Solid Carriers of Potentially Toxic Elements and Their Fate in Stream Sediments in the Area Affected by Iron Ore Mining and Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Description
2.2. Geology of the Study Area
2.3. Sample Collection and Preparation
2.4. Mineralogical Characterization and Microanalytical Single Particle Analysis
2.5. Chemical Analysis of Stream Sediments and Mine Waste Material
2.6. Chemical Analysis of Stream Water
2.7. Physico-Chemical Properties of Stream Water
3. Results and Discussion
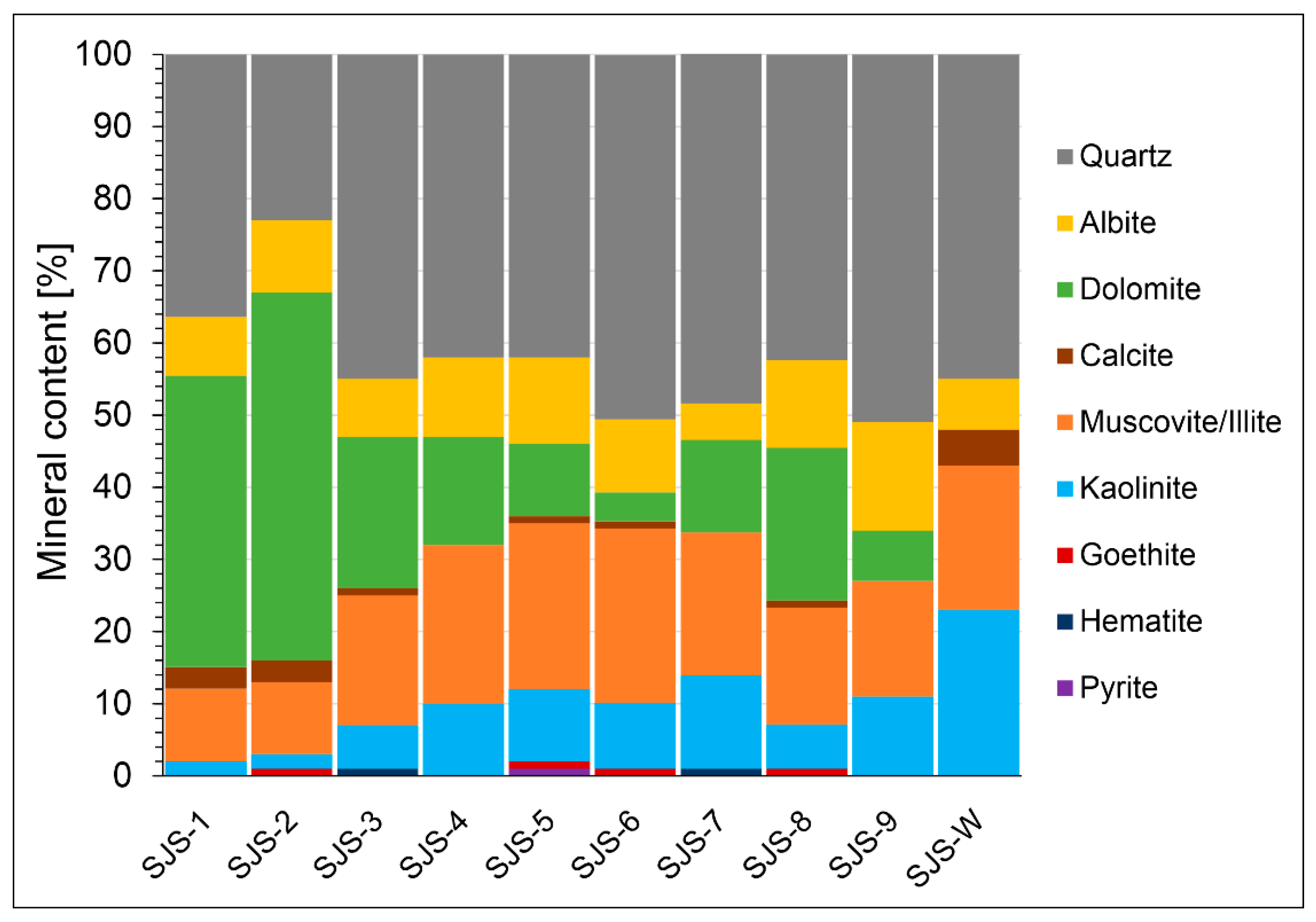
3.1. Mineralogical Characteristics of Stream Sediments and Mine Waste Material
3.2. Solid PTE Carriers and Their Characteristics
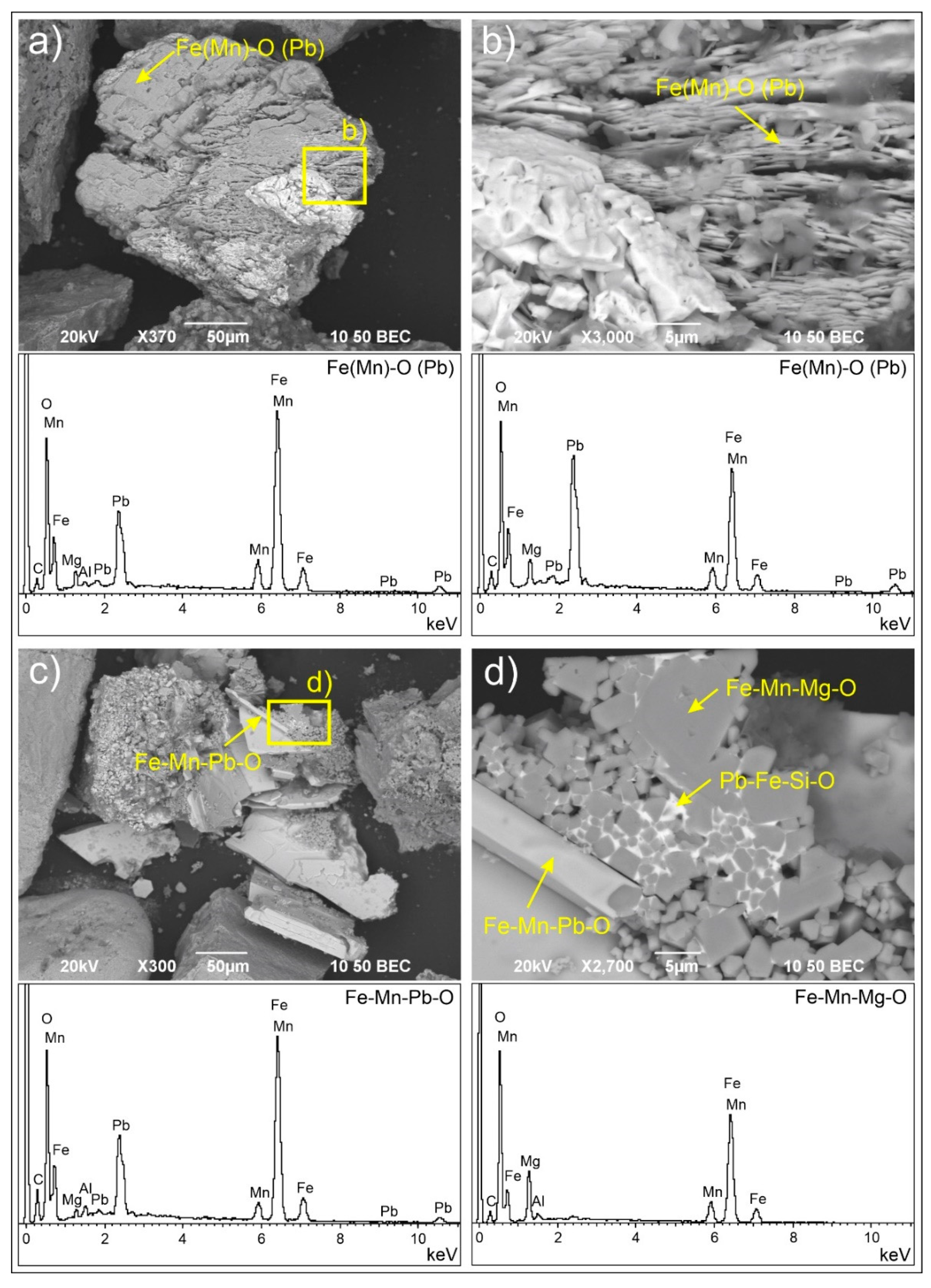
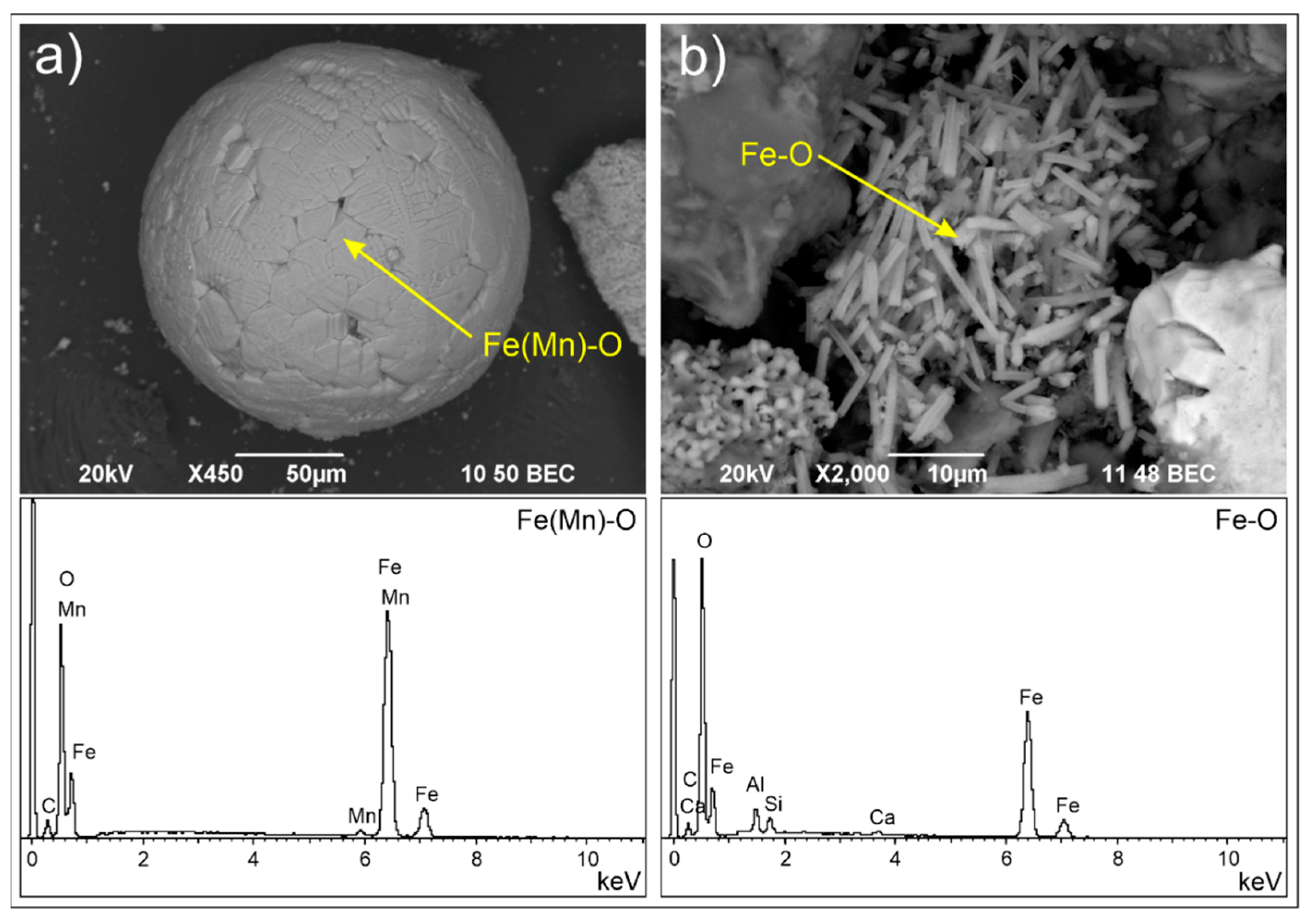
3.2.1. Solid Fe Carriers
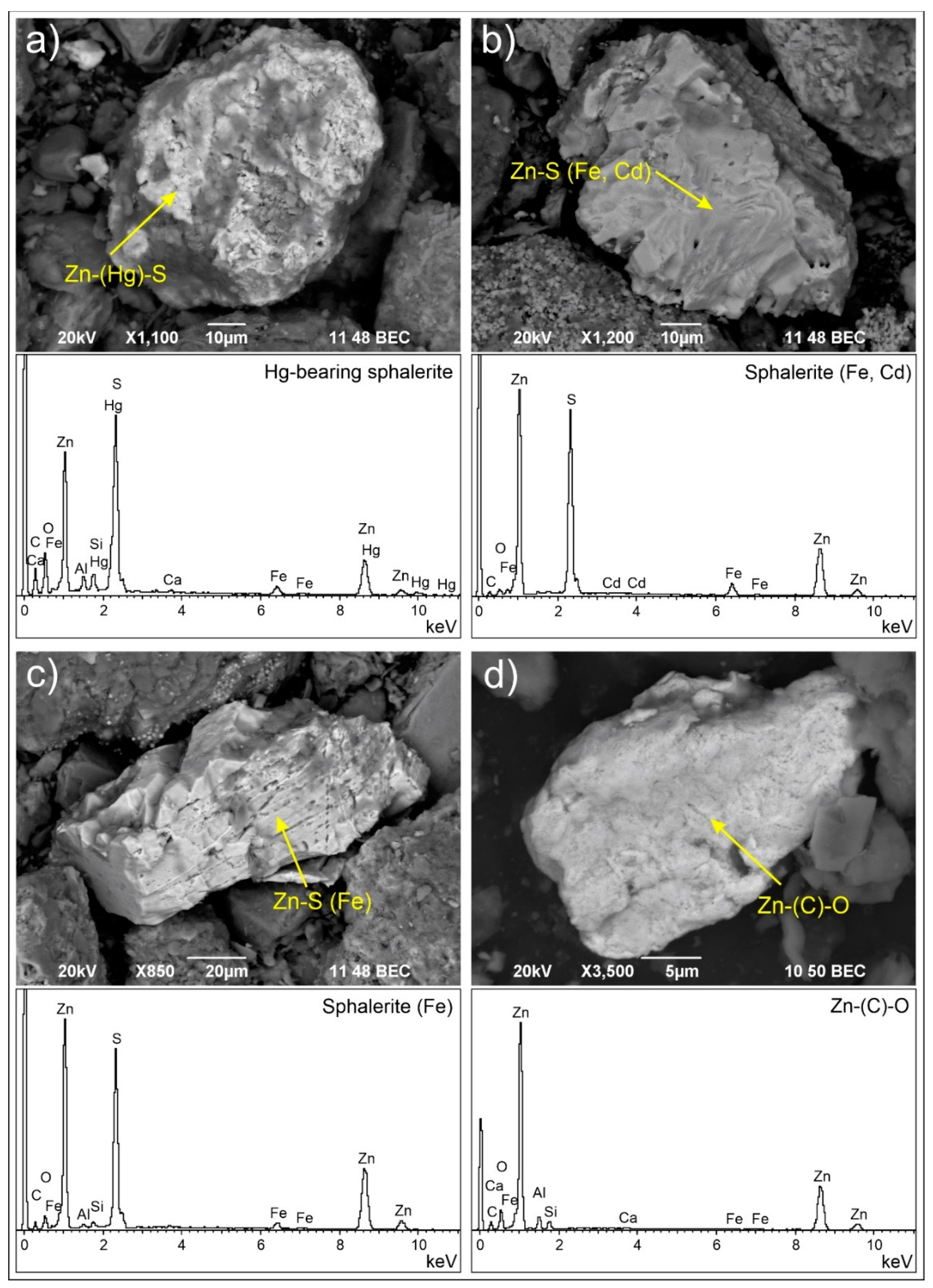
3.2.2. Solid Zn Carriers
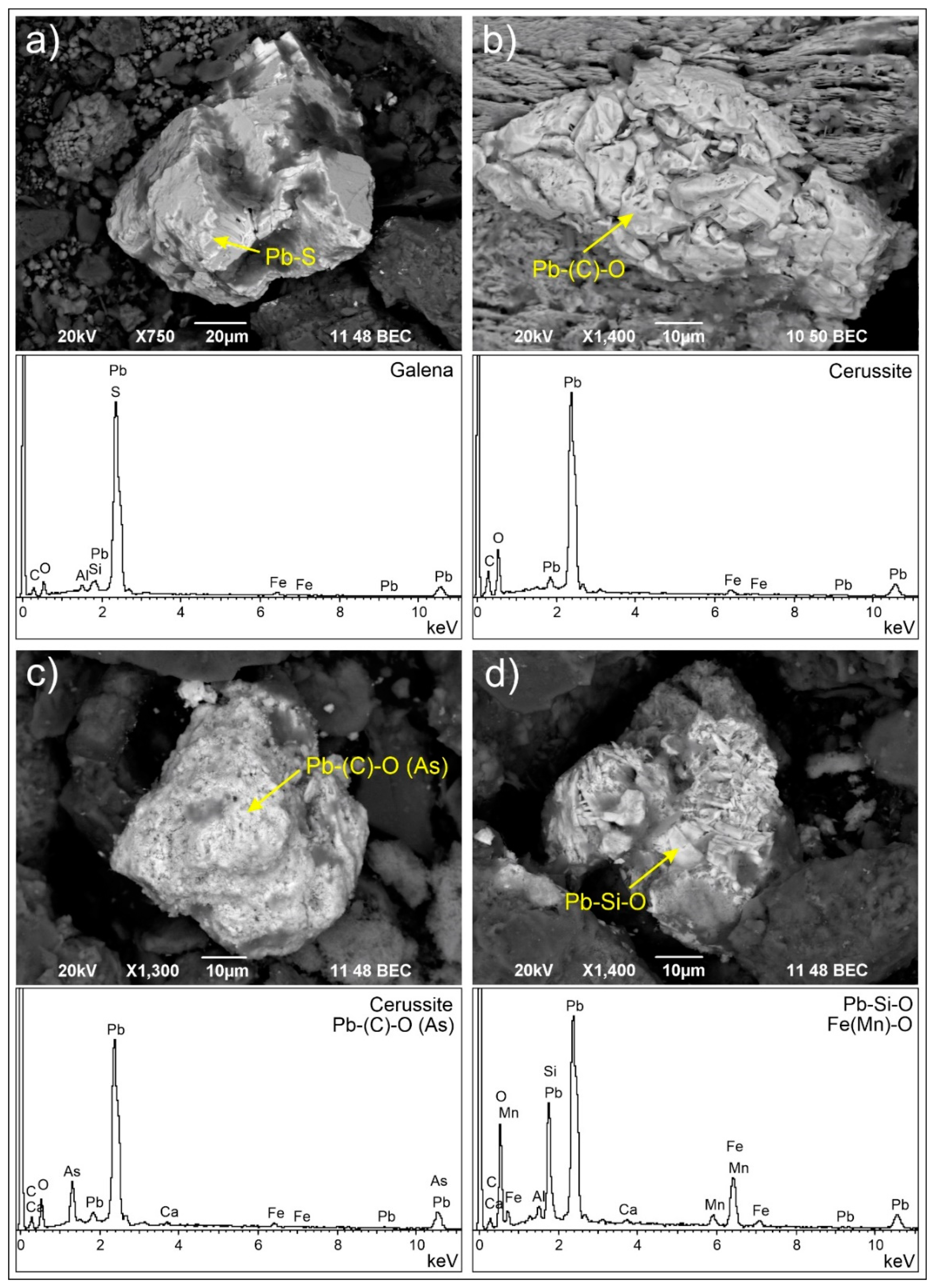
3.2.3. Solid Pb Carriers
3.3. Distribution of PTEs in the Environment
3.4. Physico-Chemical Characteristics of Stream Water
3.5. The Behavior of Solid PTE Carriers in Stream Sediment and Interaction with Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adepoju, M.O.; Adekoya, J.A. Heavy metal distribution and assessment in stream sediments of River Orle, Southwestern Nigeria. Arab. J. Geosci. 2014, 7, 743–756. [Google Scholar] [CrossRef]
- Dinelli, E.; Cortecci, G.; Lucchini, F.; Zantedeschi, E. Sources of major and trace elements in the stream sediments of the Arno River catchment (northern Tuscany, Italy). Geochem. J. 2005, 33, 531–545. [Google Scholar] [CrossRef]
