Ex and In Situ Reactivity and Sorption of Selenium in Opalinus Clay in the Presence of a Selenium Reducing Microbial Community
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of the BN Experiment
2.2. Batch Tests
2.3. In Situ Injection Tests
2.4. Chemical Analyses
2.5. Microbial Analyses
2.5.1. Microbial Cell Count
2.5.2. DNA-Based Analysis of the Microbial Community
2.6. Microscopic Analyses
2.7. Modeling
2.7.1. Se Speciation
2.7.2. Diffusion Modeling
2.7.3. Se(VI) Reduction Kinetics
2.7.4. Se(IV) Sorption onto Opalinus Clay
3. Results
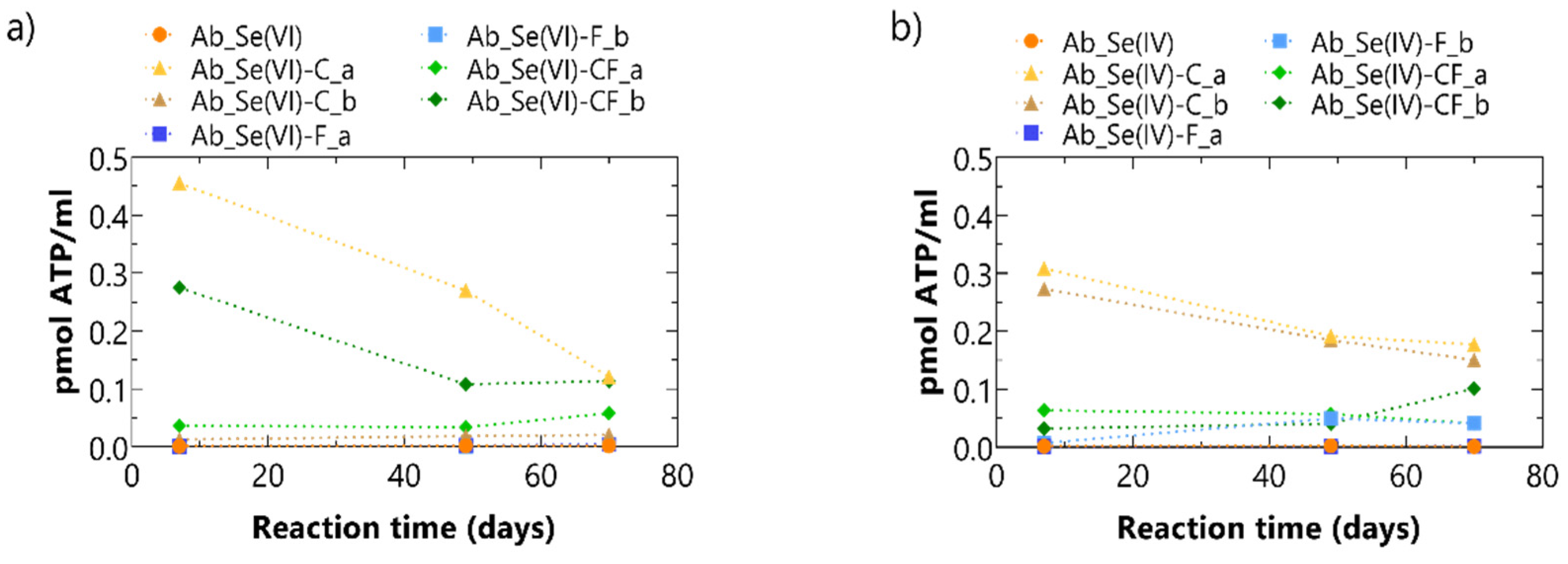
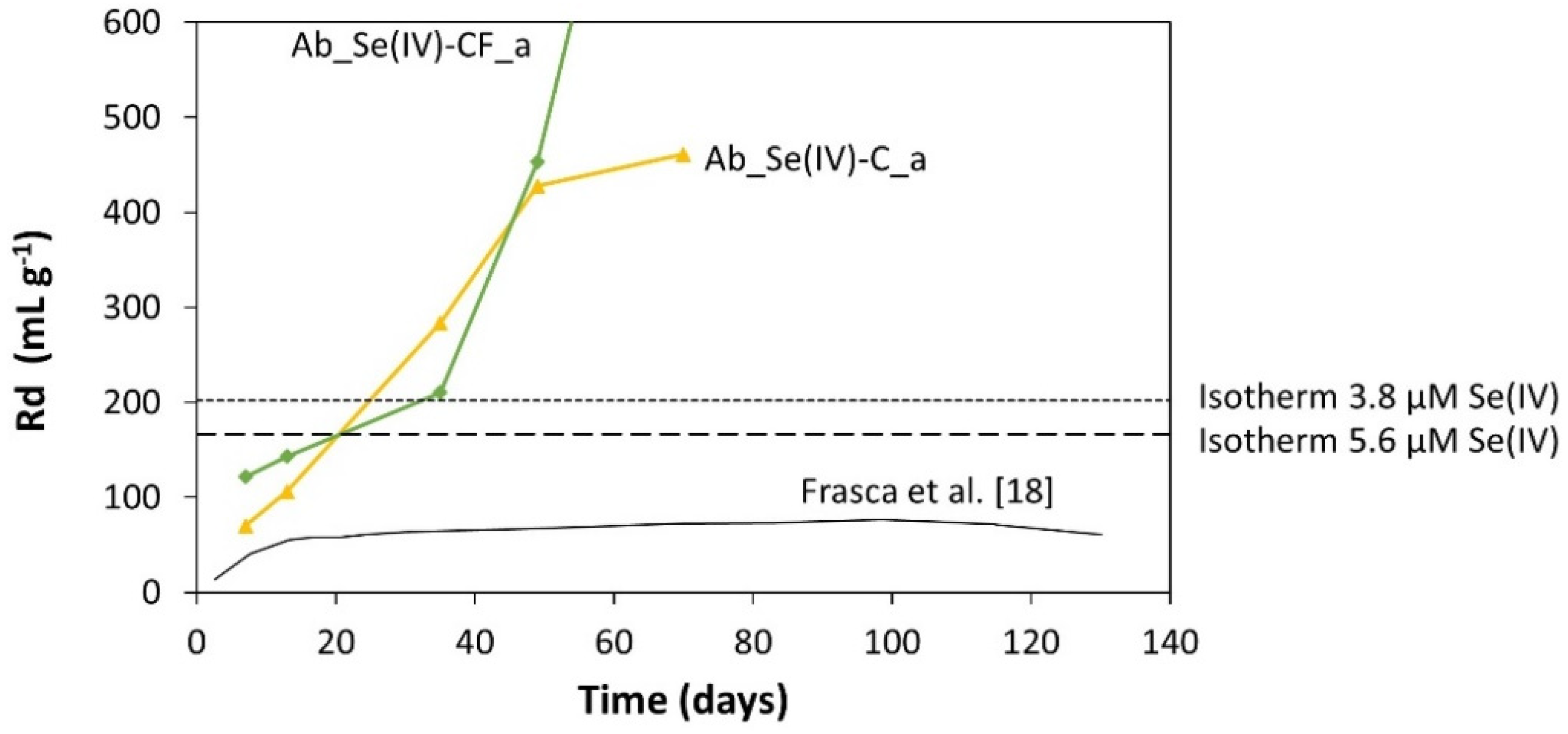
3.1. Abiotic Batch Tests
3.1.1. Experimental Results
3.1.2. Modeling Results
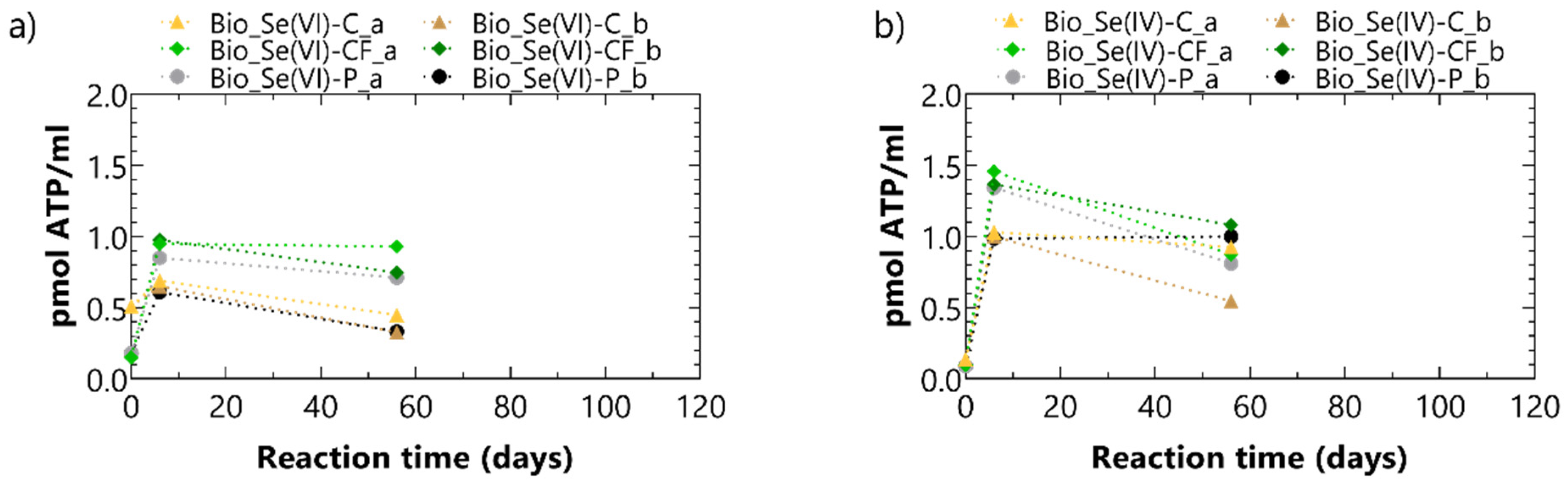
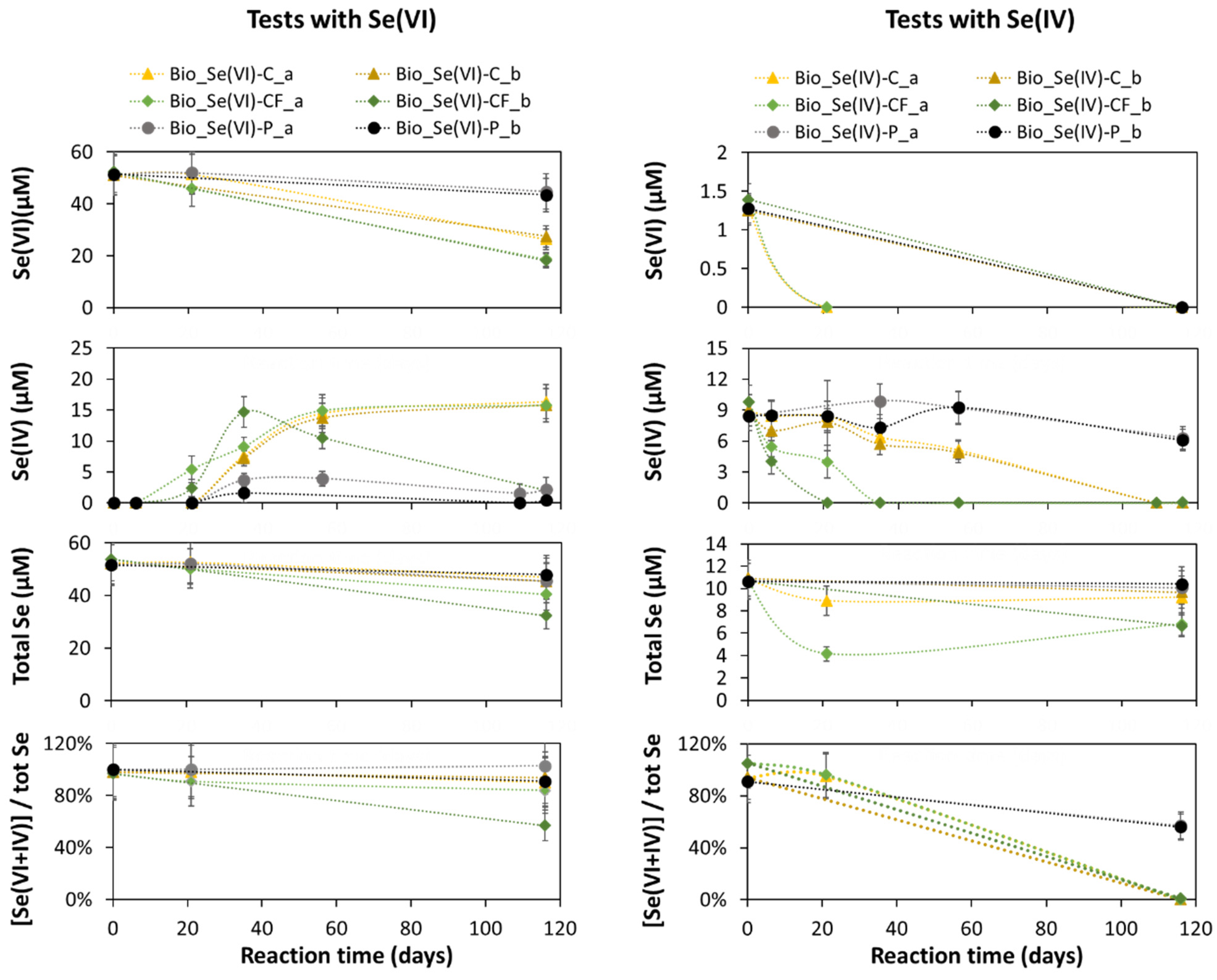
3.2. Biotic Batch Tests
3.2.1. Experimental Results
3.2.2. Modeling Results
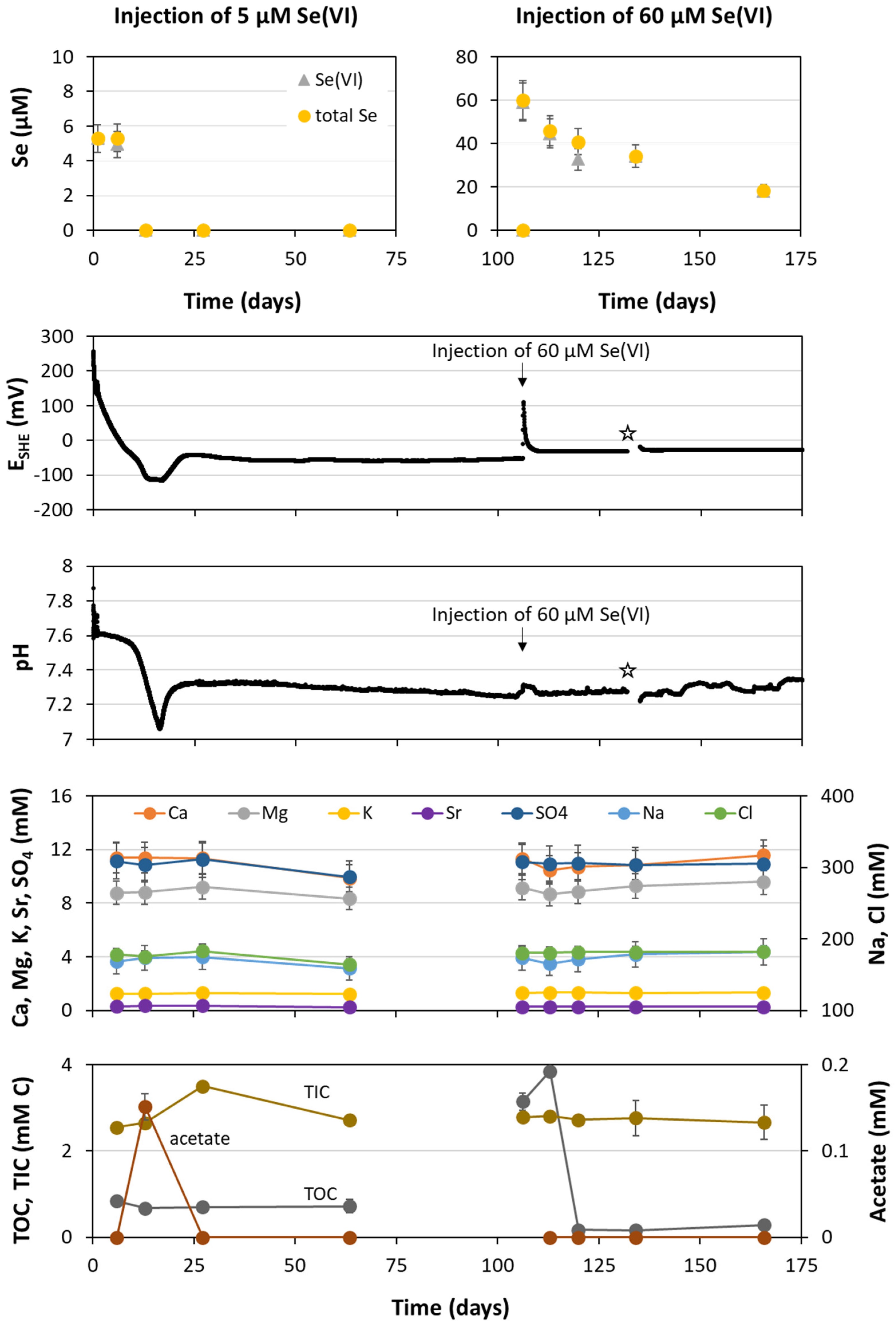
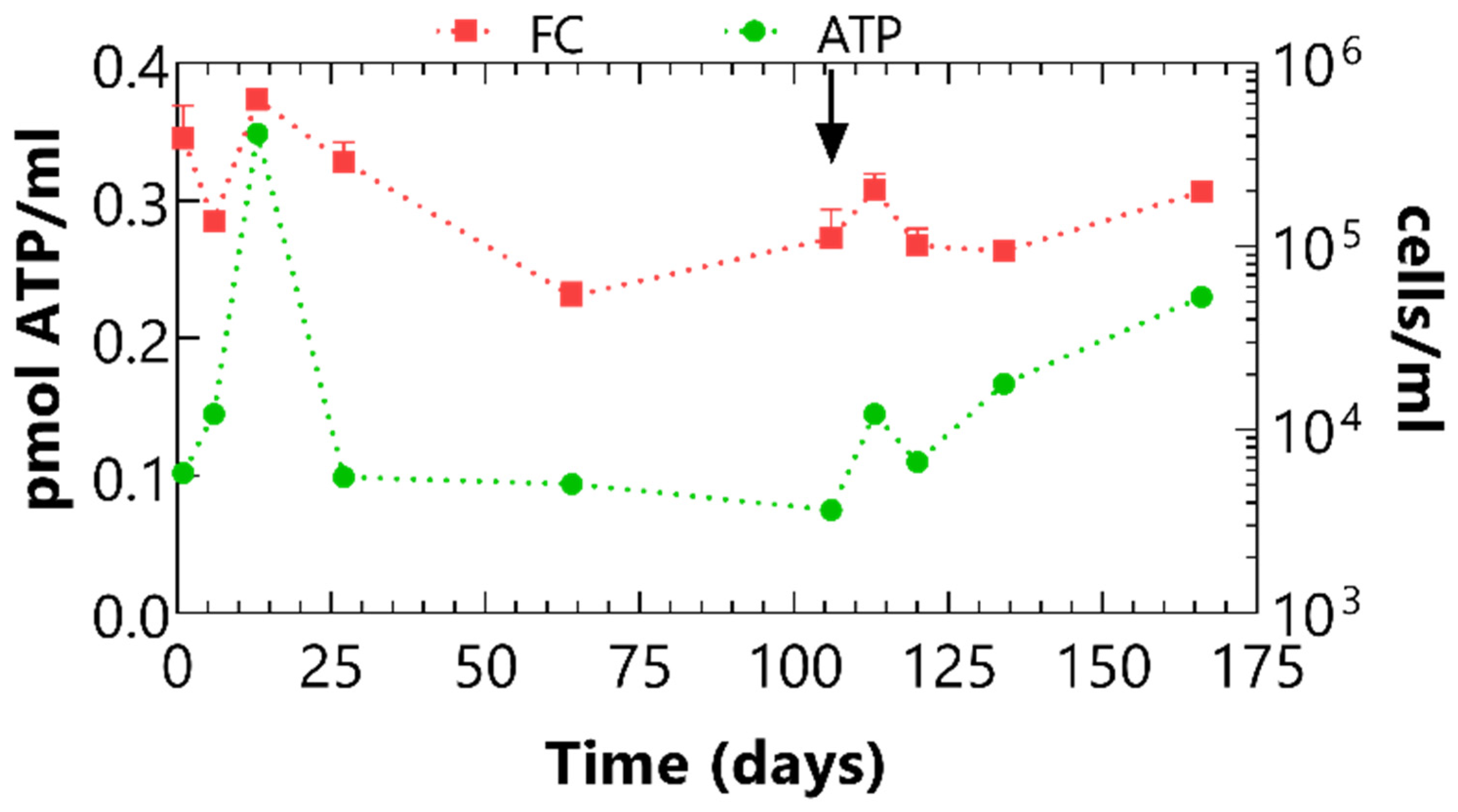
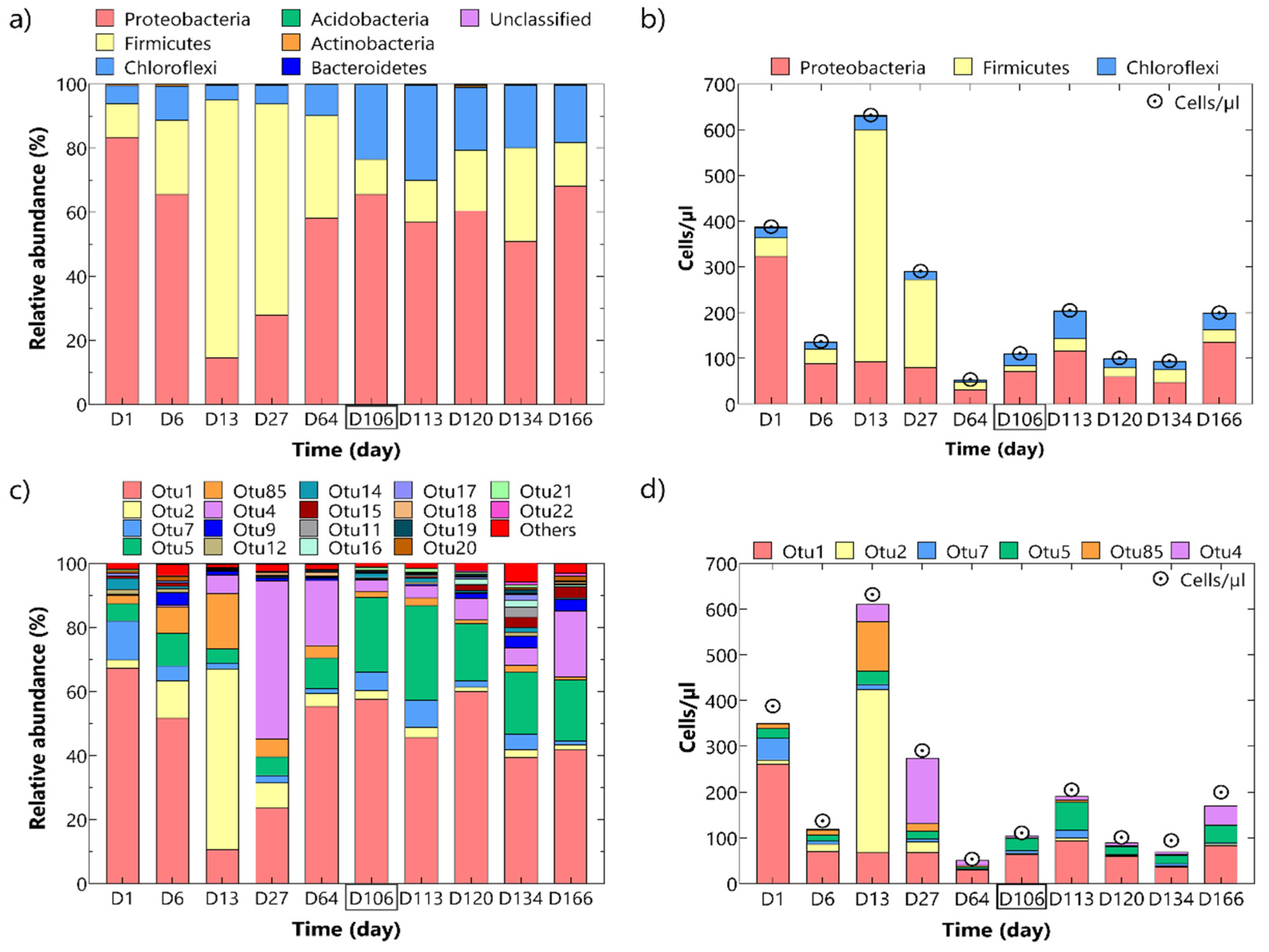
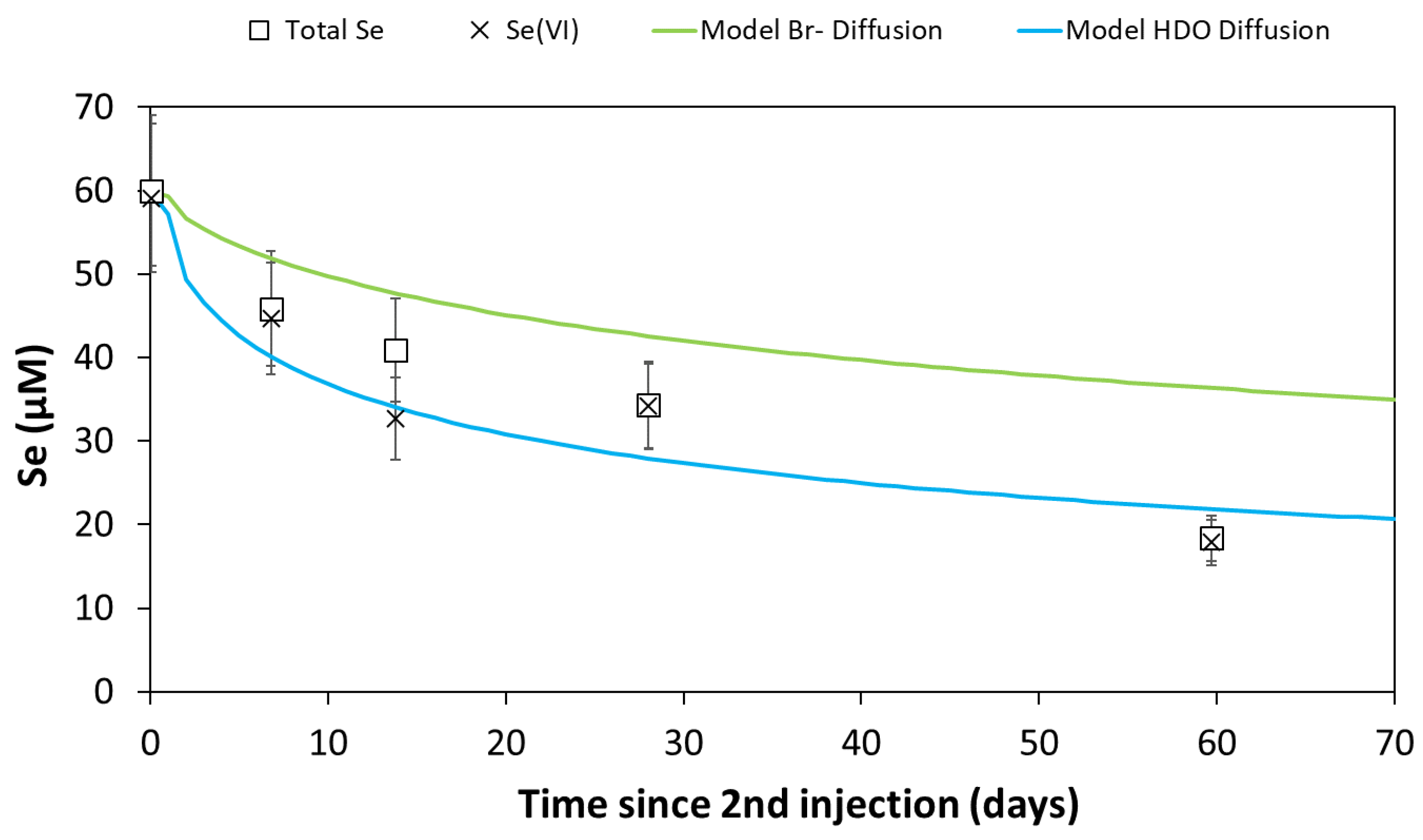
3.3. In Situ Tests
3.3.1. Chemical Analyses Results
3.3.2. Microbiological Analyses Results
3.3.3. Modeling Results
3.4. Selenium Speciation Modeling
4. Discussion
4.1. Abiotic Se Reactivity
4.2. Microbially Mediated Se Reactivity
4.3. In Situ Fate of Se(VI)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jörg, G.; Bühnemann, R.; Hollas, S.; Kivel, N.; Kossert, K.; Van Winckel, S.; Gostomski, C.L. Preparation of radiochemically pure (79)Se and highly precise determination of its half-life. Appl. Radiat. Isot. 2010, 68, 2339–2351. [Google Scholar] [CrossRef] [PubMed]
- Marivoet, J.; Weetjens, E. Impact of advanced fuel cycles on geological disposal. Mater. Res. Soc. Symp. Proc. 2009, 1193, 117. [Google Scholar] [CrossRef]
- De Cannière, P.; Maes, A.; Williams, S.; Bruggeman, C.; Beauwens, T.; Maes, N.; Cowper, M. Behaviour of Selenium in Boom Clay; External Report SCK CEN-ER-120; SCK CEN: Mol, Belgium, 2010; p. 328. [Google Scholar]
- Weetjens, E. Safety Assessment Calculations for Geological Disposal of Eurobitum Bituminised Waste in Boom Clay; Restricted Contract Report SCK CEN-R-5887; SCK CEN: Mol, Belgium, 2015; p. 83. [Google Scholar]
- Andra. Safety Options Report—Post-Closure Part; Report nr CG-TE-D-NTE-AMOA-SR2-0000-15-0062; Andra: Paris, France, 2016; p. 466. [Google Scholar]
- Bingham, P.A.; Connelly, A.J.; Cassingham, N.J.; Hyatt, N.C. Oxidation state and local environment of selenium in alkali borosilicate glasses for radioactive waste immobilisation. J. Non-Cryst. Solids 2011, 357, 2726–2734. [Google Scholar] [CrossRef]
