Explaining Colour Change in Pyrope-Spessartine Garnets
Abstract
:1. Introduction
2. Materials and Methods
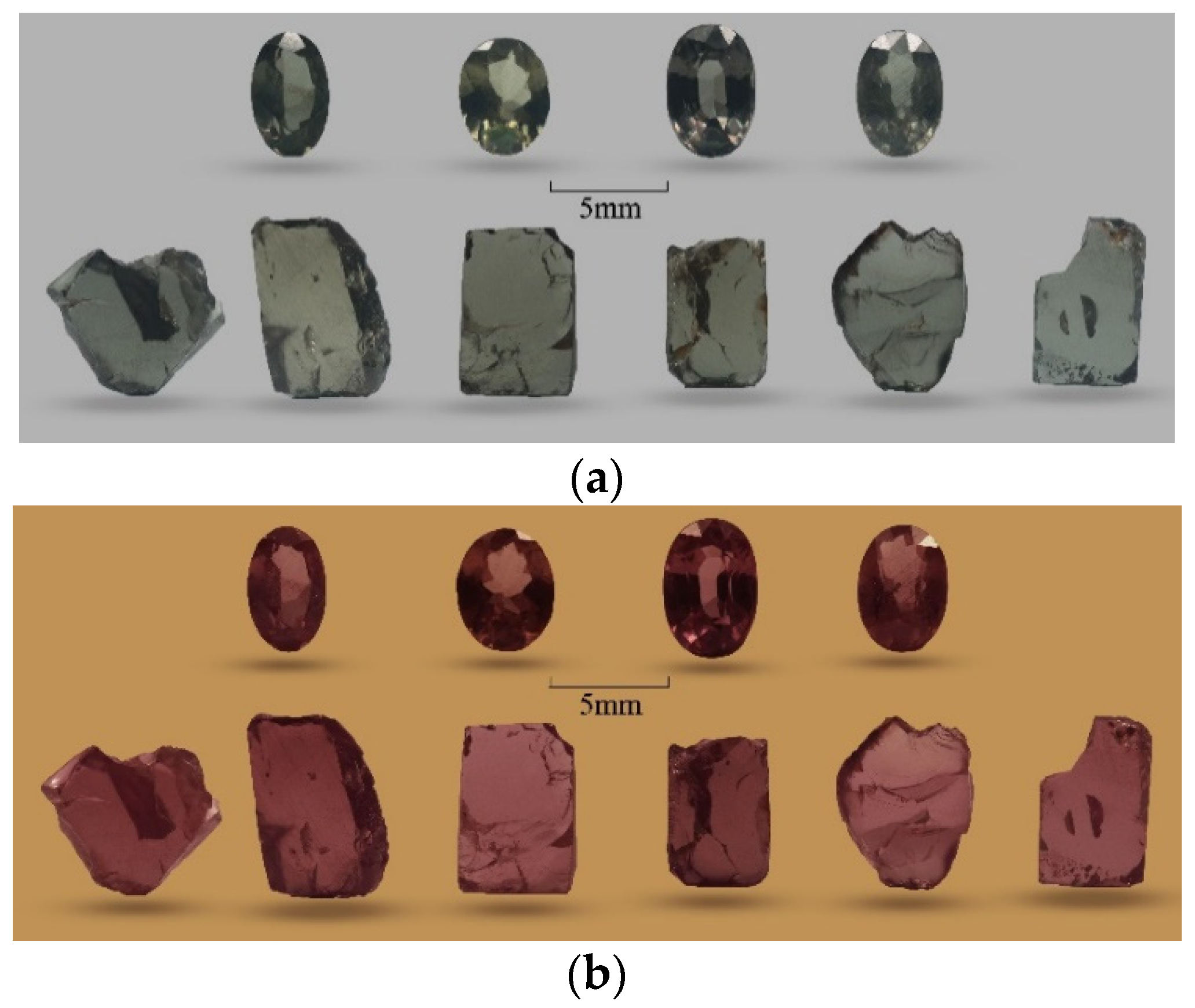
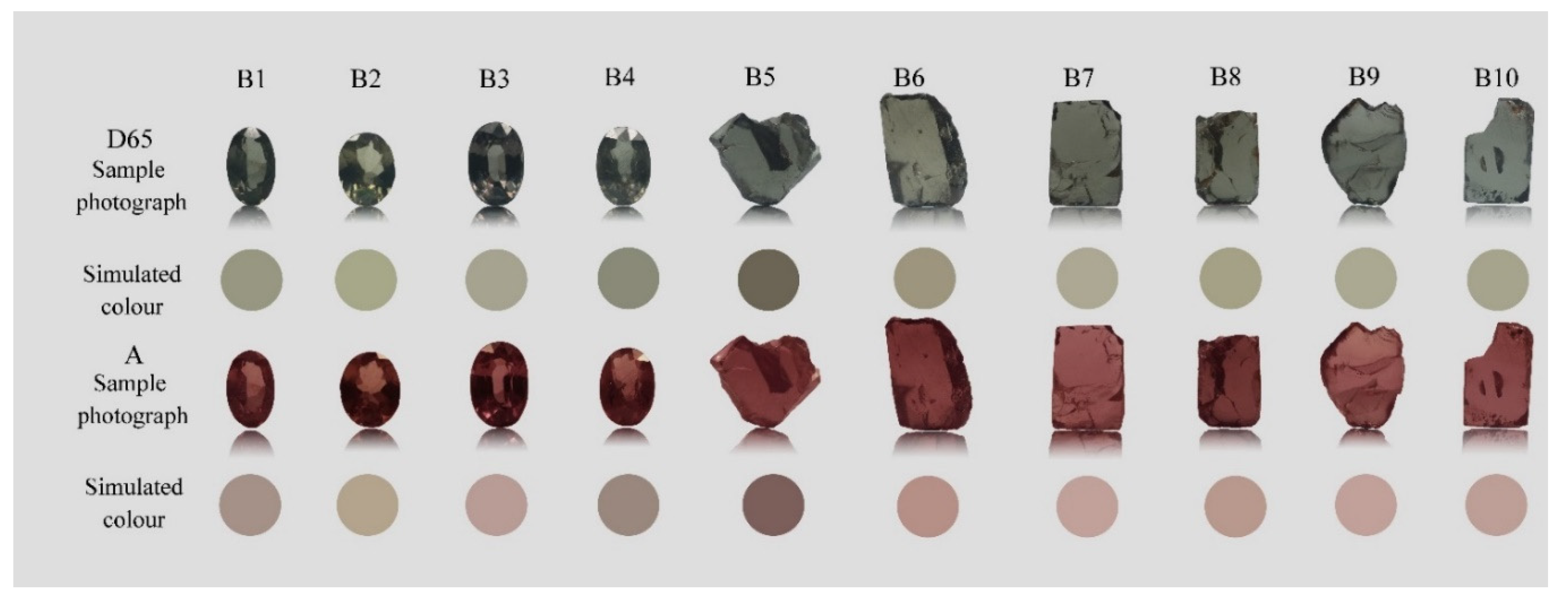
2.1. Samples
2.2. Fourier Transform Infrared (FTIR) Spectroscopy
2.3. UV-Vis Spectroscopy
2.4. Correcting the UV-Vis Spectra
2.5. CIE1931 XYZ Colour Matching Functions
2.6. CIE1976 La*b* Colour System
2.7. Colour Space Conversion
3. Results
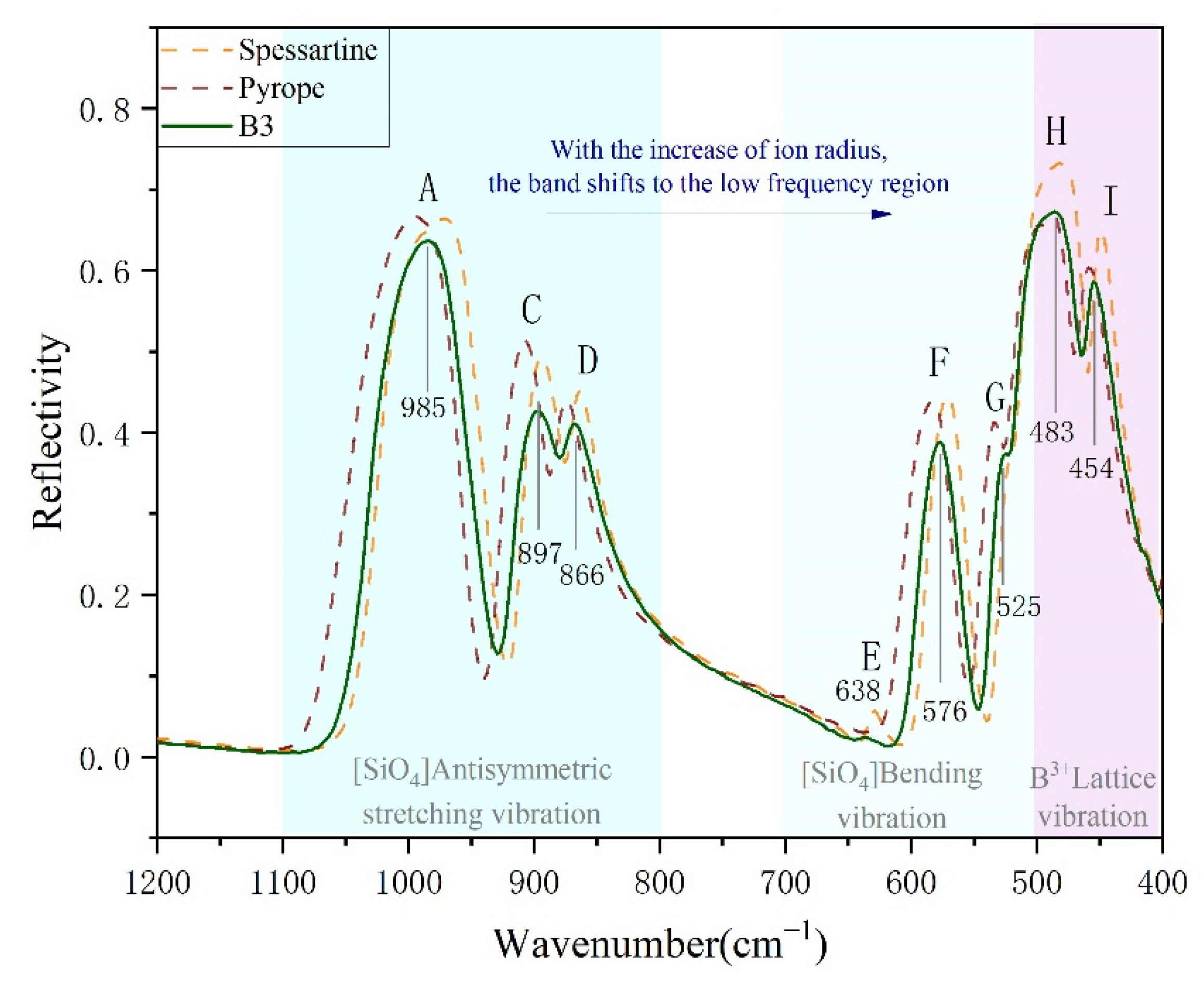
3.1. Infrared Spectral Characteristics of Colour-Changing Garnet
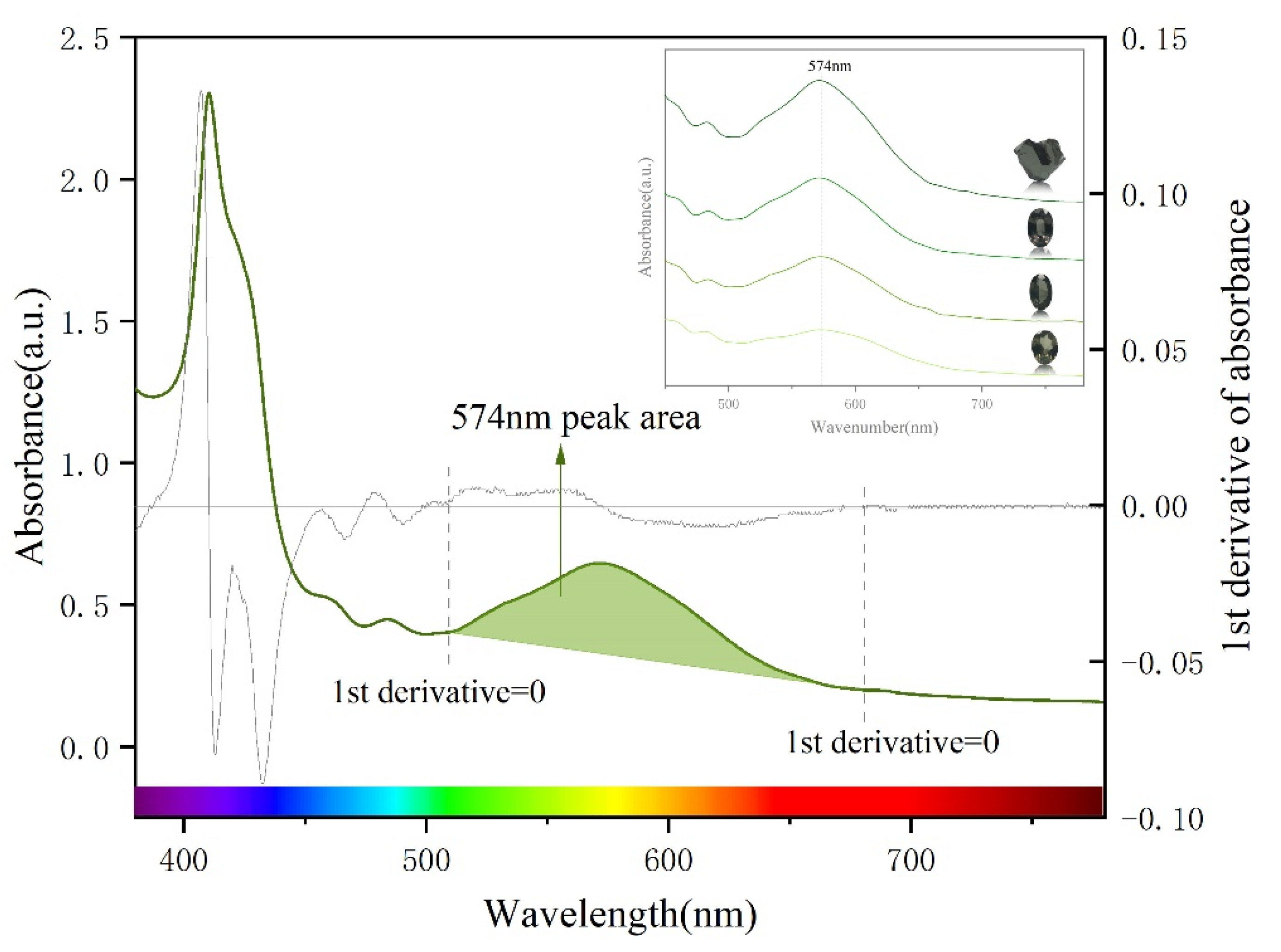
3.2. UV-Vis Spectral Characteristics of Colour-Changing Garnet
3.3. Correcting the Absorbance of UV-Vis Spectra
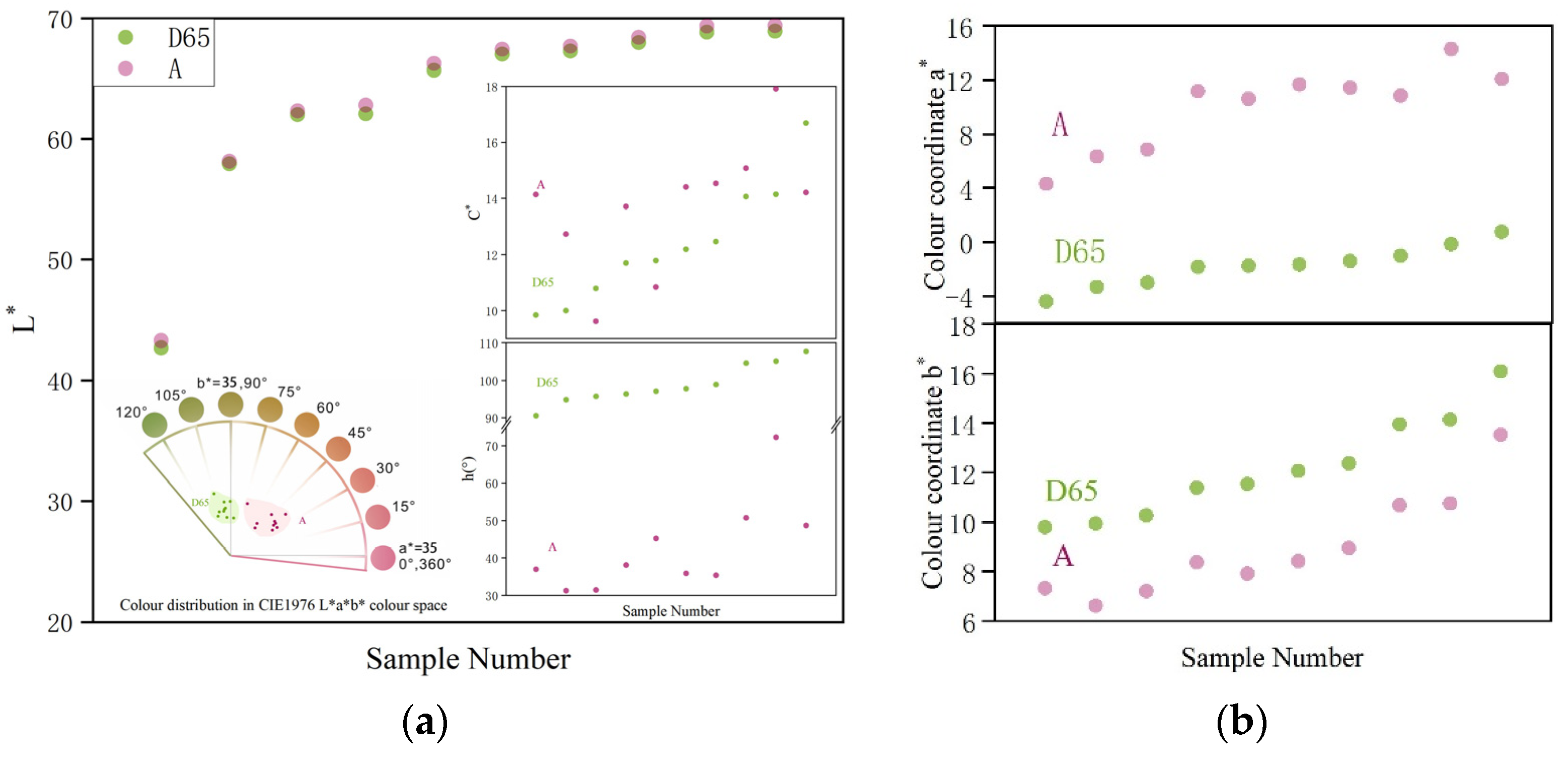
3.4. Colour Calculation by CIE1931 XYZ Colour Matching Functions
4. Discussion
4.1. Effect of Standard Light Source on Appearance of Colour-Changing Garnet
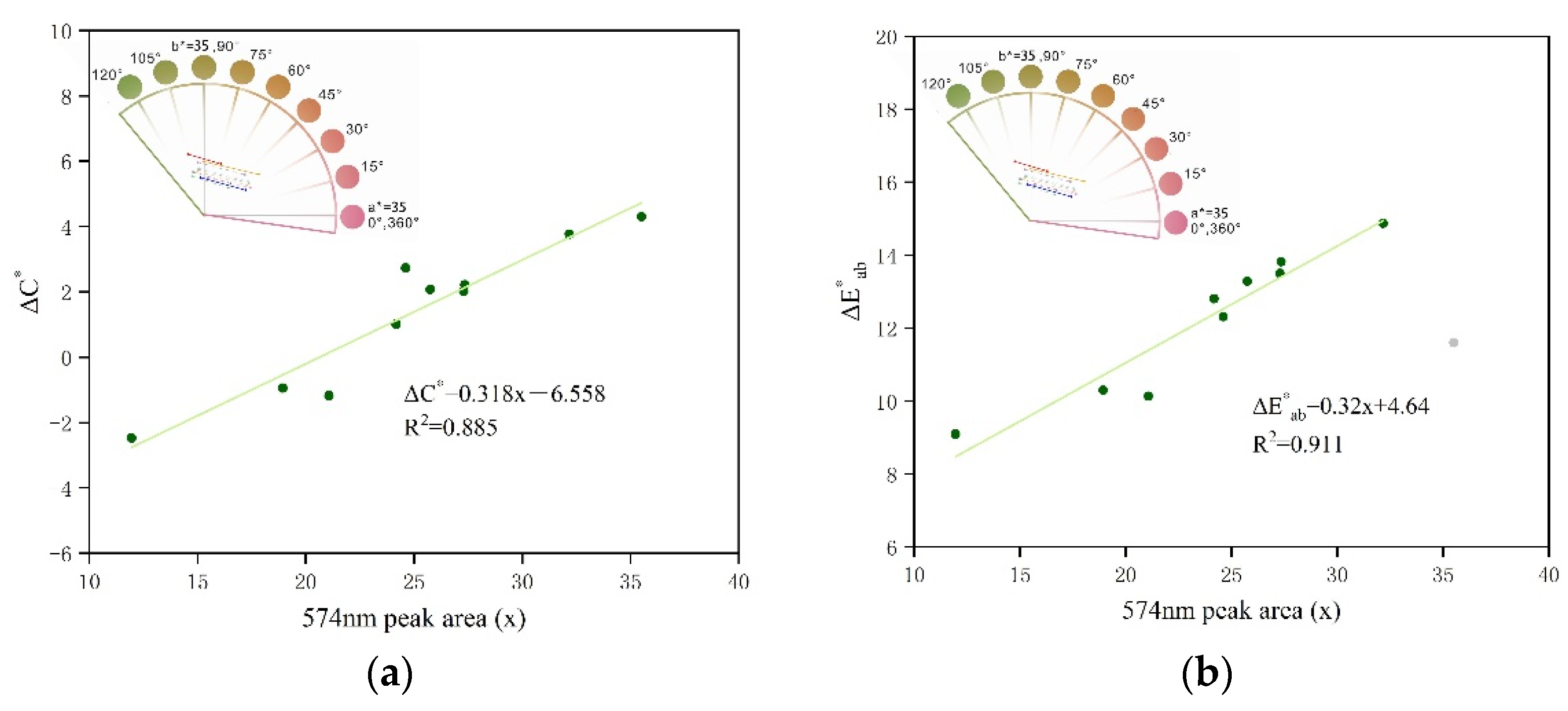
4.2. Effect of UV-Vis Absorbance Peak Area on Colorimetric Coordinate a* and Hue Angle h(°)
4.3. Effect of UV-Vis Absorbance Peak Area on Chroma Difference ΔC* and Colour Difference
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolesov, B.A.; Geiger, C.A. Raman spectra of silicate garnets. Phys. Chem. Miner. 1998, 25, 142–151. [Google Scholar] [CrossRef]
- Novak, G.A.; Gibbs, G.V. The crystal chemistry of the silicate garnets. Am. Mater. 1971, 56, 791–825. [Google Scholar]
