Rare Earth Element and Incompatible Trace Element Abundances in Emeralds Reveal Their Formation Environments
Abstract
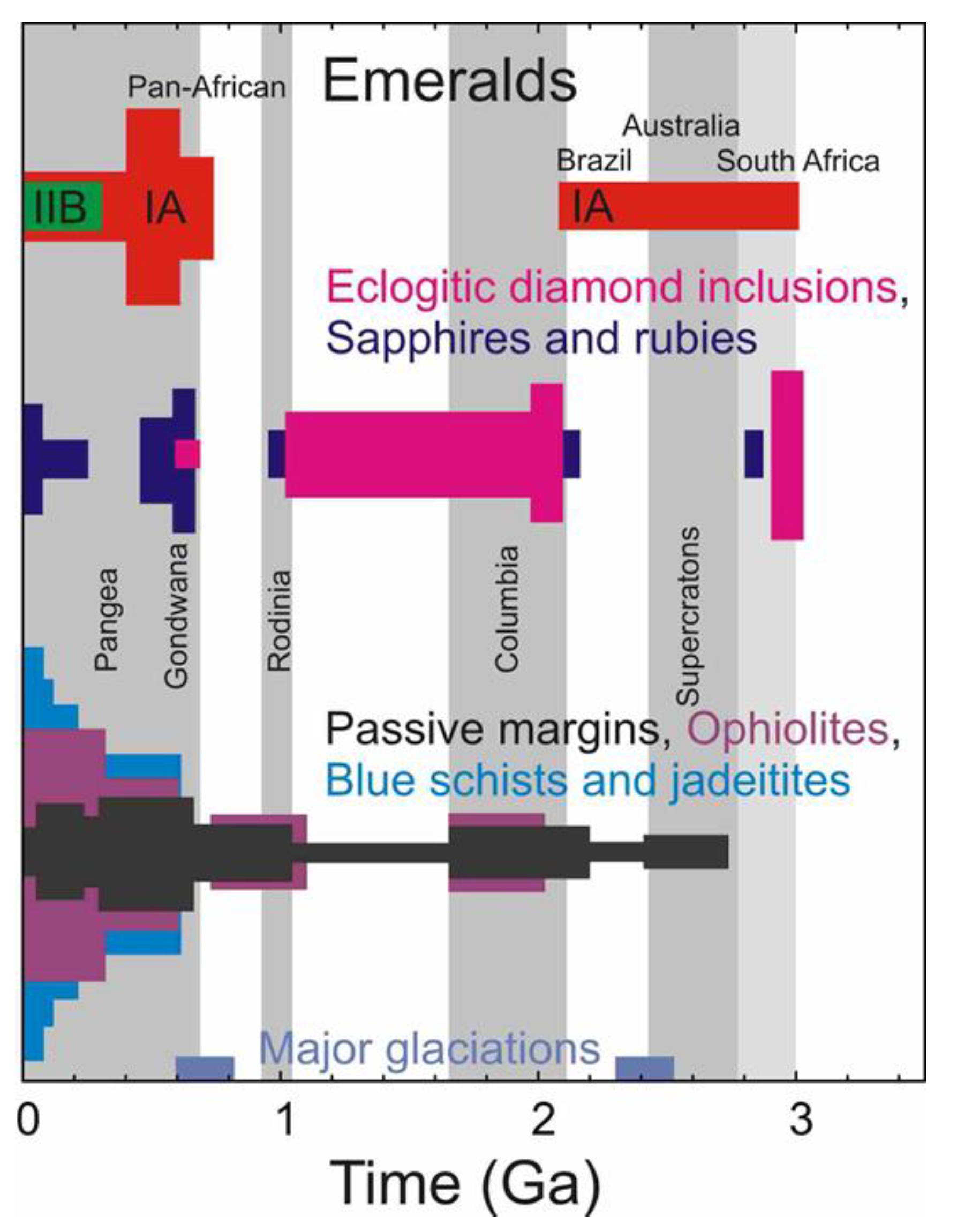
1. Introduction
2. Materials and Methods
3. Results
3.1. Major Element Composition of Emeralds
3.2. Trace Element Abundance Composition of Emeralds
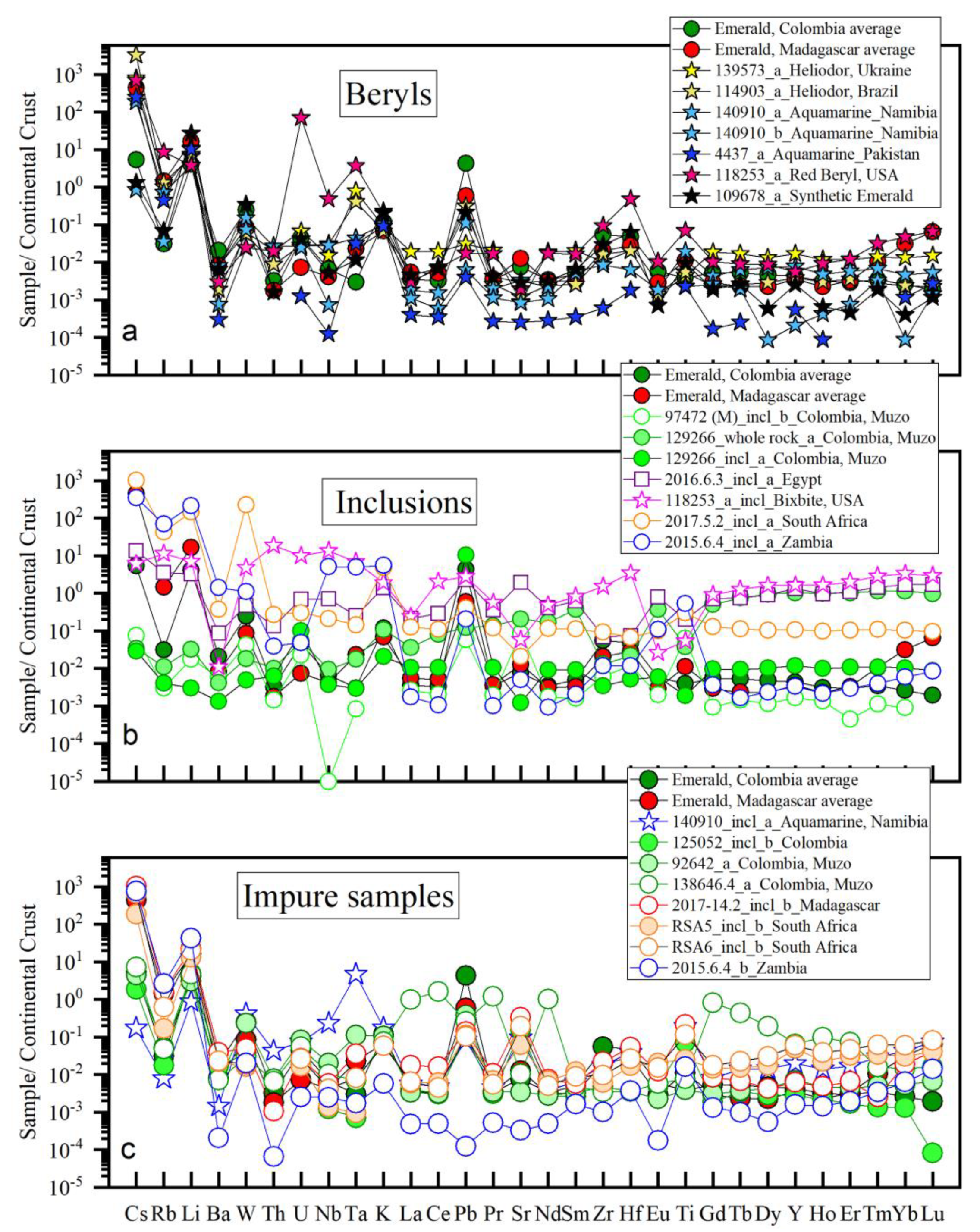
3.3. Compositions of Other Beryl Varieties and Inclusion-Rich Emeralds
3.4. Trace Element Abundances of Mananjary Deposit Whole-Rocks
4. Discussion
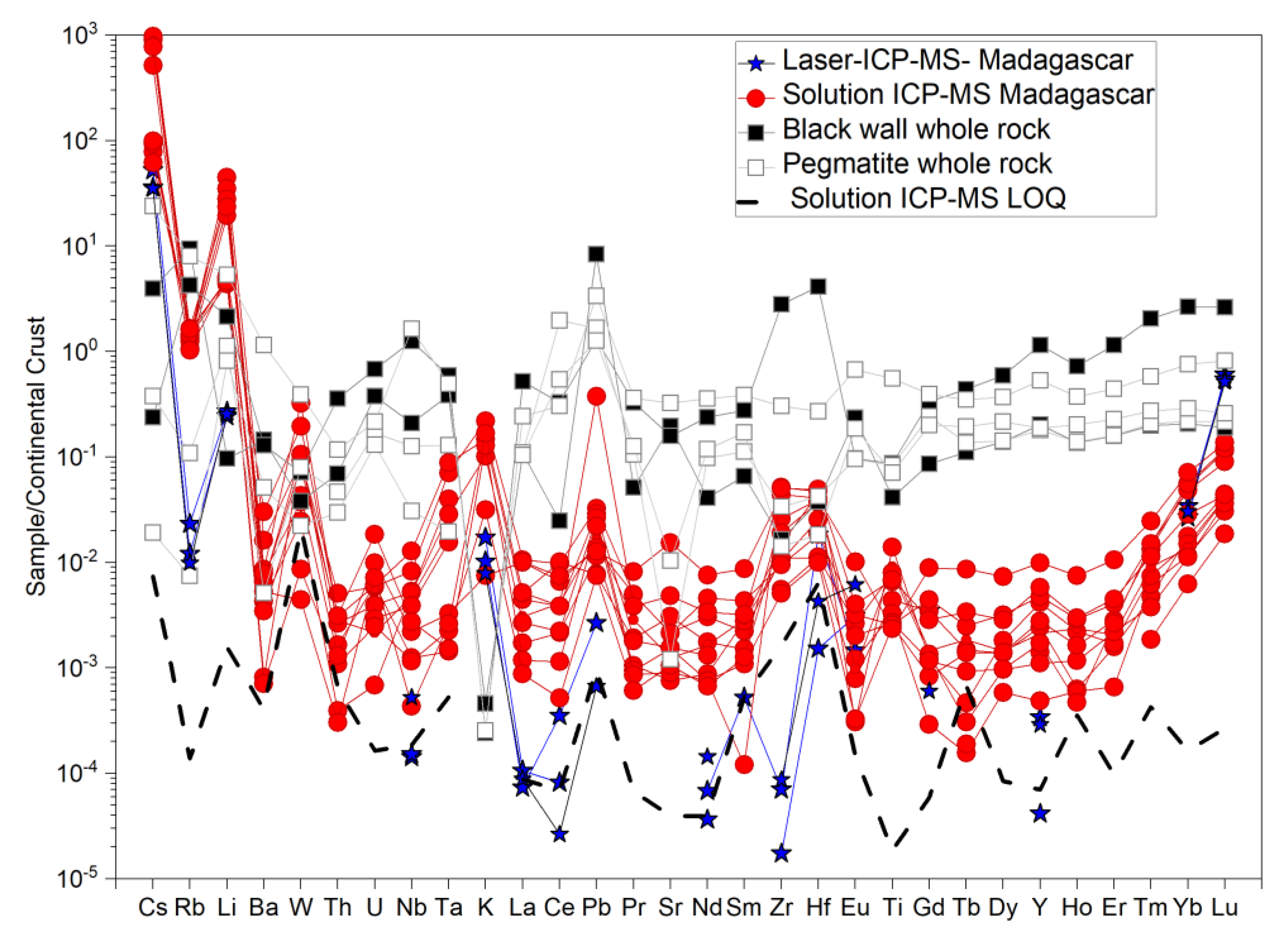
4.1. Solution Versus Laser-Ablation ICP-MS for Emeralds
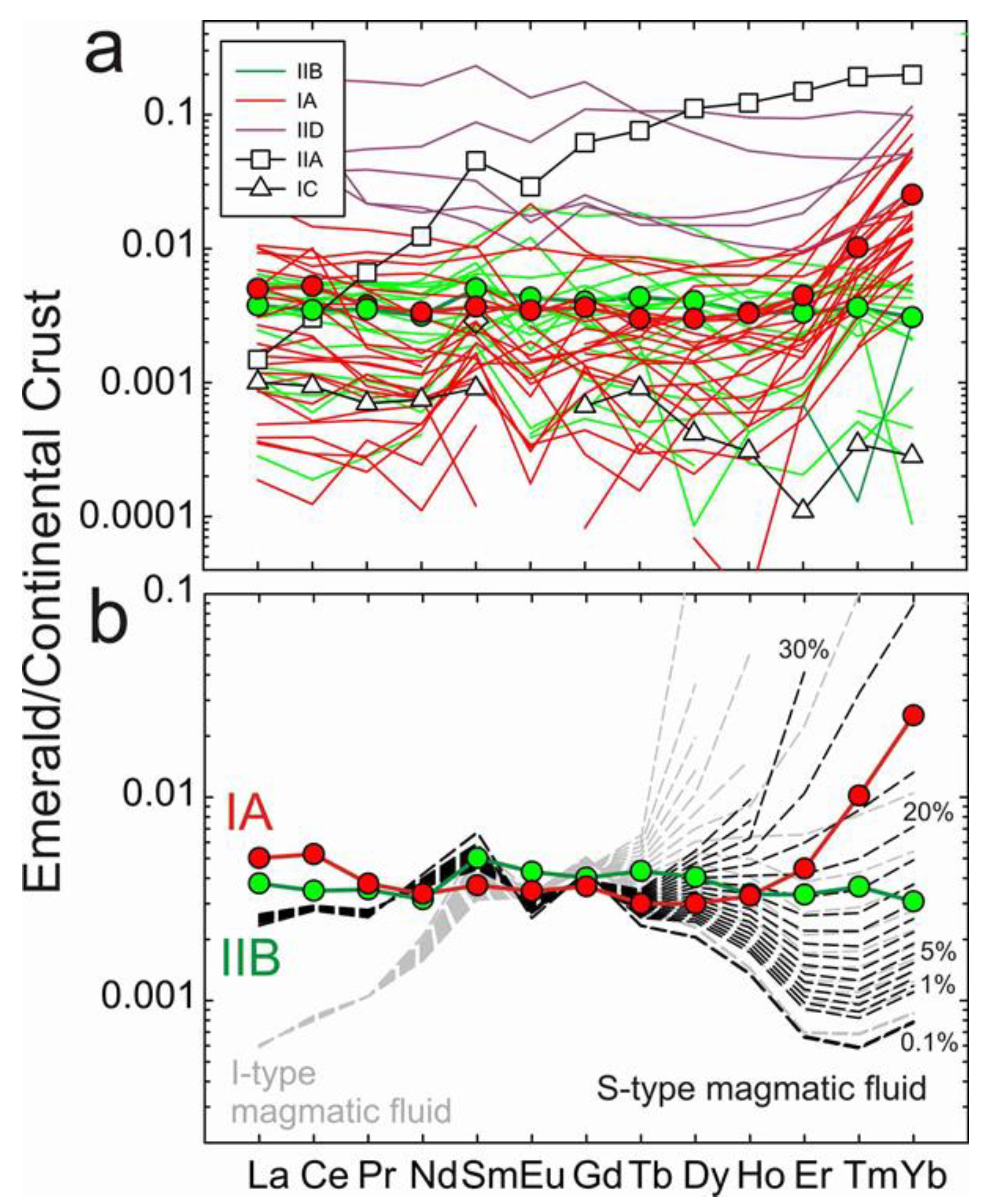
4.2. Rare Earth Element Distribution in Emeralds and Choice of Continental Crust Normalization
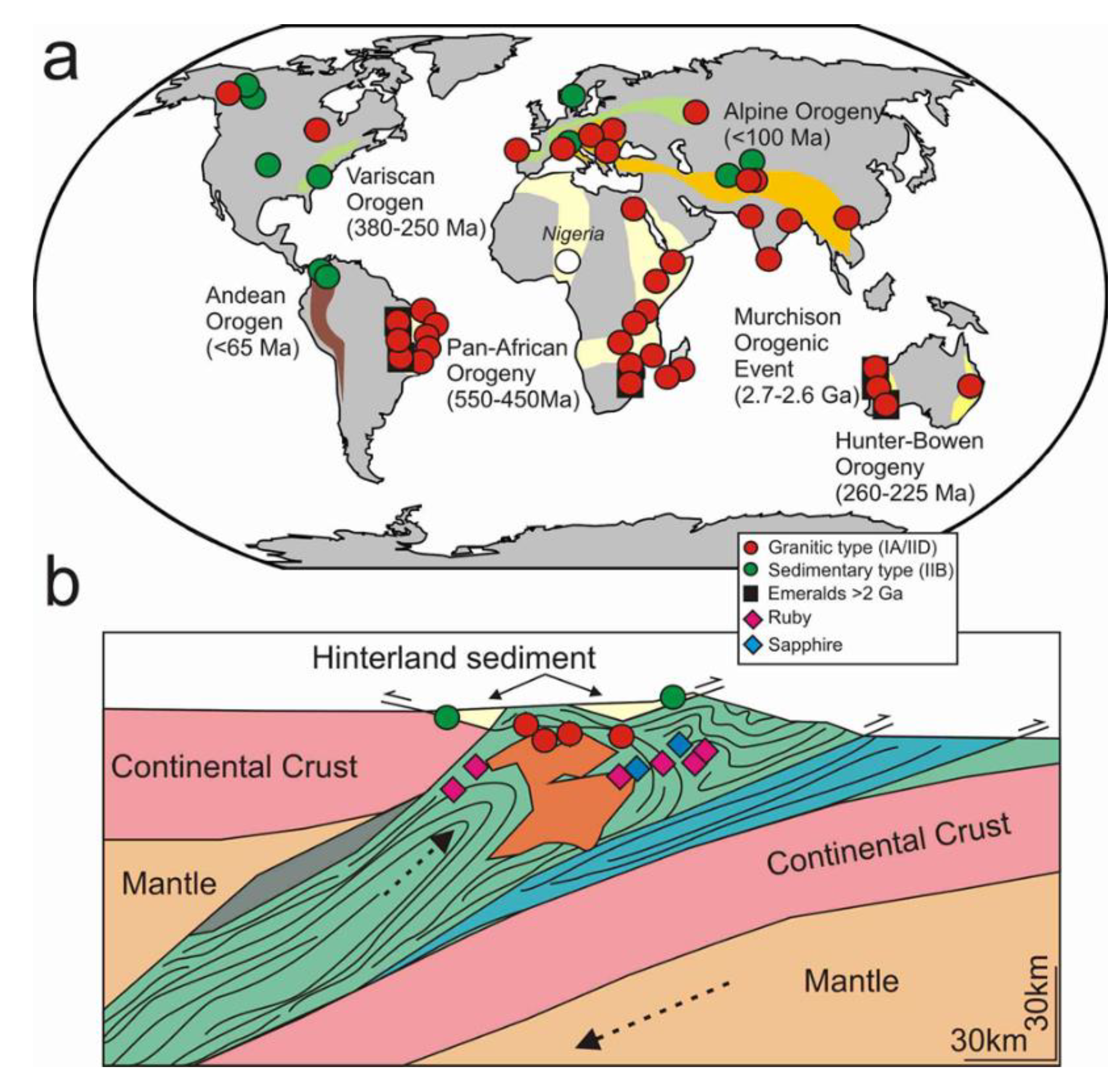
4.3. Trace Element Indicators of Origin for Colombian, Nigerian and Austrian Emeralds
4.4. Modelling the Fluid Compositions and Origins of Type IA Emeralds
4.5. Wider Implications of the REE Chemistry of Emeralds
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groat, L.A.; Giuliani, G.; Marshall, D.D.; Turner, D. Emerald deposits and occurrences: A review. Ore Geol. Rev. 2008, 34, 87–112. [Google Scholar] [CrossRef]
- Schwarz, D.; Schmetzer, K.T. The definition of emerald. Extralapis 2002, 2, 74–78. [Google Scholar]
- Giuliani, G. La spirale du temps de l’émeraude. Règne Minéral 2011, 98, 31–41. [Google Scholar]
- Grundmann, G.; Morteani, G. Emerald Mineralization during Regional Metamorphism—The Habachtal (Austria) and Leydsdorp (Transvaal, South-Africa) Deposits. Econ. Geol. 1989, 84, 1835–1849. [Google Scholar] [CrossRef]
- Zwaan, J.C. Gemmology, geology and origin of the Sandawana emerald deposits, Zimbabwe. Scripta Geol. 2006, 131, 1–212. [Google Scholar]