- Lim, W.Y.; Aris, A.Z.; Ismail, H.T. Spatial Geochemical Distribution and Sources of Heavy Metals in the Sediment of Langat River, Western Peninsular Malaysia. Environ. Forensics 2013, 14, 133–145. [Google Scholar] [CrossRef]
- Calmano, W.; Hong, J.; Förstner, U. Binding and mobilization of heavy metals in contaminated sediments affected by pH and redox potential. Water Sci. Technol. 1993, 28, 223–235. [Google Scholar] [CrossRef]
- Ashayeri, N.Y.; Keshavarzi, B. Geochemical characteristics, partitioning, quantitative source apportionment, and ecological and health risk of heavy metals in sediments and water: A case study in Shadegan Wetland, Iran. Mar. Pollut. Bull. 2019, 149, 110495. [Google Scholar] [CrossRef]
- Miranda, L.S.; Wijesiri, B.; Ayoko, G.A.; Egodawatta, P.; Goonetilleke, A. Water-sediment interactions and mobility of heavy metals in aquatic environments. Water Res. 2021, 202, 117386. [Google Scholar] [CrossRef]
- Krishna, A.; Mohan, K.R.K.; Murthy, N.N. A multivariate statistical approach for monitoring of heavy metals in sediments: A case study from Wailpalli Watershed, Nalgonda District, Andhra Pardesh, India. Res. J. Environ. Earth. Sci. 2010, 3, 103–113. Available online: https://maxwellsci.com/jp/abstract.php?jid=RJEES&no=89&abs=06 (accessed on 19 August 2022).
- Budkovič, T.; Šajn, R.; Gosar, M. Environmental impact of active and abandoned mines and metal smelters in Slovenia. Geologija 2003, 46, 135–140. [Google Scholar] [CrossRef]
