The Kaolinite Crystallinity and Influence Factors of Coal-Measure Kaolinite Rock from Datong Coalfield, China
Abstract
1. Introduction
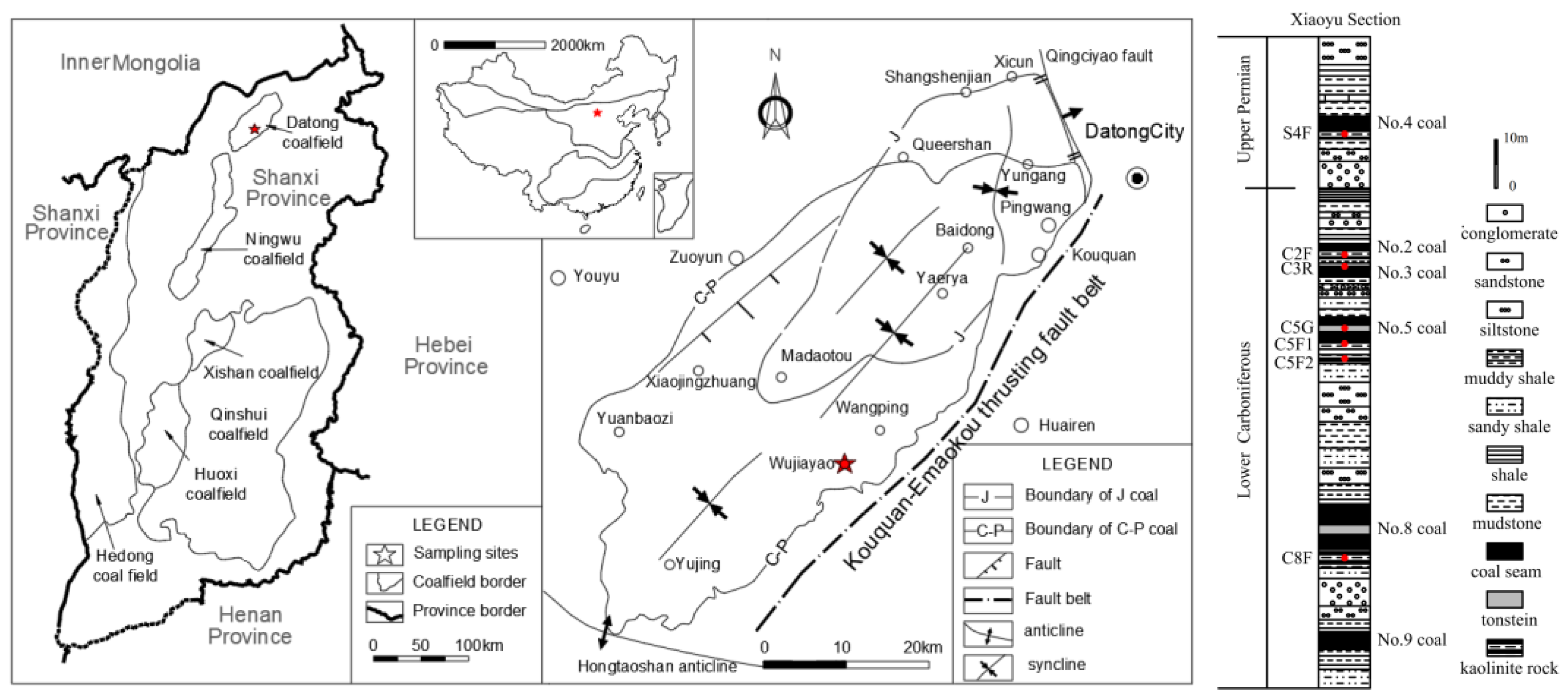
2. Geological Background
3. Materials and Methods
4. Results and Discussion
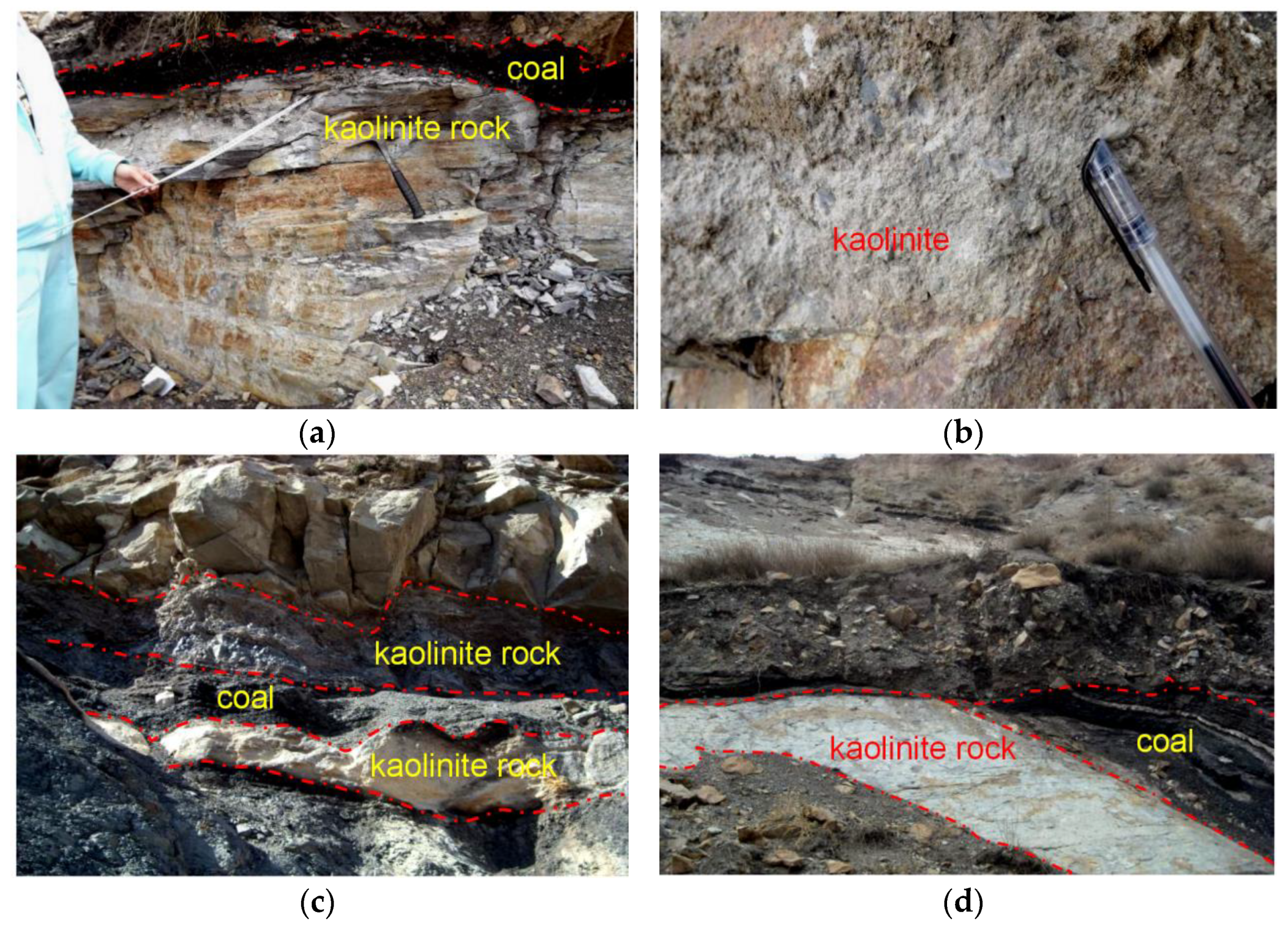
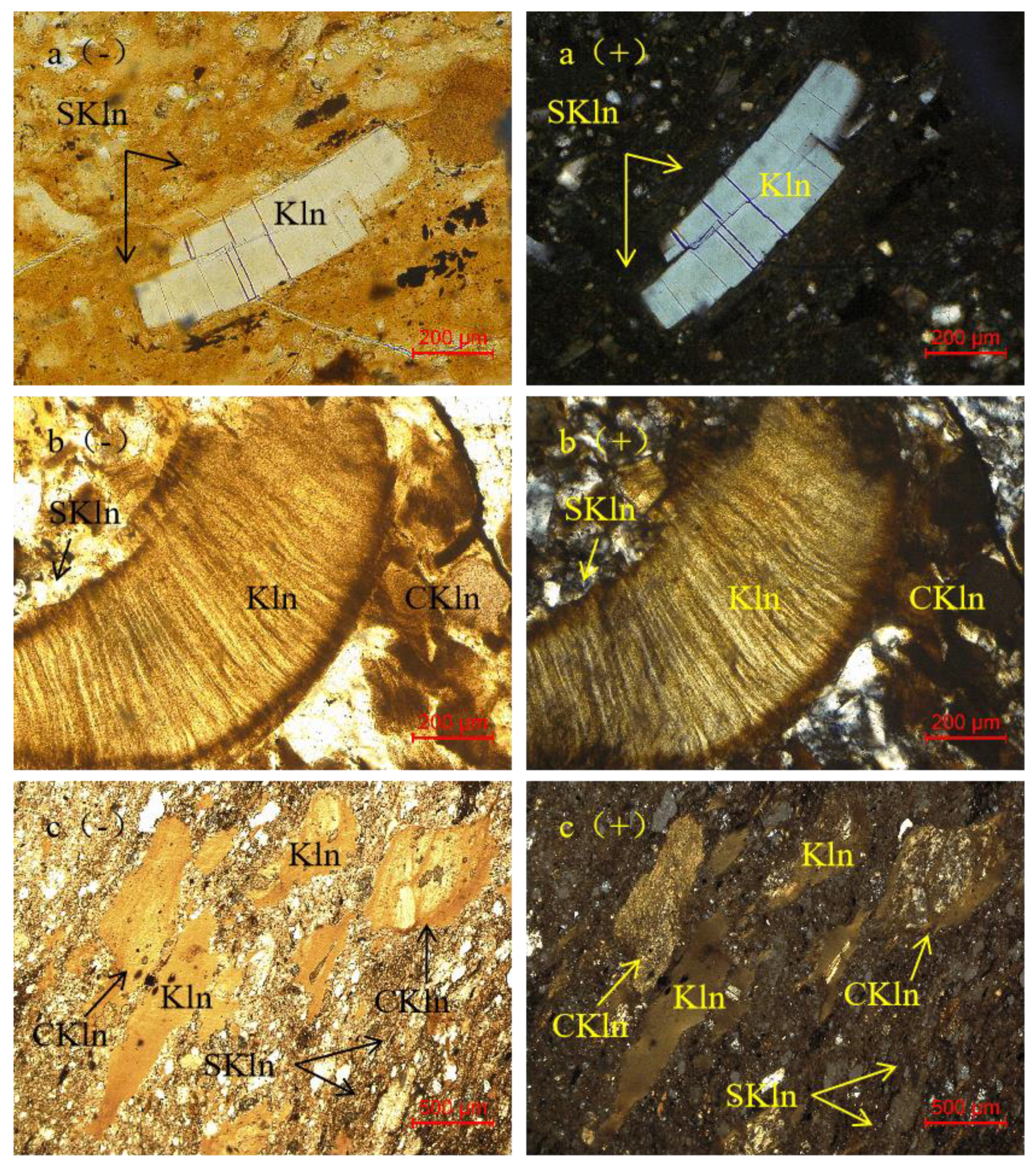
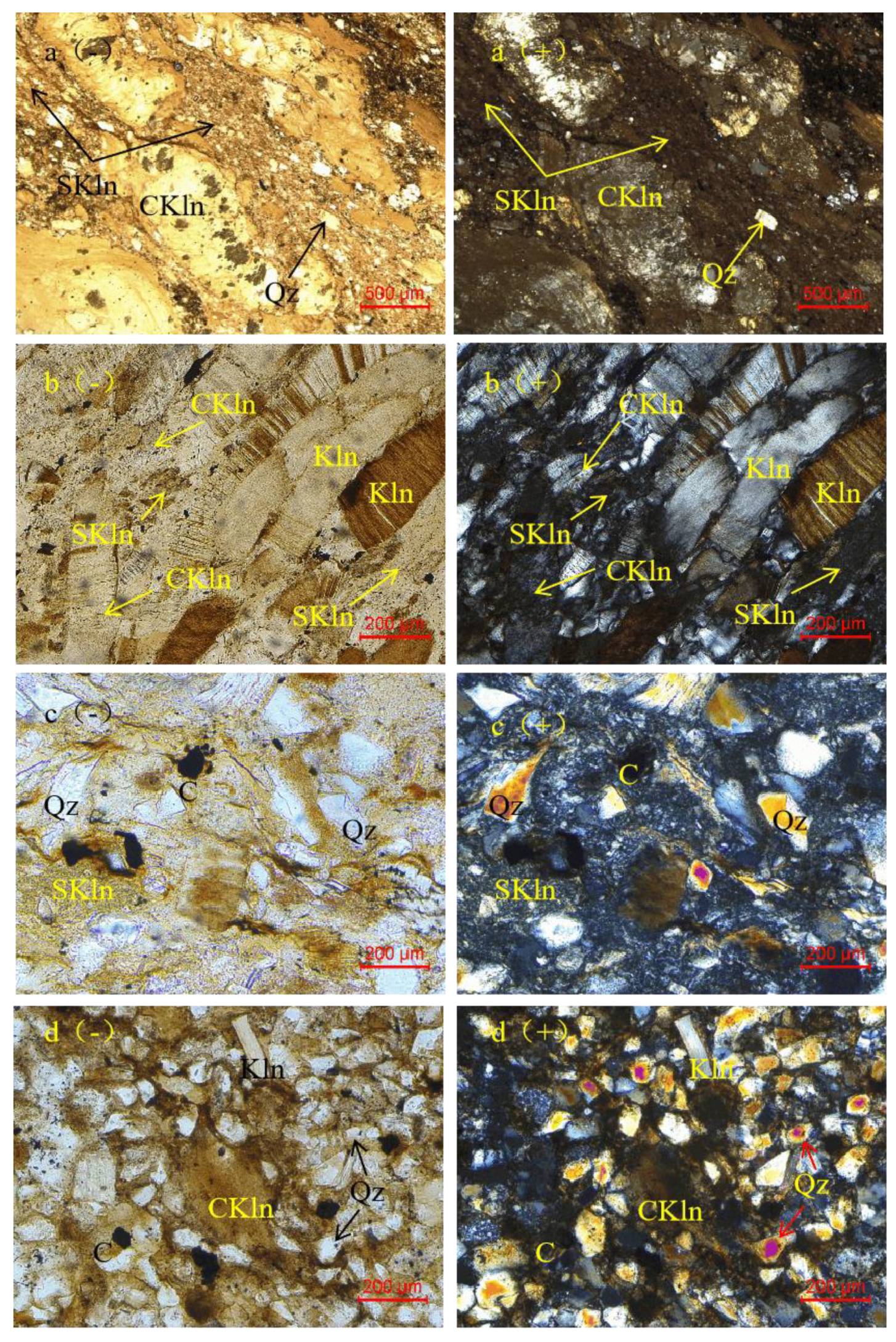
4.1. Petrography and Mineralogy
4.2. Crystallinity
4.2.1. X-ray Diffraction Analysis
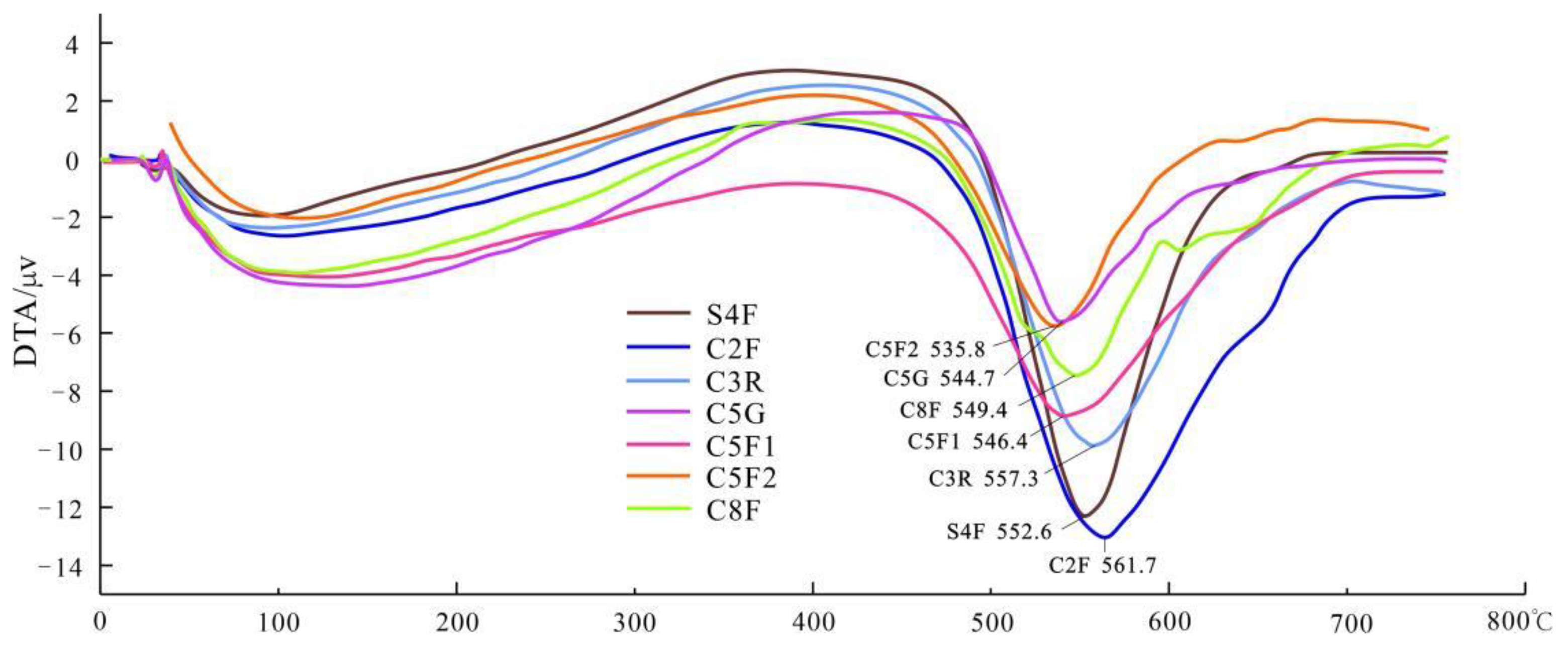
4.2.2. Differential Thermal Analysis
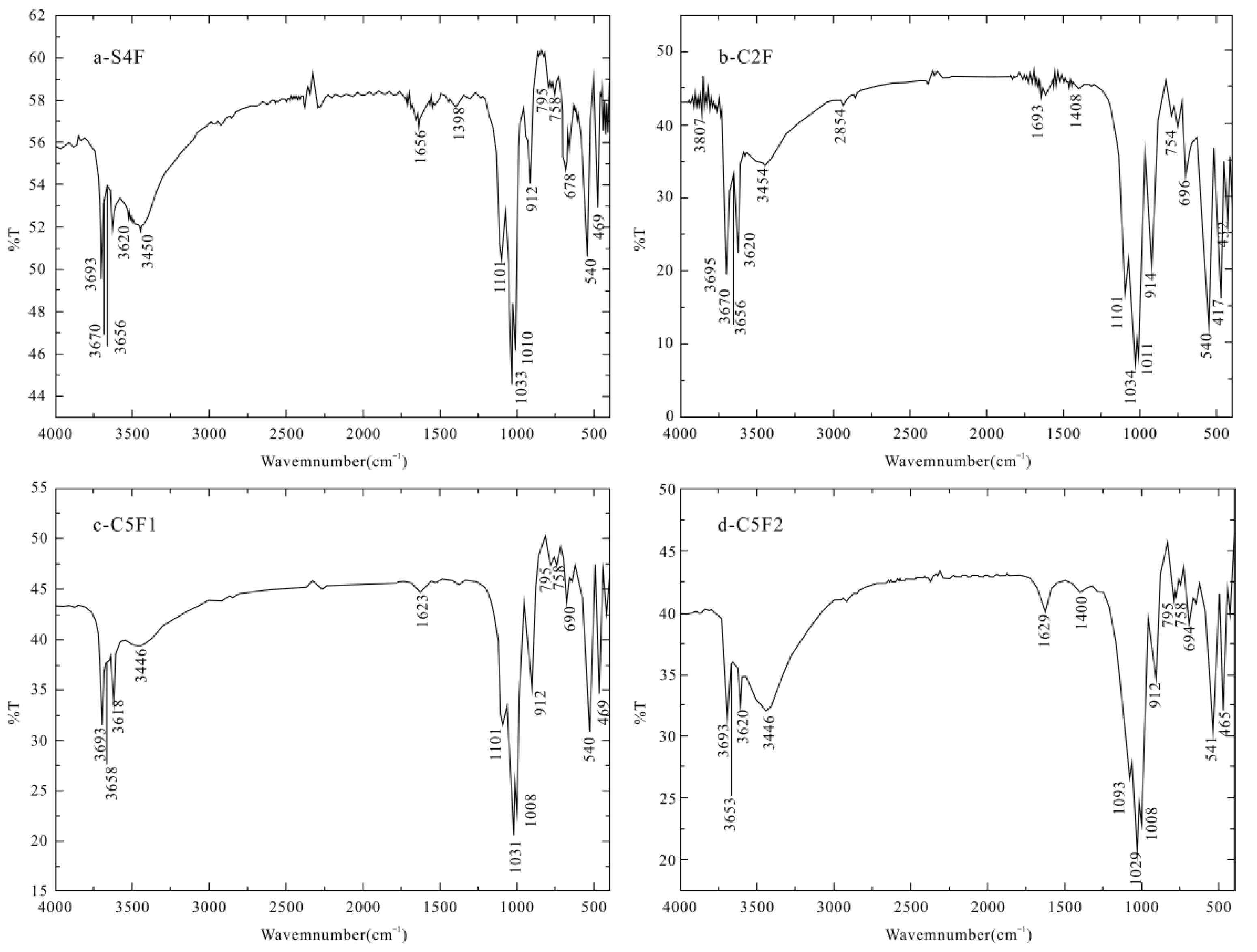
4.2.3. Infrared Spectroscopy
4.3. Geochemistry
4.4. Geological Effect Factors
4.4.1. Original Sedimentary Environment
4.4.2. Weathering
4.4.3. Organic Matter
5. Conclusions
- (1)
- The kaolinite crystallinity from the Datong coalfield can be divided into ordered, slightly disordered and disordered. Kaolinite crystallinity is positively correlated with its clay minerals content, purity and particle size, which are all affected by the original sedimentary environment and hydrodynamic conditions.
- (2)
- The kaolinite that formed in continental river facies was ordered, while that from delta facies was relatively disordered and disordered. The existence of organic matter is conducive to the stable existence and crystallinity of kaolinite.
- (3)
- Crystallinity is related to the rock SiO2/Al2O3 molar ratio and CIA; a higher weathering intensity creates a better order. With the same weathering intensity, a high purity creates a better order.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Q.F.; Zhang, P.F. Material Composition and Metallogenic Mechanism of Late Paleozoic kaolinite in North China; Ocean Press: Beijing, China, 1997. (In Chinese) [Google Scholar]
- Zhou, A.C. Geological Research on the Coal-Bearing Basin of Datong in Late Paleozoic; Coal Industry Press: Beijing, China, 2010. [Google Scholar]
- Wang, S.L.; Liang, S.X. Geochemical characteristics of clay parting from Xiaoyu coal mine of Datong, Shanxi. Coal Geol. Explor. 2011, 39, 6–10, (In Chinese with English abstract). [Google Scholar]
- Yuan, Y.; Tang, S.H.; Zhang, S.H. Geochemical and mineralogical characteristics of the middle Jurassic coals from the Tongjialiang mine in the northern Datong coalfield, Shanxi province, China. Minerals 2019, 9, 184. [Google Scholar] [CrossRef]
