Acid Mine Drainage Sources and Impact on Groundwater at the Osarizawa Mine, Japan
Abstract
1. Introduction
2. Materials and Methods
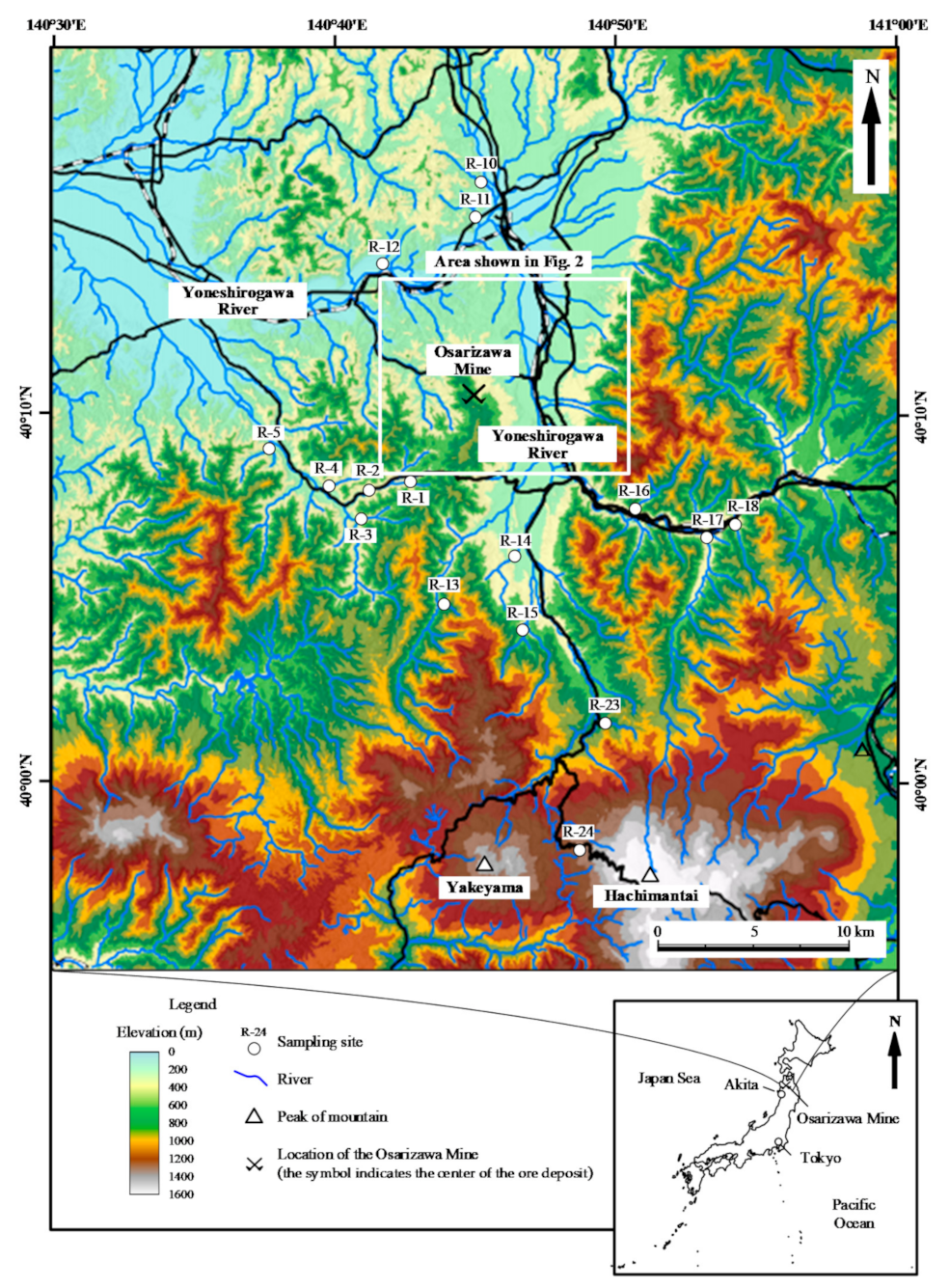
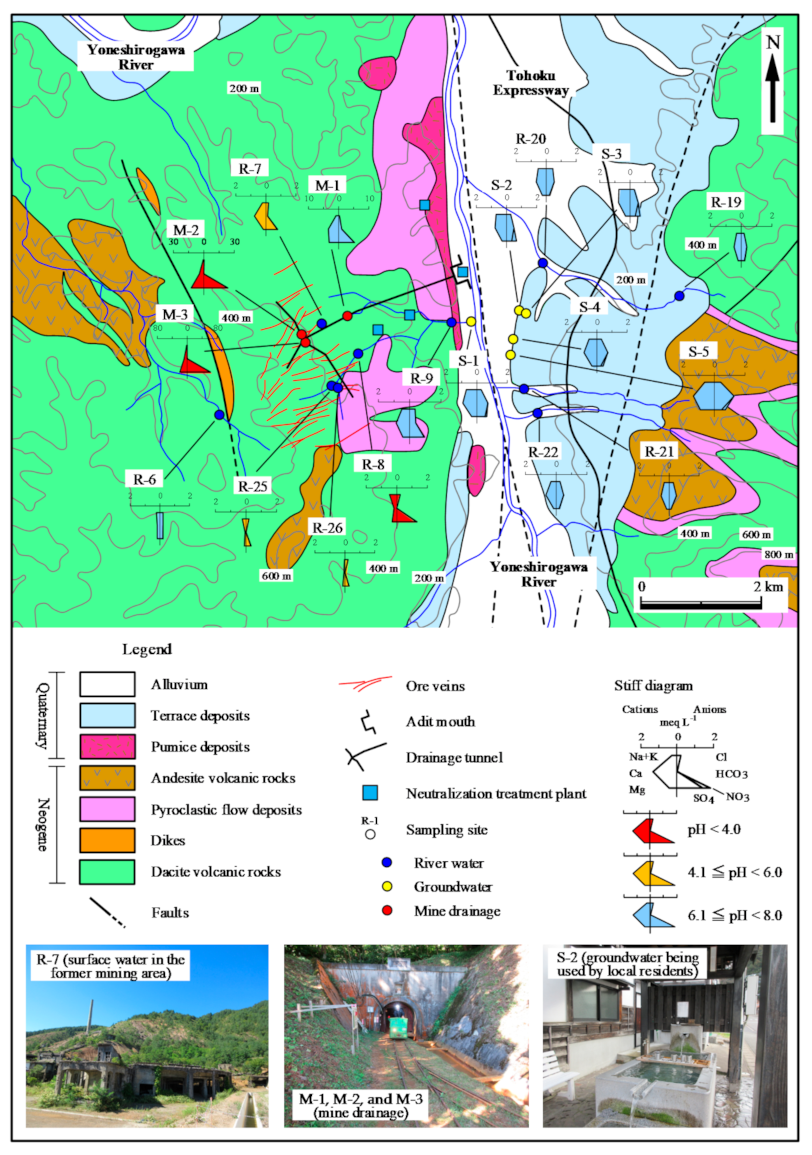
