Geochemistry and Genesis of Beryl Crystals in the LCT Pegmatite Type, Ebrahim-Attar Mountain, Western Iran
Abstract
1. Introduction
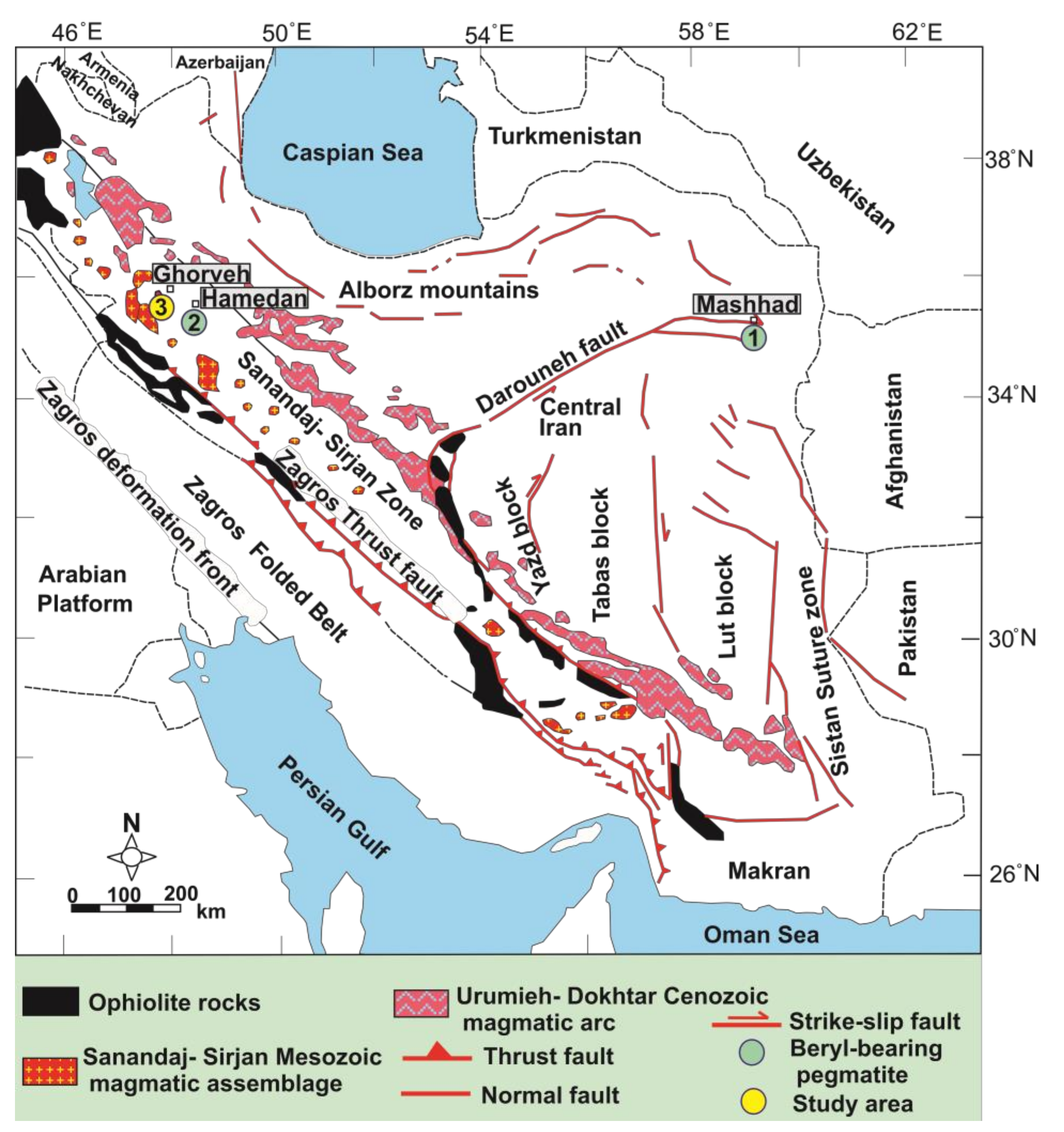
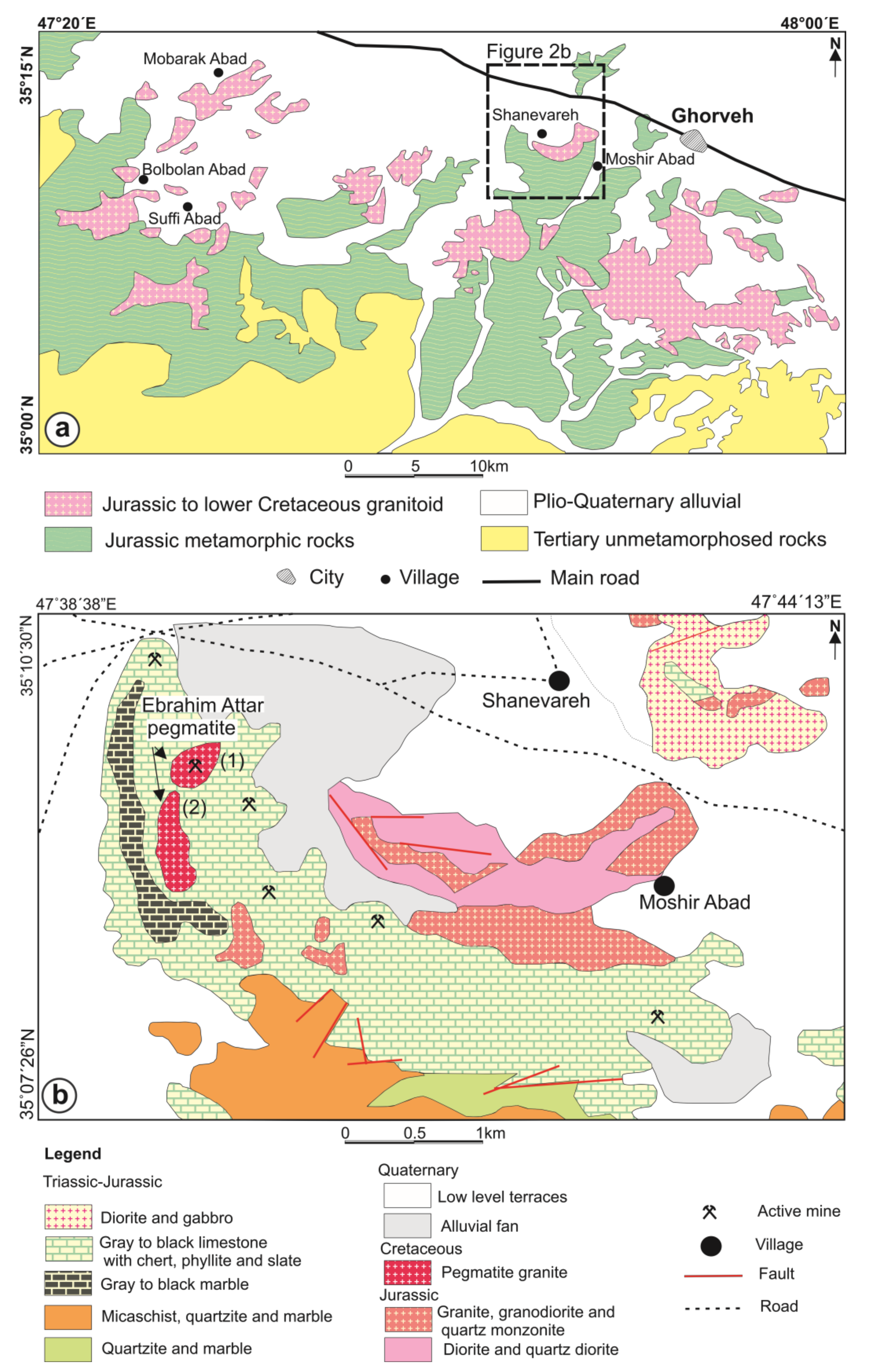
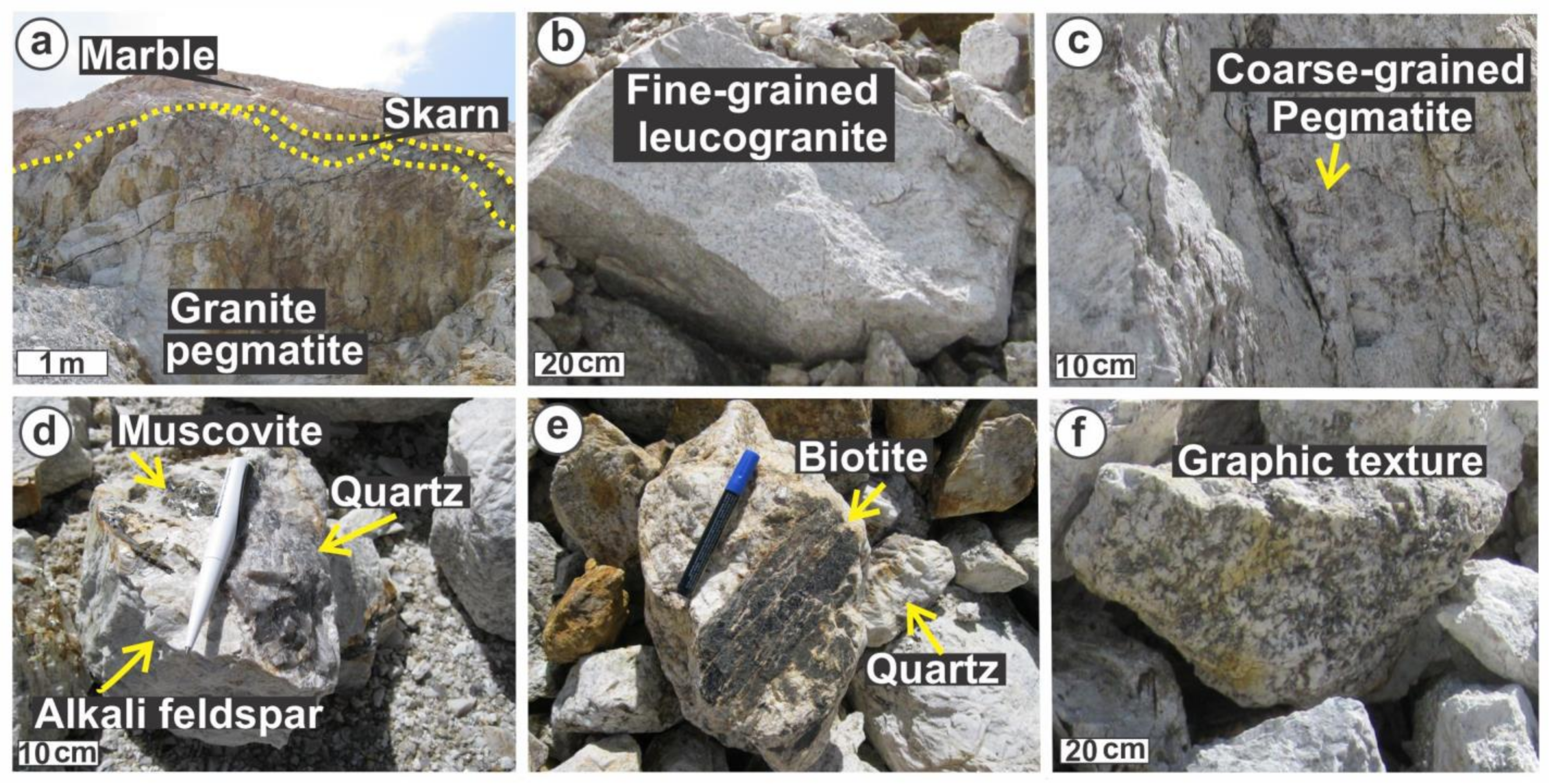