- Goberna-Ferrón, S.; Asta, M.P.; Zareeipolgardani, B.; Bureau, S.; Findling, N.; Simonelli, L.; Greneche, J.-M.; Charlet, L.; Fernández-Martínez, A. Influence of Silica Coatings on Magnetite-Catalyzed Selenium Reduction. Environ. Sci. Technol. 2021, 55, 3021–3031. [Google Scholar] [CrossRef] [PubMed]
- Bar-Yosef, B.; Meek, D. Selenium sorption by kaolinite and montmorillonite. Soil Sci. 1987, 144, 11–19. [Google Scholar] [CrossRef]
- Masscheleyn, P.H.; Delaune, R.D.; Patrick, W.H., Jr. Transformations of selenium as affected by sediment oxidation-reduction potential and pH. Environ. Sci. Technol. 1990, 24, 91–96. [Google Scholar] [CrossRef]
- Elrashidi, M.A.; Adriano, D.C.; Workman, S.M.; Lindsay, W.L. Chemical equilibria of selenium in soils: A theoretical development. Soil Sci. 1987, 144, 141–152. [Google Scholar] [CrossRef]
- Krivovichev, V.; Charykova, M.; Vishnevsky, A. The Thermodynamics of Selenium Minerals in Near-Surface Environments. Minerals 2017, 7, 188. [Google Scholar] [CrossRef] [Green Version]
- Charykova, M.V.; Krivovichev, V.G. Mineral systems and the thermodynamics of selenites and selenates in the oxidation zone of sulfide ores—A review. Mineral. Petrol. 2017, 111, 121–134. [Google Scholar] [CrossRef]
- Su, C.; Suarez, D.L. Selenate and selenite sorption on iron oxides an infrared and electrophoretic study. Soil Sci. Soc. Am. J. 2000, 64, 101–111. [Google Scholar] [CrossRef]
- Missana, T.; Alonso, U.; García-Gutiérrez, M. Experimental study and modelling of selenite sorption onto illite and smectite clays. J. Colloid Interface Sci. 2009, 334, 132–138. [Google Scholar] [CrossRef]
- Rovira, M.; Giménez, J.; Martínez, M.; Martínez-Lladó, X.; de Pablo, J.; Martí, V.; Duro, L. Sorption of selenium(IV) and selenium(VI) onto natural iron oxides: Goethite and hematite. J. Hazard. Mater. 2008, 150, 279–284. [Google Scholar] [CrossRef]
- Breynaert, E.; Scheinost, A.C.; Dom, D.; Rossberg, A.; Vancluysen, J.; Gobechiya, E.; Kirschhock, C.E.A.; Maes, A. Reduction of Se(IV) in Boom Clay: XAS solid phase speciation. Environ. Sci. Technol. 2010, 44, 6649–6655. [Google Scholar] [CrossRef]
- Hoving, A.L.; Münch, M.A.; Bruggeman, C.; Banerjee, D.; Behrends, T. Kinetics of selenite interactions with Boom Clay: Adsorption–reduction interplay. Geol. Soc. Lond. Spec. Publ. 2019, 482, 225–239. [Google Scholar] [CrossRef]
- Frasca, B.; Savoye, S.; Wittebroodt, C.; Leupin, O.; Michelot, J.-L. Comparative study of Se oxyanions retention on three argillaceous rocks: Upper Toarcian (Tournemire, France), Black Shales (Tournemire, France) and Opalinus Clay (Mont Terri, Switzerland). J. Environ. Radioact. 2014, 127, 133–140. [Google Scholar] [CrossRef]
