Comparative Study of the Mineralogy and Chemistry Properties of Elephant Bones: Implications during Diagenesis Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
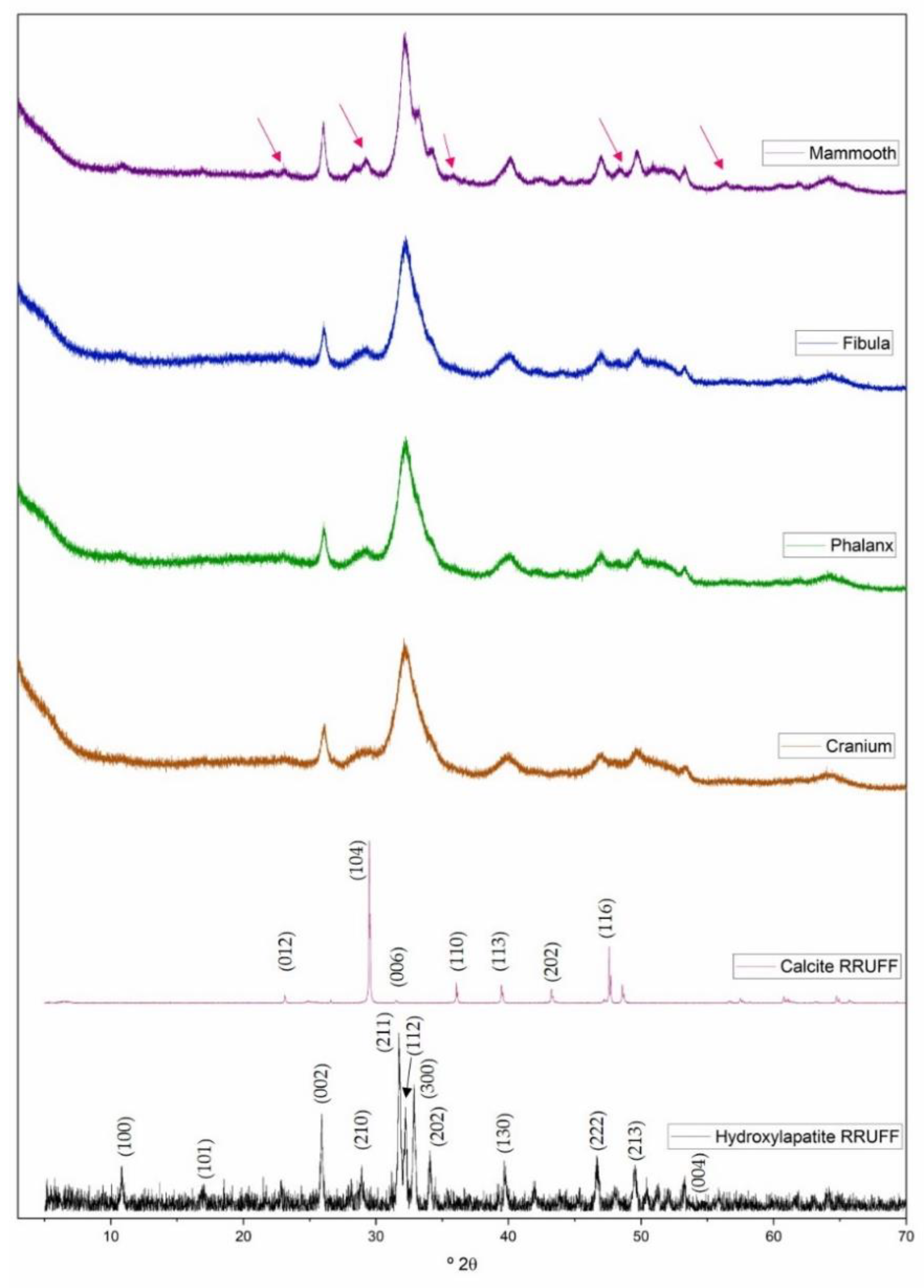
2.2.1. X-ray Powder Diffraction
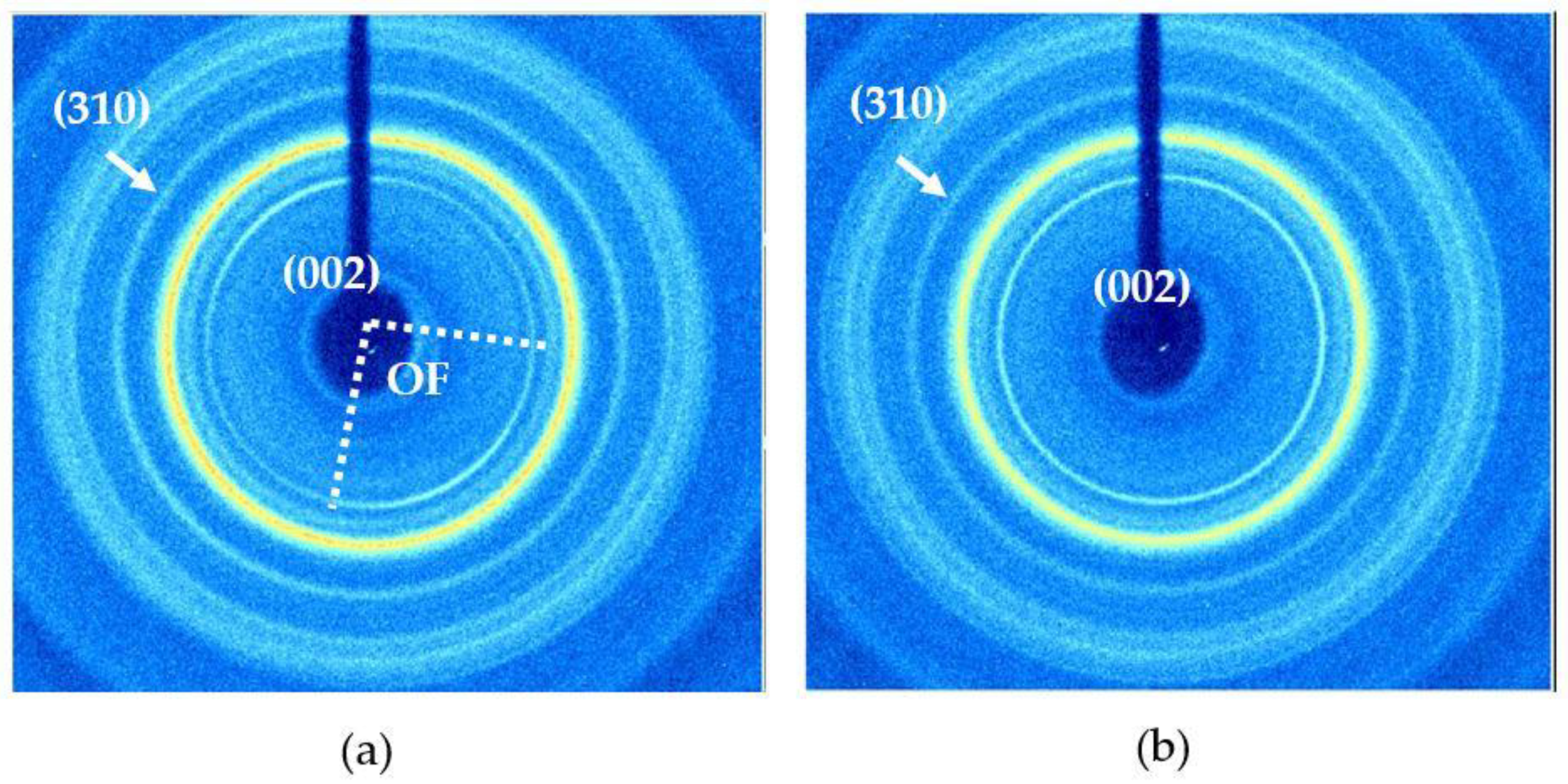
2.2.2. Two-Dimensional (2D) X-ray Diffraction
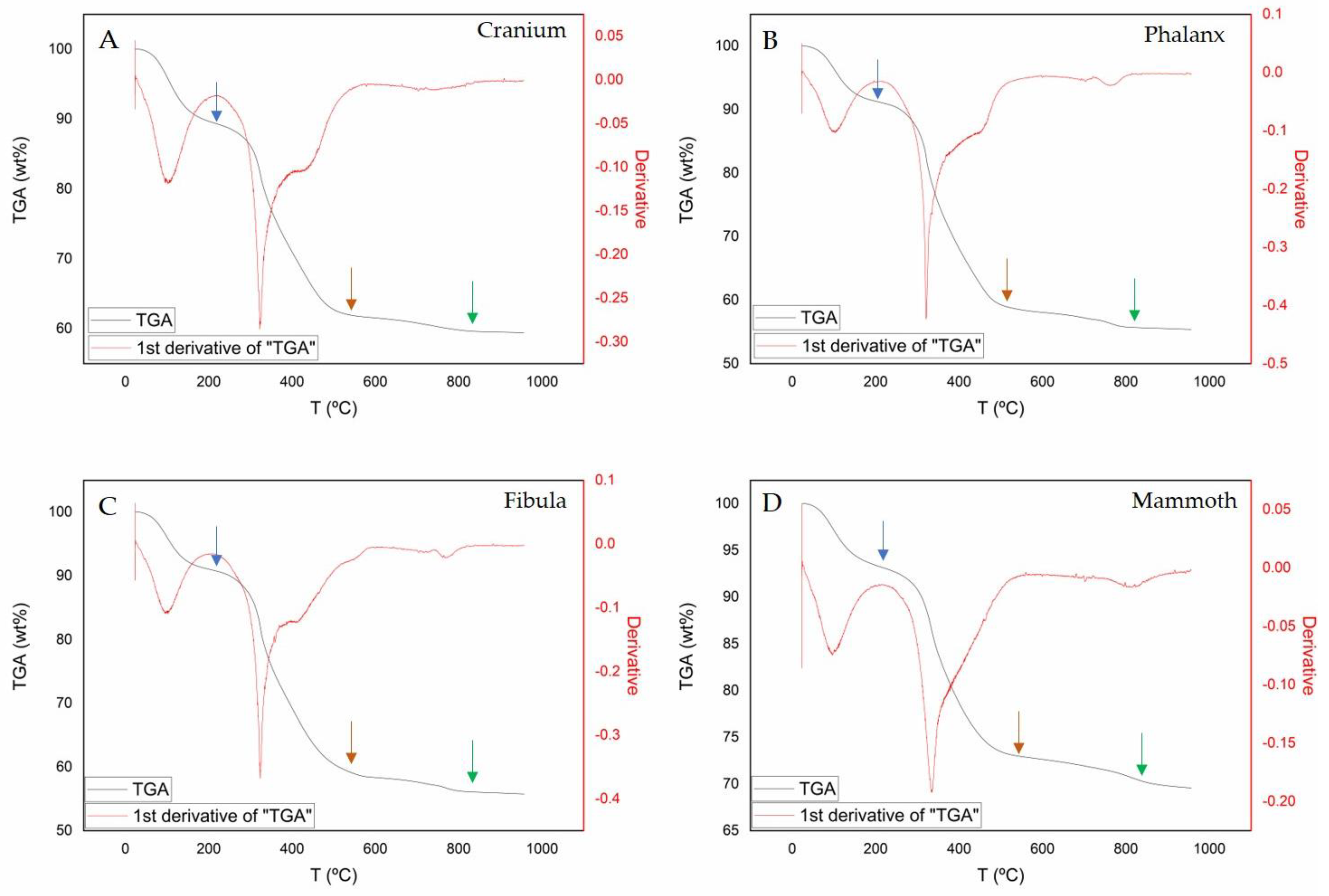
2.2.3. Thermogravimetry
2.2.4. Infrared Spectroscopy
2.2.5. Inductively Coupled Plasma Optical Emission Spectroscopy
2.2.6. Scanning Electron Microscopy
3. Results
3.1. Bone Mineralogy
3.2. Bone Chemistry
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngatia, J.N.; Lan, T.M.; Ma, Y.; Dinh, T.D.; Wang, Z.; Dahmer, T.D.; Xu, Y.C. Distinguishing extant elephants ivory from mammoth ivory using a short sequence of cytochrome b gene. Sci. Rep. 2019, 9, 18863. [Google Scholar] [CrossRef] [PubMed]
- Hauenstein, S.; Kshatriya, M.; Blanc, J.; Dormann, C.F.; Beale, C.M. African elephant poaching rates correlate with local poverty, national corruption and global ivory price. Nat. Commun. 2019, 10, 2242. [Google Scholar] [CrossRef] [PubMed]
- The IUCN Red List of Threatened Species; Version 2020; International Union for Conservation of Nature: Gland, Switzerland, 2020.