2. Geological Setting and Mineralogy
3. Analytical Method
4. Results
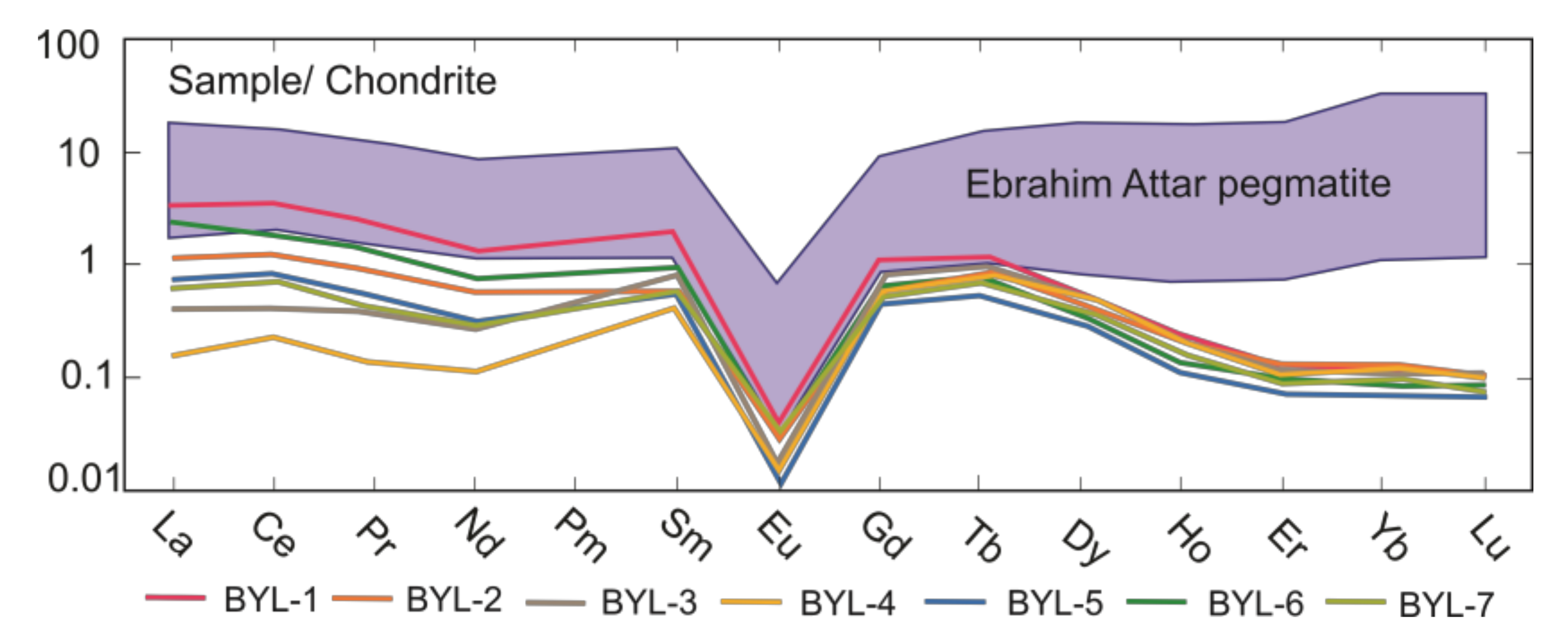
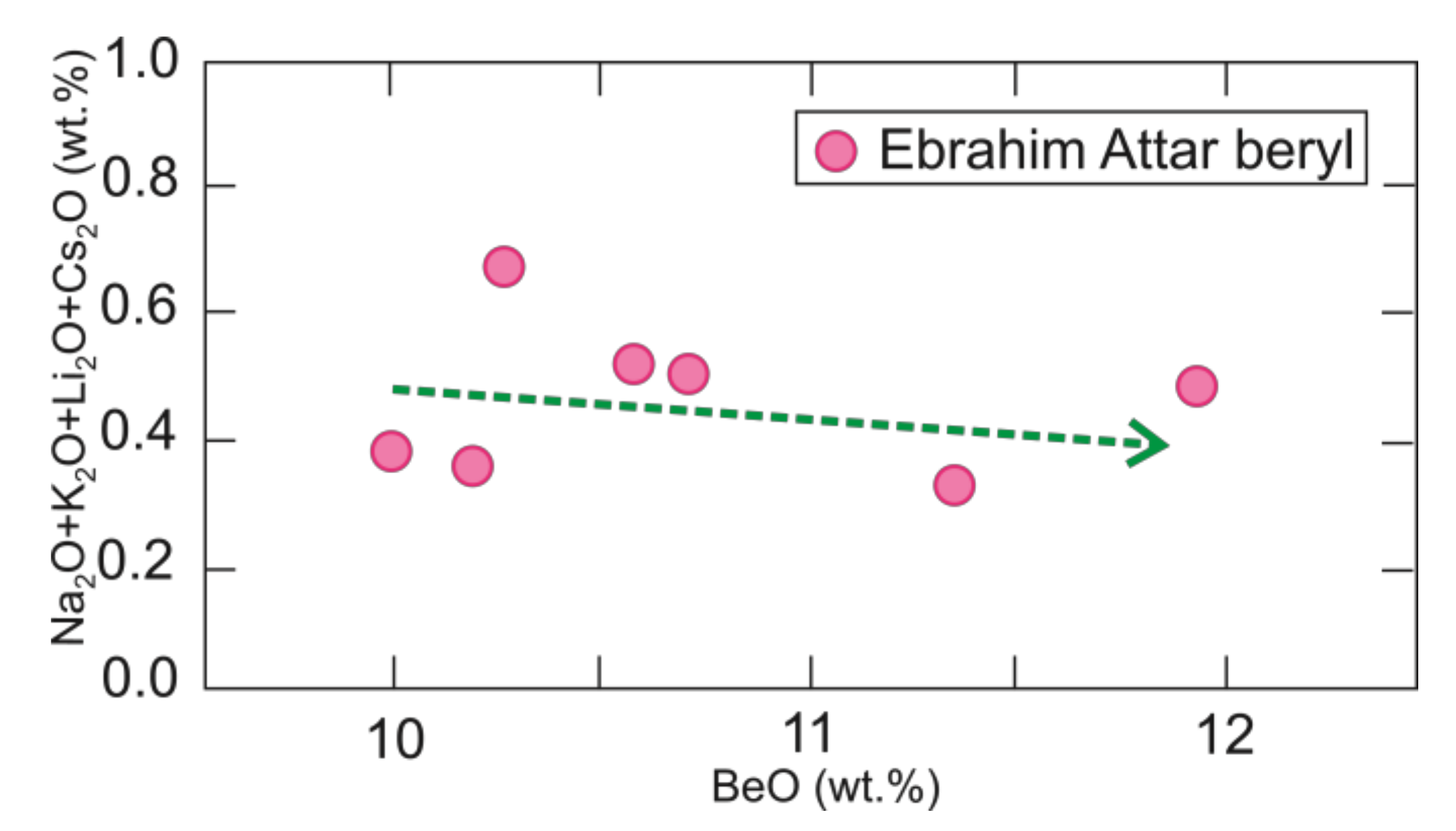
4.1. Beryl Chemistry
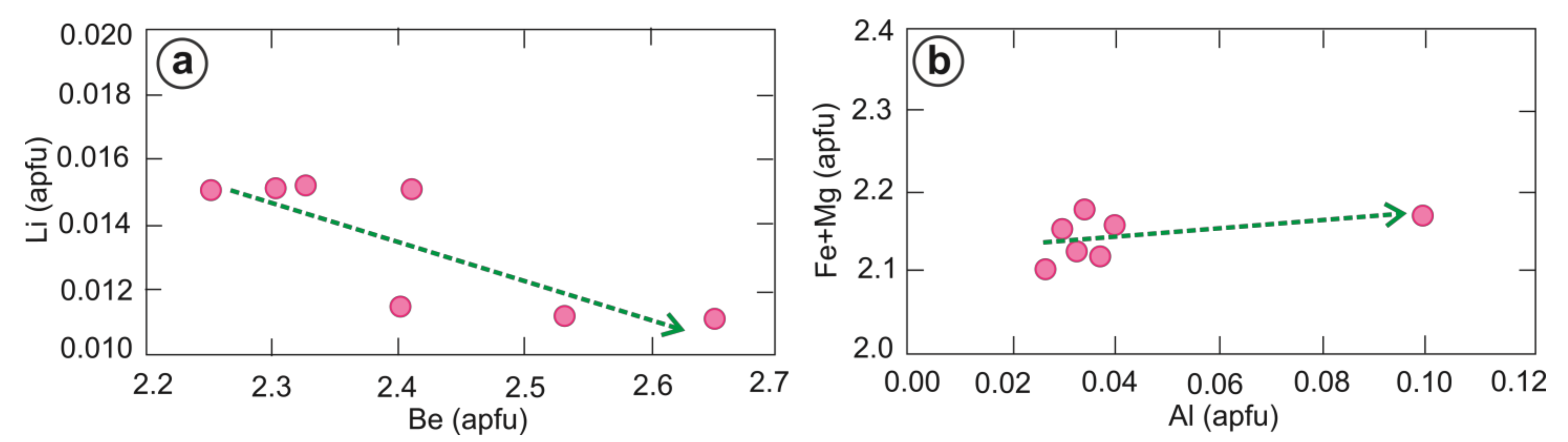
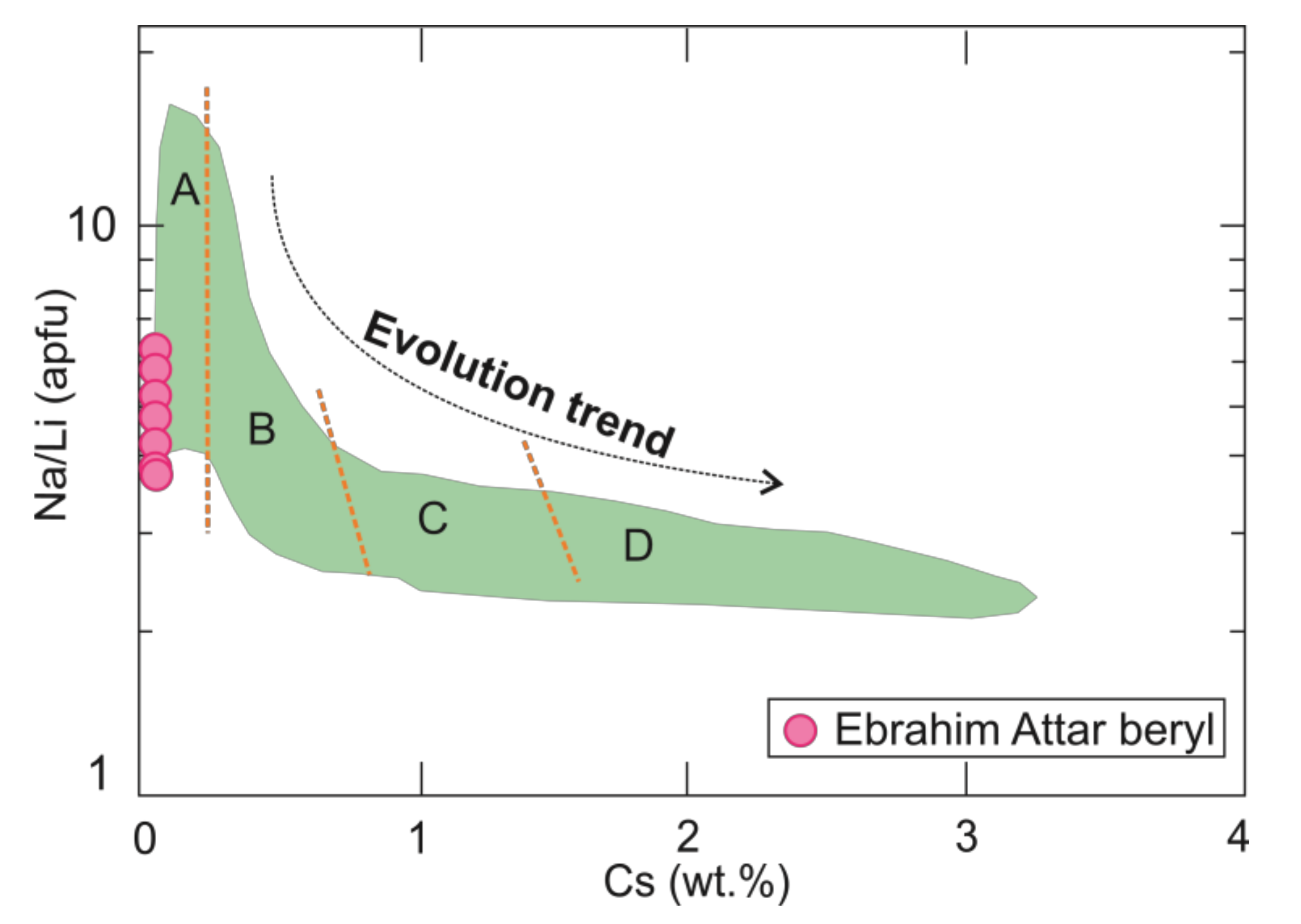
