Radiological Risk Parameters of the Phosphorite Deposits, Gebel Qulu El Sabaya: Natural Radioactivity and Geochemical Characteristics
Abstract
:1. Introduction
2. Materials and Methods
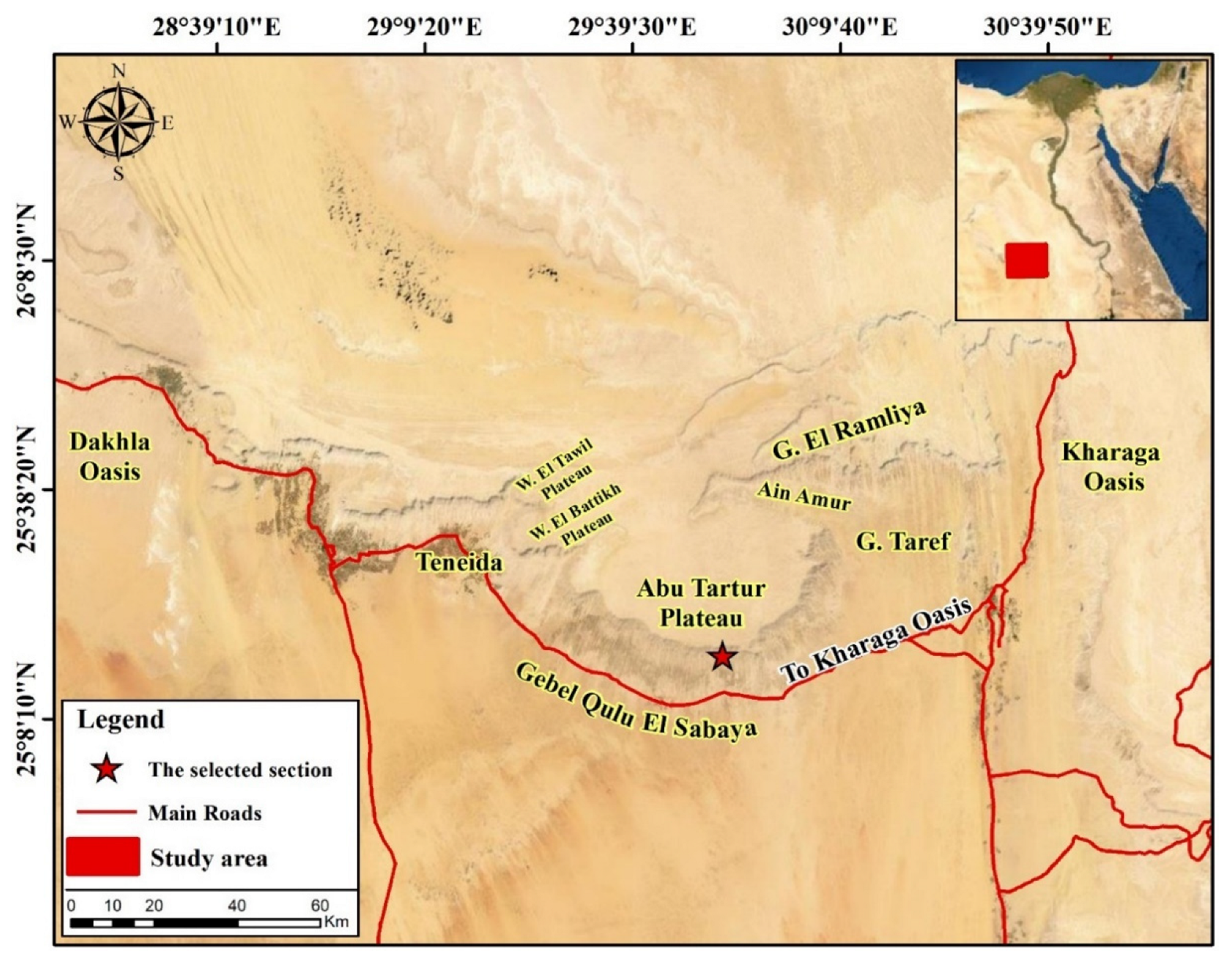
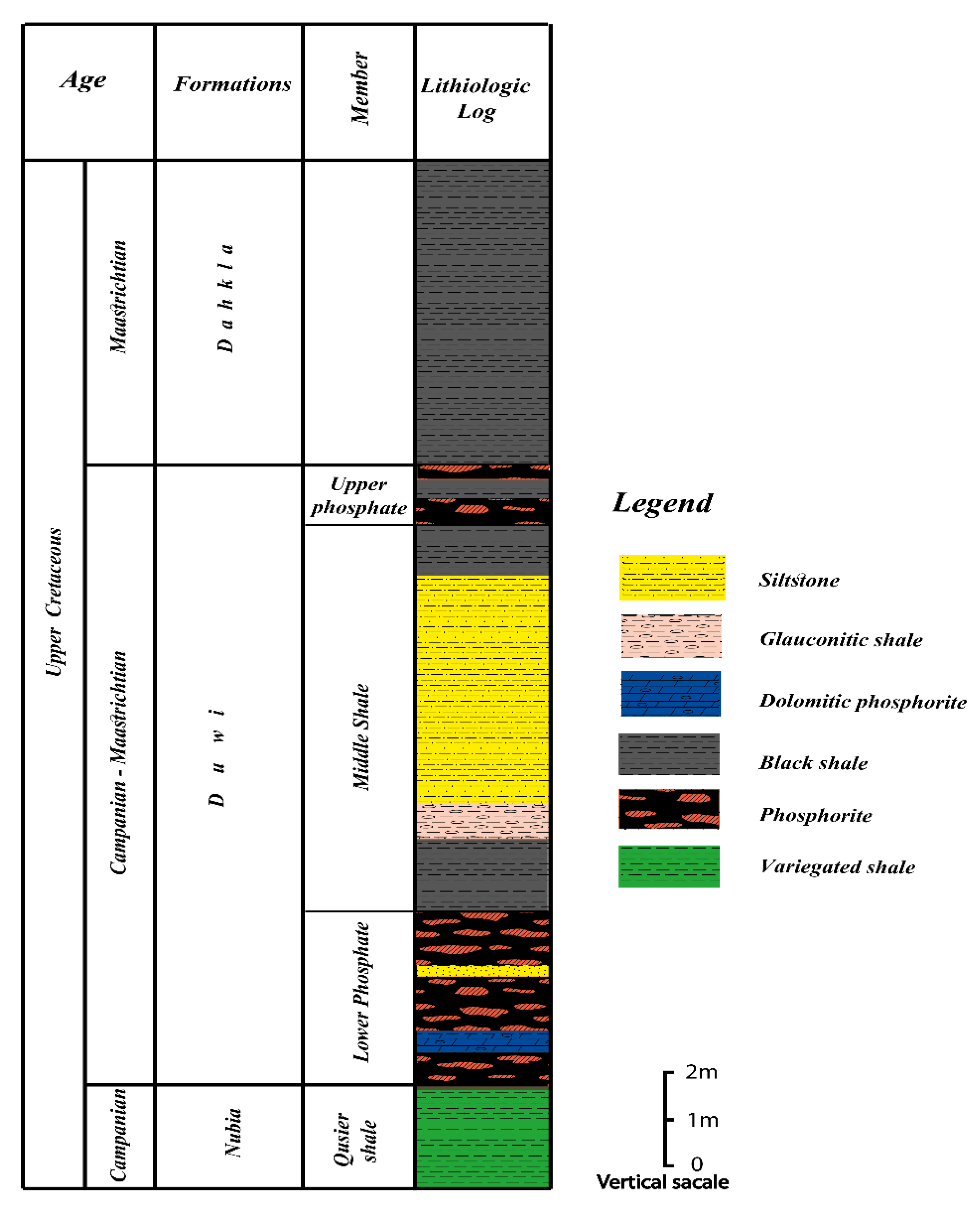
2.1. Geological Setting
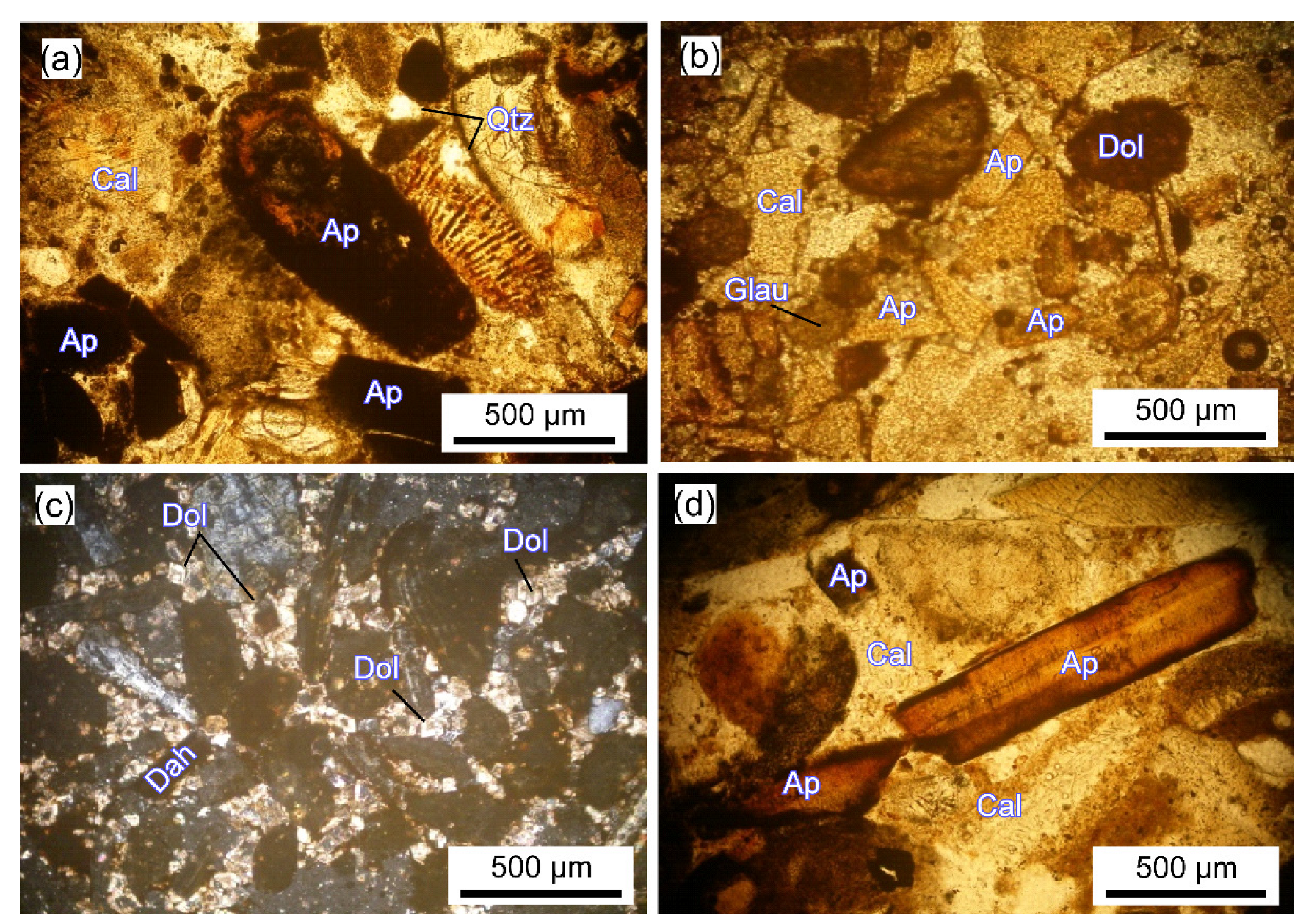
2.2. Petrography
2.3. Sampling and Analytical Techniques
2.4. Energy and Efficiency Calibration
3. Results and Discussion
3.1. Geochemical Characteristics
3.1.1. Major Oxides
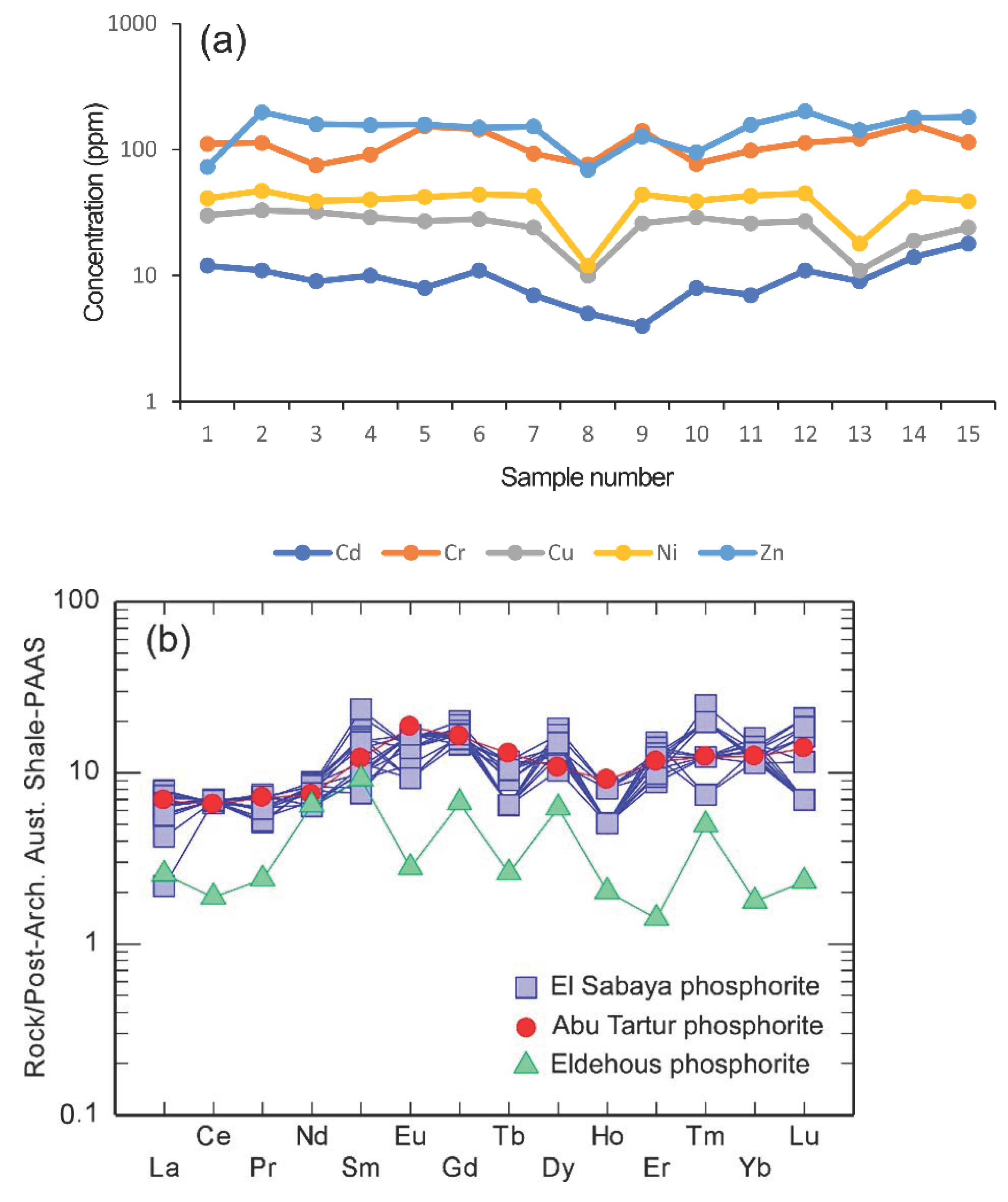
3.1.2. Trace Elements
3.1.3. Rare Earth Elements (REEs)
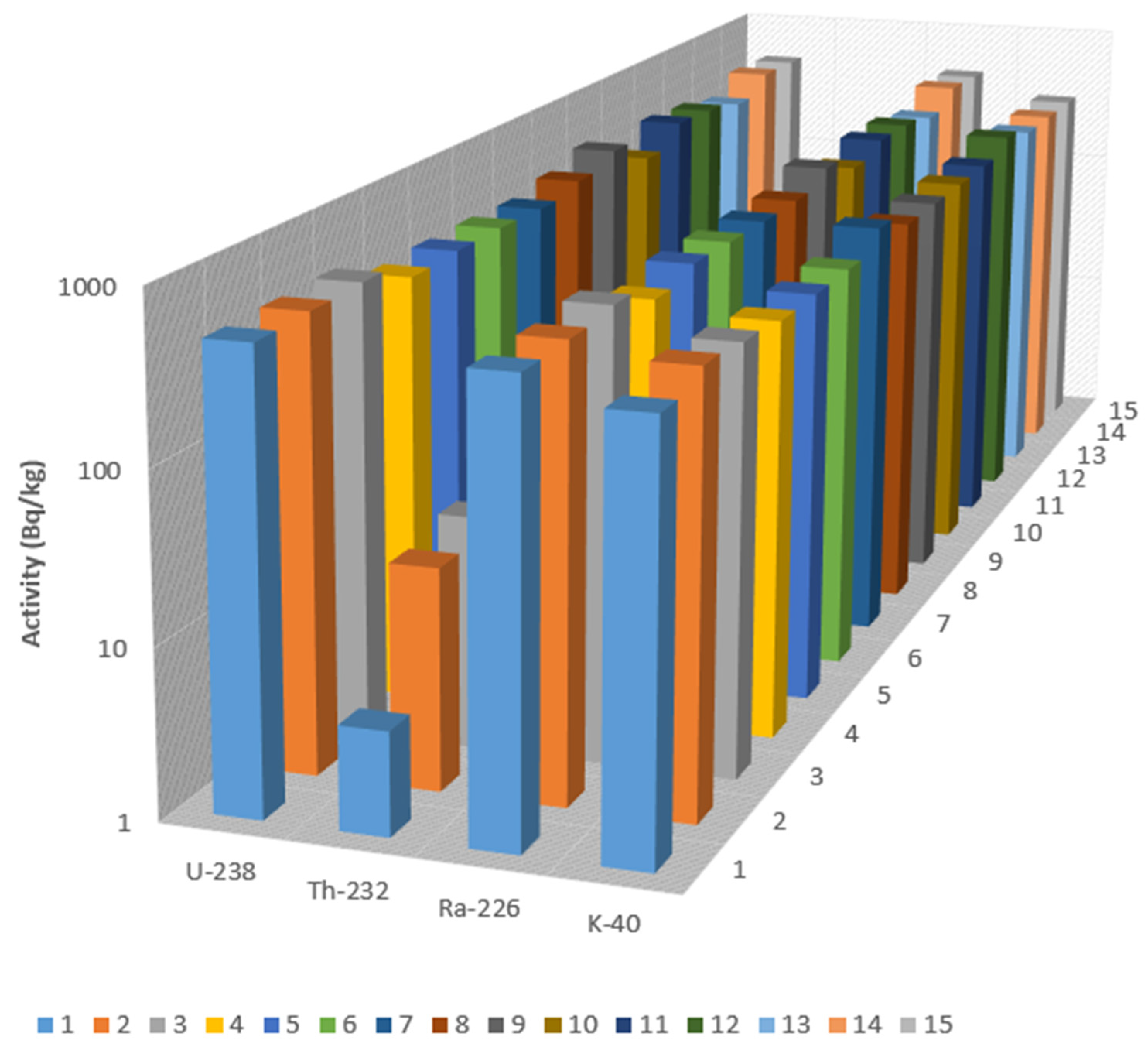
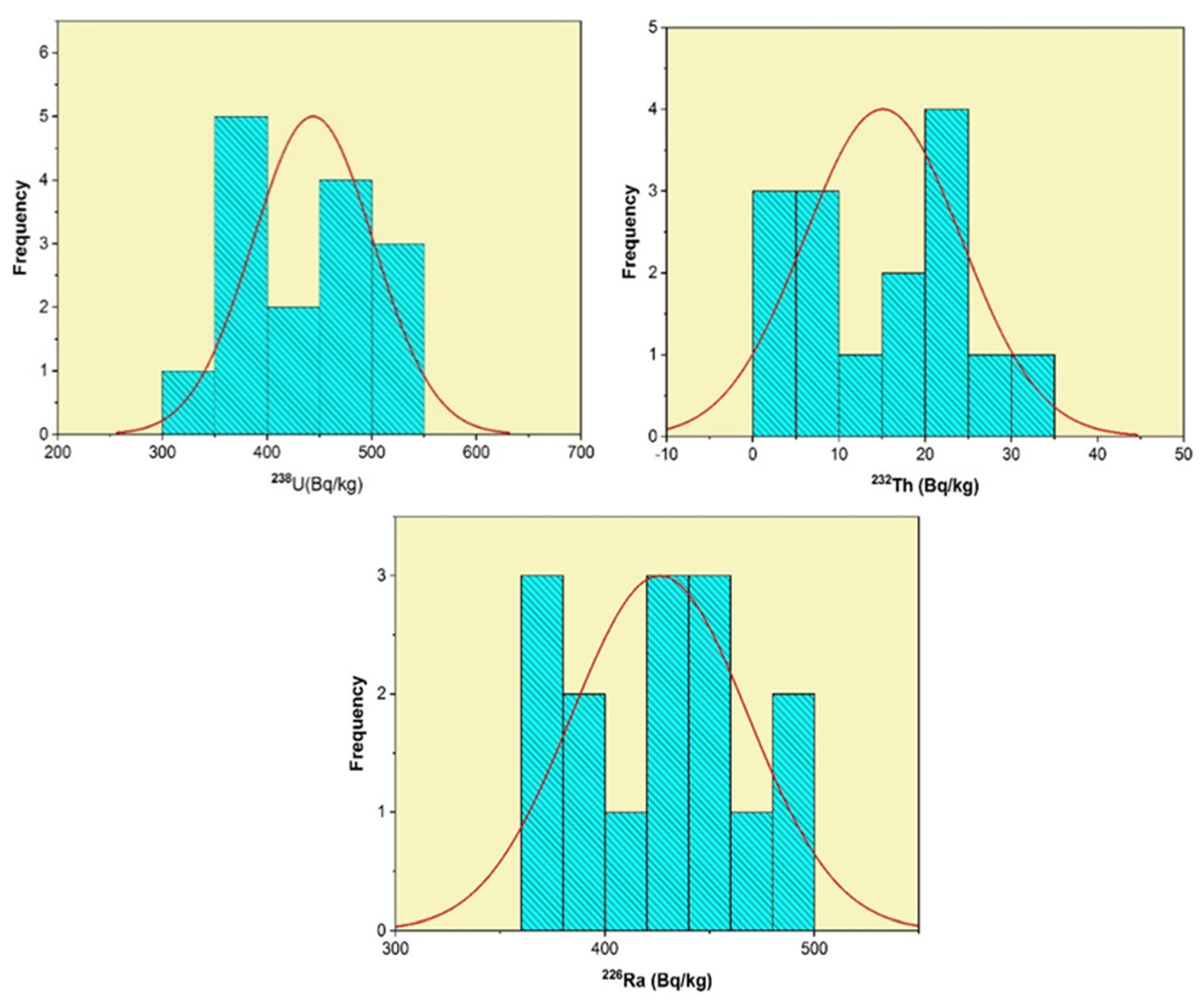
3.2. Radioactivity Concentrations
3.3. Radiological Risk Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khaleal, F.M.; Saleh, G.M.; Lasheen, E.S.R.; Lentz, D.R. Occurrences and Genesis of Emerald and Other Beryls Mineralization in Egypt: A Review. Phys. Chem. Earth Parts A B C 2022, 128, 103266. [Google Scholar] [CrossRef]
- Saleh, G.M.; Kamar, M.S.; Lasheen, E.S.R.; Ibrahim, I.H.; Azer, M.K. Whole Rock and Mineral Chemistry of the Rare Metals-Bearing Mylonitic Rocks, Abu Rusheid Borehole, South Eastern Desert, Egypt. J. Afr. Earth Sci. 2022, 196, 104736. [Google Scholar] [CrossRef]
