Innovative Magnetic Aggregates for the Removal of Transition Metals from Industrial Wastewater
Abstract
1. Introduction
2. Materials and Methods
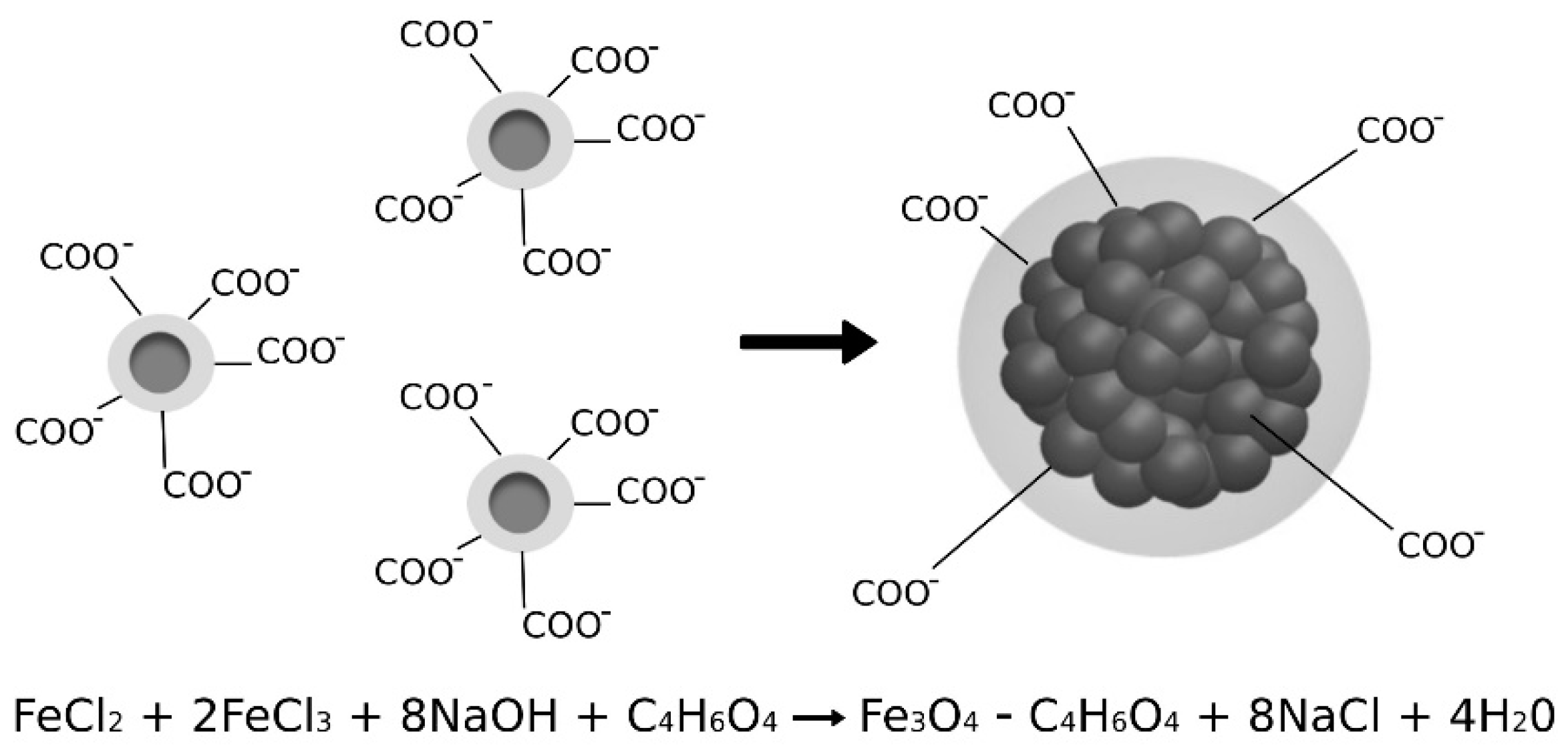
2.1. Sample Preparation
2.2. Sample Characterization
2.3. Adsorption Test
2.4. Electrochemical Characterization
3. Results and Discussion
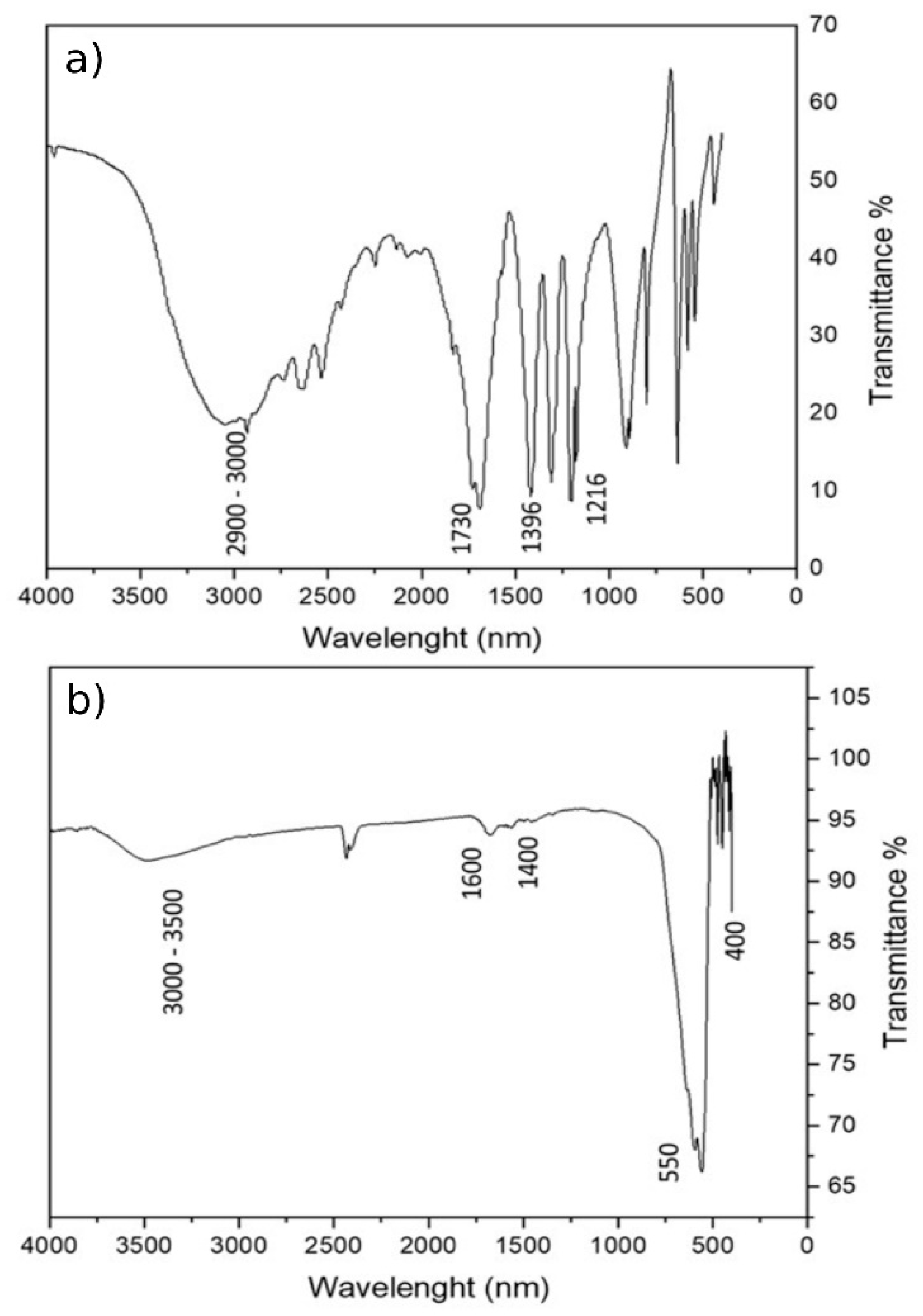
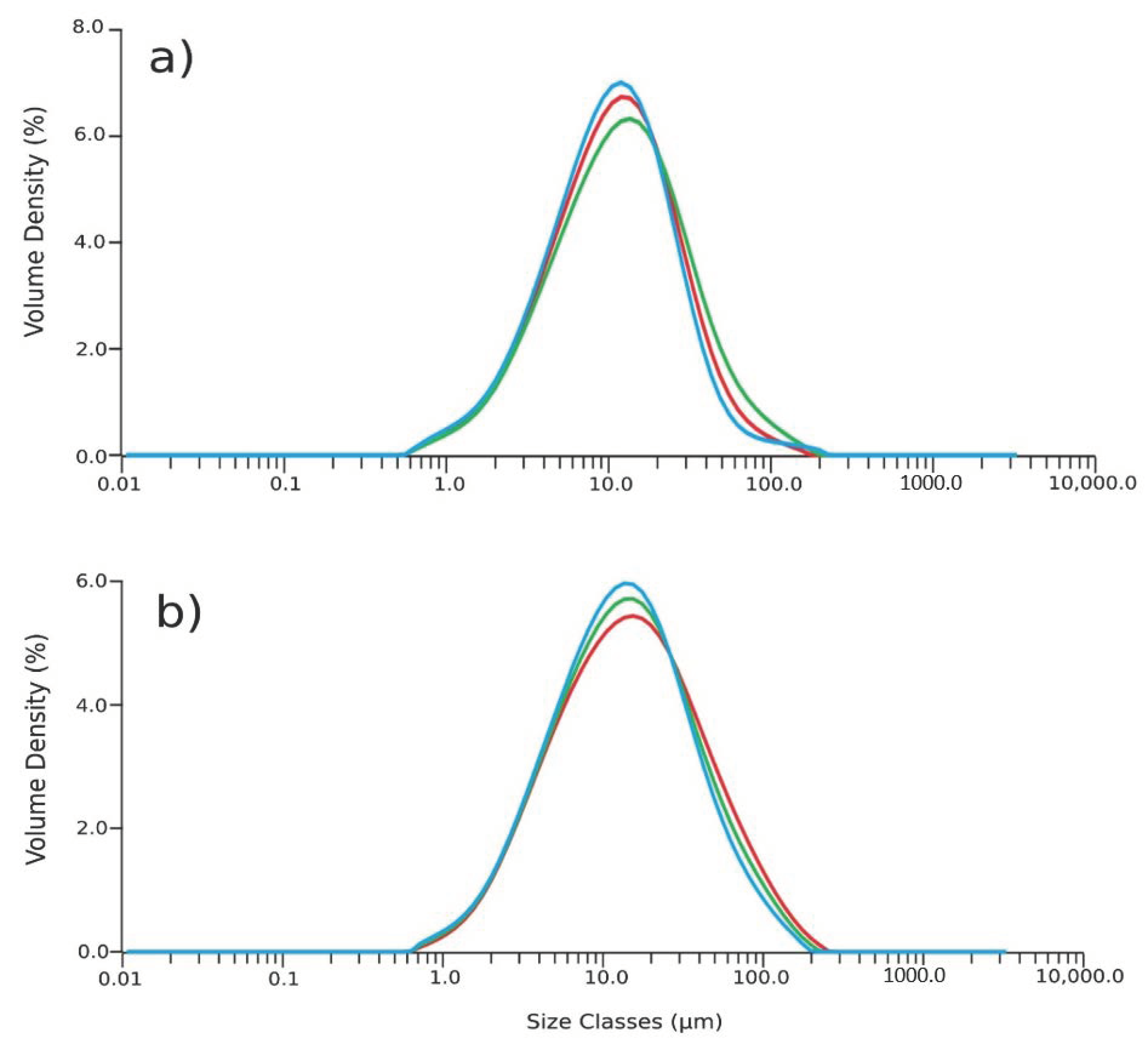
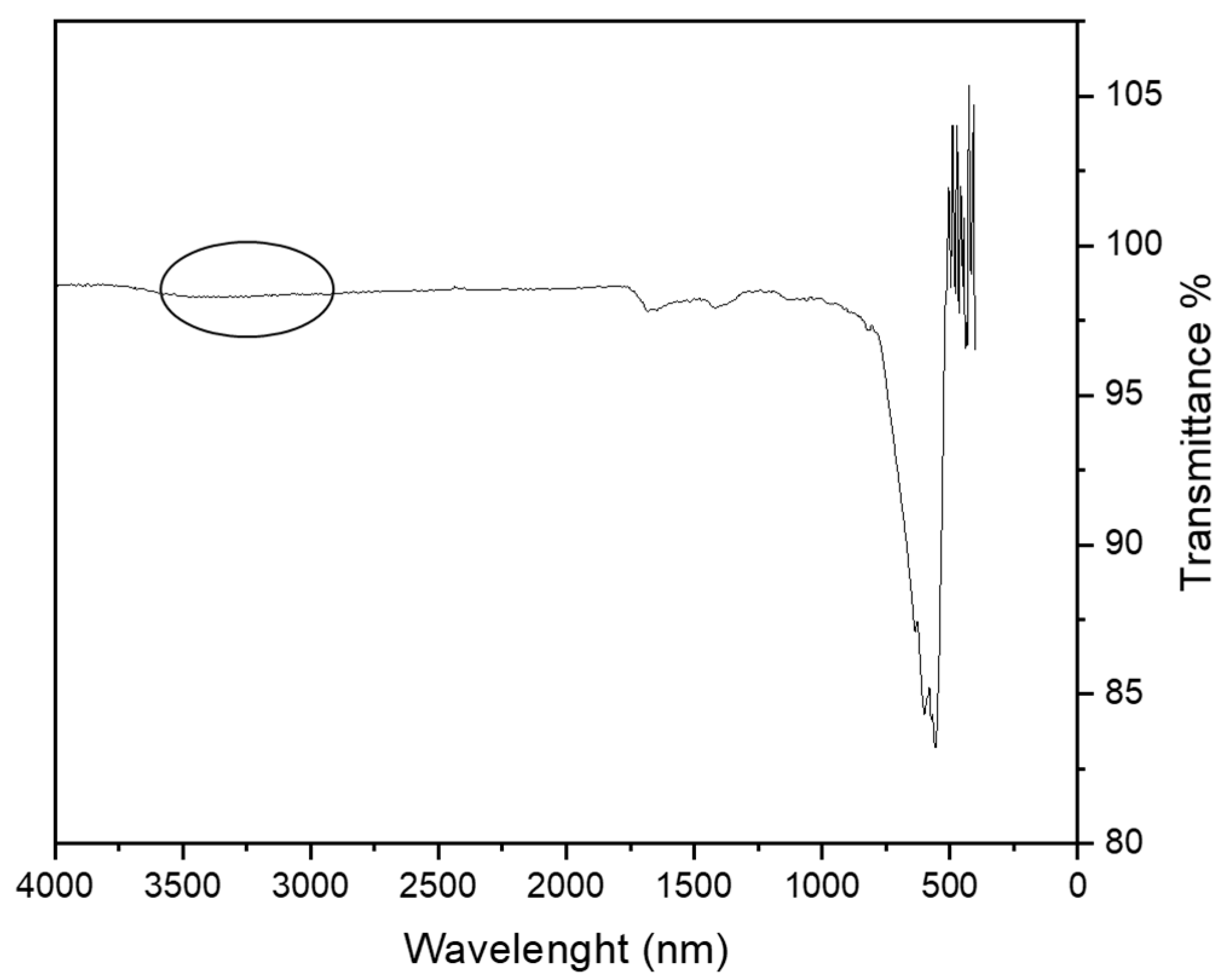
3.1. MA Characterization
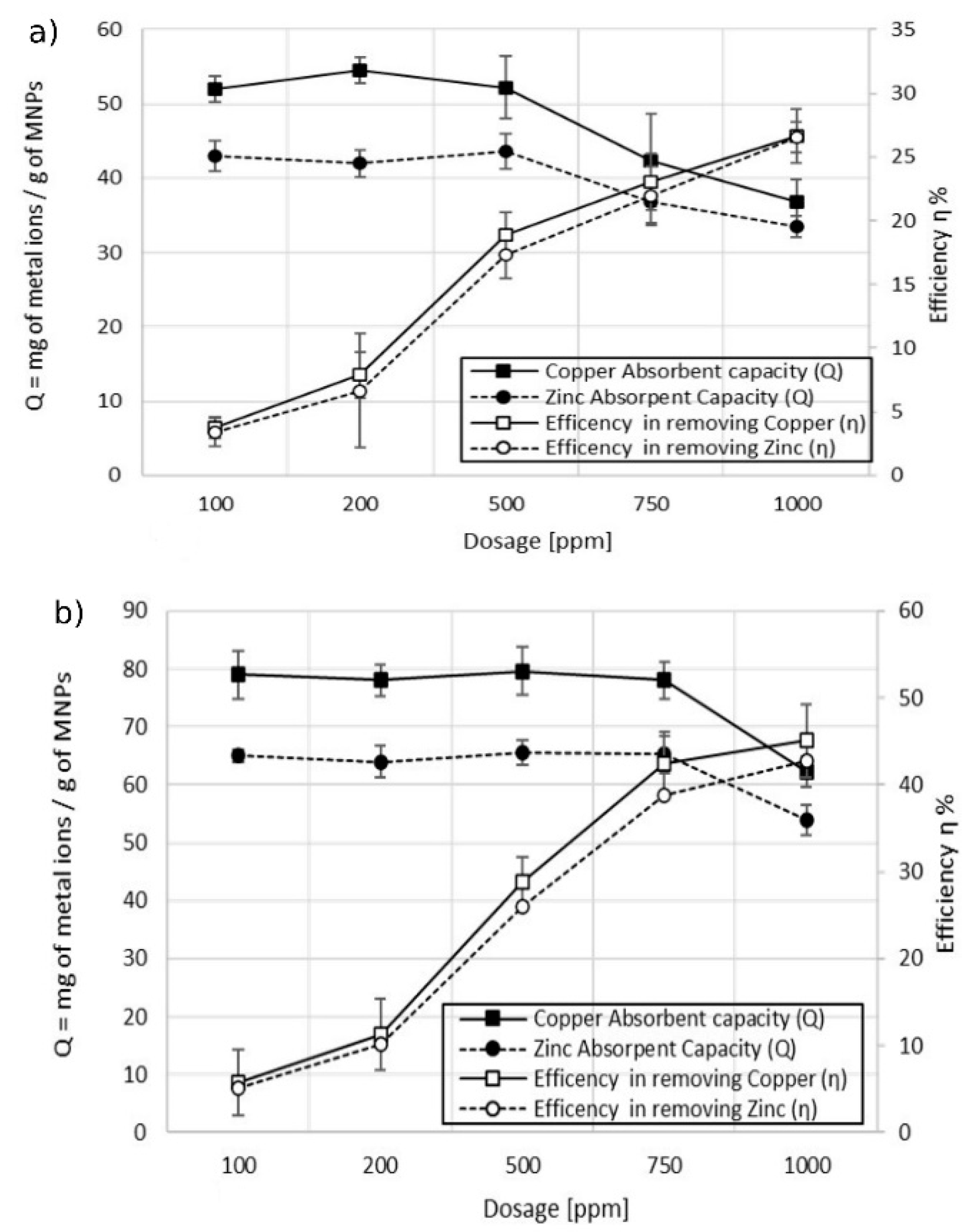
3.2. Adsorption Tests
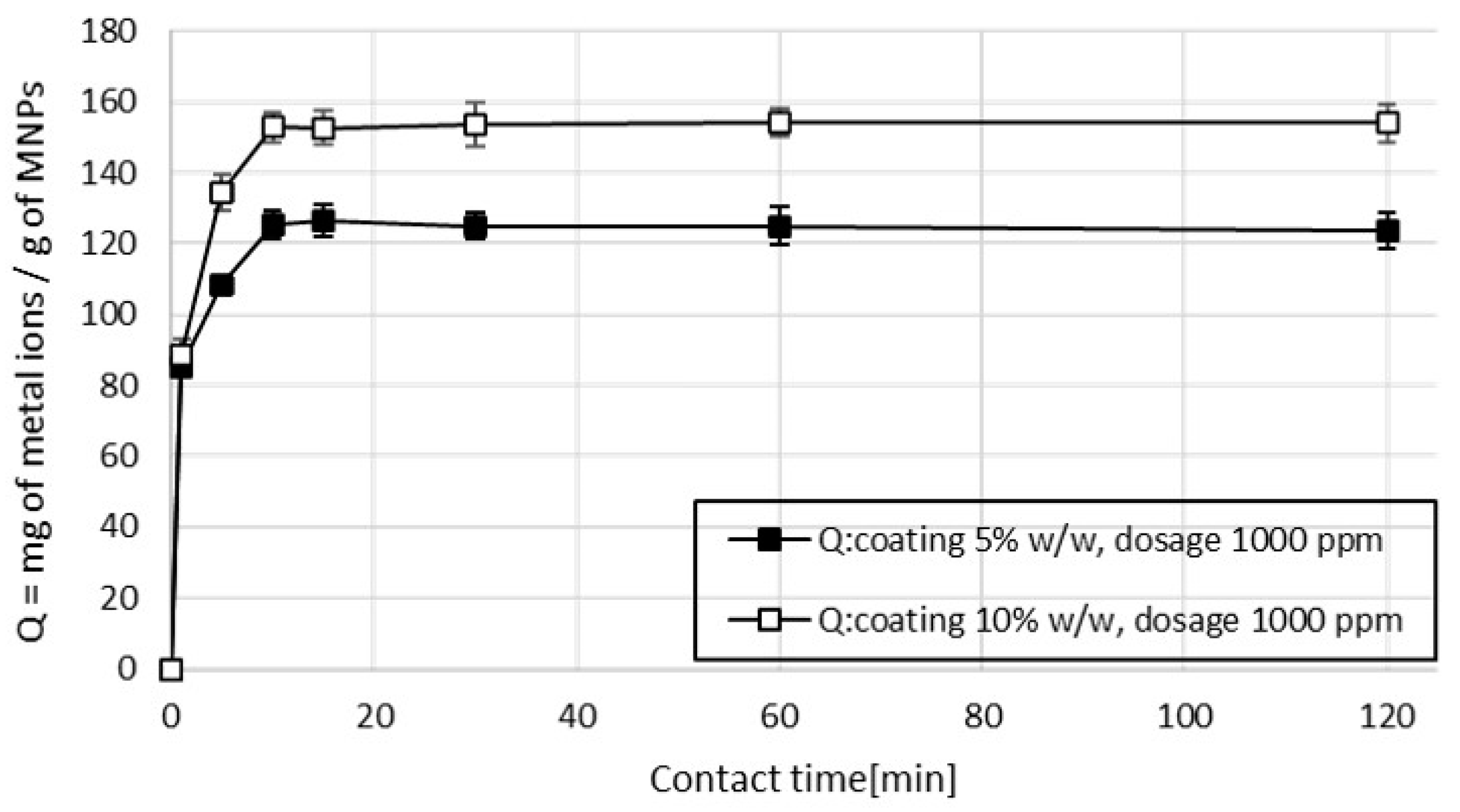
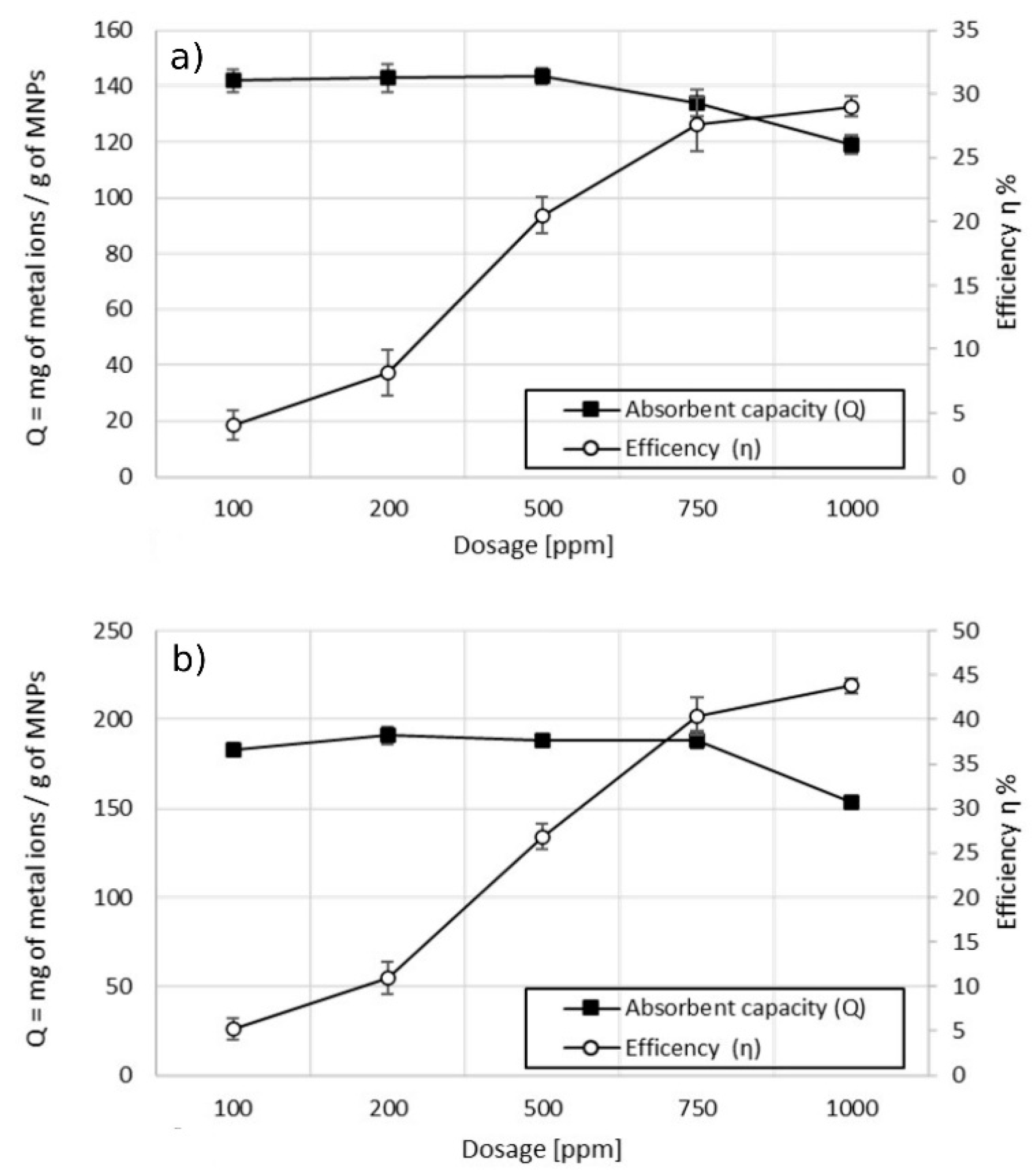
3.2.1. Single-Ion Adsorption
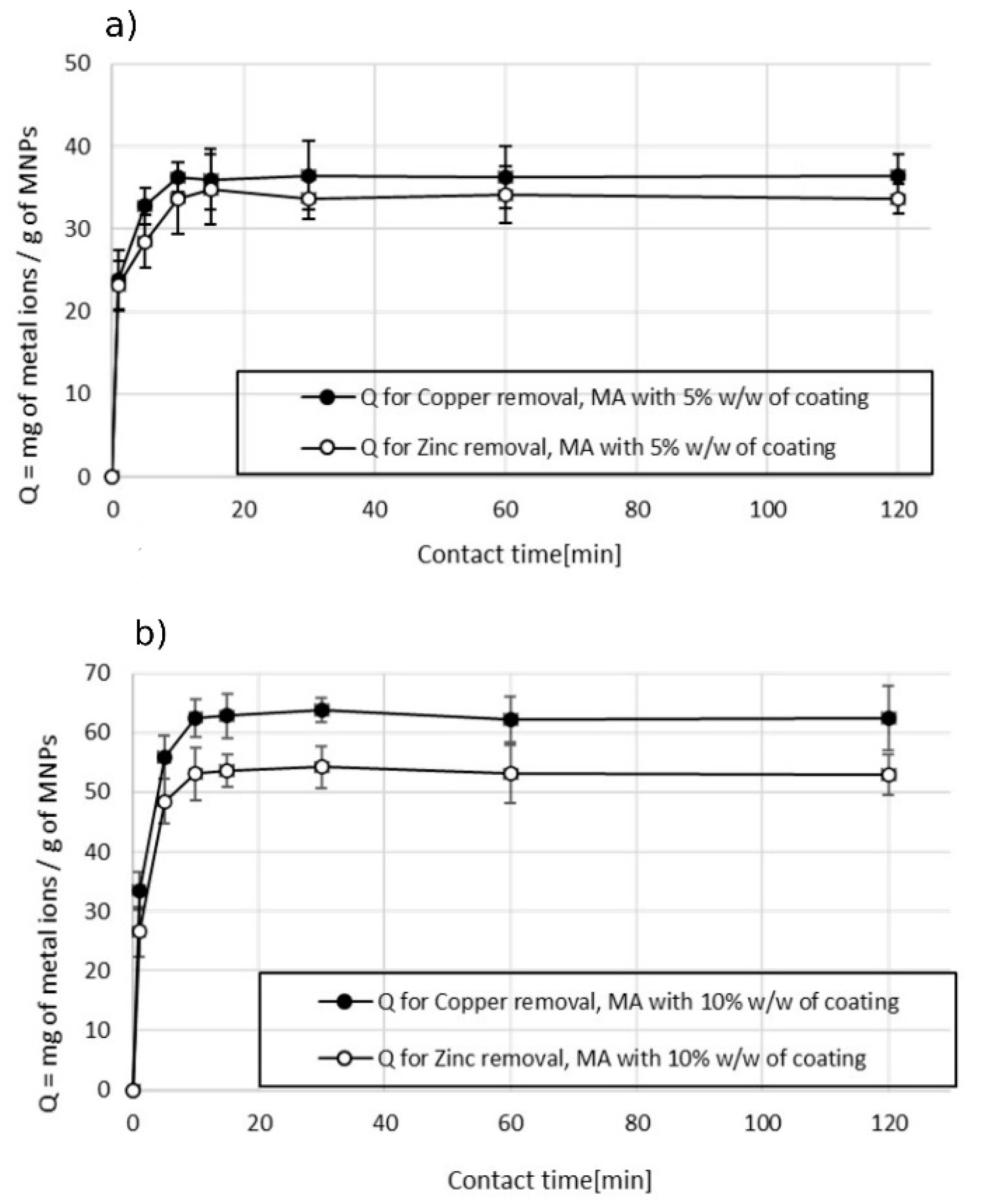
3.2.2. Double-Ion Adsorption
3.3. Adsorption Isotherm
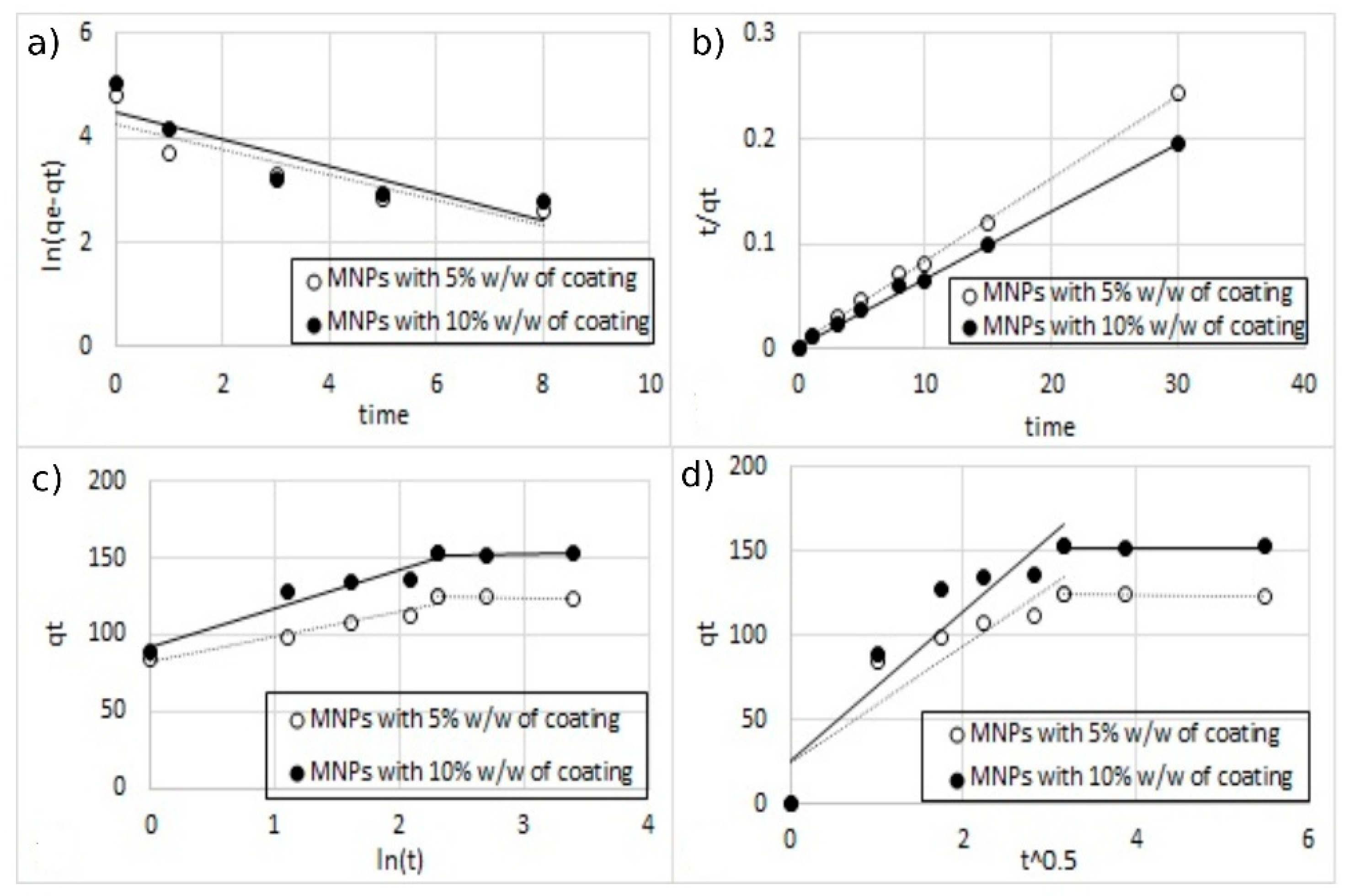
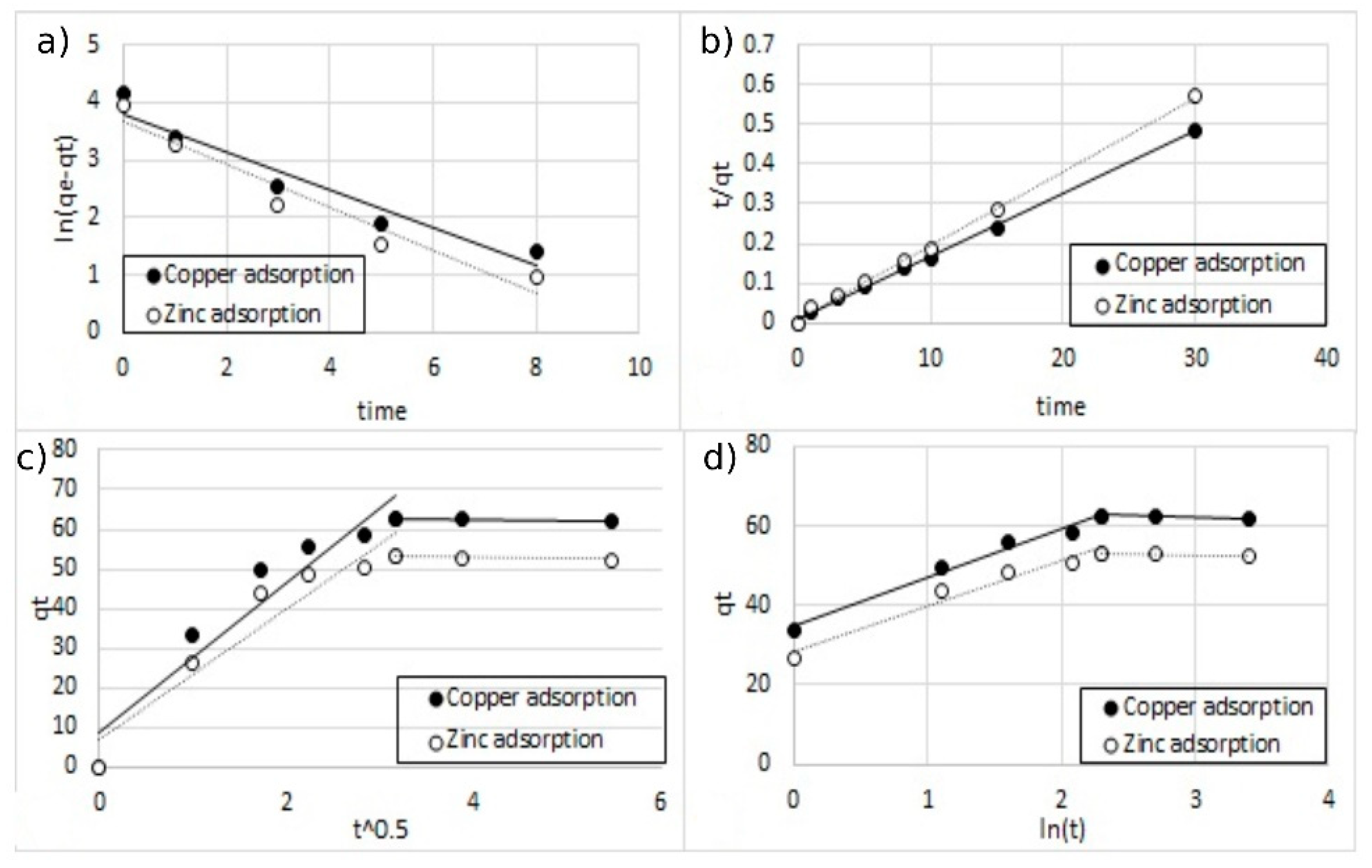
3.4. Kinetic Studies
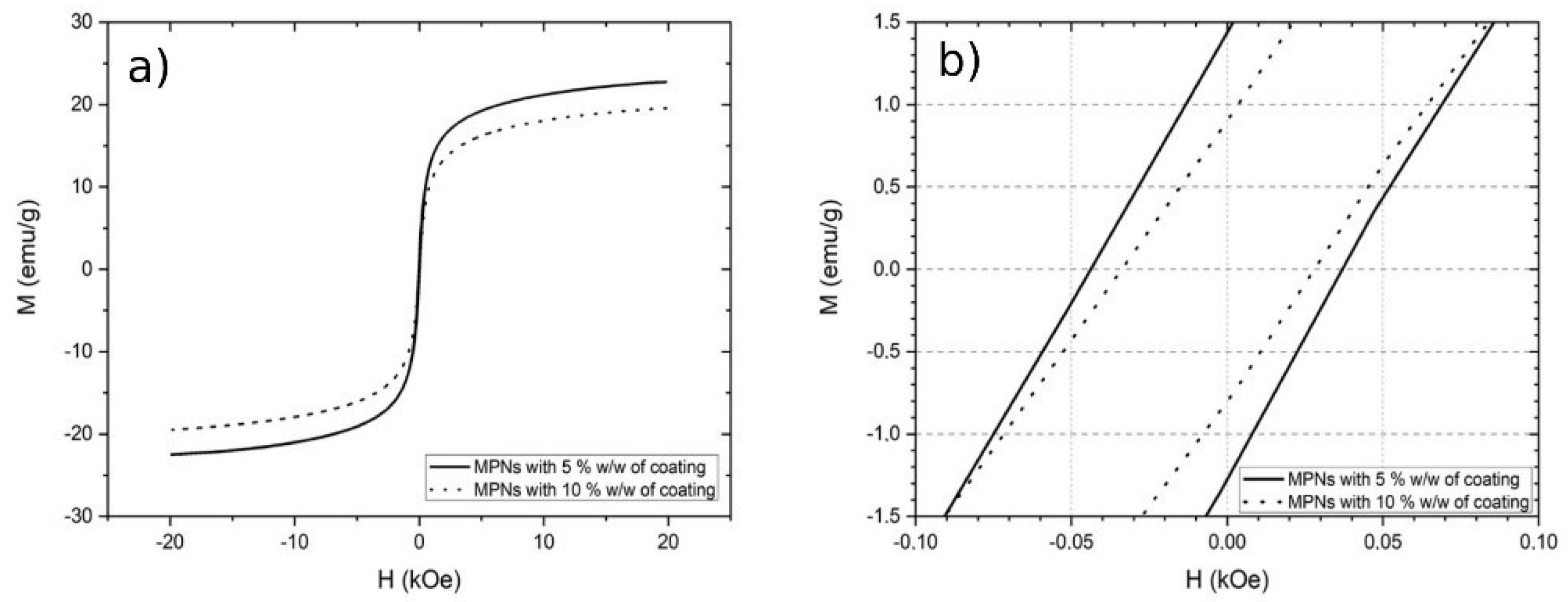
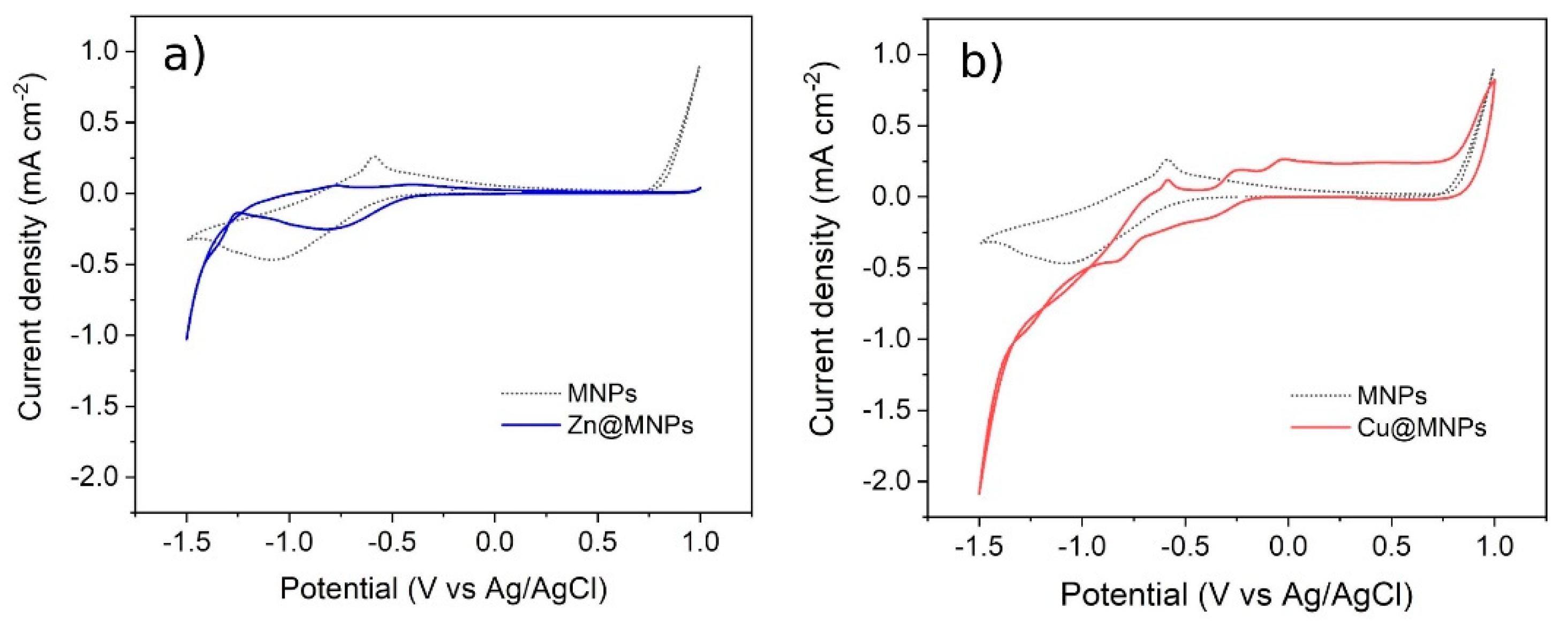
3.5. Evaluation of the Electrochemical Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Saydeh, S.A.; El-Naas, M.; Zaidi, S.J. Copper removal from industrial wastewater: A comprehensive review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Arbabi, M.; Golshani, N. Removal of copper ions Cu (II) from industrial wastewater: A review of removal methods. Int. J. Epidemiol. Res. 2016, 3, 283–293. [Google Scholar]
- Zhang, X.; Yang, F.; Ma, J.; Liang, P. Effective removal and selective capture of copper from salty solution in flow electrode capacitive deionization. Environ. Sci. Water Res. Technol. 2019, 6, 341–350. [Google Scholar] [CrossRef]
