A Real-Time, Non-Invasive Technique for Visualizing the Effects of Acid Mine Drainage (AMD) on Soybean
Abstract
1. Introduction
2. Materials and Methods
2.1. Simulated AMD and Plant Materials
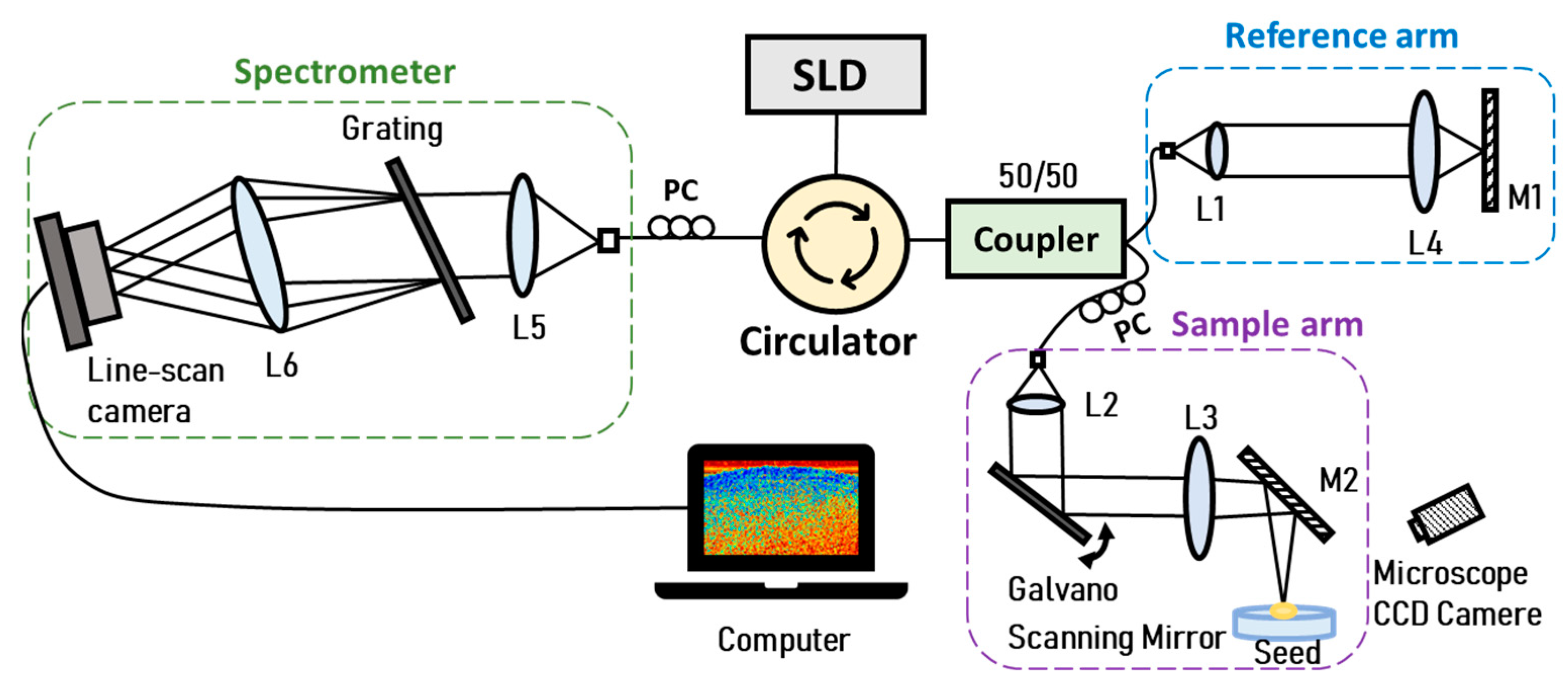
2.2. Optical Coherent Tomography (OCT) Experimental System
2.3. Biospeckle Contrast
2.4. Traditional Physiological Indicators
2.5. Data Analysis
3. Results
3.1. Comparison of OCT Structural Images and bOCT Biospeckle Contrast Images
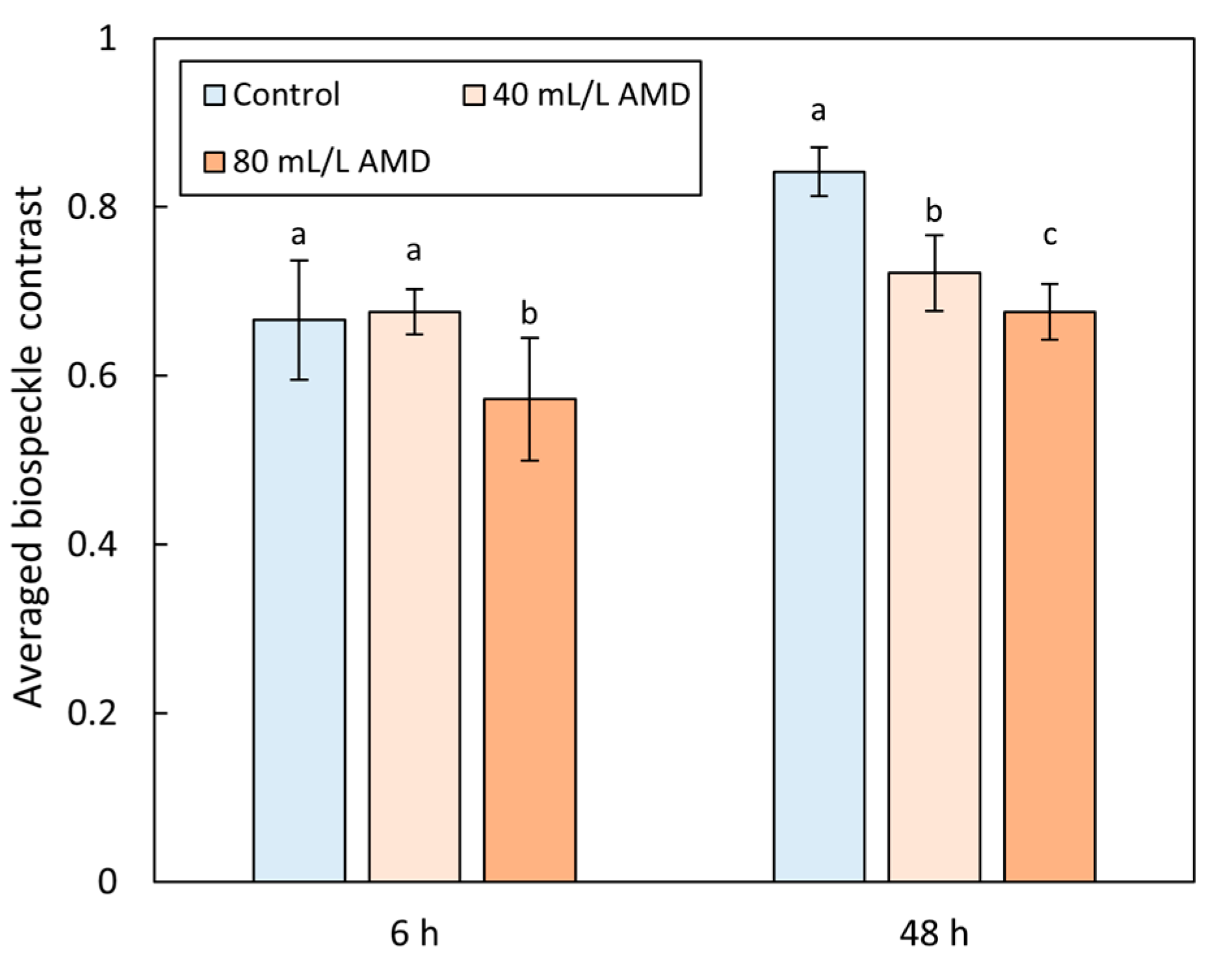
3.2. Biospeckle Contrast
3.3. Conventional Measurements
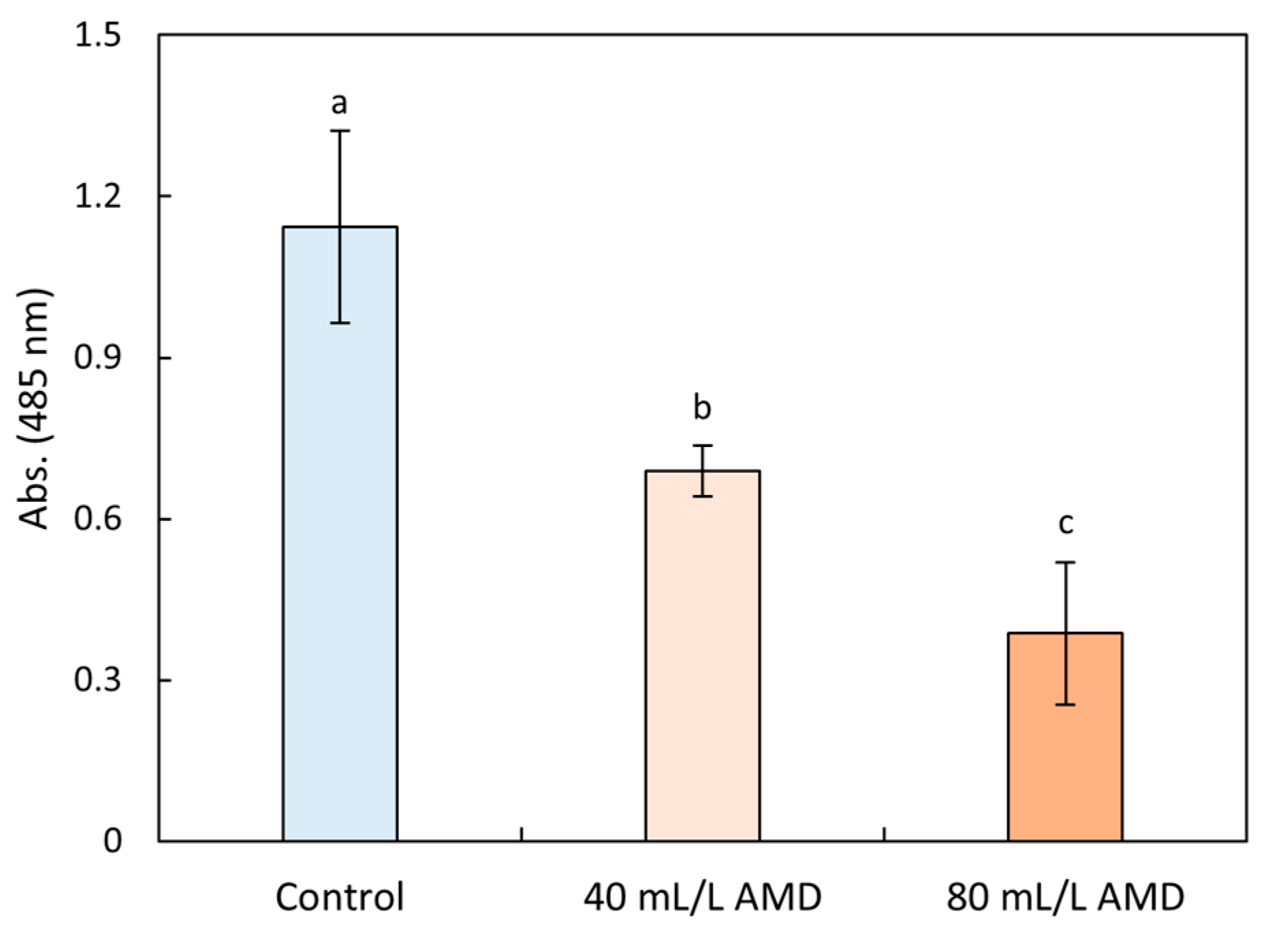
3.3.1. Seed Vigor
3.3.2. Antioxidative System Response
3.3.3. Fe Concentration
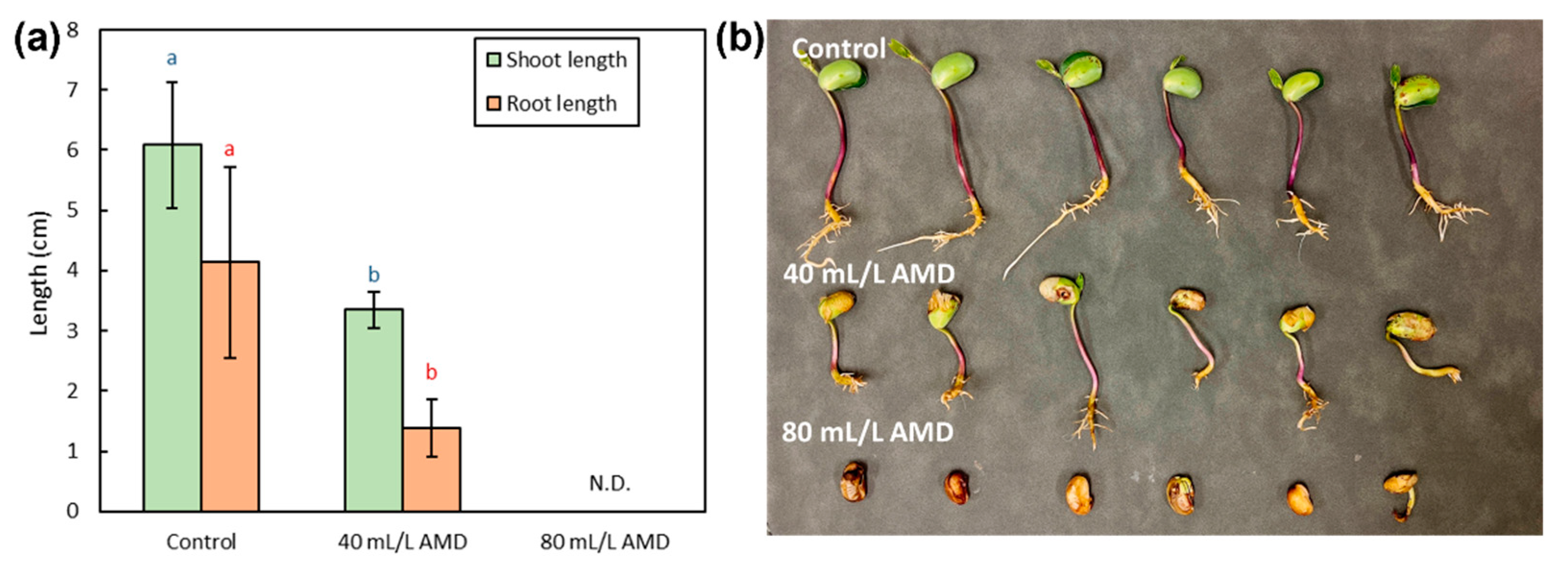
3.3.4. Length of Shoot and Root
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dang, Z.; Liu, C.; Haigh, M.J. Mobility of heavy metals associated with the natural weathering of coal mine spoils. Environ. Pollut. 2002, 118, 419–426. [Google Scholar] [CrossRef]
- Gunsinger, M.; Ptacek, C.; Blowes, D.; Jambor, J. Evaluation of long-term sulfide oxidation processes within pyrrhotite-rich tailings, Lynn Lake, Manitoba. J. Contam. Hydrol. 2006, 83, 149–170. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Thomas, G.; Sheridan, C.; Holm, P.E. A critical review of phytoremediation for acid mine drainage-impacted environments. Sci. Total Environ. 2021, 811, 152230. [Google Scholar] [CrossRef]
- Prosekov, A.Y.; Ivanova, S.A. Food security: The challenge of the present. Geoforum 2018, 91, 73–77. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, Y.; Guo, C.; Bao, Y.; Lu, G.; Reinfelder, J.R.; Dang, Z. Bacterial, archaeal, and fungal community responses to acid mine drainage-laden pollution in a rice paddy soil ecosystem. Sci. Total Environ. 2018, 616–617, 107–116. [Google Scholar] [CrossRef]
