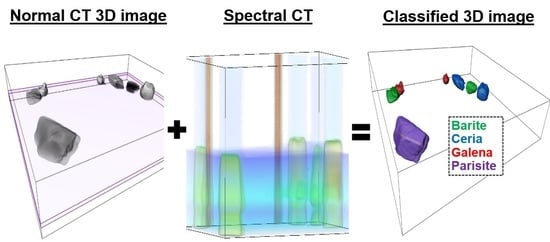
Spectral Tomography for 3D Element Detection and Mineral Analysis
Abstract
1. Introduction
2. Materials and Methods
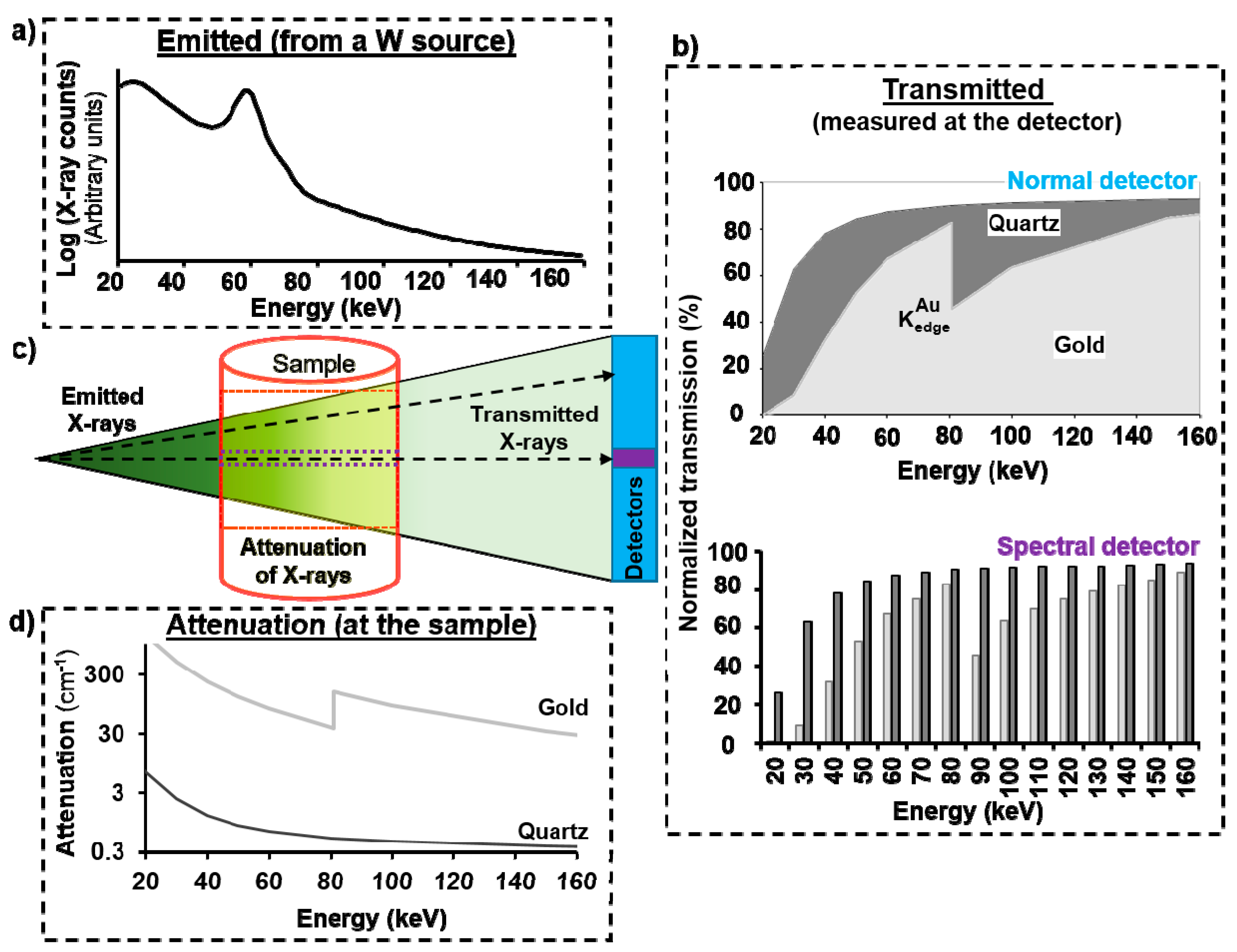
2.1. Principle of Sp-CT
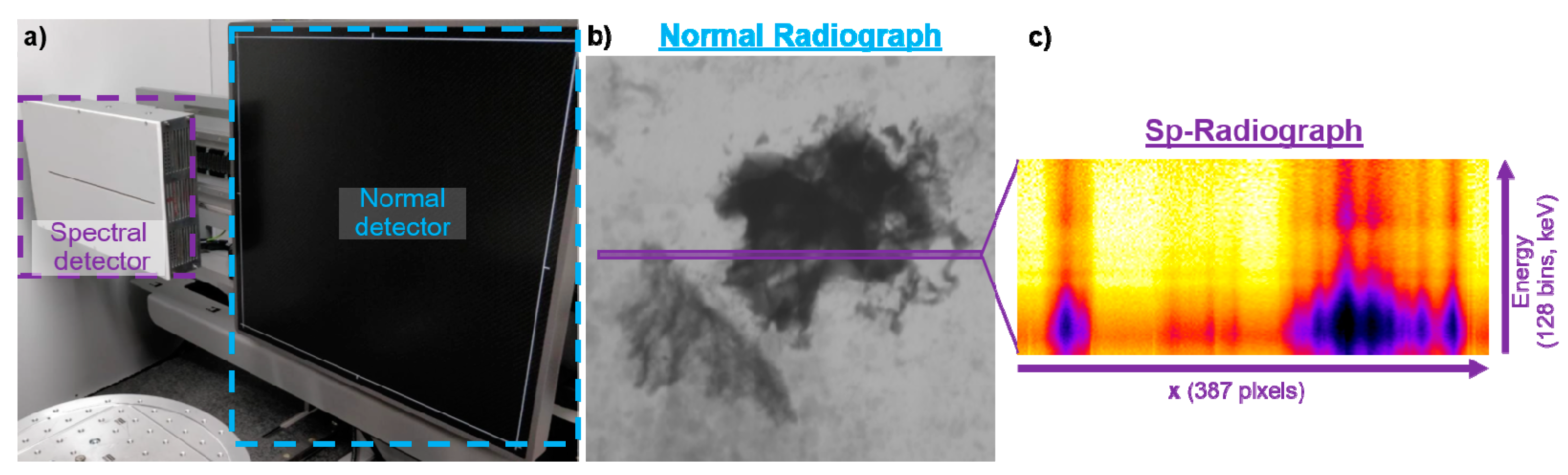
2.2. Equipment
2.3. How It Works in Practise
2.4. Experimental Conditions
3. Results
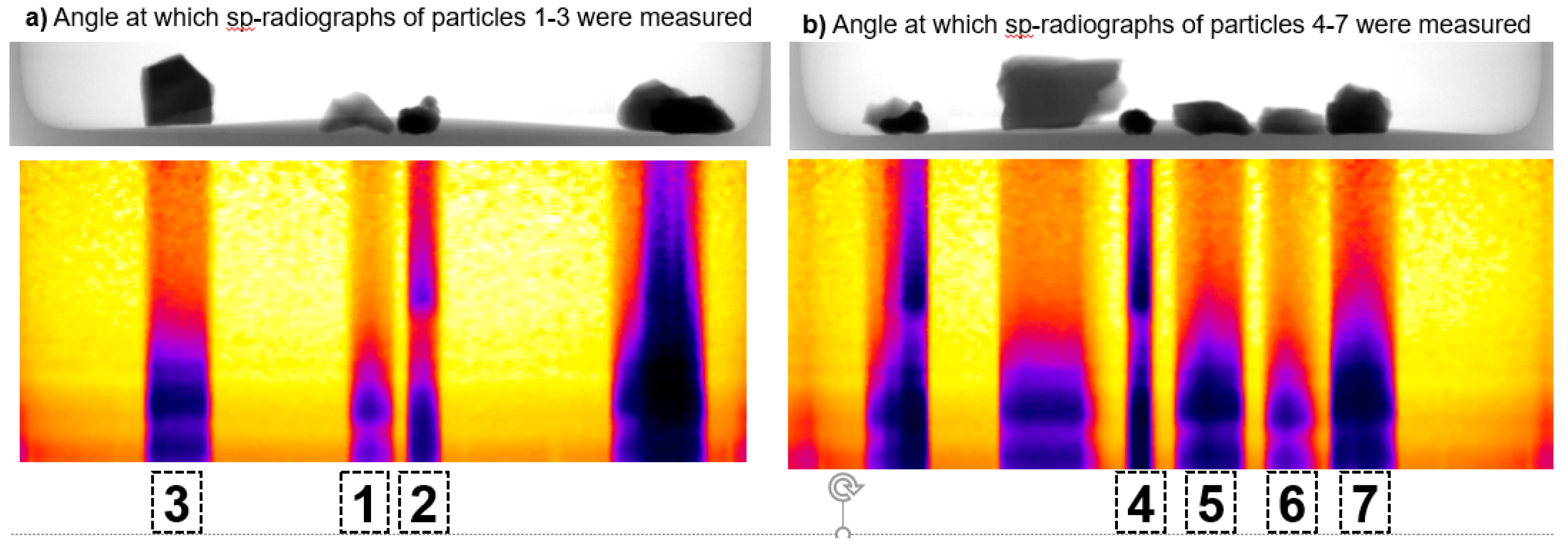
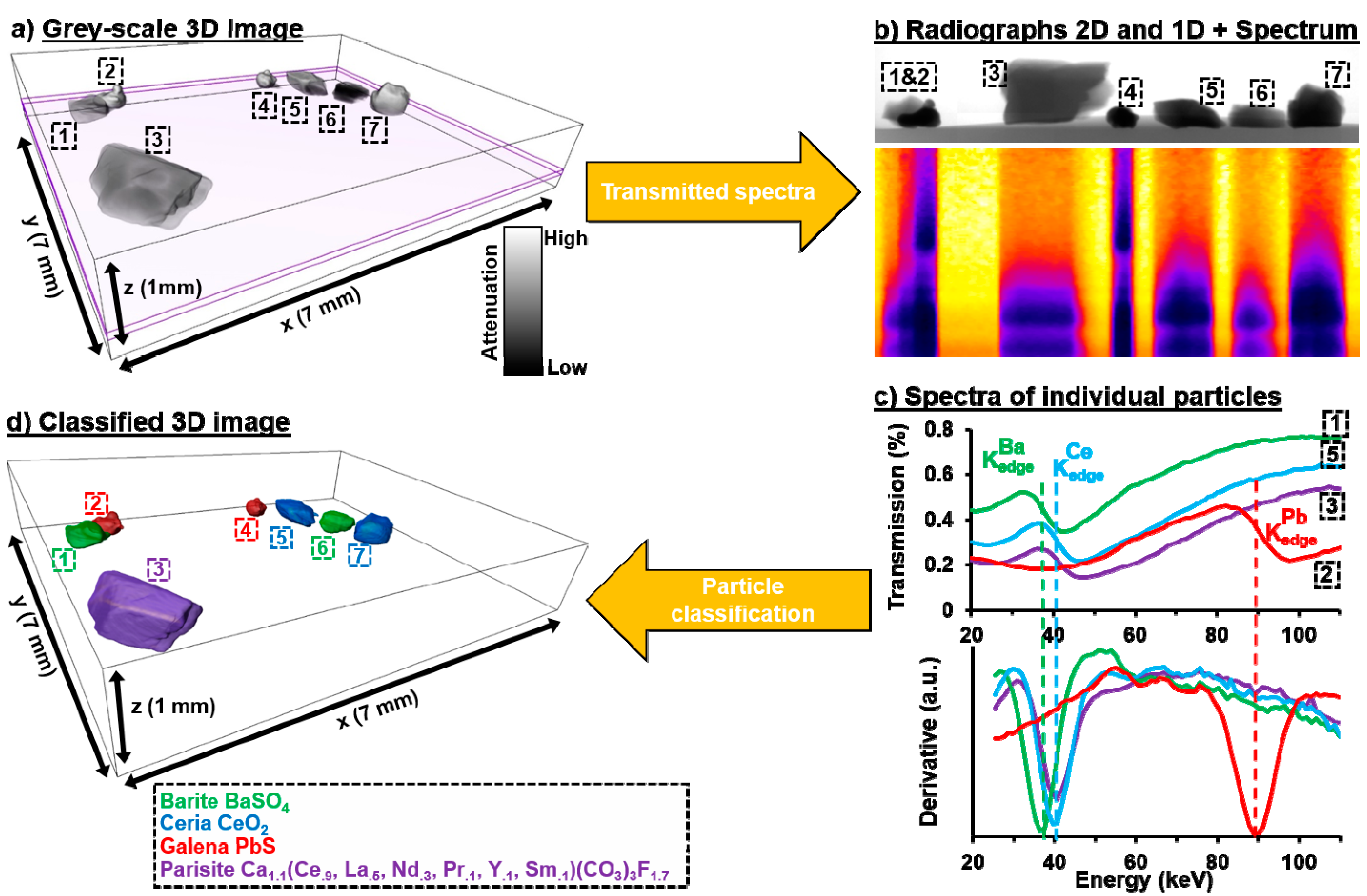
3.1. Spectral Radiographs
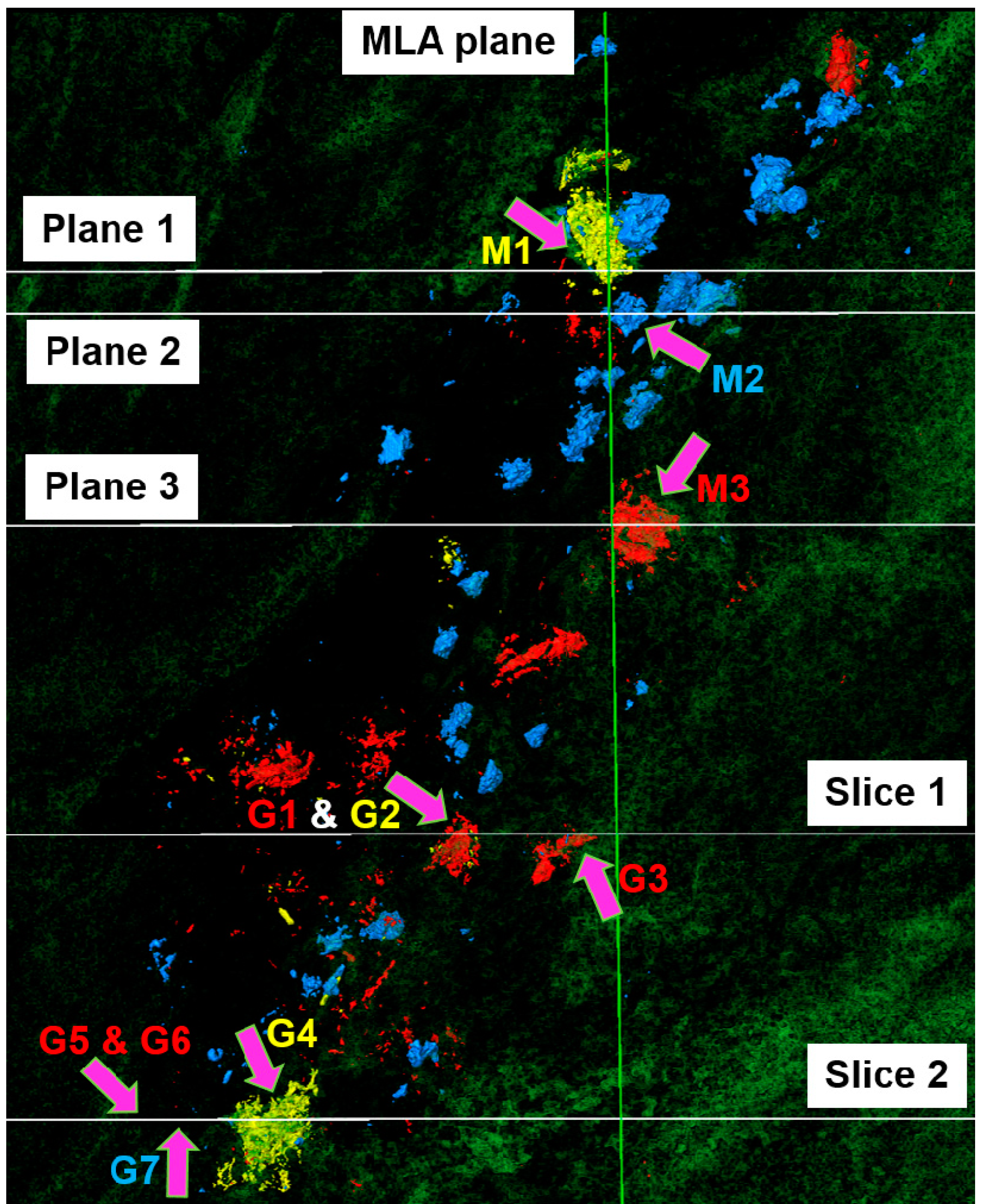
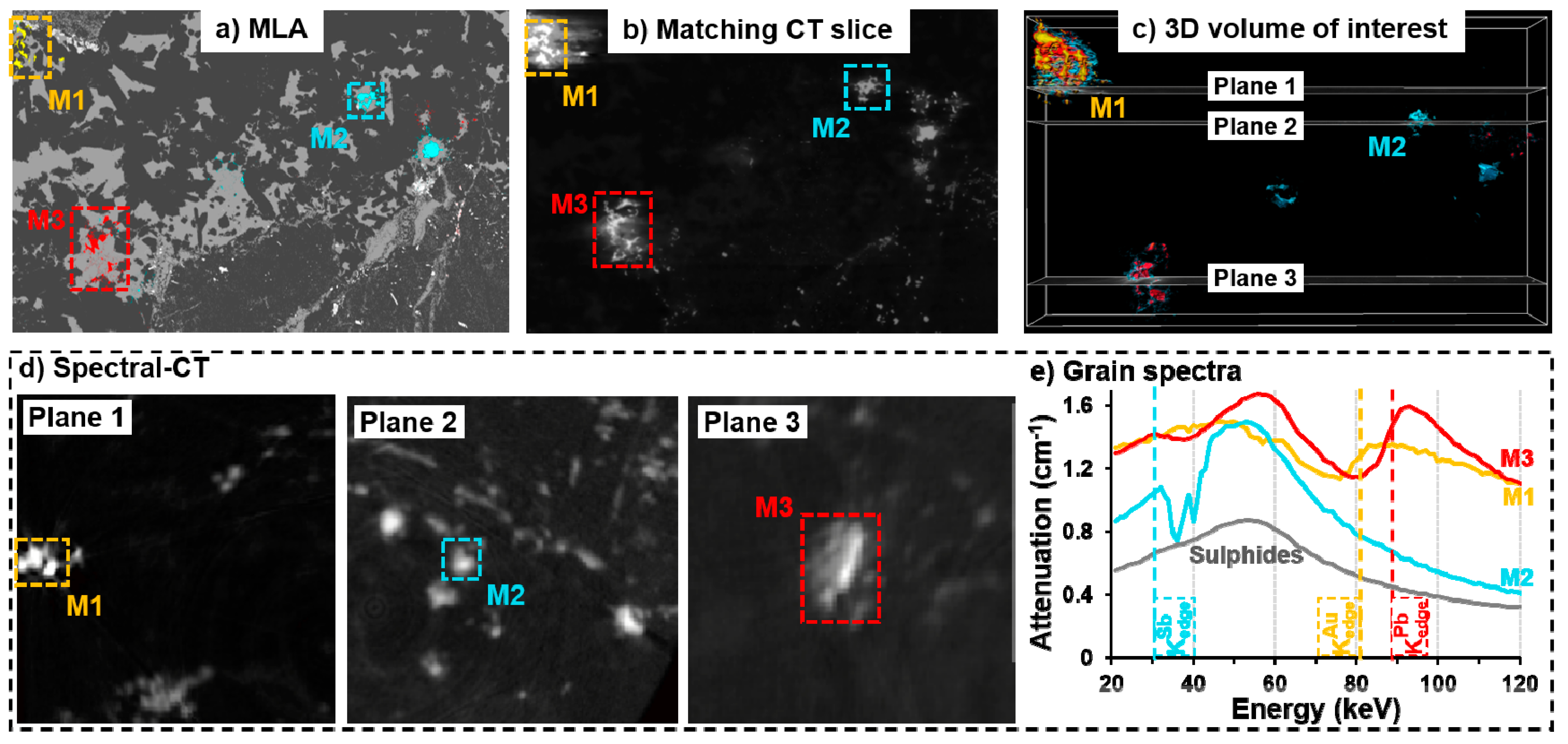
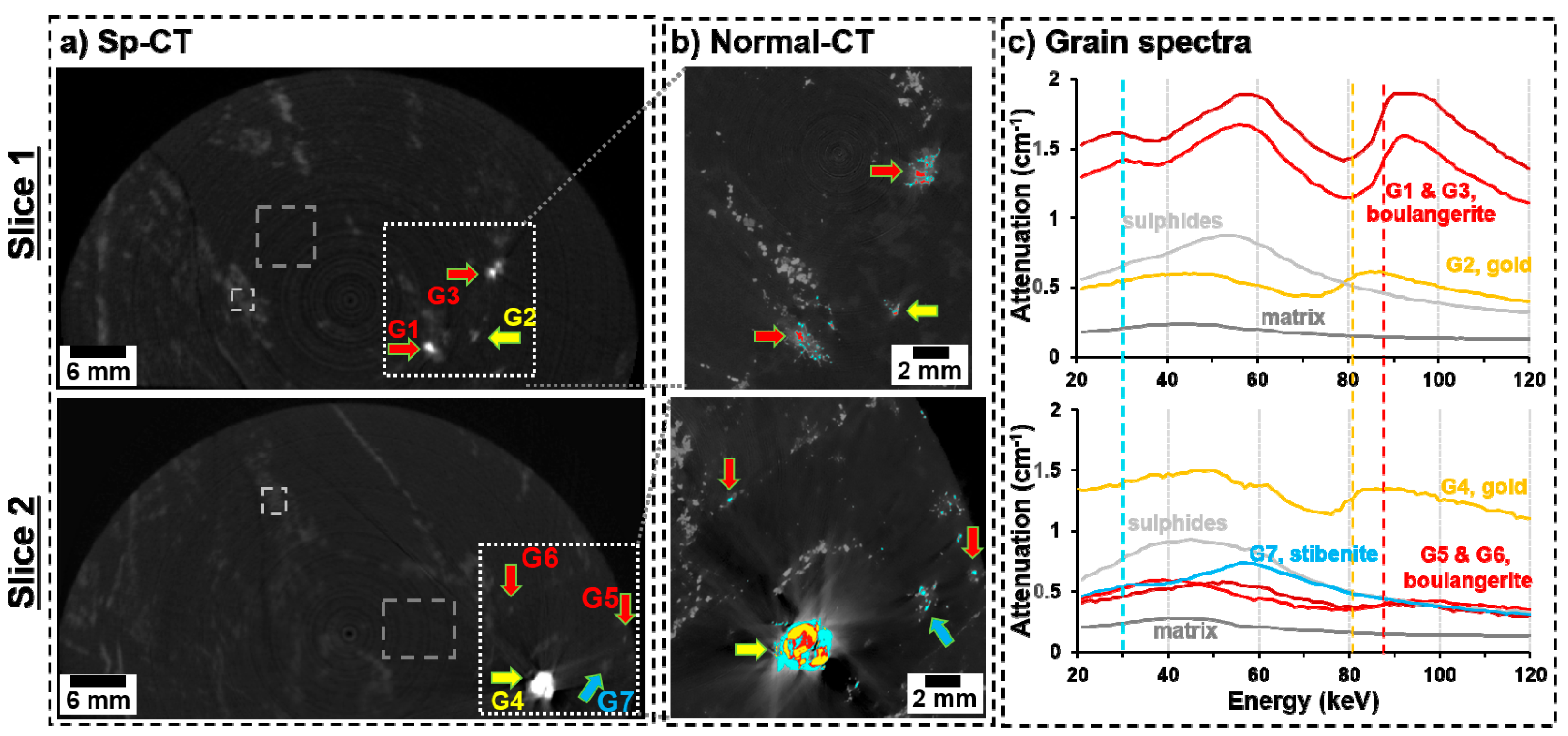
3.2. Spectral Tomography
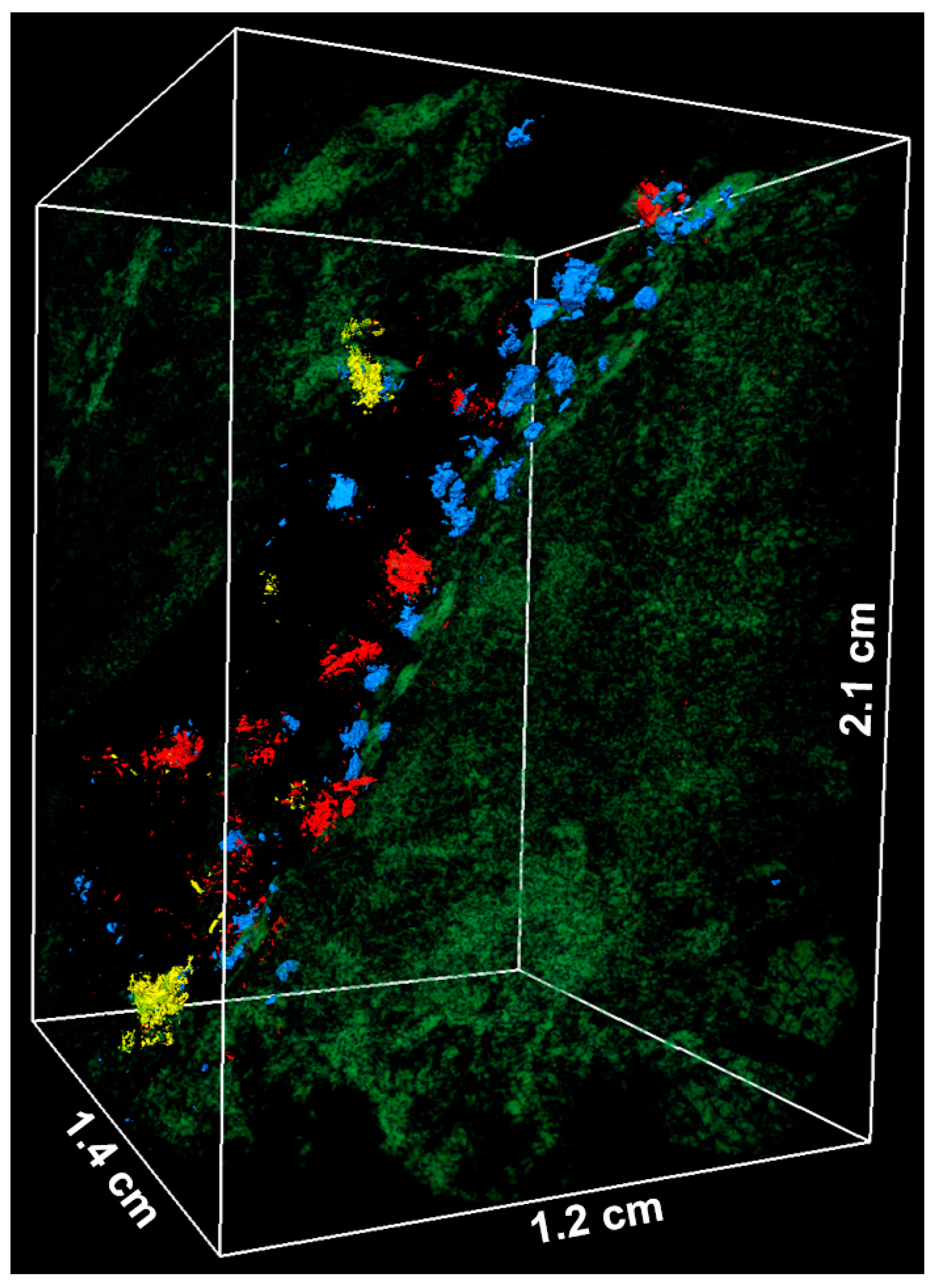
3.3. Applications and Limitations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Withers, P.J.; Bouman, C.; Carmignato, S.; Cnudde, V.; Grimaldi, D.; Hagen, C.K.; Maire, E.; Manley, M.; Du