- Andrianjakavah, P.R.; Salvi, S.; Béziat, D.; Rakotondrazafy, M.; Giuliani, G. Proximal and distal styles of pegmatite-related metasomatic emerald mineralization at Ianapera, southern Madagascar. Miner. Depos. 2009, 44, 817–835. [Google Scholar] [CrossRef]
- Loughrey, L.; Marshall, D.; Ihlen, P.; Jones, P. Boiling as a mechanism for colour zonations observed at the Byrud emerald deposit, Eidsvoll, Norway: Fluid inclusion, stable isotope and Ar-Ar studies. Geofluids 2013, 13, 542–558. [Google Scholar] [CrossRef]
- Groat, L.A.; Turner, D.J.; Evans, R.J. 13.23-Gem Deposits. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 13, pp. 595–622. [Google Scholar] [CrossRef]
- Marshall, D.; Downes, P.; Ellis, S.; Greene, R.; Loughrey, L.; Jones, P. Pressure–Temperature–Fluid Constraints for the Poona Emerald Deposits, Western Australia: Fluid Inclusion and Stable Isotope Studies. Minerals 2016, 6, 130. [Google Scholar] [CrossRef]
- Giuliani, G.; Groat, L.A.; Marshall, D.; Fallick, A.E.; Branquet, Y. Emerald Deposits: A Review and Enhanced Classification. Minerals 2019, 9, 105. [Google Scholar] [CrossRef]
- Franz, G.; Vyshnevskyi, O.; Taran, M.; Khomenko, V.; Wiedenbeck, M.; Schiperski, F.; Nissen, J. A new emerald occurrence from Kruta Balka, Western Peri-Azovian region, Ukraine: Implications for understanding the crystal chemistry of emerald. Am. Mineral. J. Earth Planet. Mater. 2020, 105, 162–181. [Google Scholar] [CrossRef]
- Aurisicchio, C.; Conte, A.M.; Medeghini, L.; Ottolini, L.; De Vito, C. Major and trace element geochemistry of emerald from several deposits: Implications for genetic models and classification schemes. Ore Geol. Rev. 2018, 94, 351–366. [Google Scholar] [CrossRef]
- Saeseaw, S.; Renfro, N.D.; Palke, A.C.; Sun, Z.; McClure, S.F. Geographic Origin determination of emeralds. Gems Gemol. 2019, 55, 614–646. [Google Scholar] [CrossRef]