- Gosar, M.; Šajn, R.; Miler, M.; Burger, A.; Bavec, Š. Overview of existing information on important closed (or in closing phase) and abandoned mining waste sites and related mines in Slovenia. Geologija 2020, 63, 221–250. [Google Scholar] [CrossRef]
- Gosar, M.; Pirc, S.; Bidovec, M. Mercury in the Idrijca river sediments as a reflection of mining and smelting activities of the mercury mine Idrija. J. Geochem. Explor. 1997, 58, 125–131. [Google Scholar] [CrossRef]
- Biester, H.; Gosar, M.; Covelli, S. Mercury speciation in sediments affected by dumped mining residues in the drainage area of the Idrija mercury mine, Slovenia. Environ. Sci. Technol. 2000, 34, 3330–3336. [Google Scholar] [CrossRef]
- Gosar, M.; Šajn, R. Mercury in soil and attic dust as a reflection of Idrija mining and mineralization (Slovenia). Geologija 2001, 44, 137–159. [Google Scholar] [CrossRef]
- Šajn, R. Factor Analysis of soil and attic-dust to separate mining and metallurgy influence, Meža valley, Slovenia. Math. Geol. 2006, 38, 735–747. [Google Scholar] [CrossRef]
- Šajn, R.; Gosar, M. Soil pollution in surroundings of Litija as a reflection of mining, metallurgy and natural conditions. Geologija 2007, 50, 131–145. [Google Scholar] [CrossRef]
- Šajn, R.; Gosar, M. Multivariate statistical approach to identify metal sources in Litija area (Slovenia). J. Geochem. Explor. 2014, 138, 8–21. [Google Scholar] [CrossRef]