- Lasheen, E.S.R.; Saleh, G.M.; Khaleal, F.M.; Alwetaishi, M. Petrogenesis of Neoproterozoic Ultramafic Rocks, Wadi Ibib–Wadi Shani, South Eastern Desert, Egypt: Constraints from Whole Rock and Mineral Chemistry. Appl. Sci. 2021, 11, 10524. [Google Scholar] [CrossRef]
- Hamdy, M.M.; Lasheen, E.S.R.; Abdelwahab, W. Gold-Bearing Listwaenites in Ophiolitic Ultramafics from the Eastern Desert of Egypt: Subduction Zone-Related Alteration of Neoproterozoic Mantle? J. Afr. Earth Sci. 2022, 193, 104574. [Google Scholar] [CrossRef]
- Lasheen, E.S.R.; Mohamed, W.H.; Ene, A.; Awad, H.A.; Azer, M.K. Implementation of Petrographical and Aeromagnetic Data to Determine Depth and Structural Trend of Homrit Waggat Area, Central Eastern Desert, Egypt. Appl. Sci. 2022, 12, 8782. [Google Scholar] [CrossRef]
- Ali, S.; Ntaflos, T.; Sami, M. Geochemistry of Khor Um-Safi Ophiolitic Serpentinites, Central Eastern Desert, Egypt: Implications for Neoproterozoic Arc-Basin System in the Arabian-Nubian Shield. Geochemistry 2021, 81, 125690. [Google Scholar] [CrossRef]
- Abdel-Karim, A.-A.; Azer, M.; Sami, M. Petrogenesis and Tectonic Implications of the Maladob Ring Complex in the South Eastern Desert, Egypt: New Insights from Mineral Chemistry and Whole-Rock Geochemistry. Int. J. Earth Sci. 2021, 110, 53–80. [Google Scholar] [CrossRef]