Plessis, A.; Stock, S.R. X-ray computed tomography. Nat. Rev. Methods Primers 2021, 1, 18. [Google Scholar] [CrossRef]
- Cnudde, V.; Boone, M.N. High-resolution X-ray computed tomography in geosciences: A review of the current technology and applications. Earth Sci. Rev. 2013, 123, 1–17. [Google Scholar] [CrossRef]
- Guntoro, P.I.; Ghorbani, Y.; Koch, P.-H.; Rosenkranz, J. X-ray Microcomputed Tomography (µCT) for Mineral Characterization: A Review of Data Analysis Methods. Minerals 2019, 9, 183. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, C.L.; Miller, J.D. Quantitative analysis of exposed grain surface area for multiphase particles using X-ray microtomography. Powder Technol. 2017, 308, 368–377. [Google Scholar] [CrossRef]
- Reyes, F.; Lin, Q.; Cilliers, J.J.; Neethling, S.J. Quantifying mineral liberation by particle grade and surface exposure using X-ray microCT. Miner. Eng. 2018, 125, 75–82. [Google Scholar] [CrossRef]
- Reyes, F.; Lin, Q.; Udoudo, O.; Dodds, C.; Lee, P.D.; Neethling, S.J. Calibrated X-ray micro-tomography for mineral ore quantification. Miner. Eng. 2017, 110, 122–130. [Google Scholar] [CrossRef]
- Godinho, J.; Kern, M.; Renno, A.D.; Gutzmer, J. Volume quantification in interphase voxels of ore minerals using 3D imaging. Miner. Eng. 2019, 144, 106016. [Google Scholar] [CrossRef]
- Miller, J.D.; Lin, C.L. X-ray tomography for mineral processing technology—3D particle characterization from mine to mill. Miner. Metall. Process. 2018, 35, 1–12. [Google Scholar] [CrossRef]