- Gakwisiri, C.; Raut, N.; Al-Saadi, A.; Al-Aisri, S.; Al-Ajmi, A. A Critical Review of Removal of Zinc from Wastewater. Proc. World Congr. Eng. 2012, 1, 627–630. [Google Scholar]
- Emadi, M.; Shams, E.; Amini, M.K. Removal of zinc from aqueous solutions by magnetite silica core-shell nanoparticles. J. Chem. 2013, 2013, 787682. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The Essential Toxin: Impact of Zinc on Human Health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed]
- Şenel, S.; Uzun, L.; Kara, A.; Denizli, A. Heavy Metal Removal from Synthetic Solutions with Magnetic Beads Under Magnetic Field. J. Macromol. Sci. Part A 2008, 45, 635–642. [Google Scholar] [CrossRef]
- Uzun, L.; Kara, A.; Osman, B.; Yılmaz, E.; Beşirli, N.; Denizli, A. Removal of heavy-metal ions by magnetic beads containing triazole chelating groups. J. Appl. Polym. Sci. 2009, 114, 2246–2253. [Google Scholar] [CrossRef]
- Purnamawati, F.; Sembiring, R. Preparation of Magnetic Chitosan Beads for Heavy Metal Ions Removal from Water. IOP Conf. Ser. Earth Environ. Sci. 2019, 276, 012004. [Google Scholar] [CrossRef]
- Rani, P.; Johar, R.; Jassal, P.S. Adsorption of nickel (II) ions from wastewater using glutaraldehyde cross-linked magnetic chitosan beads: Isotherm, kinetics and thermodynamics. Water Sci. Technol. 2020, 82, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, T.; Naushad, M.; Eldesoky, G.E.; Alqadami, A.A.; Khan, A. Synthesis and characterization of egg-albumen-formaldehyde based magnetic polymeric resin (MPR): Highly efficient adsorbent for Cd(II) ion removal from aqueous medium. J. Mol. Liq. 2019, 286, 110951. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Abdalla, M.A.; Ahamad, T.; Alothman, Z.A.; Alshehri, S.M.; Ghfar, A.A. Efficient removal of toxic metal ions from wastewater using a recyclable nanocomposite: A study of adsorption parameters and interaction mechanism. J. Clean. Prod. 2017, 156, 426–436. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Alothman, Z.A.; Alsuhybani, M.; Algamdi, M. Excellent adsorptive performance of a new nanocomposite for removal of toxic Pb(II) from aqueous environment: Adsorption mechanism and modeling analysis. J. Hazard. Mater. 2020, 389, 121896. [Google Scholar] [CrossRef] [PubMed]
- Naushad, M.; Alqadami, A.A.; Ahamad, T. Removal of Cd(II) ion from aqueous environment using triaminotriethoxysilane grafted oxidized activated carbon synthesized via activation and subsequent silanization. Environ. Technol. Innov. 2020, 18, 100686. [Google Scholar] [CrossRef]
- Alhumaimess, M.S.; Alsohaimi, I.H.; Alqadami, A.A.; Kamel, M.M.; Naushad, M.; Ahamad, T.; Alshammari, H. Synthesis of phosphorylated raw sawdust for the removal of toxic metal ions from aqueous medium: Adsorption mechanism for clean approach. J. Sol-Gel Sci. Technol. 2018, 89, 602–615. [Google Scholar] [CrossRef]
- Khan, M.A.; Alqadami, A.A.; Wabaidur, S.M.; Siddiqui, M.R.; Jeon, B.-H.; Alshareef, S.A.; Alothman, Z.A.; Hamedelniel, A.E. Oil industry waste based non-magnetic and magnetic hydrochar to sequester potentially toxic post-transition metal ions from water. J. Hazard. Mater. 2020, 400, 123247. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Barick, K.; Bahadur, D. Surface engineered magnetic nanoparticles for removal of toxic metal ions and bacterial pathogens. J. Hazard. Mater. 2011, 192, 1539–1547. [Google Scholar] [CrossRef]
