Kelyphite Rims on Garnets of Contrast Parageneses in Mantle Xenoliths from the Udachnaya-East Kimberlite Pipe (Yakutia)
Abstract
1. Introduction
2. Methods, Samples, and Abbreviations
3. Results
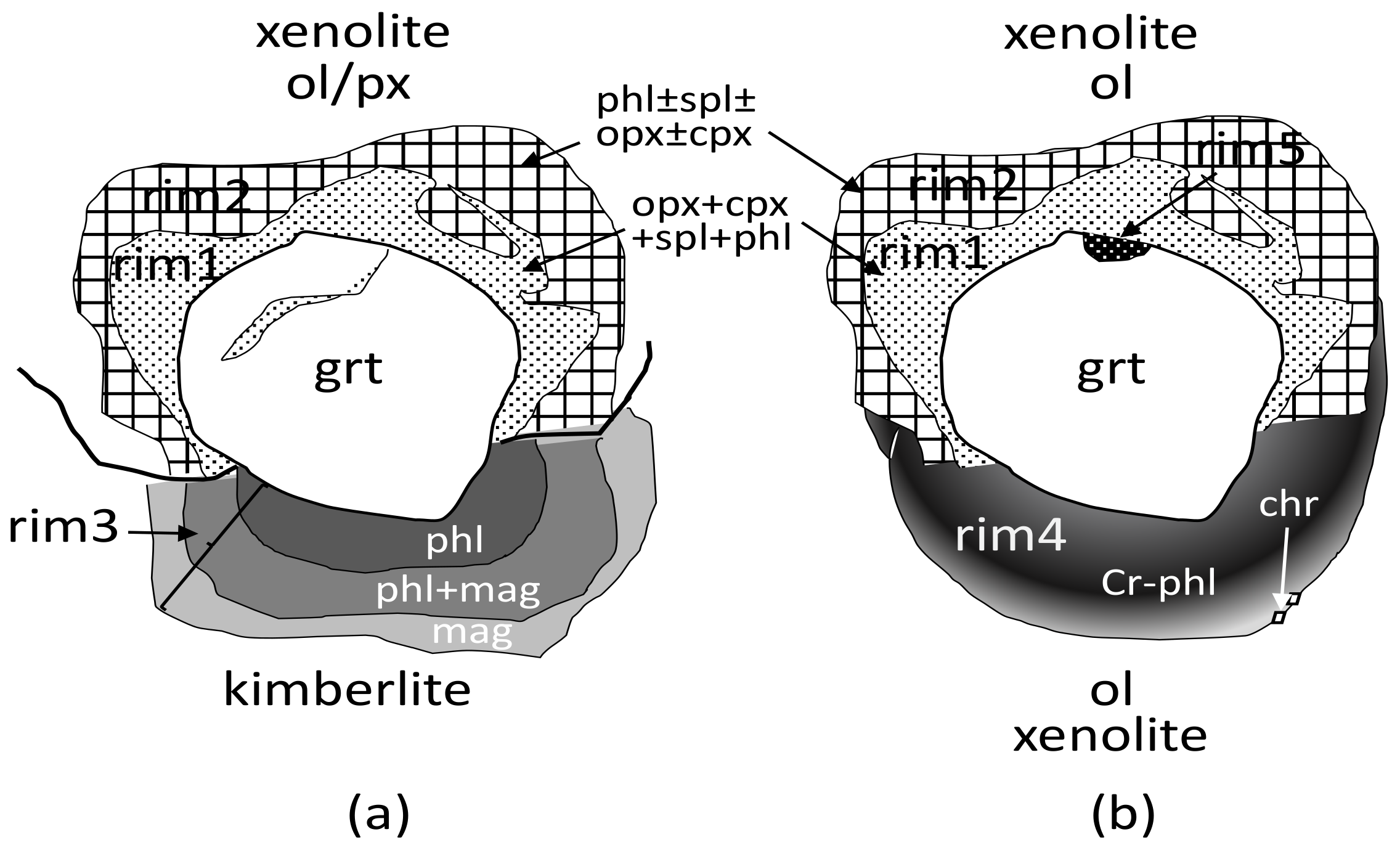
3.1. Classification
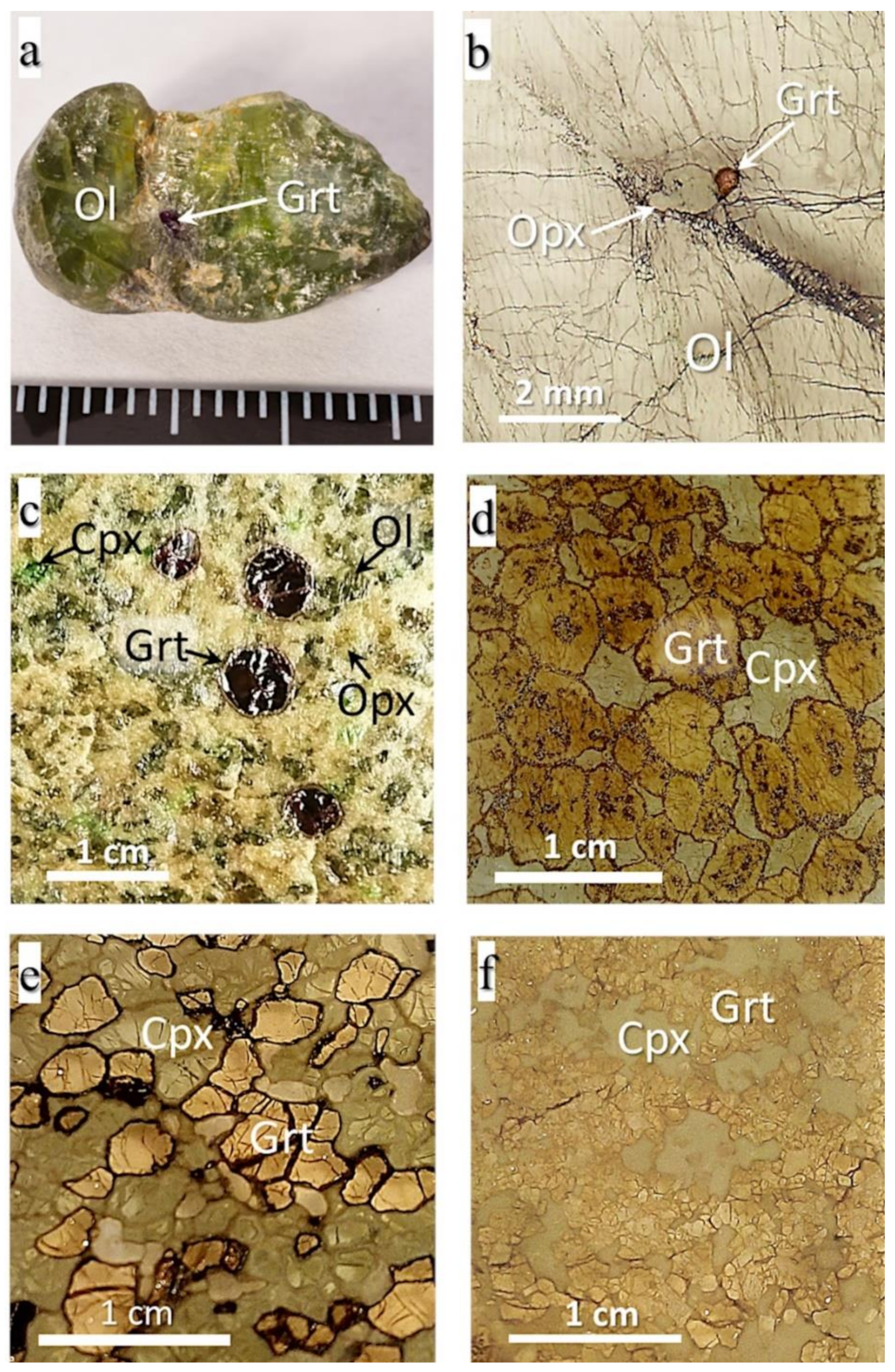
3.1.1. Kelyphite Rims in MHD Samples
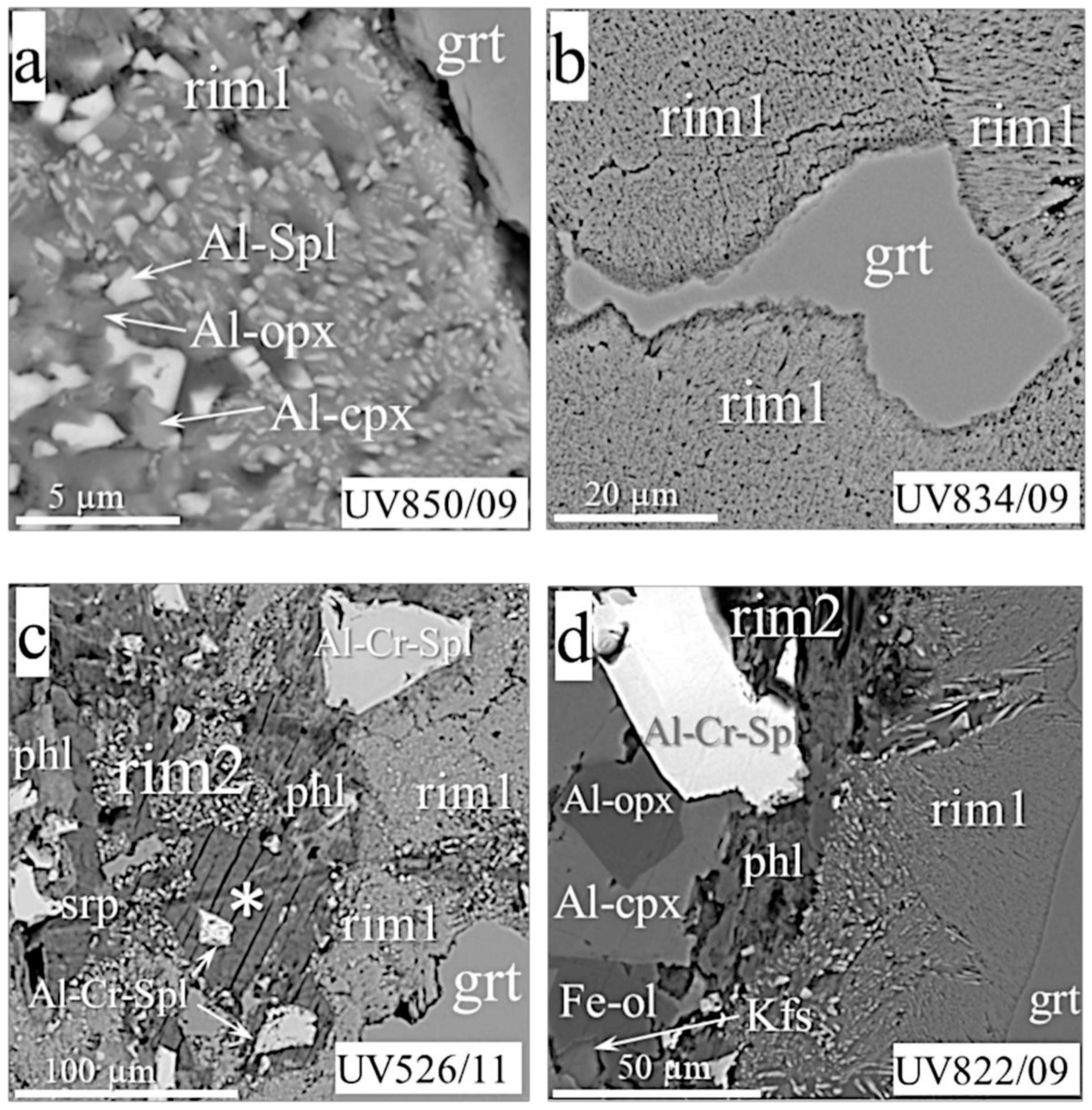
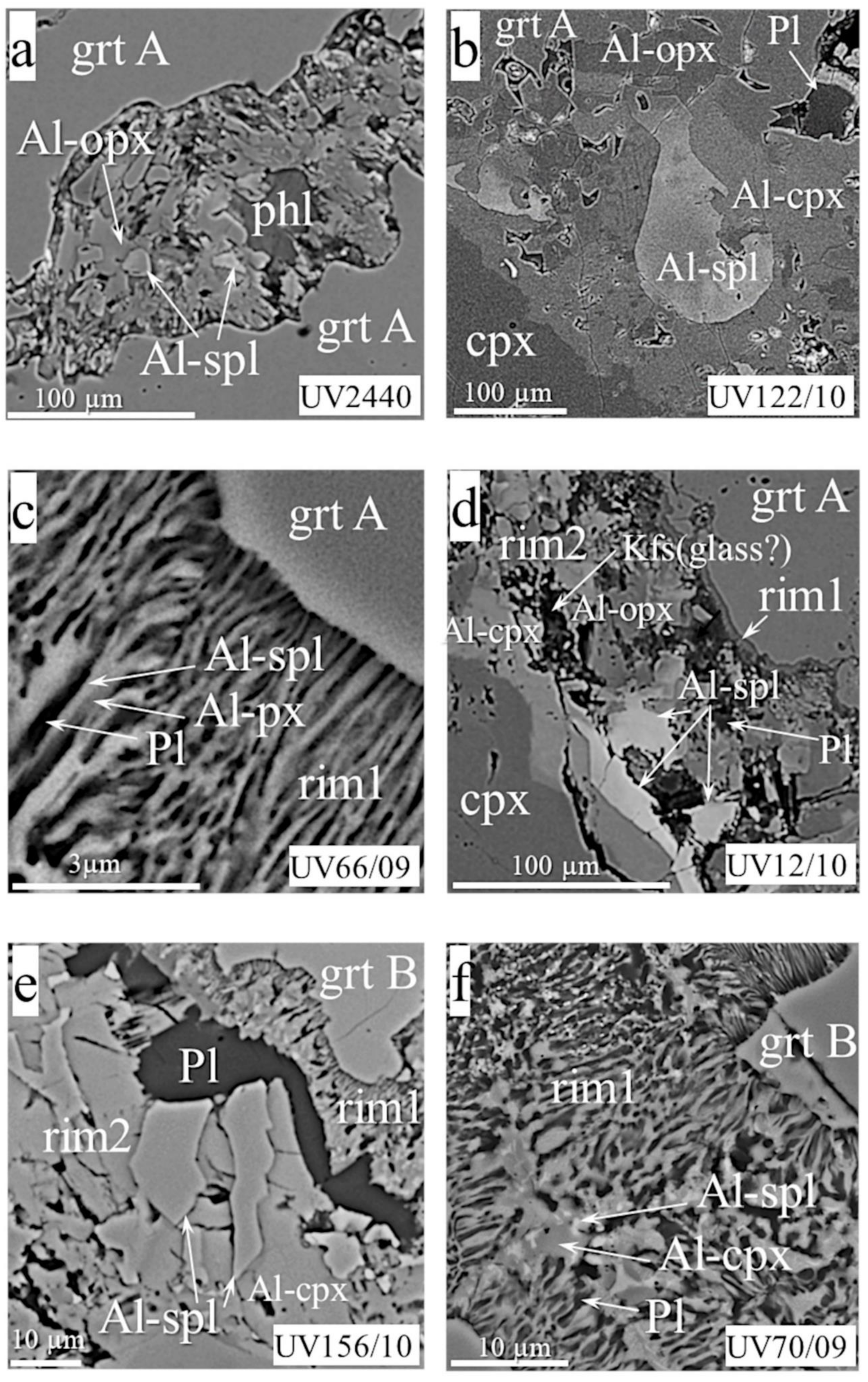
- Rim1, from a few to 180 microns thick, is located between garnet and olivine or in garnet cracks (Figure 2a–g,k,l). The rim minerals coarsen gradually or abruptly outward from garnet. The size of mineral grains rarely reaches 15–20 microns and, generally, does not exceed 10 microns. The rim assemblages usually consist of the typical intergrowths of rounded isometric or elongated randomly located grains of orthopyroxene, clinopyroxene, and spinel. Phlogopite, and rarely amphibole, sodalite, feldspar, chlorite, and serpentine, occur interstitially to those minerals. Rims of this type in MHD samples are sometimes equigranular (Figure 2b,k) or look like cones of gradually increasing intergrown grains (Figure 2a,d,f), but most often they are patched mixtures with rim2 (Figure 2c,e).