- Pinheir, M.V.B.; Basiho, M.S. The cause of colour of the blue alexandrites from Malacacheta, Minas Gerais, Brazil. J. Gemol. 2000, 27, 161–170. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Lu, T.J.; Sun, R.D.; Shi, G.H.; Chen, H.; Ke, J. Explanation of the alexandrite effect of zultanite: From the view of colorimetry and chemical analysis. Color Res. Appl. 2019, 44, 925–931. [Google Scholar] [CrossRef]
- Shen, C.; Lu, R. The color origin of gem diaspore: Correlation to corundum. Gems Gemol. 2018, 54, 394–403. [Google Scholar] [CrossRef]
- Liu, Y.; Shigley, J.; Moses, T.; Reinitz, I. The alexandrite effect of the Tavernier diamond caused by fluorescence under daylight. Color Res. Appl. 1998, 23, 323–327. [Google Scholar] [CrossRef]
- Chen, C.Y.; Huang, W.Z.; Shao, T.; Shen, C.; Li, Z.B.; Shen, A.H. The study on UV-Vis spectrum of a special color-changed sapphire. Spectrosc. Spectr. Anal. 2019, 39, 2470–2473. [Google Scholar]
- Sun, Z.Y.; Palke, A.C.; Renfro, N.; Breitzmann, H.; Hand, D.; Muyal, J. Discovery of color-change chrome grossular garnets from Ethiopia. Gems Gemol. 2018, 54, 233–236. [Google Scholar]
- Promwongnan, S.; Buathong, A. A rare nearly pure end-member grossular garnet with color-change effect. Gems Gemol. 2018, 54, 336. [Google Scholar]
- Schmetzer, K.; Hainschwang, T.; Bernhardt, H.-J.; Kiefert, L. New chromium- and vanadium-bearing garnets from Tranoroa, Madagascar. Gems Gemol. 2002, 38, 148–155. [Google Scholar] [CrossRef]