- Hermina, M.H. Review on the Phosphate Deposits of Egypt. In Proceedings of the Second Arab Conference of Mineral Resources, Santiago, Chile, 13 April–21 May 1972. [Google Scholar]
- Issawi, B.; Hassan, M.Y.; Saad, E.N. Geology of Abu Tartur Plateau, Western Desert, Egypt. Ann. Geol. Surv. Egypt 1978, 8, 91–127. [Google Scholar]
- Issawi, B.; El Hinnawi, M.; Francis, M.; Mazhar, A. The Phanerozoic Geology of Egypt. A Geodynamic Approach. Special Publ. No. 76. Geol. Surv. Cairo Egypt 1999, 76, 462. [Google Scholar]
- Fathy, D.; Wagreich, M.; Ntaflos, T.; Sami, M. Provenance Characterization of Campanian Lacustrine Organic-Rich Mudstones on the Southern Tethyan Margin, Egypt. ACS Earth Space Chem. 2021, 5, 197–209. [Google Scholar] [CrossRef]
- Zidan, I.H. Geological and Mineralogical Studies of Abu Tartur Phosphates, Western Desert-Egypt; Al-Azhar University: Cairo, Egypt, 1998. [Google Scholar]
- Zidan, I.H. Geological, Mineralogical and Geochemical Studies of Abu Tartur Area. Western Desert, Egypt; Al Azhar University: Cairo, Egypt, 2002. [Google Scholar]
- Zidan, I.H. Evaluation of Phosphorite and Uranium in Lower Member, Duwi Formation at Kummer Area South Esna, West Nile Valley, Egypt. J. Sedimentol. Soc. Egypt 2013, 21, 143–154. [Google Scholar]