- Branquet, Y.; Laumonier, B.; Cheilletz, A.; Giuliani, G. Emeralds in the Eastern Cordillera of Colombia: Two tectonic settings for one mineralization. Geology 1999, 27, 597–600. [Google Scholar] [CrossRef]
- Ottaway, T.; Wicks, F.; Bryndzia, L.; Kyser, T.; Spooner, E. Formation of the Muzo hydrothermal emerald deposit in Colombia. Nature 1994, 369, 552. [Google Scholar] [CrossRef]
- Giuliani, G.; France-Lanord, C.; Cheilletz, A.; Coget, P.; Branquet, Y.; Laumomnier, B. Sulfate reduction by organic matter in Colombian emerald deposits: Chemical and stable isotope (C, O, H) evidence. Econ. Geol. 2000, 95, 1129–1153. [Google Scholar] [CrossRef]
- Cooper, M.; Addison, F.; Alvarez, R.; Coral, M.; Graham, R.H.; Hayward, A.; Howe, S.; Martinez, J.; Naar, J.; Peñas, R. Basin development and tectonic history of the Llanos Basin, Eastern Cordillera, and middle Magdalena Valley, Colombia. AAPG Bull. 1995, 79, 1421–1442. [Google Scholar]
- Karampelas, S.; Al-Shaybani, B.; Mohamed, F.; Sangsawong, S.; Al-Alawi, A. Emeralds from the most important occurrences: Chemical and spectroscopic data. Minerals 2019, 9, 561. [Google Scholar] [CrossRef]
- Fritsch, E.; Rossman, G.R. An update on color in gems. Part 2: Colors involving multiple atoms and color centers. Gems Gemol. 1988, 24, 3–15. [Google Scholar] [CrossRef]
- Turner, D.; Groat, L.A.; Hart, C.J.; Mortensen, J.K.; Linnen, R.L.; Giuliani, G.; Wengzynowski, W. Mineralogical and geochemical study of the True Blue aquamarine showing, southern Yukon. Can. Mineral. 2007, 45, 203–227. [Google Scholar] [CrossRef]
- Nassau, K.; Wood, D. An examination of red beryl from Utah. Am. Mineral. J. Earth Planet. Mater. 1968, 53, 801–806. [Google Scholar]
- Falster, A.U.; Simmons, W.B.; Webber, K.L.; Boudreaux, A.P. Mineralogy and Geochemistry of the Erongo Sub-Volcanic Granite-Miarolitic-Pegmatite Complex, Erongo, NamibiaMiarolitic Pegmatites, Erongo. Can. Mineral. 2018, 56, 425–449. [Google Scholar] [CrossRef]