- Sharma, M.; Kalita, P.; Senapati, K.K.; Garg, A. Study on Magnetic Materials for Removal of Water Pollutants. In Emerging Pollutants-Some Strategies for the Quality Preservation of Our Environment; IntechOpen Limite: London, UK, 2018. [Google Scholar] [CrossRef]
- Luo, X.; Lei, X.; Cai, N.; Xie, X.; Xue, Y.; Yu, F. Removal of Heavy Metal Ions from Water by Magnetic Cellulose-Based Beads with Embedded Chemically Modified Magnetite Nanoparticles and Activated Carbon. ACS Sustain. Chem. Eng. 2016, 4, 3960–3969. [Google Scholar] [CrossRef]
- Baresel, C.; Schaller, V.; Jonasson, C.; Johansson, C.; Bordes, R.; Chauhan, V.; Sugunan, A.; Sommertune, J.; Welling, S. Functionalized magnetic particles for water treatment. Heliyon 2019, 5, e02325. [Google Scholar] [CrossRef]
- Baek, M.K.; Sung, T.I.; Cho, E.S.; Namkung, K.C.; Bae, D.J.; Park, I.H. Magnetic Separation for Contaminants in Wastewater Using Magnetic Micro Bead. IEEE Trans. Magn. 2012, 48, 3768–3771. [Google Scholar] [CrossRef]
- Bora, T.; Dutta, J. Applications of Nanotechnology in Wastewater Treatment. Int. J. Innov. Emerg. Res. Eng. 2015, 2, 6. [Google Scholar] [CrossRef]
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and Environmental Risks of Nanomaterials: Challenges and Future Needs. J. Environ. Sci. Health Part C 2009, 27, 1–35. [Google Scholar] [CrossRef]
- Moscatelli, D.; Masi, M.; Pesce, R.M. Amphiphilic Magnetic Nanoparticles and Aggregates to Remove Hydrocarbons and Metal Ions and Synthesis Thereof. U.S. Patent No. 10,418,159, 17 September 2019. [Google Scholar]