- Jarvis, I.; Burnett, W.; Nathan, Y. Phosphorite Geochemistry: State-of-the-Art and Environmental Concerns. Eclogae Geol. Helv. 1994, 87, 643–700. [Google Scholar]
- Slansky, M. Geology of Sedimentary Phosphates; Originally Published in French in 1980; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar] [CrossRef]
- Amin, M.M.; Zidan, I.H. Rare Earth Elements Recovery and Preparation of Phosphoric Acid from Abu-Tartur Phosphate Rock Using Penicillium Simplicissimum. Chem. Technol. Indian J. 2014, 10, 57–61. [Google Scholar]
- Ilyin, A.V. Rare-Earth Geochemistry of “old” Phosphorites and Probability of Syngenetic Precipitation and Accumulation of Phosphate. Chem. Geol. 1998, 144, 243–256. [Google Scholar] [CrossRef]
- Hannigan, R.E.; Sholkovitz, E.R. The Development of Middle Rare Earth Element Enrichments in Freshwaters: Weathering of Phosphate Minerals. Chem. Geol. 2001, 175, 495–508. [Google Scholar] [CrossRef]
- Zakaly, H.M.; Uosif, M.A.; Madkour, H.; Tammam, M.; Issa, S.; Elsaman, R.; El-Taher, A. Assessment of Natural Radionuclides and Heavy Metal Concentrations in Marine Sediments in View of Tourism Activities in Hurghada City, Northern Red Sea, Egypt. J. Phys. Sci. 2019, 30, 21–47. [Google Scholar] [CrossRef]
