Application of Lignite Combustion Waste Slag Generated in Heating Plants as a Partial Replacement for Cement. Part II: Physical–Mechanical and Physical–Chemical Characterization of Mortar and Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Concrete and Mortar Preparation
2.3. Characterization of Mortar and Concrete Samples
2.3.1. Tests on Mortar Samples
2.3.2. Tests of Physical–mechanical Properties of Concrete Samples
2.3.3. Physical–chemical Characterization of Hardened Mortar Samples
3. Results and Discussion
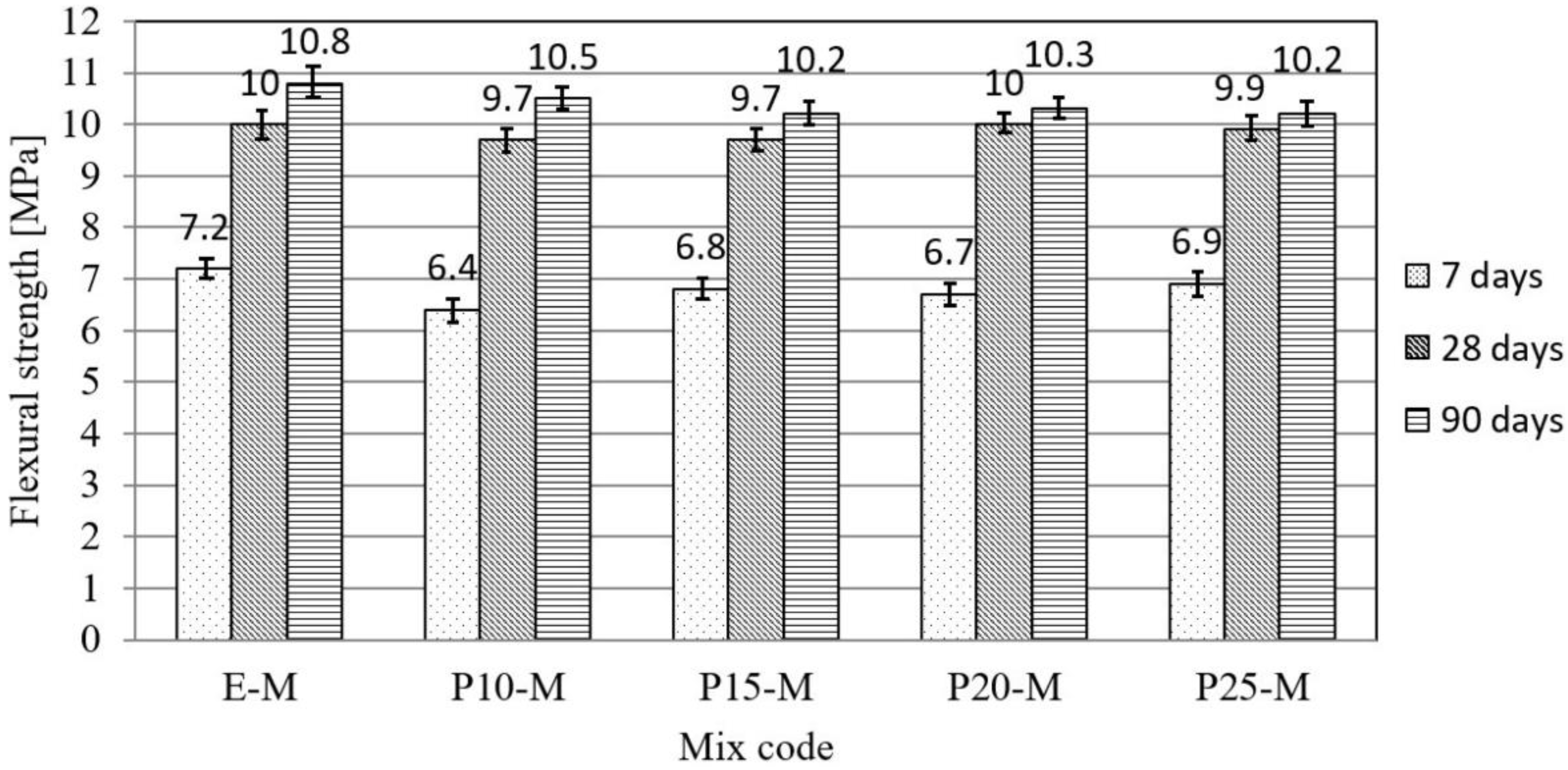
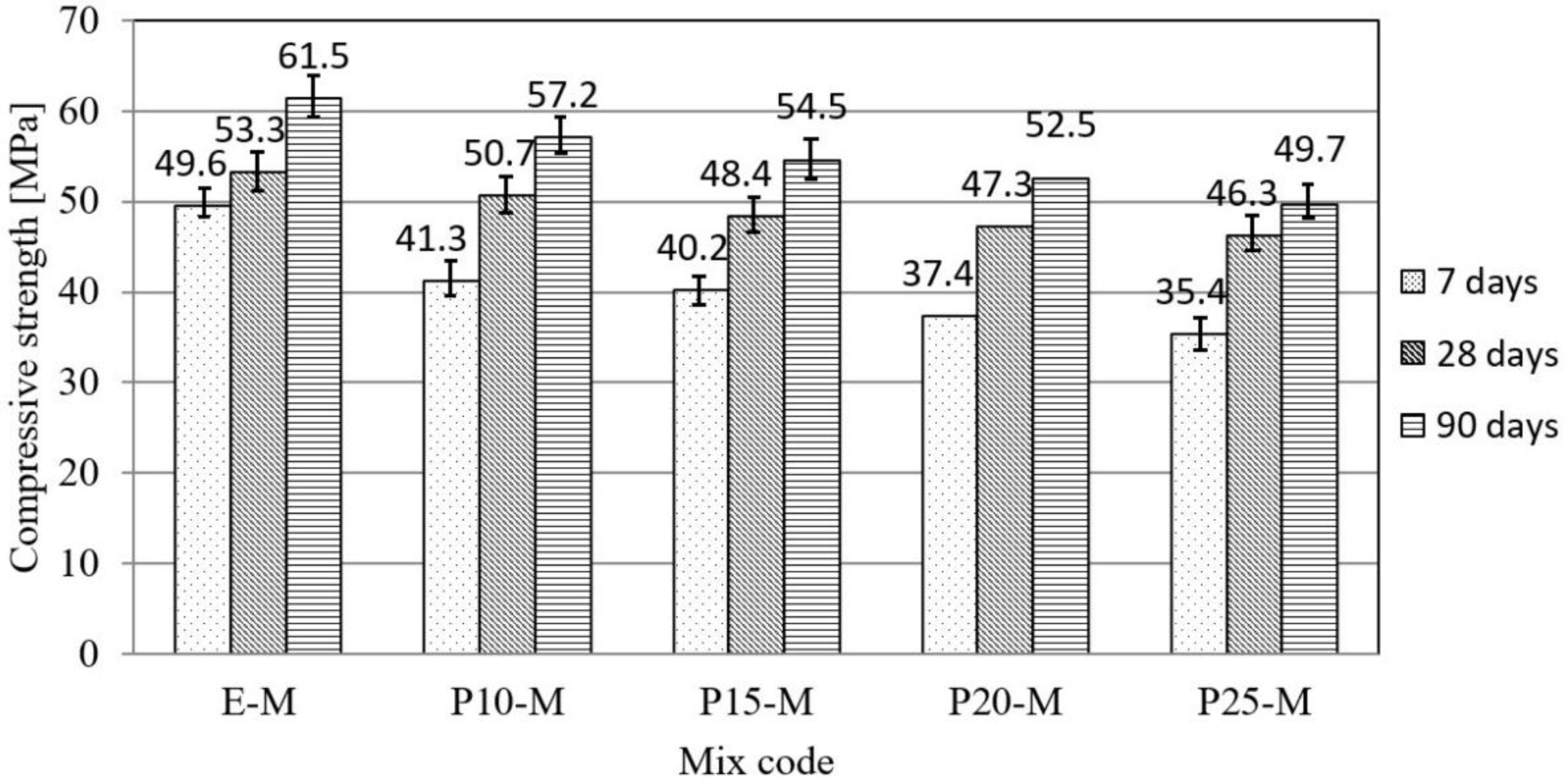
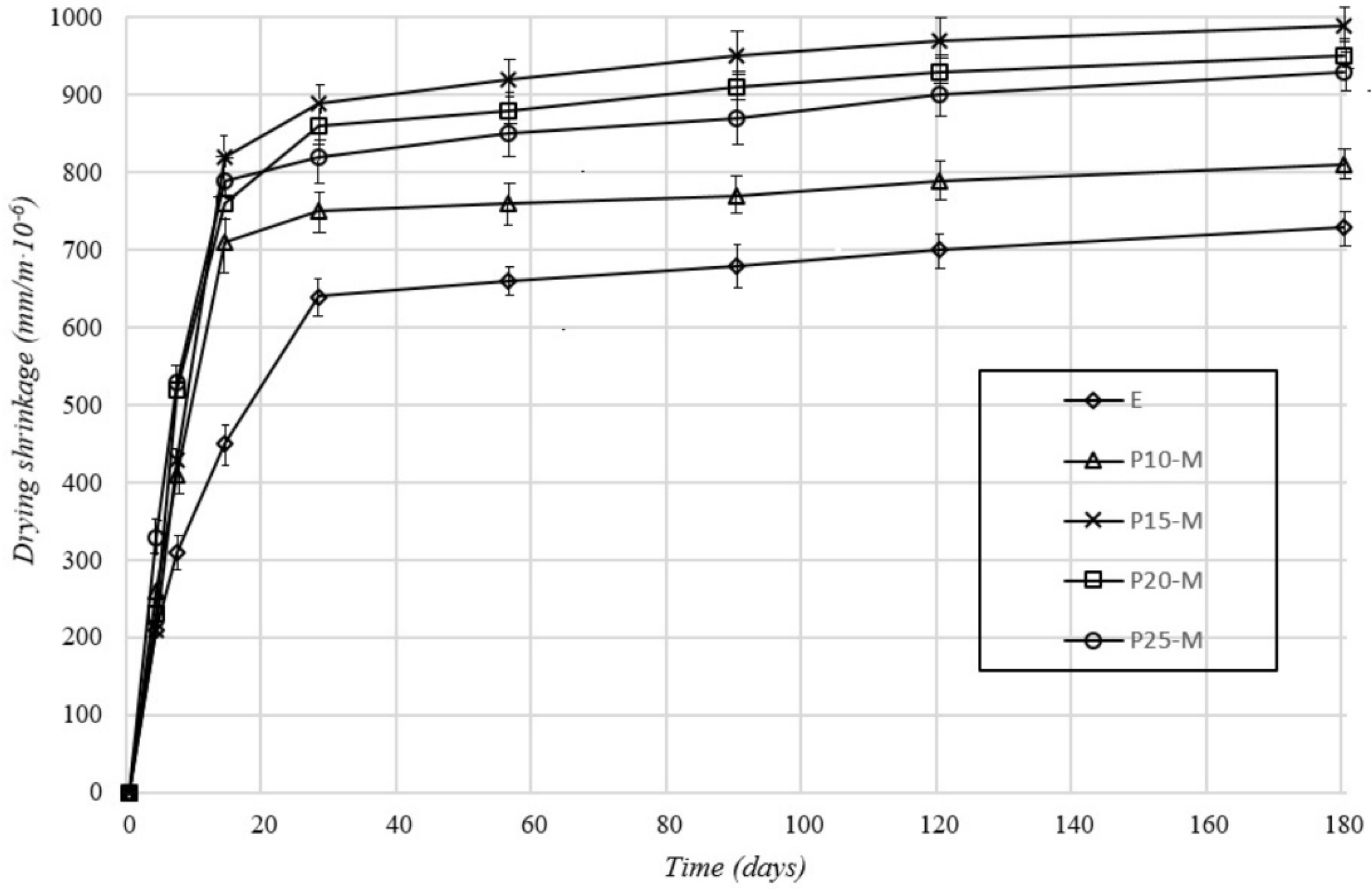
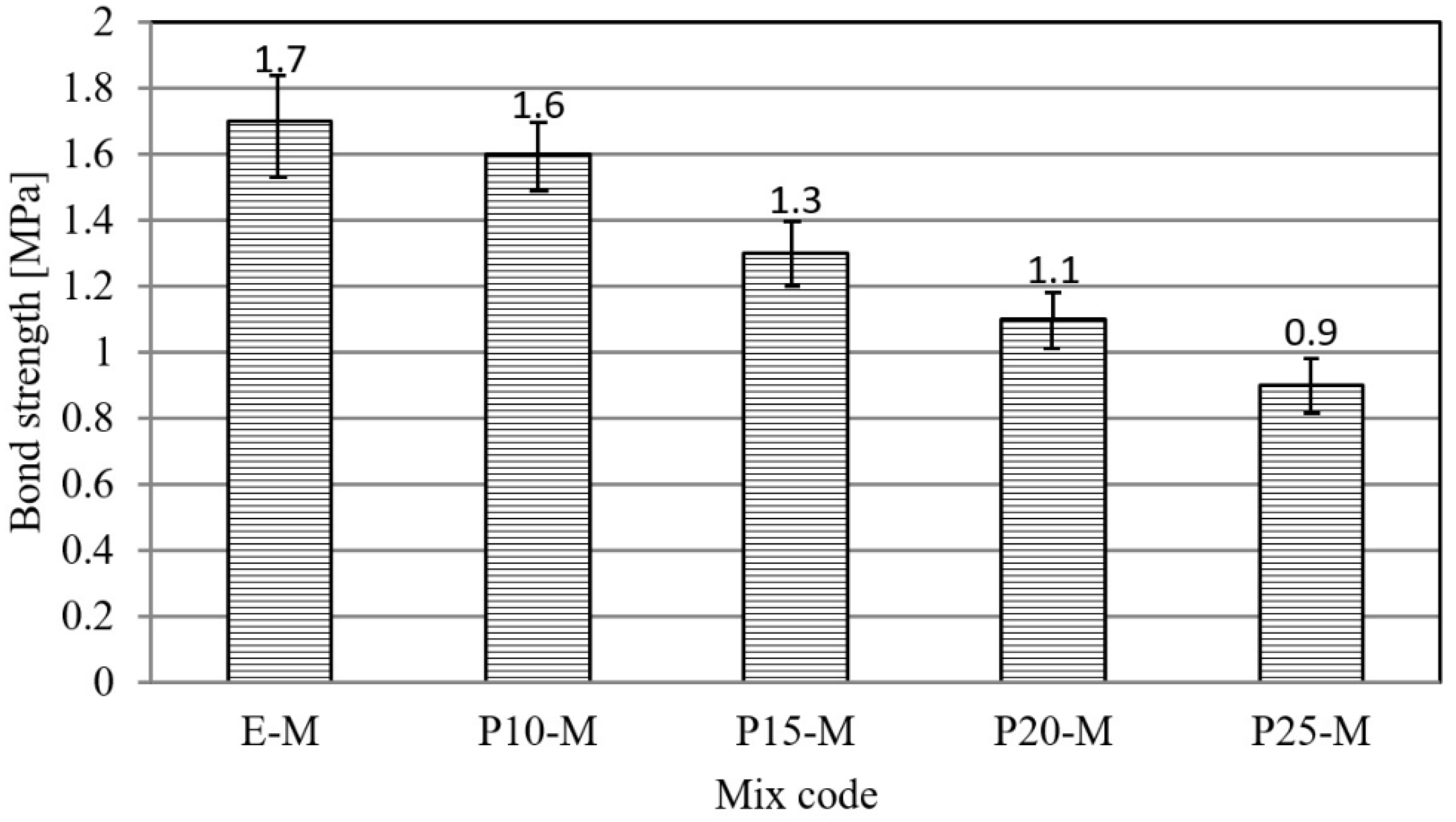
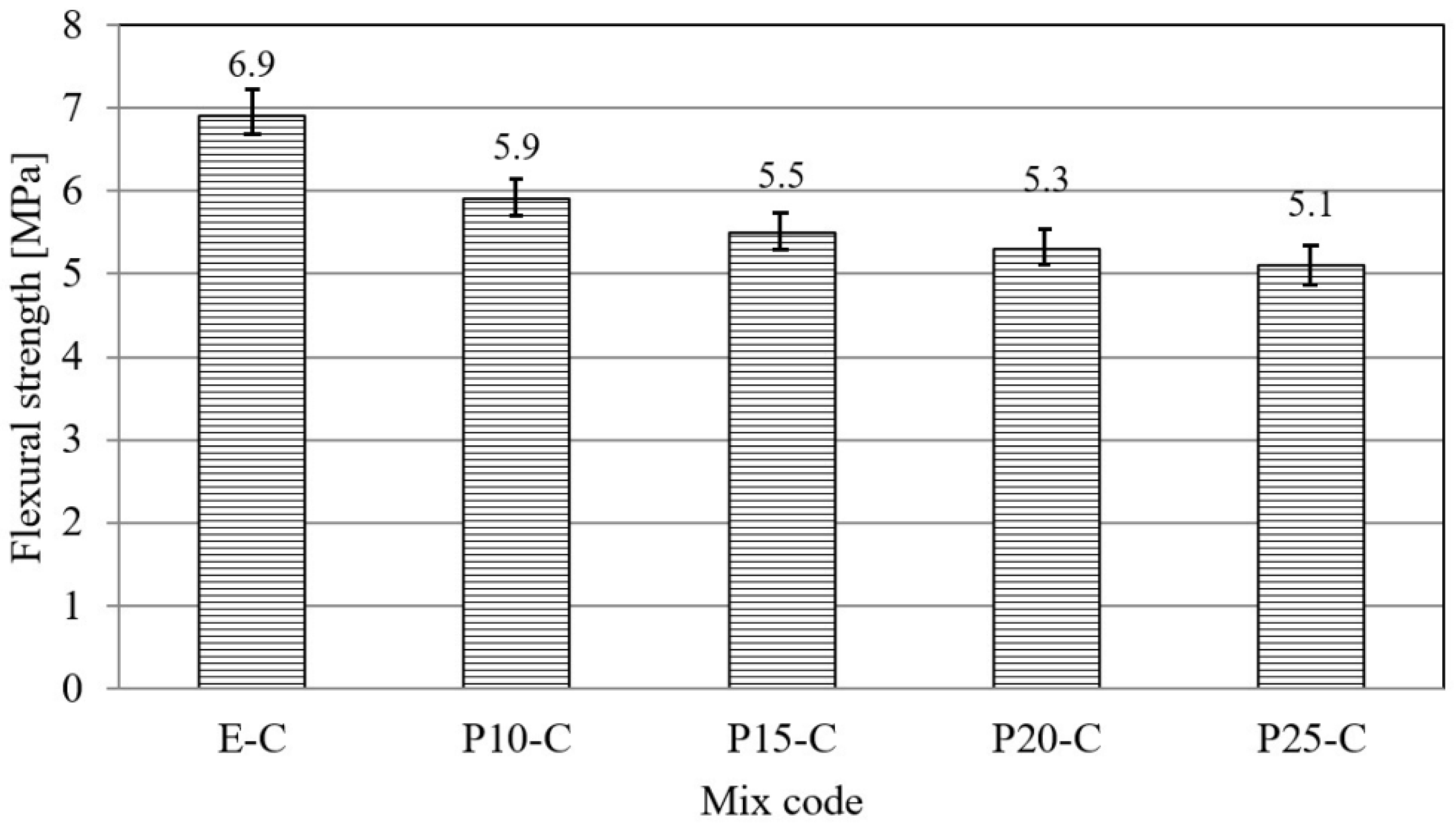
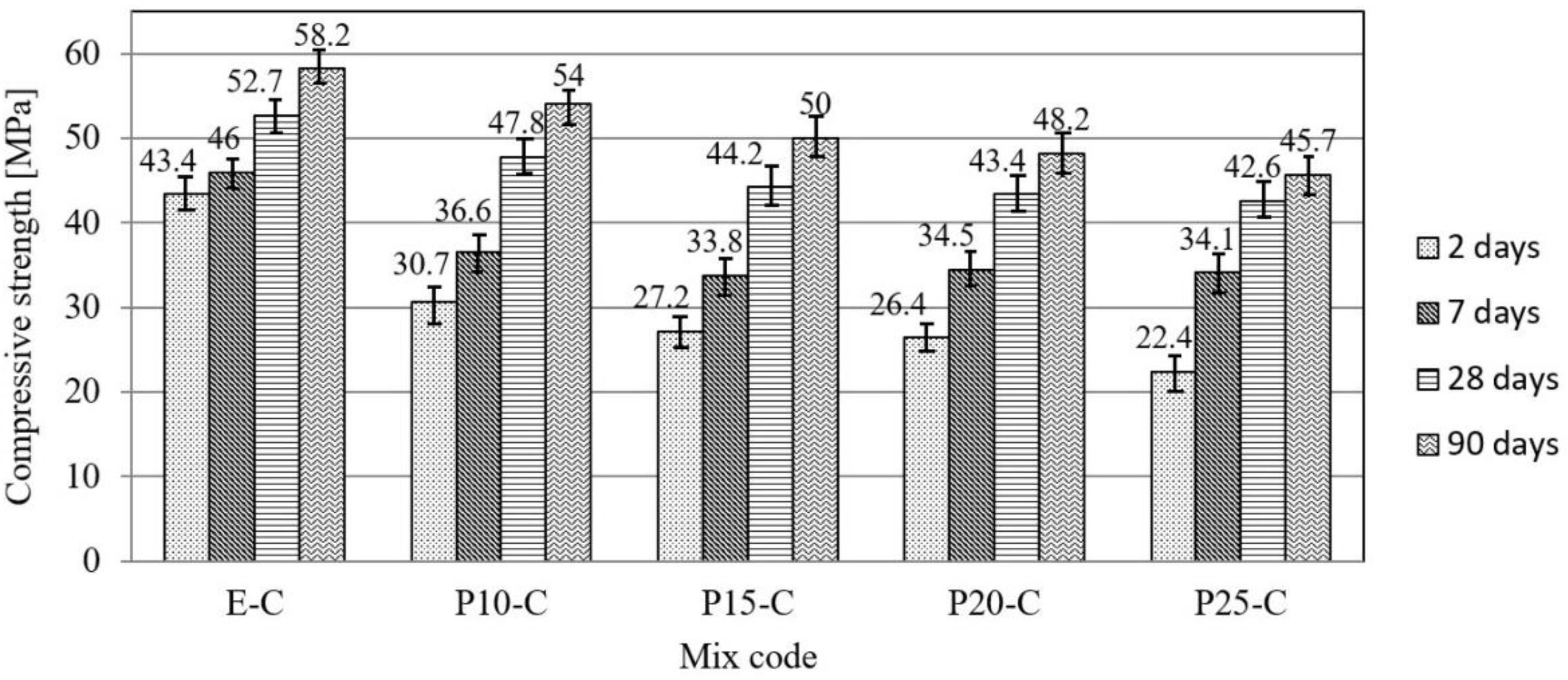
3.1. Physical–Mechanical Characterization of Mortar and Concrete
