Valorisation of Partially Oxidized Tailings in a Cover System to Reclaim an Old Acid Generating Mine Site
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Used for the Column Tests
2.1.1. Mine Site Description
2.1.2. Location and Sampling Procedure
2.1.3. Chemical and Mineralogical Composition
2.1.4. Physical and Hydraulic Properties
2.2. Column Tests
2.2.1. Setup and Instrumentation
2.2.2. Wetting and Drying Cycles
2.2.3. Chemical Analysis of the Column Leachates
3. Column Test Results
3.1. Effectiveness of the Tested CCBEs as Oxygen Barriers
3.1.1. Degree of Saturation
3.1.2. Oxygen Concentrations
3.2. Leachate Quality
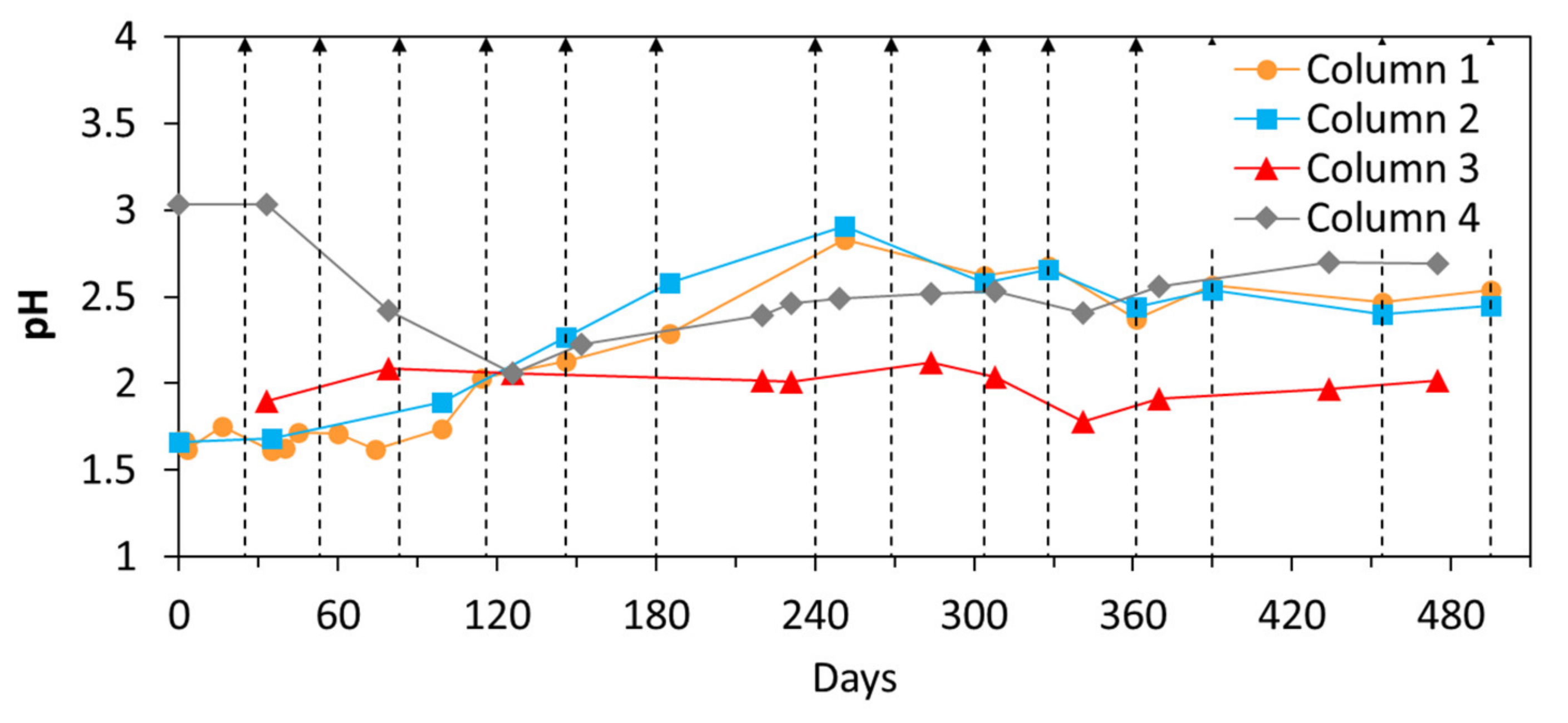
3.2.1. pH
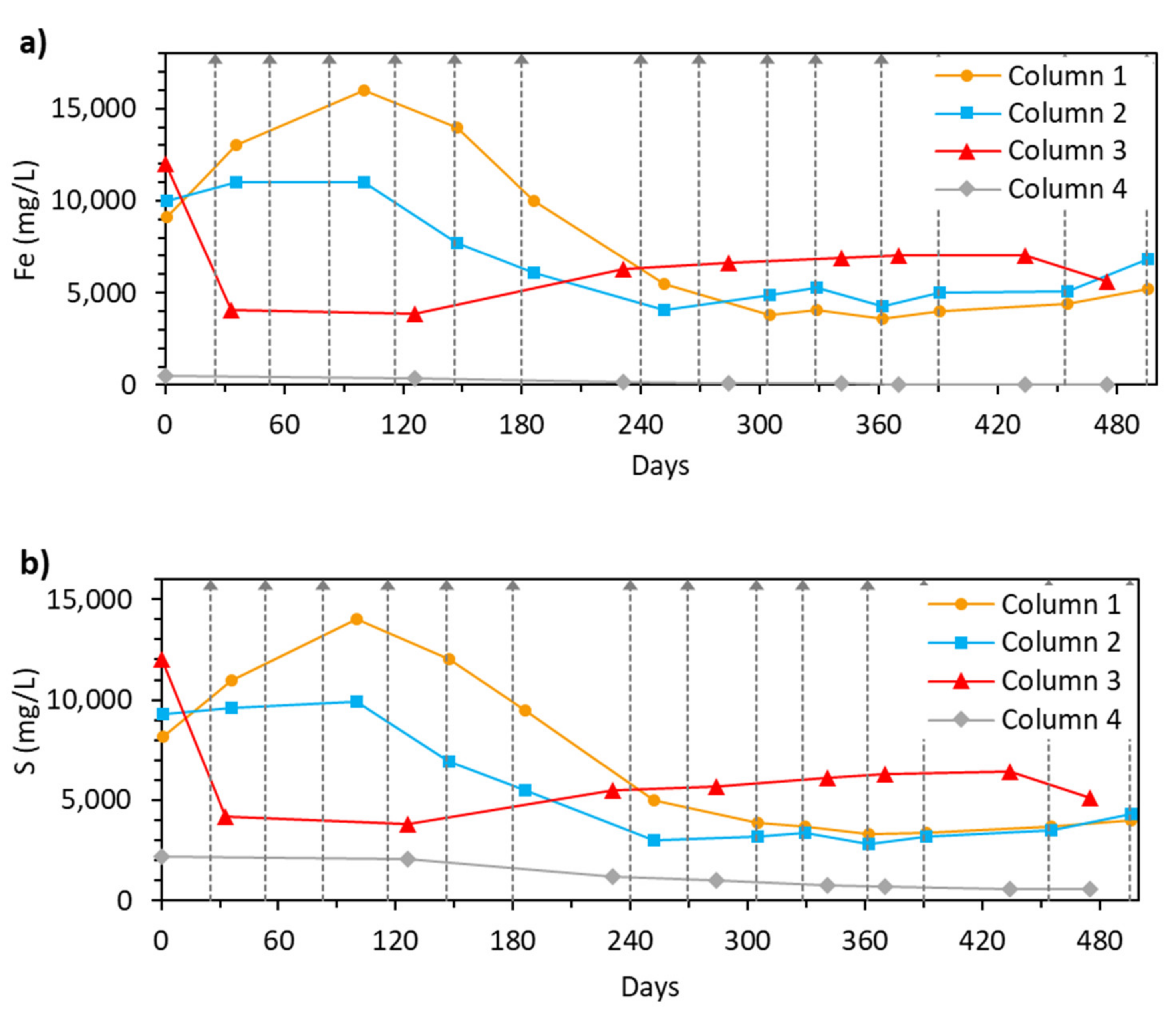
3.2.2. Fe and S Concentration
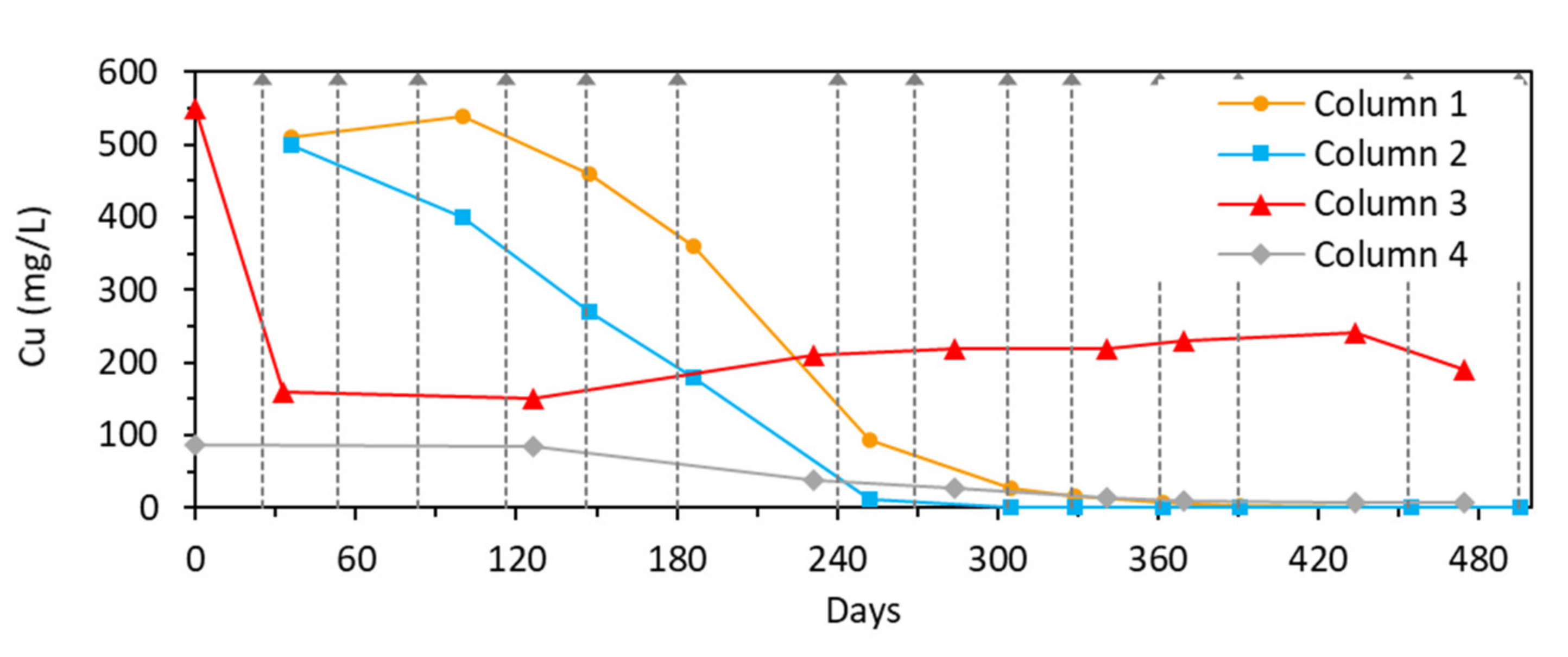
3.2.3. Cu
3.2.4. Other Elements
4. Discussion
4.1. Reactivity of HS and LS Tailings
4.2. Limited Performance of the CCBE Made of LS Tailings
4.3. Positive Effects on Cu Concentrations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spitz, K.; Trudinger, J. Mining and the Environment. From Ore to Metal, 2nd ed.; CRC Press; Taylor and Francis: London, UK, 2019; 812p. [Google Scholar]
- Hawley, P.M.; Cunning, J. Guidelines for Mine Waste Dump and Stockpile Design; CRC Press; Taylor and Francis: London, UK, 2017; 368p. [Google Scholar]
- Blowes, D.W.; Ptacek, C.J.; Jambor, J.L.; Weisener, C.G.; Paktunc, D.; Gould, W.D.; Johnson, D.B. The geochemistry of acid mine drainage. In Treatise on Geochemistry, 2nd ed.; Holland, H., Turekian, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 11, pp. 131–190. [Google Scholar]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and microbiology of mine drainage: An update. Appl. Geochem. 2015, 57, 3–16. [Google Scholar] [CrossRef]
- Neculita, C.M.; Zagury, G.J.; Bussière, B. Passive treatment of acid mine drainage in bioreactors using sulfate-reducing bacteria: Critical review and research needs. J. Environ. Qual. 2007, 36, 1–16. [Google Scholar] [CrossRef]
- Skousen, J.; Zipper, C.; Rose, A.; Ziemkiewicz, P.F.; Nairn, R.; McDonald, L.M.; Kleinmann, R.L. Review of passive systems for acid mine drainage treatment. Mine Water Environ. 2016, 36, 133–153. [Google Scholar] [CrossRef] [Green Version]
- Ben Ali, H.E.; Neculita, C.M.; Molson, J.W.; Maqsoud, A.; Zagury, G.J. Performance of passive systems for mine drainage treatment at lowtemperature and high salinity: A review. Miner. Eng. 2019, 134, 325–344. [Google Scholar] [CrossRef]