- Savoye, S.; Schlegel, M.L.; Frasca, B. Mobility of selenium oxyanions in clay-rich media: A combined batch and diffusion experiments and synchrotron-based spectroscopic investigation. Appl. Geochem. 2021, 128, 104932. [Google Scholar] [CrossRef]
- Scheinost, A.C.; Kirsch, R.; Banerjee, D.; Fernandez-Martinez, A.; Zaenker, H.; Funke, H.; Charlet, L. X-ray absorption and photoelectron spectroscopy investigation of selenite reduction by FeII-bearing minerals. J. Contam. Hydrol. 2008, 102, 228–245. [Google Scholar] [CrossRef]
- Kang, M.; Chen, F.; Wu, S.; Yang, Y.; Bruggeman, C.; Charlet, L. Effect of pH on aqueous Se (IV) reduction by pyrite. Environ. Sci. Technol. 2011, 45, 2704–2710. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, C.; Maes, A.; Vancluysen, J.; Vandemussele, P. Selenite reduction in Boom clay: Effect of FeS2, clay minerals and dissolved organic matter. Environ. Pollut. 2005, 137, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Peak, D.; Saha, U.K.; Huang, P.M. Selenite adsorption mechanisms on pure and coated montmorillonite: An EXAFS and XANES spectroscopic study. Soil Sci. Soc. Am. J. 2006, 70, 192–203. [Google Scholar] [CrossRef]
- Beauwens, T.; De Cannière, P.; Moors, H.; Wang, L.; Maes, N. Studying the migration behaviour of selenate in Boom Clay by electromigration. Eng. Geol. 2005, 77, 285–293. [Google Scholar] [CrossRef]
- Goldberg, S. Modeling selenate adsorption behavior on oxides, clay minerals, and soils using the triple layer model. Soil Sci. 2014, 179, 568–576. [Google Scholar] [CrossRef]
- Balistrieri, L.S.; Chao, T.T. Selenium adsorption by goethite. Soil Sci. Soc. Am. J. 1987, 51, 1145–1151. [Google Scholar] [CrossRef]
- Duc, M.; Lefèvre, G.; Fedoroff, M.; Jeanjean, J.; Rouchaud, J.C.; Monteil-Rivera, F.; Dumonceau, J.; Milonjic, S. Sorption of selenium anionic species on apatites and iron oxides from aqueous solutions. J. Environ. Radioact. 2003, 70, 61–72. [Google Scholar] [CrossRef]
- Staicu, L.C.; Barton, L.L. Selenium respiration in anaerobic bacteria: Does energy generation pay off? J. Inorg. Biochem. 2021, 222, 111509. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, T.C. Biosynthesis and function of selenocysteine-containing enzymes. J. Biol. Chem. 1991, 266, 16257–16260. [Google Scholar] [CrossRef]
- Heider, J.; Böck, A. Selenium metabolism in micro-organisms. Adv. Microb. Physiol. 1993, 35, 71–109. [Google Scholar] [CrossRef]