3.2. X-Ray-Structural Diffraction Analysis (XRD) of the Mortar Samples
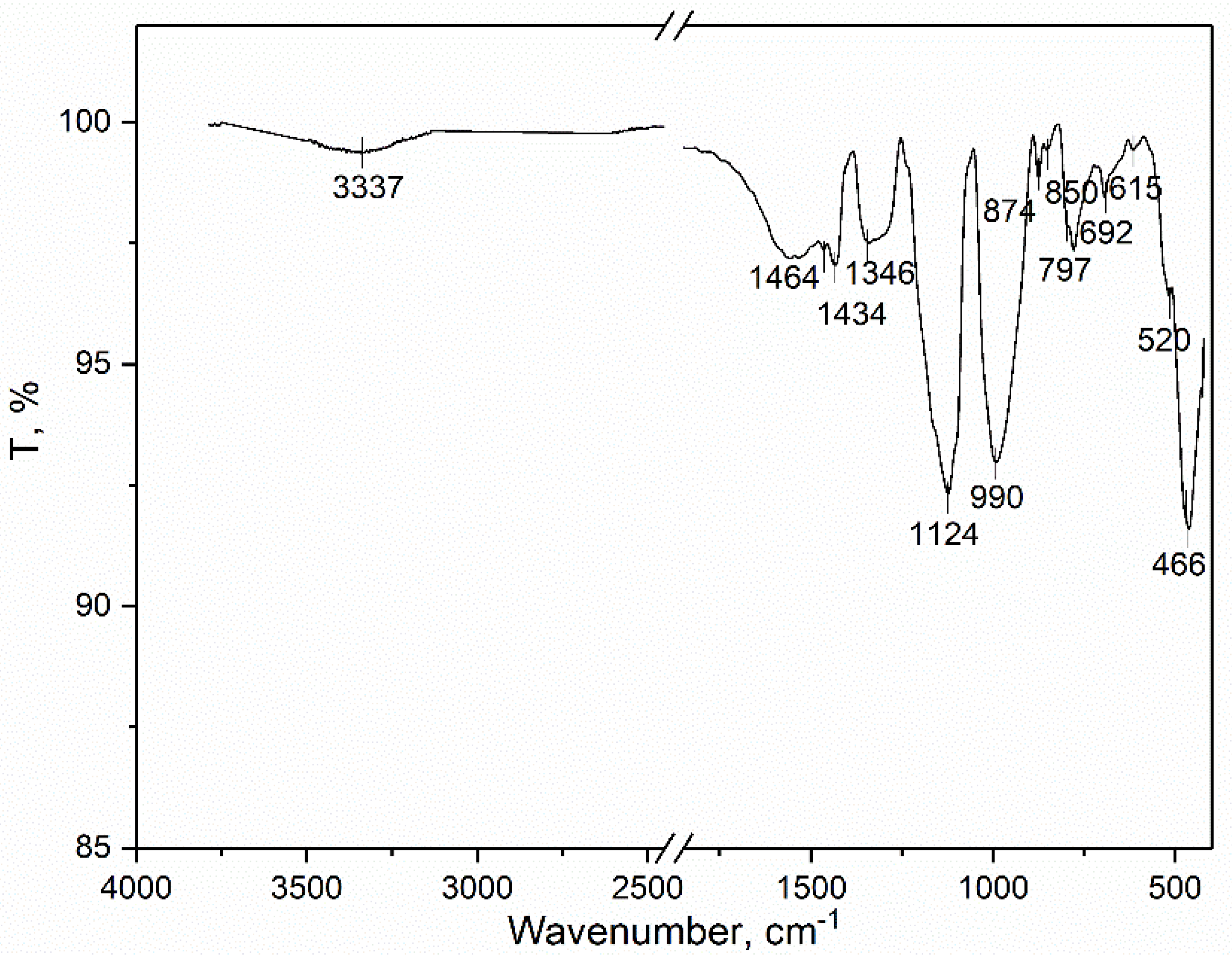
3.3. Infrared Spectroscopy with Fourier Transform (FTIR)
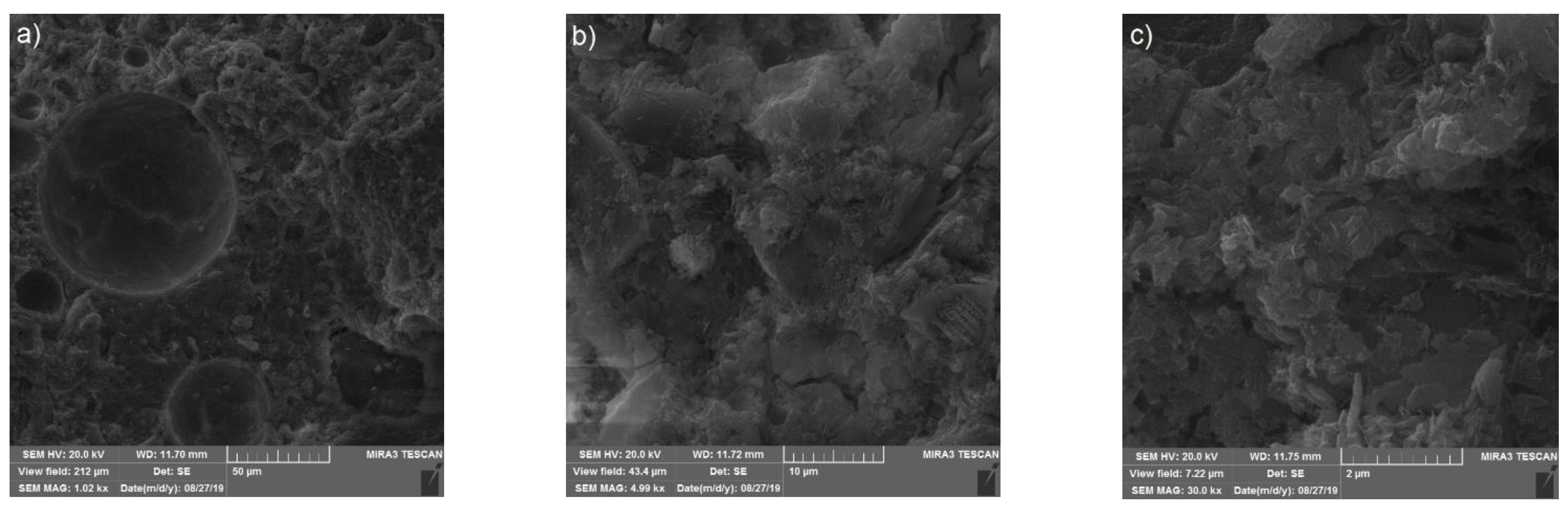
3.4. Scanning Electron Microscopy (SEM) of the Mortar Samples
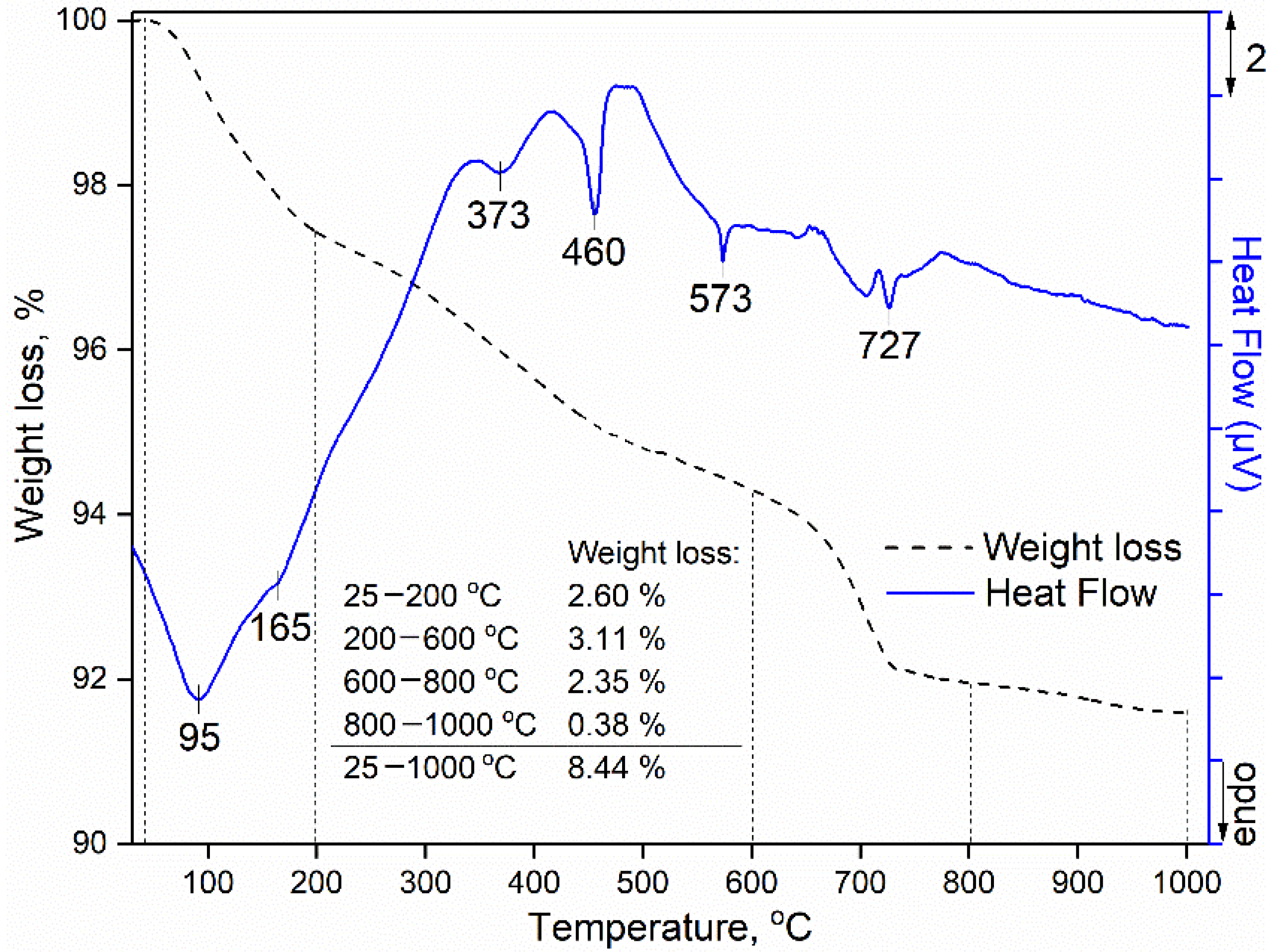
3.5. Thermal Analysis of the Hardened Mortar Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, F.; Xing, Y.; Chang, G.; Yang, Z.; Cao, Y.; Gui, X. Enhanced lignite flotation using interfacial nanobubbles based on temperature difference method. Fuel 2021, 293, 120313. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Li, Y.H.; Song, Q.; Liu, S.C.; Yan, J.; Ma, Q.X.; Zhu, X. Investigation on the thermal behavior characteristics and products composition of four pulverized coals: Its potential applications in coal cleaning. Int. J. Hydrogen Energy 2019, 44, 23620–23638. [Google Scholar] [CrossRef]
- Xia, W.C.; Xie, G.Y.; Peng, Y.L. Recent advances in beneficiation for low rank coals. Powder Technol. 2015, 277, 206–221. [Google Scholar] [CrossRef]
- American Geosciences Institute. Available online: https://www.americangeosciences.org/critical-issues/faq/what-are-the-dierent-types-of-coal (accessed on 30 May 2021).
