Ecological Risk Assessment of Cadmium in Karst Lake Sediments Based on Daphnia pulex Ecotoxicology
Abstract
1. Introduction
2. Materials and Methods
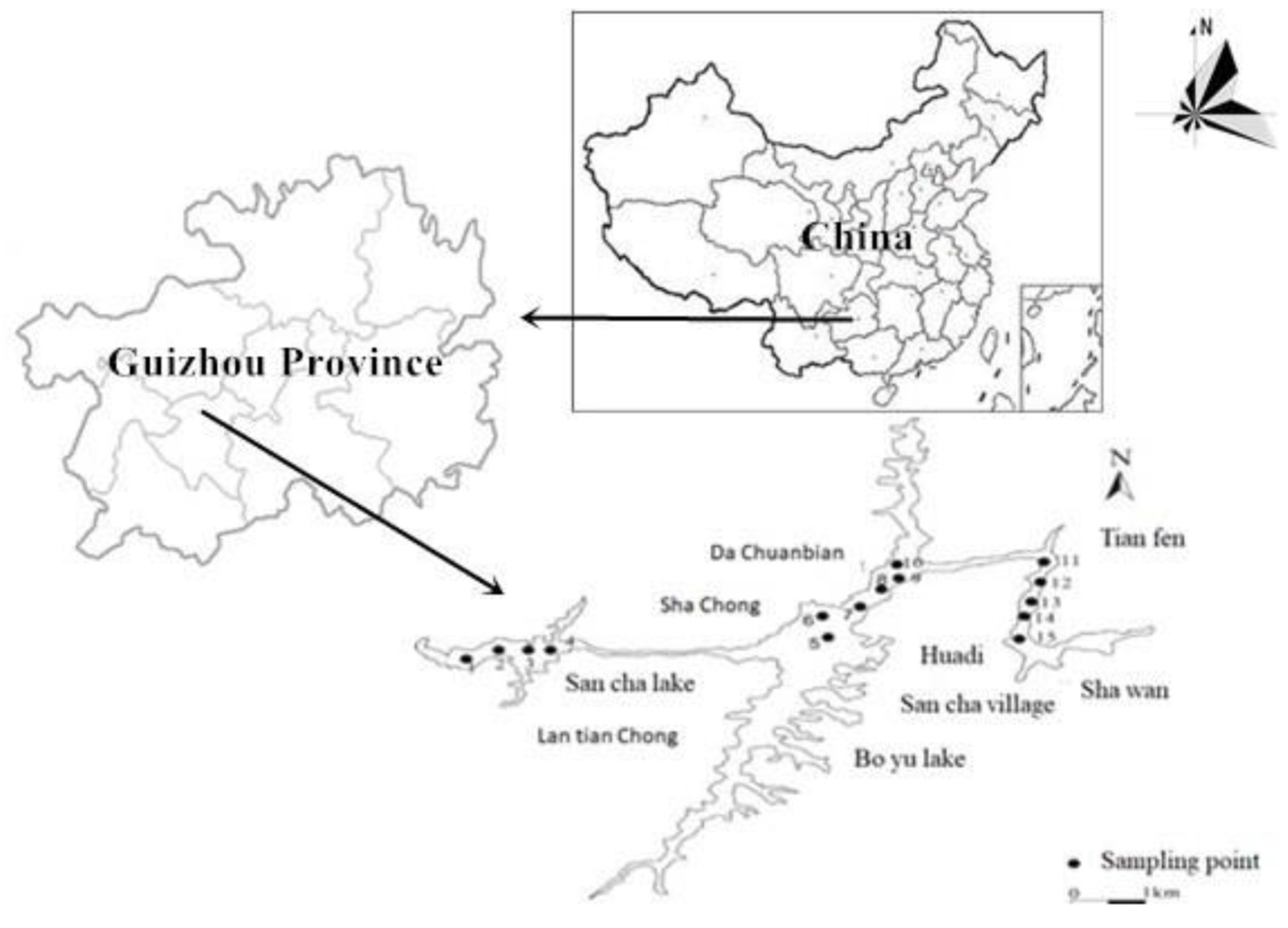
2.1. Study Area
2.2. Sample Collection
2.3. Sample Determination
2.4. Biotoxicity Experimental Design
2.4.1. Test Organism and Culture Conditions
2.4.2. Test Material and Water Dilution
2.4.3. Simulation of Sediment Samples with Different Concentration Gradients
2.4.4. Acute Toxicity Test of Cd to Daphnia pulex in Water Bodies
2.4.5. Acute Toxicity Test of Cd to Daphnia pulex in Sediments
2.4.6. Measurement of the Accumulation of Daphnia pulex Body
2.5. Risk Assessment Method for Heavy Metals in Sediments
2.5.1. Geo-Accumulation Index Method
2.5.2. Potential Ecological Risk Index Method
2.6. Data and Statistical Analysis
3. Results and Discussion
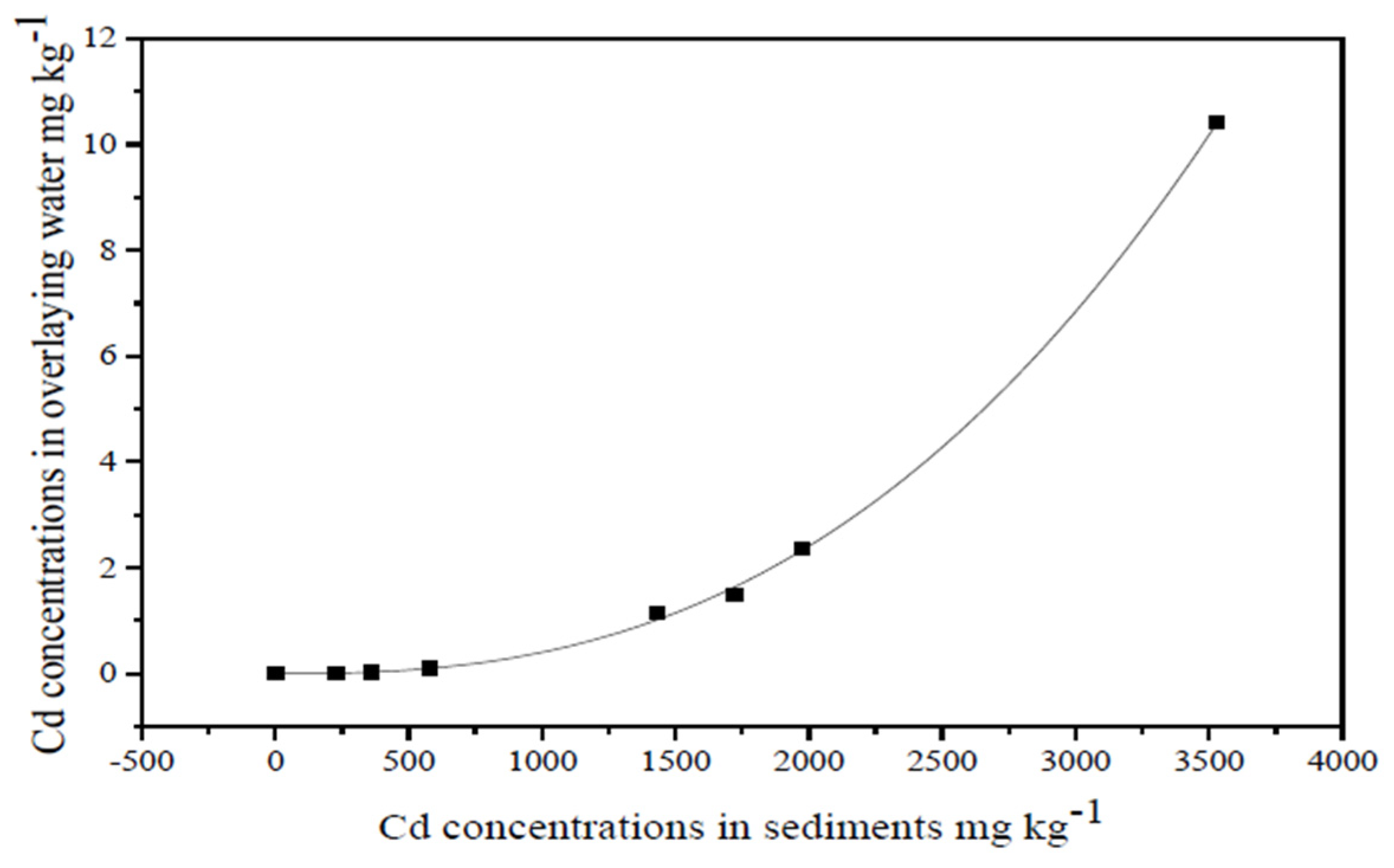
3.1. Distribution Coefficient and Fitted Model of Cd in Simulated Sediment and Overlying Water Systems
3.2. Ecological Risk Assessment Based on Daphnia pulex Bio Toxicity Test
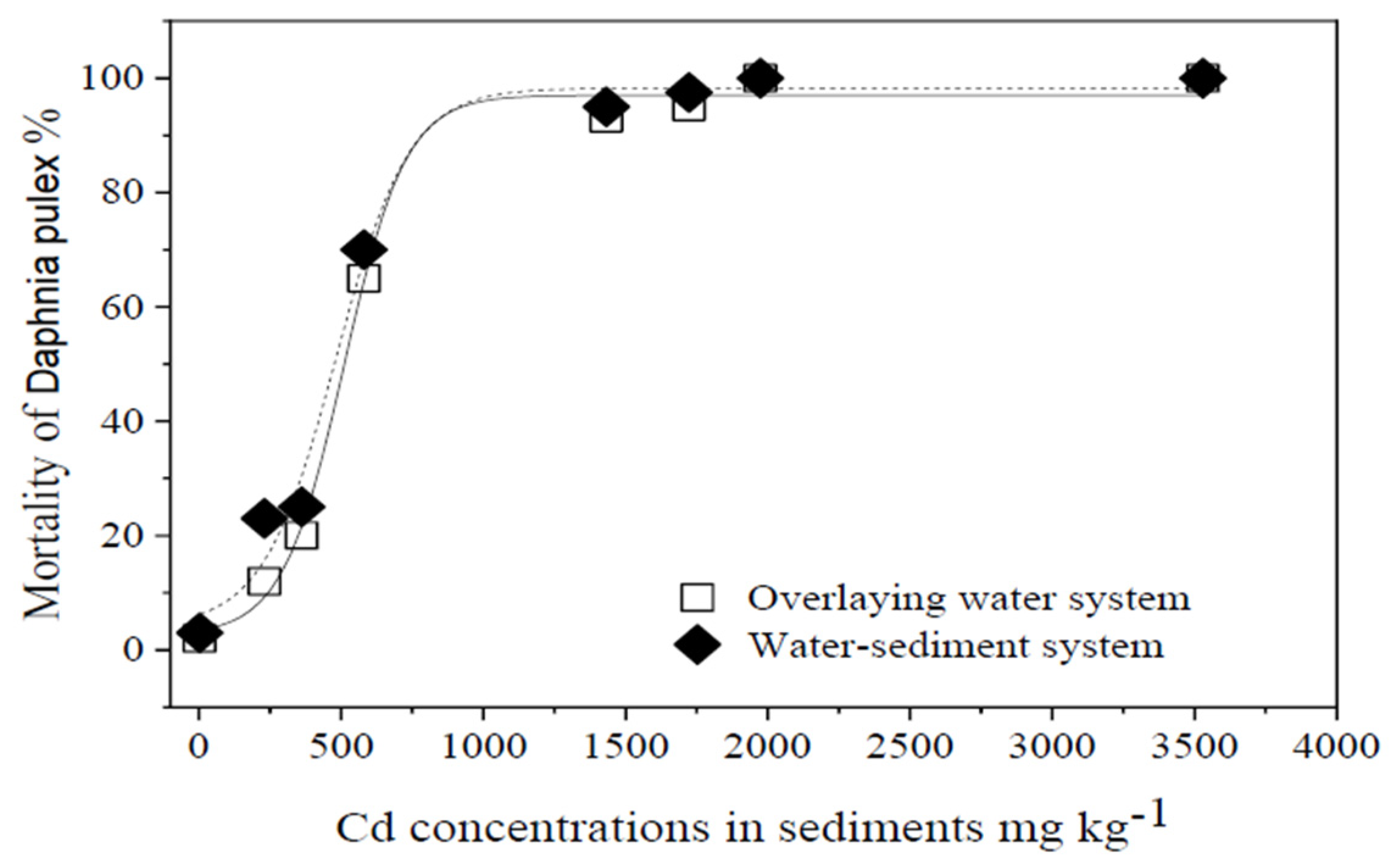
3.2.1. Lethal Effect of Cd2+ on Daphnia pulex
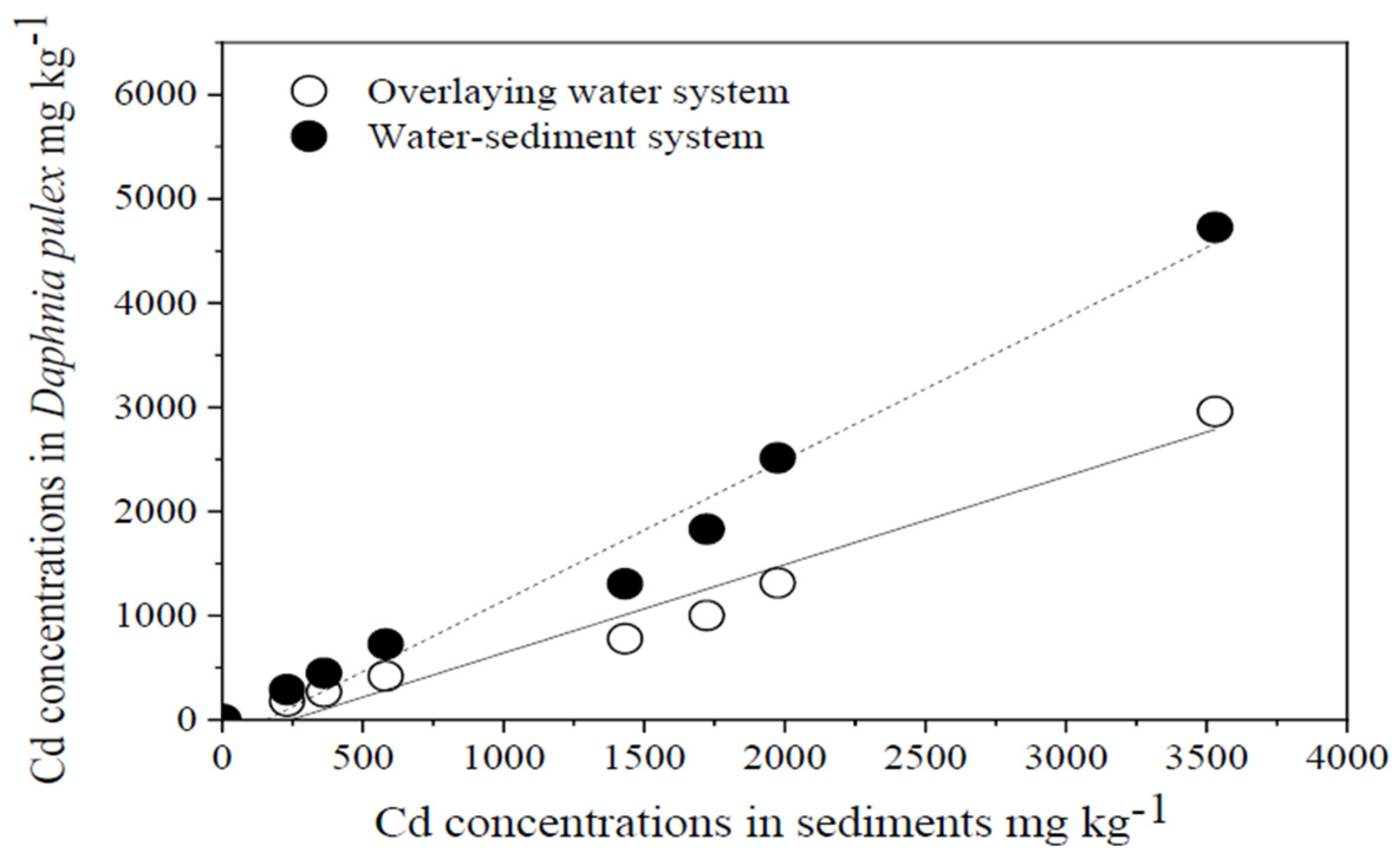
3.2.2. Mortality Rate and Cd Accumulation of Daphnia pulex after Cd Exposure in Both Systems
3.3. Cadmium Content in Sediments of Yelang Reservoir
3.4. Ecological Risk Assessment Based on Sedimentology
3.5. Analysis of the Application of Ecological Risk Assessment Methods for Cadmium in Karst Lake Reservoir Sediments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burton, G.A. Metal bioavailability and toxicity in sediments. Crit. Rev. Environ. Sci. Technol. 2010, 40, 852–907. [Google Scholar] [CrossRef]
- Hallare, A.V.; Seiler, T.B.; Hollert, H. The versatile, changing, and advancing roles of fish in sediment toxicity assessment—A review. J. Soils Sediments 2011, 11, 141–173. [Google Scholar] [CrossRef]
- Li, X.; Peng, W.; Jiang, Y.; Duan, Y.; Ren, J.; Liu, Y.; Fan, W. The Daphnia magna role to predict the cadmium toxicity of sediment: Bioaccumlation and biomarker response. Ecotoxicol. Environ. Saf. 2017, 138, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Saito, H.; Niikura, Y.; Shigeoka, T.; Nakano, Y. Embryonic development assay with Daphnia magna: Application to toxicity of aniline derivatives. Chemosphere 2001, 45, 487–495. [Google Scholar] [CrossRef]
- Hanazato, T. Growth analysis of Daphnia early juvenile stages as an alternative method to test the chronic effect of chemicals. Chemosphere 1998, 36, 1903–1909. [Google Scholar] [CrossRef]