- Aubertin, M.; Bussière, B.; Pabst, T.; James, M.; Mbonimpa, M.; Farid, A.; De, A.; Reddy, K.R.; Yesiller, N.; Zekkos, D. Review of the reclamation techniques for acid-generating mine wastes upon closure of disposal sites. In Proceedings of Geo-Chicago; Zekkos, D., Farid, A., De, A., Reddy, K.R., Yesiller, N., Eds.; American Society of Civil Engineers: Reston, VA, USA; pp. 343–358.
- Aubertin, M.; Bussière, B.; Bernier, L. Environnement et Gestion des Rejets Miniers; Presses Polytechnique Internationales: Montreal, QC, Canada, 2002. [Google Scholar]
- Bussière, B.; Guittonny, M. Hard rock mine reclamation. In Prediction to Management of Acid Mine Drainage; CRC Press; Taylor and Francis: London, UK, 2020; 408p. [Google Scholar]
- Awoh, A.S.; Mbonimpa, M.; Bussière, B. Field study of the chemical and physical stability of highly sulphide-rich tailings stored under a shallow water cover. Mine Water Environ. 2013, 32, 42–55. [Google Scholar] [CrossRef]
- Awoh, A.S.; Mbonimpa, M.; Bussiere, B. Water covers. In Hard Rock Mine Reclamation. From Prediction to Management of Acid Mine Drainage; Bussière, B., Guittonny, M., Eds.; CRC Press; Taylor and Francis: London, UK, 2020; 408p. [Google Scholar]
- Aubertin, M.; Bussière, B.; Monzon, M.; Joanes, A.M.; Gagnon, D.; Barbera, J.M.; Aachib, M.; Bédard, C.; Chapuis, R.P.; Bernier, L. Etude sur les Barrières Sèches Construites à Partir des Résidus Miniers. Phase II, Essais en Place; Research Report, Projet CDT P1899. NEDEM/MEND 2.22.2c; Bureau de la Recherche et Centre de Developpement Technologique, Ecole Polytechnique de Montreal: Montreal, QC, Canada, 1999. [Google Scholar]
- Bussière, B.; Aubertin, M.; Chapuis, R.P. The behavior of inclined covers used as oxygen barriers. Can. Geotech. J. 2003, 40, 512–535. [Google Scholar] [CrossRef]
- Demers, I.; Pabst, T. Covers with capillary barrier effects. In Hard Rock Mine Reclamation. From Prediction to Management of Acid Mine Drainage; Bussière, B., Guittonny, M., Eds.; CRC Press; Taylor and Francis: London, UK, 2020; 408p. [Google Scholar]
- Ouangrawa, M.; Molson, J.; Aubertin, M.; Bussière, B.; Zagury, G. Reactive transport modelling of mine tailings columns with capillarity-induced high water saturation for preventing sulfide oxidation. Appl. Geochem. 2009, 24, 1312–1323. [Google Scholar] [CrossRef]
- Pabst, T.; Aubertin, M.; Bussière, B.; Molson, J. Experimental and numerical evaluation of single-layer covers placed on acid-generating tailings. Geotech. Geol. Eng. 2017, 35, 1421–1438. [Google Scholar] [CrossRef]