- Schmetzer, K.; Bernhardt, H.-J. Garnets from Madagascar with a color change of blue-green to purple. Gems Gemol. 1999, 35, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.Y.; Palke, A.C.; Renfro, N. Vanadium- and chromium-bearing pink pyrope garnet: Characterization and quantitative colorimetric analysis. Gems Gemol. 2015, 51, 348–369. [Google Scholar] [CrossRef] [Green Version]
- Gübelin, E.; Schmetzer, K. Gemstones with alexandrite effect. Gems Gemol. 1982, 18, 197–203. [Google Scholar] [CrossRef]
- Liu, Y.; Shigley, J.; Fritsch, E.; Hemphill, S. The “alexandrite effect” in gemstones. Color Res. Appl. 1994, 19, 186–191. [Google Scholar] [CrossRef]
- Tang, J.; Guo, Y.; Xu, C. Color effect of light sources on peridot based on CIE1976 L*a*b* color system and round RGB diagram system. Color Res. Appl. 2019, 44, 932–940. [Google Scholar] [CrossRef]
- Cheng, R.P.; Guo, Y. Study on the effect of heat treatment on amethyst color and the cause of coloration. Sci. Rep. 2020, 10, 14927. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Guo, Y. The impact of trace metal cations and absorbed water on colour transition of turquoise. R. Soc. Open Sci. 2021, 8, 201110. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y. Quality grading system of Jadeite-Jade green based on three colorimetric parameters under CIE standard light sources D-65, CWF and A. Bulg. Chem. Commun. 2017, 49, 961–968. [Google Scholar]