- Prozorov, T.; Kataby, G.; Gedanken, A. Effect of surfactant concentration on the size of coated ferromagnetic nanoparticles. Thin Solid Films 1999, 340, 189–193. [Google Scholar] [CrossRef]
- Puentes-Vara, L.A.; Gregorio-Jauregui, K.M.; Bolarín, A.M.; Navarro-Clemente, M.E.; Dorantes, H.J.; Corea, M. Effects of surfactant and polymerization method on the synthesis of magnetic colloidal polymeric nanoparticles. J. Nanoparticle Res. 2016, 18, 212. [Google Scholar] [CrossRef]
- Huang, S.-H.; Chen, D.-H. Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent. J. Hazard. Mater. 2009, 163, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, L.; Erto, A.; Moreno-Piraján, J.C. Magnetite nanoparticles for removal of heavy metals from aqueous solutions: Synthesis and characterization. Adsorption 2013, 19, 465–474. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.; Khraisheh, M.; Kumar, A.; Almomani, T. Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP). Appl. Surf. Sci. 2020, 506, 144924. [Google Scholar] [CrossRef]
- Ren, Y.; Abbood, H.A.; He, F.; Peng, H.; Huang, K. Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: Preparation, characterization, and application in heavy metal adsorption. Chem. Eng. J. 2013, 226, 300–311. [Google Scholar] [CrossRef]
- Hao, Y.-M.; Man, C.; Hu, Z.-B. Effective removal of Cu (II) ions from aqueous solution by amino-functionalized magnetic nanoparticles. J. Hazard. Mater. 2010, 184, 392–399. [Google Scholar] [CrossRef]