- Choudhury, B.U.; Malang, A.; Webster, R.; Mohapatra, K.P.; Verma, B.C.; Kumar, M.; Das, A.; Islam, M.; Hazarika, S. Acid drainage from coal mining: Effect on paddy soil and productivity of rice. Sci. Total Environ. 2017, 583, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Tong, X.; Lu, W.; Yan, L.; Wu, Y.; Nie, C.; Chu, C.; Long, J. Environmental impacts of surface mining on mined lands, affected streams and agricultural lands in the Dabaoshan mine region, southern China. Land Degrad. Dev. 2005, 16, 463–474. [Google Scholar] [CrossRef]
- Ma, S.-C.; Zhang, H.-B.; Ma, S.-T.; Wang, R.; Wang, G.-X.; Shao, Y.; Li, C.-X. Effects of mine wastewater irrigation on activities of soil enzymes and physiological properties, heavy metal uptake and grain yield in winter wheat. Ecotoxicol. Environ. Saf. 2015, 113, 483–490. [Google Scholar] [CrossRef]
- Munyai, R.; Raletsena, M.V.; Modise, D.M. LC-MS Based Metabolomics Analysis of Potato (Solanum tuberosum L.) Cultivars Irrigated with Quicklime Treated Acid Mine Drainage Water. Metabolites 2022, 12, 221. [Google Scholar] [CrossRef]
- Schmitt, J.M. Optical coherence tomography (OCT): A review. IEEE J. Sel. Top. Quantum Electron. 1999, 5, 1205–1215. [Google Scholar] [CrossRef]
- Fujimoto, J.G.; Drexler, W.; Schuman, J.S.; Hitzenberger, C.K. Optical Coherence Tomography (OCT) in ophthalmology: Introduction. Opt. Express 2009, 17, 3978–3979. [Google Scholar] [CrossRef] [PubMed]
- de Wit, J.; Tonn, S.; Van den Ackerveken, G.; Kalkman, J. Quantification of plant morphology and leaf thickness with optical coherence tomography. Appl. Opt. 2020, 59, 10304–10311. [Google Scholar] [CrossRef]
- Larimer, C.J.; Denis, E.H.; Suter, J.D.; Moran, J.J. Optical coherence tomography imaging of plant root growth in soil. Appl. Opt. 2020, 59, 2474–2481. [Google Scholar] [CrossRef]