4.2. Crytallochemistry of Beryl
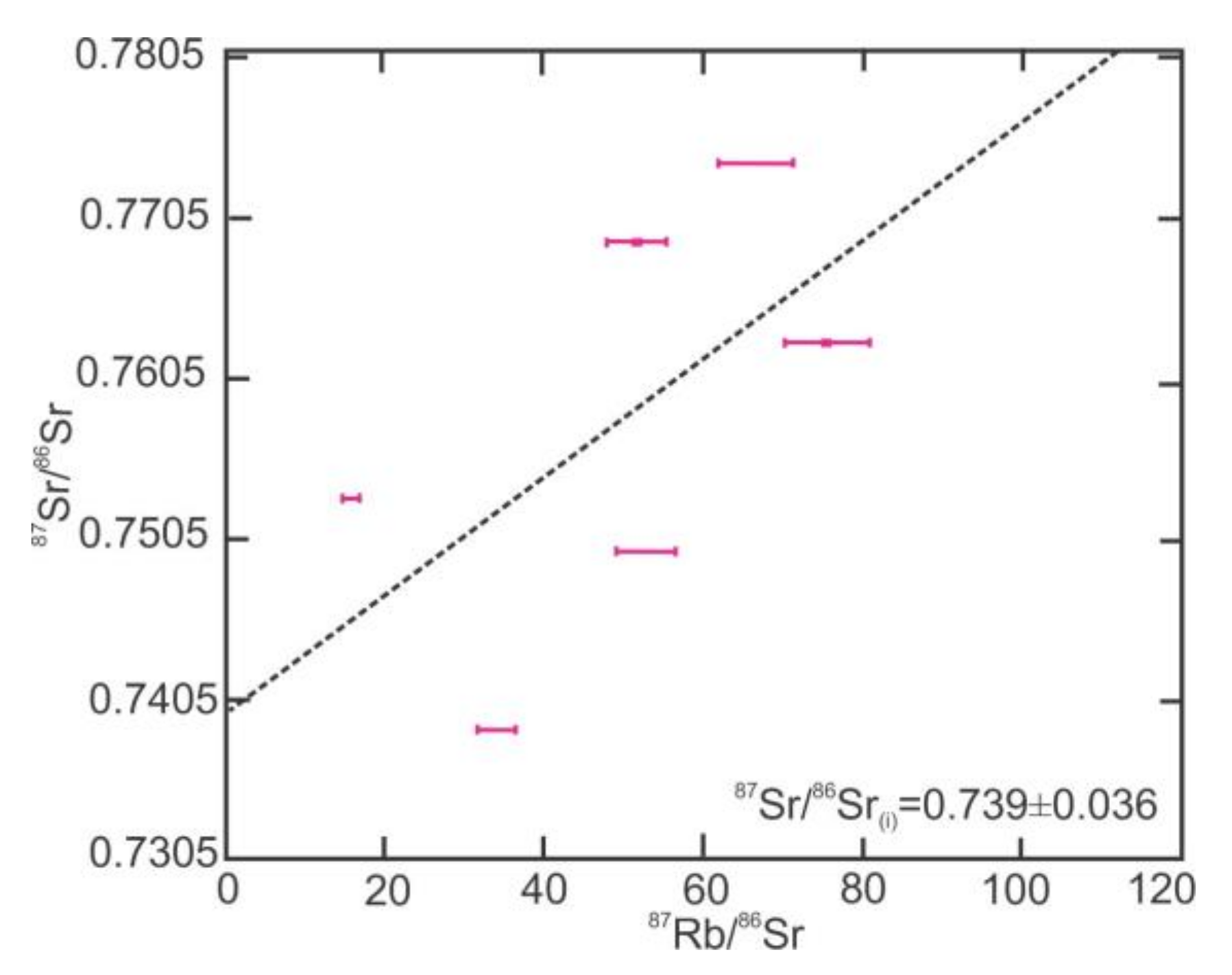
4.3. 87Sr/86Sr Isotope Ratios in the Beryl Grains
5. Discussion
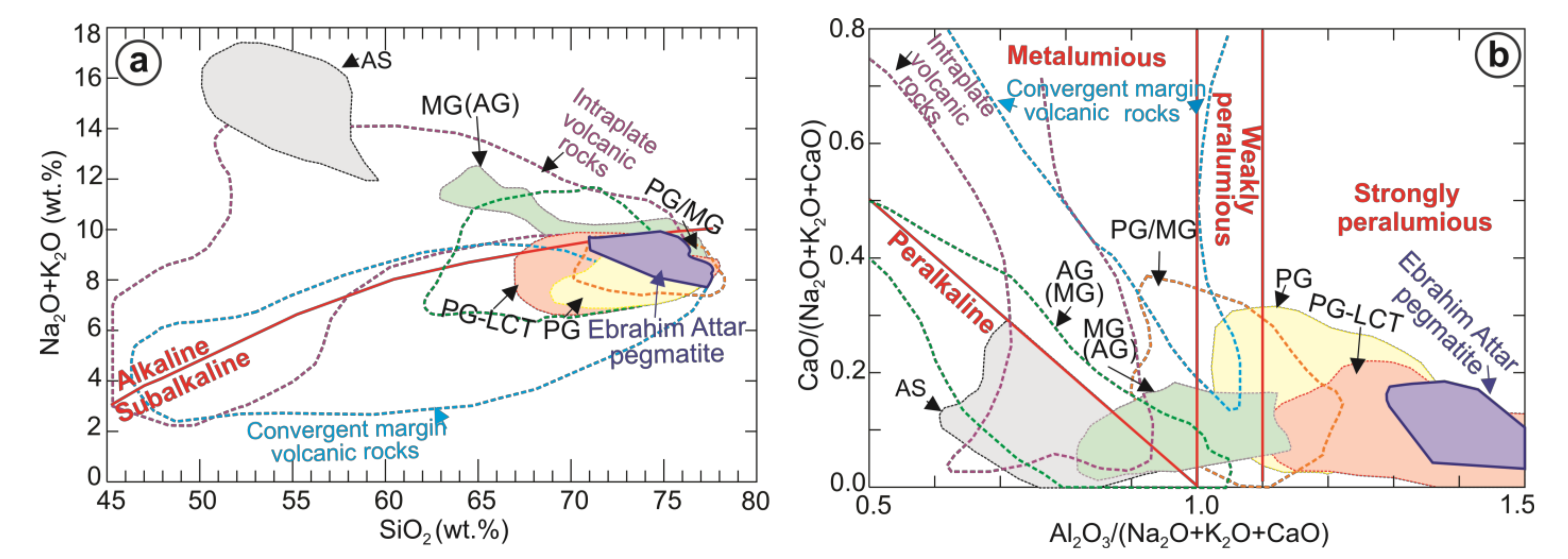
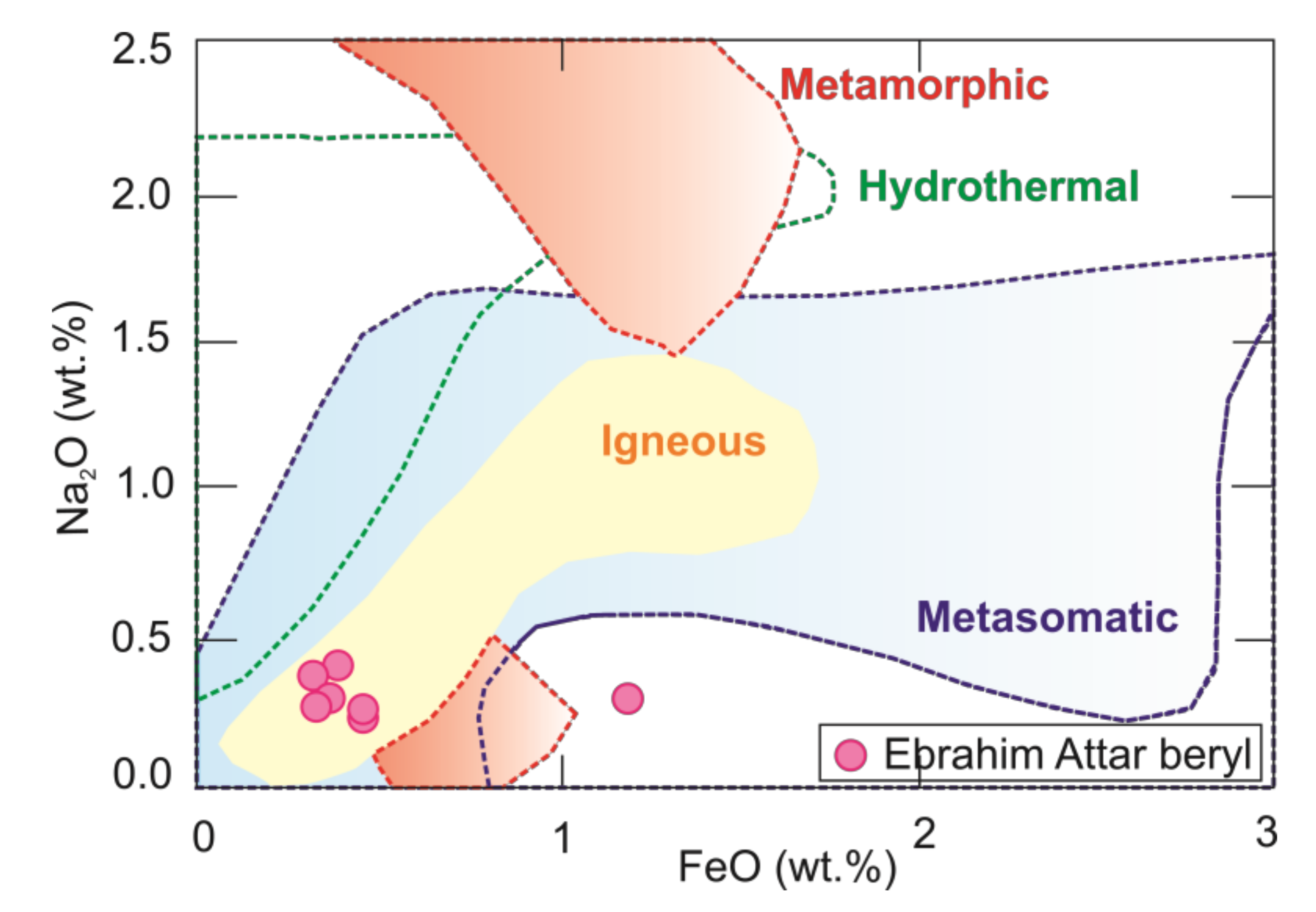
5.1. Beryl and Its Parental Magma