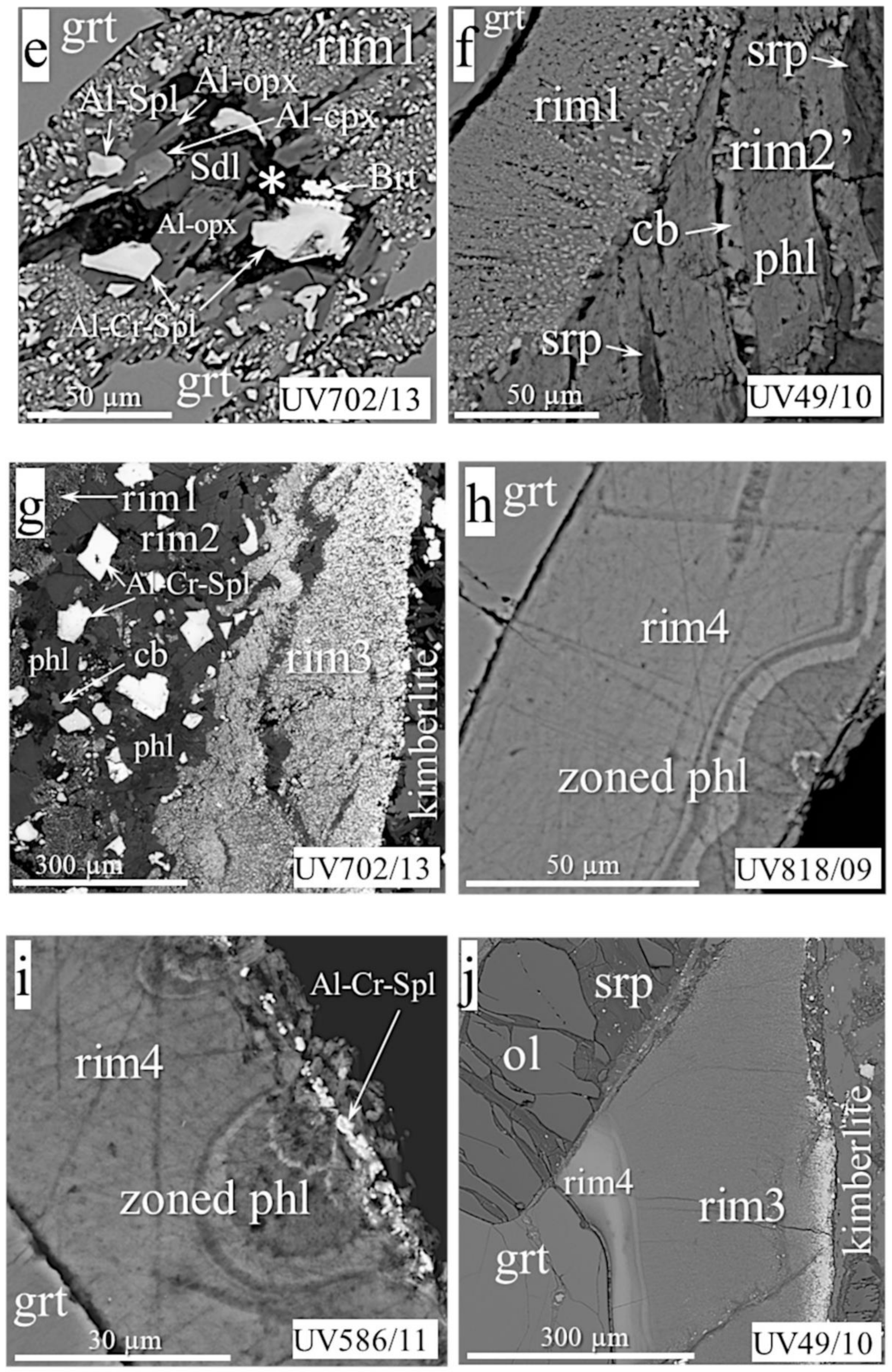
- Rim2, ≤90 microns thick, occurs as continuous or discontinuous envelopes around rim1 (Figure 2d,f,g), or as patches inside rim1 (Figure 2c,e). The grain size of rim2 is typically tens of microns. The rim often presents hypidiomorphic phlogopite plates with or without enclosed spinel grains (Figure 2f,g,k). Sometimes, it also contains ortho- and clinopyroxene, sodalite, amphibole, feldspar, plagioclase, calcite, serpentine, tetraferriphlogopite, and Fe-olivine (Figure 2d,e,f). In this case, phlogopite, serpentine, tetraferriphlogopite, and sodalite fill the intergranular space of other minerals (Figure 2c,d,e). Fe-Ni sulfides rarely occur as inclusions within serpentine at the periphery of the rim closer to olivine. Djerfisherite with a size of about 5 microns was found in phlogopite near rim1. A layered hydrous magnesium aluminosilicate phase bearing Cr and Cl was found in six samples (asterisked in Figure 2c,e). Sometimes, this is represented by large (up to 80 microns) plates, partially replaced by phlogopite, with disseminated spinel grains and local areas of rim1 (Figure 2c). In other samples, it was found in the intergranular space together with barite (Figure 2e). The rim on the UV49/10 garnet (Figure 2f; referred to as rim2′ in the Discussion) has an unusual, banded texture, consisting of large phlogopite flakes alternating with calcite lamellae. The intergrowths are located at an angle of 45° to the border rim1/rim2’. Rims1 and 2 surround garnets in most studied samples.
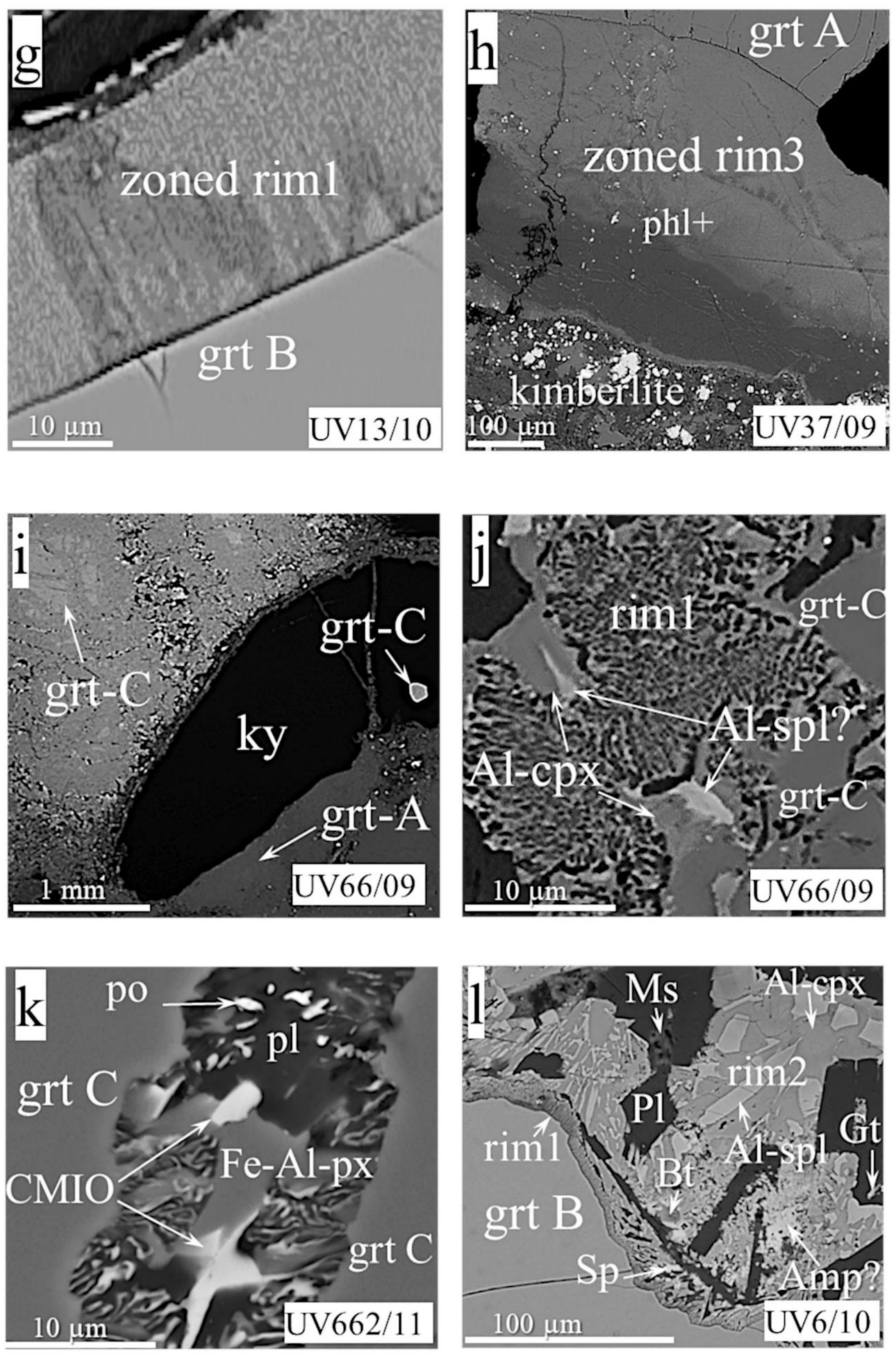
- Rim3, the phlogopite-spinel-magnetite rim, up to 350 µm thick, usually occurs be-tween garnet and kimberlite, and rarely between kimberlite and other types of rim (Figure 2g,j). It consists mostly of phlogopite with enclosed changing composition spinel grains, and has a zonal structure, with phlogopite percentages higher near garnet and more abundant magnetite near kimberlite. The size of mineral grains varies from fractions of a micron to 7–8 microns at the border with kimberlite.
- Rim4 is zoned, up to 100 microns thick, composed of cryptocrystalline high-Cr phlogopite/hydrophlogopite; rare Cr-spinel grains with a size of about one micron are sometimes located on the outer band of the rim (Figure 2i). Such rims are found in samples UV586/11 and UV818/89, instead of rim1 and rim2 (Figure 2h,i). Rim4 is partially replaced by rim3 in samples UV159/10-2 and UV49/10 (Figure 2j).
- Rim5 comprises “pockets” found between garnet and rim1 (Figure 2k,l) in three samples. They have a hemispherical (or close to) shape with a radius of 10 to 50 microns and an uneven spotty structure in the BSE mode.