- Emmanuel, E.; Keck, G.; Blanchard, J.M.; Vermande, P.; Perrodin, Y. Toxicological effects of disinfections using sodium hypochlorite on aquatic organisms and its contribution to AOX formation in hospital wastewater. Environ. Int. 2004, 30, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.; McGeer, J.C. Development of a biotic ligand model to predict the acute toxicity of cadmium to Daphnia pulex. Aquat. Toxicol. 2010, 98, 1–7. [Google Scholar] [CrossRef]
- Wong, C.K.; Wong, P.K. Life table evaluation of the effects of cadmium exposure on the freshwater cladoceran, Moina macrocopa. Bull. Environ. Contam. Toxicol. 1990, 44, 135–141. [Google Scholar] [CrossRef]
- Heugens, E.H.; Jager, T.; Creyghton, R.; Kraak, M.H.; Hendriks, A.J.; Van Straalen, N.M.; Admiraal, W. Temperature-dependent effects of cadmium on Daphnia magna: Accumulation versus sensitivity. Environ. Sci. Technol. 2003, 37, 2145–2151. [Google Scholar] [CrossRef]
- Heugens, E.H.; Tokkie, L.T.; Kraak, M.H.; Hendriks, A.J.; Van Straalen, N.M.; Admiraal, W. Population growth of Daphnia magna under multiple stress conditions: Joint effects of temperature, food, and cadmium. Environ. Toxicol. Chem. Int. J. 2006, 25, 1399–1407. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, M.; Liu, Q.; Wang, Z.; Zhao, L.; Chen, Y. Study of heavy metal pollution, ecological risk and source apportionment in the surface water and sediments of the Jiangsu coastal region, China: A case study of the Sheyang Estuary. Mar. Pollut. Bull. 2018, 137, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, L.; Liu, L.Z.; Shi, W.L.; Meng, X.Z. Comprehensive risk assessment of heavy metals in lake sediment from public parks in Shanghai. Ecotoxicol. Environ. Saf. 2014, 102, 129–135. [Google Scholar] [CrossRef]
- Singh, H.; Yadav, S.; Singh, B.K.; Dubey, B.; Tripathi, K.; Srivastava, V.; Shukla, D.N. Assessment of geochemical environment from study of river sediments in the middle stretch of river Ganga at Ghazipur, Buxar and Ballia Area. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2013, 83, 371–384. [Google Scholar] [CrossRef]
- Caumette, G.; Koch, I.; Moriarty, M.; Reimer, K.J. Arsenic distribution and speciation in daphnia pulex. Sci. Total Environ. 2012, 432, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Müller, G. Schwermetalle in den sedimenten des Rheins-Veränderungen seit 1971. Umschan 1979, 79, 778–783. [Google Scholar]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Shuhaimi-Othman, M.; Yakub, N.; Ramle, N.A.; Abas, A. Comparative toxicity of eight metals on freshwater fish. Toxicol. Ind. Health 2015, 31, 773–782. [Google Scholar] [CrossRef]
- Moore, D.W.; Farrar, D.; Altman, S.; Bridges, T.S. Comparison of acute and chronic toxicity laboratory bioassay endpoints with benthic community responses in field-exposed contaminated sediments. Environ. Toxicol. Chem. 2019, 38, 1784–1802. [Google Scholar] [CrossRef]