- Eswayah, A.S.; Smith, T.J.; Gardiner, P.H.E. Microbial transformations of selenium species of relevance to bioremediation. Appl. Environ. Microbiol. 2016, 82, 4848–4859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimmi, T.; Leupin, O.X.; Eikenberg, J.; Glaus, M.A.; Van Loon, L.R.; Waber, H.N.; Wersin, P.; Wang, H.A.O.; Grolimund, D.; Borca, C.N.; et al. Anisotropic diffusion at the field scale in a 4-year multi-tracer diffusion and retention experiment—I: Insights from the experimental data. Geochim. Cosmochim. Acta 2014, 125, 373–393. [Google Scholar] [CrossRef]
- Descostes, M.; Blin, V.; Bazer-Bachi, F.; Meier, P.; Grenut, B.; Radwan, J.; Schlegel, M.L.; Buschaert, S.; Coelho, D.; Tevissen, E. Diffusion of anionic species in Callovo-Oxfordian argillites and Oxfordian limestones (Meuse/Haute–Marne, France). Appl. Geochem. 2008, 23, 655–677. [Google Scholar] [CrossRef]
- Bleyen, N.; Smets, S.; Small, J.; Moors, H.; Leys, N.; Albrecht, A.; De Cannière, P.; Schwyn, B.; Wittebroodt, C.; Valcke, E. Impact of the electron donor on in situ microbial nitrate reduction in Opalinus Clay: Results from the Mont Terri rock laboratory (Switzerland). Swiss J. Geosci. 2017, 110, 355–374. [Google Scholar] [CrossRef] [Green Version]
- Bossart, P.; Bernier, F.; Birkholzer, J.; Bruggeman, C.; Connolli, P.; Dewonck, S.; Fukaya, M.; Herfort, M.; Jensen, M.; Matray, J.-M.; et al. Geologic setting and overview of key experiments in the Mont Terri rock laboratory. Swiss J. Geosci. 2017, 110, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Pearson, F.J.; Arcos, D.; Bath, A.; Boisson, J.Y.; Fernández, A.M.; Gabler, H.E.; Gaucher, E.; Gautschi, A.; Griffault, L.; Hernán, P.; et al. Mont Terri Project—Geochemistry of Water in the Opalinus Clay Formation at the Mont Terri Rock Laboratory; Geology Series No. 5; Federal Office for Water and Geology: Bern, Switzerland, 2003; p. 321. [Google Scholar]
- Bleyen, N.; Albrecht, A.; De Cannière, P.; Wittebroodt, C.; Valcke, E. Non-destructive on-line and long-term monitoring of in situ nitrate and nitrite reactivity in a clay environment at increasing turbidity. Appl. Geochem. 2019, 100, 131–142. [Google Scholar] [CrossRef]
- Martínez-Bravo, Y.; Roig-Navarro, A.F.; López, F.J.; Hernández, F. Multielemental determination of arsenic, selenium and chromium(VI) species in water by high-performance liquid chromatography–inductively coupled plasma mass spectrometry. J. Chromatogr. A 2001, 926, 265–274. [Google Scholar] [CrossRef]
- Walkinson, J.H. Fluorometric determination of selenium in biological material with 2,3-diaminonaphthalene. Anal. Chem. 1966, 38, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Props, R.; Monsieurs, P.; Mysara, M.; Clement, L.; Boon, N. Measuring the biodiversity of microbial communities by flow cytometry. Methods Ecol. Evol. 2016, 7, 1376–1385. [Google Scholar] [CrossRef]