- Teršič, T.; Gosar, M.; Šajn, R. Impact of mining activities on soils and sediments at the historical mining area in Podljubelj, NW Slovenia. J. Geochem. Explor. 2009, 1, 1–10. [Google Scholar] [CrossRef]
- Teršič, T.; Miler, M.; Gaberšek, M.; Gosar, M. Contents of arsenic and some other elements in stream sediments and waters of the Medija drainage basin, central Slovenia. Geologija 2018, 61, 5–35. [Google Scholar] [CrossRef]
- Gosar, M.; Žibret, G. Mercury contents in the vertical profiles through alluvial sediments as a reflection of mining in Idrija (Slovenia). J. Geochem. Explor. 2011, 110, 81–91. [Google Scholar] [CrossRef]
- Miler, M.; Gosar, M. Characteristics and potential environmental influences of mine waste in the area of the closed Mežica Pb-Zn mine (Slovenia). J. Geochem. Explor. 2012, 112, 152–160. [Google Scholar] [CrossRef]
- Miler, M.; Bavec, Š.; Gosar, M. The environmental impact of historical Pb-Zn mining waste deposits in Slovenia. J. Environ. Manag. 2022, 308, 114580. [Google Scholar] [CrossRef]
- Šorn, J. Bucelleniji in Ruardi na Savi pri Jesenicah. Ljubljana. Kronika 1976, 24, 69–74. Available online: http://www.dlib.si/?URN=URN:NBN:SI:doc-BW51LH1L (accessed on 13 September 2022).
- Češmiga, I. Rudarstvo LR Slovenije; Nova Proizvodnja: Ljubljana, Slovenia, 1959. [Google Scholar]
- Rjazancev, A. Razpad fužin in nastanek metalurške industrije na Jesenicah. Jeklo Ljud. 1964, 1, 16380162. Available online: http://www.dlib.si/?URN=URN:NBN:SI:DOC-PHHC39IL (accessed on 13 September 2022).