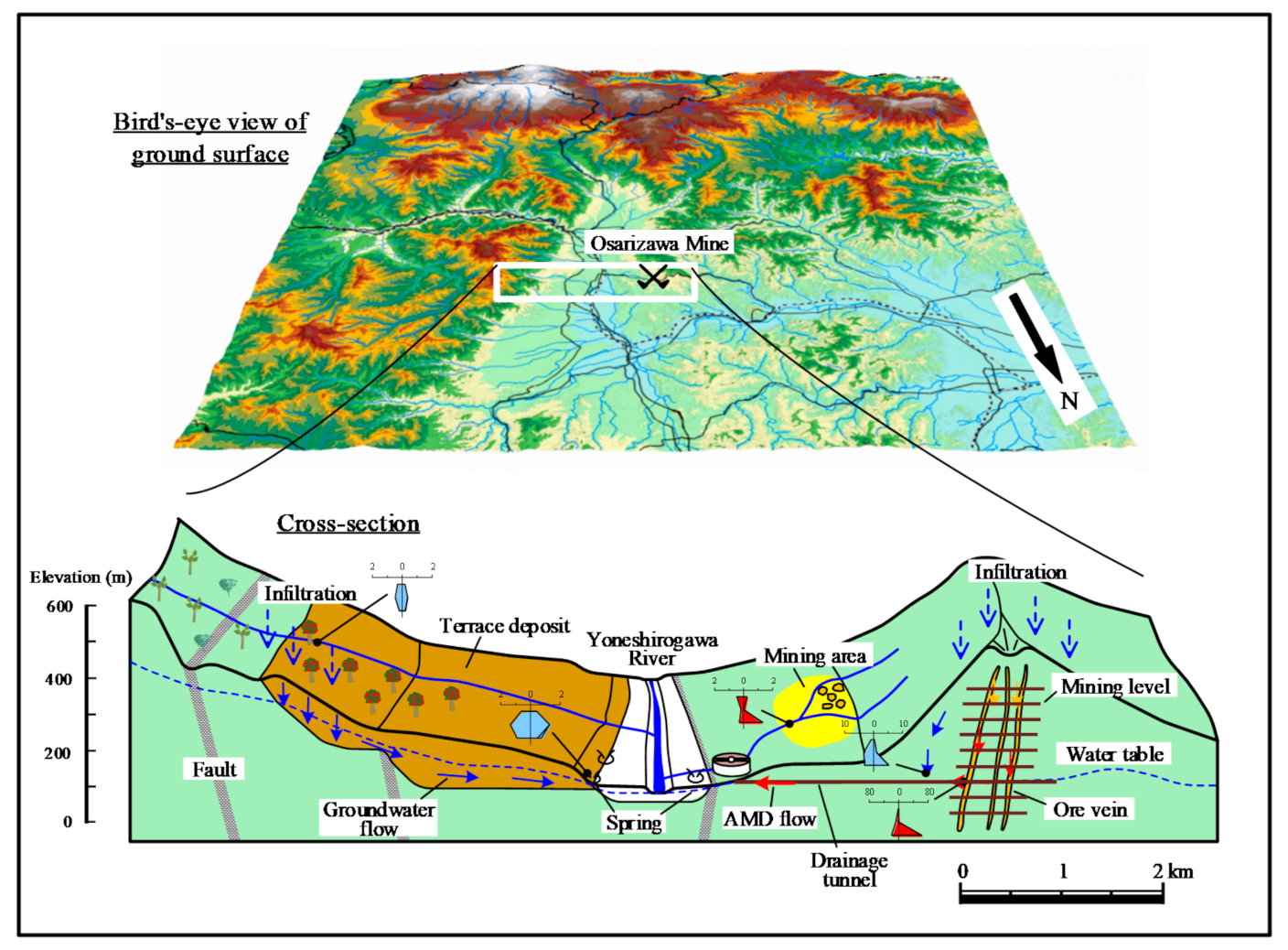
2.1. Geology and History of the Osarizawa Mine
2.2. Water Sample Collection and Analysis
2.3. Principal Component Analysis
3. Results and Discussion