- Roca, A.L.; Ishida, Y.; Brandt, A.L.; Benjamin, N.R.; Zhao, K.; Georgiadis, N.J. Elephant natural history: A genomic perspective. Annu. Rev. Anim. Biosci. 2015, 3, 139–167. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.; Drautz, D.I.; Ratan, A.; Pusey, B.; Qi, J.; Lesk, A.M.; Tomsho, L.P.; Packard, M.D.; Zhao, F.; Sher, A. Sequencing the nuclear genome of the extinct woolly mammoth. Nature 2008, 456, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Lima-Ribeiro, M.S.; Nogués-Bravo, D.; Marske, K.A.; Fernandez, F.A.S.; Araujo, B.; Diniz-Filho, J.A.F. Human arrival scenarios have a strong influence on interpretations of the late Quaternary extinctions. Proc. Natl. Acad. Sci. USA 2012, 109, E2409–E2410. [Google Scholar] [CrossRef]
- Fiedel, S. Sudden deaths: The chronology of terminal Pleistocene megafaunal extinction. In American Megafaunal Extinctions at the End of the Pleistocene; Springer: Berlin/Heidelberg, Germany, 2009; pp. 21–37. [Google Scholar]
- Moss, C.J. The demography of an African elephant (Loxodonta africana) population in Amboseli, Kenya. J. Zool. 2001, 255, 145–156. [Google Scholar] [CrossRef]
- Locke, M. Structure of ivory. J. Morphol. 2008, 269, 423–450. [Google Scholar] [CrossRef]
- Schmidt-Nielsen, K.; Knut, S.-N. Scaling: Why Is Animal Size So Important? Cambridge University Press: Singapore, 1984; ISBN 0521319870. [Google Scholar]
- Dove, P.M.; De Yoreo, J.J.; Weiner, S. Biomineralization; Mineralogical Society of America: Chantilly, VA, USA, 2003; Volume 54, p. 381. [Google Scholar]
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef]
- Dominguez-Gasca, N.; Benavides-Reyes, C.; Sánchez-Rodríguez, E.; Rodríguez-Navarro, A.B. Changes in avian cortical and medullary bone mineral composition and organization during acid-induced demineralization. Eur. J. Mineral. 2019, 31, 209–216. [Google Scholar] [CrossRef]
- Grunenwald, A.; Keyser, C.; Sautereau, A.-M.; Crubézy, E.; Ludes, B.; Drouet, C. Revisiting carbonate quantification in apatite (bio) minerals: A validated FTIR methodology. J. Archaeol. Sci. 2014, 49, 134–141. [Google Scholar] [CrossRef]
- Hedges, R.E.M. Bone diagenesis: An overview of processes. Archaeometry 2002, 44, 319–328. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, A.B.; McCormack, H.M.; Fleming, R.H.; Alvarez-Lloret, P.; Romero-Pastor, J.; Dominguez-Gasca, N.; Prozorov, T.; Dunn, I.C. Influence of physical activity on tibial bone material properties in laying hens. J. Struct. Biol. 2018, 201, 36–45. [Google Scholar] [CrossRef]
- Álvarez-Lao, D.J.; Kahlke, R.-D.; García, N.; Mol, D. The Padul mammoth finds—On the southernmost record of Mammuthus primigenius in Europe and its southern spread during the Late Pleistocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 278, 57–70. [Google Scholar] [CrossRef]
- García-Alix, A.; Huertas, A.D.; Suárez, E.M. Unravelling the Late Pleistocene habitat of the southernmost woolly mammoths in Europe. Quat. Sci. Rev. 2012, 32, 75–85. [Google Scholar] [CrossRef]
- ICDD PDF-2 Database of the International Center for Diffraction Data (Pennsylvania, USA). 1996. Available online: https://www.icdd.com/pdf-2/ (accessed on 18 August 2022).