- Anwar, J.; Shafique, U.; Zaman, W.-U.; Salman, M.; Dar, A.; Anwar, S. Removal of Pb(II) and Cd(II) from water by adsorption on peels of banana. Bioresour. Technol. 2010, 101, 1752–1755. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Balasubramanian, R.; Iyer, C. Evaluation of the marine algae Ulva fasciata and Sargassum sp. for the biosorption of Cu(II) from aqueous solutions. Bioresour. Technol. 2007, 98, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Alsuhybani, M.; Alshahrani, A.; Algamdi, M.; Al-Kahtani, A.A.; Alqadami, A.A. Highly efficient removal of Pb(II) from aqueous systems using a new nanocomposite: Adsorption, isotherm, kinetic and mechanism studies. J. Mol. Liq. 2020, 301, 112393. [Google Scholar] [CrossRef]
- Erentürk, S.; Malkoç, E. Removal of lead(II) by adsorption onto Viscum album L.: Effect of temperature and equilibrium isotherm analyses. Appl. Surf. Sci. 2007, 253, 4727–4733. [Google Scholar] [CrossRef]
- Predescu, A.; Matei, E.; Berbecaru, A.; Vidu, R. Synthesis of Magnetic Nanoparticles for the Removal of Heavy Metal Ions from Wastewaters. ARA Annu. Congr. Proc. 2014. [Google Scholar] [CrossRef]
- Mobasherpour, I.; Salahi, E.; Pazouki, M. Comparative of the removal of Pb2+, Cd2+ and Ni2+ by nano crystallite hydroxyapatite from aqueous solutions: Adsorption isotherm study. Arab. J. Chem. 2012, 5, 439–446. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Jiang, Q.; Zhao, L. Water-soluble Fe3O4 nanoparticles with high solubility for removal of heavy-metal ions from waste water. Dalton Trans. 2012, 41, 4544–4551. [Google Scholar] [CrossRef] [PubMed]

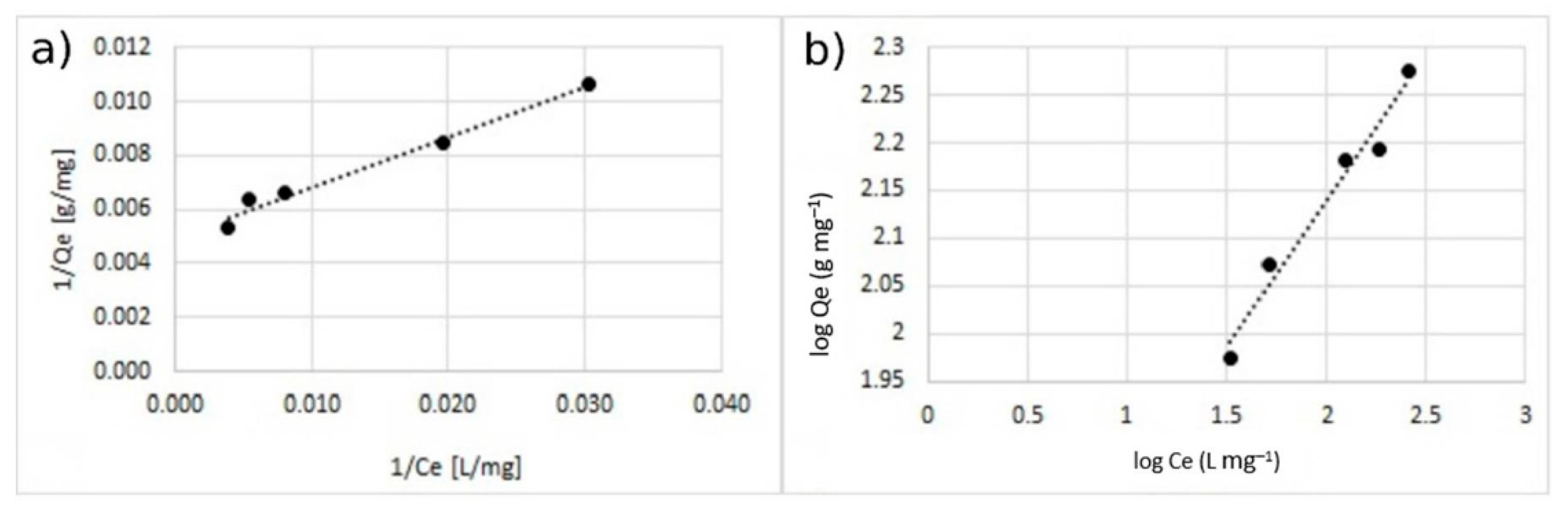
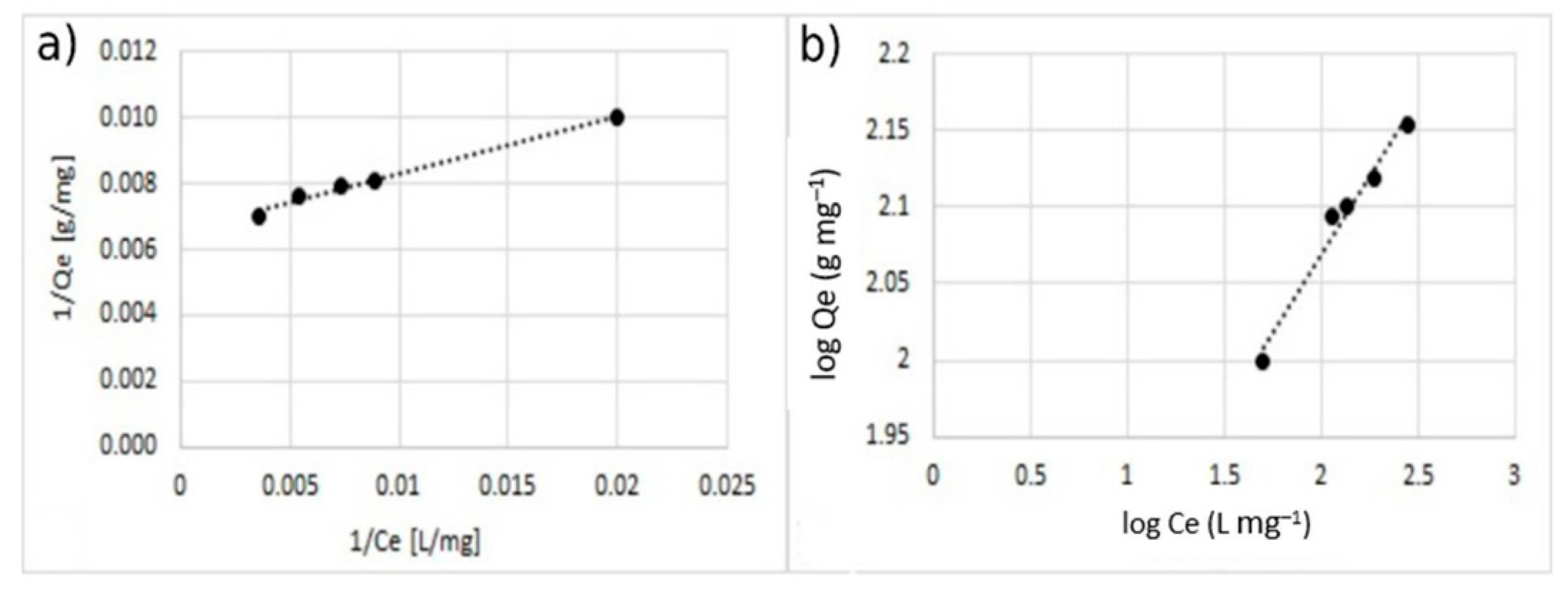

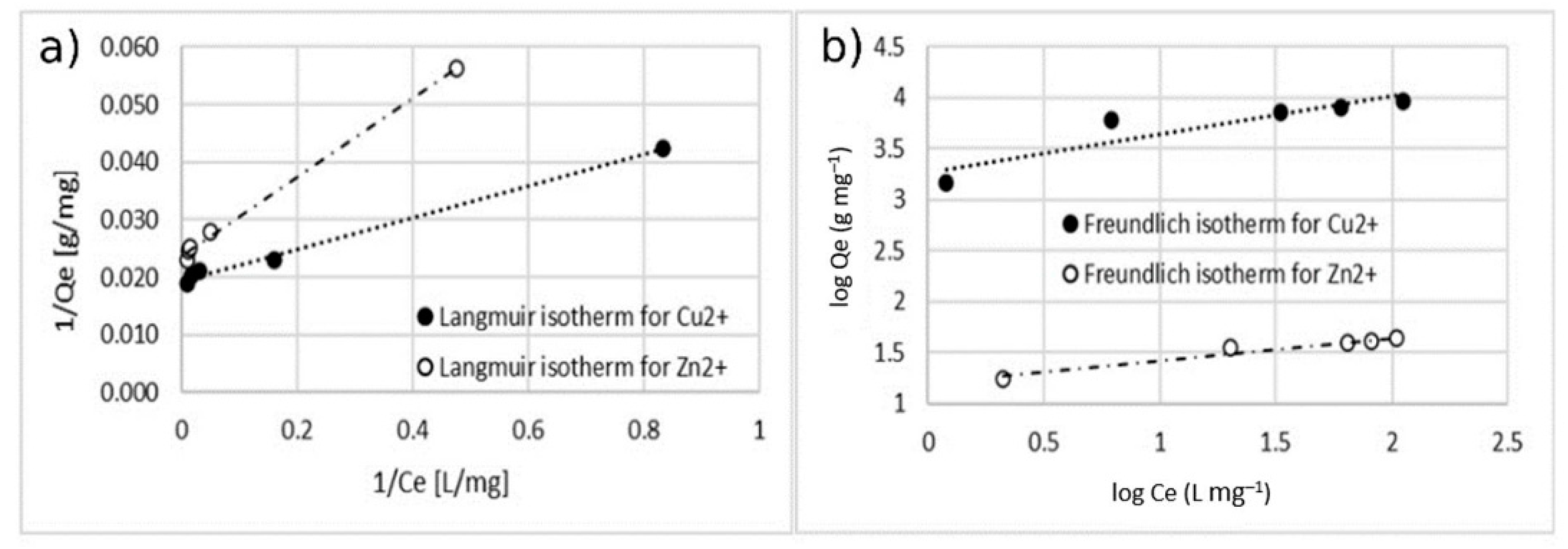

| % Coating w/w | Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|---|
| Qmax (mg g−1) | KL (L m−1) | RL Range | r2 | n | KF | r2 | |