- Erkoyuna, H.; Kadir, S.; Huggett, J. Occurrence and genesis of tonsteins in the Miocene lignite, Tunbilek Basin, Kütahya, western Turkey. Int. J. Coal Geol. 2019, 202, 46–68. [Google Scholar] [CrossRef]
- Guo, F.Y.; Zhou, M.; Xu, J.C.; Fein, J.B.; Yu, Q.; Wang, Y.W.; Huang, Q.Y.; Rong, X.M. Glyphosate adsorption onto kaolinite and kaolinite—humic acid composites: Experimental and molecular dynamics studies. Chemosphere 2021, 263, 127979. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.F.; Yang, X.J.; Ding, S.D.; Ge, L.M. Geochemistry of trace elements and REE on kaolinite rocks in Late-Palaeozoic measures, North China. Geochimica 1998, 27, 196–202. (In Chinese) [Google Scholar]
- Painter, P.C.; Snyder, R.W.; Youtcheff, J.; Given, P.H.; Gong, H.; Suhr, N. Analysis of kaolinite in coal by infrared spectroscopy. Fuel 1980, 59, 364–366. [Google Scholar]
- Ufer, K.; Kleeberg, R.; Monecke, T. Quantification of stacking disordered Si–Al layer silicates by the Rietveld method: Application to exploration for high-sulphidation epithermal gold deposits. Powder Diffr. 2015, 30, S111–S118. [Google Scholar] [CrossRef]
- Kogure, T.; Inoue, A. Determination of defect structures in kaolin minerals by high-resolution transmission electron microscopy (HRTEM). Am. Mineral. 2005, 90, 85–89. [Google Scholar] [CrossRef]
- Liu, Q.F.; Xu, H.L.; Zhang, P.F. Crystallinity difference for various origin of kaolinites in coal measures. J. China Coal Soc. 2000, 25, 576–580, (In Chinese with English abstract). [Google Scholar]
- Yang, X.J.; Liu, D.M.; Chu, L.K.; Chen, K.H. Mineralization mechanism of kaolinite rocks in coal measures. J. China Coal Soc. 2006, 31, 85–89, (In Chinese with English abstract). [Google Scholar]
- Kogure, T.; Elzea-Kogel, J.; Johnston, C.T.; Bish, D.L. Stacking disorder in a sedimentary kaolinite. Clays Clay Miner. 2010, 58, 62–71. [Google Scholar] [CrossRef]
- Chen, R.X.; Dong, X.S.; Fan, Y.P.; Ma, X.M.; Dong, Y.D.; Chang, M. Interaction between STAC and coal/kaolinite in tailing dewatering: An experimental and molecular-simulation study. Fuel 2020, 279, 118224. [Google Scholar] [CrossRef]