- Yu, J.L.; Tahmasebi, A.; Han, Y.N.; Yin, F.K.; Li, X.C. A review on water in low rank coals: The existence, interaction with coal structure and effects on coal utilization. Fuel Process Technol. 2013, 106, 9–20. [Google Scholar] [CrossRef]
- Available online: https://iea.blob.core.windows.net/assets/c40f0317-f8e6-4f00-b183-27a24c7b6a8f/Coal_Information_2019_Overview.pdf (accessed on 30 May 2021).
- Aydin, S.; Baradan, B. Efect of pumice and fly ash incorporation on high temperature resistance of cement based mortars. Cem. Concr. Res. 2007, 37, 988–995. [Google Scholar] [CrossRef]
- European List of Waste, the Classification of Waste Based on: The European List of Waste (Commission Decision 2000/532/EC—Consolidated Version) and Annex III to Directive 2008/98/EC (Consolidated Version). Available online: http://data.europa.eu/eli/dec/2000/532/2015-06-01 (accessed on 25 May 2021).
- Berryman, C.; Zhu, J.; Tadros, M. High-percentage replacement of cement with fly ash for reinforced concrete pipe. Cem. Concr. Res. 2005, 35, 1088–1091. [Google Scholar] [CrossRef]
- Ray, T.; Mohanta, N.R.; Kumar, M.H.; Macharyulu, I.S.; Samantaray, S. Study of effect of temperature on behavior of alkali activated slag concrete. Mater. Today Proc. 2021, 43, 1352–1357. [Google Scholar] [CrossRef]
- Czop, M.; Łaźniewska-Piekarczyk, B. Use of Slag from the Combustion of Solid Municipal Waste as a Partial Replacement of Cement in Mortar and Concrete. Materials 2020, 13, 1593. [Google Scholar] [CrossRef] [Green Version]
- Rivera, J.F.; Cristelo, N.; Fernández-Jiménez, A.; de Gutiérrez, R.M. Synthesis of alkaline cements based on fly ash and metallurgic slag: Optimisation of the SiO2/Al2O3 and Na2O/SiO2 molar ratios using the response surface methodology. Constr. Build. Mater. 2019, 213, 424–433. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Ni, W.; Yan, Q.; Gao, W.; Li, Y. Immobilisation of high-arsenic-containing tailings by using metallurgical slag-cementing materials. Chemosphere 2019, 223, 117–123. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Villacorte-Tabelin, M.; Park, I.; Phengsaart, T.; Jeon, S.; Hiroyoshi, N. Copper and critical metals production from porphyry ores and E-wastes: A review of resource availability, processing/recycling challenges, socio-environmental aspects, and sustainability issues. Resour. Conserv. Recycl. 2021, 170, 105610. [Google Scholar] [CrossRef]
- Opiso, E.M.; Tabelin, C.B.; Maestre, C.V.; Aseniero, J.P.J.; Park, I.; Illacorte-Tabelin, M. Synthesis and characterization of coal fly ash and palm oil fuel ash modified artisanal and small-scale gold mine (ASGM) tailings based geopolymer using sugar mill lime sludge as Ca-based activator. Heliyon 2021, 7, e06654. [Google Scholar] [CrossRef]
- Nedeljković, A.; Stojmenović, M.; Gulicovski, J.; Ristić, N.; Milićević, S.; Krstić, J.; Kragović, M. Waste Slag from Heating Plants as a Partial Replacement for Cement in Mortar and Concrete Production. Part I—Physical–Chemical and Physical–Mechanical Characterization of Slag. Minerals 2020, 10, 992. [Google Scholar]
- Cheah, C.B.; Ramli, M. Mechanical strength, durability and drying shrinkage ofstructural mortar containing HCWA as partial replacement of cement. Constr. Build. Mater. 2012, 30, 320–329. [Google Scholar] [CrossRef]
- Garcia, M.D.L.; Sousa-Coutinho, J. Strength and durability of cement with forest waste bottom ash. Constr. Build. Mater. 2013, 2013 41, 897–910. [Google Scholar] [CrossRef]
- Grdić, D.; Ristić, N.; Topličić-Ćurčić, G.; Đorđević, D.; Krstić, N. Effects of addition of finely ground CRT glass on the properties of cement paste and mortar. Građevinar 2020, 72, 1–10. [Google Scholar]
- Mafalda, A.; Sousa-Coutinho, J. Durability of mortar using waste glass powder as cement replacement. Constr. Build. Mater 2012, 36, 205–215. [Google Scholar]
- Chavan, S.P.; Salokhe, S.A.; Nadagauda, P.A.; Patil, S.T.; Mane, K.M. An investigational study on properties of concrete produced with industrial waste red mud. Mater. Today Proc. 2021, 42, 733–738. [Google Scholar] [CrossRef]
- Anirudh, M.; Rekha, K.S.; Venkatesh, C.; Nerella, R. Characterization of red mud based cement mortar; mechanical and microstructure studies. Mater. Today Proc. 2021, 43, 1587–1591. [Google Scholar] [CrossRef]
- Sadiqul, I.G.M.; Rahman, M.H.; Kazi, N. Waste glass powder as partial replacement of cement for sustainable concrete practice. Int. J. Sustain. Built Environ. 2017, 6, 37–44. [Google Scholar]
- Seo, J.; Kim, S.; Park, S.; Yoon, H.N.; Lee, H.K. Carbonation of calcium sulfoaluminate cement blended with blast furnace slag. Cem. Concr. Compos 2021, 118, 103918. [Google Scholar] [CrossRef]