- Mysara, M.; Njima, M.; Leys, N.; Raes, J.; Monsieurs, P. From reads to operational taxonomic units: An ensemble processing pipeline for MiSeq amplicon sequencing data. Gigascience 2017, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Mysara, M.; Leys, N.; Raes, J.; Monsieurs, P. IPED: A highly efficient denoising tool for Illumina MiSeq Paired-end 16S rRNA gene amplicon sequencing data. BMC Bioinform. 2016, 17, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mysara, M.; Saeys, Y.; Leys, N.; Raes, J.; Monsieurs, P. CATCh, an ensemble classifier for chimera detection in 16S rRNA sequencing studies. Appl. Environ. Microbiol. 2015, 81, 1573–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. In U.S. Geological Survey Techniques and Methods; U.S. Geological Survey: Reston, VA, USA, 2013; p. 497, Book 6, Chapter A43. [Google Scholar]
- Giffaut, E.; Grive, M.; Blanc, P.; Vieillard, P.; Colas, E.; Gailhanou, H.; Gaboreau, S.; Marty, N.; Made, B.; Duro, L. Andra thermodynamic database for performance assessment: ThermoChimie. Appl. Geochem. 2014, 49, 225–236. [Google Scholar] [CrossRef]
- Olin, A.; Nolang, B.; Osadchii, E.G.; Ohman, L.O.; Rosen, E. Chemical Thermodynamics of Selenium; NEA North Holland Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 2015; Volume 7, p. 893. [Google Scholar]
- Kinniburgh, D.; Cooper, D. PhreePlot: Creating Graphical Output with PHREEQC. 2011. Available online: http://www.phreeplot.org (accessed on 24 June 2020).
- Appelo, C.A.J.; Wersin, P. Multicomponent diffusion modeling in clay systems with application to the diffusion of tritium, iodide, and sodium in Opalinus Clay. Environ. Sci. Technol. 2007, 41, 5002–5007. [Google Scholar] [CrossRef]
- Tournassat, C.; Alt-Epping, P.; Gaucher, E.C.; Gimmi, T.; Leupin, O.X.; Wersin, P. Biogeochemical processes in a clay formation in situ experiment: Part F–Reactive transport modelling. Appl. Geochem. 2011, 26, 1009–1022. [Google Scholar] [CrossRef] [Green Version]
- Mijnendonckx, K.; Van Gompel, A.; Coninx, I.; Bleyen, N.; Leys, N. Water-soluble bitumen degradation products can fuel nitrate reduction from non-radioactive bituminized waste. Appl. Geochem. 2020, 114, 104525. [Google Scholar] [CrossRef]
- Bleyen, N.; Hendrix, K.; Moors, H.; Durce, D.; Vasile, M.; Valcke, E. Biodegradability of dissolved organic matter in Boom Clay pore water under nitrate-reducing conditions: Effect of additional C and P sources. Appl. Geochem. 2018, 92, 45–58. [Google Scholar] [CrossRef]