- Miller, J.D.; Lin, C.-L.; Hupka, L.; Al-Wakeel, M.I. Liberation-limited grade/recovery curves from X-ray micro CT analysis of feed material for the evaluation of separation efficiency. Int. J. Miner. Process. 2009, 93, 48–53. [Google Scholar] [CrossRef]
- Gajjar, P.; Jørgensen, J.S.; Godinho, J.R.A.; Johnson, C.G.; Ramsey, A.; Withers, P.J. New software protocols for enabling laboratory based temporal CT. Rev. Sci. Instrum. 2018, 89, 93702. [Google Scholar] [CrossRef]
- Bam, L.; Miller, J.; Becker, M. A Mineral X-ray Linear Attenuation Coefficient Tool (MXLAC) to Assess Mineralogical Differentiation for X-ray Computed Tomography Scanning. Minerals 2020, 10, 441. [Google Scholar] [CrossRef]
- Fandrich, R.; Gu, Y.; Burrows, D.; Moeller, K. Modern SEM-based mineral liberation analysis. Int. J. Miner. Process. 2007, 84, 310–320. [Google Scholar] [CrossRef]
- Tuşa, L.; Kern, M.; Khodadadzadeh, M.; Blannin, R.; Gloaguen, R.; Gutzmer, J. Evaluating the performance of hyperspectral short-wave infrared sensors for the pre-sorting of complex ores using machine learning methods. Miner. Eng. 2020, 146, 106150. [Google Scholar] [CrossRef]
- Pankhurst, M.J.; Fowler, R.; Courtois, L.; Nonni, S.; Zuddas, F.; Atwood, R.C.; Davis, G.R.; Lee, P.D. Enabling three-dimensional densitometric measurements using laboratory source X-ray micro-computed tomography. SoftwareX 2018, 7, 115–121. [Google Scholar] [CrossRef]
- Sittner, J.; Godinho, J.R.A.; Renno, A.D.; Cnudde, V.; Boone, M.; de Schryver, T.; van Loo, D.; Merkulova, M.; Roine, A.; Liipo, J. Spectral X-ray computed micro tomography: 3-dimensional chemical imaging. X-Ray Spectrom 2021, 50, 92–105. [Google Scholar] [CrossRef]