- Liao, Y.; Jiang, G.; Wang, K.; Al Qunaynah, S.; Yuan, W. Effect of steel slag on the hydration and strength development of calcium sulfoalumi-nate cement. Constr. Build. Mater. 2020, 265, 120301. [Google Scholar] [CrossRef]
- Sengül, K.; Erdogan, S.T. Influence of ground perlite on the hydration and strength develop-ment of calcium aluminate cement mortars. Constr. Build. Mater. 2021, 266, 120943. [Google Scholar] [CrossRef]
- Mojet, B.L.; Ebbesenz, S.D.; Lefferts, L. Light at the interface: The potential of attenuated total reflection infrared spectroscopy for understanding heterogeneous catalysis in water. Chem. Soc. Rev. 2010, 39, 4643–4655. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Danilushkina, A.A.; Evtugyn, V.G.; Lazzara, G.; Milioto, S.; Parisi, F.; Rozhina, E.V.; Fakhrullin, R.F. Halloysite Nanotubes: Controlled Access and Release by Smart Gates. Nanomaterials 2017, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Abdolmohammadi, S.; Siyamak, S.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Rahman, M.Z.A.; Azizi, S.; Fatehi, A. Enhancement of Mechanical and Thermal Properties of Polycaprolactone/Chitosan Blend by Calcium Carbonate Nanoparticles. Int. J. Mol. Sci. 2012, 13, 4508–4522. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Pham, T.V.A.; Le, M.L.P.; Nguyen, T.P.T.; Tran, V.M. Synthesis of amorphous silica and sulfonic acid functionalized silica used as reinforced phase for polymer electrolyte membrane. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 045007. [Google Scholar] [CrossRef] [Green Version]
- Tabelin, C.B.; Veerawattananun, S.; Ito, M.; Hiroyoshi, N.; Igarashi, T. Pyrite oxidation in the presence of hematite and alumina: I. Batch leaching experiments and kinetic modeling calculations. Sci. Total Environ. 2017, 580, 687–698. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Magaribuchi, K.; Seno, K.; Ito, M.; Hiroyoshi, N. Suppression of the release of arsenic carrier-microencapsulation from arsenopyrite by using Ti-catechol complex. J. Hazard. Mater. 2018, 344, 322–332. [Google Scholar] [CrossRef]
- Handke, M.; Ptak, W. IR and Raman studies of the stabilization beta-Ca2SiO4. Ceramurgia international 1978, 4, 75. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Z.; Zhang, L.; Shang, Y.; Wang, H. Experimental research on drying control condition with minimal effect on concrete strength. Constr. Build. Mater 2017, 135, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Schelz, J.P. The detection of quartz in clay minerals by differential thermal analysis. Thermochimicu Acta 1976, 15, 17-B. [Google Scholar] [CrossRef]
- Parvan, M.-G.; Voicu, G.; Badanoiu, A.-I.; Nicoara, A.-I.; Vasile, E. CO2 Sequestration in the Production of Portland Cement Mortars with Calcium Carbonate Additions. Nanomaterials 2021, 11, 875. [Google Scholar] [CrossRef] [PubMed]

| Properties/Content | Cement | Slag | Silica Fume |
|---|---|---|---|
| ρ, g/cm3 | 3.15 | 2.24 | 2.21 |
| SBET, m2/g | 1.22 | 25.00 | 28.14 |
| SiO2, % | 21.62 | 21.20 | 93.60 |
| Fe2O3, % | 2.60 | 10.81 | 0.21 |
| Al2O3, % | 7.00 | 15.70 | 0.27 |
| CaO, % | 60.16 | 6.32 | 0.05 |
| MgO, % | 2.34 | 2.50 | 0.05 |
| SO3, % | 2.55 | 1.78 | 0.80 |
| Na2O, % | 0.33 | 2.67 | 0.23 |
| K2O, % | 0.66 | 0.63 | 0.50 |
| P2O5, % | - | 0.036 | - |
| LOI, % | 2.68 | 37.45 | 2.40 |
| Mineral composition | brownmillerite, calcium silicate oxide, calcite, larnite, magnesium silicate, calcium hydroxide | quartz, olivine, magnetite, pyrite, tridymite, feldspars | quartz |
| Component | E-M | P10-M | P15-M | P20-M | P25-M |
|---|---|---|---|---|---|
| Cement [g (cm3)] | 450.00 (142.86) | 402.34 (127.73) | 378.73 (120.23) | 355.27 (112.78) | 331.96 (105.38) |
| Slag [g (cm3)] | - | 39.79 (17.76) | 59.48 (26.55) | 79.05 (35.29) | 98.48 (43.96) |
| Silica fumes [g (cm3)] | - | 4.90 (2.23) | 7.36 (3.33) | 9.77 (4.42) | 12.18 (5.51) |
| River sand 0/2 mm [g (cm3)] | 1350.00 (517.24) | 1341.14 (513.85) | 1336.70 (512.15) | 1332.26 (510.44) | 1327.83 (508.75) |
| Water [g] | 225.00 (225.00) | 223.52 (223.52) | 222.78 (222.78) | 222.04 (222.04) | 221.30 (221.30) |
| Superplasticizer [g (cm3)] | 3.60 (3.30) | 3.58 (3.28) | 3.56 (3.27) | 3.55 (3.26) | 3.54 (3.25) |
| SUM [g (cm3)] | 2028.60 (888.40) | 2015.29 (888.37) | 2008.62 (888.24) | 2001.94 (888.24) | 1995.28 (888.16) |
| Component | E-C | P10-C | P15-C | P20-C | P25-C |
|---|---|---|---|---|---|
| Cement [kg (m3)] | 375.00 (0.119) | 336.32 (0.107) | 316.78 (0.101) | 297.32 (0.094) | 277.67 (0.088) |
| Slag [kg] | - | 33.16 (0.015) | 49.65 (0.022) | 66.08 (0.029) | 82.34 (0.037) |
| Silica fumes [kg (m3)] | - | 4.15 (0.002) | 6.21 (0.003) | 8.26 (0.004) | 10.30 (0.005) |
| River sand 0/4 mm [kg (m3)] | 752.00 (0.287) | 748.26 (0.286) | 746.77 (0.285) | 745.29 (0.284) | 743.08 (0.284) |