- Pabst, T.; Molson, J.; Aubertin, M.; Bussière, B. Reactive transport modelling of the hydro-geochemical behaviour of partially oxidized acid-generating mine tailings with a monolayer cover. Appl. Geochem. 2017, 78, 219–233. [Google Scholar] [CrossRef]
- Pabst, T. Elevated water table with monolayer covers. In Hard Rock Mine Reclamation. From Prediction to Management of Acid Mine Drainage; Bussière, B., Guittonny, M., Eds.; CRC Press; Taylor and Francis: London, UK, 2020; 408p. [Google Scholar]
- Collin, M.; Rasmuson, A. A comparison of gas diffusivity models for unsaturated porous media. Soil Sci. Soc. Am. J. 1988, 52, 1559–1565. [Google Scholar] [CrossRef]
- Mbonimpa, M.; Boulanger-Martel, V.; Bussière, B.; Maqsoud, A. Water, gas, and heat movement in cover materials. In Hard Rock Mine Reclamation. From Prediction to Management of Acid Mine Drainage; Bussière, B., Guittonny, M., Eds.; CRC Press; Taylor and Francis: London, UK, 2020; 408p. [Google Scholar]
- Nicholson, R.V.; Gillham, R.W.; Cherry, J.A.; Reardon, E.J. Reduction of acid generation through the use of moisture-retaining cover layers as oxygen barriers. Can. Geotech. J. 1997, 26, 1–8. [Google Scholar] [CrossRef]
- Ricard, J.; Aubertin, M.; Firlotte, F.; Knapp, R.; McMullen, J.; Julien, M. Design and construction of a dry cover made of tailings for the closure of Les Terrains Aurifères site, Malartic, Québec, Canada. In Proceedings of the 4th International Conference on Acid Rock Drainage, Vancouver, BC, Canada, 31 May–6 June 1997; pp. 1515–1530. [Google Scholar]
- Bussière, B.; Maqsoud, A.; Aubertin, M.; Martschuk, J.; McMullen, J.; Julien, M. Performance of the oxygen limiting cover at the LTA site, Malartic, Quebec. CIM Bull. 2006, 1, 1–11. [Google Scholar]
- Dagenais, A.-M.; Aubertin, M.; Bussière, B.; Cyr, J.; Fontaine, R. Auscultation et suivi du recouvrement multicouche construit au site minier Lorraine, Latulipe, Québec. In Proceedings of the Défis & Perspectives: Symposium sur l’Environnement et les Mines, Rouyn-Noranda, QC, Canada, 3–5 November 2002; pp. 3–5. [Google Scholar]
- Dagenais, A.-M. Techniques de Contrôle du Drainage Minier Acide Basées sur les Effets Capillaires. Ph.D. Thesis, École Polytechnique de Montréal, Montreal, QC, Canada, 2005. [Google Scholar]
- Dagenais, A.M.; Aubertin, M.; Bussière, B. Parametric study on the water content profiles and oxidation rates in nearly saturated tailings above the water table. In Proceedings of the 7th International Conference on Acid Rock Drainage, St-Louis, MO, USA, 27–30 March 2006; pp. 405–420. [Google Scholar]