5.2. Geochemical Characteristics of Beryl Grains
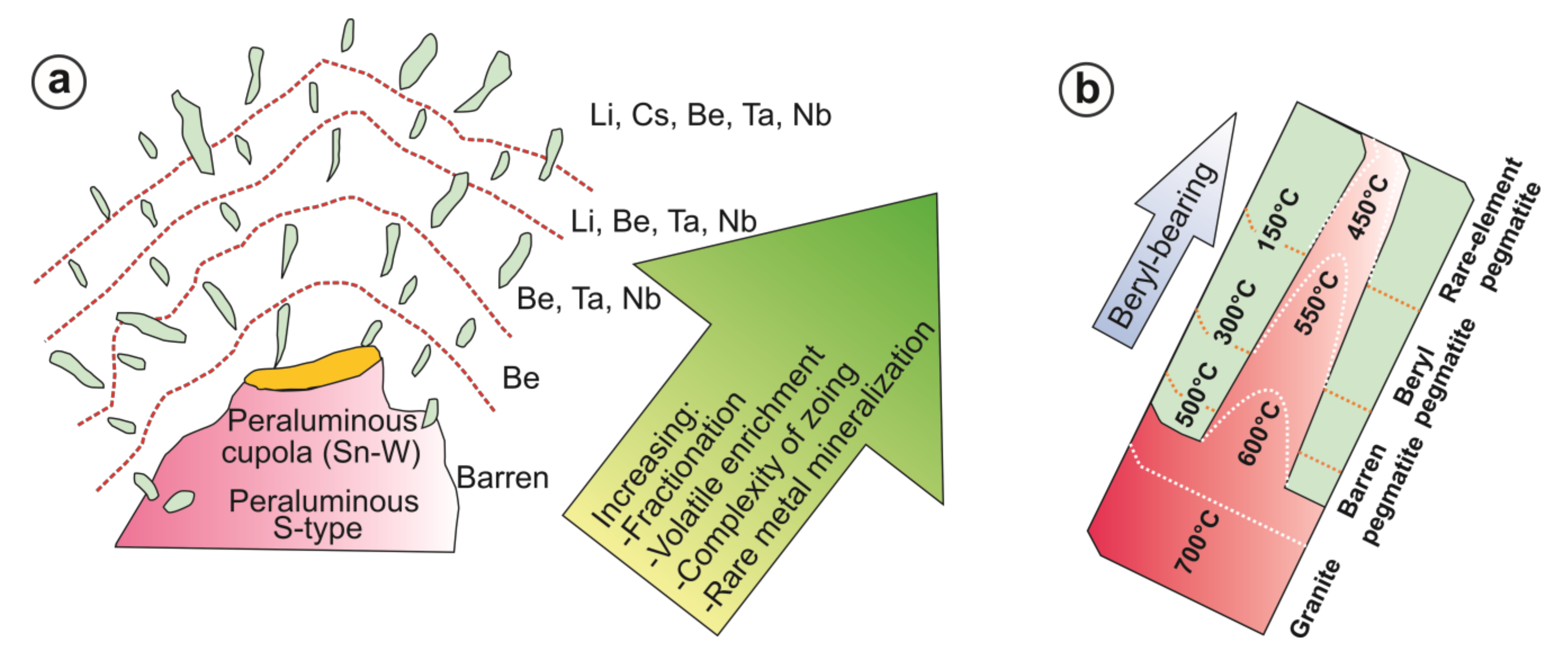
5.3. Beryl Mineralization
6. Conclusions
- According to the mineral sequence, beryl is the only Be-bearing mineral, and its host rock is classified as Be-pegmatite in the Ebrahim-Attar area.