- Guo, Y.; Zong, X.; Qi, M. Feasibility study on quality evaluation of Jadeite-jade color green based on GemDialogue color chip. Multimed. Tools Appl. 2019, 78, 841–856. [Google Scholar] [CrossRef]
- Tooms, M.S. Exploiting colorimetric relationships in characterizing the spectral response functions of the human visual system directly from colour matching functions. Color Res. Appl. 2020, 45, 782–795. [Google Scholar] [CrossRef]
- Kasajima, I.; Sasaki, K. Dichromatism causes color variations in leaves and spices. Color Res. Appl. 2015, 40, 605–611. [Google Scholar] [CrossRef]
- Kasajima, I. Alexandrite-like effect in purple flowers analyzed with newly devised round RGB diagram. Sci. Rep. 2016, 6, 29630. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.Y.; Palke, A.C.; Muyal, J.; McMurtry, R. How to facet gem-quality chrysoberyl: Clues from the relationship between color and pleochroism, with spectroscopic analysis and colorimetric parameters. Am. Miner. 2017, 102, 1747–1758. [Google Scholar] [CrossRef]
- Xie, F.; Cao, Y.; Ranchon, C.; Hart, A.; Hansen, R. Explanation of the colour change in alexandrites. Sci. Rep. 2020, 10, 6130. [Google Scholar] [CrossRef] [Green Version]
- Fairchild, M.D. Color appearance models and complex visual stimuli. J. Dent. 2010, 38, e25–e33. [Google Scholar] [CrossRef]
- DiDomenico, M.; Wemple, S.H. Oxygen-octahedra ferroelectrics. I. Theory of electro-optical and nonlinear optical effects. J. Appl. Phys. 1969, 40, 720–734. [Google Scholar] [CrossRef]