- Peng, Z.Z.; Dan, X.; Yan, H.L.; Rong, H.T.; Ping, L.Y. Adsorption and desorption characteristics of cadmium in yellow soil and limestone soil. Guizhou Agric. Sci. 2015, 436, 83–86. (In Chinese) [Google Scholar]
- Luo, K.; Liu, H.; Yu, E.; Tu, Y.; Gu, X.; Xu, M. Distribution and release mechanism of heavy metals in sediments of Yelang Lake by DGT. Stoch. Environ. Res. Risk Assess. 2020, 34, 793–805. [Google Scholar] [CrossRef]
- Ling, H.S.; Lin, L.C.; Zhong, L.Y.; Guang, H.D.; Sheng, Y.Y.; Wei, Y.S. Geochemistry and environment of the cadmium in the soil and sediments at the surface of Guizhou province. Guizhou Geol. 2004, 4, 245–250. (In Chinese) [Google Scholar]
- Ying, L.Z.; Zhong, C.Z.; Sheng, Y.G.; Ya, C.Z. Concentrations of 39 elements in stream sediment in different landscape zones of china. Earth Sci. Front. 2015, 225, 226–230. (In Chinese) [Google Scholar]
- Fang, Y. Study on the Mass Balance of Mercury in Reservoirs at Different Evolution Stages in the Wujiang River Basin. Ph.D. Thesis, Southwest University, Chongqing, China, 2009. (In Chinese). [Google Scholar]
- Wu, Y.G.; Xiong, Y.; Lin, C.X.; Yuan, L. The joint-biotoxicity effect of the different forms of phosphorus on heavy metal (Cu, Zn, Cd). Acta Sci. Circumstantiae 2006, 12, 2045–2051. (In Chinese) [Google Scholar]
- Wu, Y.G.; Huang, J.G.; Yuan, L. Applying phototactic behaviour of Daphnia to monitor water quality. China Environ. Sci. 2004, 243, 336–339. (In Chinese) [Google Scholar]
- Chinese Environmental Protection Administration (CEPA). Water Quality-Determination of the Acute Toxicity of Substance to Daphnia (Daphnia magna straus) (GB/T 13266-1991); China Standards Press: Beijing, China, 1991. (In Chinese) [Google Scholar]
- Cairns, M.A.; Nebeker, A.V.; Gakstatter, J.H.; Griffis, W.L. Toxicity of copper-spiked sediments to freshwater invertebrates. Environ. Toxicol. Chem. 1984, 3, 435–445. [Google Scholar] [CrossRef]
- ISO. Water Quality-Determination of the Inhibition of the Mortality of Daphnia magna Straus (Cladocera: Crustacea); Technical Report 6241; International Standards Organisation: Geneva, Switzerland, 1982. [Google Scholar]
- Zhou, Y.X.; Zhang, Z.S. Test Method for Toxicity of Aquatic Organisms; Agricultural Press: Beijing, China, 1989; pp. 24–145. (In Chinese) [Google Scholar]
- Daam, M.A.; Moutinho, M.F.; Espíndola, E.L.G.; Schiesari, L. Lethal toxicity of the herbicides acetochlor, ametryn, glyphosate and metribuzin to tropical frog larvae. Ecotoxicology 2019, 28, 707–715. [Google Scholar] [CrossRef]
- Huan, L.Y.; Hong, L. Acute toxicity study of cadmium on scallops in the bay. Mar. Fish. Res. 2006, 6, 80–83. (In Chinese) [Google Scholar]