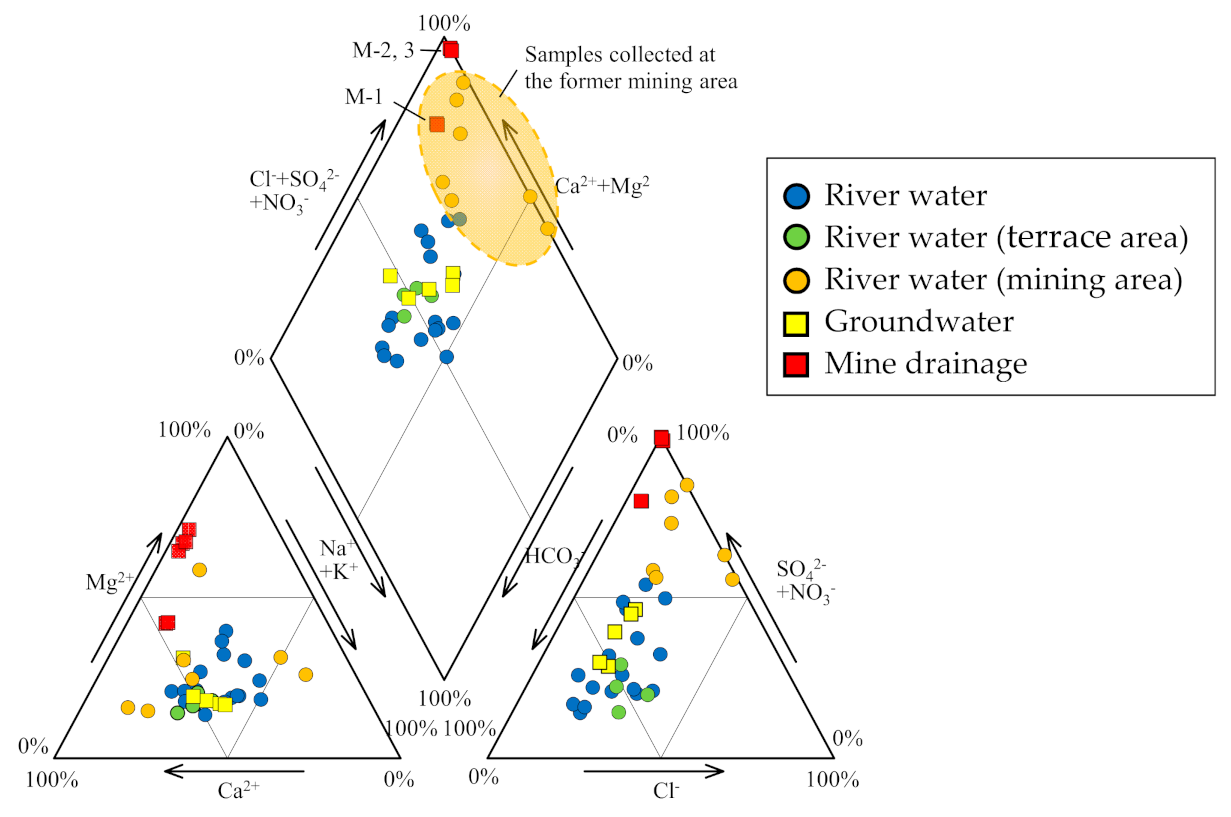
3.1. Geochemistry of Water Samples
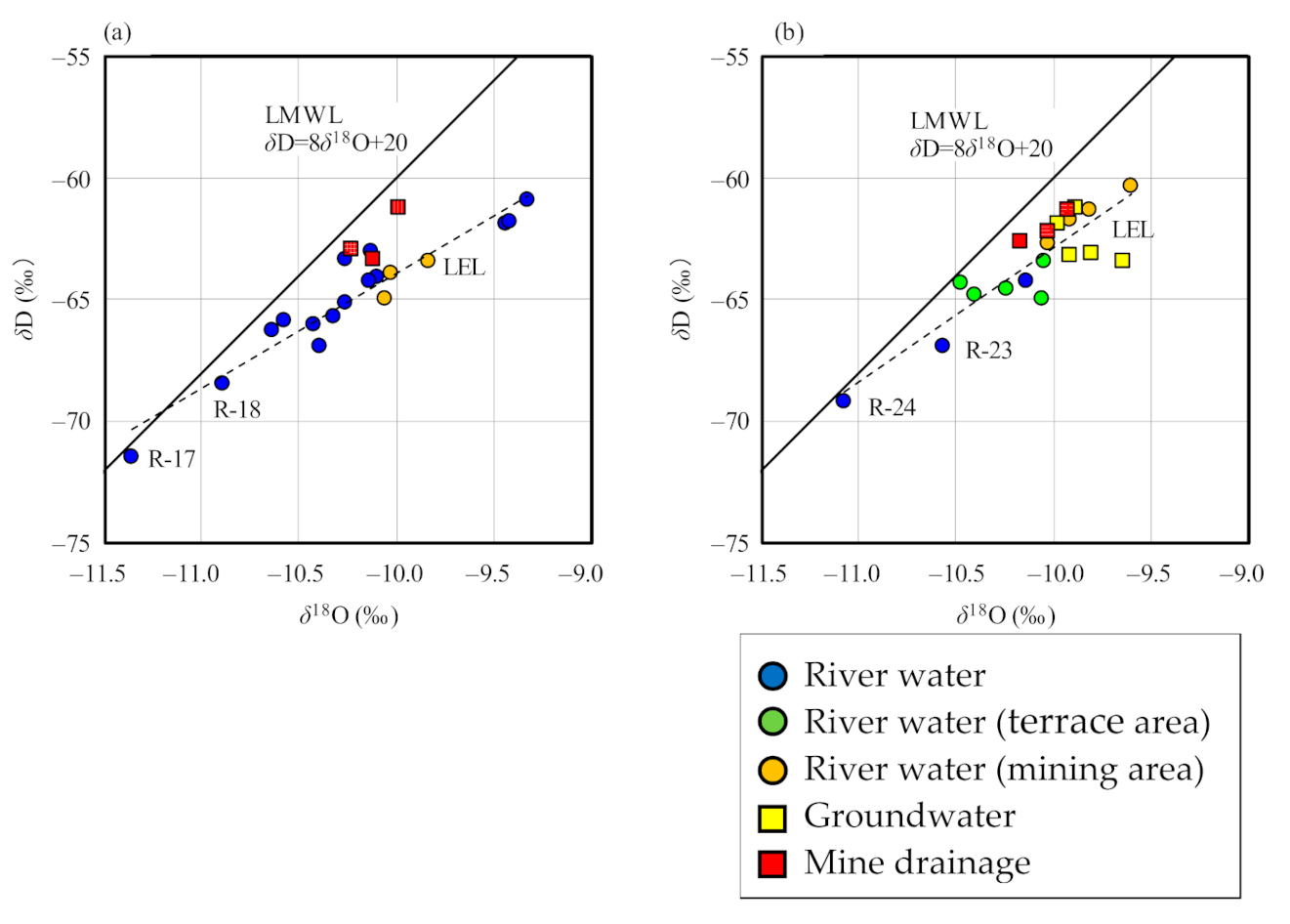
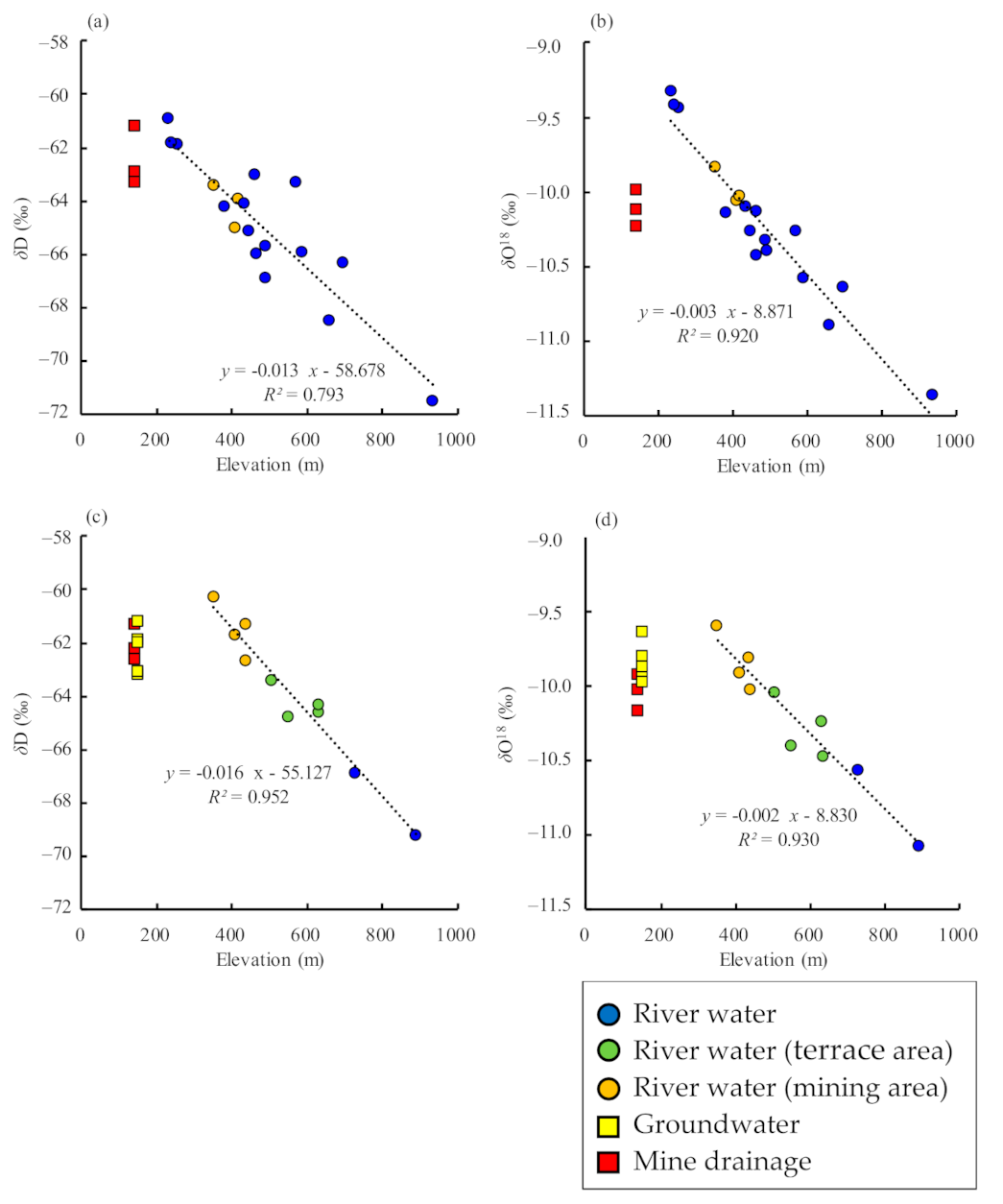
3.2. H–O Isotopic Ratios
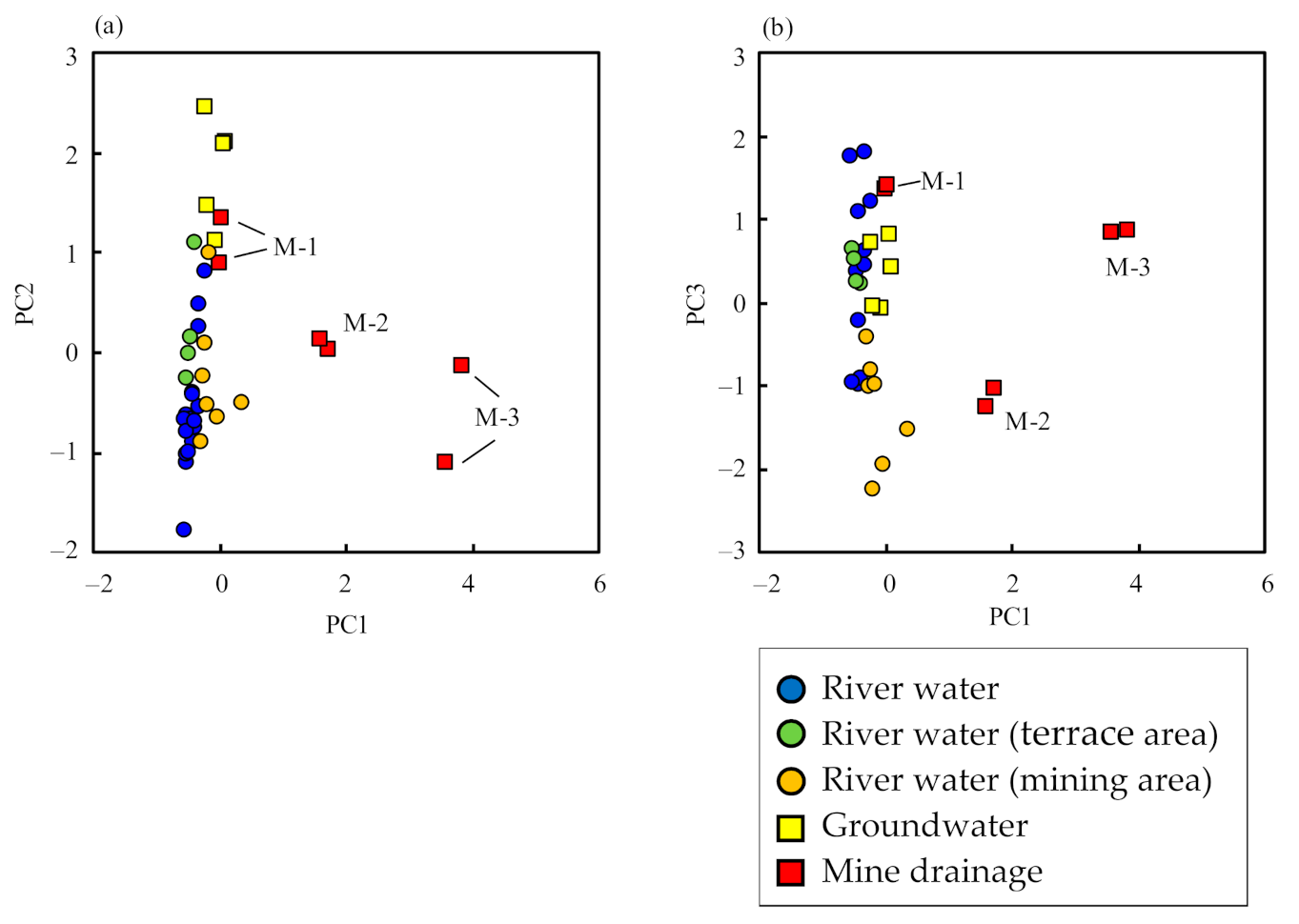
3.3. Principal Component Analysis
3.4. Groundwater Flow and Discharge
4. Conclusions
- (1)
- AMD is formed through interactions between groundwater and sulfide minerals in excavated areas.