- Wemple, S.H.; DiDomenico, M. Behavior of the electronic dielectric constant in covalent and ionic materials. Phys. Rev. B 1971, 3, 1338–1351. [Google Scholar] [CrossRef]
- Wemple, S.H. Optical oscillator strengths and excitation energies in solids, liquids, and molecules. J. Chem. Phys. 1977, 67, 2151–2168. [Google Scholar] [CrossRef]
- Medenbach, O.; Shannon, R.D. Refractive indices and optical dispersion of 103 synthetic and mineral oxides and silicates measured by a small-prism technique. J. Opt. Soc. Am. B 1997, 14, 3299–3318. [Google Scholar] [CrossRef]
- Han, Z.Y.; Sun, D.L.; Zhang, H.L.; Luo, J.Q. Investigation on the growth and properties of six garnet single crystals with large lattice constants. Cryst. Res. Technol. 2021, 56, 2000221. [Google Scholar] [CrossRef]
- Shen, C. Color quantification of gem corundum. Master’s Thesis, China University of Geosciences, Wuhan, China, 2019. [Google Scholar]
- Liao, N.F.; Shi, J.S.; Wu, W.M. An Introduction to Digital Color Management System; Beijing Institute of Technology Press: Beijing, China, 2009. [Google Scholar]
- ISO/CIE 11664-3:2012(E) Colorimetry—Part 3: CIE Tristimulus Values; ISO: Geneva, Switzerland; CIE: Vienna, Austria, 2012.
- ISO/CIE 11664-4:2019(E) Colorimetry—Part 4: CIE 1976 L*a*b* Colour Space; ISO: Geneva, Switzerland; CIE: Vienna, Austria, 2019.
- CIE International Commission on Illumination. CIE15-2004: Colorimetry, 3rd ed.; CIE: Vienna, Austria, 2004. [Google Scholar]