3.1.2. Kelyphite Rims in ShP Samples
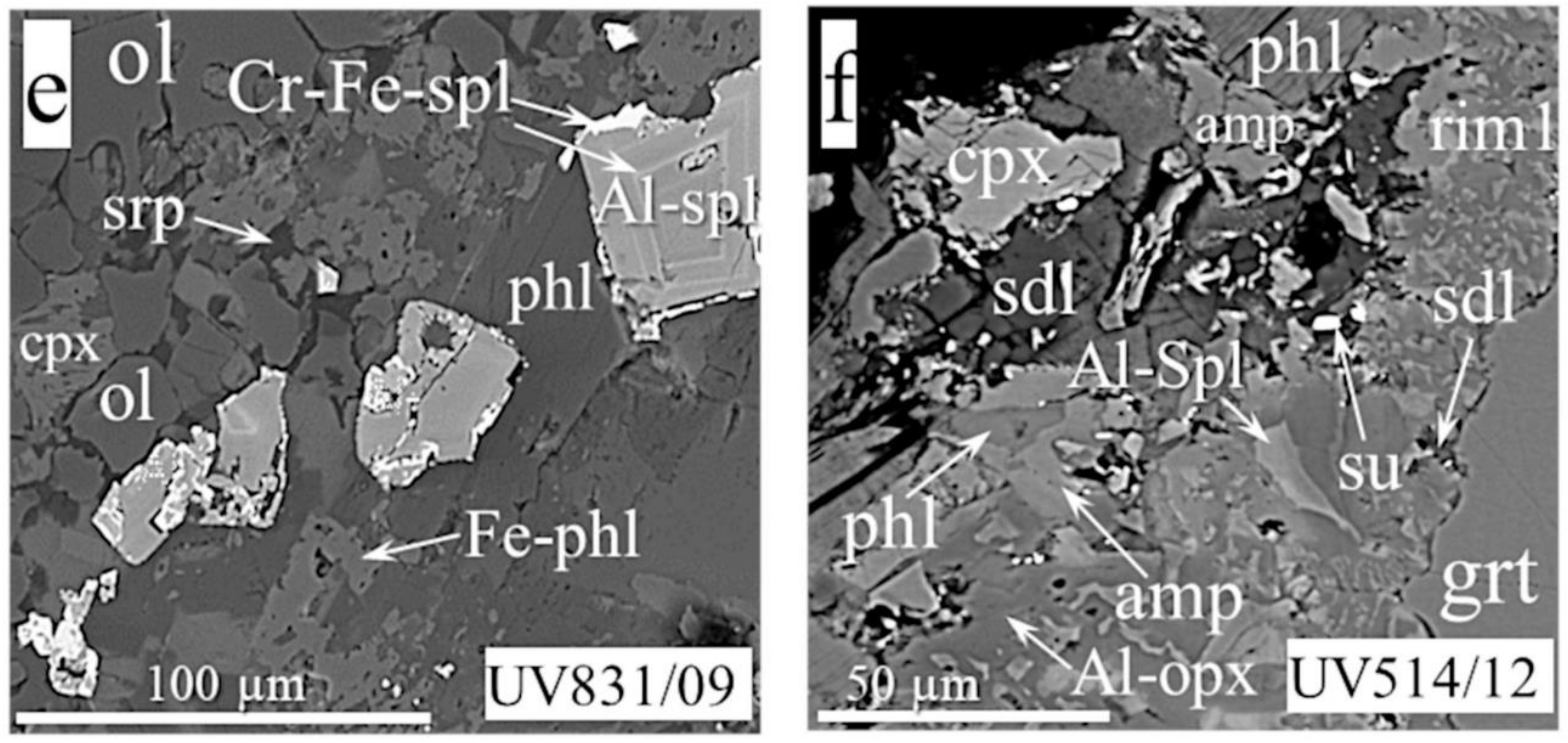
- Rim1 (Figure 3a–d,f) comprises intergrowths of spinel, orthopyroxene, clinopyroxene, phlogopite, amphibole, sodalite, and K-Na-feldspar in various combinations, or, more rarely, are all present in one rim. Phlogopite, sodalite, and feldspar usually occur interstitially (Figure 3b,f). Fe-olivine was found as xenomorphic grains located around Al-spinel in the rim of sample UV831/09. This rim at the contact with garnet is more similar texturally to rim2 than to rim1 (Figure 3b), with coarser grains (tens of microns) of Al-pyroxenes and spinel. Fe-Ni-sulfides and djerfisherite in the form of individual grains or inter-growths were in the space between the main minerals in the outer part of the rim on the border with recrystallized olivine of the ground mass.
- Rim2 (Figure 3a,c,e,f) contains the same minerals as rim1 (two pyroxenes and spinel) but with coarser grain sizes. The mineral assemblage also includes phlogopite as hypidiomorphic plates (Figure 3c) and a lower abundance of amphibole (Figure 3a,f), feldspar, sodalite (Figure 3f), calcite, and serpentine (Figure 3e). Intergrowths of Fe-olivine and Al-pyroxenes surround zoned crystals of Al-spinel in rim2, which develops within the crack in one of the garnets of sample UV831/09. Baritocelestite (with different proportions of barite and celestine components) fills cracks and cavities in Al-spinel of sample UV33/10. Rim2 of sample UV831/09 consists of intergrown phlogopite of variable Al-Fe compositions (phlogopite and tetraferriphlogopite) and aluminous spinel with distinct zones of Cr-Fe spinel (appearing as light-color bands in a gray spinel crystal in Figure 3e). In the same rim, Ni sulfide (sometimes with a djerfisherite edging) was noted in the form of inclusions in spinel or phlogopite at the outer border of the rim.
- Rim3 is identical in appearance to the corresponding rim on MHD garnets. Apparently, the mineral association is the same.
3.1.3. Kelyphite Rims in A, B, C Ecl Samples
- Rim1, 10–15 µm (Figure 4c–f,k) thick, rarely more (Figure 4g,l), with prominent symplectite structures of vermicular or elongated grains perpendicular to the “garnet–rim” boundary (Figure 4c,e–g). The rim mineralogy differs in different groups, but aluminous spinel is present in almost all rim assemblages (Table 1). Additionally, typical rim phases in Ecl A are clinopyroxene and orthopyroxene, sometimes amphibole instead of or after pyroxene. Phlogopite, sodalite, and plagioclase occur interstitially. Chlorite is the product of their replacement. Ecl B have rims with slightly different relative percentages of phases and high Fe content in the lat. The mineralogy consists of Al-Fe-pyroxenes/amphibole and Al-spinel symplectites, interstitial phlogopite, plagioclase, K-feldspar, or sodalite, and phases stoichiometrically corresponding to tourmaline and staurolite (sample UV156/10). Magnetite and Fe-Ni-Cu sulfides occur on this type of rim periphery as individual interstitial grains (samples UV211/10, UV13/10, UV139/09). The rim in Ecl C lacks orthopyroxene and phlogopite and consists of Al-Fe-clinopyroxene, plagioclase, spinel, and rare amphibole. The garnet rim in sample UV662/11, instead of spinel, contains OCMI (see Abbreviations) intergrowth with the pyroxene (Figure 4k). Pyrrhotite enclosed within the plagioclase was found in that rim. The rim on the garnet of diamond-bearing Ecl A (sample UV2440) looks like this on garnet of ShP sample UV831/09, described above (Figure 4a and Figure 3b, respectively). Rim1 in the Ecl B sample UV13/10 is broader than this in other Ecl. Dark and light areas (in BSE mode) attest to the unequal distribution of minerals in the rim (Figure 4g).
- Rim2 was found in three samples of Ecl A, three samples of Ecl B, and one sample of Ecl C (Figure 4b,d,e,l). Practically all of these rims contain Al-spinel, Al-Fe-clinopyroxene, and plagioclase, which is either intergrown with two other minerals or occurs as a band between rim1 and rim2 (Figure 4e,l). Rim2 in sample UV13/10 contains the spots of the rim1 symplectites. Al-orthopyroxene or Fe-olivine rarely occur together or instead of Al-clinopyroxene in the rim of Ecl A and B (Figure 4b,d). Feldspar, plagioclase, amphibole, phlogopite, and calcite usually fill the intergranular space (Figure 4b,d). Additionally, pyrite, pyrrhotite, and djerfisherite, which surrounds it as a ragged strip, are present as inclusions in feldspar in rim2 of Ecl A and B (e.g., sample UV139/09). A phase stoichiometrically similar to Fe-amphibole appears in the rim of sample UV6/10, which also contains biotite and muscovite in different parts, in addition to a few grains of sphalerite (wurtzite) and galena (Figure 4l). The rim of sample UV66/09-C bears interstitial calcite and a phase identified as scapolite.
- Rim3, ~450 microns thick, between garnet and kimberlite in Ecl A (Figure 4h), looks like the respective rim in MHD samples. Rims of this type comprise three visible zones of light, dark, and exceptionally light colors (outward from garnet); the latter is a very thin band that contours the rim at the boundary with kimberlite. All zones consist of compositionally variable phlogopite and spinels occurring as inclusions in cracks.
3.2. Analytical Data
3.2.1. Chemistry of Main Rim Minerals
- Rim1. The difference in the composition of the same-named rim minerals of peridotite parageneses (MHD and ShP) is due to the paragenetic features of the garnet composition. Although having similar contents of the main components, orthopyroxenes in MHD and ShP rims1 show different Cr2O3 (and, respectively, Al2O3) (Table 2). The minerals of eclogite rims show much higher iron content and an almost complete absence of chromium. Al2O3 participates in the formation of the Mg-Tschermak component (MgAlVIAlIVSiO6) in both orthopyroxene and clinopyroxene (although the latter normally contains the Ca-Tschermak component (CaAlVIAlIVSiO6)). This is a general trend for the three studied parageneses. Both pyroxenes are present in this rim type in peridotites and eclogites, whereas as rock-forming ones, they are simultaneously present only in the ShP. MHD contain only orthopyroxene (in harzburgites), whereas Ecl contain only clinopyroxene. Moreover, the rock-forming clinopyroxene of Ecl C has more Al2O3 than clinopyroxene in the rim (Table 2), but practically does not contain a Tschermak component, because all aluminum is bound with sodium to form a Jadeite component. Both pyroxenes in the rims of three studied parageneses show notably lower Mg # values in comparison with those of primary pyroxenes. The Cr2O3 content in the spinel of this type of rim varies from 31 wt.% in MHD up to 0.2 wt.% in Ecl (Table 2). In addition, spinel of the rim contains less Cr and slightly more Fe than the primary Cr-spinel present in some MHD. The phlogopite compositions of the peridotite parageneses rims are similar, except for the enrichment of phlogopite in the ShP with titanium (TiO2 1.73 wt.%, Table 2). The phlogopite composition of the Ecl rim differs from that of MHD and ShP in the absence of Cr2O3 (about 2 wt.% in the phlogopite of the peridotite garnets rims) and a 3–4-fold excess of FeO (Table 2). Kelyphites in twenty studied MHD garnets contained Al-clinopyroxene, whose presence generally correlates with CaO content in garnet (according to this study, the threshold CaO content in garnet is approximately 1.6 wt.%). However, this is not always the case. For instance, the rim of megacrystalline harzburgite pyrope with 5.49 wt.% CaO (sample UV162/10-1) consists of phlogopite and spinel, whereas rim1 of the same paragenesis pyrope with 6.96 wt.% CaO (sample UV654/11) contains amphibole, in addition to phlogopite and spinel, instead of pyroxenes. Some MHD rims are nearly identical to garnets in terms of the bulk composition of the main components (samples UV12/01-1, UV26/10-1, UV667/11); others have slight differences, which are expressed as a lower SiO2 content (by 1.6–6.46 wt.%) at a higher MgO content (by 0.9–1.81 wt.%), and in the presence of certain amounts of K2O (up to 1.85 wt.%) and Na2O (up to 0.82 wt.%) (Table 3, MHD). The rims also often have a lower sum (the difference is up to 2.7 wt.%) than in garnet. This is most likely due to the presence of a hydrous phase in these rims. The prevalence of K2O over Na2O indicates the priority presence of phlogopite and potassium feldspar in these rims. Amphibole, sodalite, and plagioclase are found in smaller quantities. In addition, sometimes a deficiency of CaO (up to 1 wt.%), and an excess of chromium (up to 1.62 wt.%) and iron (up to 1.1 wt.%), in relation to the composition of the MHD garnet can be found in the rims. This can be caused by focusing the central part of the analyzer on spinels. The bulk composition of the rims of the ShP garnets, analyzed in three samples, in general, has more pronounced components differences from the composition of garnet (Table 3, ShP). The excess in MgO in the rim reaches 2.54 wt.%, and the deficit of Al2O3, which is almost invisible in the MHD rims, ranges from 1.72 to 2.55 wt.%. The amounts of K2O and Na2O in the rim are close: Up to 1.37 and 1.8 wt %, respectively, which indicates a noticeable role of Na-containing phases, in addition to K-containing ones. The rim1 bulk composition of the Ecl A garnet (UV66/09-A) is identical to that of the garnet. In the rims of Ecl garnets B (six samples) and C (two samples), a decrease in FeO (by 0.7–7.9 wt %) with respect to the composition of garnet is noticeable. The amounts of silica, alumina, and calcium in these rims are sometimes higher than those in garnets. An impurity of Na2O (up to 2.2 wt.%) is observed in almost all rims, whereas noticeable amounts of K2O (2.04 wt.%) were noted only in Ecl B UV156/10 (Table 3, Ecl). This confirms that plagioclase, amphibole, and sodalite are more abundant in Ecl B, and especially C, than phlogopite and potassium feldspar.