- Agheem, M.H.; Shah, M.T.; Khan, T.; Laghari, A.; Dars, H. Field features and petrography used as indicators for the classification of Shigar valley pegmatites, Gilgit-Baltistan region of Pakistan. Himal J. Earth Sci. Univ. Peshawar. 2011, 44, 1–7. [Google Scholar]
- Nassau, K. Synthetic emerald: The confusing history and the current technologies. J. Cryst. Growth 1976, 35, 211–222. [Google Scholar] [CrossRef]
- Hattingh, R.; Alonso-Perez, R.; Palke, A.C.; Groat, L.; Day, J.M.D. Comparison of Laser Ablation and Solution ICP-MS Analyses of Emeralds. Gems Gemol. 2018, 55, 297. [Google Scholar]
- Day, J.; Peters, B.J.; Janney, P.E. Oxygen isotope systematics of South African olivine melilitites and implications for HIMU mantle reservoirs. Lithos 2014, 202, 76–84. [Google Scholar] [CrossRef]
- Tait, K.T.; Day, J.M.D. Chondritic late accretion to Mars and the nature of shergottite reservoirs. Earth Planet. Sci. Lett. 2018, 494, 99–108. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. Treatise Geochem. 2003, 3, 659. [Google Scholar]
- Poujol, M. U-Pb isotopic evidence for episodic granitoid emplacement in the Murchison greenstone belt, South Africa. J. Afr. Earth Sci. 2001, 33, 155–163. [Google Scholar] [CrossRef]
- Schwarz, D.; Kanis, J.; Kinnaird, J. Emerald and green beryl from Central Nigeria. J. Gemol. Lond. 1996, 25, 117–141. [Google Scholar] [CrossRef]
- Schmetzer, K.; Gilg, H.A.; Vaupel, E. Synthetic Emeralds Grown by W. Zerfass: Historical Account, Growth Technology and Properties. J. Gemmol. 2017, 35, 120–125. [Google Scholar] [CrossRef]
- Bersani, D.; Azzi, G.; Lambruschi, E.; Barone, G.; Mazzoleni, P.; Raneri, S.; Longobardo, U.; Lottici, P.P. Characterization of emeralds by micro-Raman spectroscopy. J. Raman Spectrosc. 2014, 45, 1293–1300. [Google Scholar] [CrossRef]