- Ethier, M.-P.; Bussière, B.; Broda, S.; Aubertin, M. Three-dimensional hydrogeological modeling to assess the elevated-water-table technique for controlling acid generation from an abandoned tailings site in Quebec, Canada. Hydrogeol. J. 2018, 26, 1201–1219. [Google Scholar] [CrossRef]
- Pabst, T.; Bussière, B.; Aubertin, M.; Molson, J. Comparative performance of cover systems to prevent acid mine drainage from pre-oxidized tailings: A numerical hydro-geochemical assessment. J. Contam. Hydrol. 2018, 214, 39–53. [Google Scholar] [CrossRef]
- Williamson, M.A.; Rimstidt, J. The kinetics and electrochemical rate-determining step of aqueous pyrite oxidation. Geochim. Cosmochim. Acta 1994, 58, 5443–5454. [Google Scholar] [CrossRef]
- Nordstrom, D.K. Advances in the hydrogeochemistry and microbiology of acid mine waters. Int. Geol. Rev. 2000, 42, 499–515. [Google Scholar] [CrossRef]
- Moses, C.O.; Nordstrom, D.K.; Herman, J.S.; Mills, A.L. Aqueous pyrite oxidation by dissolved oxygen and by ferric iron. Geochim. Cosmochim. Acta 1987, 51, 1561–1571. [Google Scholar] [CrossRef]
- Sasaki, K.; Tsunekawa, M.; Ohtsuka, T.; Konno, H. Confirmation of a sulfur-rich layer on pyrite after oxidative dis-solution by Fe (lIl) ions around pH2. Geochim. Cosmochim. Acta 1995, 59, 3155–3158. [Google Scholar] [CrossRef]
- Mazumdar, A.; Goldberg, T.; Strauss, H. Abiotic oxidation of pyrite by Fe(III) in acidic media and its implications for sulfur isotope measurements of lattice-bound sulfate in sediments. Chem. Geol. 2008, 253, 30–37. [Google Scholar] [CrossRef]
- Janzen, M.P.; Nicholson, R.V.; Scharer, J.M. Pyrrhotite reaction kinetics: Reaction rates for oxidation by oxygen, ferric iron, and for nonoxidative dissolution. Geochim. Cosmochim. Acta 2000, 64, 1511–1522. [Google Scholar] [CrossRef]
- Bussière, B.; Aubertin, M. Clean tailings as cover material for preventing acid mine drainage: An in situ experiment. In Proceedings of the Sudbury 2003, Mining and the Environment Conference, Sudbury, ON, Canada, 5–28 May 2003; Volume 99, pp. 19–28. [Google Scholar]
- Bussière, B.; Aubertin, M.; Mbonimpa, M.; Molson, J.; Chapuis, R.P. Field experimental cells to evaluate the hydrogeological behaviour of oxygen barriers made of silty materials. Can. Geotech. J. 2007, 44, 245–265. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Bussière, B.; Kongolo, M.; McLaughlin, J.; Marion, P. Environmental desulphurization of four Canadian mine tailings using froth flotation. Int. J. Miner. Proc. 2000, 60, 57–74. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Bussière, B.; Demers, I.; Aubertin, M.; Fried, E.; Blier, A. Integrated mine tailings management by combining environmental desulphurization and cemented paste backfill: Application to mine Doyon, Quebec, Canada. Miner. Eng. 2008, 21, 330–340. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Aubertin, M.; Mbonimpa, M. A laboratory study of covers made of low-sulphide tailings to prevent acid mine drainage. Environ. Earth Sci. 2003, 45, 609–622. [Google Scholar] [CrossRef]
- Bjerkgård, T.; Scack, S.P.; Siedlecka, A. Folldal. Berggrunnskart; Folldal; 15192; 1:50 000; Foreløpig Utgave Plotteversjon. In Folldal. Berggrunnskart; Folldal; 15192; 1:50 000; Foreløpig Utgave Plotteversjon; Norges Geologiske Undersokelse: Trondheim, Norway, 2002. [Google Scholar]
- Folldal Verk. Folldal Verk Gjennom 240 År: Historiske Trekk, 1748–1988; Folldal verk: Folldal, Norway, 1988; 116p, Available online: https://urn.nb.no/URN:NBN:no-nb_digibok_2011011103034 (accessed on 16 August 2021).
- Streitlien, I.A. Bygdebok for Folldal. B.3: Geologi, Arkeologi m.m.; Trykk AS: Elverum, Norway, 1980; 326p, Available online: https://urn.nb.no/URN:NBN:no-nbdigibok2014052606018 (accessed on 16 August 2021).
- Iversen, E.; Knudsen, C.H. Miljøsikringsfondet Folldal verk. Utredning av Forurensningsbegrensende Tiltak i Gruveområdet i Folldal Sentrum; Norsk Institutt for Vannforskning: Oslo, Norway, 2002. [Google Scholar]
- Norwegian Environmental Agency. Faktaark Vannregistreringer og Måledata, Vannlokalitet: Folla Oppstrøms Folla Sentrum ved Skytebanen (FO5), 1970 to 2020. Available online: https://vannmiljofaktaark.miljodirektoratet.no/Home/Details/44732 (accessed on 14 June 2021).
- Norwegian Environmental Agency. Faktaark Vannregistreringer og Måledata, Vannlokalitet: Folla Nedstrøms Gruve (F3), 2015 to 2020. Available online: https://vannmiljofaktaark.miljodirektoratet.no/Home/Details/92634 (accessed on 16 August 2021).
- Miljødirektoratet. Forurensning fra tidligere gruvedrift i folldal sentrum; Miljødirektoratet, Nærings- og Handelsdepartementet, Eds.; Environmental Protection Agency: Oslo, Norway, 2003. [Google Scholar]
- De Caritat, P.; Reimann, C.; Smith, D.; Wang, X. Chemical elements in the environment: Multi-element geochemical datasets from continental- to national-scale surveys on four continents. Appl. Geochem. 2017, 89, 150–159. [Google Scholar] [CrossRef]
- Sobeck, A.A.; Schuller, W.A.; Freeman, J.R.; Smith, R.M. Field and Laboratory Methods Applicable to Overburdens and Minesoils; EPA-600/2-78-054; U.S. Environmental Protection Agency: Washington, DC, USA, 1978.