| 5 | 148.5 | 0.039 | 0.068–0.205 | 0.991 | 4.95 | 46.2 | 0.9681 |
| 10 | 192.3 | 0.028 | 0.093–0.311 | 0.982 | 3.24 | 33.34 | 0.9612 |
| % Coating w/w | Langmuir Model | Freundlich Model | ||||||
|---|---|---|---|---|---|---|---|---|
| ion | Qmax (mg g−1) | KL (L m−1) | RL Range | r2 | n | KF | r2 | |
| 5 | copper | 51.82 | 0.699 | 0.01–0.099 | 0.994 | 6.131 | 2.241 | 0.841 |
| zinc | 42.19 | 0.346 | 0.022–0.207 | 0.996 | 4.524 | 15.958 | 0.951 | |
| 10 | copper | 74.07 | 0.224 | 0.031–0.255 | 0.989 | 3.161 | 19.961 | 0.968 |
| zinc | 58.14 | 0.239 | 0.032–0.278 | 0.9853 | 3.262 | 15.653 | 0.961 | |
| Kinetic Parameters | Single-Ion Adsorption | Double-Ion Adsorption | ||||
|---|---|---|---|---|---|---|
| Coating | 5% w/w | 10% w/w | 5% w/w | 10% w/w | ||
| Copper | Copper | Zinc | Copper | Zinc | ||
| Qe (exp) (mg L−1) | 125.2 | 152.8 | 37.2 | 33.7 | 62.6 | 53.1 |
| wPseudo-first order model | ||||||
| Qe (calc) (mg L−1) | 72.588 | 91.049 | 24.501 | 20.166 | 44.768 | 39.064 |
| K1 (min−1) | 0.247 | 0.261 | 0.298 | 0.263 | 0.331 | 0.374 |
| R2 | 0.805 | 0.781 | 0.907 | 0.849 | 0.936 | 0.944 |
| SSE | 53.733 | 62.255 | 12.710 | 13.752 | 17.920 | 13.813 |
| Pseudo-second order model | ||||||
| Qe (calc) (mg L−1) | 126.582 | 156.250 | 37.736 | 33.784 | 63.291 | 53.476 |
| K2 ( mg L−1) | 0.016 | 0.011 | 0.044 | 0.059 | 0.032 | 0. 042 |
| R2 | 0.998 | 0.986 | 0.991 | 0.989 | 0.992 | 0.988 |
| SSE | 5.391 | 6.076 | 1.244 | 1.335 | 3.661 | 3.900 |
| Intra | ||||||
| Qe (calc) (mg L−1) | 127.610 | 152.190 | 34.209 | 33.644 | 63.645 | 54.068 |
| Kintra (mg g−1 min−1/2) | 34.966 | 44.399 | 10.585 | 9.431 | 19.008 | 16.506 |