- Zidan, I.H. Evaluation of Phosphorite and Uranium Resources of Duwi Formation at Helal Area, South Esna, East Nile Valley, Egypt. Sedimentol. Egypt 2017, 23, 63–80. [Google Scholar]
- Negm, S.H. Mineralogical and Geochemical Studies of the Duwi Formation Phosphatic Rock in El-Sibaiya. East Area. Nile Vally Egypt; Minufiya University: Al Minufiyah, Egypt, 2009. [Google Scholar]
- Altschuler, Z.A. The Geochemistry of Trace Elements in Marine Phosphorites, Part I. Characteristic Abundances and Enrichment. In Marine Phosphorites; The Society for Sedimentary Geology: Tulsa, OK, USA, 1980; pp. 19–30. [Google Scholar] [CrossRef]
- El-Kammar, A. Comparative Geochemical and Mineralogical Studies on Egyptian Phosphorites; Cairo University: Cairo, Egypt, 1974. [Google Scholar]
- Dardir, A.A.; Kobtan, S.M. Preliminary Evaluation of the Rare-Earth Elements in Sector Abu Tartur Phosphorites. Ann. Geol. Surv. Egypt 1980, 10, 909–921. [Google Scholar]
- El-Sankary, M.; Mahdy, A. The Heavy Minerals Associating Phosphorites of the Western Desert of Egypt: Possible Supplier of Trace Elements and provenance indicator. In Proceedings of the 7th International Conference on Geochemistry, Alexandria, Egypt, 6–7 September 2006; Volume II, pp. 15–30. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; U.S. Department of Energy: Germantown, MD, USA, 1985.