- The host rock has an LCT pegmatite and is a less-evolved pegmatite with high values of Nb/Ta (4.79–16.3) and Zr/Hf (12.2–19.1).
- Pale-green to white-colored beryl in the host granite appears as euhedral crystals and is alkali-poor beryl with low amounts of Na2O, K2O, Li2O, and Cs2O.
- Concentrations of Cs and Na/Li values in the beryl grains imply a magmatic signature and suggest a relation to less-evolved granite.
- High 87Sr/86Sr ratios of both the beryl and its host granite are consistent with continental crust contributions from their sources.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Study of Inter Atomic Distances in Halides and Chalcogenides in Halides and Chaleogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Uher, P.; Bačík, P.; Fridrichová, J. Beryllium silicate minerals in granite-pegmatite suites: Tracers of magmatic to hydrothermal and tectonic evolution (examples from Western Carpathians). Carpathica 2019, 106–107. [Google Scholar] [CrossRef]
- Foley, N.K.; Jaskula, B.W.; Piatak, N.M.; Schulte, R.F. Beryllium; Schulz, K.J., DeYoung John, J.H., Jr., Seal, R.R., II, Bradley, D.C., Eds.; U.S. Geological Survey: Reston, VA, USA, 2017. [Google Scholar]
- Barton, M.D.; Young, S. Non-pegmatitic Deposits of Beryllium: Mineralogy, Geology, Phase Equilibria and Origin. Rev. Mineral. Geochem. 2002, 50, 591–691. [Google Scholar] [CrossRef]
- Sardi, F.E.G. Pegmatitic beryl as indicator of melt evolution: Example from the Velaco district, Pampeana pegmatite province, Argentina and review of worldwide occurrences. Can. Mineral. 2014, 52, 809–836. [Google Scholar] [CrossRef]
- Hörmann, P. Beryllium. In Handbook of Geochemistry; Wedepohl, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1978; p. 458. [Google Scholar]
- Bradley, D.; Mccauley, A. A Preliminary Deposit Model for Lithium-Cesium-Tantalum (LCT) Pegmatites; U.S. Geological Survey: Reston, VA, USA, 2016. [Google Scholar]
- Foley, N.K.; Hofstra, A.H.; Lindsey, D.A.; Seal, R.R., II; Jaskula, B.W.; Piatak, N.M. Occurrence Model for Volcanogenic Beryllium Deposits; U.S. Geological Survey: Reston, VA, USA, 2012. [Google Scholar]
- Černý, P. Mineralogy of beryllium in granitic pegmatites. Mineral. Geochem. 2002, 50, 405–444. [Google Scholar] [CrossRef]
- Prikryl, J.; Novák, M.; Filip, J.; Gadas, P.; Vašinová, M.G. Iron-Bearing Beryl from Granitic Pegmatites; EMPA, LA-ICP-MS, Mössbauer Spectroscopy and Powder XRD Study. In Proceedings of the PEG 2013: The 6th International Symposium on Granitic-Pegmatites, New Orleans, LA, USA, 26 May–2 June 2013; pp. 115–116. [Google Scholar]
- Uher, P.; Chudík, P.; Bačík, P.; Vaculovič, T.; Galiová, M. Beryl composition and evolution trends: An example from granitic pegmatites of the beryl-columbite subtype, Western Carpathians, Slovakia. J. Geosci. 2010, 55, 69–80. [Google Scholar] [CrossRef][Green Version]
- London, D. Reading Pegmatites: Part 1—What Beryl Says. Rocks Miner. 2015, 90, 138–153. [Google Scholar] [CrossRef]
- Grew, E.S. Mineralogy, Petrology and Geochemistry of Beryllium: An Introduction and List of Beryllium Minerals. Rev. Mineral. Geochem. 2002, 50, 1–76. [Google Scholar] [CrossRef]
- Cerny, P.; Turnock, A.C. Beryl from the granitic pegmatites at Greer Lake, southeastern Manitoba. Can. Mineral. 1975, 13, 55–61. [Google Scholar]
- Neiva, A.M.R.; Neiva, J.M.C. Beryl from the granitic pegmatite at Namivo, Alto Ligonha, Mozambique. J. Mineral. Geochem. 2005, 181, 173–182. [Google Scholar] [CrossRef]
- Linnen, R.L.; Van Lichtervelde, M.; Černý, P. Granitic pegmatites as sources of strategic metals. Elements 2012, 8, 275–280. [Google Scholar] [CrossRef]
- Thomas, R.; Webster, J.D.; Davidson, P. Be-daughter minerals in fluid and melt inclusions: Implications for the enrichment of Be in granite-pegmatite systems. Contrib. Mineral. Petrol. 2011, 161, 483–495. [Google Scholar] [CrossRef]
- Evensen, J.M.; London, D. Experimental silicate mineral/melt partition coefficients for beryllium and the crustal Be cycle from migmatite to pegmatite. Geochim. Cosmochim. Acta 2002, 66, 2239–2265. [Google Scholar] [CrossRef]
- Rao, C.; Wang, R.C.; Hu, H. Paragenetic assemblage of beryllium silicates and phosphates from the Nanping granite pegmatite dyke; Fujian province southeastern China. Can. Mineral. 2011, 49, 1175–1187. [Google Scholar] [CrossRef]
- Schilling, J.; Bingen, B.; Skar, Q.; Wenzel, T.; Markl, G. Formation and evolution of the Høgtuva beryllium deposit, Norway. Contrib. Mineral. Petrol. 2015, 170, 1–21. [Google Scholar] [CrossRef]
- Lyalina, L.M.; Selivanva, E.A.; Zozulya, D.; Ivanyuk, G.Y. Beryllium mineralogy of the Kola Peninsula, Russia—A review. Minerals 2019, 9, 12. [Google Scholar] [CrossRef]
- London, D.; Kontak, D.J. Granitic Pegmatites: Scientific Wonders and Economic Bonanzas. Elements 2012, 8, 257–261. [Google Scholar] [CrossRef]
- Glover, A.S.; Rogers, W.Z.; Barton, J.E. Industrial Minerals. Elements 2012, 8, 269–274. [Google Scholar] [CrossRef]
- Černý, P.; London, D.; Novák, M. Granitic pegmatites as reflections of their sources. Elements 2012, 8, 289–294. [Google Scholar] [CrossRef]
- Tkachev, A.V. Evolution of metallogeny of granitic pegmatites associated with orogens throughout geological time. Geol. Soc. London Spec. Publ. 2011, 350, 7–23. [Google Scholar] [CrossRef]
- Sheikhi, F.; Alaminia, Z.; Tabakh Shabani, A.A. Seranjic skarn geothermomtery (SW Ghorveh, Kurkistan province). Iran. J. Crystallogr. Mineral. 2012, 20, 343–354. [Google Scholar]