| C | 24.269 | 25.640 | 6.567 | 6.575 | 8.649 | 6.936 |
| R2 | 0.876 | 0.546 | 0.782 | 0.543 | 0.906 | 0.941 |
| SSE | 14.535 | 16.440 | 3.941 | 3.919 | 5.599 | 5.260 |
| Elovich | ||||||
| Qe (calc) (mg L−1) | 128.850 | 152.270 | 33.436 | 33.960 | 64.189 | 54.612 |
| alpha (mg g−1 min−1) | 2975.211 | 1025.352 | 402.383 | 879.825 | 205.754 | 141.845 |
| beta (g mg−1 min−1) | 0.063 | 0.040 | 0.184 | 0.237 | 0.081 | 0.089 |
| R2 | 0.937 | 0.579 | 0.804 | 0.623 | 0.967 | 0.969 |
| SSE | 2.82 | 4.812 | 0.597 | 0.763 | 1.129 | 1.668 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesce, R.; Accogli, A.; Kostoula, C.; Ilare, J.; Panzeri, G.; Perecin, C.J.; Magagnin, L. Innovative Magnetic Aggregates for the Removal of Transition Metals from Industrial Wastewater. Minerals 2021, 11, 643. https://doi.org/10.3390/min11060643
Pesce R, Accogli A, Kostoula C, Ilare J, Panzeri G, Perecin CJ, Magagnin L. Innovative Magnetic Aggregates for the Removal of Transition Metals from Industrial Wastewater. Minerals. 2021; 11(6):643. https://doi.org/10.3390/min11060643
Chicago/Turabian StylePesce, Ruggiero, Alessandra Accogli, Chrysavgi Kostoula, Juri Ilare, Gabriele Panzeri, Caio Josè Perecin, and Luca Magagnin. 2021. "Innovative Magnetic Aggregates for the Removal of Transition Metals from Industrial Wastewater" Minerals 11, no. 6: 643. https://doi.org/10.3390/min11060643
APA StylePesce, R., Accogli, A., Kostoula, C., Ilare, J., Panzeri, G., Perecin, C. J., & Magagnin, L. (2021). Innovative Magnetic Aggregates for the Removal of Transition Metals from Industrial Wastewater. Minerals, 11(6), 643. https://doi.org/10.3390/min11060643