- Pignatelli, I.; Giuliani, G.; Morlot, C.; Rouer, O.; Claiser, N.; Chatagnier, P.-Y.; Goubert, D. Recent Advances in Understanding the Similarities and Differences of Colombian Euclases. Can. Mineral. 2017, 55, 799–820. [Google Scholar] [CrossRef]
- Zwaan, J.C.; Jacob, D.E.; Häger, T.; Neto, M.T.O. Emeralds from the Fazenda Bonfim Region, Rio Grande do Norte, Brazil. Gems Gemol. 2012. [Google Scholar] [CrossRef]
- Saeseaw, S.; Pardieu, V.; Sangsawong, S. Three-phase inclusions in emerald and their impact on origin determination. Gems Gemol. 2014, 50, 114–132. [Google Scholar] [CrossRef]
- Bačík, P.; Fridrichová, J. The Site Occupancy Assessment in Beryl Based on Bond-Length Constraints. Minerals 2019, 9, 641. [Google Scholar] [CrossRef]
- McLennan, S.M. Relationships between the trace element composition of sedimentary rocks and upper continental crust. Geochem. Geophys. Geosyst. 2001, 2. [Google Scholar] [CrossRef]
- Kinnaird, J. Hydrothermal alteration and mineralization of the alkaline anorogenic ring complexes of Nigeria. J. Afr. Earth Sci. 1985, 3, 229–251. [Google Scholar] [CrossRef]
- Giuliani, G.; France-Lanord, C.; Zimmermann, J.; Cheilletz, A.; Arboleda, C.; Charoy, B.; Coget, P.; Fontan, F.; Giard, D. Fluid composition, δD of channel H2O, and δ18O of lattice oxygen in beryls: Genetic implications for Brazilian, Colombian, and Afghanistani emerald deposits. Int. Geol. Rev. 1997, 39, 400–424. [Google Scholar] [CrossRef]
- Giuliani, G.; France-Lanord, C.; Coget, P.; Schwarz, D.; Cheilletz, A.; Branquet, Y.; Giard, D.; Martin-Izard, A.; Alexandrov, P.; Piat, D. Oxygen isotope systematics of emerald: Relevance for its origin and geological significance. Miner. Depos. 1998, 33, 513–519. [Google Scholar] [CrossRef]