- Srimal, L.; Kadono, H.; Rajagopalan, U. Optical Coherence Tomography Biospeckle Imaging for Fast Monitoring Varying Surface Responses of a Plant Leaf Under Ozone Stress. In Proceedings of the Sensing Technologies for Biomaterial, Food, and Agriculture 2013, Yokohama, Japan, 23–25 April 2013; pp. 96–101. [Google Scholar]
- Srimal, L.; Rajagopalan, U.; Kadono, H. Functional Optical Coherence Tomography (fOCT) Biospeckle Imaging to Investigate Response of Plant Leaves to Ultra-Short Term Exposure of Ozone. J. Phys. Conf. Ser. 2015, 664, 012013. [Google Scholar] [CrossRef]
- Lim, Y.; Funada, K.; Kadono, H. Monitor biological activities in seed germination by biospeckle optical coherence tomography. In Proceedings of the Dynamics and Fluctuations in Biomedical Photonics XVI, San Francisco, CA, USA, 2–3 February 2019; pp. 42–47. [Google Scholar]
- Rajagopalan, U.M.; Kabir, M.; Lim, Y.; Kadono, H. Biospeckle optical coherence tomography in speedy visualizing effects of foliar application of plant growth hormone to Chinese chives leaves. BMC Res. Notes 2020, 13, 377. [Google Scholar] [CrossRef] [PubMed]
- De Silva, Y.S.K.; Rajagopalan, U.M.; Li, D.; Kadono, H. Optical screening method to observe the biological activities of lentil (Lens culinaris) seeds quantitatively under the exposure of polyethylene microplastics (PEMPs) using ultrahigh accurate biospeckle optical coherence tomography. In Proceedings of the Tissue Optics and Photonics II, Strasbourg, France, 3–7 April 2022; pp. 70–77. [Google Scholar]
- De Silva, Y.S.K.; Rajagopalan, U.M.; Kadono, H.; Li, D. Effects of microplastics on lentil (Lens culinaris) seed germination and seedling growth. Chemosphere 2022, 303, 135162. [Google Scholar] [CrossRef] [PubMed]