- El-Kammar, A.; El-Kammar, M. On the Trace Elements Composition of the Egyptian Phosphorites: A New Approach. In Proceedings of the 6th International Conference on the Geology of the Arab World, Giza, Egypt, 21–24 February 2002; Cairo University: Cairo, Egypt, 2002; pp. 227–244. [Google Scholar]
- Elderfield, H.; Greaves, M.J. The Rare Earth Elements in Seawater. Nature 1982, 296, 214–219. [Google Scholar] [CrossRef]
- Kolodny, Y. Phosphorite. In Encyclopedia of Paleoclimatology and Ancient Environments; Gornitz, V., Ed.; Encyclopedia of Earth Sciences Series; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- McArthur, J.M.; Walsh, J.N. Rare-Earth Geochemistry of Phosphorites. Chem. Geol. 1984, 47, 191–220. [Google Scholar] [CrossRef]
- De Baar, H.J.W.; Bacon, M.P.; Brewer, P.G.; Bruland, K.W. Rare Earth Elements in the Pacific and Atlantic Oceans. Geochim. Cosmochim. Acta 1985, 49, 1943–1959. [Google Scholar] [CrossRef]
- Piper, D.; Baedecker, P.; Crock, J.; Geology, W.B.-M. Undefined Rare Earth Elements in the Phosphatic-Enriched Sediment of the Peru Shelf; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Bonnot-Courtois, C.; Flicoteaux, R. Distribution of Rare-Earth and Some Trace Elements in Tertiary Phosphorites from the Senegal Basin and Their Weathering Products. Chem. Geol. 1989, 75, 311–328. [Google Scholar] [CrossRef]
- El Gemmizi, M.A.; Ali, M.M. Contribution of heavy minerals to the trace elements from East Sebaiya phosphorites, Egypt. Proc. 2nd Int. Conf. Geol. Arab World, Cairo Univ. 1999; 145–153. [Google Scholar]
- Hassan, F.; El-Kammar, A. Environmental Conditions Affecting the Distribution of Uranium and Rare Earth Elements in Egyptian Phosphorites. Egypt. J. Pet. 1975, 19, 169–178. [Google Scholar]
- Lucas, J.; Flicoteaux, R.; Nathan, Y.; Prevot, L.; Shahar, Y. Different Aspects of Phosphorite Weathering. In Marine Phosphorites; The Society for Sedimentary Geology: Tulsa, OK, USA, 1980; pp. 41–51. [Google Scholar] [CrossRef]
- Guirgues, N.R. Geological and Geochemical Studies on Some Phosphate Exposures, East-Central Sinai, Egypt; Mansoura University: Mansoura, Egypt, 2005. [Google Scholar]
- Hussein, A.H. Lecture Course in Nuclear Geology; NMA Egypt; Ain Shams University: Cairo, Egypt, 1978; 101p. [Google Scholar]
- Hansink, J.D. Equilibrium Analysis of a Sandstone Roll-Front Uranium Deposit. 1976. Available online: https://inis.iaea.org (accessed on 20 August 2022).
- Wignall, P.B.; Myers, K.J. Interpreting Benthic Oxygen Levels in Mudrocks: A New Approach. Geology 1988, 16, 452–455. [Google Scholar] [CrossRef]
- UNSCEAR. Sources and Effects of Ionizing Radiation; UNSCEAR 2000 Report to the General Assembly, with Scientific Annexes; United Nations Scientific Committee on the Effects of Atomic Radiation: Vienna, Austria, 2000; ISBN 92-1-142238-8. [Google Scholar]
- UNSCEAR. Sources and Effects of Ionizing Radiation; United Nations Scientific Committee on the Effects of Atomic Radiation: Vienna, Austria, 2008; Volume 1, ISBN 9789211422740. [Google Scholar]
- Ravisankar, R.; Chandramohan, J.; Chandrasekaran, A.; Prince Prakash Jebakumar, J.; Vijayalakshmi, I.; Vijayagopal, P.; Venkatraman, B. Assessments of Radioactivity Concentration of Natural Radionuclides and Radiological Hazard Indices in Sediment Samples from the East Coast of Tamilnadu, India with Statistical Approach. Mar. Pollut. Bull. 2015, 97, 419–430. [Google Scholar] [CrossRef]
- Kamar, M.S.; Salem, I.A.; El-Aassy, I.E.; El-Sayed, A.A.; Zakaly, H.M.H.; Alzahrani, A.M.; Saeed, E.; Lasheen, R. An Extended Investigation of High-Level Natural Radioactivity and Geochemistry of Neoproterozoic Dokhan Volcanics: A Case Study of Wadi Gebeiy, Southwestern Sinai, Egypt. Sustainability 2022, 14, 9291. [Google Scholar] [CrossRef]
- Abbasi, A. Calculation of Gamma Radiation Dose Rate and Radon Concentration Due to Granites Used as Building Materials in Iran. Radiat. Prot. Dosim. 2013, 155, 335–342. [Google Scholar] [CrossRef]