- Wei, X.U.; Mu, F.H.; Sun, Y.T.; Cao, Z.Q. Effect of simulated ocean acidification on the acute toxicity of Cu and Cd to Tigriopus japonicus. Acta Ecol. Sin. 2014, 3414, 3879–3884. (In Chinese) [Google Scholar]
- Qi, X.Z.; Jun, N.S.; Guo, T.X.; Jiang, Z.C. Calculation of toxicity coefficients of heavy metals in potential ecological hazard index method. Environ. Sci. Technol. 2008, 2, 112–115. (In Chinese) [Google Scholar]
- Qiang, M.Z.; Jiang, F.; Mi, W. Environmental Toxicology; China Environmental Science Press: Beijing, China, 2000; pp. 120–275. (In Chinese) [Google Scholar]
- Liang, Y. Study on Biological Toxicity of Toxic Pollutants in Acid Wastewater of Coal Mines in Karst Areas. Ph.D. Thesis, Guizhou University, Guizhou, China, 2009. (In Chinese). [Google Scholar]
- Yan, H.-J.; Zhang, H.-Y.; Shi, Y.-J.; Zhou, P.; Li, H.; Wu, D.-L.; Liu, L. Simulation on release of heavy metals Cd and Pb in sediments. Trans. Nonferrous Met. Soc. China 2021, 31, 277–287. [Google Scholar] [CrossRef]
- National Standards of People’s Republic of China. GB/T 21851-2008 (Chemicals-Adsorption-Desorption Using a Batch Equilibrium Method), GB/T 21805-2008 (Chemicals-Alga Growth Inhibition Test), GB/T 21830-2008 (Chemicals-Daphnia sp., Acute Immobilisation Test); Standardization Administration of the People’s Republic of China: Beijing, China, 2008. [Google Scholar]
- MEP. Environmental Quality Standards for Surface Water; GB 3838; Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2002. (In Chinese) [Google Scholar]
- Zidour, M.; Boubechiche, Z.; Pan, Y.J.; Bialais, C.; Cudennec, B.; Grard, T.; Drider, D.; Flahaut, C.; Ouddane, B.; Souissi, S. Population response of the estuarine copepod eurytemora affinis to your bioaccumulation of trace metals. Chemosphere 2019, 220, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.X.; Hui, P.Y.; Chao, L.C.; Hui, Q.Z. Acute toxicity study of copper and cadmium against Aspergillus. Fish. Sci. 2015, 3402, 95–99. (In Chinese) [Google Scholar]
- Min, Z.Y.; Lei, Z.; Wen, Q.Y.; Hui, Z.B. Bio-toxicity of cadmium-spiked sediments to misgurnus anguillicaudatus. Asian J. Econ. 2011, 601, 80–86. (In Chinese) [Google Scholar]
- Ling, P.; Ning, Z.J.; Zhen, C.Q.; Wei, H.; Ping, D. Effects of cadmium on antioxidant enzyme activity in gill of Mytilus coruscus and acute toxicity of cadmium on Mytilus coruscus. Environ. Sci. Technol. 2015, 38, 13–24. (In Chinese) [Google Scholar]
- Xue, P.; Liang, H.X.; Han, C.S.; Jing, C.S.; Ping, Y.X. Single and joint acute toxicity of Cd+2 and Hg+2 to chinese mitten handed crab eriochersinensis. Fish. Sci. 2015, 34, 220–226. (In Chinese) [Google Scholar]
- Zeng, Y.Y.; Lai, Z.N.; Yang, W.L.; Gao, Y.; Li, Y.F.; Pang, S.X. Toxicities and potential ecological effects of copper and cadmium to natural fish larvae and juveniles from the pearl river. Asian J. Econ. 2014, 91, 49–55. (In Chinese) [Google Scholar]