- Mariën, A.; Bleyen, N.; Aerts, S.; Valcke, E. The study of abiotic reduction of nitrate and nitrite in Boom Clay. Phys. Chem. Earth 2011, 36, 1639–1647. [Google Scholar] [CrossRef]
- Props, R.; Kerckhof, F.-M.; Rubbens, P.; De Vrieze, J.; Hernandez Sanabria, E.; Waegeman, W.; Monsieurs, P.; Hammes, F.; Boon, N. Absolute quantification of microbial taxon abundances. ISME J. 2017, 11, 584–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wersin, P.; Leupin, O.; Mettler, S.; Gaucher, E.C.; Mäder, U.; De Cannière, P.; Vinsot, A.; Gäbler, H.; Kunimaro, T.; Kiho, K. Biogeochemical processes in a clay formation in situ experiment: Part A—Overview, experimental design and water data of an experiment in the Opalinus Clay at the Mont Terri Underground Research Laboratory, Switzerland. Appl. Geochem. 2011, 26, 931–953. [Google Scholar] [CrossRef]
- Steinberg, N.A.; Oremland, R.S. Dissimilatory selenate reduction potentials in a diversity of sediment types. Appl. Environ. Microbiol. 1990, 56, 3550–3557. [Google Scholar] [CrossRef] [Green Version]
- Oremland, R.S.; Herbel, M.J.; Blum, J.S.; Langley, S.; Beveridge, T.J.; Ajayan, P.M.; Sutto, T.; Ellis, A.V.; Curran, S. Structural and spectral features of selenium nanospheres produced by Se-respiring bacteria. Appl. Environ. Microbiol. 2004, 70, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avendaño, R.; Chaves, N.; Fuentes, P.; Sánchez, E.; Jiménez, J.I.; Chavarría, M. Production of selenium nanoparticles in Pseudomonas putida KT2440. Sci. Rep. 2016, 6, 37155. [Google Scholar] [CrossRef] [Green Version]
- Courdouan, A.; Christl, I.; Meylan, S.; Wersin, P.; Kretzschmar, R. Characterization of dissolved organic matter in anoxic rock extracts and in situ pore water of the Opalinus Clay. Appl. Geochem. 2007, 22, 2926–2939. [Google Scholar] [CrossRef]
- Eichinger, F.; Lorenz, G.; Eichinger, L. WS-H Experiment: Water Sampling from Borehole BWS-H2—Report on Physical-Chemical and Isotopic Analyses; Mont Terri Technical Note TN 2010-49; Swisstopo: Wabern, Switzerland, 2011; p. 24. [Google Scholar]
- Van Loon, L.R.; Leupin, O.X.; Cloet, V. The diffusion of SO42− in Opalinus Clay: Measurements of effective diffusion coefficients and evaluation of their importance in view of microbial mediated reactions in the near field of radioactive waste repositories. Appl. Geochem. 2018, 95, 19–24. [Google Scholar] [CrossRef]
- Li, Y.-H.; Gregory, S. Diffusion of ions in sea water and in deep-sea sediments. Geochim. Cosmochim. Acta 1974, 38, 703–714. [Google Scholar] [CrossRef]