- Moore, R.K.; White, W.B.; Long, T.V. Vibrational spectra of the common silicates: I. The garnets. Am. Miner. 1971, 56, 54–71. [Google Scholar]
- Hofmeister, A.M.; Fagan, T.J.; Campbell, K.M.; Schaal, R.B. Single-crystal IR spectroscopy of pyrope-almandine garnets with minor amounts of Mn and Ca. Am. Mineral. 1996, 81, 418–429. [Google Scholar] [CrossRef]
- Weng, S.F.; Xu, Y.Z. Fourier Transform Infrared Spectroscopy Analysis, 3rd ed.; Chemical Industry Press: Beijing, China, 2016; pp. 10–429. (In Chinese) [Google Scholar]
- Schmetzer, K.; Bernhardt, H.-J.; Bosshart, G.; Hainschwang, T. Colour-change garnets from Madagascar: Variation of chemical, spectroscopic and colorimetric properties. J. Gemol. 2010, 31, 235–282. [Google Scholar] [CrossRef]
- Moore, R.K.; White, W.B. Electronic spectra of transition metal ions in silicate garnets. Can. Mineral. 1972, 11, 791–811. [Google Scholar]
- Taran, M.N.; Dyar, M.D.; Matsyuk, S.S. Optical absorption study of natural garnets of almandine-skiagite composition showing intervalence Fe2+ + Fe3+→Fe3+ + Fe2+ charge-transfer transition. Am. Miner. 2008, 92, 753–760. [Google Scholar] [CrossRef]
- Krambrock, K.; Guimarães, F.S.; Pinheiro, M.V.B. Purplish-red almandine garnets with alexandrite-like effect: Causes of colors and color-enhancing treatments. Phys. Chem. Miner. 2013, 40, 555–562. [Google Scholar] [CrossRef]