- (2)
- The groundwater recharge area for AMD formation is located on the mountain slope at 400–500 m a.s.l.
- (3)
- Groundwater infiltrating from the terrace and mountain surface at 350–450 m a.s.l. reacts with felspars in terrace deposits and is discharged as spring water.
- (4)
- Based on a conceptual model of discharge and groundwater flow at the mine area, the area affected by AMD is limited to near the mine area, with no effect on surrounding groundwater.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simate, G.S.; Ndlovu, S. Acid mine drainage: Challenges and opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Anawar, H.M. Sustainable rehabilitation of mining waste and acid mine drainage using geochemistry, mine type, mineralogy, texture, ore extraction and climate knowledge. J. Environ. Manag. 2015, 158, 111–121. [Google Scholar] [CrossRef]
- Kefeni, K.; Msagati, T.; Mamba, B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Vriens, B.; Plante, B.; Seigneur, N.; Jamieson, H. Review Mine Waste Rock: Insights for Sustainable Hydrogeochemical Management. Minerals 2020, 10, 728. [Google Scholar] [CrossRef]
- JOGMEC (Japan Oil, Gas and Metals National Corporation). Mine Pollution Control. Available online: http://www.jogmec.go.jp/english/mp_control/index.html (accessed on 11 May 2021).
- Ministry of Economy, Trade and Industry. Safety of Mines. Available online: https://www.meti.go.jp/english/policy/safety_security/industrial_safety/index.html#mines (accessed on 11 May 2021).
- Kondo, S. On fissures and percolation of water in the rock formation. Bull. Geol. Suev. Japan 1958, 9, 67–76. Available online: https://www.gsj.jp/data/bull-gsj/09-02_01.pdf (accessed on 11 May 2021). (In Japanese, with English abstract).
- Demura, O. Practice of mine water at the Yanahara Mine. J. MMIJ 1961, 77, 312–318, (In Japanese, with English abstract). [Google Scholar] [CrossRef][Green Version]
- Imai, H.; Takenouchi, S. Some hot springs flowing out from the gold-Quartz veins in Japan. J. MMIJ 1961, 77, 305–311, (In Japanese, with English abstract). [Google Scholar] [CrossRef][Green Version]
- Ito, J.; Zimpo, K.; Makiuchi, S.; Fuchimoto, H. Underground water of the Makimine Main. J. MMIJ 1961, 77, 327–331, (In Japanese, with English abstract). [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef]
- Tokoro, C.; Fukaki, K.; Kadokura, M.; Fuchida, S. Forecast of AMD quantity by a series tank model in three stages: Case studies in two closed Japanese mines. Minerals 2020, 10, 430. [Google Scholar] [CrossRef]
- Iwasaki, K.; Fukaya, K.; Fuchida, S.; Matsumoto, S.; Araoka, D.; Tokoro, C.; Yasutaka, T. Projecting future changes in element concentrations of approximately100 untreated discharges from legacy mines in Japan by a hierarchicallog-linear model. Sci. Total Environ. 2021, 786, 147500. [Google Scholar] [CrossRef]
- Khoeurn, K.; Sasaki, A.; Tomiyama, S.; Igarashi, T. Distribution of zinc, copper, and iron in the tailings dam of an abandoned mine in Shimokawa, Hokkaido, Japan. Mine Water Environ. 2019, 38, 119–129. [Google Scholar] [CrossRef]
- Khoeurn, K.; Sakaguchi, A.; Tomiyama, S.; Igarashi, T. Long-term acid generation and heavy metal leaching from the tailings of Shimokawa mine, Hokkaido, Japan: Column study under natural condition. J. Geochem. Explor. 2019, 201, 1–12. [Google Scholar] [CrossRef]
- Sasaki, K.; Nakamuta, Y.; Hirajima, T.; Tuovinen, O.H. Raman characterization of secondary minerals formed during chalcopyrite leaching with Acidithiobacillus ferrooxidans. Hydrometallurgy 2009, 95, 153–158. [Google Scholar] [CrossRef]
- Tokoro, C.; Yatsugi, Y.; Sasaki, H.; Owada, S. A quantitative modeling of co-precipitation phenomena in wastewater containing dilute anions with ferrihydrite using a surface complexation model. Resour. Process. 2008, 55, 3–8. [Google Scholar] [CrossRef][Green Version]
- Tokoro, C.; Yatsugi, Y.; Koga, H.; Owada, S. Sorption mechanisms of arsenate during coprecipitation with ferrihydrite in aqueous solution. Environ. Sci. Technol. 2010, 44, 638–643. [Google Scholar] [CrossRef]
- Tokoro, C. Removal mechanism in anionic co-precipitation with hydroxides in acid mine drainage treatment. Resour. Process. 2015, 62, 3–9. [Google Scholar] [CrossRef][Green Version]