- Voss, W. Die Mineralien des Herzogthums Krain; Kleinmayr & Fed: Bamberg, Germany, 1895; pp. 10–81. [Google Scholar]
- Schroll, E. Ein Beitrag zur geochemischen Analyse Ostalpiner Blei-Zink Erze. Mitt. Der Osterr. Mineral. Gesselschaft 1954, 3, 41–43. [Google Scholar]
- Slovenian Environmental Agency, Climate. Available online: https://meteo.arso.gov.si/uploads/probase/www/climate/table/sl/by_location/planina-pod-golico/climate-normals_81-10_Planina-pod-Golico.pdf (accessed on 21 October 2022).
- Mohorič, N.; Grigillo, D.; Jemec Auflič, M.; Mikoš, M.; Celarc, B. Longitudinal profiles of torrential Channels in the Western Karavanke mountains. Geologija 2016, 59, 273–286. [Google Scholar] [CrossRef]
- Buser, S.; Cajhen, J. Osnovna Geološka Karta SFRJ. L 33-52, Celovec (Klagenfurt); [Kartografsko gradivo]. 1:100,000; Zvezni Geološki Zavod Beograd: Beograd, Serbia, 1978. [Google Scholar]
- Iskra, M. The geologic features of the Savske jame iron ore deposit. Geologija 1965, 8, 279–298. Available online: https://www.geologija-revija.si/index.php/geologija/article/view/223 (accessed on 12 September 2022).
- Drovenik, M.; Drovenik, F.; Pleničar, M. The origin of Slovenian ore deposits. Geologija 1980, 23, 1–157. Available online: https://www.geologija-revija.si/index.php/geologija/article/view/1743 (accessed on 13 September 2022).
- Mikuž, V.; Vidrih, R.; Grm, M. Minerali Savskih jam in okolice. Scopolia Suppl. 2006, 3, 78–83. Available online: http://www.dlib.si/?URN=URN:NBN:SI:doc-048L4JBH (accessed on 13 September 2022).
- De Groot, A.J.; Zschuppe, K.H.; Salomons, W. Standardization of methods of analysis for heavy metals in sediments. Hydrobiologia 1982, 92, 689–695. [Google Scholar] [CrossRef]
- Noack, C.W.; Jain, J.C.; Stegmeier, J.; Hakala, J.A.; Karamalidis, A.K. Rare earth element geochemistry of outcrop and core samples from the Marcellus Shale. Geochem. Trans. 2015, 16, 6. [Google Scholar] [CrossRef]
- Goldstein, J.; Newbury, D.; Joy, D. Scanning Electron Microscopy and X-ray Microanalysis, 3rd ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003. [Google Scholar]
- Oxford Instruments. INCA Energy Operator Manual; Oxford Instruments Analytical Ltd.: High Wycombe, UK, 2006. [Google Scholar]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. The Handbook of Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 2009; Available online: http://www.handbookofmineralogy.org/ (accessed on 26 July 2022).
- The Mineralogy Database. 2012. Available online: http://webmineral.com/ (accessed on 26 July 2022).
- Reimann, C.; Demetriades, A.; Eggen, O.A.; Filzmoser, P. EuroGeoSurveys Geochemistry Expert Group. The EuroGeoSurveys Geochemical Mapping of Agricultural and Grazing Land Soil Project (GEMAS)–Evaluation of Quality Control Results of Aqua Regia Extraction Analysis; NGU Report 2009.049; Geological Survey of Norway: Trondheim, Norway, 2009; p. 94. [Google Scholar]
- Oreas, OREAS 45P. Available online: https://www.oreas.com/crm/oreas-45p/ (accessed on 13 September 2022).
- Inorganic Ventures–Products. Available online: https://www.inorganicventures.com/coa/Letterhead/IV/IV-STOCK-1643_T2-MEB720866.pdf (accessed on 13 September 2022).
- Usher, C.R.; Cleveland, C.A.; Strongin, D.R.; Schoonen, M.A. Origin of oxygen in sulfate during pyrite oxidation with water and dissolved oxygen: An in situ horizontal attenuated total reflectance infrared spectroscopy isotope study. Environ. Sci. Technol. 2004, 38, 5604–5606. [Google Scholar] [CrossRef]
- Huggins, F.E.; Huffman, G.P.; Kosmack, D.A.; Lowenhaupt, D.E. Mossbauer detection of goethite (α-FeOOH) in coal and its potential as an indicator of coal oxidation. Int. J. Coal Geol. 1980, 1, 75–81. [Google Scholar] [CrossRef]
- Sileo, E.E.; Alvarez, M.; Rueda, E.H. Structural studies on the manganese for iron substitution in the synthetic goethite-jacobsite system. Int. J. Inorg. Mater. 2001, 3, 271–279. [Google Scholar] [CrossRef]