- Rim2. AlIV content in minerals in the rims of this type of peridotite and eclogite parageneses is lower than for similar minerals in rim1, but higher than in rock-forming minerals (Table 2). of pyroxenes and phlogopite is higher than in rim1, whereas the opposite trend is observed for the spinel of all parageneses due to the sharply increasing Fe component. This is most clearly manifested in spinel of Ecl C. In rim2 spinels of peridotite parageneses, a sharp increase in TiO2 was observed compared to similar spinels of rim1 (from 0.5 to 2 wt.%). The same was observed for phlogopite of all three parageneses: The TiO2 variations in rims 1 and 2 were (wt.%): 0.4–1, 1.7–2.2, and 0.7–2.1 for MHD, ShP, and Ecl, respectively. The chemical composition of the exotic layered phase (shown as an asterisk in Figure 2c,e and described in the section Classification) varied in different samples (wt.%): SiO2 12–28; TiO2 0–0.4; Al2O3 3–10; Cr2O3 0.5–3; FeO 1.5–12; MnO 0–0.3; MgO 32–44; CaO 0–1.7; Na2O 0–0.4; K2O 0–0.8; SO3 0–1.8; Cl 3–6.5. Unfortunately, the phase was impossible to identify according to the available databases.
- Rim 3. Average values for main components of the spinel and phlogopite of this rim for MHD and Ecl A are shown in Table 2. However, the composition of the minerals changes markedly from the border with the garnet to the border with kimberlite. In spinel, the content of iron and titanium increases, and an increase in ulvöspinel and magnetite molecules occurs [42]. The phlogopite chemistry of MHD varies as follows: Decreasing Al2O3 (15.1 to 8.73 wt.%), Cr2O3 (4.86 to 0 wt.%), and FeO (5.85 to 3.73 wt.%), but increasing MgO (21.9 to 27.4 wt.%) and BaO (0 to 6.51 wt.%), as the analysis of sample UV159/10-2 shows. Phlogopite with the highest BaO component contains 1.34 wt.% F, but phlogopite from kimberlite, adjacent to rim3, contains 0.9 wt.% Ba0, and is free from F. Rims of this type occur in three other samples; phlogopite in one of them (sample UV702/13) contains 5.43 wt.% Ca0 and 4.74 wt.% Cl, possibly due to fine ingrowths of calcite and an unidentified chlorine-containing phase (asterisk in Figure 2c,e); the probability of the presence of sodalite is excluded, because phlogopite does not contain Na2O. In the phlogopite of the Ecl A rim, the major-element composition changes gradually from garnet to kimberlite: Increasing Al2O3 (11.5 to 15.9 wt.%), MgO (from 19.1 to 25.6 wt.%), and BaO (0.55 to 3.74 wt.% in the outer zone) but decreasing FeO (9.13 to 2.86 wt.% in the outer zone). Phlogopite from kimberlite adjacent to the rim contains 0.9 wt.% BaO. Phlogopite from the rim encloses fine calcite grains that become fewer toward kimberlite: CaO decreasing from 7.5 to 0 wt.%. The minerals of rim3 on Ecl garnet are free from Cr2O3.
- MHD rim4. The Cr2O3 content in some rim zones is higher than that in garnet: Cr2O3 variations (wt.%) in garnets and their rims are 9.31–10.58 and 6.36–11.93, respectively (analyses of two points with extreme Cr2O3 content are shown in Table 2). Some rims have chains of spinel grains on the periphery (Figure 2i), but their sizes (about one micron or less) are too small for good analysis.
- MHD rim5. Seven different areas of rim5 were analyzed and the following results were obtained (wt.%): SiO2 29.87–37.89; TiO2 0–0.3; Al2O3 5.03–10.56; Cr2O3 6.85–10.76; FeO 6.56–8.10; MnO 0–0.49; MgO 6.52–15.60; CaO 11.46–24.09; Na2O 0–0.27; K2O 2.10–3.99; total 82.32–96.51. The deficit of the sum indicates the presence of water (most obvious in this situation), and volatile (fluorine, chlorine, etc.) or unaccounted components. This deficit cannot be explained by hydrogarnet due to the presence of 3 wt.% K2O; furthermore, it cannot be amphibole due to the large amount of CaO present, in addition to the complete absence of Na2O in the presence of K2O. Moreover, proceeding from the inhomogeneity (light and dark areas in the BSE mode), this is not one phase, but a mixture (apparently, very thin intergrowths) of several phases. The quantitative variations of the components make it possible to assume that these are the same phases, but in different proportions. The large amount of CaO suggests that one of the phases is calcite. The presence of 34–37 wt.% SiO2 simultaneously with 3–4 wt.% K2O and the deficiency of the sum indicates a high probability of the presence of phlogopite. However, the combination of calcite and phlogopite will result in a much larger deficit of the sum than in our analyses. In addition, the presence of calcite greatly reduces the silicon content. Therefore, potassium feldspar (a phase with high content of K, Si, and a normal sum of components), in addition to alumina clinopyroxene and Ca-plagioclase (phases with high content of Ca, Si, and a normal sum of components) are probably present here. The quantity of 7–9 wt.% Cr2O3, in addition to similar amounts of FeO and Al2O3, allows a small amount of Cr-spinel. Thus, according to our reasoning, rim5 comprises very thin intergrowths of calcite, phlogopite, and Cr-spinel. This is the most likely set of minerals based on common sense and the mineralogy of the other types of rim. Simple calculations give quite wide possible variations in the combinations of the above minerals: 5cpx + 4phl + 1cat + 1spl; 6cpx + 2phl + 3cat + 2spl + kfs; 4cpx + 5phl + 3cat + 2spl + Ca-pl; 3cpx + 2cat + 1spl + 2kfs, etc.
3.2.2. Rare-Earth Data
4. Discussion
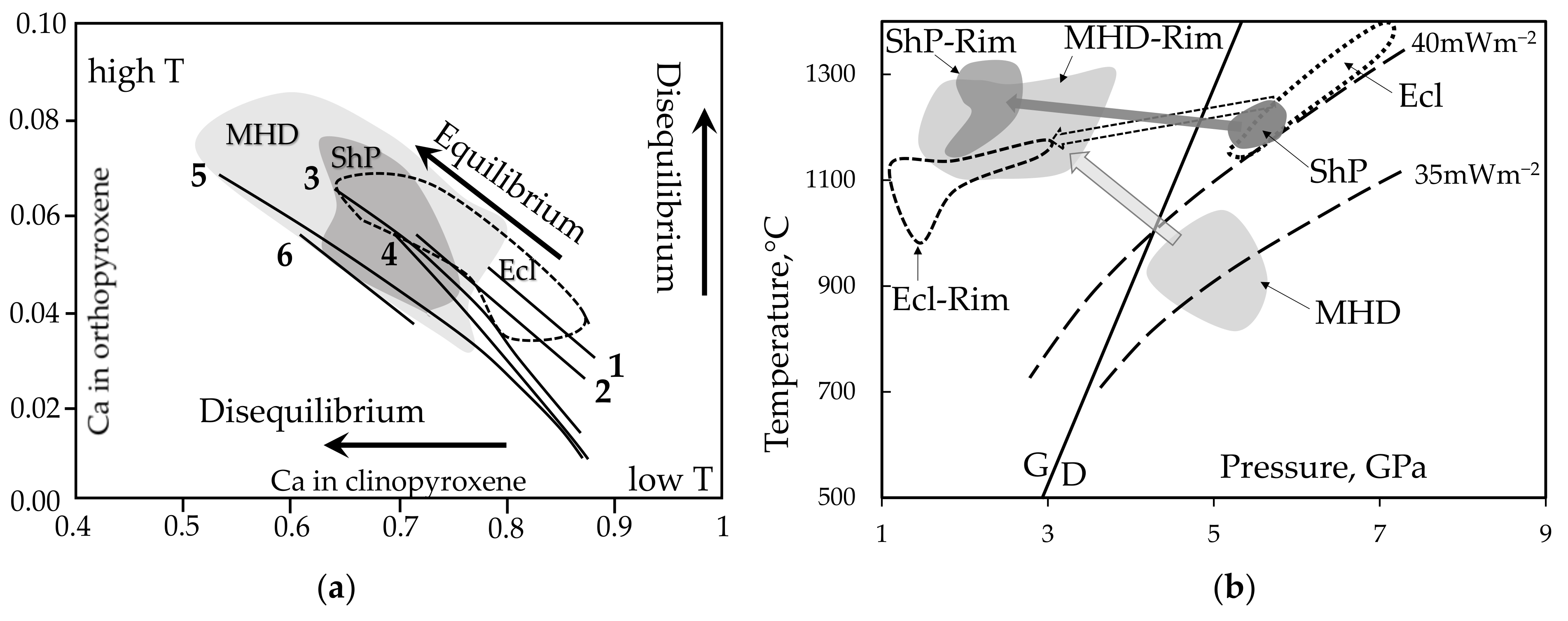
4.1. Distribution of Main Components in Minerals of Kelyphite Rims
4.2. Possible Rim Forming Reactions and Trends
4.3. PT Parameters of Phase Equilibrium in Rims
4.4. Geochemistry of Rim1/2, 3
5. Conclusions
- Garnets from different parageneses are surrounded by similar kelyphite rims of several types. Rims on garnets from MHD, ShP, and Ecl A and B samples are composed of the same main minerals.
- Garnets from the most depleted samples (MHD) have the most diverse rim types.
- Small sizes (µm) of mineral grains in rims, their zoning, and lack of equilibrium, idicate a high rate of formation. Variations in the compositions of minerals with the same name, and the variety of formation reactions with a change in the redox situation in some of these, attest to the multi-stage formation of kelyphite rims and the inversion of the fluid regime within small mantle areas.
- Rims 1 and 2 formed before the intrusion of kimberlite in the conditions of the active migration of alkali- and water-rich metasomatic fluids along cracks in mantle rocks. The presence of sodalite and an unidentified Cl-bearing silicate phase in rims of this type indicates the participation of chlorine during their formation.
- Rim4 formed at higher activity of K and water. The high chromium content indicates on the effect of the reduced fluid.
- Rim3 formed when kimberlites entrapped xenoliths.
- The origin of rim5 (“pockets” in rim1) remains unclear. These rims can represent zones of local carbonatization at the reaction front, which is consistent with inference (3).
