cu2+ | 5 | 2500 | 25 | 52.63 | [44] |
| Geopolymer/alginate hybrid spheres | Cu2+ | 5 | 2500 | 25 | 60.8 | [7] |
| CTAB/geopolymer | Cu2+ | 5 | 60 | 30 | 147.2 | [45] |
| Jordanian zeolite | Cu2+ | 6 | 20 | - | 14.3 | [46] |
| Cd2+ | 6 | 20 | - | 25.9 | ||
| Metakaolin geopolymer powder | Cd2+ | 5 | 400 | 25 | 70.3 | [11] |
| Fly ash-chitosan | Cd2+ | 8 | 180 | - | 87.72 | [43] |
| Geopolymer | Cu2+ | 6.8 | 60 | 25 | 172.83 | This work |
| Cd2+ | 6.8 | 100 | 25 | 271.66 | ||
| Geopolymer-NanoTiO2 | Cu2+ | 6.8 | 150 | 25 | 1708.2 | |
| Cd2+ | 6.8 | 20 | 25 | 706.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatib, K.; Lahmyed, L.; El Azhari, M. Synthesis, Characterization, and Application of Geopolymer/TiO2 Nanoparticles Composite for Efficient Removal of Cu(II) and Cd(II) Ions from Aqueous Media. Minerals 2022, 12, 1445. https://doi.org/10.3390/min12111445
Khatib K, Lahmyed L, El Azhari M. Synthesis, Characterization, and Application of Geopolymer/TiO2 Nanoparticles Composite for Efficient Removal of Cu(II) and Cd(II) Ions from Aqueous Media. Minerals. 2022; 12(11):1445. https://doi.org/10.3390/min12111445
Chicago/Turabian StyleKhatib, Khalid, Loubna Lahmyed, and Mohamed El Azhari. 2022. "Synthesis, Characterization, and Application of Geopolymer/TiO2 Nanoparticles Composite for Efficient Removal of Cu(II) and Cd(II) Ions from Aqueous Media" Minerals 12, no. 11: 1445. https://doi.org/10.3390/min12111445
APA StyleKhatib, K., Lahmyed, L., & El Azhari, M. (2022). Synthesis, Characterization, and Application of Geopolymer/TiO2 Nanoparticles Composite for Efficient Removal of Cu(II) and Cd(II) Ions from Aqueous Media. Minerals, 12(11), 1445. https://doi.org/10.3390/min12111445