- Schmetzer, K.; Hainschwang, T.; Kiefert, L.; Bernhardt, H.-J. Pink to pinkish orange Malaya garnets from Bekily, Madagascar. Gems Gemol. 2001, 37, 296–308. [Google Scholar] [CrossRef]
- Heidi, B.; Breitzmann, H.; Sun, Z.Y.; Palke, A.C. Color-change spessartine garnet: A first report. Gems Gemol. 2019, 55, 254–257. [Google Scholar]
- Geiger, C.A.; Stahl, A.; Rossman, G.R. Single-crystal IR- and UV/VIS-spectroscopic measurements on transition-metal-bearing pyrope: The incorporation of hydroxide in garnet. Eur. J. Mineral. 2000, 12, 259–271. [Google Scholar] [CrossRef]
- Li, L.P.; Ye, D. Role of Cr and V in colour change effect of gemstones. Gems Gemol. 2003, 5, 17–21, (In Chinese with English abstract). [Google Scholar]
- Liu, Y.; Shigley, J.; Fritsch, E.; Hemphill, S. Abnormal hue-angle change of the gemstone tanzanite between CIE illuminants D65 and a in CIELAB color space. Color Res. Appl. 1995, 20, 245–250. [Google Scholar] [CrossRef]
- CIE International Commission on Illumination. Available online: http://cie.co.at/technical-work/technical-resources. (accessed on 2 March 2021).





| Sample | D65 | A | Δh(°) | ΔC* | ΔE*ab | ||||
|---|---|---|---|---|---|---|---|---|---|
| L* | C* | h(°) | L* | C* | h(°) | ||||
| B1 | 62.04 | 11.79 | 104.64 | 62.34 | 10.84 | 50.71 | 53.93 | −0.94 | 10.30 |
| B2 | 68.01 | 16.69 | 105.18 | 68.44 | 14.22 | 72.22 | 32.97 | −2.48 | 9.10 |
| B3 | 67.04 | 10.00 | 95.70 | 67.46 | 12.73 | 31.43 | 64.27 | 2.73 | 12.31 |