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B Condens. Matter Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Rey, C.; Collins, B.; Goehl, T.; Dickson, I.R.; Glimcher, M.J. The carbonate environment in bone mineral: A resolution-enhanced Fourier transform infrared spectroscopy study. Calcif. Tissue Int. 1989, 45, 157–164. [Google Scholar] [CrossRef]
- Donnelly, E.; Boskey, A.L.; Baker, S.P.; Van Der Meulen, M.C.H. Effects of tissue age on bone tissue material composition and nanomechanical properties in the rat cortex. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 92, 1048–1056. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. 1. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; De Gruyter (O): Berlin, Germany, 2015; pp. 1–30. ISBN 3110417103. [Google Scholar]
- Peters, F.; Schwarz, K.; Epple, M. The structure of bone studied with synchrotron X-ray diffraction, X-ray absorption spectroscopy and thermal analysis. Thermochim. Acta 2000, 361, 131–138. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Sandhyarani, M.; Nellaippan, T.A.; Rameshbabu, N. Estimation of crystallite size, lattice strain and dislocation density of nanocrystalline carbonate substituted hydroxyapatite by X-ray peak variance analysis. Procedia Mater. Sci. 2014, 5, 212–221. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Ruiz-Agudo, E.; Luque, A.; Rodriguez-Navarro, A.B.; Ortega-Huertas, M. Thermal decomposition of calcite: Mechanisms of formation and textural evolution of CaO nanocrystals. Am. Mineral. 2009, 94, 578–593. [Google Scholar] [CrossRef]
- Lozano, L.F.; Pena-Rico, M.A.; Heredia, A.; Ocotlan-Flores, J.; Gomez-Cortes, A.; Velazquez, R.; Belio, I.A.; Bucio, L. Thermal analysis study of human bone. J. Mater. Sci. 2003, 38, 4777–4782. [Google Scholar] [CrossRef]
- Mackie, E.J. Osteoblasts: Novel roles in orchestration of skeletal architecture. Int. J. Biochem. Cell Biol. 2003, 35, 1301–1305. [Google Scholar] [CrossRef]
- Burger, E.H.; Klein-Nulend, J. Mechanotransduction in bone—Role of the lacunocanalicular network. FASEB J. 1999, 13, S101–S112. [Google Scholar] [CrossRef]
- Weinbaum, S.; Cowin, S.C.; Zeng, Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 1994, 27, 339–360. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; Bacabac, R.G.; Mullender, M.G. Mechanobiology of bone tissue. Pathol. Biol. 2005, 53, 576–580. [Google Scholar] [CrossRef]
- McNamara, L.M. 2.10 Bone as a Material. Compr. Biomater. 2017, 2, 202–227. [Google Scholar]
- Benavides-Reyes, C.; Rodriguez-Navarro, A.B.; McCormack, H.A.; Eusemann, B.K.; Dominguez-Gasca, N.; Alvarez-Lloret, P.; Fleming, R.H.; Petow, S.; Dunn, I.C. Comparative analysis of the morphology, chemistry and structure of the tibiotarsus, humerus and keel bones in laying hens. Br. Poult. Sci. 2021, 62, 795–803. [Google Scholar] [CrossRef]
- Torres Pérez-Hidalgo, T.J. Un nuevo sondeo de investigación paleoambiental del Pleistoceno y Holoceno en la turbera del Padul (Granada, Andalucía). Geogaceta 1998, 23, 99–102. [Google Scholar]
- Ortiz, J.E.; Torres, T.; Delgado, A.; Julia, R.; Lucini, M.; Llamas, F.J.; Reyes, E.; Soler, V.; Valle, M. The palaeoenvironmental and palaeohydrological evolution of Padul Peat Bog (Granada, Spain) over one million years, from elemental, isotopic and molecular organic geochemical proxies. Org. Geochem. 2004, 35, 1243–1260. [Google Scholar] [CrossRef]