- Yue, L.Y.; Lin, Z.Z.; Long, D.B.; Yang, F.J.; Mei, L.D. Acute toxicity of cadmium to allogynogenetic crucian carp. J. Fish. 2018, 3106, 36–39. (In Chinese) [Google Scholar]
- Yang, L.; Xi, C.; Hui, Q.J. Acute toxic effect of cadmium on mosquito-eating fish (Gambusia affinis). J. Saf. Environ. 2015, 1503, 362–366. (In Chinese) [Google Scholar]
- Long, W.R.; Zhi, M.G.; Qiang, Z.F. Safety Assessment and Acute toxicity of copper, cadmium and zinc to white clound mountain minnow tanichthys albonubes. Fish. Sci. 2006, 3, 117–120. (In Chinese) [Google Scholar]
- Shou, S.; Lin, S.X.; Yan, W.G. Ecological toxicity of cadmium chloride on grass carp (Ctenopharyngodon idellus). J. Shenyang Ligong Univ. 2015, 34, 65–68, 72. (In Chinese) [Google Scholar]
- Shuhaimi-Othman, M.; Yakub, N.; Umirah, N.S.; Abas, A. Toxicity of eight metals to malaysian freshwater midge larvae chironomus javanus (diptera, chironomidae). Toxicol. Ind. Health 2011, 27, 879–886. [Google Scholar] [CrossRef]
- Martinez-Haro, M.; Moreira-Santos, M.; Marques, J.C.; Ribeiro, R. A short-term laboratory and in situ sediment assay based on the postexposure feeding of the estuarine isopod cyathura carinata. Environ. Res. 2014, 134, 242–250. [Google Scholar] [CrossRef]
- Bielmyer-Fraser, G.K.; Harper, B.; Picariello, C.; Albritton-Ford, A. The influence of salinity and water chemistry on acute toxicity of cadmium to two euryhaline fish species. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2018, 214, 23–27. [Google Scholar] [CrossRef]
- Shuhaimi-Othman, M.; Nadzifah, Y.; Nur-Amalina, R.; Umirah, N.S. Deriving freshwater quality criteria for copper, cadmium, aluminum and manganese for protection of aquatic life in Malaysia. Chemosphere 2013, 90, 2631–2636. [Google Scholar] [CrossRef]
- Wigginton, A.J.; Birge, W.J. Toxicity of cadmium to six species in two genera of crayfish and the effect of cadmium on molting success. Environ. Toxicol. Chem. 2007, 26, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.R.; Colbourne, J.K.; Glaholt, S.P.; Turner, E.; Folt, C.L.; Chen, C.Y. Dynamics of cadmium acclimation in daphnia pulex: Linking fitness costs, cross-tolerance, an hyper-inaction of metallothionein. Environ. Sci. Technol. 2019, 53, 14670–14678. [Google Scholar] [CrossRef]
- Shaw, J.R.; Colbourne, J.K.; Davey, J.C.; Glaholt, S.P.; Hampton, T.H.; Chen, C.Y.; Folt, C.L.; Hamilton, J.W. Gene response profiles for daphnia pulex exposed to the environmental stressor cadmium reveals novel crustacean metallothioneins. BMC Genom. 2007, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yuan, L.; Tang, Y. Monitoring sodium pentachlorophenate and cadmium in aquatic solutions by phototactic behavior of Daphnia carinata clone. Acta Sci. Circumstantiae 2006, 266, 1011–1015. (In Chinese) [Google Scholar]