- Zakaly, H.M.H.; Uosif, M.A.M.; Issa, S.A.M.; Tekin, H.O.; Madkour, H.; Tammam, M.; El-Taher, A.; Alharshan, G.A.; Mostafa, M.Y.A. An Extended Assessment of Natural Radioactivity in the Sediments of the Mid-Region of the Egyptian Red Sea Coast. Mar. Pollut. Bull. 2021, 171, 112658. [Google Scholar] [CrossRef]
- UNSCEAR. 2008 Sources and Effects of Ionising Radiation; Report to the General Assembly with Scientific Annexes; UNSCEAR: New York, NY, USA, 2010. [Google Scholar]
- Lasheen, E.S.R.; Azer, M.K.; Ene, A.; Abdelwahab, W.; Zakaly, H.M.H.; Awad, H.A.; Kawady, N.A. Radiological Hazards and Natural Radionuclide Distribution in Granitic Rocks of Homrit Waggat Area, Central Eastern Desert, Egypt. Materials 2022, 15, 4069. [Google Scholar] [CrossRef]
- Alharshan, G.A.; Kamar, M.S.; Lasheen, E.S.R.; Ene, A.; Uosif, M.A.M.; Awad, H.A.; Issa, S.A.M.; Zakaly, H.M.H. Distribution of Radionuclides and Radiological Health Assessment in Seih-Sidri Area, Southwestern Sinai. Int. J. Environ. Res. Public. Health 2022, 19, 10717. [Google Scholar] [CrossRef] [PubMed]

| S.NO. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO | 37 | 38 | 39 | 41 | 38 | 36 | 40 | 37 | 38 | 39 | 38 | 37 | 38 | 39 | 41 |
| P2O5 | 22 | 24 | 26 | 27 | 24 | 23 | 27 | 23 | 26 | 25 | 25 | 24 | 25 | 25 | 28 |
| SiO2 | 9 | 8 | 6 | 5 | 8 | 9 | 6 | 9 | 7 | 6 | 7 | 7 | 7 | 7 | 6 |
| Fe2O3 | 5 | 4 | 4 | 4 | 5 | 5 | 4 | 5 | 4 | 4 | 3 | 5 | 3 | 5 | 3 |
| MgO | 6 | 5 | 3 | 3 | 5 | 5 | 3 | 5 | 3 | 4 | 3 | 4 | 3 | 4 | 3 |
| F | 2 | 1 | 2 | 3 | 1 | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 1 | 2 |
| Al2O3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 |
| Na2O | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 1 |
| K2O | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| L.O.I. | 10 | 9 | 11 | 12 | 11 | 7 | 15 | 9 | 10 | 7 | 9 | 16 | 12 | 14 | 11 |
| Cd | 12 | 11 | 9 | 10 | 8 | 11 | 7 | 5 | 4 | 8 | 7 | 11 | 9 | 14 | 18 |
| Cr | 111 | 113 | 75 | 91 | 153 | 145 | 93 | 76 | 141 | 77 | 98 | 113 | 122 | 157 | 114 |
| Cu | 30 | 33 | 32 | 29 | 27 | 28 | 24 | 10 | 26 | 29 | 26 | 27 | 11 | 19 | 24 |
| Ni | 41 | 47 | 39 | 40 | 42 | 44 | 43 | 12 | 44 | 39 | 43 | 45 | 18 | 42 | 39 |
| Zn | 73 | 198 | 159 | 156 | 158 | 149 | 152 | 69 | 127 | 95 | 157 | 201 | 143 | 179 | 181 |
| Pb | 2 | 6 | 10 | 9 | 5 | 7 | 3 | 2 | 6 | 11 | 3 | 6 | 3 | 9 | 12 |
| U | 33 | 29 | 35 | 35 | 30 | 26 | 22 | 27 | 36 | 24 | 27 | 29 | 32 | 30 | 28 |
| Y | 615 | 611 | 265 | 236 | 228 | 217 | 211 | 255 | 100 | 218 | 268. | 548 | 603 | 611 | 605 |
| La | 240 | 208 | 285 | 301 | 276 | 271 | 247 | 161 | 83 | 298 | 280 | 221 | 254 | 250 | 216 |
| Ce | 554 | 549 | 543 | 533 | 543 | 555 | 535 | 540 | 540 | 526 | 542 | 546 | 552 | 550 | 549 |
| Pr | 45 | 55 | 63 | 55 | 62 | 64 | 45 | 66 | 48 | 55 | 64 | 53 | 54 | 46 | 54 |
| Nd | 288 | 273 | 283 | 220 | 298 | 218 | 290 | 215 | 230 | 283 | 280 | 268 | 283 | 285 | 258 |
| Sm | 85 | 118 | 90 | 60 | 87 | 56 | 63 | 51 | 54 | 45 | 55 | 42 | 130 | 77 | 84 |
| Eu | 18 | 15 | 10 | 18 | 15 | 18 | 10 | 12 | 18 | 17 | 15 | 18 | 15 | 18 | 10 |
| Gd | 75 | 78 | 70 | 81 | 78 | 76 | 79 | 75 | 68 | 93 | 88 | 78 | 82 | 73 | 78 |
| Tb | 7 | 5 | 9 | 5 | 8 | 5 | 9 | 5 | 8 | 7 | 5 | 7 | 5 | 9 | 8 |
| Dy | 65 | 58 | 65 | 78 | 60 | 85 | 53 | 48 | 65 | 65 | 63 | 68 | 73 | 78 | 70 |
| Ho | 5 | 5 | 5 | 8 | 5 | 8 | 8 | 5 | 8 | 5 | 5 | 5 | 5 | 8 | 5 |
| Er | 33 | 25 | 35 | 28 | 30 | 35 | 33 | 38 | 33 | 43 | 27 | 28 | 40 | 37 | 35 |
| Tm | 5 | 5 | 3 | 8 | 5 | 8 | 8 | 5 | 8 | 3 | 5 | 10 | 5 | 8 | 5 |
| Yb | 41 | 42 | 38 | 35 | 45 | 38 | 33 | 38 | 34 | 33 | 35 | 40 | 37 | 33 | 32 |
| Lu | 3 | 5 | 3 | 7 | 3 | 8 | 3 | 9 | 3 | 7 | 3 | 9 | 3 | 8 | 5 |
| TREEs | 1464 | 1441 | 1502 | 1437 | 1515 | 1445 | 1416 | 1268 | 1200 | 1480 | 1467 | 1393 | 1538 | 1480 | 1409 |
| Areas | CaO | P2O5 | SiO2 | Fe2O3 | MgO | Al2O3 | Na2O | K2O |
|---|---|---|---|---|---|---|---|---|
| Gebel Qulu El Sabaya (present study) | 38.35 | 24.92 | 7.19 | 4.18 | 3.99 | 1.84 | 1.33 | 0.22 |