- Each type of kelyphite demonstrates a clear enrichment with a certain component: Rim1—MgO and alkalis; rim2—TiO2; rim3—FeO and TiO2; rim4—Cr2O3; and rim5—Ca0, suggesting the stage-by-stage metasomatic enrichment of upper mantle rocks.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Paragenesis | Sample | SiO2 | TiO2 | Al2O3 | Cr2O3 | FeO | MnO | MgO | CaO | Na2O | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MHD | UV2/09 | 41.17 | 0.083 | 14.63 | 11.12 | 6.803 | 0.455 | 20.82 | 4.450 | 0.031 | 99.57 |
| UV12/01-1 | 41.36 | 0.000 | 16.55 | 8.540 | 7.186 | 0.376 | 21.9 | 3.377 | 0.012 | 99.30 | |
| UV26/10-1 | 41.67 | 0.002 | 16.22 | 8.894 | 6.906 | 0.403 | 22.64 | 2.351 | 0.015 | 99.12 | |
| UV49/10 | 41.79 | 0.017 | 16.73 | 9.309 | 6.592 | 0.432 | 23.65 | 1.227 | 0.030 | 99.79 | |
| UV70/01 | 41.79 | 0.044 | 16.35 | 9.418 | 6.673 | 0.351 | 22.83 | 1.754 | 0.042 | 99.27 | |
| UV71/10 | 41.24 | 0.036 | 15.41 | 10.76 | 6.911 | 0.363 | 22.75 | 1.643 | 0.00 | 99.12 | |
| UV112/09 | 41.59 | 0.411 | 18.43 | 5.664 | 7.277 | 0.358 | 20.71 | 4.537 | 0.122 | 99.12 | |
| UV113/09 | 41.65 | 0.000 | 16.65 | 9.322 | 6.275 | 0.342 | 22.93 | 2.507 | 0.040 | 99.72 | |
| UV134/09 | 41.11 | 0.052 | 15.96 | 9.838 | 6.569 | 0.354 | 23.39 | 2.176 | 0.039 | 99.51 | |
| UV157/10-2 | 40.96 | 0.003 | 14.31 | 11.85 | 7.017 | 0.434 | 23.35 | 1.682 | 0.056 | 99.67 | |
| UV159/10-1 | 41.6 | 0.033 | 16.14 | 9.206 | 6.684 | 0.438 | 22.8 | 2.041 | 0.052 | 99.01 | |
| UV159/10-2 | 41.02 | 0.033 | 15.07 | 10.58 | 6.927 | 0.416 | 19.99 | 5.485 | 0.021 | 99.55 | |
| UV162/10-1 | 40.09 | 0.119 | 13.13 | 13.13 | 6.903 | 0.439 | 18.92 | 6.536 | 0.054 | 99.32 | |
| UV162/10-2 | 41.44 | 0.007 | 16.02 | 9.924 | 6.994 | 0.35 | 22.72 | 2.252 | 0.003 | 99.71 | |
| UV167/09 | 41.05 | 0.000 | 15.04 | 11.10 | 6.856 | 0.363 | 23.45 | 2.131 | 0.012 | 100.0 | |
| UV177/09 | 40.55 | 0.441 | 14.68 | 9.680 | 7.015 | 0.263 | 20.3 | 6.384 | 0.032 | 99.36 | |
| UV233/10 | 40.32 | 0.091 | 14.84 | 10.72 | 6.900 | 0.393 | 20.98 | 4.788 | 0.070 | 99.09 | |
| UV343/08 | 40.74 | 0.016 | 14.56 | 11.47 | 7.281 | 0.410 | 22.01 | 3.375 | 0.065 | 99.94 | |
| UV517/11 | 41.14 | 0.171 | 15.20 | 10.18 | 6.561 | 0.371 | 20.72 | 4.677 | 0.052 | 99.09 | |
| UV524/11 | 40.51 | 0.073 | 14.41 | 11.67 | 6.785 | 0.400 | 19.82 | 4.890 | 0.043 | 98.60 | |
| UV528/11 | 40.33 | 0.007 | 16.06 | 9.688 | 7.139 | 0.437 | 21.97 | 3.496 | 0.033 | 99.17 | |
| UV586/11 | 41.16 | 0.113 | 15.80 | 9.992 | 7.212 | 0.394 | 21.26 | 3.697 | 0.052 | 99.67 | |
| UV588/11 | 40.79 | 0.021 | 15.25 | 10.56 | 7.010 | 0.423 | 22.36 | 2.859 | 0.018 | 99.29 | |
| UV658/11 | 41.37 | 0.043 | 16.42 | 8.719 | 7.000 | 0.413 | 20.77 | 4.579 | 0.002 | 99.33 | |
| UV667/11 | 41.08 | 0.061 | 15.13 | 10.88 | 6.96 | 0.401 | 21.37 | 3.540 | 0.000 | 99.43 | |
| UV708/11 | 41.11 | 0.090 | 15.35 | 10.04 | 6.478 | 0.32 | 22.78 | 3.123 | 0.040 | 99.36 | |
| UV803/09 | 40.79 | 0.078 | 14.19 | 12.2 | 7.182 | 0.408 | 21.04 | 3.797 | 0.020 | 99.70 | |
| UV818/09 | 41.14 | 0.135 | 15.94 | 9.754 | 7.061 | 0.415 | 21.12 | 3.612 | 0.077 | 99.26 | |
| UV823/09 | 41.17 | 0.019 | 16.44 | 9.285 | 6.868 | 0.416 | 23.51 | 1.793 | 0.000 | 99.50 | |
| UV833/09 | 40.64 | 0.050 | 15.70 | 10.72 | 6.980 | 0.36 | 20.36 | 5.000 | 0.030 | 99.88 | |
| UV837/11 | 40.74 | 0.769 | 14.83 | 8.675 | 6.846 | 0.311 | 21.03 | 6.083 | 0.073 | 99.37 | |
| UV850/09 | 41.32 | 0.089 | 15.78 | 9.956 | 6.385 | 0.324 | 22.26 | 3.002 | 0.048 | 99.18 | |
| ShP | UV831/09 | 42.55 | 0.520 | 20.99 | 1.910 | 7.870 | 0.000 | 21.77 | 4.390 | 0.000 | 100.0 |
| UV71/09 | 41.49 | 1.273 | 16.41 | 6.422 | 8.503 | 0.289 | 20.01 | 5.201 | 0.116 | 99.72 | |
| UV163/09 | 40.76 | 1.466 | 14.18 | 9.064 | 8.057 | 0.367 | 19.23 | 6.358 | 0.106 | 99.61 | |
| UV862/09 | 41.58 | 0.959 | 18.24 | 4.581 | 7.942 | 0.292 | 20.96 | 5.292 | 0.113 | 99.98 | |
| UV207/10 | 40.54 | 0.820 | 14.89 | 9.500 | 7.140 | 0.350 | 19.60 | 6.350 | 0.000 | 99.19 |
| Paragenesis | Sample | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MHD | UV2/09 | 0.370 | 2.859 | 0.543 | 3.037 | 0.754 | 0.231 | 0.812 | 0.063 | 0.304 | 0.076 | 0.250 | 0.034 | 0.017 | |
| UV12/01-1 | 0.303 | 4.293 | 1.149 | 8.144 | 0.869 | 0.246 | 0.418 | 0.027 | 0.155 | 0.044 | 0.168 | 0.036 | 0.523 | 0.186 | |
| UV26/10-1 | 0.128 | 2.170 | 0.643 | 4.265 | 0.612 | 0.131 | 0.532 | 0.002 | 0.195 | 0.012 | 0.008 | 0.007 | 0.032 | ||
| UV49/10 | 1.091 | 9.535 | 1.087 | 4.258 | 0.376 | 0.131 | 0.447 | 0.034 | 0.197 | 0.028 | 0.033 | 0.005 | 0.292 | 0.049 | |
| UV70/01 | 0.587 | 6.138 | 0.839 | 4.141 | 0.75 | 0.151 | 0.461 | 0.066 | 0.110 | 0.027 | 0.074 | 0.003 | 0.252 | 0.041 | |
| UV71/10 | 2.220 | 4.882 | 0.512 | 2.616 | 0.321 | 0.101 | 0.376 | 0.079 | 0.149 | 0.031 | 0.171 | 0.021 | 0.213 | 0.057 | |
| UV112/09 | 0.035 | 0.249 | 0.119 | 1.902 | 1.005 | 0.509 | 1.26 | 0.199 | 1.174 | 0.143 | 0.594 | 0.171 | 1.206 | 0.249 | |
| UV113/09 | 0.491 | 6.793 | 1.839 | 12.88 | 3.634 | 0.978 | 2.161 | 0.162 | 0.612 | 0.112 | 0.219 | 0.028 | 0.181 | 0.027 | |
| UV134/09 | 0.482 | 4.648 | 0.986 | 3.954 | 0.817 | 0.219 | 0.337 | 0.041 | 0.148 | 0.036 | 0.106 | 0.032 | 0.32 | 0.075 | |
| UV157/10-2 | 0.851 | 7.82 | 1.461 | 8.406 | 0.703 | 0.128 | 0.461 | 0.105 | 0.05 | 0.107 | 0.033 | 0.327 | 0.059 | ||
| UV159/10-1 | 0.928 | 14.56 | 3.859 | 22.73 | 3.55 | 0.842 | 1.606 | 0.154 | 0.515 | 0.126 | 0.283 | 0.041 | 0.509 | 0.131 | |
| UV159/10-2 | 1.245 | 7.679 | 1.454 | 8.59 | 1.238 | 0.181 | 0.826 | 0.085 | 0.319 | 0.068 | 0.286 | 0.031 | 0.815 | 0.067 | |
| UV162/10-1 | 0.468 | 1.693 | 0.303 | 1.499 | 0.283 | 0.122 | 0.527 | 0.081 | 0.507 | 0.149 | 0.575 | 0.075 | 1.096 | 0.208 | |
| UV162/10-2 | 0.099 | 2.126 | 0.675 | 5.256 | 0.756 | 0.179 | 0.283 | 0.003 | 0.015 | 0.003 | 0.112 | 0.034 | 0.286 | 0.066 | |
| UV167/09 | 0.741 | 4.695 | 0.829 | 2.297 | 0.202 | 0.048 | 0.163 | 0.02 | 0.067 | 0.016 | 0.056 | 0.019 | 0.132 | 0.005 | |
| UV177/09 | 0.490 | 2.187 | 0.446 | 4.235 | 1.876 | 0.532 | 1.121 | 0.033 | 0.289 | 0.093 | 0.285 | 0.044 | 0.547 | 0.159 | |
| UV233/10 | 0.133 | 1.919 | 0.529 | 3.534 | 2.672 | 0.823 | 1.828 | 0.136 | 0.152 | 0.044 | 0.027 | 0.014 | 0.127 | 0.199 | |
| UV343/08 | 0.535 | 4.069 | 1.058 | 9.185 | 2.002 | 0.334 | 0.648 | 0.054 | 0.260 | 0.087 | 0.100 | 0.043 | 0.387 | 0.171 | |
| UV517/11 | 0.347 | 4.683 | 1.284 | 8.685 | 2.666 | 0.86 | 2.599 | 0.310 | 1.563 | 0.210 | 0.600 | 0.085 | 0.725 | 0.023 | |
| UV524/11 | 0.702 | 4.283 | 0.62 | 3.046 | 0.536 | 0.264 | 0.807 | 0.107 | 0.661 | 0.206 | 0.599 | 0.073 | 0.863 | 0.081 | |
| UV528/11 | 1.256 | 12.469 | 2.734 | 15.283 | 2.255 | 0.324 | 0.822 | 0.026 | 0.275 | 0.106 | 0.290 | 0.057 | 0.667 | 0.117 | |
| UV586/11 | 0.249 | 2.861 | 0.844 | 6.512 | 1.154 | 0.321 | 0.868 | 0.087 | 0.627 | 0.106 | 0.214 | 0.053 | 0.715 | 0.093 | |
| UV588/11 | 0.761 | 8.799 | 2.074 | 10.39 | 1.214 | 0.238 | 0.529 | 0.037 | 0.213 | 0.045 | 0.116 | 0.02 | 0.436 | 0.234 | |
| UV658/11 | 0.269 | 2.402 | 0.356 | 1.560 | 0.071 | 0.031 | 0.253 | 0.039 | 0.247 | 0.105 | 0.273 | 0.042 | 0.660 | 0.108 | |
| UV667/11 | 1.844 | 14.39 | 2.459 | 11.68 | 1.496 | 0.285 | 0.755 | 0.051 | 0.302 | 0.133 | 0.346 | 0.049 | 0.597 | 0.096 | |
| UV708/11 | 0.093 | 1.281 | 0.501 | 4.910 | 3.047 | 0.896 | 1.830 | 0.188 | 0.764 | 0.073 | 0.191 | 0.035 | 0.150 | 0.311 | |
| UV803/09 | 1.911 | 5.423 | 0.357 | 1.441 | 0.35 | 0.104 | 0.444 | 0.047 | 0.416 | 0.138 | 0.531 | 0.081 | 0.950 | 0.141 | |
| UV818/09 | 0.299 | 3.464 | 0.862 | 5.683 | 1.165 | 0.262 | 0.843 | 0.088 | 0.631 | 0.082 | 0.324 | 0.042 | 0.472 | 0.072 | |
| UV823/09 | 0.152 | 1.305 | 0.196 | 1.359 | 0.434 | 0.037 | 0.129 | 0.014 | 0.098 | 0.011 | 0.032 | 0.026 | 0.253 | 0.256 | |
| UV833/09 | 0.303 | 1.606 | 0.227 | 0.897 | 0.208 | 0.049 | 0.13 | 0.031 | 0.229 | 0.049 | 0.168 | 0.029 | 0.320 | 0.078 | |
| UV837/11 | 0.190 | 2.170 | 0.669 | 6.254 | 1.407 | 0.468 | 0.762 | 0.120 | 0.654 | 0.009 | 0.054 | 0.315 | 1.919 | 0.098 | |
| UV850/09 | 0.106 | 2.220 | 0.711 | 7.060 | 3.071 | 0.879 | 2.049 | 0.225 | 1.034 | 0.186 | 0.619 | 0.081 | 0.910 | 0.164 | |
| ShP | UV831/09 | 0.093 | 0.476 | 0.116 | 0.986 | 0.614 | 0.298 | 1.501 | 0.296 | 2.584 | 0.593 | 1.932 | 0.308 | 2.050 | 0.345 |
| UV71/09 | 0.426 | 1.630 | 0.341 | 2.682 | 1.668 | 0.728 | 3.212 | 0.634 | 4.897 | 1.026 | 2.86 | 0.399 | 2.439 | 0.374 | |
| UV163/09 | 0.127 | 1.190 | 0.321 | 2.978 | 1.891 | 0.835 | 3.413 | 0.615 | 4.212 | 0.805 | 2.157 | 0.276 | 1.665 | 0.249 | |
| UV862/09 | 0.030 | 0.451 | 0.148 | 1.497 | 1.070 | 0.489 | 2.205 | 0.431 | 3.806 | 0.863 | 2.814 | 0.415 | 3.139 | 0.469 | |
| UV207/10 | 0.119 | 0.927 | 0.265 | 2.172 | 1.061 | 0.403 | 1.308 | 0.174 | 0.946 | 0.146 | 0.325 | 0.045 | 0.309 | 0.057 |
| Paragenesis | Sample | Rim | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MHD | UV49/10 | 1-2 | 0.051 | 0.545 | 0.063 | 0.347 | 0.05 | 0.007 | 0.017 | 0.006 | ||||||
| UV 71/10 | 1-2 | 2.938 | 5.299 | 0.463 | 2.145 | 0.218 | 0.099 | 0.366 | 0.056 | 0.152 | 0.03 | 0.171 | 0.01 | 0.311 | 0.056 | |
| UV667/11 | 1-2 | 6.56 | 9.995 | 0.598 | 1.881 | 0.137 | 0.163 | 0.103 | 0.005 | |||||||
| UV26/10-1 | 1-2 | 6.364 | 11.72 | 1.027 | 3.673 | 0.381 | 0.158 | 0.297 | 0.001 | 0.117 | 0.031 | 0.005 | 0.118 | |||
| UV12/01-1 | 1-2 | 0.078 | 0.586 | 0.168 | 1.189 | 0.04 | 0.044 | 0.055 | ||||||||
| UV134/09 | 1-2 | 0.549 | 1.076 | 0.079 | 0.142 | 0.131 | 0.041 | |||||||||
| UV157/10-2 | 3 | 18.24 | 36 | 3.233 | 9.079 | 2.053 | 0.647 | 1.695 | 0.2 | 1.186 | 0.192 | 0.501 | 0.058 | 0.276 | 0.019 | |