- Villafane, O.R.S.; Igarashi, T.; Kuroswa, M.; Takase, T. Comparison of potentially toxic metals leaching from weathered rocks at a closed mine site between laboratory columns and field observation. Appl. Geochem. 2012, 27, 2271–2279. [Google Scholar] [CrossRef]
- Villafane, O.R.S.; Igarashi, T.; Kuroswa, M.; Takase, T. Porewater Monitoring Under Different Layer Systems on a Sloping Surface at a Closed Mine Sit. Water Air Soil Pollut. 2013, 224, 1480. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Tomiyama, S.; Metugi, H.; Ii, H.; Ueda, A. Flow and geochemical modeling of drainage from Tomitaka mine, Miyazaki, Japan. J. Environ. Sci. 2015, 36, 130–143. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Tomiyama, S.; Igarashi, T.; Yamagata, S.; Ebato, M.; Sakoda, M. Effects of backfilling excavated underground space on reducing acid mine drainage in an abandoned mine. Minerals 2020, 10, 777. [Google Scholar] [CrossRef]
- Jiang, C.; Gao, X.; Hou, B.; Zhang, S.; Zhang, J.; Li, C.; Wang, W. Occurrence and Environmental Impact of Coal Mine Goaf Water in Karst Areas in China. J. Clean. Prod. 2020, 275, 123813. [Google Scholar] [CrossRef]
- Ren, K.; Zeng, J.; Liang, J.; Yuan, D.; Jiao, Y.; Peng, C.; Pan, X. Impacts of acid mine drainage on karst aquifers: Evidence from hydrogeochemistry, stable sulfur and oxygen isotopes. Sci. Total Environ. 2020, 10, 143223. [Google Scholar] [CrossRef] [PubMed]
- Runkel, R.L.; Kimball, B.A.; Nimick, D.A.; Walton-Day, K. Effects of flow regime on metal concentrations and the attainment of water quality standards in a remediated stream reach, Butte, Montana. Environ. Sci. Technol. 2016, 50, 12641–12649. [Google Scholar] [CrossRef]
- Runkel, R.L.; Verplanck, P.L.; Kimball, B.A.; Walton-Day, K. Cinnamon Gulch revisited: Another look at separating natural and mining-impacted contributions to instream metal load. Appl. Geochem. 2018, 95, 206–217. [Google Scholar] [CrossRef]
- Skierszkan, E.K.; Mayer, K.U.; Weis, D.; Beckie, R.D. Molybdenum and zinc stable isotope variation in mining waste rock drainage and waste rock at the Antamina mine, Peru. Sci. Total Environ. 2016, 550, 103–113. [Google Scholar] [CrossRef]
- Salifu, M.; Hallstrom, L.; Aiglsperger, T.; Alakangas, L. A simple model for evaluating isotopic (18O, 2H and 87Sr/86Sr) mixing calculations of mine—impacted surface waters. J. Contam. Hydrol. 2020, 232, 103640. [Google Scholar] [CrossRef]
- Lahmira, B.; Lefebvre, R.; Michel Aubertin, M.; Bussière, B. Effect of heterogeneity and anisotropy related to the construction method on transfer processes in waste rock piles. J. Contam. Hydrol. 2016, 184, 35–49. [Google Scholar] [CrossRef]
- Ethier, M.P.; Bussière, B.; Broda, S.; Aubertin, M. Three-dimensional hydrogeological modeling to assess the elevated-water-table technique for controlling acid generation from an abandoned tailings site in Quebec, Canada. Hydrogeol. J. 2018, 26, 1201–1219. [Google Scholar] [CrossRef]
- Gongzalez-Quiros, A.; Fernandez-Alvarez, J.P. Conceptualization and finite element groundwater flow modeling of a flooded underground mine reservoir in the Asturian Coal Basin, Spain. J. Hydrol. 2019, 578, 124036. [Google Scholar] [CrossRef]
- Tomiyama, S.; Igarashi, T.; Tabelin, C.B.; Tangviroon, P.; Ii, H. Modeling of the groundwater flow system in excavated areas of an abandoned mine. J. Contam. Hydrol. 2020, 230, 103617. [Google Scholar] [CrossRef]
- Tomiyama, S.; Igarashi, T.; Tabelin, C.B.; Tangviroon, P.; Ii, H. Acid mine drainage sources and hydrogeochemistry at the Yatani mine, Yamagata, Japan: A geochemical and isotopic study. J. Contam. Hydrol. 2020, 225, 103502. [Google Scholar] [CrossRef]
- Shimizu, H.; Matsunaga, E. Observations on the Tanosawa Veins at the Osarizawa Mine. J. MMIJ 1964, 14, 126–133, (In Japanese, with English abstract). [Google Scholar] [CrossRef]
- Takahashi, H.; Yasuda, K. Osarizawa Mine. J. MMIJ 1967, 83, 1640–1646. (In Japanese) [Google Scholar] [CrossRef]
- Saito, S. The Changes of a Community with the Decline of its Mining Industry–A Case of the Osarizawa Mining Industry Ltd. Ann. Tohoku Geogr. Assoc. 1979, 31, 1–7, (In Japanese, with English abstract). [Google Scholar] [CrossRef][Green Version]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdisciplinary Reviews. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Barrio-Parra, F.; Izquierdo-Díaz, M.; Fernández-Gutiérrez del Álamo, L.J.; Biosca, B.; De Miguel, E. Modelling the Transference of Trace Elements between Environmental Compartments in Abandoned Mining Areas. Int. J. Environ. Res. Public Health 2020, 17, 5117. [Google Scholar] [CrossRef]