- Niyogi, S.; Wood, C.M. Biotic ligand model, a flexible tool for developing site-specific water quality guidelines for metals. Environ. Sci. Technol. 2004, 38, 6177–6192. [Google Scholar] [CrossRef]
- Dallinger, R.; Höckner, M. Evolutionary concepts in ecotoxicology: Tracing the genetic background of differential cadmium sensitivities in invertebrate lineages. Ecotoxicology 2013, 22, 767–778. [Google Scholar] [CrossRef]
- Tan, Q.G.; Wang, W.X. Acute toxicity of cadmium in daphnia magna under different calcium and PH conditions: Importance of influx rate. Environ. Sci. Technol. 2011, 45, 1970–1976. [Google Scholar] [CrossRef]
- Chen, S.; Nichols, K.M.; Poynton, H.C.; Sepúlveda, M.S. MicroRNAs are involved in cadmium tolerance in daphnia pulex. Aquat. Toxicol. 2016, 175, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Colbourne, J.K.; Pfrender, M.E.; Gilbert, D.; Thomas, W.K.; Tucker, A.; Oakley, T.H.; Tokishita, S.; Aerts, A.; Arnold, G.J.; Basu, M.K.; et al. The ecoresponsive genome of daphnia pulex. Science 2011, 331, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Wen-Hong, F.; Tang, G.; Zhao, C.M.; Duan, Y.; Zhang, R. Metal accumulation and biomarker responses in daphnia magna following cadmium and zinc exposure. Environ. Toxicol. Chem. 2009, 28, 305–310. [Google Scholar]
- CMEE (China Ministry of Ecology and Environment). Chinese Soil Environmental Quality: Risk Control Standard for Soil Contamination of Agricultural Land (GB 15618-2018); China Environmental Science Press: Beijing, China, 2018. (In Chinese) [Google Scholar]
- Vukosav, P.; Mlakar, M.; Cukrov, N.; Kwokal, Ž.; Pižeta, I.; Pavlus, N.; Špoljarić, I.; Vurnek, M.; Brozinčević, A.; Omanović, D. Heavy metal contents in water, sediment and fish in a karst aquatic ecosystem of the plitvice lakes national park (Croatia). Environ. Sci. Pollut. Res. 2014, 21, 3826–3839. [Google Scholar] [CrossRef]
- Liu, P.; Zheng, C.; Wen, M.; Luo, X.; Wu, Z.; Liu, Y.; Chai, S.; Huang, L. Ecological risk assessment and contamination history of heavy metals in the sediments of Chagan Lake, Northeast China. Water 2021, 13, 894. [Google Scholar] [CrossRef]
- Shi, J.B.; Wu, Y.G.; Yan, L.; Liu, F.; Yu, Y.H. The acute biological toxicity of Pb and Zn caused by zinc oxide industrial waste in karst areas in Guizhou. Guizhou Agric. Sci. 2009, 37, 89–92. (In Chinese) [Google Scholar]
- Xiong, X.Q.; Luo, S.; Wu, B.L.; Wu, B.L.; Wang, J.W. Acute toxicity of cadmium and copper to gobiocypris rarus under different water hardness. Asian J. Econ. 2016, 11, 316–322. (In Chinese) [Google Scholar]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019. [Google Scholar] [CrossRef]