- Cheng, H.F.; Zhang, S.; Liu, Q.F.; Li, X.G.; Frost, R.L. The molecular structure of kaolinite-potassium acetate intercalation complexes: A combined experimental and molecular dynamic simulation study. Appl. Clay Sci. 2015, 116–117, 273–280. [Google Scholar] [CrossRef]
- Ma, X.M.; Fan, Y.P.; Dong, X.S.; Chen, R.X.; Li, H.L.; Sun, D.; Yao, S.L. Impact of clay minerals on the dewatering of coal slurry: An experimental and molecular-simulation study. Minerals 2018, 8, 400. [Google Scholar] [CrossRef]
- Artioli, G.; Belloto, M.; Gualtieri, A.; Pavese, A. Nature of stacking disorder in natural kaolinites: A new model based on computer simulation of powder diffraction data and electrostatic energy calculations. Clays Clay Miner. 1995, 43, 438–445. [Google Scholar] [CrossRef]
- Zhang, X.L.; Song, B.X.; Ren, D.W. The Study of Carbon on Kaolin Tonstein. China Non-Met. Min. Ind. Her. 2009, 6, 27–30, (In Chinese with English abstract). [Google Scholar]
- Liu, C.L.; Liu, Q.F. Advance on the relation of orders of kaolinite crystallization in coal series to the origin. Contrib. Geol. Miner. Resour. Res. 2002, 17, 73–81, (In Chinese with English abstract). [Google Scholar]
- Ma, J.; Xiao, L.; Zhang, K.; Jiao, Y.; Wang, Z.; Li, J.; Guo, W.; Gao, P.; Qin, S.; Zhao, C. Geochemistry of Carboniferous-Permian Coal from the Wujiawan mine, Datong coalfield, Northern China: Modes of occurrence, origin of valuable trace elements, and potential industrial utilization. Minerals 2020, 10, 776. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, D.N.; Zhou, A.C.; Zou, Y. Provenance analyses of early Mesozoic sediments in the Ningwu basin: Implications for the tectonic–palaeogeographic evolution of the north-central North China Craton. Int. Geol. Rev. 2019, 61, 86–108. [Google Scholar] [CrossRef]
- Li, J.C.; Wang, Z.; Shen, Y.K. Characters and origin of Kaolin mine in the coal field of Datong, Shanxi. J. Guilin Univ. Technol. 2005, 25, 146–151, (In Chinese with English abstract). [Google Scholar]
- Zhu, R. A Study on the defect texture of kaolinite. Acta Mineral. Sin. 1996, 16, 245–252. (In Chinese) [Google Scholar]
- Zhang, Z.; Lu, G.; Chen, Y.; Zhang, T.; Liu, Y. Alumina extraction from kaolinite via calcification-carbonation process. Russ. J. Non-Ferr. Met. 2020, 61, 248–256. [Google Scholar] [CrossRef]