- De Silva, Y.S.K.; Rajagopalan, U.M.; Kadono, H.; Li, D. Positive and negative phenotyping of increasing Zn concentrations by Biospeckle Optical Coherence Tomography in speedy monitoring on lentil (Lens culinaris) seed germination and seedling growth. Plant Stress 2021, 2, 100041. [Google Scholar] [CrossRef]
- Li, D.; Rajagopalan, U.M.; De Silva, Y.S.K.; Liu, F.; Kadono, H. Biospeckle optical coherence tomography (BOCT) in the speedy assessment of the responses of the seeds of Raphanus sativus L. (Kaiware Daikon) to acid mine drainage (AMD). Appl. Sci. 2021, 12, 355. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.; Mamba, B.B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, F.; Qiao, X.; Zhou, L.; Bi, W. Effects of acid mine drainage on calcareous soil characteristics and Lolium perenne L. germination. Int. J. Environ. Res. Public Health 2018, 15, 2742. [Google Scholar] [CrossRef] [PubMed]
- Fetisova, N. Arsenic speciation and sorption in acid mine drainage and the polluted water of the Kosva river basin, Russia. IOP Conf. Ser. Earth Environ. Sci. 2022, 962, 012050. [Google Scholar] [CrossRef]
- Seyfried, M.; Fukshansky, L. Light gradients in plant tissue. Appl. Opt. 1983, 22, 1402–1408. [Google Scholar] [CrossRef]
- Aizu, Y.; Asakura, T. Bio-speckle phenomena and their application to the evaluation of blood flow. Opt. Laser Technol. 1991, 23, 205–219. [Google Scholar] [CrossRef]
- Draijer, M.; Hondebrink, E.; van Leeuwen, T.; Steenbergen, W. Review of laser speckle contrast techniques for visualizing tissue perfusion. Lasers Med. Sci. 2009, 24, 639–651. [Google Scholar] [CrossRef]