- Rahimi, S.; Moattari, R.M.; Rajabi, L.; Derakhashan, A.A.; Keyhani, M. Iron oxide/hydroxide (α,γ-FeOOH) nanoparticles as high potential adsorbents for lead removal from polluted aquatic media. J. Ind. Eng. Chem. 2015, 23, 33–43. [Google Scholar] [CrossRef]
- Caraballo, M.A.; Asta, M.P.; Perez, J.P.H.; Hochella, M.F. Past, present and future global influence and technological applications of iron-bearing metastable nanominerals. Gondwana Res. 2022, 110, 283–304. [Google Scholar] [CrossRef]
- Lin, M.; Tng, L.; Lim, T.; Choo, M.; Zhang, J.; Tan, H.R.; Bai, S. Hydrothermal synthesis of octahedral hematite (α-Fe2O3) nanoparticles: An epitaxial growth from goethite (α-Fe2O3). J. Phys. Chem. 2014, 118, 10903–10910. [Google Scholar] [CrossRef]
- Katsuki, H.; Choi, E.K.; Lee, W.J.; Hwang, K.T.; Cho, W.S.; Huang, W.; Komarneni, S. Ultrafast microwave-hydrothermal synthesis of hexagonal plates of hematite. Mater. Chem. Phys. 2018, 205, 210–216. [Google Scholar] [CrossRef]
- Pino, M.; Abarzúa, A.M.; Astorga, G.A.; Martel-Cea, A.; Cossio-Montecinos, N.; Navarro, R.X.; Lira, M.P.; Labarca, R.; Lecompte, M.A.; Adedeji, V.; et al. Sedimentary record from Patagonia, southern Chile supports cosmic-impact triggering of biomass burning, climate change, and megafaunal extinctions at 12.8 ka. Sci. Rep. 2019, 9, 4413. [Google Scholar] [CrossRef]
- Hashimoto, H.; Yokoyama, S.; Asaoka, H.; Kusano, Y.; Ikeda, Y.; Seno, M.; Takada, J.; Fujii, T.; Nakanishi, M.; Murakami, R. Characteristics of hollow microtubes consisting of amorphous iron oxide nanoparticles produced by iron oxidizing bacteria, Leptothrix ochracea. J. Magn. Magn. Mater. 2007, 310, 2405–2407. [Google Scholar] [CrossRef]
- Schwartz, M.O. Mercury in Zinc Deposits: Economic Geology of a Polluting Element. Inter. Geol. Rev. 1997, 39, 905–923. [Google Scholar] [CrossRef]
- Radosavljević, S.A.; Stojanović, J.N.; Pačevski, A.M. Hg-bearing sphalerite from the Rujevac polymetallic ore deposit, Podrinje metallogenic district, Serbia: Compositional variations and zoning. Chem. Erde. 2012, 72, 237–244. [Google Scholar] [CrossRef]
- Lee, P.; Kang, M.-J.; Choi, S.-H.; Touray, J.-C. Sulfide oxidation and the natural attenuation of arsenic and trace metals in the waste rocks of the abandoned Seobo tungsten mine, Korea. Appl. Geochem. 2005, 20, 1687–1703. [Google Scholar] [CrossRef]
- Szczerba, M.; Sawlowicz, Z. Remarks on the origin of cerussite in the Upper Silesian Zn-Pb deposits, Poland. Mineralogia 2009, 40, 53–64. [Google Scholar] [CrossRef]
- Sotlar, K. Potočni Sediment Kot Vzorčno Sredstvo za Izdelavo Geokemične Karte Slovenije. Diploma Thesis, University of Ljubljana, Ljubljana, Slovenia, 4 December 1995. [Google Scholar]
- Salminen, R. Part 1: Background Information, Methodology and Maps. In Geochemical Atlas of Europe; Salminen, R., Ed.; Geological Survey of Finland: Espoo, Finland, 2006. [Google Scholar]
- Lintern, A.; Webb, J.A.; Ryu, D.; Liu, S.; Bende-Michl, U.; Waters, D.; Leahy, P.; Wilson, P.; Western, A.W. Key factors influencing differences in stream water quality across space. WIREs Water 2017, 5, e1260. [Google Scholar] [CrossRef]
- Chuman, T.; Hruška, J.; Oulehle, F.; Gürtlerová, P.; Majer, V. Does stream water chemistry reflect watershed characteristics? Environ. Monit. Assess. 2013, 185, 5683–5701. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.C.; Ferrier, R.C.; Miller, J.D. The contribution of sulphate to total sulphur in a range of natural water samples. Hydrol. Sci. J. 1992, 37, 277–283. [Google Scholar] [CrossRef]
- Likus-Cieślik, J.; Smoliński, A.; Pietrzykowski, M.; Bąk, A. Sulphur contamination impact on seasonal and surface water chemistry on reforested area of a former sulphur mine. Land Degrad. Dev. 2019, 30, 212–225. [Google Scholar] [CrossRef]
- Du Laing, G.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F.M. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: A review. Sci. Total. Enivron. 2009, 407, 3972–3985. [Google Scholar] [CrossRef]