- Rebuffel, V.; Tartare, M.; Brambilla, A.; Moulin, V.; Verger, L. Multi-energy X-ray Techniques for NDT: A New Challenge. In Proceedings of the 11th European Conference on Non-Destructive Testing, Prague, Czech Republic, 6–10 October 2014. [Google Scholar]
- Roessl, E.; Cormode, D.; Brendel, B.; Jürgen Engel, K.; Martens, G.; Thran, A.; Fayad, Z.; Proksa, R. Preclinical spectral computed tomography of gold nano-particles. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2011, 648, S259–S264. [Google Scholar] [CrossRef]
- Cuadros, A.; Ma, X.; Arce, G.R. Compressive spectral X-ray tomography based on spatial and spectral coded illumination. Opt. Express 2019, 27, 10745–10764. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.; Lima, I.; Lopes, R.T. Methodology for attainment of density and effective atomic number through dual energy technique using microtomographic images. Appl. Radiat. Isot. 2014, 89, 6–12. [Google Scholar] [CrossRef]
- Egan, C.K.; Jacques, S.D.M.; Wilson, M.D.; Veale, M.C.; Seller, P.; Beale, A.M.; Pattrick, R.A.D.; Withers, P.J.; Cernik, R.J. 3D chemical imaging in the laboratory by hyperspectral X-ray computed tomography. Sci. Rep. 2015, 5, 15979. [Google Scholar] [CrossRef] [PubMed]
- Roessl, E.; Proksa, R. K-edge imaging in x-ray computed tomography using multi-bin photon counting detectors. Phys. Med. Biol. 2007, 52, 4679–4696. [Google Scholar] [CrossRef] [PubMed]
- Warr, R.; Ametova, E.; Cernik, R.J.; Fardell, G.; Handschuh, S.; Jørgensen, J.S.; Papoutsellis, E.; Pasca, E.; Withers, P.J. Enhanced Hyperspectral Tomography for Bioimaging by Spatiospectral Reconstruction. 2021. Available online: http://arxiv.org/pdf/2103.04796v1 (accessed on 10 March 2021).