- Khaska, M.; La Salle CL, G.; Sassine, L.; Cary, L.; Bruguier, O.; Verdoux, P. Arsenic and metallic trace elements cycling in the surface water-groundwater-soil continuum down-gradient from a reclaimed mine area: Isotopic imprints. J. Hydrol. 2018, 558, 341–355. [Google Scholar] [CrossRef]
- Holland, H.D. The Chemistry of the Atomosphere and Oceans; Wiley: New York, NY, USA, 1978; p. 351. [Google Scholar]
- Walter, J.; Chesnaux, R.; Cloutier, V.; Gaboury, D. The influence of water/rock—water/clay interactions and mixing in the salinization processes of groundwater. J. Hydro. Reg. Stud. 2017, 13, 168–188. [Google Scholar] [CrossRef]
- Mizota, C.; Kusakabe, M. Spatial distribution of δD–δ18O values of surface and shallow groundwater from Japan, south Korea and east China. Geochem. J. 1994, 28, 387–410. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–438. [Google Scholar] [CrossRef]
- Singer, P.C.; Stumm, W. Acidic mine drainage: The rate-determining step. Science 1970, 167, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Moses, C.O.; Nordstrom, D.K.; Herman, J.S.; Mills, A.L. Aqueous pyrite oxidation by dissolved oxygen and ferric iron. Geochim. Cosmochim. Acta 1987, 51, 1561–1571. [Google Scholar] [CrossRef]
| Sample 1 | pH | EC (mS m−1) | Eh (mV) | Temp. (°C) | Concentration (mg L−1) | Stable-Isotope Ratios (‰) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | Mg2+ | Cl− | HCO3− | SO42− | NO3− | Si | T-Fe | Cu | Zn | δD | δ18O | |||||
| R-1 | 6.3 | 9.88 | 423 | 18.5 | 4.3 | 1.2 | 4.5 | 3.5 | 6.1 | 15.4 | 23.4 | 0.6 | 7.1 | 0.17 | <0.01 | <0.01 | −66.9 | −10.4 |
| R-2 | 6.5 | 10.1 | 465 | 18.9 | 4.4 | 1.1 | 4.6 | 2.6 | 6.9 | 10.7 | 17.2 | 0.6 | 6.6 | <0.01 | <0.01 | <0.01 | −65.1 | −10.3 |
| R-3 | 6.8 | 10.4 | 402 | 19.1 | 4.7 | 0.9 | 7.1 | 2.6 | 6.2 | 32.2 | 8.5 | 1.4 | 5.3 | <0.01 | <0.01 | <0.01 | −66.0 | −10.4 |
| R-4 | 6.8 | 8.43 | 431 | 18.8 | 4.8 | 0.8 | 4.3 | 1.3 | 7.6 | 18.6 | 5.3 | 1.2 | 5.8 | <0.01 | <0.01 | <0.01 | −64.1 | −10.1 |
| R-5 | 7.1 | 5.51 | 445 | 20.6 | 3.6 | 0.8 | 2.1 | 1.1 | 6.1 | 11.7 | 4.3 | 0.4 | 4.5 | 0.18 | <0.01 | <0.01 | −63.0 | −10.1 |
| R-6 | 6.9 | 8.79 | 500 | 18.8 | 4.1 | 2.4 | 3.6 | 2.2 | 7.6 | 13.2 | 9.5 | 0.5 | 7.0 | <0.01 | <0.01 | <0.01 | −64.2 | −10.1 |
| R-7 | 6.3 | 16.5 | 426 | 23.8 | 3.2 | 1.3 | 18.1 | 2.5 | 6.0 | 5.1 | 52.2 | 0.9 | 6.1 | 0.66 | 0.43 | 0.51 | −65.0 | −10.1 |
| R-8 | 3.5 | 29.9 | 608 | 17.9 | 7.1 | 6.3 | 3.6 | 3.6 | 8.3 | <0.01 | 61.5 | 2.8 | 9.0 | <0.01 | 1.2 | <0.01 | −63.9 | −10.0 |
| R-9 | 6.8 | 18.4 | 504 | 22.1 | 6.4 | 1.7 | 13.7 | 5.4 | 10.4 | 22.2 | 43 | 1.6 | 9.5 | <0.01 | 0.11 | 0.15 | −63.4 | −9.8 |
| R-10 | 6.7 | 8.19 | 410 | 20.4 | 5.4 | 1.2 | 4.5 | 1.4 | 8.1 | 20.7 | 6.3 | 1.5 | 10.8 | <0.01 | <0.01 | <0.01 | −61.9 | −9.4 |
| R-11 | 6.8 | 9.00 | 427 | 22.8 | 5.7 | 1.3 | 4.9 | 1.5 | 9.2 | 29.5 | 11.4 | 1.5 | 13.1 | <0.01 | <0.01 | <0.01 | −61.8 | −9.4 |
| R-12 | 6.5 | 12.1 | 428 | 22.2 | 7.6 | 1.9 | 7.9 | 2.0 | 9.9 | 35.9 | 10.5 | 0.6 | 15.5 | 0.15 | <0.01 | <0.01 | −60.9 | −9.3 |
| R-13 | 7.0 | 10.0 | 426 | 19.8 | 4.3 | 1.9 | 5.2 | 3.5 | 7.7 | 20.5 | 15.2 | 0.8 | 6.1 | <0.01 | <0.01 | <0.01 | −63.3 | −10.3 |
| R-14 | 7.5 | 8.63 | 441 | 23.0 | 3.8 | 1.1 | 6.2 | 1.6 | 6.0 | 34.9 | 5.2 | 0.9 | 6.1 | <0.01 | <0.01 | <0.01 | −65.7 | −10.3 |
| R-15 | 7.3 | 8.96 | 440 | 20.3 | 3.4 | 0.9 | 7.9 | 1.8 | 4.7 | 17.3 | 17.0 | 0.3 | 6.3 | <0.01 | <0.01 | <0.01 | −66.3 | −10.6 |
| R-16 | 7.1 | 7.61 | 451 | 19.5 | 4.1 | 0.8 | 6.3 | 1.2 | 5.2 | 36.9 | 6.9 | 0.5 | 6.7 | <0.01 | <0.01 | <0.01 | −65.9 | −10.6 |
| R-17 | 7.0 | 8.16 | 441 | 22.4 | 3.4 | 1.0 | 6.7 | 1.6 | 3.7 | 15.6 | 16.0 | 0.4 | 11.0 | <0.01 | <0.01 | <0.01 | −71.5 | −11.4 |
| R-18 | 7.3 | 7.52 | 445 | 17.7 | 4.6 | 0.9 | 6.0 | 1.0 | 5.1 | 27.8 | 5.0 | 0.6 | 10.4 | <0.01 | <0.01 | <0.01 | −68.5 | −10.9 |
| M-1 | 7.2 | 60.5 | 262 | 13.8 | 12.6 | 9.6 | 60.0 | 32.6 | 9.7 | 59.2 | 237 | 2.4 | 9.0 | <0.01 | <0.01 | <0.01 | −63.3 | −10.1 |
| M-2 | 4.1 | 183 | 591 | 13.7 | 10.3 | 8.4 | 107 | 147 | 10.2 | <0.01 | 1040 | 2.7 | 14.3 | 35.7 | 1.4 | 47.2 | −61.2 | −10.0 |
| M-3 | 3.2 | 387 | 603 | 14.5 | 17.1 | 11.6 | 177 | 217 | 1.7 | <0.01 | 3237 | <0.01 | 16.5 | 522 | 3.8 | 55.5 | −62.9 | −10.2 |