| Crushed aggr. (4/8 mm) [kg (m3)] | 432.00 (0.149) | 429.85 (0.149) | 429.00 (0.148) | 428.15 (0.148) | 426.88 (0.148) |
| Crushed aggr. (8/16 mm) [kg (m3)] | 692.00 (0.239) | 688.56 (0.238) | 687.19 (0.238) | 685.83 (0.237) | 683.79 (0.237) |
| Water [kg (m3)] | 182.25 (0.182) | 181.34 (0.181) | 180.98 (0.181) | 180.62 (0.181) | 180.09 (0.180) |
| Superplasticizer [kg (m3)] | 3.75 (0.003) | 3.73 (0.003) | 3.72 (0.003) | 3.72 (0.003) | 3.71 (0.003) |
| SUM | 2437 (0.981) | 2425.37 (0.981) | 2420.31 (0.981) | 2415.26 (0.981) | 2407.86 (0.981) |
| Assumed air content | ~2% | ~2% | ~2% | ~2% | ~2% |
| Component | E-C | P10-C | P15-C | P20-C | P25-C |
| Cement [kg (m3)] | 375.00 (0.119) | 336.32 (0.107) | 316.78 (0.101) | 297.32 (0.094) | 277.67 (0.088) |
| Slag [kg] | - | 33.16 (0.015) | 49.65 (0.022) | 66.08 (0.029) | 82.34 (0.037) |
| Silica fumes [kg (m3)] | - | 4.15 (0.002) | 6.21 (0.003) | 8.26 (0.004) | 10.30 (0.005) |
| River sand 0/4 mm [kg (m3)] | 752.00 (0.287) | 748.26 (0.286) | 746.77 (0.285) | 745.29 (0.284) | 743.08 (0.284) |
| Crushed aggr. (4/8 mm) [kg (m3)] | 432.00 (0.149) | 429.85 (0.149) | 429.00 (0.148) | 428.15 (0.148) | 426.88 (0.148) |
| Crushed aggr. (8/16 mm) [kg (m3)] | 692.00 (0.239) | 688.56 (0.238) | 687.19 (0.238) | 685.83 (0.237) | 683.79 (0.237) |
| Water [kg (m3)] | 182.25 (0.182) | 181.34 (0.181) | 180.98 (0.181) | 180.62 (0.181) | 180.09 (0.180) |
| Superplasticizer [kg (m3)] | 3.75 (0.003) | 3.73 (0.003) | 3.72 (0.003) | 3.72 (0.003) | 3.71 (0.003) |
| SUM | 2437 (0.981) | 2425.37 (0.981) | 2420.31 (0.981) | 2415.26 (0.981) | 2407.86 (0.981) |
| Assumed aer content | ~2% | ~2% | ~2% | ~2% | ~2% |
| Parameter | Unit | E-M | P10-M | P15-M | P20-M | P25-M |
|---|---|---|---|---|---|---|
| Consistency: by flow table | mm | 205 ± 7.9 | 187 ± 7.2 | 169 ± 7.5 | 152 ± 6.4 | 136 ± 6.0 |
| Bulk density of fresh mortar | kg/m3 | 2248 ± 12.3 | 2215 ± 10.4 | 2186 ± 12.0 | 2158 ± 9.1 | 2126 ± 10.9 |
| Dry bulk density of hardened mortar | kg/m3 | 2176 ± 11.5 | 2132 ± 12.4 | 2073 ± 12.0 | 2055 ± 9.7 | 2031 ± 10.2 |
| Water absorption (water absorption at atmospheric pressure) | % (m/m) | 7.1 ± 0.19 | 7.5 ± 0.15 | 8.2 ± 0.17 | 8.1 ± 0.14 | 7.9 ± 0.18 |
| Water absorption (coefficient due to capillary action of hardened mortar): for mortars other than renovation mortars | m2 × min−0.5 | 0.25 ± 0.016 | 0.21 ± 0.018 | 0.23 ± 0.014 | 0.23 ±0.020 | 0.19 ±0.015 |
| Water absorption (coefficient due to capillary action of hardened mortar): for renovation mortars | kg/m2 | 7.65 ± 0.104 | 5.19 ± 0.122 | 5.88 ± 0.134 | 5.67 ± 0.115 | 5.11 ± 0.128 |
| Parameter | Unit | E-C | P10-C | P15-C | P20-C | P25-C |
|---|---|---|---|---|---|---|
| Consistency: slump test | mm | 240 ± 12.3 | 180 ± 11.6 | 140 ± 10.4 | 90 ± 8.2 | 20 ± 6.5 |
| Air content in fresh concrete | % | 2.5 ± 0.25 | 3.4 ± 0.32 | 4.1 ± 0.34 | 5.0 ± 0.33 | 5.6 ± 0.35 |
| Density of fresh concrete | kg/m3 | 2473 ± 17.7 | 2432 ± 16.2 | 2369 ± 15.5 | 2349 ± 16.0 | 2341 ± 14.3 |
| Density of hardened concrete (water-saturated | kg/m3 | 2465 ± 16.9 | 2422 ± 17.5 | 2360 ± 14.7 | 2338 ± 15.4 | 2331 ± 15.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kragović, M.; Ristić, N.; Gulicovski, J.; Nedeljković, A.; Pašalić, S.; Ristović, I.; Stojmenović, M. Application of Lignite Combustion Waste Slag Generated in Heating Plants as a Partial Replacement for Cement. Part II: Physical–Mechanical and Physical–Chemical Characterization of Mortar and Concrete. Minerals 2021, 11, 925. https://doi.org/10.3390/min11090925
Kragović M, Ristić N, Gulicovski J, Nedeljković A, Pašalić S, Ristović I, Stojmenović M. Application of Lignite Combustion Waste Slag Generated in Heating Plants as a Partial Replacement for Cement. Part II: Physical–Mechanical and Physical–Chemical Characterization of Mortar and Concrete. Minerals. 2021; 11(9):925. https://doi.org/10.3390/min11090925
Chicago/Turabian StyleKragović, Milan, Nenad Ristić, Jelena Gulicovski, Andrijana Nedeljković, Snežana Pašalić, Ivica Ristović, and Marija Stojmenović. 2021. "Application of Lignite Combustion Waste Slag Generated in Heating Plants as a Partial Replacement for Cement. Part II: Physical–Mechanical and Physical–Chemical Characterization of Mortar and Concrete" Minerals 11, no. 9: 925. https://doi.org/10.3390/min11090925
APA StyleKragović, M., Ristić, N., Gulicovski, J., Nedeljković, A., Pašalić, S., Ristović, I., & Stojmenović, M. (2021). Application of Lignite Combustion Waste Slag Generated in Heating Plants as a Partial Replacement for Cement. Part II: Physical–Mechanical and Physical–Chemical Characterization of Mortar and Concrete. Minerals, 11(9), 925. https://doi.org/10.3390/min11090925