- Samadi, R.; Miller, N.R.; Mirnejad, H.; Harris, C. Origin of garnet in aplite and pegmatite from Khajeh Morad in northeastern Iran: A major, trace element, and oxygen isotope approach Ramin. Lithos 2014, 208, 378–392. [Google Scholar] [CrossRef]
- Sepahi, A.A.; Salami, S.; Lentz, D.R.; McFardand, C.R.M. Petrography, Geochemistry, and U–Pb Geochronology of Pegmatites and Aplites Associated with the Alvand Intrusive Complex in the Hamedan Region, Sanandaj–Sirjan Zone, Zagros Orogen (Iran). Int. J. Earth Sci. 2018, 107, 1059–1096. [Google Scholar] [CrossRef]
- Azizi, H.; Nouri, F.; Stern, R.J.; Azizi, M.; Lucci, F.; Asahara, Y.; Zarinkoub, M.H.; Chung, S.L. New evidence for Jurassic continental rifting in the northern Sanandaj Sirjan Zone, western Iran: The Ghalaylan seamount, southwest Ghorveh. Int. Geol. Rev. 2020, 62, 1635–1657. [Google Scholar] [CrossRef]
- Azizi, H.; Mohammadi, K.; Asahara, Y.; Tsuboi, M.; Daneshvar, N.; Mehrabi, B. Strongly peraluminous leucogranite (Ebrahim-Attar granite) as evidence for extensional tectonic regime in the Cretaceous, Sanandaj Sirjan zone, northwest Iran. Chem. Erde Geochem. 2016, 76, 529–541. [Google Scholar] [CrossRef]
- Nouri, F.; Stern, R.J.; Azizi, H. The Jurassic tourmaline–garnet–beryl semi-gemstone province in the Sanandaj–Sirjan Zone, western Iran. Int. Geol. Rev. 2018, 1–25. [Google Scholar] [CrossRef]
- Salami, S.; Sepahi, A.A.; Maanijou, M. The study of beryl pegmatites of Ebrahim Attar and related skarn (south west of Ghorveh). In Proceedings of the 23rd, Symposium of Crystallography and Minelogy of Iran, Dagman, Iran, 28–29 January 2013; pp. 825–829. [Google Scholar]
- Stöcklin, J. Structural history and tectonics of Iran1: A review. Am. Assoc. Pet. Geol. Bull. 1968, 52, 1229–1258. [Google Scholar] [CrossRef]
- Alavi, M. Regional stratigraphy of the Zagros fold-thrust belt of Iran and its proforeland evolution. Am. J. Sci. 2004, 304, 1–20. [Google Scholar] [CrossRef]
- Ghasemi, A.; Talbot, C.J. A new tectonic scenario for the Sanandaj–Sirjan Zone (Iran). J. Asian Earth Sci. 2006, 26, 683–693. [Google Scholar] [CrossRef]
- Azizi, H.; Zanjefili-Beiranvand, M.; Asahara, Y. Zircon U–Pb ages and petrogenesis of a tonalite–trondhjemite–granodiorite (TTG) complex in the northern Sanandaj–Sirjan zone, northwest Iran: Evidence for Late Jurassic arc–continent collision. Lithos 2015, 216–217, 178–195. [Google Scholar] [CrossRef]
- Azizi, H.; Asahara, Y.; Minami, M.; Anma, R. Sequential magma injection with a wide range of mixing and mingling in Late Jurassic plutons, southern Ghorveh, western Iran. J. Asian Earth Sci. 2020, 104469. [Google Scholar] [CrossRef]
- Yajam, S.; Scarrow, J.H.; Ghalamghash, J.; Bea, F. The spatial and compositional evolution of the Late Jurassic Ghorveh- Dehgolan plutons of the Zagros Orogen, Iran: SHRIMP zircon U-Pb and Sr and Nd isotope evidence. Geologica Acta 2015, 13, 25–43. [Google Scholar] [CrossRef]
- Azizi, H.; Asahara, Y. Juvenile granite in the Sanandaj–Sirjan Zone, NW Iran: Late Jurassic–Early Cretaceous. Int. Geol. Rev. 2013, 55, 1523–1540. [Google Scholar] [CrossRef]
- Sartibi, H. Geological Map of Sanandaj (1/100000); Geological Survey of Iran: Tehran, Iran, 2005. [Google Scholar]
- Hosseiny, M.; Mosawery, F.; Karimynia, M. 1:100.000 Geological Map of Ghorveh; Geological Survey of Iran: Tehran, Iran, 1999. [Google Scholar]
- Eshraghi, A.A. Geological Map of Iran Sheet 5559-Songhor, Scale 1:100,000; Geological Survey of Iran: Tehran, Iran, 1996. [Google Scholar]
- Selway, J.B.; Breaks, F.W.; Road, R.L.; Pe, O.N.; Tindle, A.G. A review of rare-element (Li-Cs-Ta) pegmatite exploration techniques for the Superior Province, Canada, and large worldwide tantalum deposits. Explor. Min. Geol. 2005, 14, 1–30. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. London Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Rollinson, H. Using Geochemical Data: Evaluation Presentation, Interpreation; Routledge: Abingdon, UK, 1993. [Google Scholar]
- Bragg, W.L.; West, J. The structure of beryl, Be3Al2Si6O18. Proc. R. Soc. London. Ser. A Contain. Pap. Math. Phys. Character 1926, 111, 691–714. [Google Scholar]
- Aurisicchio, C.; Fioravanti, G.; Grubessi, O.; Zanazzi, P.F. Reappraisal of the crystal chemistry of beryl. Am. Mineral. 1988, 73, 826–837. [Google Scholar]
- Belov, N.V. Essays on structural mineralogy IX. Miner. Sb. Geol Soc Lvov 1958, 12, 15–42. [Google Scholar]
- Thomas, Z.; Link, K. In-situ Rb-Sr dating of beryl. In Proceedings of the Goldschmidt, Barcelona, Spain, 18–23 August 2019; p. 1. [Google Scholar]
- Groat, L.A. Crystal chemistry of dark blue aquamarine from the true showing, Yukan Territory, Canada. Can. Mineral. 2010, 48, 597–613. [Google Scholar] [CrossRef]
- Novák, M.; Filip, J. Unusual (Na, Mg)-enriched beryl and its breakdown products (beryl II, bazzite, bavenite) from euxenite-type NYF pegmatite related to the orogenic ultrapotassic Pluton, Czech Republic. Can. Mineral. 2010, 48, 615–628. [Google Scholar] [CrossRef]
- Černý, P.; Menizer, R. Fertile granites in the Archean and Proterozoic fields of rare element pegmatites: Crustal environment, geochemistry and petrogenetic relationships. In Recent Advances in the Geology of Granite-related Mineral Deposits. Can. Inst. Min. Metall. 1988, 39, 170–206. [Google Scholar]
- Černý, P. Exploration strategy and methods for pegmatite deposits of tantalum. In Lanthanides, Tantalum and Niobium; Springer: Berlin/Heidelber, Germany, 1989; pp. 274–302. [Google Scholar]