- Zdunek, A.; Adamiak, A.; Pieczywek, P.M.; Kurenda, A. The biospeckle method for the investigation of agricultural crops: A review. Opt. Lasers Eng. 2014, 52, 276–285. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Mayookha, V.; Kothakota, A.; Ramesh, S.; Thirumdas, R.; Juvvi, P. Biospeckle laser technique–A novel non-destructive approach for food quality and safety detection. Trends Food Sci. Technol. 2020, 97, 1–13. [Google Scholar] [CrossRef]
- Pajuelo, M.; Baldwin, G.; Rabal, H.; Cap, N.; Arizaga, R.; Trivi, M. Bio-speckle assessment of bruising in fruits. Opt. Lasers Eng. 2003, 40, 13–24. [Google Scholar] [CrossRef]
- Braga, R.A.; Dupuy, L.; Pasqual, M.; Cardoso, R. Live biospeckle laser imaging of root tissues. Eur. Biophys. J. 2009, 38, 679–686. [Google Scholar] [CrossRef]
- Liu, J.J.; Wei, Z.; Li, J.H. Effects of copper on leaf membrane structure and root activity of maize seedling. Bot. Stud. 2014, 55, 47. [Google Scholar] [CrossRef]
- Davis, D.G.; Swanson, H.R. Activity of stress-related enzymes in the perennial weed leafy spurge (Euphorbia esula L.). Environ. Exp. Bot. 2001, 46, 95–108. [Google Scholar] [CrossRef]
- Ren, X.; Zhu, J.; Liu, H.; Xu, X.; Liang, C. Response of antioxidative system in rice (Oryza sativa) leaves to simulated acid rain stress. Ecotoxicol. Environ. Saf. 2018, 148, 851–856. [Google Scholar] [CrossRef]
- Kos, V.; Budič, B.; Hudnik, V.; Lobnik, F.; Zupan, M. Determination of heavy metal concentrations in plants exposed to different degrees of pollution using ICP-AES. Fresenius J. Anal. Chem. 1996, 354, 648–652. [Google Scholar] [CrossRef]
- Tajima, R.; Kato, Y. 【Short Report】 A Quick Method to Estimate Root Length in Each Diameter Class Using Freeware ImageJ. Plant Prod. Sci. 2013, 16, 9–11. [Google Scholar] [CrossRef]
- Williams, L.J.; Abdi, H. Fisher’s least significant difference (LSD) test. Encycl. Res. Des. 2010, 218, 840–853. [Google Scholar]