- Bagnoud, A.; de Bruijn, I.; Andersson, A.F.; Diomidis, N.; Leupin, O.X.; Schwyn, B.; Bernier-Latmani, R. A minimalistic microbial food web in an excavated deep subsurface clay rock. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef] [Green Version]
- Ficker, M.; Krastel, K.; Orlicky, S.; Edwards, E. Molecular characterization of a toluene-degrading methanogenic consortium. Appl. Environ. Microbiol. 1999, 65, 5576–5585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, B.; Wang, L.Y.; Mbadinga, S.M.; Liu, J.F.; Yang, S.Z.; Gu, J.D.; Mu, B.Z. Anaerolineaceae and Methanosaeta turned to be the dominant microorganisms in alkanes-dependent methanogenic culture after long-term of incubation. AMB Express 2015, 5, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIlroy, S.J.; Kirkegaard, R.H.; Dueholm, M.S.; Fernando, E.; Karst, S.M.; Albertsen, M.; Nielsen, P.H. Culture-independent analyses reveal novel Anaerolineaceae as abundant primary fermenters in anaerobic digesters treating waste activated sludge. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Toyonaga, M.; Ohashi, A.; Matsuura, N.; Tourlousse, D.M.; Meng, X.-Y.; Tamaki, H.; Hanada, S.; Cruz, R.; Yamaguchi, T.; et al. Isolation and characterization of Flexilinea flocculi gen. nov., sp. nov., a filamentous, anaerobic bacterium belonging to the class Anaerolineae in the phylum Chloroflexi. Int. J. Syst. Evol. 2016, 66, 988–996. [Google Scholar] [CrossRef]
- Yamada, T.; Sekiguchi, Y. Cultivation of uncultured Chloroflexi subphyla: Significance and ecophysiology of formerly uncultured Chloroflexi ‘subphylum I’ with natural and biotechnological relevance. Microbes Environ. 2009, 24, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Gil, G.; Lens, P.N.L.; Saikaly, P.E. Selenite reduction by anaerobic microbial aggregates: Microbial community structure, and proteins associated to the produced selenium spheres. Front. Microbiol. 2016, 7, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolz, J.F.; Basu, P.; Santini, J.M.; Oremland, R.S. Arsenic and selenium in microbial metabolism. Annu. Rev. Microbiol. 2006, 60, 107–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knotek-Smith, H.M.; Crawford, D.L.; Möller, G.; Henson, R.A. Microbial studies of a selenium-contaminated mine site and potential for on-site remediation. J. Ind. Microbiol. Biotechnol. 2006, 33, 897–913. [Google Scholar] [CrossRef] [PubMed]




| Chemical Species | Concentration (mM) | ||
|---|---|---|---|
| APW | Interval 1 | Interval 3 | |
| Na+ | 162 | 181 | 177 |
| K+ | 1.1 | 1.2 | 1.3 |
| Mg2+ | 8.6 | 9.71 | 10.2 |
| Ca2+ | 12.6 | 11.7 | 10.9 |
| Sr2+ | 0.38 | 0.32 | 0.32 |