| Sample 1 | pH | EC (mS m−1) | Eh (mV) | Temp. (°C) | Concentration (mg L−1) | Stable-Isotope Ratios (‰) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | Mg2+ | Cl− | HCO3− | SO42− | NO3− | Si | T-Fe | Cu | Zn | δD | δ18O | |||||
| S-1 | 6.3 | 18.4 | 446 | 13.0 | 13.6 | 3.7 | 18.6 | 4.3 | 10.0 | 44.2 | 25.5 | 7.6 | 11.2 | 0.17 | <0.01 | <0.01 | −63.2 | −9.6 |
| S-2 | 6.3 | 17.1 | 429 | 10.3 | 14.4 | 2.5 | 15.3 | 3.6 | 10.0 | 30.0 | 16.5 | 19.9 | 28.3 | <0.01 | <0.01 | <0.01 | −61.9 | −9.9 |
| S-3 | 6.4 | 17.1 | 412 | 10.1 | 15.5 | 2.4 | 15.2 | 3.6 | 9.9 | 32.7 | 16.5 | 19.9 | 28.7 | <0.01 | <0.01 | <0.01 | −63.1 | −10.0 |
| S-4 | 6.5 | 15.7 | 424 | 9.5 | 11.8 | 0.4 | 17.3 | 4.0 | 10.4 | 44.2 | 12.0 | 9.8 | 15.8 | <0.01 | <0.01 | <0.01 | −61.2 | −9.8 |
| S-5 | 6.7 | 25.6 | 411 | 8.3 | 14.1 | 0.4 | 27.2 | 10.9 | 15.1 | 78.1 | 22.6 | 15.7 | 15.6 | <0.01 | <0.01 | <0.01 | −62.0 | −9.9 |
| R-7 | 6.4 | 13.3 | 657 | 4.8 | 4.8 | 0.9 | 15.3 | 2.1 | 6.3 | 6.8 | 36.1 | 2.1 | 4.4 | 0.01 | 0.26 | 0.36 | −61.7 | −9.9 |
| R-9 | 7.1 | 18.8 | 449 | 5.1 | 10.5 | 1.6 | 17.3 | 5.4 | 12.4 | 24.2 | 44.4 | 2.1 | 8.4 | <0.01 | 0.26 | 0.17 | −60.3 | −9.6 |
| R-19 | 7.4 | 8.8 | 342 | 5.5 | 6.8 | 0.3 | 9.4 | 2.4 | 6.6 | 22.5 | 10.2 | 1.1 | 6.2 | <0.01 | <0.01 | <0.01 | −64.8 | −10.4 |
| R-20 | 7.3 | 13.5 | 339 | 6.8 | 11.9 | 0.6 | 13.3 | 3.2 | 15.6 | 32.5 | 9.8 | 2.2 | 9.0 | 0.01 | <0.01 | <0.01 | −63.4 | −10.1 |
| R-21 | 7.5 | 9.31 | 415 | 3.9 | 7.5 | 0.3 | 10.9 | 2.1 | 9.5 | 29.3 | 5.3 | 0.94 | 7.8 | <0.01 | 0.11 | 0.15 | −64.6 | −10.2 |
| R-22 | 7.5 | 10.3 | 402 | 5.0 | 7.5 | 0.2 | 13.4 | 2.0 | 8.7 | 29.8 | 9.2 | 1.2 | 6.8 | <0.01 | <0.01 | <0.01 | −64.3 | −10.5 |
| R-23 | 5.6 | 7.1 | 390 | 3.1 | 7.5 | 0.7 | 4.2 | 1.5 | 5.4 | 23.9 | 11.6 | 0.82 | 9.2 | <0.01 | <0.01 | <0.01 | −66.9 | −10.6 |
| R-24 | 6.4 | 8.4 | 381 | 4.3 | 6.2 | 0.7 | 10.7 | 2.1 | 3.7 | 29.3 | 9.1 | 0.92 | 9.2 | 0.15 | <0.01 | <0.01 | −69.2 | −11.1 |
| R-25 | 4.3 | 9.2 | 657 | 4.6 | 5.3 | 0.5 | 1.2 | 1.3 | 6.9 | <0.01 | 15.0 | 1.4 | 4.0 | <0.01 | <0.01 | <0.01 | −62.7 | −10.0 |
| R-26 | 5.0 | 6.9 | 610 | 5.3 | 0.5 | 0.3 | 1.4 | 1.7 | 7.7 | 0.5 | 13.2 | 0.48 | 4.6 | <0.01 | <0.01 | <0.01 | −61.3 | −19.8 |
| M-1 | 7.2 | 61.8 | 252 | 12.2 | 17.1 | 4.7 | 67.6 | 37.8 | 9.9 | 62.7 | 249 | 4.6 | 8.9 | <0.01 | <0.01 | <0.01 | −62.2 | −10.0 |
| M-2 | 3.8 | 183 | 609 | 13.4 | 10.6 | 5.5 | 89.2 | 151 | 10.1 | <0.01 | 1128 | 4.4 | 14.9 | 31.2 | 0.61 | 45.7 | −61.3 | −9.9 |
| M-3 | 3.4 | 347 | 605 | 14.4 | 23.1 | 10.4 | 165 | 238 | 8.6 | <0.01 | 2981 | 4.7 | 17.3 | 462 | 8.4 | 63.5 | −62.6 | −10.2 |
| Variable | PC1 | PC2 | PC3 | |
|---|---|---|---|---|
| Temperature | −0.03 | −0.32 | −0.05 | |
| EC | 0.98 | −0.06 | 0.10 | |
| pH | −0.79 | −0.14 | 0.34 | |
| Eh | 0.56 | −0.32 | −0.60 | |
| Chemical composition | Na+ | 0.67 | 0.63 | 0.33 |
| K+ | 0.90 | 0.04 | 0.07 | |
| Ca2+ | 0.95 | 0.05 | 0.17 | |
| Mg2+ | 0.97 | −0.06 | 0.06 | |
| Cl− | −0.04 | 0.83 | –0.23 | |
| SO42− | 0.97 | −0.12 | 0.12 | |
| HCO3− | −0.39 | 0.63 | 0.50 | |
| NO3− | 0.09 | 0.79 | 0.16 | |
| Si | 0.42 | 0.61 | 0.21 | |
| Total Fe | 0.90 | 0.15 | 0.18 | |
| Cu | 0.88 | −0.11 | 0.10 | |
| Zn | 0.95 | −0.10 | –0.01 | |
| Stable-isotope ratio | δD | 0.32 | 0.64 | –0.61 |
| δ18O | 0.13 | 0.60 | –0.66 | |
| Rate of contribution (%) | 49.5 | 19.7 | 10.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimoto, N.; Yamamoto, Y.; Yamagata, S.; Igarashi, T.; Tomiyama, S. Acid Mine Drainage Sources and Impact on Groundwater at the Osarizawa Mine, Japan. Minerals 2021, 11, 998. https://doi.org/10.3390/min11090998
Nishimoto N, Yamamoto Y, Yamagata S, Igarashi T, Tomiyama S. Acid Mine Drainage Sources and Impact on Groundwater at the Osarizawa Mine, Japan. Minerals. 2021; 11(9):998. https://doi.org/10.3390/min11090998
Chicago/Turabian StyleNishimoto, Naoto, Yosuke Yamamoto, Saburo Yamagata, Toshifumi Igarashi, and Shingo Tomiyama. 2021. "Acid Mine Drainage Sources and Impact on Groundwater at the Osarizawa Mine, Japan" Minerals 11, no. 9: 998. https://doi.org/10.3390/min11090998
APA StyleNishimoto, N., Yamamoto, Y., Yamagata, S., Igarashi, T., & Tomiyama, S. (2021). Acid Mine Drainage Sources and Impact on Groundwater at the Osarizawa Mine, Japan. Minerals, 11(9), 998. https://doi.org/10.3390/min11090998