- Ortner, D.J.; Turner-Walker, G. The biology of skeletal tissues. In Identification of Pathological Conditions in Human Skeletal Remains; Elsevier: Amsterdam, The Netherlands, 2003; pp. 11–35. [Google Scholar]
- Sillen, A.; LeGeros, R. Solubility profiles of synthetic apatites and of modern and fossil bones. J. Archaeol. Sci. 1991, 18, 385–397. [Google Scholar] [CrossRef]
- Von Endt, D.W.; Ortner, D.J. Experimental effects of bone size and temperature on bone diagenesis. J. Archaeol. Sci. 1984, 11, 247–253. [Google Scholar] [CrossRef]
- Lyman, R.L. Bone density and bone attrition. In Manual of Forensic Taphonomy; CRC Press: Boca Raton, FL, USA, 2014; pp. 51–72. [Google Scholar]
- Trueman, C.N.G.; Behrensmeyer, A.K.; Tuross, N.; Weiner, S. Mineralogical and compositional changes in bones exposed on soil surfaces in Amboseli National Park, Kenya: Diagenetic mechanisms and the role of sediment pore fluids. J. Archaeol. Sci. 2004, 31, 721–739. [Google Scholar] [CrossRef]
- Smith, C.I.; Craig, O.E.; Prigodich, R.V.; Nielsen-Marsh, C.M.; Jans, M.M.E.; Vermeer, C.; Collins, M.J. Diagenesis and survival of osteocalcin in archaeological bone. J. Archaeol. Sci. 2005, 32, 105–113. [Google Scholar] [CrossRef]
- Trueman, C.N. Chemical taphonomy of biomineralized tissues. Palaeontology 2013, 56, 475–486. [Google Scholar] [CrossRef]
- Reiche, I.; Vignaud, C.; Menu, M. The crystallinity of ancient bone and dentine: New insights by transmission electron microscopy. Archaeometry 2002, 44, 447–459. [Google Scholar] [CrossRef]
- Weiner, S.; Bar-Yosef, O. States of preservation of bones from prehistoric sites in the Near East: A survey. J. Archaeol. Sci. 1990, 17, 187–196. [Google Scholar] [CrossRef]
- Qi, L.; Yuan, X.; Cao, S. Representation and Application of Infrared Reflection Spectra of Gems. J. Gems Gemmol. 2005, 7, 21–25. [Google Scholar]
- Yin, Z.; Zhang, P.; Chen, Q.; Luo, Q.; Zheng, C.; Li, Y. A Comparison of Modern and Fossil Ivories Using Multiple Techniques. Gems Gemol. 2013, 49, 16–27. [Google Scholar] [CrossRef]
- Labs-Hochstein, J.; MacFadden, B.J. Quantification of diagenesis in Cenozoic sharks: Elemental and mineralogical changes. Geochim. Cosmochim. Acta 2006, 70, 4921–4932. [Google Scholar] [CrossRef]
- Rey, C.; Marsan, O.; Combes, C.; Drouet, C.; Grossin, D.; Sarda, S. Characterization of calcium phosphates using vibrational spectroscopies. Adv. Calcium Phosphate Biomater. 2014, 229–266. [Google Scholar] [CrossRef]
- Madupalli, H.; Pavan, B.; Tecklenburg, M.M.J. Carbonate substitution in the mineral component of bone: Discriminating the structural changes, simultaneously imposed by carbonate in A and B sites of apatite. J. Solid State Chem. 2017, 255, 27–35. [Google Scholar] [CrossRef]
- Roche, D.; Ségalen, L.; Balan, E.; Delattre, S. Preservation assessment of Miocene–Pliocene tooth enamel from Tugen Hills (Kenyan Rift Valley) through FTIR, chemical and stable-isotope analyses. J. Archaeol. Sci. 2010, 37, 1690–1699. [Google Scholar] [CrossRef]
- Pfretzschner, H.-U. Fossilization of Haversian bone in aquatic environments. Comptes Rendus Palevol 2004, 3, 605–616. [Google Scholar] [CrossRef]
- Machel, H.G. Bacterial and thermochemical sulfate reduction in diagenetic settings—Old and new insights. Sediment. Geol. 2001, 140, 143–175. [Google Scholar] [CrossRef]
- Pfretzschner, H.-U. Pyrite formation in Pleistocene bones-a case of very early mineral formation during diagenesis. Neues Jahrb. Für Geol. Paläontologie-Abhandlungen 2000, 217, 143–160. [Google Scholar] [CrossRef]