- Lawrence, R.W.; Wang, Y. Determination of neutralization potential in the prediction of acid rock drainage. In Proceedings of the 4th International Conference on Acid Rock Drainage, Vancouver, BC, Canada, 31 May–6 June 1997; Volume 1, pp. 449–464. [Google Scholar]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef] [Green Version]
- Fredlund, D.; Xing, A. Equations for the soil-water characteristic curve. Can. Geotech. J. 1994, 31, 521–532. [Google Scholar] [CrossRef]
- Bussière, B. Colloquium 2004: Hydro-geotechnical properties of hard rock tailings from metal mines and emerging geo-environmental disposal approaches. Can. Geotech. J. 2007, 44, 1019–1052. [Google Scholar] [CrossRef]
- Demers, I.; Bussière, B.; Benzaazoua, M.; Mbonimpa, M.; Blier, A. Column test investigation on the performance of monolayer covers made of desulphurized tailings to prevent acid mine drainage. Miner. Eng. 2008, 21, 317–329. [Google Scholar] [CrossRef]
- Aubertin, M.; Chapuis, R.P.; Aachib, M.; Bussière, B.; Ricard, J.F.; Tremblay, L. Evaluation en Laboratoire de Barrières Sèches Construites à Partir de Résidus Miniers; NEDEM/MEND Projet 2.22.2a; Ecole Polytechnique de Montréal: Montreal, QC, Canada, 1995. [Google Scholar]
- Demers, I.; Bussière, B.; Aachib, M.; Aubertin, M. Repeatability evaluation of instrumented column tests in cover efficiency evaluation for the prevention of acid mine drainage. Water Air Soil Pollut. 2010, 219, 113–128. [Google Scholar] [CrossRef]
- Pabst, T.; Aubertin, M.; Bussière, B.; Molson, J. Column tests to characterise the hydrogeochemical response of pre-oxidised acid-generating tailings with a monolayer cover. Water Air Soil Pollut. 2014, 225, 1–21. [Google Scholar] [CrossRef]
- Lieber, E.; Demers, I.; Pabst, T.; Bresson, E. Simulating the effect of climate change on the performance of a monolayer cover combined with an elevated water table placed on acid generating mine tailings. Can. Geotech. J. 2021, in press. [Google Scholar] [CrossRef]
- Topp, G.C.; Davis, J.L.; Annan, A.P. Electromagnetic determination of soil water content: Measurements in coaxial transmission lines. Water Resour. Res. 1980, 16, 574–582. [Google Scholar] [CrossRef] [Green Version]
- Demers, I.; Bussière, B.; Mbonimpa, M.; Benzaazoua, M. Oxygen diffusion and consumption in low-sulphide tailings covers. Can. Geotech. J. 2009, 46, 454–469. [Google Scholar] [CrossRef]