- Zhong, H.R.; Sun, Y.; Yang, Y.Q.; Wang, D.H.; Huang, F.; Zhao, Z. Bauxite (aluminum)—type lithium resources and analysis of its development and utilization potential. Miner. Depos. 2019, 38, 898–916, (In Chinese with English abstract). [Google Scholar]
- Li, Z.H.; Dong, S.W.; Qu, H.J. Timing of the initiation of the Jurassic Yanshan movement on the North China Craton: Evidence from sedimentary cycles, heavy minerals, geochemistry, and zircon U-Pb geochronology. Int. Geol. Rev. 2014, 56, 288–312. [Google Scholar] [CrossRef]
- Shao, J.A.; Zhang, Y.B.; Zhang, L.Q.; Mu, B.L.; Wang, P.Y.; Guo, F. Early Mesozoic dike swarms of carbonatites and lamprophyres in Datong area. Acta Petrol. Sin. 2003, 19, 93–104. [Google Scholar]
- Liu, D.N.; Zhou, A.C.; Chang, Z.G. Geochemistry characteristics of major and rare earth elements in No. 8 raw and weathered coal from Taiyuan Formation of Datong Coalfield. J. China Coal Soc. 2015, 40, 422–430, (In Chinese with English Abstract). [Google Scholar]
- Li, Z.H.; Qu, H.J.; Gong, W.B. Late Mesozoic basin development and tectonic setting of the northern North China Craton. J. Asian Earth Sci. 2015, 114, 115–139. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, D.; Zhou, A.; Zou, Y.; Xie, J. A synthesis of late Paleozoic and early Mesozoic sedimentary provenances and constraints on the tectonic evolution of the northern North China Craton. J. Asian Earth Sci. 2019, 185, 104029. [Google Scholar] [CrossRef]
- Liu, S.; Feng, C.X.; Hu, R.Z.; Lai, S.C.; Yang, Y.H. Geochemical, Sr-Nd isotope, and zircon U-Pb geochronological constraints on the origin of Early Cretaceous carbonatite dykes, northern Shanxi Province, China. Acta Petrol. Sin. 2014, 30, 350–360. [Google Scholar]
- Guo, W.Z.; Cheng, Y.H.; Gao, Y.P.; Chen, Z.G.; Zhang, Z.W.; Wang, C. Structural characteristics and coal-bearing boundaries of Taiyuan Formation in Datong coalfield. Coal Geol. Explor. 2015, 43, 1–7. [Google Scholar]
- Hinckley, D.N. Variability in “crystallinity” values among the kaolin deposits of the coastal plain of Georgia and south Carolina. Clays Clay Miner. 1963, 11, 229–235. [Google Scholar] [CrossRef]
- Balan, E.; Saitta, A.M.; Mauri, F.; Calas, G. First-principles modeling of the infrared spectrum of kaolinite. Am. Mineral. 2001, 86, 1321–1330. [Google Scholar] [CrossRef]