- Godinho, J.R.; Piazolo, S.; Stennett, M.C.; Hyatt, N.C. Sintering of CaF2 pellets as nuclear fuel analog for surface stability experiments. J. Nucl. Mater. 2011, 419, 46–51. [Google Scholar] [CrossRef]
- Schulz, B.; Sandmann, D.; Gilbricht, S. SEM-Based Automated Mineralogy and Its Application in Geo- and Material Sciences. Minerals 2020, 10, 1004. [Google Scholar] [CrossRef]
- Groves, D.; Phillips, G.N.; Ho, S.; Houstoun, S.M.; Standing, C.A. Craton-scale distribution of Archean greenstone gold deposits; predictive capacity of the metamorphic model. Econ. Geol. 1987, 82, 2045–2058. [Google Scholar] [CrossRef]
- Sibson, R.H.; Robert, F.; Poulsen, K.H. High-angle reverse faults, fluid-pressure cycling, and mesothermal gold-quartz deposits. Geology 1988, 16, 551. [Google Scholar] [CrossRef]
- Lin, Q.; Neethling, S.J.; Dobson, K.J.; Courtois, L.; Lee, P.D. Quantifying and minimising systematic and random errors in X-ray micro-tomography based volume measurements. Comput. Geosci. 2015, 77, 1–7. [Google Scholar] [CrossRef]
- Hoal, K.O.; Stammer, J.G.; Appleby, S.K.; Botha, J.; Ross, J.K.; Botha, P.W. Research in quantitative mineralogy: Examples from diverse applications. Miner. Eng. 2009, 22, 402–408. [Google Scholar] [CrossRef]
- Ueda, T.; Oki, T.; Koyanaka, S. Experimental analysis of mineral liberation and stereological bias based on X-ray computed tomography and artificial binary particles. Adv. Powder Technol. 2018, 29, 462–470. [Google Scholar] [CrossRef]
- Kusne, A.G.; Yu, H.; Wu, C.; Zhang, H.; Hattrick-Simpers, J.; DeCost, B.; Sarker, S.; Oses, C.; Toher, C.; Curtarolo, S.; et al. On-the-fly closed-loop materials discovery via Bayesian active learning. Nat. Commun. 2020, 11, 5966. [Google Scholar] [CrossRef]
- Furat, O.; Leißner, T.; Ditscherlein, R.; Šedivý, O.; Weber, M.; Bachmann, K.; Gutzmer, J.; Peuker, U.; Schmidt, V. Description of Ore Particles from X-Ray Microtomography (XMT) Images, Supported by Scanning Electron Microscope (SEM)-Based Image Analysis. Microsc. Microanal. 2018, 24, 461–470. [Google Scholar] [CrossRef]
- Voigt, M.; Miller, J.A.; Mainza, A.N.; Bam, L.C.; Becker, M. The Robustness of the Gray Level Co-Occurrence Matrices and X-Ray Computed Tomography Method for the Quantification of 3D Mineral Texture. Minerals 2020, 10, 334. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godinho, J.R.A.; Westaway-Heaven, G.; Boone, M.A.; Renno, A.D. Spectral Tomography for 3D Element Detection and Mineral Analysis. Minerals 2021, 11, 598. https://doi.org/10.3390/min11060598
Godinho JRA, Westaway-Heaven G, Boone MA, Renno AD. Spectral Tomography for 3D Element Detection and Mineral Analysis. Minerals. 2021; 11(6):598. https://doi.org/10.3390/min11060598
Chicago/Turabian StyleGodinho, Jose R. A., Gabriel Westaway-Heaven, Marijn A. Boone, and Axel D. Renno. 2021. "Spectral Tomography for 3D Element Detection and Mineral Analysis" Minerals 11, no. 6: 598. https://doi.org/10.3390/min11060598
APA StyleGodinho, J. R. A., Westaway-Heaven, G., Boone, M. A., & Renno, A. D. (2021). Spectral Tomography for 3D Element Detection and Mineral Analysis. Minerals, 11(6), 598. https://doi.org/10.3390/min11060598