| ShP | UV831/09 | 1-2 | 2.761 | 7.502 | 0.865 | 4.034 | 1.287 | 0.554 | 1.959 | 0.366 | 2.338 | 0.473 | 1.385 | 0.225 | 1.362 | 0.257 |
| UV71/09 | 1-2 | 2.843 | 6.985 | 0.922 | 4.146 | 1.275 | 0.483 | 2.174 | 0.402 | 3.51 | 0.778 | 2.657 | 0.383 | 2.672 | 0.446 | |
| UV163/09 | 1-2 | 0.305 | 0.903 | 0.159 | 1.076 | 0.483 | 0.33 | 1.602 | 0.333 | 3.179 | 0.791 | 2.56 | 0.387 | 3.094 | 0.481 | |
| UV862/09 | 1-2 | 1.433 | 4.574 | 0.645 | 3.153 | 1.269 | 0.5 | 1.975 | 0.354 | 2.84 | 0.663 | 1.92 | 0.278 | 2.148 | 0.294 | |
| UV207/10 | 3 | 23.61 | 70.26 | 7.44 | 26.88 | 6.193 | 1.652 | 4.907 | 0.618 | 3.153 | 0.643 | 1.697 | 0.261 | 1.699 | 0.319 |
References
- Müller, H. Geognostische Skizze der Greifendorfer Serpentin Partie. In Neues Jahrbuch für Mineralogie, Geognosie, Geologie und Petrefaktenkunde; Schweizerbarfscke Verlagshandlung und Druckerei: Stuttgart, Germany, 1846; pp. 257–288. [Google Scholar]
- Delesse, A. Mémoire sur la constitution minéralogique et chimique des roches des Vosges. Serpentine des Vosges. Ann. Mines 1850, 18, 309–356. [Google Scholar]
- Hochstetter, F. Geognostische Studien aus dem Böhmerwalde. Jahrb. K. K. Geol. Reichsanst. 1854, 5, 1–67. [Google Scholar]
- Schrauf, A. Beiträge zur Kenntniss des Associations Kreises der Magnesia Silicate. Z. Krist. Miner. 1882, 6, 321–388. [Google Scholar]
- Hezner, L. Ein Beitrag zur Kenntnis der Eklogite und Amphibolite mit besonderer Berücksichtigung der Vorkommnisse des mittleren Oetztals. Tschermaks Miner. Petrogr. Mitt. 1903, 2, 437–471. [Google Scholar]
- Godard, G.; Martin, S. Petrogenesis of kelyphites in garnet peridotites: A case study from the Ulten zone, Italian Alps. J. Geodyn. 2000, 30, 117–145. [Google Scholar] [CrossRef]
- Obata, M. Kelyphite and symplectite: Textural and mineralogical diversities and universality, and a new dynamic view of their structural formation. In New Frontiers in Tectonic Research—General Problems, Sedimentary Basins and Island Arcs; Sharkov, E., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-595-2. Available online: http://www.intechopen.com/books/new-frontiers-in-tectonic-research-general-problems-sedimentarybasins-and-island-arcs/kelyphite-and-symplectite-textural-and-mineralogical-diversities-and-universality-and-anew-dynamic1 (accessed on 4 June 2021). [CrossRef]
- Obata, M. Material transfer and local equilibria in a zoned kelyphite from a garnet pyroxenite, Ronda, Spain. J. Petrol. 1994, 35, 271–287. [Google Scholar] [CrossRef]
- Altherr, R.; Kalt, A. Metamorphic evolution of ultrahigh-pressure garnet peridotite from the Variscan Vosges Mts. (France). Chem. Geol. 1996, 134, 27–47. [Google Scholar] [CrossRef]
- Dégi, J.; Abart, R.; Kalman, T.; Bali, E.; Wirth, R.; Rhede, D. Symplectite formation during decompression induced garnet breakdown in lower crustal mafic granulite xenoliths: Mechanisms and rates. Contrib. Mineral. Petrol. 2010, 159, 293–314. [Google Scholar] [CrossRef]
- Zang, Q.; Enami, M.; Suwa, K. Aluminian orthopyroxene in pyrometamorphosed garnet megacryst from Liaoning and Shandong provinces, northeast China. Eur. J. Mineral. 1993, 5, 153–164. [Google Scholar] [CrossRef]
- Fermor, L.L. Preliminary note on the origin of meteorites. J. Proc. Asiat. Soc. Bengal 1912, 8, 315–324. [Google Scholar]
- Sobolev, N.V.; Lodocnikova, V.S. Mineralogy of garnet peridotites. Rus. Geol. Geophys. 1962, 6, 52–59. (In Russian) [Google Scholar]
- Fediukova, E. Kelyphitic reaction rims in garnet peridotites. Acta Univ. Carol. Geol. 1979, 3–4, 185–192. [Google Scholar]
- Fiala, Y.; Padera, K. The chemistry of the minerals of the pyrope dunite from Borehole T-7 near Stare (Bohemia). TMPM 1977, 24, 205–219. [Google Scholar] [CrossRef]
- Kharkiv, A.D.; Vishnevsky, A.A. Features of garnet kellitization from xenoliths of deep rocks in kimberlites. Zap. Vsesoyuznogo Mineral. Obs. 1989, 118, 27–37. (In Russian) [Google Scholar]
- Dawson, Y.B.; Smith, I.V. Occurense of diamond in a micagarnet lhersolite xenoliths from kimberlite. Nature 1975, 5501, 580–581. [Google Scholar] [CrossRef]
- Treneva, N.V.; Vasilieva, G.L.; Ilupin, I.P. New data on garnets and keliphite rims from kimberlites of Yakutia. Dokl. Earth Sci. 1979, 247, 1471–1474. (In Russian) [Google Scholar]
- Spetsius, Z.V.; Griffin, W.L. Trace element composition of garnet kelyphites in xenoliths from Udachnaya as evidence of their origin. In Proceedings of the 7th International Kimberlite Conference, Cape Town, South Africa, 13–17 April 1998; Red Roof Design: Cape Town, South Africa, 1999; Volume 7, pp. 853–855. [Google Scholar]
- Foley, S.F.; Andronikov, A.V.; Jacob, D.E.; Melzer, S. Evidence from Antarctic mantle peridotite xenoliths for changes in mineralogy, geochemistry and geothermal gradients beneath a developing rift. Geochim. Cosmochim. Acta 2006, 70, 3096–3120. [Google Scholar] [CrossRef]
- Ilupin, I.P.; Levshov, P.P.; Rovsha, V.S. On the composition and genesis of kelyphite shells on garnet-pyrope in kimberlites of Yakutia. Uchenye Zap. Nauchno Issledovatelskogo Inst. Geol. Arktiki. Reg. Geol. 1969, 16, 45–52. (In Russian) [Google Scholar]
- Fiala, J. The distribution of elements in mineral phases of some garnet peridotites from the Bohemian massif. Krystalinikum 1966, 4, 31–53. [Google Scholar]
- Van der Wal, D.; Vissers, R.L.M. Structural petrology of the Ronda peridotite, SW Spain: Deformation history. J. Petrol. 1996, 37, 23–43. [Google Scholar] [CrossRef]
- Pokhilenko, N.P.; Sobolev, N.V.; Boyd, F.R.; Pearson, D.G.; Shimizu, N. Megacrystalline pyrope peridotites in the lithosphere of the Siberian Platform: Mineralogy, geochemical peculiarities and the problem of their origin. Rus. Geol. Geophys. 1993, 34, 1–84. [Google Scholar]
- Pokhilenko, L.N.; Malkovets, V.G.; Kuzmin, D.V.; Pokhilenko, N.P. New Data on the Mineralogy of Megacrystalline Pyrope Peridotite from the Udachnaya Kimberlite Pipe, Siberian Craton, Yakutian Diamondiferous Province. Dokl. Earth Sci. 2014, 454, 179–184. [Google Scholar] [CrossRef]
- Howarth, G.H.; Barry, P.H.; Pernet-Fisher, J.F.; Baziotis, I.P.; Pokhilenko, N.P.; Pokhilenko, L.N.; Bodnar, R.J.; Taylor, L.A.; Agashev, A.M. Superplume metasomatism: Evidence from Siberian mantle xenoliths. Lithos 2014, 184–185, 209–224. [Google Scholar] [CrossRef]
- Coleman, R.G.; Lee, D.E.; Beatty, L.B.; Brannock, W.W. Eclogites and eclogites: Their differences and similarities. Geol. Soc. Am. Bull. 1965, 76, 483–508. [Google Scholar] [CrossRef]
- Taylor, L.A.; Neal, C.R. Eclogites with oceanic crustal and mantle signatures from the Bellsbank kimberlite, South Africa, part I: Mineralogy, petrography, and whole-rock chemistry. J. Geol. 1989, 97, 551–567. [Google Scholar] [CrossRef]
- MacGregor, I.D.; Carter, J.L. The chemistry of clinopyroxenes and garnets of eclogite and peridotite xenoliths from the Roberts Victor mine, South Africa. Phys. Earth Planet Int. 1970, 3, 391–397. [Google Scholar] [CrossRef]
- MaCandless, T.E.; Gurney, J.J. Sodium in garnet and potassium in clinopyroxene: Criteria for classifying mantle eclogites. In Kimberlite and Related Rocks: Proceedings of the Fourth International Kimberlite Conference (4 IKC), Perth, Australia, August 10–15 1986, Volume 2: Their Mantle/Crust Setting, Diamonds and Diamond Exploration; Geological Society of Australia Inc.: Hornsby, Australia, 1989; pp. 827–832. [Google Scholar]
- Agashev, A.M.; Pokhilenko, L.N.; Pokhilenko, N.P.; Shchukina, E.V. Geochemistry of eclogite xenoliths from the Udachnaya Kimberlite Pipe: Section of ancient oceanic crust sampled. Lithos 2018, 314–315, 187–200. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Kamenetsky, M.B.; Sharygin, V.V.; Faure, K.; Golovin, A.V. Chloride and carbonate immiscible liquids at the closure of the kimberlite magma evolution (Udachnaya-East kimberlite, Siberia). Chem. Geol. 2007, 237, 384–400. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Kamenetsky, M.B.; Golovin, A.V.; Sharygin, V.V.; Maas, R. Ultrafresh salty kimberlite of the Udachnaya–East pipe (Yakutia, Russia): A petrological oddity or fortuitous discovery? Lithos 2012, 152, 173–186. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Golovin, A.V.; Maas, R.; Giuliani, A.; Kamenetsky, M.B.; Weiss, Y. Towards a new model for kimberlite petrogenesis: Evidence from unaltered kimberlites and mantle minerals. Earth Sci. Rev. 2014, 139, 145–167. [Google Scholar] [CrossRef]
- Pokhilenko, L.N.; Golovin, A.V.; Sharygin, I.S.; Pokhilenko, N.P. Accessory Minerals of Mantle Xenoliths: First Finds of Cl-free K-Fe Sulfides. Dokl. Earth Sci. 2011, 440, 1404–1409. [Google Scholar] [CrossRef]
- Sharygin, I.S.; Golovin, A.V.; Pokhilenko, N.P. Djerfisherite in Kimberlites of the Kuoikskoe field as an indicator of enrichment of Kimberlite melts in chlorine. Dokl. Earth Sci. 2011, 436, 301–307. [Google Scholar] [CrossRef]
- Korolyuk, V.N.; Lavrent’ev, Y.G.; Usova, L.V.; Nigmatulina, E.N. JXA-8100 microanalyzer: Accuracy of analysis of rock-forming minerals. Rus. Geol. Geophys. 2008, 49, 165–168. [Google Scholar] [CrossRef]
- Lavrentev, Y.G.; Karmanov, N.S.; Usova, L.V. Electron probe microanalysis of minerals: Microanalyzer or scanning electron microscope? Rus. Geol. Geophys. 2015, 56, 1154–1161. [Google Scholar] [CrossRef]
- Norman, M.D.; Pearson, N.J.; Sharma, A.; Griffin, W.L. Quantitative Analysis of Trace Elements in Geological Materials by Laser Ablation ICPMS: Instrumental Operating Conditions and Calibration Values of NIST Glasses. Geostand. Newsl. 1996, 20, 247–261. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Available online: https://www.mindat.org/min-52356.html (accessed on 4 June 2021).