- Lopez Del Egido, L.; Navarro-Miró, D.; Martinez-Heredia, V.; Toorop, P.E.; Iannetta, P.P. A spectrophotometric assay for robust viability testing of seed batches using 2, 3, 5-triphenyl tetrazolium chloride: Using Hordeum vulgare L. as a model. Front. Plant Sci. 2017, 8, 747. [Google Scholar] [CrossRef]
- Alia, F.J.; Shamshuddin, J.; Fauziah, C.I.; Husni, M.H.A.; Panhwar, Q.A. Effects of aluminum, iron and/or low pH on rice seedlings grown in solution culture. Int. J. Agric. Biol. 2015, 17, 702–710. [Google Scholar] [CrossRef]
- Lim, Y.; Kadono, H. Evaluation of germination ability of seeds at different temperatures by biospeckle optical coherence tomography. In Proceedings of the Tissue Optics and Photonics, Online, 6–10 April 2020; pp. 27–32. [Google Scholar]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development; Sinauer Associates Incorporated: Sunderland, MA, USA, 2015. [Google Scholar]
- Perico, C.; Sparkes, I. Plant organelle dynamics: Cytoskeletal control and membrane contact sites. New Phytol. 2018, 220, 381–394. [Google Scholar] [CrossRef]
- Wada, M.; Suetsugu, N. Plant organelle positioning. Curr. Opin. Plant Biol. 2004, 7, 626–631. [Google Scholar] [CrossRef]
- Zhao, P.; Zhu, Y.; Wang, W. Evaluation and improvement of spectrophotometric assays of TTC reduction: Maize (Zea mays) embryo as an example. Acta Physiol. Plant. 2010, 32, 815–819. [Google Scholar] [CrossRef]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Skłodowska, M. Differential biochemical responses of wheat shoots and roots to nickel stress: Antioxidative reactions and proline accumulation. Plant Growth Regul. 2008, 54, 179–188. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Silva, J.A.; Fujita, M. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In Crop Stress and Its Management: Perspectives and Strategies; Springer: Berlin/Heidelberg, Germany, 2012; pp. 261–315. [Google Scholar]