- Cravotta, C.A., III. Secondary iron-sulfate minerals as sources of sulfate and acidity. In Environmental Geochemistry of Sulfide Oxidation; Alpers, C.N., Blowes, D.W., Eds.; Imperial College Press: London, UK, 1994; Volume 550, pp. 345–364. [Google Scholar]
- Welch, S.A.; Kirste, D.; Christy, A.G.; Beavis, F.R.; Beavis, S.G. Jarosite dissolution II—Reaction kinetics, stoichi-ometry and acid flux. Chem. Geol. 2008, 254, 73–86. [Google Scholar] [CrossRef]
- Desborough, G.A.; Smith, K.S.; Lowers, H.A.; Swayze, G.A.; Hammarstrom, J.M.; Diehl, S.F.; Leinz, R.W.; Driscoll, R.L. Mineralogical and chemical characteristics of some natural jarosites. Geochim. Cosmochim. Acta 2010, 74, 1041–1056. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-España, J.; Yusta, I.; López, G.A. Schwertmannite to jarosite conversion in the water column of an acidic mine pit lake. Mineral. Mag. 2012, 76, 2659–2682. [Google Scholar] [CrossRef]
- Bigham, J.; Schwertmann, U.; Traina, S.; Winland, R.; Wolf, M. Schwertmannite and the chemical modeling of iron in acid sulfate waters. Geochim. Cosmochim. Acta 1996, 60, 2111–2121. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Directive 2000/60/EC of the European Parliament and the Council Establishing a Framework for the Community Action in the Field of Water Policy; EU: Brussels, Belgium, 2000. [Google Scholar]
- Tao, H.; Dongwei, L. Presentation on mechanisms and applications of chalcopyrite and pyrite bioleaching in biohydrometallurgy—A presentation. Biotechnol. Rep. 2014, 4, 107–119. [Google Scholar] [CrossRef] [Green Version]

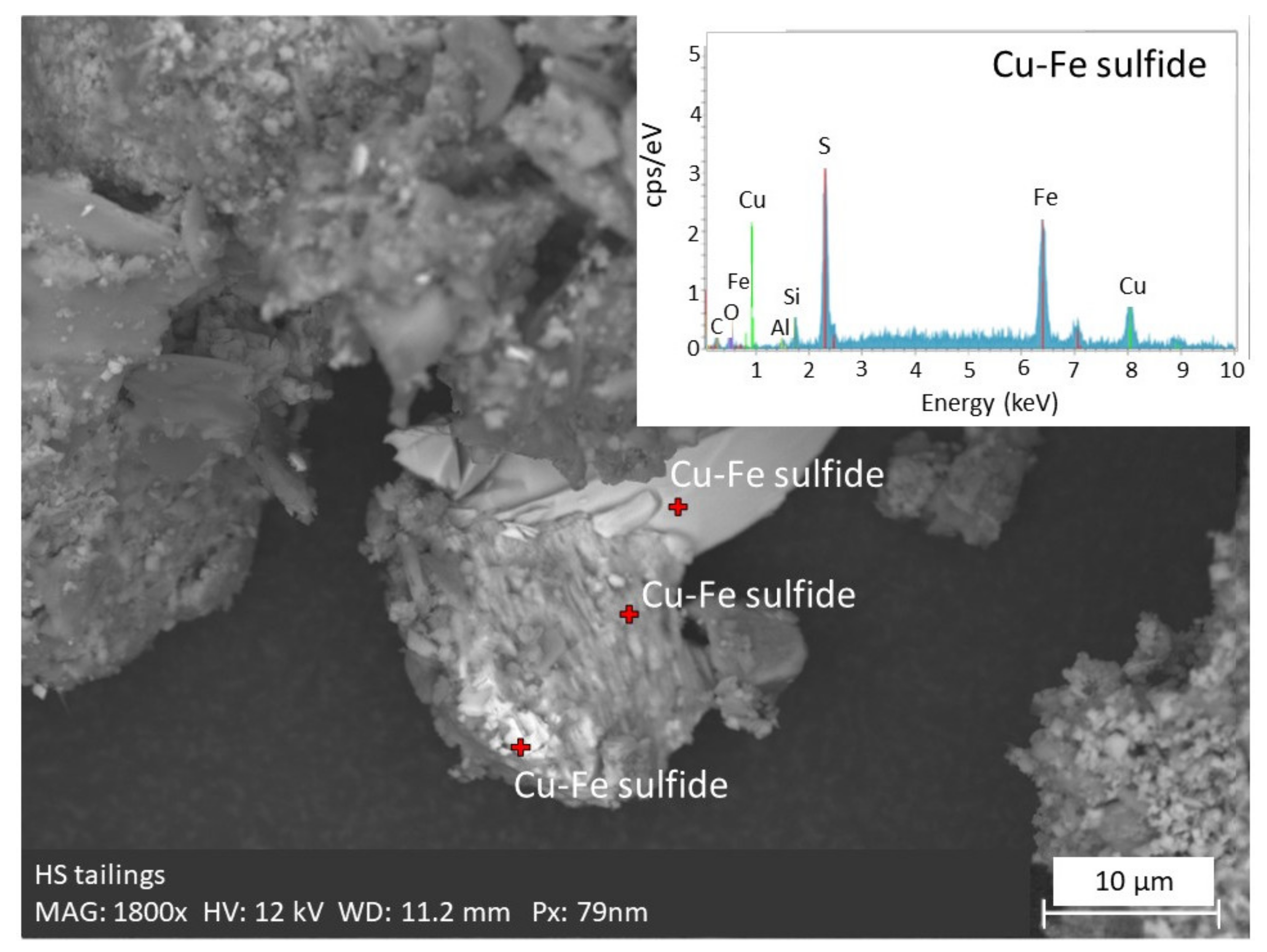
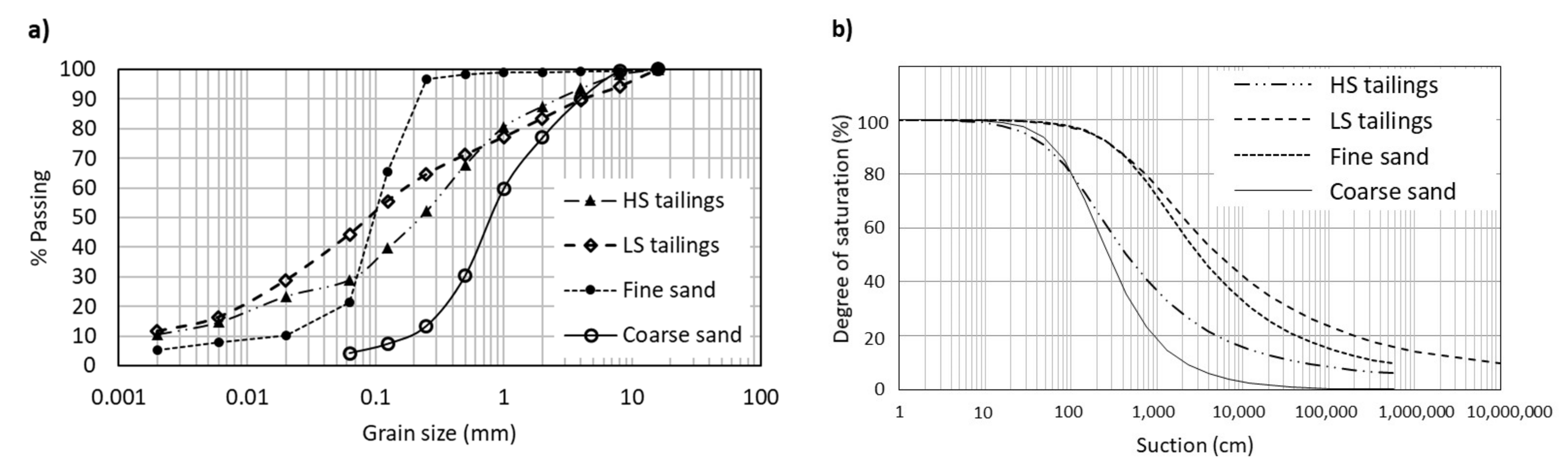




| Parameter | Units | HS Tailings | LS Tailings | Fine Sand | Coarse Sand |
|---|---|---|---|---|---|
| Fe | wt% | 14.7 | 3.98 | 0.47 | 0.18 |
| STotal | wt% | 5.1 | 0.4 | <0.003 | <0.003 |
| Al | wt% | 0.21 | 0.89 | 0.23 | 0.12 |
| As | mg/kg | 83 | 2.8 | 0.8 | <0.5 |
| Ba | mg/kg | 30 | 62 | 72 | 68 |
| Be | mg/kg | <0.01 | 0.04 | 0.04 | 0.09 |
| Cd | mg/kg | <0.1 | <0.1 | <0.1 | <0.1 |
| Co | mg/kg | 48 | 7.2 | 3.0 | 1.0 |
| Cr | mg/kg | 6.5 | 31 | 9.3 | 4.1 |
| Cu | mg/kg | 3340 | 119 | 8.1 | 2.2 |
| Hg | mg/kg | <0.20 | <0.20 | <0.20 | <0.20 |
| Mn | mg/kg | 26 | 126 | 95 | 62 |
| Mo | mg/kg | 38 | 0.7 | <0.4 | <0.4 |
| Ni | mg/kg | <5.0 | 16 | 6 | <5.0 |
| P | mg/kg | 80 | 468 | 258 | 192 |
| Pb | mg/kg | 74 | 5 | 2 | 2 |
| Sr | mg/kg | 5.5 | 7.3 | 9.1 | 6.8 |
| V | mg/kg | 25 | 46 | 8.2 | 2.7 |
| Zn | mg/kg | 49 | 48 | 7 | 4 |
| Li | mg/kg | <1.0 | 6 | 2 | 3 |
| TIC | wt% | 0.02 | 0.01 | <0.010 | <0.010 |
| CO3 | wt% | 0.12 | 0.06 | <0.050 | <0.050 |