- Marshall, D.; Meisser, N.; Ellis, S.; Jones, P.; Bussy, F.; Mumenthaler, T. Formational Conditions for the Binntal Emerald Occurrence, Valais, Switzerland: Fluid Inclusion, Chemical Composition, and Stable Isotope Studies. Can. Mineral. 2017, 55, 725–741. [Google Scholar] [CrossRef]
- Dill, H.G. The “chessboard” classification scheme of mineral deposits: Mineralogy and geology from aluminum to zirconium. Earth-Sci. Rev. 2010, 100, 1–420. [Google Scholar] [CrossRef]
- Chappell, B.; White, A. I-and S-type granites in the Lachlan Fold Belt. Earth Environ. Sci. Trans. R. Soc. Edinb. 1992, 83, 1–26. [Google Scholar]
- Adam, J.; Green, T. Trace element partitioning between mica-and amphibole-bearing garnet lherzolite and hydrous basanitic melt: 1. Experimental results and the investigation of controls on partitioning behaviour. Contrib. Mineral. Petrol. 2006, 152, 1–17. [Google Scholar] [CrossRef]
- Bea, F.; Pereira, M.; Stroh, A. Mineral/leucosome trace-element partitioning in a peraluminous migmatite (a laser ablation-ICP-MS study). Chem. Geol. 1994, 117, 291–312. [Google Scholar] [CrossRef]
- Clemens, J.; Buick, I.; Kisters, A. The Donkerhuk batholith, Namibia: A giant S-type granite emplaced in the mid crust, in a fore-arc setting. J. Geol. Soc. Lond. 2017, 174, 157–169. [Google Scholar] [CrossRef]
- Hanson, G.N. Rare earth elements in petrogenetic studies of igneous systems. Annu. Rev. Earth Planet. Sci. 1980, 8, 371–406. [Google Scholar] [CrossRef]
- Chappell, B.W.; White, A.J. Two contrasting granite types: 25 years later. Aust. J. Earth Sci. 2001, 48, 489–499. [Google Scholar] [CrossRef]
- Grew, E.S.; Hazen, R.M. Beryllium mineral evolution. Am. Mineral. 2014, 99, 999–1021. [Google Scholar] [CrossRef]
- Dill, H.G. Pegmatites and aplites: Their genetic and applied ore geology. Ore Geol. Rev. 2015, 69, 417–561. [Google Scholar] [CrossRef]