- Lim, K.Y.; Zakaria, N.A.; Foo, K.Y. Geochemistry pollution status and ecotoxicological risk assessment of heavy metals in the Pahang river sediment after the high magnitude of flood event. Hydrol. Res. 2021, 52, 107–124. [Google Scholar] [CrossRef]
- Tian, K.; Wu, Q.; Liu, P.; Hu, W.; Huang, B.; Shi, B.; Zhou, Y.; Kwon, B.O.; Choi, K.; Ryu, J.; et al. Ecological risk assessment of heavy metals in sediments and water from the coastal areas of the Bohai Sea and the Yellow Sea. Environ. Int. 2020, 136, 105512. [Google Scholar] [CrossRef] [PubMed]

| Total Reservoir Capacity/km3 | Normal Reservoir Capacity/km3 | Normal Catchment Area/km2 | Flow/km3 | Distance/km | Age/a | Evolution Stage |
|---|---|---|---|---|---|---|
| 4.2 | 2.48 | 19.25 | 33.8 | 238.4 | 24 | Middle level |
| pH | EC/μs cm−1 | T/℃ | DO/mg L−1 | Ca2+/mg L−1 | Mg2+/mg L−1 | HCO3−/mg L−1 |
|---|---|---|---|---|---|---|
| 7.92 | 535 | 24.6 | 8.35 | 56.44 | 15.15 | 151.87 |
| Geoaccumulation Index (Igeo) | Rank | Pollution Level |
|---|---|---|
| Igeo ≤ 0 | 0 | Unpolluted |
| 0 < Igeo ≤ 1 | 1 | Unpolluted to moderately polluted |
| 1 < Igeo ≤ 2 | 2 | Moderately polluted |
| 2 < Igeo ≤ 3 | 3 | Moderately to strongly polluted |
| 3 < Igeo ≤ 4 | 4 | Strongly polluted |
| 4 < Igeo ≤ 5 | 5 | Strongly to extremely polluted |
| 5 < Igeo ≤ 10 | 6 | Extremely polluted |
| Rank | Individual Potential Ecological Risk Index () | Individual Potential Ecological Risk Level |
|---|---|---|
| 1 | <40 | Slight |
| 2 | 40–80 | Medium |
| 3 | 80–160 | High |
| 4 | 160–320 | Very High |
| 5 | ≥320 | Extremely High |
| Exposure time/h | LC50/mg L−1 | 95% Confidence Ìnterval | Toxicology Regression Equation | Coefficient of Correlation R2 | Chi-Square |
|---|---|---|---|---|---|
| 24 | 1.17 | 0.85~1.60 | y = 4.89 + 1.66x | 0.98 | 0.01 |
| 48 | 0.50 | 0.23~1.03 | y = 6.38 + 4.47x | 0.92 | 0.68 |
| 72 | 0.24 | 0.15~0.38 | y = 7.16 + 3.46x | 0.97 | 0.23 |
| 96 | 0.12 | 0.04~0.29 | y = 7.70 + 2.80x | 0.93 | 0.3 |
| Species | 96 h-LC50/mg L−1 | Author(s) | Safe Concentration/mg L−1 |
|---|---|---|---|
| Sipunculus nudus | 24.328 | [40] | 0.24 |
| Misgurnus anguillicaudatus | 1753.8 | [41] | 17.54 |
| Argopecten irradiams | 3.45 | [31] | 0.03 |
| Mytilus coruscus | 3.1 | [42] | 0.03 |
| Eriocheir sinensis | 40.279 | [43] | 0.40 |
| Megalobrama terminalis | 3.2 | [44] | 0.03 |
| Carassius auratus gibelio | 26.51 | [45] | 0.27 |
| Gambusia affinis | 22.55 | [46] | 0.23 |
| Tanichthys albonubes | 4.447 | [47] | 0.04 |
| Ctenopharyngodon idella | 23.51 | [48] | 0.24 |
| Tigriopus japonicus | 6.31 | [32] | 0.06 |
| Chironomus javanus | 0.06 | [49] | 6 × 10−4 |
| Cyathura carinata | 37 | [50] | 0.37 |
| Rasbora sumatrana | 0.1 | [17] | 0.001 |