| SO4 | wt% | 4.2 | 1.3 | <0.1 | <0.1 |
| Ca | wt% | 0.04 | 0.17 | - | - |
| HS Tailings | LS Tailings | |
|---|---|---|
| Ilite + Mica | 3 | 9 |
| Chlorite | 14 | 7 |
| Quartz | 36 | 42 |
| K-Feldspar | <1 | 6 |
| Plagioclase | 17 | 25 |
| Amphibole | 15 | 10 |
| Calcite | 2 | <1 |
| Dolomite | 2 | <1 |
| Jarosite | 2 | 1 |
| Pyrite | 9 | <1 |
| Material | D10 (mm) | D60 (mm) | CU (-) | Gs (-) | n (-) | ksat-KC (m/s) | AEV (cm) |
|---|---|---|---|---|---|---|---|
| HS tailings | 0.002 | 0.35 | 177 | 3.02 | 0.50 | 3 × 10−7 | 35 |
| LS tailings | 0.002 | 0.18 | 88 | 2.80 | 0.37 | 7 × 10−8 | 200 |
| Coarse sand | 0.17 | 1.01 | 6 | 2.69 | 0.38 | 2 × 10−4 | 55 |
| Fine sand | 0.02 | 0.12 | 6 | 2.69 | 0.43 | 4 × 10−6 | 220 |
| Labsolute (mg/kg) a | Linitial (wt%) b | Performance (%) c | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | S | Cu | Fe | S | Cu | Fe | S | Cu | |
| HS tailings (column 3) | 15,441 | 13,965 | 525 | 11 | 27 | 16 | |||
| HS tailings covered with LS tailings (column 1) | 6071 | 5311 | 146 | 4% | 10% | 4% | 61% | 62% | 72% |
| HS tailings covered with fine sand (column 2) | 5370 | 4177 | 102 | 4% | 8% | 3% | 65% | 70% | 81% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dublet-Adli, G.; Pabst, T.; Okkenhaug, G.; Sætre, C.; Vårheim, A.M.; Tvedten, M.K.; Gelena, S.K.; Smebye, A.B.; Kvennås, M.; Breedveld, G.D. Valorisation of Partially Oxidized Tailings in a Cover System to Reclaim an Old Acid Generating Mine Site. Minerals 2021, 11, 987. https://doi.org/10.3390/min11090987
Dublet-Adli G, Pabst T, Okkenhaug G, Sætre C, Vårheim AM, Tvedten MK, Gelena SK, Smebye AB, Kvennås M, Breedveld GD. Valorisation of Partially Oxidized Tailings in a Cover System to Reclaim an Old Acid Generating Mine Site. Minerals. 2021; 11(9):987. https://doi.org/10.3390/min11090987
Chicago/Turabian StyleDublet-Adli, Gabrielle, Thomas Pabst, Gudny Okkenhaug, Christian Sætre, Anna M. Vårheim, Mari K. Tvedten, Samuel K. Gelena, Andreas B. Smebye, Marianne Kvennås, and Gijs D. Breedveld. 2021. "Valorisation of Partially Oxidized Tailings in a Cover System to Reclaim an Old Acid Generating Mine Site" Minerals 11, no. 9: 987. https://doi.org/10.3390/min11090987
APA StyleDublet-Adli, G., Pabst, T., Okkenhaug, G., Sætre, C., Vårheim, A. M., Tvedten, M. K., Gelena, S. K., Smebye, A. B., Kvennås, M., & Breedveld, G. D. (2021). Valorisation of Partially Oxidized Tailings in a Cover System to Reclaim an Old Acid Generating Mine Site. Minerals, 11(9), 987. https://doi.org/10.3390/min11090987