- Sakharov, B.A.; Drits, V.A.; Mccarty, D.K.; Walker, G.M. Modeling powder X-ray diffraction patterns of the clay minerals society kaolinite standards: Kga-1, kga-1b, and kga-2. Clays Clay Miner. 2016, 64, 312–333. [Google Scholar] [CrossRef]
- Olaseinde, A.; Shongwe, M.B.; Babalola, J.; Adisa, A.L. Instrumental characterization of pretoria clay soil by XRF, XRD and SEM. J. Miner. Mater. Charact. Eng. 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Mackenzie, R.C.; Rahman, A.A. Interaction of kaolinite with calcite on heating. I. instrumental and procedural factors for one kaolinite in air and nitrogen. Thermochim. Acta 1987, 121, 51–69. [Google Scholar] [CrossRef]
- Cheng, H.F.; Liu, Q.F.; Cui, X.N.; Zhang, Q.; Zhang, Z.L.; Frost, R.L. Mechanism of dehydroxylation temperature decrease and high temperature phase transition of coal-bearing strata kaolinite intercalated by potassium acetate. J. Colloid Interface Sci. 2012, 376, 47–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Drits, V.A.; Zviagina, B.B.; Sakharov, B.A.; Dorzhieva, O.V.; Savichev, A.T. New insight into the relationships between structural and FTIR spectroscopic features of kaolinites. Clays Clay Miner. 2021, 69, 366–388. [Google Scholar] [CrossRef]
- Iflikli, M. Hydrothermal alteration-related kaolinite/dickite occurrences in ignimbrites: An example from miocene ignimbrite units in avanos, central turkey. Arab. J. Geosci. 2020, 13, 1–18. [Google Scholar]
- Zhang, A.; Kang, L.; Zhang, Y.; Ding, D.; Zhang, Y. Thermal behaviors and kinetic analysis of two natural kaolinite samples selected from Qingshuihe region in Inner Mongolia in china. J. Therm. Anal. Calorim. 2021, 145, 3281–3291. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, T.; Liu, J.; Liu, Q.; Kuo, L.I.; Liu, D. Genesis of kaolinite deposits in Jungar coalfield, North China: Petrological, mineralogical and geochemical evidence. Acta Geol. Sin. Engl. Ed. 2021, 95, 517–530. [Google Scholar] [CrossRef]
- Dominguez, E.; Iglesias, C.; Dondi, M. The geology and mineralogy of a range of kaolins from the Santa Cruz and Chubut Provinces, Patagonia (Argentina). Appl. Clay Sci. 2008, 1, 124–142. [Google Scholar] [CrossRef]