- Nielsen-Marsh, C.M.; Hedges, R.E.M. Patterns of diagenesis in bone I: The effects of site environments. J. Archaeol. Sci. 2000, 27, 1139–1150. [Google Scholar] [CrossRef]
- Lyman, R.L. Bone density and differential survivorship of fossil classes. J. Anthropol. Archaeol. 1984, 3, 259–299. [Google Scholar] [CrossRef]
- Nicholson, R.A. Bone degradation, burial medium and species representation: Debunking the myths, an experiment-based approach. J. Archaeol. Sci. 1996, 23, 513–533. [Google Scholar] [CrossRef]
- Kendall, C.; Eriksen, A.M.H.; Kontopoulos, I.; Collins, M.J.; Turner-Walker, G. Diagenesis of archaeological bone and tooth. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 491, 21–37. [Google Scholar] [CrossRef]


| Sample | a (Å) | c (Å) | GoF | Crystallite Size (nm) |
|---|---|---|---|---|
| Cranium | 9.4493 | 6.8834 | 4 | 17.64 |
| Phalanx | 9.4474 | 6.8878 | 4.1 | 17.64 |
| Fibula | 9.4465 | 6.8885 | 4.2 | 17.35 |
| Mammoth | 9.4444 | 6.8907 | 4.8 | 17.63 |
| Cranium | Phalanx | Fibula Trans | Fibula Long | Mammoth | |
|---|---|---|---|---|---|
| c-axis angular spread (deg) | 123.88 ± 5.80 | 136.03 ± 4.25 | 114.79 ± 7.11 | 65.43 ± 3.84 | 73.38 ± 4.81 |
| Oriented fraction (Rho) | 0.244 ± 0.08 | 0.349 ± 0.08 | 0.275 ± 0.08 | 0.622 ± 0.04 | 0.634 ± 0.10 |
| Water (wt%) | O.M. (wt%) | Carbonates (wt%) | Phosphates (wt%) | |
|---|---|---|---|---|
| Cranium | 10.77 | 27.5 | 2.19 | 59.54 |
| Phalanx | 8.87 | 32.37 | 3.09 | 55.67 |
| Fibula | 9.10 | 32.53 | 2.53 | 55.84 |
| Mammoth | 6.80 | 20.09 | 3.45 | 69.66 |
| Bone | Ca (mmol/L) | Fe (mmol/L) | Mg (mmol/L) | Na (mmol/L) | P (mmol/L) | Sr (mmol/L) | Ca/P |
|---|---|---|---|---|---|---|---|
| Cranium | 574.10 | 1.31 | 16.57 | 21.76 | 370.64 | 0.37 | 1.55 |
| Phalanx | 596.23 | 0.88 | 10.69 | 21.70 | 357.84 | 0.20 | 1.67 |
| Fibula | 590.32 | 1.58 | 10.79 | 23.80 | 362.63 | 0.16 | 1.63 |
| Mammoth | 689.16 | 5.90 | 11.82 | 12.37 | 379.56 | 0.59 | 1.82 |
| PO4/Amide I | CO3_1415/Amide I | CO3_1415/PO4 | CO3_1415/CO3_870 | |
|---|---|---|---|---|
| Cranium | 4.716 | 1.686 | 0.358 | 5.146 |
| Phalanx | 2.686 | 2.029 | 0.755 | 9.388 |
| Fibula | 3.219 | 1.756 | 0.546 | 7.908 |
| Mammoth | 8.938 | 2.452 | 0.274 | 3.047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monasterio-Guillot, L.; Crespo-López, L.; Rodríguez Navarro, A.B.; Álvarez-Lloret, P. Comparative Study of the Mineralogy and Chemistry Properties of Elephant Bones: Implications during Diagenesis Processes. Minerals 2022, 12, 1384. https://doi.org/10.3390/min12111384
Monasterio-Guillot L, Crespo-López L, Rodríguez Navarro AB, Álvarez-Lloret P. Comparative Study of the Mineralogy and Chemistry Properties of Elephant Bones: Implications during Diagenesis Processes. Minerals. 2022; 12(11):1384. https://doi.org/10.3390/min12111384
Chicago/Turabian StyleMonasterio-Guillot, Luis, Laura Crespo-López, Alejandro B. Rodríguez Navarro, and Pedro Álvarez-Lloret. 2022. "Comparative Study of the Mineralogy and Chemistry Properties of Elephant Bones: Implications during Diagenesis Processes" Minerals 12, no. 11: 1384. https://doi.org/10.3390/min12111384
APA StyleMonasterio-Guillot, L., Crespo-López, L., Rodríguez Navarro, A. B., & Álvarez-Lloret, P. (2022). Comparative Study of the Mineralogy and Chemistry Properties of Elephant Bones: Implications during Diagenesis Processes. Minerals, 12(11), 1384. https://doi.org/10.3390/min12111384