| Poecilia reticulata | 1.06 | 0.0106 | |
| Kryptolebias marmoratus | 6.43 × 10−3 | [51] | 6.43 × 10−5 |
| Fundulus heteroclitus | 2.94 × 10−3 | 2.94 × 10−5 | |
| Macrobrachium lanchesteri | 0.007 | [52] | 7 × 10−5 |
| Stenocypris major | 0.013 | 1.3 × 10−4 | |
| Nais elinguis | 0.027 | 2.7 × 10−4 | |
| Chironomus javanus | 0.06 | 6 × 10−4 | |
| Rasbora sumatrana | 0.1 | 1 × 10−3 | |
| Poecilia reticulata | 0.17 | 1.7 × 10−3 | |
| Duttaphrynus melanostictus | 0.32 | 3.2 × 10−3 | |
| Melanoides tuberculata | 1.49 | 1.49 × 10−2 | |
| Eurytemora affinis | Male 127.8 | [39] | 1.28 |
| Female 90.0 | 0.09 | ||
| Orconectes juvenilis | 0.06 | [53] | 6 × 10−4 |
| Orconectes placidus | 0.037 | 3.7 × 10−4 | |
| Orconectes virilis | 3.3 | 0.03 | |
| Procambarus acutus | 0.368 | 3.68 × 10−3 | |
| Procambarus alleni | 3.07 | 0.03 | |
| Procambarus clarkii | 0.624 | 6.24 × 10−3 |
| Sampling Site | Concentration of Cd/mg kg−1 | Igeo | Classification of Geoaccumulation Index | Potential Ecological Risk Level | |
|---|---|---|---|---|---|
| 1 | 4.13 | 3.15 | 4 | 399.3 | Extremely strong |
| 2 | 3.9 | 3.068 | 4 | 377.4 | Extremely strong |
| 3 | 4.19 | 3.17 | 4 | 405.1 | Extremely strong |
| 4 | 4.18 | 3.168 | 4 | 404.5 | Extremely strong |
| 5 | 4.25 | 3.191 | 4 | 410.9 | Extremely strong |
| 6 | 4.45 | 3.259 | 4 | 430.6 | Extremely strong |
| 7 | 3.69 | 2.989 | 3 | 357.2 | Very strong |
| 8 | 4.43 | 3.253 | 4 | 429.1 | Extremely strong |
| 9 | 2.74 | 2.557 | 3 | 264.8 | Very strong |
| 10 | 4.22 | 3.18 | 4 | 407.9 | Extremely strong |
| 11 | 2.51 | 2.432 | 3 | 242.8 | Very strong |
| 12 | 5.23 | 3.491 | 4 | 505.9 | Extremely strong |
| 13 | 2.8 | 2.592 | 3 | 271.3 | Very strong |
| 14 | 5.04 | 3.439 | 4 | 488.1 | Extremely strong |
| 15 | 3.43 | 2.884 | 3 | 332.1 | Extremely strong |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinis, F.; Liu, H.; Liu, Q.; Wang, X.; Xu, M. Ecological Risk Assessment of Cadmium in Karst Lake Sediments Based on Daphnia pulex Ecotoxicology. Minerals 2021, 11, 650. https://doi.org/10.3390/min11060650
Dinis F, Liu H, Liu Q, Wang X, Xu M. Ecological Risk Assessment of Cadmium in Karst Lake Sediments Based on Daphnia pulex Ecotoxicology. Minerals. 2021; 11(6):650. https://doi.org/10.3390/min11060650
Chicago/Turabian StyleDinis, Faustino, Hongyan Liu, Qingdong Liu, Xuewen Wang, and Meng Xu. 2021. "Ecological Risk Assessment of Cadmium in Karst Lake Sediments Based on Daphnia pulex Ecotoxicology" Minerals 11, no. 6: 650. https://doi.org/10.3390/min11060650
APA StyleDinis, F., Liu, H., Liu, Q., Wang, X., & Xu, M. (2021). Ecological Risk Assessment of Cadmium in Karst Lake Sediments Based on Daphnia pulex Ecotoxicology. Minerals, 11(6), 650. https://doi.org/10.3390/min11060650