- Pokhilenko, L.N. Formation Sequence of Different Spinel Species in Megacrystalline Peridotites of the Udachnaya-East Kimberlite Pipe (Yakutia): Evidence for the Metasomatism of Depleted Mantle. Minerals 2019, 9, 607. [Google Scholar] [CrossRef]
- Pokhilenko, N.P.; Agashev, A.M.; Litasov, K.D.; Pokhilenko, L.N. Carbonatite metasomatism of peridotite lithospheric mantle: Implications for diamond formation and carbonatite-kimberlite magmatism. Rus. Geol. Geophys. 2015, 56, 280–295. [Google Scholar] [CrossRef]
- Agashev, A.M.; Ionov, D.A.; Pokhilenko, N.P.; Golovin, A.V.; Cherepanova, Y.; Sharygin, I.S. Metasomatism in lithospheric mantle roots: Constraints from whole-rock and mineral chemical composition of deformed peridotite xenoliths from kimberlite pipe Udachnaya. Lithos 2013, 160–161, 201–215. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S. The composition of the earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Aoki, K. Origin of phlogopite and potassic-richteritebearing peridotite xenoliths from South Africa. Contrib. Mineral. Petrol. 1975, 53, 145–156. [Google Scholar] [CrossRef]
- Safonov, O.; Butvina, V.; Limanov, E. Phlogopite-Forming Reactions as Indicators of Metasomatism in the Lithospheric Mantle. Minerals 2019, 9, 685. [Google Scholar] [CrossRef]
- Yudin, D.S.; Tomilenko, A.A.; Alifirova, T.A.; Travin, A.V.; Murzintsev, N.G.; Pokhilenko, N.P. Results of 40Ar/39Ar Dating of Phlogopites from Kelyphitic Rims around Garnet Grains (Udachnaya-Vostochnaya Kimberlite Pipe). Dokl. Earth Sci. 2016, 469, 728–731. [Google Scholar] [CrossRef]
- Brey, G.P. Fictive conductive geotherms beneath the Kaapvaal craton. In Proceedings of the 5th Intern Kimberlite Conference: Extended Abstracts; CPRM: Brasilia, Brazil, 1991; pp. 23–25. [Google Scholar]
- Becker, H. Petrological constraints on the cooling history of high-temperature garnet peridotite massifs in lower Austria. Contrib. Mineral. Petrol. 1997, 128, 272–286. [Google Scholar] [CrossRef]
- Brey, G.P.; Kohler, T.; Nickel, K.G. Geothermobarometry in Four-phase Lherzolites I. Experimental Results from 10 to 60 kb. J. Petrol 1990, 31, 1313–1352. [Google Scholar] [CrossRef]
- Brey, G.P.; Kohler, T. Geothermobarometry in four phase Iherzolites II. New thermobarometers. and practical assessment of existing thermobarometers. J. Petrol. 1990, 31, 1352–1378. [Google Scholar] [CrossRef]
- MacGregor, I.D. The system MgO-Al2O3-SiO2: Solubility of Al2O3 in enstatite for spinel and garnet peridotite compositions. Am. Mineral. 1974, 59, 110–119. [Google Scholar]
- Finnerty, A.A.; Boyd, F.R. Thermobarometry for garnet-periodotite xenoliths: A basis for upper-mantle stratigraphy. In Mantle Xenoliths; Nixon, P.H., Ed.; John Wiley and Sons: New York, NY, USA, 1987; pp. 381–402. [Google Scholar]
- O’Neill, H.S.C.; Wood, B.J. An experimental study of Fe-Mg partitioning between garnet and olivine and its calibration as a geothermometer. Contrib. Mineral. Petrol. 1979, 70, 59–70. [Google Scholar] [CrossRef]
- Grütter, H.S.; Latti, D.; Menzies, A. Cr-saturation arrays in concentrate garnet compositions from kimberlite and their use in mantle barometry. J. Petrol. 2006, 47, 801–820. [Google Scholar] [CrossRef]
- Kennedy, C.S.; Kennedy, G.C. The Equilibrium Boundary Between Graphite and Diamond. J. Geophys. Res. 1976, 81, 2467–2470. [Google Scholar] [CrossRef]
- Pollack, H.N.; Chapman, D.S. On the regional variation of heat flow, geotherms and lithospheric thickness. Tectonophysics 1977, 38, 279–296. [Google Scholar] [CrossRef]
- Vishnevsky, A.A. Kelyphites on garnets in mantle xenoliths and kimberlites: Compositions, genesis, petrological implications. In Extended Abstracts of 5th International Kimberlite Conference, Araxa, Brasilia, June 1991; CRPM: Brasilia, Brazil, 1991; pp. 571–572. [Google Scholar]
- Franz, L.; Brey, G.P.; Okrusch, M. Metasomatic reequilibration of mantle xenoliths from the Gibeon kimberlite province (Namibia). In International Kimberlite Conference: Extended Abstracts; United Institute of Geology, Geophysics and Mineralology, Siberian Branch of Russian Academy of Sciences: Novosibirsk, Russia, 1995; Volume 6, pp. 169–171. [Google Scholar]
- Vyshnevsky, O.A. In-situ kelyphitisation of pyrope inclusion in chromspinel from kimberlites (kimberlite pipe “Pivdenna”, Eastern Pryazovya). Notes Ukr. Mineral. Partnersh. 2009, 6, 107–112. (In Ukrainian) [Google Scholar]
- Pokhilenko, N.P.; Sobolev, N.V.; Kuligin, S.S.; Shimizu, N. Peculiarities of distribution of pyroxenite paragenesis garnets in Yakutian kimberlite and some aspects of the Evolution of the Siberian Craton lithospheric mantle. In Proceedings of the 7th International Kimberlite Conference, Cape Town, South Africa, 11–17 April 1998; Red Roof Design: Cape Town, South Africa, 1999; Volume 2, pp. 689–698. [Google Scholar]
- Misra, K.C.; Anand, M.; Taylor, L.A.; Sobolev, N.V. Multi-stage metasomatism of diamondiferous eclogite xenoliths from the Udachnaya kimberlite pipe, Yakutia, Siberia. Contrib. Mineral. Petrol. 2004, 146, 696–714. [Google Scholar] [CrossRef]
- Liu, Y.; Taylor, L.A.; Sarbadhikari, A.B.; Valley, J.W.; Ushikubo, T.; Spicuzza, M.J.; Kita, N.; Ketchum, R.; Carlson, W.; Shatsky, V.S.; et al. Metasomatic origin of diamonds in the world’s largest diamondiferous eclogite. Lithos 2009, 112, 1014–1024. [Google Scholar] [CrossRef]




| Rim | P/g | n | Opx | Cpx | Spl | Phl | Amp | Kfs | Pl | Sdl | Chl | Srp | Cal | Mag * | Su | Fe * | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rim1 | MHD | 27 | + | + | + | + | + | + | + | + | + | ||||||
| ShP | 5 | + | + | + | + | + | + | + | + | ||||||||
| Ecl-A | 8 | + | + | + | + | + | + | + | + | ||||||||
| Ecl-B | 5 | + | + | + | + | + | + | + | + | + | + | + | |||||
| Ecl-C | 4 | + | + | + | + | + | + | ||||||||||
| rim2 | MHD | 25 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| ShP | 5 | + | + | + | + | + | + | + | + | + | + | + | |||||
| Ecl-A | 3 | + | + | + | + | + | + | + | |||||||||
| Ecl-B | 3 | + | + | + | + | + | + | + | + | + | + | + | |||||
| Ecl-C | 1 | + | + | + | + | + | |||||||||||
| rim3 | MHD | 5 | + | + | inc | + | |||||||||||
| Ecl-A | 2 | + | inc | + | |||||||||||||
| rim4 | MHD | 4 | + | + | + | + | + | + | + | + | + | ||||||
| rim5 | MHD | 3 | + | + | + | + | + | + | + | + |
| P | L | M/n | SiO2 | TiO2 | Al2O3 | Cr2O3 | FeO | MnO | MgO | CaO | Na2O | K2O | Total | Mg # | Cr # | Ts |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MHD | rock | opx/2 | 58.5 | 0.01 | 0.41 | 0.32 | 4.39 | 0.06 | 36.1 | 0.07 | 0.09 | 0.00 | 100 | 0.94 | 0.34 | 0.01 |
| spl/8 | 0.00 | 0.34 | 7.00 | 61.2 | 19.6 | 0.23 | 11.5 | 0.00 | 0.00 | 0.00 | 99.8 | 0.57 | 0.85 | n.c. | ||
| rim1 | opx/39 | 49.3 | 0.05 | 8.19 | 4.21 | 7.05 | 0.45 | 28.7 | 1.75 | 0.12 | 0.09 | 99.9 | 0.88 | 0.26 | 0.28 | |
| cpx/44 | 49.4 | 0.24 | 8.00 | 3.67 | 3.97 | 0.40 | 16.0 | 16.8 | 1.24 | 0.14 | 100 | 0.88 | 0.24 | 0.11 | ||
| spl/16 | n.d. | 0.52 | 34.0 | 31.1 | 16.0 | 0.63 | 16.5 | 0.05 | n.d. | n.d. | 98.8 | 0.71 | 0.38 | n.c. | ||
| phl/11 | 41.1 | 0.38 | 10.9 | 2.16 | 4.73 | 0.05 | 24.2 | 0.49 | 0.37 | 9.09 | 93.5 | 0.90 | 0.12 | n.c. | ||
| rim2 | opx/42 | 53.5 | 0.05 | 5.13 | 2.17 | 6.15 | 0.38 | 30.6 | 1.62 | 0.18 | 0.05 | 99.8 | 0.90 | 0.22 | 0.16 | |
| cpx/35 | 52.7 | 0.26 | 4.86 | 2.45 | 3.62 | 0.35 | 16.8 | 17.4 | 1.49 | 0.02 | 100 | 0.89 | 0.25 | 0.10 | ||
| spl/48 | n.d. | 1.88 | 22.8 | 39.4 | 20.6 | 0.37 | 14.3 | 0.26 | n.d. | n.d. | 99.4 | 0.64 | 0.54 | n.c. | ||
| phl/45 | 40.4 | 1.02 | 12.8 | 2.17 | 4.20 | 0.04 | 23.4 | 0.22 | 0.36 | 9.36 | 93.9 | 0.91 | 0.10 | n.c. | ||
| rim3 | spl/29 | 0.09 | 12.7 | 5.36 | 8.01 | 54.7 | 2.23 | 12.6 | 0.09 | 0.00 | 0.00 | 95.8 | 0.59 | 0.53 | n.c. | |
| phl/21 | 37.0 | 0.89 | 13.7 | 3.69 | 5.25 | 0.10 | 22.9 | 0.18 | 0.09 | 9.51 | 93.2 | 0.89 | 0.11 | n.c. | ||
| p-g | 37.0 | 0.55 | 15.1 | 4.86 | 5.85 | 0.23 | 21.9 | 0.00 | 0.00 | 10.0 | 95.5 | 0.87 | 0.18 | n.c. | ||
| p-k | 40.4 | 0.00 | 8.73 | 0.00 | 3.73 | 0.00 | 27.4 | 0.00 | 0.00 | 9.5 | 96.3 | 0.93 | 0.00 | n.c. | ||
| rim4 | phl/11 | 33.2 | 0.47 | 11.2 | 10.3 | 6.05 | 0.44 | 21.1 | 0.06 | 0.04 | 8.29 | 91.1 | 0.64 | 0.00 | n.c. | |
| Crmin | 37.0 | 0.37 | 12.0 | 6.36 | 3.42 | 0.00 | 23.0 | 0.00 | 0.00 | 9.14 | 91.3 | 0.92 | 0.26 | n.c. | ||
| Crmax | 32.2 | 0.52 | 11.6 | 11.9 | 5.36 | 0.45 | 20.2 | 0.00 | 0.00 | 7.95 | 90.4 | 0.87 | 0.41 | n.c. | ||
| ShP | rock | opx/5 | 58.6 | 0.00 | 0.70 | 0.27 | 5.53 | 0.00 | 34.6 | 1.03 | 0.25 | 0.03 | 101 | 0.92 | 0.21 | 0.02 |
| cpx/4 | 54.5 | 0.40 | 2.38 | 0.92 | 3.42 | 0.00 | 18.3 | 18.9 | 1.25 | 0.00 | 99.9 | 0.90 | 0.21 | 0.01 | ||
| rim1 | opx/7 | 48.3 | 0.45 | 10.8 | 2.82 | 7.82 | 0.35 | 27.6 | 1.89 | 0.00 | 0.00 | 100 | 0.86 | 0.15 | 0.30 | |