- Evensen, J.M.; London, D. Experimental silicate mineral/melt partition coefficients for beryllium and the crustal be cycle from migmatite to pegmatite. Geochim. Cosmochim. Acta 2002, 66, 2239–2265. [Google Scholar] [CrossRef]
- Stern, R.J.; Tsujimori, T.; Harlow, G.; Groat, L.A. Plate tectonic gemstones. Geology 2013, 41, 723–726. [Google Scholar] [CrossRef]
- Morteani, G. The multi-fluid metasomatic genesis of the Archean Poona emerald deposit (Murchison Province, Western Australia): Microtextures, geochemistry and oxygen isotope composition. Period. Mineral. 2017, 86, 279–300. [Google Scholar]
- Shirey, S.B.; Richardson, S.H. Start of the Wilson cycle at 3 Ga shown by diamonds from subcontinental mantle. Science 2011, 333, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.C. Passive margins through earth history. Earth Sci. Rev. 2008, 91, 1–26. [Google Scholar] [CrossRef]
- Sobolev, S.V.; Brown, M. Surface erosion events controlled the evolution of plate tectonics on Earth. Nature 2019, 570, 52. [Google Scholar] [CrossRef] [PubMed]
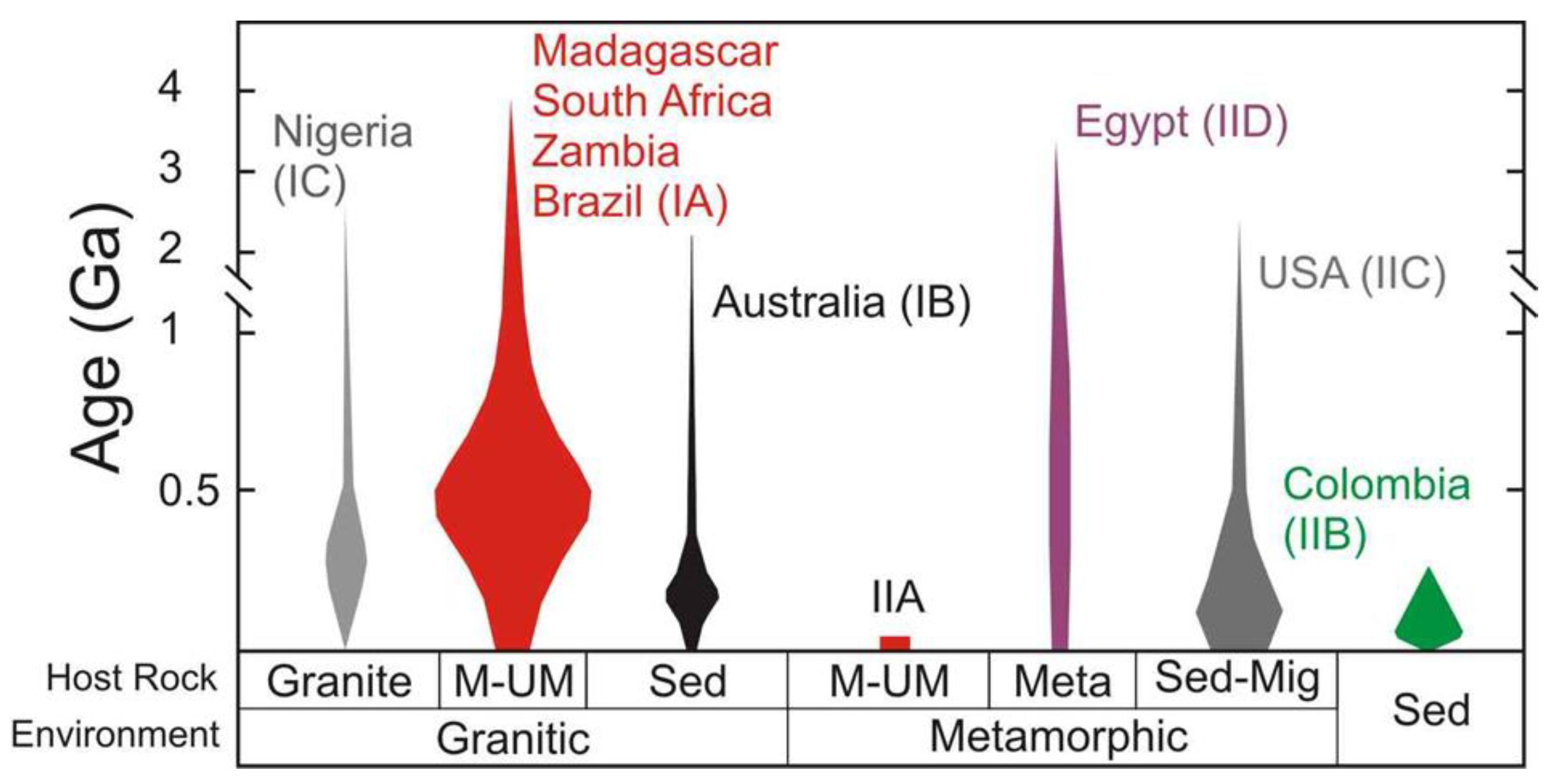
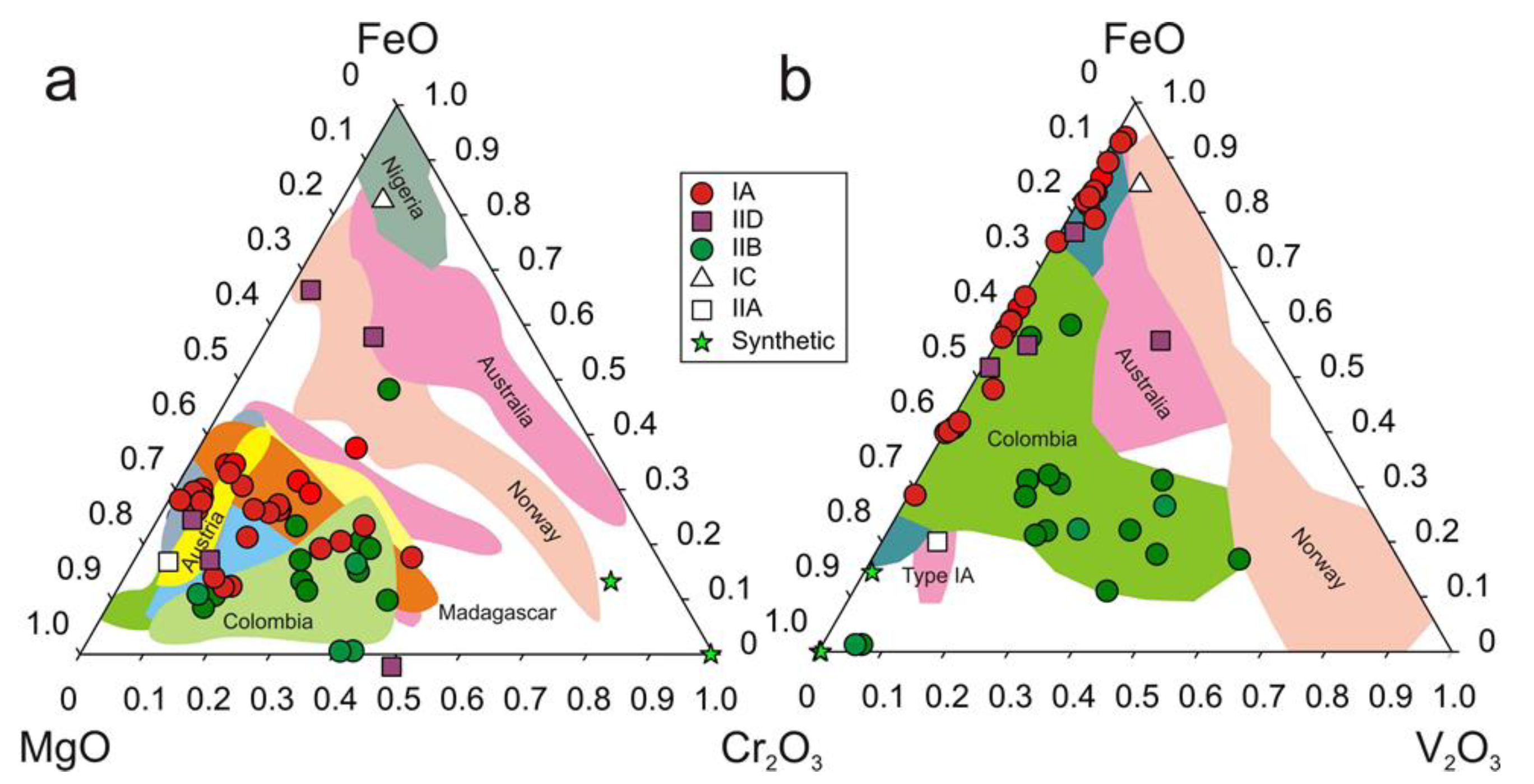
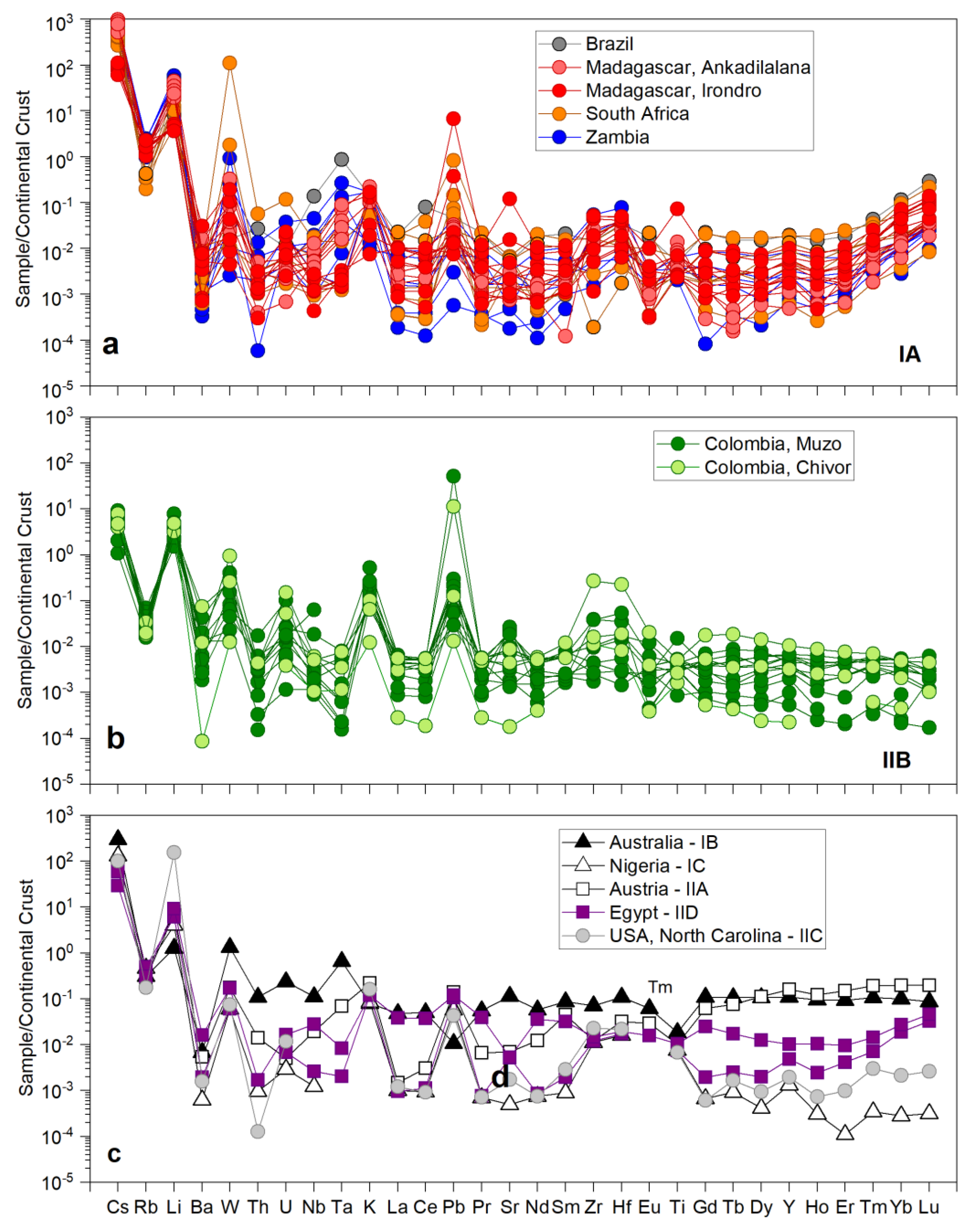
| Region | Class | Samples Number | Locality | Geology | Metamorphic Facies | Temp. °C | P, bar | Age Ma | Age/Method |
|---|---|---|---|---|---|---|---|---|---|
| Brazil | IA | L2017.27.5 | Bonfim farm, Caiçara do Rio do Vento, Borborema mineral province, Rio Grande do Norte | Pegmatitic bodies hosted by amphibolites in a succession of talc, talc-amphibole, and biotite ± amphibole schists and within a shear zone. | Greenschist to low-amphibolite | 330–470 | 200–600 | 553 | Blackwall zone, mica 40Ar/39Ar method |