- Zhang, J.S.; Jin, C.; Xing, L.C.; He, H.T.; Zhao, Y.Y.; Xin, Y.M.; Xu, Y.; Zhao, C.L.; Sun, P.F. Mineralogy and geochemistry of the coal seam of Shanxi Formation in Guotun mine, Juye coalfield, North China. Energy Explor. Exploit. 2019, 37, 1779–1803. [Google Scholar] [CrossRef]
- Kadir, S.; Karakas, Z. Mineralogy, chemistry and origin of halloysite, kaolinite and smectite from Miocene ignimbrites, Konya, Turkey. Neues Jahrb. Für Mineral.-Abh. 2002, 177, 113–132. [Google Scholar] [CrossRef]
- Ruiz-Cruz, M.D.; Reyes, E. Kaolinite and dickite formation during shale diagenesis: Isotopic data. Appl. Geochem. 1998, 13, 95–104. [Google Scholar] [CrossRef]
- Santos, A.; Rossetti, D. Origin of the Rio Capim Kaolin based on optical (petrographic and SEM) data. J. S. Am. Earth Sci. 2008, 26, 329–341. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Young, G.M. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Wang, P.; Du, Y.; Yu, W. The chemical index of alteration (CIA) as a proxy for climate change during glacial-interglacial transitions in Earth history. Earth-Sci. Rev. 2020, 201, 103032. [Google Scholar] [CrossRef]
- Huang, J.; Feng, L.; Lu, D.; Zhang, Q.; Sun, T.; Chu, X. Multiple climate cooling prior to Sturtian glaciations: Evidence from chemical index of alteration of sediments in South China. Sci. Rep. 2014, 4, 6868. [Google Scholar] [CrossRef]
- Feng, L.J.; Chu, X.L.; Zhang, Q.Y. Chemical index of alteration (CIA) and its applications in the Neoproterozoic clastic rocks. Earth Sci. Front. 2003, 10, 539–544, (In Chinese with English abstract). [Google Scholar]
- Goldberg, K.; Humayun, M. The applicability of the Chemical Index of Alteration as a paleoclimatic indicator: An example from the Permian of the Paraná basin, Brazil. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 293, 175–183. [Google Scholar] [CrossRef]
- Zhang, A.; Kang, L.L.; Zhang, Y.M.; Ding, D.Q.; Zhang, Y.F. Study on the influence of kaolinite particle size on its crystal structure and thermal evolution behavior. Bull. Chin. Ceram. Soc. 2019, 38, 3964–3971, (In Chinese with English abstract). [Google Scholar]
- Lu, Y.P.; Li, J.R.; Liu, Q.F. Effect of grain size and crystallinity of kaolinite from coal on kaolinite intercalation. Acta Mineral. Sin. 2015, 35, 209–213. [Google Scholar]