| Total dissolved Fe | — | 0.016 | 0.0015 |
| Cl− | 181 | 202 | 197 |
| SO42− | 12.3 | 11.9 | 11.7 |
| NO3− | — | 0.04 | 0.06 |
| NO2− | — | <0.1 | <0.1 |
| CH3COO− | — | <0.02 | <0.02 |
| TIC | 2.8 | 0.53 | 2.2 |
| TOC | — | <0.83 | <0.83 |
| pH | 7.8 | 7.7 | n.a. 1 |
| Test Code | Clay (g) | Stainless Steel (mm × mm) 1 | Na2HPO4 (mM) | Abiotic/Biotic |
|---|---|---|---|---|
| Tests with 50 µM selenate | ||||
| Ab_Se(VI) | — | — | — | abiotic |
| Ab_Se(VI)-C | 0.1 g | — | — | abiotic |
| Ab_Se(VI)-F | — | 5 × 5 | — | abiotic |
| Ab_Se(VI)-CF | 0.1 g | 5 × 5 | — | abiotic |
| Bio_Se(VI)-C | 0.1 g | — | — | biotic |
| Bio_Se(VI)-CF | 0.1 g | 5 × 5 | — | biotic |
| Bio_Se(VI)-P | — | — | 0.1 | biotic |
| Tests with 10 µM selenite | ||||
| Ab_Se(IV) | — | — | — | abiotic |
| Ab_Se(IV)-C | 0.1 g | — | — | abiotic |
| Ab_Se(IV)-F | — | 5 × 5 | — | abiotic |
| Ab_Se(IV)-CF | 0.1 g | 5 × 5 | — | abiotic |
| Bio_Se(IV)-C | 0.1 g | — | — | biotic |
| Bio_Se(IV)-CF | 0.1 g | 5 × 5 | — | biotic |
| Bio_Se(IV)-P | — | — | 0.1 | biotic |
| Sorption Site | KL (L mol−1) | Smax (mol kg−1) |
|---|---|---|
| Site 1 | 2.1 × 105 | 1.7 × 10−3 |
| Site 2 | 1.9 × 107 | 1.4 × 10−5 |
| Site 1 fitted (†) | 7.5 × 104 | 7.5 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bleyen, N.; Small, J.S.; Mijnendonckx, K.; Hendrix, K.; Albrecht, A.; De Cannière, P.; Surkova, M.; Wittebroodt, C.; Valcke, E. Ex and In Situ Reactivity and Sorption of Selenium in Opalinus Clay in the Presence of a Selenium Reducing Microbial Community. Minerals 2021, 11, 757. https://doi.org/10.3390/min11070757
Bleyen N, Small JS, Mijnendonckx K, Hendrix K, Albrecht A, De Cannière P, Surkova M, Wittebroodt C, Valcke E. Ex and In Situ Reactivity and Sorption of Selenium in Opalinus Clay in the Presence of a Selenium Reducing Microbial Community. Minerals. 2021; 11(7):757. https://doi.org/10.3390/min11070757
Chicago/Turabian StyleBleyen, Nele, Joe S. Small, Kristel Mijnendonckx, Katrien Hendrix, Achim Albrecht, Pierre De Cannière, Maryna Surkova, Charles Wittebroodt, and Elie Valcke. 2021. "Ex and In Situ Reactivity and Sorption of Selenium in Opalinus Clay in the Presence of a Selenium Reducing Microbial Community" Minerals 11, no. 7: 757. https://doi.org/10.3390/min11070757
APA StyleBleyen, N., Small, J. S., Mijnendonckx, K., Hendrix, K., Albrecht, A., De Cannière, P., Surkova, M., Wittebroodt, C., & Valcke, E. (2021). Ex and In Situ Reactivity and Sorption of Selenium in Opalinus Clay in the Presence of a Selenium Reducing Microbial Community. Minerals, 11(7), 757. https://doi.org/10.3390/min11070757