- Wilson, M. Review Of Igneous Petrogenesis: Aglobal Tectonic Approach. Terra Nov. 1989, 1, 218–222. [Google Scholar] [CrossRef]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Černý, P.; Meintzer, R.E.; Anderson, A.J. Extreme fractionation in rare- element granitic pegmaite: Selected examples of data and nechanism. Can. Mineral. 1985, 23, 381–421. [Google Scholar]
- Beal, K.; Lentz, D.R.; Hall, D.C.; Dunning, G. Mineralogical, geochronological and geochemical characterization of Early Devonian aquamarine-bearing dykes of the Zealand Station beryl and molybdenite deposit, west central New Brunswick. Can. J. Earth Sci. 2010, 47, 859–874. [Google Scholar] [CrossRef]
- Zhu, Y.; Zeng, Y.; Gu, L. Geochemistry of the rare metal-bearing pegmatite No. 3 vein and related granites in the Keketuohai region, Altay Mountains, northwest China. J. Asian Earth Sci. 2006, 27, 61–77. [Google Scholar] [CrossRef]
- Dostal, J.; Chatterjee, A.K. Contrasting behaviour of Nb/Ta and Zr/Hf ratios in a peraluminous granitic pluton (Nova Scotia, Canada). Chem. Geol. 2000, 163, 207–218. [Google Scholar] [CrossRef]
- Steiner, B.M. Tools and workflows for grassroots Li–Cs–Ta (LCT) pegmatite exploration. Minerals 2019, 9, 499. [Google Scholar] [CrossRef]
- Watson, E.B.; Harrison, T.M. Zircon saturation revisited: Temperature and composition effects in a variety of crustal magma types. Earth Planet. Sci. Lett. 1983, 64, 295–304. [Google Scholar] [CrossRef]
- Acosta-Vigil, A.; London, D.; Morgan, G.B.; Dewers, T. Solubility of excess alumina in hydrous granitic melts in equilibrium with peraluminous minerals at 700–800 °C and 200 MPa, and applications of the aluminum saturation index. Contrib. Miner. Pet. 2003, 146, 100–119. [Google Scholar] [CrossRef]
- Yang, X.M.; Lentz, D.R.; Chi, G. Ferric-ferrous iron oxide ratios: Effect on crystallization pressure of granites estimated by Qtz-geobarometry. Lithos 2021, 380–381, 105920. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.-S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Aurisicchio, C.; Conte, A.M. Beryl from miarolotic pockets of granitic pegmatites, Elba, Italy: Characterization of crystal chemistry by means of EMP and SIMS analyses. Can. Mineral. 2012, 50, 1467–1488. [Google Scholar] [CrossRef]
- Beus, A.A. Geochemistry of Berllium and Genetic Types of Beryllium Deposits; Freemand and Co.: San Francisco, CA, USA, 1966. [Google Scholar]
- Hawthorne, F.C.; Cerny, P. The alkali-metal positions in Cs-Li beryl. Can. Mineral. 1977, 15, 414–421. [Google Scholar]
- Markl, G.; Schumcher, J.C. Beryl stability in local hydrothermal and chemical environments in a mineralized granite. Am. Mineral. 1997, 82, 194–202. [Google Scholar] [CrossRef]
- Cerny, P.; Anderson, A.J.; Tomascak, P.B.; Chapman, R. Geochemical and morphological features of beryl from the Bikita granitic pegmatite, Zimbabwe. Can. Mineral. 2003, 41, 1003–1011. [Google Scholar] [CrossRef]
- Wang, R.C.; Che, X.D.; Zhang, W.L.; Zhang, A.C.; Zhang, H. Geochemical evolution and late re-equilibration of Na-Cs-rich beryl from the Koktokay #3 pegmatite (Altai, NW China). Eur. J. Mineral. 2009, 21, 795–809. [Google Scholar]
- Liu, Y.; Deng, J.; Sun, D.; Zhou, Y. Morphology and gensis typomorphism of minerals in W-Sn-Be deposit of Huya, Sichuan. Geosci. J. 2007, 32, 75–81. [Google Scholar]
- Sarbajna, C.; Sinha, R.P.; Krishnamurthy, P.; Krishna, K.V.G.; Viswanathan, R.; Banerjee, D. Metal bearing pegmatite of Marlagalla-Allapatna, Mandya district, Karnataka. Geol. Soc. India 1999, 599–608. [Google Scholar]
- Trueman, D.L.; Cerny, P. Exploration for rare-element granitic pegmatites. In Proceedings of the Short Course in Granitic Pegmatites in Science and Industry, Winnipeg, MB, Canada, 13–16 May 1982; Mineralogical Association of Canada: Quebec, QC, Canada, 1982; pp. 463–493. [Google Scholar]
- Merino, E.; Villaseca, C.; Orejana, D.; Jeffries, T. Gahnite, chrysoberyl and beryl co-occurrence as accessory minerals in a highly evolved peraluminous pluton: The Belvís de Monroy leucogranite (Cáceres, Spain). Lithos 2013, 179, 137–156. [Google Scholar] [CrossRef]
- Evensen, J.M.; London, D.; Wendlandt, R.F. Solubility and stability of beryl in granitic melts. Am. Mineral. 1999, 84, 733–745. [Google Scholar] [CrossRef]
- Charoy, B. Beryllium speciation in evolved granitic magmas: Phosphates versus silicates. Eur. J. Mineral. 1999, 11, 135–148. [Google Scholar] [CrossRef]
- London, D. Pegmatites, the Canadian Mineralogist; Special Publication 10; The Mineralogical Association of Canada: Quebec, QC, Canada, 2008. [Google Scholar]
- Wahlberg, J.S.; Fishman, M.J. Adsorption of Cesium on Clay Minerals; US Government Printing Office: Washington, DC, USA, 1962. [Google Scholar]
- London, D. Ore-forming processes within granitic pegmatites. Ore. Geol. Rev. 2018, 101, 349–383. [Google Scholar] [CrossRef]
- Baker, D.R. Tracer versus trace element diffusion: Diffusional decoupling of Sr concentration from Sr isotope composition. Geochim. Cosmochim. Acta 1989, 53, 3015–3023. [Google Scholar] [CrossRef]
- Černý, P. Rare-element granitic pegmatites. Part I: Anatomy and internal evolution of pegmatitic deposits. Geosci. Canada 1991, 11, 49–67. [Google Scholar]
- Simmons, W. Pegmatology: Pegmatite Mineralogy, Petrology and Petrogenesis; Rubellite Press: New Orleans, LA, USA, 2003. [Google Scholar]
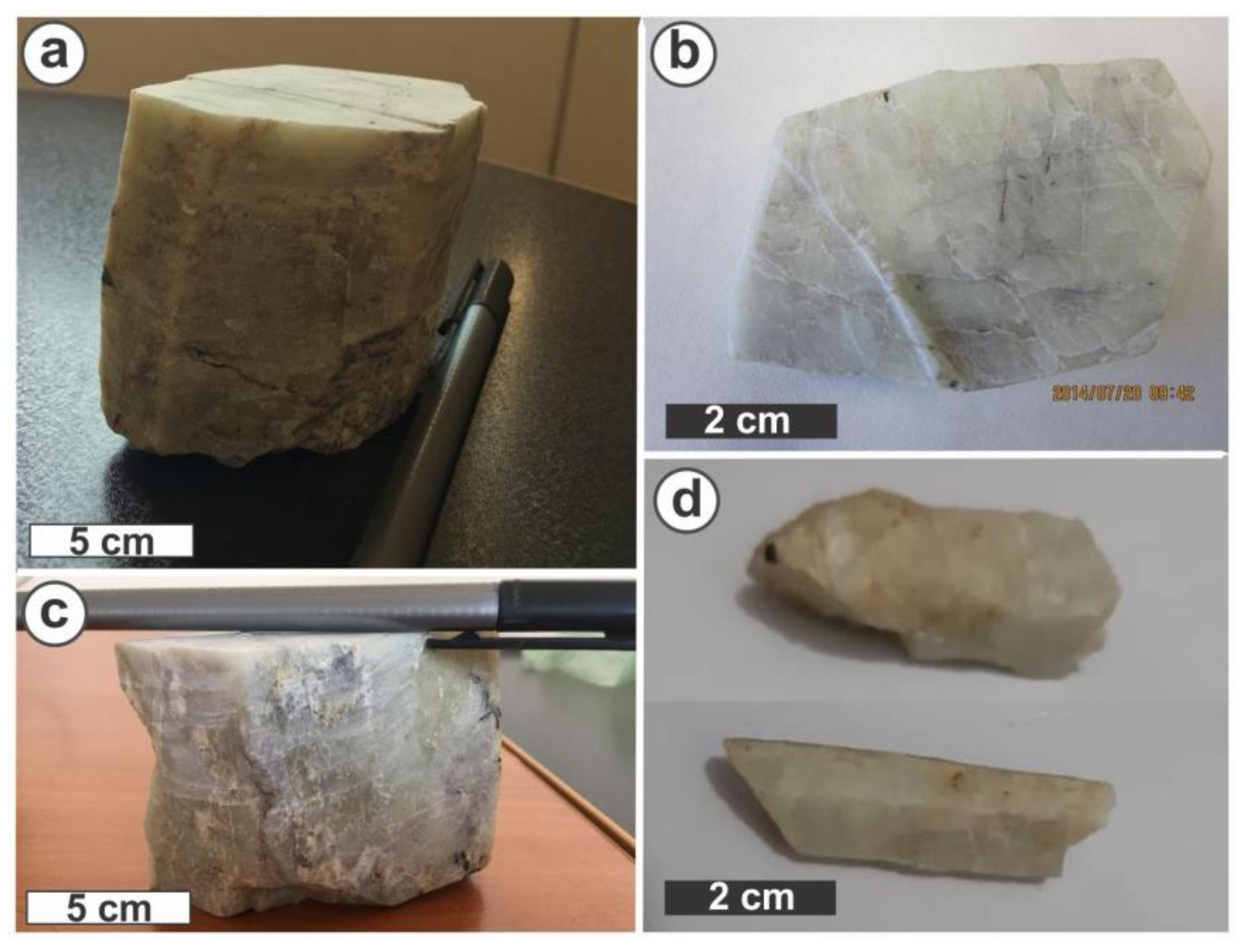
| Sample Name | BYL1 | BYL2 | BYL3 | BYL4 | BYL5 | BYL6 | BYL7 | Blank |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 66.6 | 66.8 | 66.2 | 67.2 | 65.9 | 66.2 | 67.0 | |
| Al2O3 | 18.9 | 19.0 | 18.7 | 19.1 | 19.3 | 19.2 | 19.0 | |