- Brookins, D.G. Eh-pH Diagrams for Geochemistry; Springer: Berlin/Heidelberg, Germany, 1988; pp. 16–55. [Google Scholar]
- Trick, J.K.; Stuart, M.; Reeder, S. Contaminated groundwater sampling and quality control of water analyses. In Environmental Geochemistry; De Vivo, B., Belkin, E.H., Lima, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 29–57. [Google Scholar]
- Lecomte, K.L.; García, M.G.; Formica, S.; Depetris, P.J. Influence of geomorphological variables on mountainous stream water chemistry (Sierras Pampeanas, Córdoba, Argentina). Geomorphology 2009, 110, 195–202. [Google Scholar] [CrossRef]
- Reichert, J.; Borg, G. Numerical simulation and a geochemical model of supergene carbonate hosted non-sulphide zinc deposits. Ore Geol. Rev. 2008, 33, 134–151. [Google Scholar] [CrossRef]
- Lin, Z. Mineralogical and chemical characterization of wastes from a sulfuric acid industry in Falun Sweden. Environ. Geol. 1997, 30, 153–162. [Google Scholar] [CrossRef]
- Jackson, T.A.; Bistricki, T. Selective scavenging of copper, zinc, lead, and arsenic by iron and manganese oxyhydroxide coatings on plankton in lakes polluted with mine and smelter wastes: Results of energy dispersive X-ray micro-analysis. J. Geochem. Explor. 1995, 52, 97–125. [Google Scholar] [CrossRef]
- Hou, D.; He, J.; Lu, C.; Ren, L.; Fan, Q.; Wang, J. Distribution characteristics and potential ecological risk assessment of heavy metals (Cu, Pb, Zn, Cd) in waters and sediments from Lake Dalinouer, China. Ecotoxicol. Environ. Saf. 2013, 93, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Fendorf, S.; Nico, P.; Kocar, B.D.; Masue, Y.; Tufano, K.J. Arsenic Chemistry in Soils and Sediments; Developments in Soil, Science; Singh, B., Gräfe, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 34, pp. 357–378. [Google Scholar] [CrossRef]



| Phase | Minor Constituent Elements | Relative Abundance | Mineral Equivalent |
|---|---|---|---|
| Fe-S | ***** | Pyrite (FeS2) | |
| Fe-S-O | Ag, As, Cu, Pb | ** | Szomolnokite (FeSO4·H2O) |
| Cu-Fe-S | * | Chalcopyrite (CuFeS2) | |
| Fe(Mn)-O | Pb, Zn | ***** | Siderite (FeCO3)/ferrihydrite (Fe3+10O14(OH)2/goethite (α-Fe3+O(OH))/ lepidocrocite (γ-Fe3+O(OH))/hematite (α-Fe2O3)/magnetite (Fe2+Fe23+O4) |
| Fe-O | As, Cr, Pb, Ni, Zn | **** | Ferrihydrite/goethite/lepidocrocite/ hematite/magnetite |
| Fe-Pb-Mn-O | * | Plumboferrite (Pb2(Fe3+, Mn2+, Mg)11O19) | |
| Fe-Mn-Mg-O | Pb | * | Jacobsite (Mn2+Fe3+2O4) |
| Zn-S | Cd, Fe, Hg | **** | Sphalerite (ZnS) |
| Zn-(C)-O | * | Zincite (ZnO)/hydrozincite (Zn5(CO3)2(OH)6)/smithsonite (ZnCO3) | |
| Pb-S | *** | Galena (PbS) | |
| Pb-S-O | *** | Anglesite (PbSO4) | |
| Pb-(C)-O | As, Cu | *** | Cerussite (PbCO3)/litharge (PbO2)/massicot (PbO2) |
| Pb-Si-O | * | Alamosite (PbSiO3) |
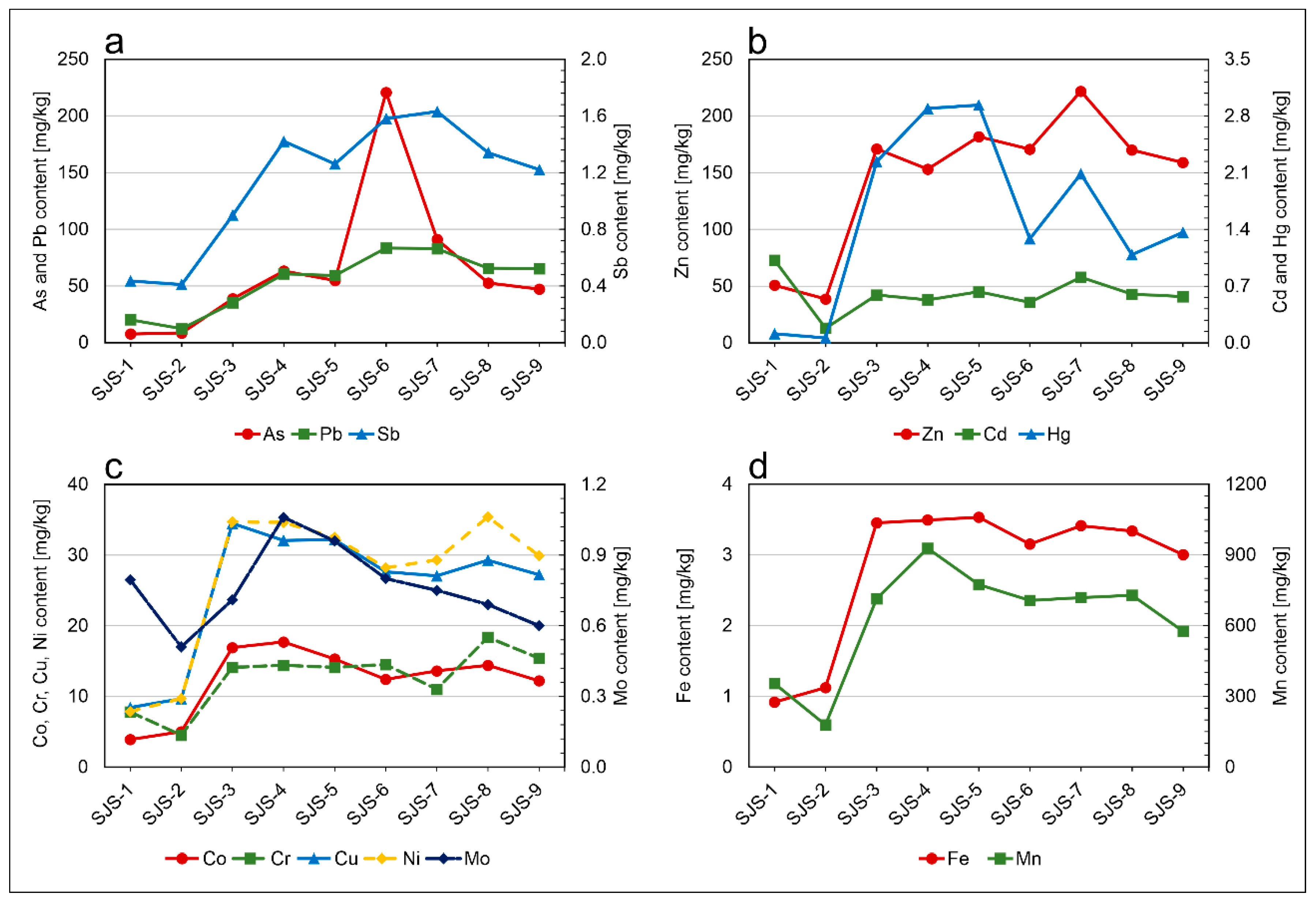
| Sampling Location | Sample | As | Cd | Co | Cr | Cu | Hg | Mo | Ni | Pb | Sb | Zn | Fe | Mn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Local background | SJS-1 | 7.6 | 1 | 3.9 | 7.8 | 8.4 | 0.1 | 0.80 | 7.9 | 20.1 | 0.4 | 50.8 | 0.9 | 354 |
| SJS-2 | 8.4 | 0.2 | 5 | 4.5 | 9.7 | 0.06 | 0.5 | 9.7 | 12.2 | 0.4 | 38.6 | 1.1 | 178 | |
| Mining area | SJS-3 | 38.7 | 0.6 | 16.9 | 14.1 | 34.5 | 2.2 | 0.7 | 34.7 | 35.0 | 0.9 | 170.9 | 3.45 | 713 |
| SJS-4 | 63.2 | 0.5 | 17.7 | 14.4 | 32.1 | 2.9 | 1.06 | 34.6 | 60.6 | 1.4 | 153.0 | 3.5 | 927 | |
| SJS-5 | 54.8 | 0.6 | 15.3 | 14.1 | 32.2 | 2.9 | 0.96 | 32.5 | 59.2 | 1.3 | 181.7 | 3.5 | 773 | |
| SJS-6 | 220.7 | 0.5 | 12.4 | 14.5 | 27.6 | 1.2 | 0.8 | 28.2 | 83.5 | 1.6 | 170.6 | 3.15 | 706 | |
| SJS-7 | 91 | 0.8 | 13.6 | 11 | 27.1 | 2.1 | 0.8 | 29.3 | 82.8 | 1.6 | 221.7 | 3.4 | 718 | |
| SJS-8 | 52.6 | 0.6 | 14.4 | 18.4 | 29.3 | 1.1 | 0.7 | 35.4 | 65.5 | 1.3 | 169.9 | 3.3 | 728 | |
| SJS-9 | 47.1 | 0.6 | 12.2 | 15.4 | 27.2 | 1.4 | 0.6 | 29.9 | 65.2 | 1.2 | 158.9 | 3 | 575 | |
| Mean | 81.1 | 0.6 | 14.6 | 14.6 | 30.0 | 2.0 | 0.8 | 32.1 | 64.5 | 1.3 | 175.3 | 3.3 | 665 | |