- He, H.P. Study on the Interaction between Clay Minerals and Metalions; Petroleum Industry Press: Beijing, China, 2001. (In Chinese) [Google Scholar]
- Zhang, H.F.; Gao, S. Geochemistry; Geological Publishing House: Beijing, China, 2012. (In Chinese) [Google Scholar]
- Oggiano, G.; Mameli, P. Tectonic and litho-stratigraphic controls on kaolin deposits within volcanic successions: Insights from the kaoliniferous district of north-western Sardinia (Italy). Ore Geol. Rev. 2012, 48, 151–164. [Google Scholar] [CrossRef]
- Aparicio, P.; Galan, E. Mineralogical interference on kaolinite cry stallinity index measurements. Clays Clay Miner. 1999, 47, 12–27. [Google Scholar] [CrossRef]
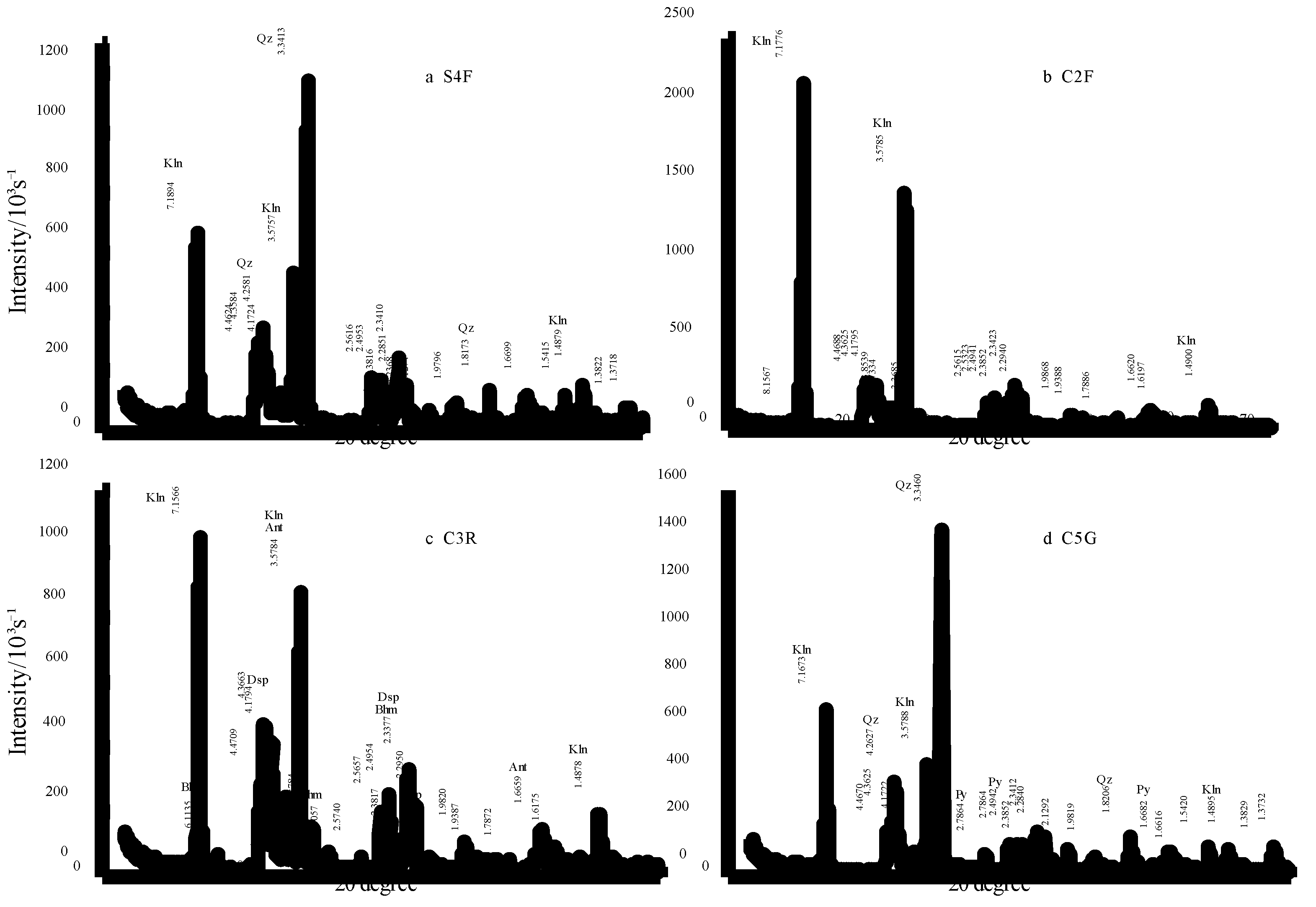
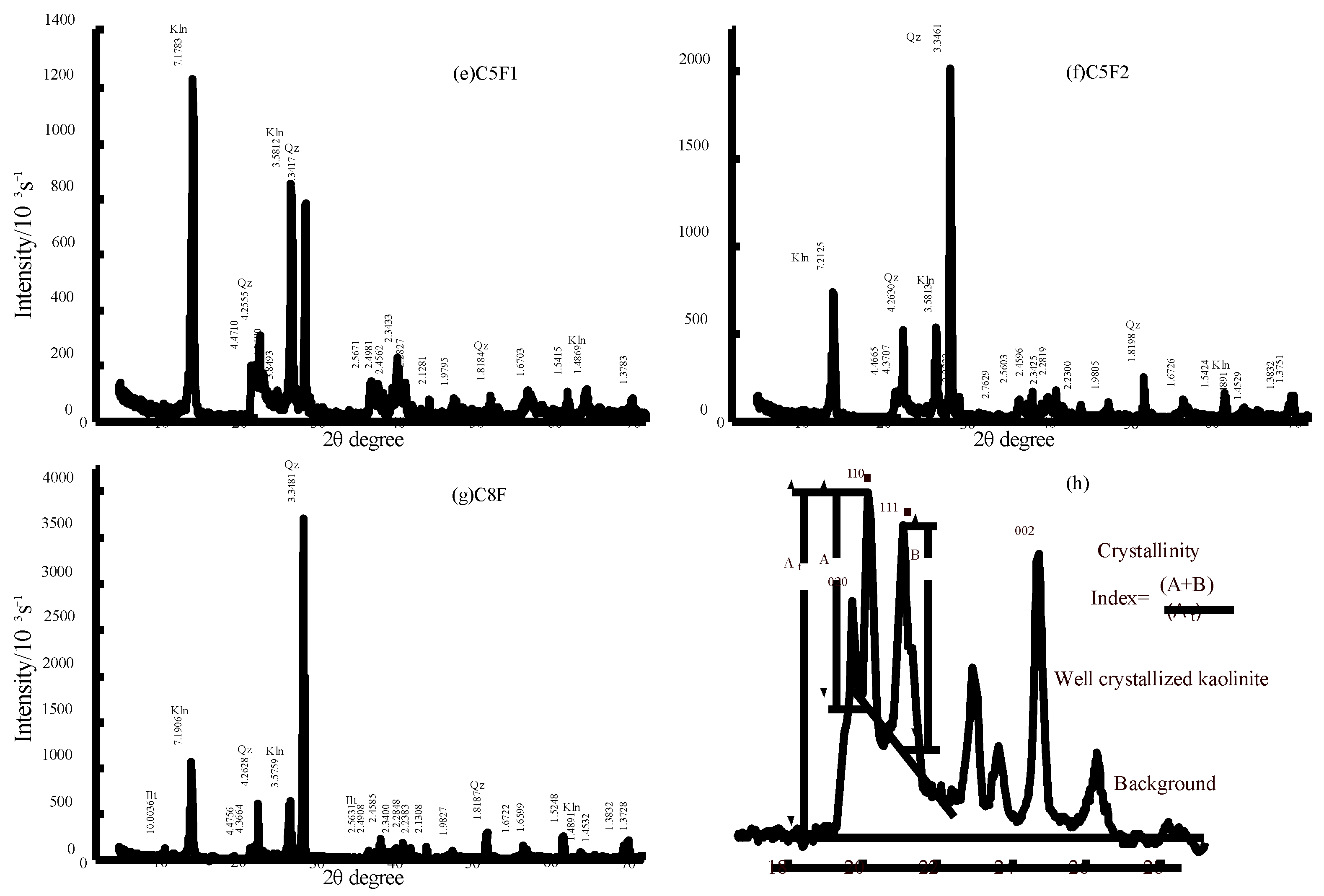
| Samples | Lithology | Thin Section | HI | Minerals |
|---|---|---|---|---|
| S4F | Compact kaolinite rock | 4 | 1.12 | Kaolinite, quartz |
| C2F | Phaneritic kaolinite rock | 3 | 1.13 | Kaolinite |
| C3R | Clastic kaolinite rock | 5 | 1.29 | Kaolinite, diaspore, boehmite, anatase |
| C5G | Clastic kaolinite rock | 3 | 0.81 | Kaolinite, quartz, pyrite |
| C5F1 | Sand-bearing kaolinite rock | 4 | 0.82 | Kaolinite, quartz |
| C5F2 | Sandy kaolinite rock | 6 | 0.68 | Kaolinite, quartz |
| C8F | Silty kaolinite rock | 7 | 0.65 | Kaolinite, quartz, illite |
| Sample | Dehydroxy Temperature (°C) | Heat Absorption Valley Peak Value (°C) |
|---|---|---|
| S4F | 520–590 | 552.6 |
| C2F | 516–650 | 561.7 |
| C3R | 510–600 | 557.3 |
| C5G | 510–560 | 544.7 |
| C5F1 | 510–600 | 546.4 |
| C5F2 | 510–560 | 535.8 |
| C8F | 516–580 | 549.4 |
| Sample | υOH and H2O Stretching Vibration | H2O Bending Vibration | Si–O Stretching Vibration | Si–O Bending Vibration |
|---|---|---|---|---|
| Theoretical wave number | 3603 | - | 1100 | 468 |
| S4F | 3620 | 1656 | 1101 | 469 |
| C2F | 3618 | 1693 | 1101 | 471 |
| C3R | 3620 | 1629 | 1103 | 473 |
| C5G | 3618 | 1631 | 1095 | 465 |
| C5F1 | 3618 | 1623 | 1101 | 469 |
| C5F2 | 3620 | 1629 | 1093 | 465 |
| C8F | 3620 | 1638 | 1092 | 464 |
| Sample | SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | K2O | MnO | TiO2 | P2O5 | FeO | CIA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S4F | 52.97 | 31.49 | 0.56 | 0.115 | 0.054 | 0.645 | 0.015 | 0.460 | 0.032 | 0.38 | 2.85 | 97.48 |