- Liu, T.T.; Wu, P.; Wang, L.H.; Zhou, Q. Response of soybean seed germination to cadmium and acid rain. Biol. Trace Elem. Res. 2011, 144, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Wyrwicka, A.; Skłodowska, M. Influence of repeated acid rain treatment on antioxidative enzyme activities and on lipid peroxidation in cucumber leaves. Environ. Exp. Bot. 2006, 56, 198–204. [Google Scholar] [CrossRef]
- Sahrawat, K.L. Elemental composition of the rice plant as affected by iron toxicity under field conditions. Commun. Soil Sci. Plant Anal. 2000, 31, 2819–2827. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, V.; Shukla, A.K.; Kaur, J.; Verma, V.; Kaur, M.; Singh, P.; Kaur, L.; Verma, G.; Singh, J. Biofortification of Soybean (Glycine max L.) through FeSO4· 7H2O to Enhance Yield, Iron Nutrition and Economic Outcomes in Sandy Loam Soils of India. Agriculture 2022, 12, 586. [Google Scholar] [CrossRef]
- Heitholt, J.; Sloan, J.; MacKown, C.; Cabrera, R. Soybean growth on calcareous soil as affected by three iron sources. J. Plant Nutr. 2003, 26, 935–948. [Google Scholar] [CrossRef]
- Suthipradit, S.; Alva, A. Aluminum and pH limitations for germination and radicle growth of soybean. J. Plant Nutr. 1986, 9, 67–73. [Google Scholar] [CrossRef]
- Uguru, M.I.; Oyiga, B.C.; Jandong, E.A. Responses of some soybean genotypes to different soil pH regimes in two planting seasons. Afr. J. Plant Sci. Biotechnol. 2012, 6, 26–37. [Google Scholar]
- Kuswantoro, H.; Zen, S. Performance of acid-tolerant soybean promising lines in two planting seasons. Int. J. Biol. 2013, 5, 49. [Google Scholar] [CrossRef][Green Version]
- Blanco, F.F.; Folegatti, M.V.; Gheyi, H.R.; Fernandes, P.D. Emergence and growth of corn and soybean under saline stress. Sci. Agric. 2007, 64, 451–459. [Google Scholar] [CrossRef][Green Version]