| Median | 54.8 | 0.6 | 14.4 | 14.4 | 29.3 | 2.1 | 0.8 | 32.5 | 65.2 | 1.3 | 170.6 | 3.4 | 723 | |
| St. dev. | 63.7 | 0.1 | 2.1 | 2.2 | 2.9 | 0.8 | 0.2 | 2.9 | 16.4 | 0.2 | 22.5 | 0.2 | 105 | |
| Mine waste | SJS-W | 188 | 2.0 | 22.9 | 26.4 | 56.7 | 8.8 | 3.3 | 77.9 | 153.1 | 11.8 | 253.9 | 4.7 | 1871 |
| SSM | Median | 7 | 0.4 | 12 | 66 | 23 | 0.09 | - | 37 | 22 | 0.4 | 81 | 3.04 | 695 |
| ESM | Median | 6 | 0.28 | 8 | 21 | 14 | 0.038 | 0.63 | 16 | 14 | 0.62 | 60 | 1.97 | 382 |
| Sample | Al | Ca | K | Mg | Na | S | Si | T | pH | Eh | DO | EC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SJS-1 | <LOD | 51.0 | 0.19 | 18.9 | 0.41 | 1 | 1.1 | 12.0 | 7.9 | 146.0 | 8.9 | 384.5 |
| SJS-2 | <LOD | 46.7 | 0.66 | 15.9 | 0.66 | 2 | 1.7 | 13.9 | 8.5 | 142.0 | 9.0 | 331.6 |
| SJS-3 | 0.003 | 58.1 | 0.45 | 18 | 0.60 | 1 | 1.3 | 11.9 | 8.5 | 122.7 | 9.3 | 371.4 |
| SJS-4 | 0.009 | 50.6 | 0.66 | 15.5 | 1.1 | 3 | 1.7 | 13.5 | 8.5 | 129.5 | 9.0 | 331.7 |
| SJS-5 | 0.004 | 51.5 | 0.62 | 15.3 | 1.44 | 3 | 1.8 | 12.9 | 8.5 | 127.0 | 9.1 | 359.6 |
| SJS-6 | 0.003 | 50.4 | 9.8 | 12.1 | 1.46 | 4 | 2 | 14.9 | 8.0 | 138.1 | 8.4 | 330.8 |
| SJS-7 | 0.003 | 52.5 | 3.76 | 15.1 | 1.74 | 4 | 1.9 | 13.0 | 8.5 | 125.2 | 9.1 | 357.0 |
| SJS-8 | 0.003 | 51.7 | 1.33 | 15.2 | 1.74 | 4 | 1.9 | 15.0 | 8.5 | 150.3 | 8.9 | 338.4 |
| SJS-9 | 0.004 | 42.0 | 5.33 | 13.3 | 1.74 | 4 | 1.9 | 13.7 | 8.5 | 129.1 | 9.2 | 339.3 |
| Mean | 0.0041 | 50.5 | 2.53 | 15.47 | 1.21 | 2.9 | 1.7 | 13.4 | 8.4 | 134.4 | 9.0 | 349.4 |
| Median | 0.003 | 51.0 | 0.66 | 15.30 | 1.44 | 3.0 | 1.8 | 13.5 | 8.5 | 129.5 | 9.0 | 339.3 |
| St. dev. | 0.0022 | 4.3 | 3.24 | 2.08 | 0.53 | 1.3 | 0.3 | 1.1 | 0.24 | 9.9 | 0.27 | 19.6 |
| Sample | As | Cd | Co | Cr | Cu | Hg | Mo | Ni | Pb | Sb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SJS-1 | 0.25 | 0.01 | 0.011 | <LOD | 0.2 | <LOD | 0.2 | <LOD | 0.04 | 0.08 | 0.6 |
| SJS-2 | 0.14 | 0.01 | 0.026 | <LOD | 0.3 | <LOD | 0.2 | <LOD | 0.07 | 0.06 | 0.9 |
| SJS-3 | 0.24 | 0.01 | 0.011 | <LOD | 0.3 | <LOD | 0.2 | <LOD | 0.09 | 0.07 | 1.9 |
| SJS-4 | 7.84 | <LOD | 0.011 | <LOD | 0.2 | <LOD | 0.2 | <LOD | 0.03 | 0.1 | <LOD |
| SJS-5 | 2.36 | <LOD | 0.01 | <LOD | 0.3 | <LOD | 0.2 | <LOD | 0.03 | 0.11 | 1.3 |
| SJS-6 | 2.64 | 0.01 | 0.013 | <LOD | 0.8 | 0.5 | 0.1 | 0.4 | 0.23 | 0.17 | 17.5 |
| SJS-7 | 2.23 | 0.02 | 0.011 | <LOD | 0.4 | <LOD | 0.4 | <LOD | 0.15 | 0.16 | 4.3 |
| SJS-8 | 1.05 | <LOD | 0.011 | <LOD | 0.5 | <LOD | 0.3 | <LOD | 0.1 | 0.15 | 7 |
| SJS-9 | 0.94 | <LOD | 0.011 | <LOD | 0.4 | 0.3 | 0.3 | <LOD | 0.11 | 0.15 | 2.2 |
| Mean | 1.97 | 0.01 | 0.01 | - | 0.38 | - | 0.23 | - | 0.09 | 0.12 | 4.46 |
| Median | 1.05 | 0.01 | 0.01 | - | 0.30 | - | 0.20 | - | 0.09 | 0.11 | 2.05 |
| St. dev. | 2.4 | 0.0 | 0.0 | - | 0.2 | - | 0.1 | - | 0.1 | 0.04 | 5.7 |
| ESWM | 0.63 | 0.01 | 0.16 | 0.38 | 0.88 | - | 0.22 | 1.91 | 0.093 | 0.07 | 2.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kos, S.; Zupančič, N.; Gosar, M.; Miler, M. Solid Carriers of Potentially Toxic Elements and Their Fate in Stream Sediments in the Area Affected by Iron Ore Mining and Processing. Minerals 2022, 12, 1424. https://doi.org/10.3390/min12111424
Kos S, Zupančič N, Gosar M, Miler M. Solid Carriers of Potentially Toxic Elements and Their Fate in Stream Sediments in the Area Affected by Iron Ore Mining and Processing. Minerals. 2022; 12(11):1424. https://doi.org/10.3390/min12111424
Chicago/Turabian StyleKos, Saša, Nina Zupančič, Mateja Gosar, and Miloš Miler. 2022. "Solid Carriers of Potentially Toxic Elements and Their Fate in Stream Sediments in the Area Affected by Iron Ore Mining and Processing" Minerals 12, no. 11: 1424. https://doi.org/10.3390/min12111424
APA StyleKos, S., Zupančič, N., Gosar, M., & Miler, M. (2022). Solid Carriers of Potentially Toxic Elements and Their Fate in Stream Sediments in the Area Affected by Iron Ore Mining and Processing. Minerals, 12(11), 1424. https://doi.org/10.3390/min12111424