| El Ziat phosphorite [19] | 38.72 | 24.75 | 7.3 | 3.38 | 3.4 | 1.7 | 0.92 | 0.2 |
| Eldehous phosphorites [10] | 35.3 | 22.7 | 10.6 | 4.8 | 6.9 | 1.9 | 1.8 | 0.2 |
| Abu Tartur [13] | 40 | 25 | 5 | 3 | 3 | 2 | 0.6 | 0.14 |
| Sibaiya Phosphorite [13] | 36 | 25 | 18 | 2.23 | 2.4 | 1.79 | 0.88 | 0.22 |
| S.NO. | eU | eTh | eRa | eU/eTh | eU/eRa | cU/eU | cU |
|---|---|---|---|---|---|---|---|
| 1 | 39 | 1 | 40 | 39 | 1 | 1 | 33 |
| 2 | 40 | 5 | 41 | 8 | 1 | 1 | 29 |
| 3 | 41 | 6 | 44 | 7 | 1 | 1 | 35 |
| 4 | 31 | 4 | 33 | 8 | 1 | 1 | 35 |
| 5 | 32 | 5 | 38 | 6 | 1 | 1 | 30 |
| 6 | 32 | 8 | 37 | 4 | 1 | 1 | 26 |
| 7 | 31 | 2 | 36 | 16 | 1 | 1 | 22 |
| 8 | 35 | 7 | 36 | 5 | 1 | 1 | 27 |
| 9 | 42 | 1 | 44 | 42 | 1 | 1 | 36 |
| 10 | 28 | 2 | 33 | 14 | 1 | 1 | 24 |
| 11 | 38 | 1 | 39 | 38 | 1 | 1 | 27 |
| 12 | 36 | 5 | 38 | 7 | 1 | 1 | 29 |
| 13 | 31 | 4 | 33 | 8 | 1 | 1 | 32 |
| 14 | 41 | 3 | 43 | 14 | 1 | 1 | 30 |
| 15 | 40 | 2 | 41 | 20 | 1 | 1 | 28 |
| Min. | 28 | 1 | 33 | 4 | 1 | 1 | 22 |
| Max. | 42 | 8 | 44 | 42 | 1 | 1 | 36 |
| Average | 36 | 4 | 38 | 16 | 1 | 1 | 30 |
| El Ziat phosphorite [19] | 27 | - | 25 | - | - | - | - |
| Eldehous phosphorites [10] | 35 | - | 35 | - | - | - | - |
| Abu Tartur phosphorites [13] | 23 | - | 19 | - | - | - | - |
| Sibaiya phosphorites [13] | 85 | - | 89 | - | - | - | - |
| Red Sea phosphorites [13]) | 68 | - | 69 | - | - | - | - |
| Um | Absorbed | Hin | Hex | AEDout | Raeq (Bq/kg) | ELCR | |
|---|---|---|---|---|---|---|---|
| S.No | nGy/h | (mSv) | |||||
| 1 | 10.86 | 223.78 | 2.69 | 1.39 | 0.27 | 473.88 | 0.96 |
| 2 | 10 | 242.51 | 2.84 | 1.50 | 0.30 | 512.91 | 1.04 |
| 3 | 10 | 249.22 | 2.91 | 1.54 | 0.31 | 549.57 | 1.07 |
| 4 | 7.71 | 189.20 | 2.21 | 1.17 | 0.23 | 413.51 | 0.81 |
| 5 | 7.71 | 197.22 | 2.29 | 1.22 | 0.24 | 474.79 | 0.85 |
| 6 | 6.86 | 205.30 | 2.33 | 1.26 | 0.25 | 481.02 | 0.88 |
| 7 | 8.29 | 187.76 | 2.19 | 1.15 | 0.23 | 442.49 | 0.81 |
| 8 | 8 | 218.60 | 2.52 | 1.35 | 0.27 | 464.14 | 0.94 |
| 9 | 11.71 | 239.78 | 2.90 | 1.49 | 0.29 | 518.28 | 1.03 |
| 10 | 7.43 | 167.82 | 1.97 | 1.03 | 0.21 | 401.96 | 0.72 |
| 11 | 10.57 | 218.45 | 2.63 | 1.35 | 0.27 | 462.78 | 0.94 |
| 12 | 8.86 | 221.31 | 2.57 | 1.36 | 0.27 | 479.85 | 0.95 |
| 13 | 7.71 | 189.20 | 2.21 | 1.17 | 0.23 | 413.51 | 0.81 |
| 14 | 10.86 | 239.83 | 2.86 | 1.49 | 0.29 | 518.73 | 1.03 |
| 15 | 10.86 | 231.81 | 2.78 | 1.44 | 0.28 | 490.76 | 1.00 |
| Min | 7.71 | 189.20 | 2.21 | 1.17 | 0.23 | 413.51 | 0.81 |
| Max | 10.86 | 239.83 | 2.86 | 1.49 | 0.29 | 518.73 | 1.03 |
| Av. | 9.81 | 220.28 | 2.61 | 1.36 | 0.27 | 474.33 | 0.95 |
| Sd | 1.48 | 22.22 | 0.29 | 0.14 | 0.03 | 44.50 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasheen, E.S.R.; Zakaly, H.M.H.; Alotaibi, B.M.; Saadawi, D.A.; Ene, A.; Fathy, D.; Awad, H.A.; El Attar, R.M. Radiological Risk Parameters of the Phosphorite Deposits, Gebel Qulu El Sabaya: Natural Radioactivity and Geochemical Characteristics. Minerals 2022, 12, 1385. https://doi.org/10.3390/min12111385
Lasheen ESR, Zakaly HMH, Alotaibi BM, Saadawi DA, Ene A, Fathy D, Awad HA, El Attar RM. Radiological Risk Parameters of the Phosphorite Deposits, Gebel Qulu El Sabaya: Natural Radioactivity and Geochemical Characteristics. Minerals. 2022; 12(11):1385. https://doi.org/10.3390/min12111385
Chicago/Turabian StyleLasheen, El Saeed R., Hesham M. H. Zakaly, B. M. Alotaibi, Diaa A. Saadawi, Antoaneta Ene, Douaa Fathy, Hamdy A. Awad, and Raafat M. El Attar. 2022. "Radiological Risk Parameters of the Phosphorite Deposits, Gebel Qulu El Sabaya: Natural Radioactivity and Geochemical Characteristics" Minerals 12, no. 11: 1385. https://doi.org/10.3390/min12111385
APA StyleLasheen, E. S. R., Zakaly, H. M. H., Alotaibi, B. M., Saadawi, D. A., Ene, A., Fathy, D., Awad, H. A., & El Attar, R. M. (2022). Radiological Risk Parameters of the Phosphorite Deposits, Gebel Qulu El Sabaya: Natural Radioactivity and Geochemical Characteristics. Minerals, 12(11), 1385. https://doi.org/10.3390/min12111385