| B4 | 57.94 | 10.80 | 107.80 | 58.16 | 9.62 | 48.67 | 59.13 | −1.18 | 10.13 |
| B5 | 42.70 | 9.84 | 85.22 | 43.30 | 14.14 | 31.26 | 53.96 | 4.30 | 11.60 |
| B6 | 62.11 | 14.15 | 90.54 | 62.83 | 17.92 | 36.92 | 53.62 | 3.77 | 14.87 |
| B7 | 68.87 | 12.46 | 96.37 | 69.38 | 14.54 | 38.06 | 58.31 | 2.08 | 13.29 |
| B8 | 65.70 | 14.07 | 97.08 | 66.26 | 15.08 | 45.18 | 51.90 | 1.01 | 12.80 |
| B9 | 68.95 | 12.19 | 97.75 | 69.42 | 14.41 | 35.85 | 61.89 | 2.22 | 13.82 |
| B10 | 67.29 | 11.70 | 98.91 | 67.71 | 13.71 | 35.34 | 63.57 | 2.02 | 13.50 |
| Colour Parameters | df | Mean Square | F | p |
|---|---|---|---|---|
| L* | 1 | 1.070 | 0.017 | 0.898 |
| a* | 1 | 688.632 | 116.762 | 0.000 |
| b* | 1 | 50.444 | 11.696 | 0.003 |
| C* | 1 | 9.146 | 1.888 | 0.186 |
| h(°) | 1 | 15,320.707 | 152.244 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Guo, Y. Explaining Colour Change in Pyrope-Spessartine Garnets. Minerals 2021, 11, 865. https://doi.org/10.3390/min11080865
Qiu Y, Guo Y. Explaining Colour Change in Pyrope-Spessartine Garnets. Minerals. 2021; 11(8):865. https://doi.org/10.3390/min11080865
Chicago/Turabian StyleQiu, Yan, and Ying Guo. 2021. "Explaining Colour Change in Pyrope-Spessartine Garnets" Minerals 11, no. 8: 865. https://doi.org/10.3390/min11080865
APA StyleQiu, Y., & Guo, Y. (2021). Explaining Colour Change in Pyrope-Spessartine Garnets. Minerals, 11(8), 865. https://doi.org/10.3390/min11080865