| cpx/7 | 46.5 | 1.26 | 11.3 | 3.05 | 4.78 | 0.18 | 14.8 | 16.9 | 0.65 | 0.06 | 99.5 | 0.85 | 0.15 | 0.16 | ||
| spl/28 | n.d. | 0.55 | 44.2 | 20.6 | 14.13 | 0.09 | 18.4 | 0.01 | n.d. | n.d. | 97.9 | 0.76 | 0.24 | n.c. | ||
| phl/6 | 40.5 | 1.73 | 13.7 | 2.05 | 3.39 | 0.00 | 23.2 | 0.07 | 0.39 | 9.8 | 94.9 | 0.92 | 0.09 | n.c. | ||
| rim2 | opx/4 | 52.1 | 0.13 | 5.59 | 1.95 | 6.74 | 0.49 | 30.2 | 1.55 | 0.00 | 0.00 | 98.8 | 0.89 | 0.19 | 0.18 | |
| cpx/4 | 49.5 | 1.16 | 7.15 | 1.87 | 3.93 | 0.16 | 15.3 | 19.1 | 1.09 | 0.00 | 99.2 | 0.87 | 0.15 | 0.11 | ||
| spl/24 | n.d. | 2.10 | 23.8 | 38.7 | 19.7 | 0.13 | 15.0 | 0.04 | n.d. | n.d. | 99.4 | 0.67 | 0.53 | n.c. | ||
| phl/9 | 40.0 | 2.15 | 14.6 | 2.09 | 3.81 | 0.00 | 22.6 | 0.08 | 0.53 | 9.35 | 95.3 | 0.91 | 0.09 | n.c. | ||
| Ecl A | rock | cpx/19 | 55.0 | 0.21 | 3.03 | 0.16 | 4.16 | 0.08 | 15.5 | 19.2 | 2.40 | 0.03 | 99.8 | 0.87 | 0.03 | 0.00 |
| Ecl B | cpx/20 | 55.1 | 0.41 | 7.64 | 0.06 | 4.59 | 0.05 | 11.7 | 15.3 | 4.92 | 0.12 | 99.9 | 0.82 | 0.01 | 0.01 | |
| Ecl C | cpx/7 | 56.1 | 0.30 | 14.0 | 0.06 | 2.99 | 0.02 | 7.20 | 11.6 | 7.67 | 0.03 | 100 | 0.81 | 0.00 | 0.01 | |
| EclA, B | rim1 | opx/12 | 48.7 | 0.45 | 9.75 | 0.11 | 14.2 | 0.45 | 24.4 | 1.68 | 0.02 | 0.01 | 99.8 | 0.75 | 0.01 | 0.25 |
| cpx/15 | 46.7 | 0.84 | 11.0 | 0.10 | 9.74 | 0.34 | 12.6 | 18 | 0.67 | 0.04 | 100 | 0.7 | 0.01 | 0.24 | ||
| spl/16 | n.d. | 0.22 | 58.4 | 0.19 | 23.1 | 0.14 | 15.3 | 0.33 | n.d. | n.d. | 97.7 | 0.61 | 0.00 | n.c. | ||
| phl/13 | 37.5 | 0.7 | 17.6 | 0.02 | 14.0 | 0.38 | 14.2 | 0.54 | 0.45 | 8.14 | 93.5 | 0.64 | 0.00 | n.c. | ||
| rim2 | opx/2 | 52.0 | 0.15 | 6.41 | 0.00 | 12.0 | 0.53 | 27.4 | 1.61 | 0.00 | 0.00 | 100 | 0.8 | 0.00 | 0.18 | |
| cpx/12 | 48.4 | 0.61 | 7.95 | 0.17 | 10.3 | 0.45 | 13.9 | 17.0 | 0.72 | 0.00 | 99.4 | 0.71 | 0.01 | 0.18 | ||
| spl/27 | n.d. | 0.32 | 55.7 | 0.40 | 26.3 | 0.24 | 13.9 | 0.4 | n.d. | n.d. | 97.4 | 0.57 | 0.01 | n.c. | ||
| phl/4 | 37.8 | 2.1 | 15.3 | 0.55 | 7.48 | 0.00 | 20.4 | 0.00 | 0.55 | 9.21 | 93.3 | 0.83 | 0.02 | n.c. | ||
| rim3 | phl/10 | 39.3 | 0.54 | 12.8 | 0.18 | 5.83 | 0.08 | 23.4 | 3.39 | 0.16 | 8.83 | 94.5 | 0.87 | 0.01 | n.c. | |
| p-g | 37.7 | 0.62 | 11.5 | 0.00 | 9.13 | 0.45 | 19.1 | 7.58 | 0.26 | 7.28 | 94.2 | 0.79 | 0.00 | n.c. | ||
| p-k | 37.5 | 0.00 | 15.9 | 0.00 | 2.86 | 0.00 | 25.6 | 0.00 | 0.00 | 8.73 | 94.3 | 0.94 | 0.00 | n.c. | ||
| Ecl C | rim1 | cpx/7 | 44.9 | 0.68 | 11.4 | 0.00 | 14.5 | 0.45 | 8.52 | 18.8 | 0.49 | 0.03 | 99.7 | 0.51 | 0.00 | 0.26 |
| spl/2 | n.d. | 0.48 | 54.2 | 0.21 | 36.1 | 0.14 | 8.04 | 0.14 | n.d. | n.d. | 99.2 | 0.34 | 0.00 | n.c. | ||
| rim2 | cpx/1 | 46.8 | 0.33 | 7.78 | 0.00 | 14.7 | 0.21 | 10.2 | 18.7 | 0.00 | 0.00 | 98.8 | 0.55 | 0.00 | 0.19 | |
| spl/2 | n.d. | 0.71 | 47.3 | 0.15 | 44.0 | 0.37 | 5.31 | 0.10 | n.d. | n.d. | 97.9 | 0.24 | 0.00 | n.c. |
| Paragenesis | Sample | G/R | SiO2 | TiO2 | Al2O3 | Cr2O3 | FeO | MnO | MgO | CaO | Na2O | K2O | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MHD | UV12/01-1 | Grt | 41.4 | 0.00 | 16.6 | 8.54 | 7.19 | 0.38 | 21.9 | 3.38 | 0.01 | 0.00 | 99.3 |
| rim1 | 41.9 | 0.01 | 17.0 | 8.31 | 7.16 | 0.42 | 21.3 | 3.28 | 0.02 | 0.02 | 99.5 | ||
| UV26/10-1 | Grt | 41.7 | 0.00 | 16.2 | 8.89 | 6.91 | 0.40 | 22.6 | 2.35 | 0.02 | 0.01 | 99.1 | |
| rim1 | 41.8 | 0.00 | 16.1 | 9.23 | 6.97 | 0.36 | 22.4 | 2.28 | 0.00 | 0.00 | 99.2 | ||
| UV667/11 | Grt | 41.1 | 0.06 | 15.1 | 10.9 | 6.96 | 0.40 | 21.4 | 3.54 | 0.00 | 0.01 | 99.4 | |
| rim1 | 41.3 | 0.04 | 15.7 | 9.94 | 6.91 | 0.39 | 21.6 | 3.47 | 0.03 | 0.01 | 99.4 | ||
| UV159/10-2 | Grt | 41.1 | n.d. | 15.4 | 11.3 | 6.93 | 0.41 | 19.3 | 5.41 | 0.00 | 0.00 | 99.9 | |
| rim1 | 39.4 | 0.02 | 14.7 | 11.2 | 7.18 | 0.55 | 20.2 | 5.20 | 0.33 | 0.52 | 99.3 | ||
| UV157/10-1 | Grt | 41.4 | n.d. | 16.9 | 8.86 | 7.80 | 0.51 | 20.9 | 3.67 | 0.03 | 0.00 | 100 | |
| rim1 | 39.9 | 0.02 | 16.4 | 9.53 | 7.76 | 0.49 | 21.9 | 3.51 | 0.06 | 0.50 | 100 | ||
| UV526/11 | Grt | 41.0 | n.d. | 15.6 | 10.8 | 6.99 | 0.48 | 21.6 | 3.41 | 0.00 | 0.00 | 99.9 | |
| rim1 | 38.9 | n.d. | 14.8 | 10.9 | 7.28 | 0.66 | 22.5 | 3.29 | 0.10 | 1.22 | 99.7 | ||
| UV71/10 | Grt | 41.4 | n.d. | 15.3 | 10.8 | 7.50 | 0.44 | 22.7 | 1.67 | 0.00 | 0.00 | 99.7 | |
| rim1 | 37.9 | n.d. | 14.6 | 11.8 | 7.54 | 0.58 | 23.7 | 1.25 | 0.22 | 1.25 | 98.8 | ||
| UV850/09 | Grt | 41.4 | n.d. | 15.8 | 10.5 | 6.95 | 0.37 | 22.1 | 2.96 | 0.00 | 0.00 | 100 | |
| rim1 | 39.8 | n.d. | 15.6 | 10.6 | 6.96 | 0.00 | 22.1 | 1.90 | 0.43 | 1.61 | 98.9 | ||
| UV524/11 | Grt | 40.7 | n.d. | 14.1 | 11.9 | 7.50 | 0.67 | 20.0 | 4.69 | 0.00 | 0.00 | 99.6 | |
| rim1 | 37.0 | n.d. | 13.8 | 13.3 | 7.68 | 0.53 | 21.4 | 4.21 | 0.50 | 1.04 | 99.3 | ||
| UV113/09 | Grt | 41.8 | n.d. | 16.5 | 9.56 | 6.24 | 0.40 | 22.7 | 2.36 | 0.00 | 0.00 | 99.5 | |
| rim1 | 38.0 | n.d. | 15.7 | 9.85 | 7.02 | 0.62 | 24.4 | 1.59 | 0.72 | 1.85 | 99.7 | ||
| UV702/13 | Grt | 41.1 | n.d. | 15.0 | 11.3 | 7.06 | 0.52 | 22.1 | 2.42 | 0.00 | 0.00 | 99.6 | |
| rim1 | 36.8 | n.d. | 14.2 | 12.2 | 7.20 | 0.44 | 23.1 | 2.64 | 0.73 | 1.08 | 98.5 | ||
| UV704/13 | Grt | 41.1 | n.d. | 15.3 | 11.6 | 6.82 | 0.00 | 22.7 | 1.58 | 0.00 | 0.00 | 99.1 | |
| rim1 | 39.3 | n.d. | 14.7 | 11.3 | 6.99 | 0.53 | 23.1 | 1.44 | 0.54 | 0.73 | 98.8 | ||
| UV180/10 | Grt | 40.6 | n.d. | 13.6 | 13.1 | 7.10 | 0.52 | 17.5 | 7.79 | 0.00 | 0.00 | 101 | |
| rim1 | 37.0 | n.d. | 12.8 | 14.7 | 8.20 | 0.53 | 18.4 | 6.80 | 0.82 | 1.19 | 100 | ||
| UV86/01 | Grt | 42.4 | n.d. | 18.2 | 8.16 | 6.97 | 0.43 | 23.5 | 0.97 | 0.00 | 0.00 | 101 | |
| rim1 | 35.9 | n.d. | 18.0 | 8.73 | 7.90 | 0.43 | 25.3 | 0.74 | 0.45 | 0.49 | 98.0 | ||
| ShP | UV207/10 | Grt | 40.5 | 0.82 | 14.9 | 9.50 | 7.14 | 0.35 | 19.6 | 6.35 | 0.00 | 0.00 | 99.2 |
| rim1 | 38.5 | 0.87 | 13.2 | 9.66 | 7.26 | 0.52 | 20.0 | 6.52 | 0.66 | 0.70 | 97.9 | ||
| UV33/10 | Grt | 41.8 | 0.00 | 17.3 | 7.67 | 7.33 | 0.36 | 20.4 | 5.92 | 0.00 | 0.00 | 101 | |
| rim1 | 39.6 | 0.52 | 15.5 | 7.40 | 8.39 | 0.48 | 23.0 | 4.87 | 0.38 | 0.14 | 100 | ||
| UV831/09 | Grt | 42.9 | 0.45 | 21.3 | 1.94 | 8.01 | 0.34 | 22.1 | 4.44 | 0.00 | 0.00 | 101 | |
| rm1 | 39.2 | 0.93 | 18.8 | 2.48 | 7.09 | 0.25 | 22.9 | 5.43 | 1.79 | 1.37 | 101 | ||
| Ecl | UV66/09 | Grt-A | 41.4 | 1.23 | 22.1 | 0.00 | 11.4 | 0.32 | 17.1 | 6.03 | 0.28 | 0.00 | 100 |
| rim1 | 41.3 | 1.13 | 22.1 | 0.00 | 11.2 | 0.45 | 17.2 | 5.47 | 0.43 | 0.00 | 99.3 | ||
| UV156/10 | Grt-B | 40.0 | 0.17 | 22.0 | 0.00 | 13.7 | 0.32 | 12.8 | 9.51 | 0.00 | 0.00 | 98.4 | |
| rim1 | 39.2 | 0.52 | 18.0 | 0.00 | 11.8 | 0.37 | 13.2 | 7.47 | 1.20 | 2.04 | 93.8 | ||
| UV6/10 | Grt-B | 39.9 | n.d. | 22.1 | 0.00 | 16.2 | 0.30 | 9.63 | 11.0 | 0.00 | 0.00 | 99.1 | |
| rim1 | 40.4 | n.d. | 21.7 | 0.00 | 14.9 | 0.28 | 9.60 | 11.2 | 0.92 | 0.00 | 98.9 | ||
| UV13/10 | Grt-B | 40.2 | n.d. | 22.4 | 0.00 | 18.4 | 0.48 | 9.68 | 10.0 | 0.00 | 0.00 | 101 | |
| rim1 | 39.8 | n.d. | 22.2 | 0.00 | 17.7 | 0.37 | 9.63 | 10.2 | 0.97 | 0.00 | 101 | ||
| UV211/10 | Grt-B | 40.4 | 0.52 | 22.1 | 0.00 | 16.3 | 0.44 | 11.7 | 9.30 | 0.00 | 0.00 | 101 | |
| rim1 | 42.3 | 0.75 | 23.9 | 0.00 | 8.40 | 0.00 | 7.78 | 11.0 | 2.20 | 0.30 | 96.9 | ||
| UV70/09 | Grt-B | 41.4 | 0.70 | 22.5 | 0.00 | 11.9 | 0.00 | 13.0 | 10.8 | 0.00 | 0.00 | 100 | |
| rim1 | 39.7 | 0.62 | 22.3 | 0.00 | 11.1 | 0.00 | 13.6 | 11.4 | 0.50 | 0.00 | 99.2 | ||
| UV150/09 | Grt-B | 40.1 | 0.30 | 22.2 | 0.15 | 14.1 | 0.23 | 11.0 | 11.7 | 0.00 | 0.00 | 99.8 | |
| rim1 | 40.0 | 0.22 | 22.5 | 0.15 | 13.5 | 0.30 | 10.6 | 10.6 | 1.39 | 0.00 | 99.3 | ||
| UV134/10 | Grt-C | 39.8 | 0.00 | 22.4 | 0.00 | 11.2 | 0.00 | 4.69 | 22.1 | 0.00 | 0.00 | 100 | |
| rim1 | 39.8 | 0.38 | 22.6 | 0.00 | 10.9 | 0.00 | 4.53 | 21.8 | 0.55 | 0.00 | 101 | ||
| UV662/11 | Grt-C | 39.0 | 0.20 | 21.6 | 0.00 | 19.0 | 0.40 | 5.62 | 13.6 | 0.00 | 0.00 | 99.5 | |
| rim1 | 39.0 | 0.00 | 20.9 | 0.00 | 16.9 | 0.49 | 4.96 | 14.8 | 0.00 | 0.00 | 97.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokhilenko, L. Kelyphite Rims on Garnets of Contrast Parageneses in Mantle Xenoliths from the Udachnaya-East Kimberlite Pipe (Yakutia). Minerals 2021, 11, 615. https://doi.org/10.3390/min11060615
Pokhilenko L. Kelyphite Rims on Garnets of Contrast Parageneses in Mantle Xenoliths from the Udachnaya-East Kimberlite Pipe (Yakutia). Minerals. 2021; 11(6):615. https://doi.org/10.3390/min11060615
Chicago/Turabian StylePokhilenko, Lyudmila. 2021. "Kelyphite Rims on Garnets of Contrast Parageneses in Mantle Xenoliths from the Udachnaya-East Kimberlite Pipe (Yakutia)" Minerals 11, no. 6: 615. https://doi.org/10.3390/min11060615
APA StylePokhilenko, L. (2021). Kelyphite Rims on Garnets of Contrast Parageneses in Mantle Xenoliths from the Udachnaya-East Kimberlite Pipe (Yakutia). Minerals, 11(6), 615. https://doi.org/10.3390/min11060615