- Samarakoon, U.; Weerasinghe, P.; Weerakkody, W. Effect of electrical conductivity (EC) of the nutrient solution on nutrient uptake, growth and yield of leaf lettuce (Lactuca sativa L.) in stationary culture. Trop. Agric. Res. 2006, 18, 3. [Google Scholar]

| Treatment | SOD (U·g−1 FW) | CAT (U·g−1 FW·min−1) | H2O2 (μmol·g−1 FW) | MDA (μmol·g−1 FW) |
|---|---|---|---|---|
| Control | 898.5 ± 64.3 a | 0.23 ± 0.03 a | 794.4 ± 170.3 b | 22.2 ± 1.2 b |
| 40 mL/L AMD | 621.5 ± 101.5 b | 0.16 ± 0.01 b | 1180.4 ± 117.2 a | 35.9 ± 3.2 a |
| 80 mL/L AMD | N.D. | N.D. | N.D. | N.D. |
| Treatment | Fe Concentration (mg·kg −1) |
|---|---|
| Control | 46.7 ± 3.4 b |
| 40 mL/L AMD | 68.3 ± 4.5 a |
| 80 mL/L AMD | 71.8 ± 4.8 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Rajagopalan, U.M.; Kadono, H.; De Silva, Y.S.K. A Real-Time, Non-Invasive Technique for Visualizing the Effects of Acid Mine Drainage (AMD) on Soybean. Minerals 2022, 12, 1194. https://doi.org/10.3390/min12101194
Li D, Rajagopalan UM, Kadono H, De Silva YSK. A Real-Time, Non-Invasive Technique for Visualizing the Effects of Acid Mine Drainage (AMD) on Soybean. Minerals. 2022; 12(10):1194. https://doi.org/10.3390/min12101194
Chicago/Turabian StyleLi, Danyang, Uma Maheswari Rajagopalan, Hirofumi Kadono, and Y. Sanath K. De Silva. 2022. "A Real-Time, Non-Invasive Technique for Visualizing the Effects of Acid Mine Drainage (AMD) on Soybean" Minerals 12, no. 10: 1194. https://doi.org/10.3390/min12101194
APA StyleLi, D., Rajagopalan, U. M., Kadono, H., & De Silva, Y. S. K. (2022). A Real-Time, Non-Invasive Technique for Visualizing the Effects of Acid Mine Drainage (AMD) on Soybean. Minerals, 12(10), 1194. https://doi.org/10.3390/min12101194