| Fe2O3 | 0.31 | 0.49 | 0.40 | 0.50 | 1.31 | 0.42 | 0.35 | |
| MnO | 0.005 | 0.006 | 0.006 | 0.004 | 0.008 | 0.005 | 0.005 | |
| MgO | 0.022 | 0.007 | 0.020 | 0.019 | 0.017 | 0.019 | 0.023 | |
| CaO | 0.071 | 0.069 | 0.068 | 0.070 | 0.072 | 0.087 | 0.079 | |
| Na2O | 0.36 | 0.24 | 0.28 | 0.25 | 0.29 | 0.41 | 0.26 | |
| K2O | 0.072 | 0.053 | 0.186 | 0.088 | 0.132 | 0.217 | 0.052 | |
| P2O5 | 0.009 | 0.007 | 0.007 | 0.006 | 0.008 | 0.013 | 0.007 | |
| BeO | 11.9 | 11.3 | 10.6 | 10.0 | 10.7 | 10.3 | 10.2 | <10 ng |
| LOI | 1.61 | 1.63 | 1.63 | 1.65 | 1.69 | 1.70 | 1.68 | |
| Total | 99.9 | 99.7 | 98.1 | 98.9 | 99.4 | 98.5 | 98.6 | |
| Li | 155 | 143 | 145 | 164 | 173 | 178 | 179 | 40 ng |
| V | 1.29 | 0.58 | 0.74 | 0.63 | 0.85 | 0.93 | 0.89 | 3 ng |
| Zn | 55.8 | 40.8 | 33.0 | 52.9 | 31.9 | 62.4 | 51.9 | 70 ng |
| Rb | 66 | 53 | 55 | 15 | 85 | 72 | 44 | 10 µg |
| Sr | 3.61 | 3.01 | 2.26 | 2.73 | 3.26 | 3.12 | 3.73 | 1 ng |
| Nb | 0.90 | 11.8 | 6.92 | 0.98 | 2.90 | 2.43 | 0.52 | 2 ng |
| Cs | 199 | 253 | 228 | 106 | 260 | 121 | 167 | 5 ng |
| Y | 0.833 | 0.565 | 0.547 | 0.588 | 0.351 | 0.489 | 0.511 | 90 pg |
| La | 0.869 | 0.274 | 0.098 | 0.039 | 0.174 | 0.566 | 0.145 | 60 pg |
| Ce | 2.05 | 0.778 | 0.269 | 0.136 | 0.532 | 1.219 | 0.444 | 140 pg |
| Pr | 0.224 | 0.087 | 0.035 | 0.014 | 0.047 | 0.131 | 0.038 | 30 pg |
| Nd | 0.661 | 0.280 | 0.132 | 0.057 | 0.153 | 0.387 | 0.134 | 50 pg |
| Sm | 0.307 | 0.184 | 0.131 | 0.067 | 0.095 | 0.176 | 0.087 | 11 pg |
| Eu | 0.0026 | 0.0018 | 0.0009 | 0.0007 | 0.0007 | 0.0017 | 0.0022 | <5 pg |
| Gd | 0.220 | 0.164 | 0.154 | 0.113 | 0.094 | 0.141 | 0.105 | 6 pg |
| Tb | 0.045 | 0.037 | 0.037 | 0.031 | 0.021 | 0.029 | 0.027 | 2 pg |
| Dy | 0.144 | 0.120 | 0.129 | 0.113 | 0.076 | 0.097 | 0.104 | 6 pg |
| Ho | 0.0122 | 0.0112 | 0.0114 | 0.0110 | 0.0064 | 0.0082 | 0.0097 | 5 pg |
| Er | 0.020 | 0.020 | 0.019 | 0.018 | 0.012 | 0.017 | 0.015 | 14 pg |
| Tm | 0.0023 | 0.0027 | 0.0027 | 0.0027 | 0.0014 | 0.0023 | 0.0028 | 5 pg |
| Yb | 0.018 | 0.021 | 0.016 | 0.018 | 0.013 | 0.014 | 0.017 | 15 pg |
| Lu | 0.0023 | 0.0027 | 0.0021 | 0.0019 | 0.0017 | 0.0022 | 0.0028 | 3 pg |
| ΣREE | 5.41 | 2.55 | 1.58 | 1.21 | 1.58 | 3.28 | 1.64 | |
| ΣLREE | 4.33 | 1.77 | 0.82 | 0.43 | 1.10 | 2.62 | 0.96 | |
| ΣHREE | 0.244 | 0.213 | 0.217 | 0.195 | 0.131 | 0.170 | 0.178 | |
| ΣHREE/ΣLREE | 0.056 | 0.121 | 0.264 | 0.458 | 0.119 | 0.065 | 0.187 | |
| (Ce/La)N | 0.913 | 1.10 | 1.06 | 1.34 | 1.19 | 0.835 | 1.18 | |
| (La/Yb)N | 34.8 | 9.59 | 4.33 | 1.57 | 9.96 | 28.0 | 6.14 | |
| (La/Sm)N | 1.83 | 0.963 | 0.484 | 0.381 | 1.18 | 2.078 | 1.08 | |
| Eu/Eu* | 0.030 | 0.031 | 0.019 | 0.025 | 0.021 | 0.032 | 0.072 |
| Sample Name | BYL1 | BYL2 | BYL3 | BYL4 | BYL5 | BYL6 | BYL7 | Average |
|---|---|---|---|---|---|---|---|---|
| Si | 6.292 | 6.337 | 6.396 | 6.449 | 6.307 | 6.387 | 6.435 | 6.372 |
| Al | 2.103 | 2.122 | 2.128 | 2.157 | 2.174 | 2.179 | 2.154 | 2.145 |
| Fe | 0.023 | 0.035 | 0.029 | 0.037 | 0.096 | 0.031 | 0.026 | 0.040 |
| Mn | 0.0004 | 0.0005 | 0.0005 | 0.0003 | 0.0007 | 0.0004 | 0.0004 | 0.000 |
| Mg | 0.003 | 0.001 | 0.003 | 0.003 | 0.002 | 0.003 | 0.003 | 0.003 |
| Ca | 0.0071 | 0.0069 | 0.0070 | 0.0071 | 0.0073 | 0.0089 | 0.0080 | 0.007 |
| Na | 0.064 | 0.043 | 0.051 | 0.044 | 0.053 | 0.075 | 0.046 | 0.054 |
| K | 0.0084 | 0.0062 | 0.0222 | 0.0104 | 0.0156 | 0.0259 | 0.0062 | 0.014 |
| Be | 2.651 | 2.531 | 2.403 | 2.253 | 2.411 | 2.328 | 2.303 | 2.411 |
| Li | 0.011 | 0.011 | 0.011 | 0.015 | 0.015 | 0.015 | 0.015 | 0.013 |
| Cs | 0.0008 | 0.0012 | 0.0008 | 0.0004 | 0.0012 | 0.0004 | 0.0008 | 0.001 |
| Na/Li | 5.75 | 3.84 | 4.51 | 2.94 | 3.49 | 4.93 | 3.04 | 4.07 |
| Sample Name | BRYL1 | BRYL2 | BRYL3 | BRYL4 | BRYL5 | BRYL6 | BRYL7 |
|---|---|---|---|---|---|---|---|
| 87Sr/86Sr | 0.749177 | 0.768480 | 0.806010 | 0.752527 | 0.762224 | 0.773416 | 0.738121 |
| Error (1SE) | 0.000007 | 0.000016 | 0.000006 | 0.000008 | 0.000007 | 0.000006 | 0.000007 |
| Rb (ppm) | 66 | 53 | 55 | 15 | 85 | 72 | 44 |
| Sr (ppm) | 3.61 | 3.01 | 2.26 | 2.73 | 3.26 | 3.12 | 3.73 |
| 87Rb/86Sr | 53 | 52 | 71 | 16 | 76 | 67 | 34 |
| Name | Ebrahim-Attar Granitic Pegmatite | Zealand Station Dyke |
|---|---|---|
| Rb | 145–440 | 218–327 |
| Cs | 0.6–4.7 | 6–22 |
| Nb | 9.3–71.4 | 12.0–21.3 |
| Ta | 0.57–9.14 | 3–14 |
| Rb/Sr | 4.5–60 | 4.1–15.8 |
| Nb/Ta | 4.8–16.3 | 2.6–7.1 |
| Zr/Hf | 12.2–19.1 | 12.6–76.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daneshvar, N.; Azizi, H.; Asahara, Y.; Tsuboi, M.; Minami, M.; Mohammad, Y.O. Geochemistry and Genesis of Beryl Crystals in the LCT Pegmatite Type, Ebrahim-Attar Mountain, Western Iran. Minerals 2021, 11, 717. https://doi.org/10.3390/min11070717
Daneshvar N, Azizi H, Asahara Y, Tsuboi M, Minami M, Mohammad YO. Geochemistry and Genesis of Beryl Crystals in the LCT Pegmatite Type, Ebrahim-Attar Mountain, Western Iran. Minerals. 2021; 11(7):717. https://doi.org/10.3390/min11070717
Chicago/Turabian StyleDaneshvar, Narges, Hossein Azizi, Yoshihiro Asahara, Motohiro Tsuboi, Masayo Minami, and Yousif O. Mohammad. 2021. "Geochemistry and Genesis of Beryl Crystals in the LCT Pegmatite Type, Ebrahim-Attar Mountain, Western Iran" Minerals 11, no. 7: 717. https://doi.org/10.3390/min11070717
APA StyleDaneshvar, N., Azizi, H., Asahara, Y., Tsuboi, M., Minami, M., & Mohammad, Y. O. (2021). Geochemistry and Genesis of Beryl Crystals in the LCT Pegmatite Type, Ebrahim-Attar Mountain, Western Iran. Minerals, 11(7), 717. https://doi.org/10.3390/min11070717