| C2F | 44.97 | 38.48 | 0.16 | 0.079 | 0.025 | 0.080 | 0.005 | 0.386 | 0.016 | 0.12 | 1.99 | 99.52 |
| C3R | 42.63 | 38.87 | 0.47 | 0.099 | 0.086 | 0.202 | 0.002 | 1.270 | 0.049 | 0.30 | 1.90 | 99.01 |
| C5G | 52.17 | 30.76 | 2.28 | 0.115 | 0.060 | 0.636 | 0.037 | 0.849 | 0.029 | 1.25 | 2.88 | 95.80 |
| C5F1 | 61.48 | 24.03 | 1.51 | 0.098 | 0.103 | 0.972 | 0.026 | 0.923 | 0.033 | 0.78 | 4.35 | 97.31 |
| C5F2 | 57.17 | 25.92 | 2.73 | 0.265 | 0.088 | 0.783 | 0.014 | 0.488 | 0.024 | 2.10 | 3.75 | 95.35 |
| C8F | 67.97 | 20.15 | 0.86 | 0.122 | 0.015 | 0.720 | 0.015 | 1.170 | 0.029 | 0.70 | 5.73 | 95.92 |
| Kaolinite Crystallinity | Kaolinite Content | SiO2/Al2O3 | CIA (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Type | XRD | DTA | IR | ||||||
| Ordered | C3R C2F S4F | C3R C2F S4F | C3R C2F S4F | C2F C3R S4F | >95% | C2F C3R | 1.90–1.99 | C2F C3R | 99.01–99.5 |
| Slightly disordered | C5F1 C5G | C5G C5F1 C8F | C5F1 | C5G C5F1 | 80–90% | S4F C5G | 2.85–2.88 | S4F C5F1 | 97.31–97.48 |
| Disordered | C5F2 C8F | C5F2 | C5G C5F2 C8F | C5F2 C8F | 60–70% | C5F1 C5F2 C8F | 3.75–5.73 | C5G C5F2 C8F | 95.35–95.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Zhang, Y.; Zhou, A.; Nnachi, E.N.; Huo, S.; Zhang, Q. The Kaolinite Crystallinity and Influence Factors of Coal-Measure Kaolinite Rock from Datong Coalfield, China. Minerals 2022, 12, 54. https://doi.org/10.3390/min12010054
Liu D, Zhang Y, Zhou A, Nnachi EN, Huo S, Zhang Q. The Kaolinite Crystallinity and Influence Factors of Coal-Measure Kaolinite Rock from Datong Coalfield, China. Minerals. 2022; 12(1):54. https://doi.org/10.3390/min12010054
Chicago/Turabian StyleLiu, Dongna, Yun Zhang, Anchao Zhou, Emmanuel N. Nnachi, Shuting Huo, and Qi Zhang. 2022. "The Kaolinite Crystallinity and Influence Factors of Coal-Measure Kaolinite Rock from Datong Coalfield, China" Minerals 12, no. 1: 54. https://doi.org/10.3390/min12010054
APA StyleLiu, D., Zhang, Y., Zhou, A., Nnachi, E. N., Huo, S., & Zhang, Q. (2022). The Kaolinite Crystallinity and Influence Factors of Coal-Measure Kaolinite Rock from Datong Coalfield, China. Minerals, 12(1), 54. https://doi.org/10.3390/min12010054