| Madagascar | IA | 2017-14.1, 2017-14.2, 2017-14.3 | Ankadilalana emerald mine, Ambalahosy Nord Commune, Mananjary District, Vatovavy Fitovinany | Black-wall reaction zones (amphibolite-phlogopite-rich rocks occasionally pillowed) between migmatitic gneiss, talc-schist and lenses of chromite-bearing serpentinites within a shear zone | Amphibolite | 250–500 | 150–200 | 490 | Phlogopite, 40Ar/39Ar method |
| Madagascar | IA | 2017.9.3-1, 2017.9.3-2, 2017.9.3-3, 2017.9.4 | Irondro mine, Andonabe Commune, Mananjary District, Vatovavy Fitovinany | Black-wall reaction zones (amphibolite-phlogopite-rich rocks occasionally pillowed) between migmatitic gneiss, talc-schist and lenses of chromite-bearing serpentinites within a shear zone | Amphibolite | 250–500 | 150–200 | 490 | Phlogopite, 40Ar/39Ar method |
| South Africa | IA | 2017.5.2, RSA1, RSA2, RSA3, RSA4 | Gravelotte Emerald Mine, Gravelotte, Murchison Range, Limpopo Province | Black-wall zones associated with ultramafic rocks; Archean tonalitic gneisses with talc-chlorite, actinolite, and biotite schist, interpreted as a tectonic mélange | Green-schist | 450–500 | 400 | 2.97 | Granitoid zircon, U-Pb method |
| Zambia | IA | 2015.6, 2015.6.1, 2015.6.4, 2015.6.5 | Kagem Emerald Mine, Kafubu Emerald District, Lufwanyama, Copperbelt | Talc-chlorite ± actinolite ± magnetite metabasites identified as metamorphosed komatiites and metasomatized by Be-bearing fluids derived from hydrothermal veins | Amphibolite | 360–390 | 400–450 | 452–447 | Muscovite, 40Ar/39Ar method |
| Australia | IB | 2016.6.13 | New South Wales, Clive Co.,Torrington | At the contact between I-Type granite and sediments- pegmatite and aplite into mudstone and silstone | Amphibolite to Green-schist | 350–400 | 150–250 | 298–236 | Rb/Sr whole rock method |
| Nigeria | IC | L2017.27.4 | Jos Plateau, Plateau | Miarolitic cavities subjected to autometasomatic alteration | Amphibolite | 400–450 | 200–300 | 600–450; 190 –144 | Regional terranes |
| Austria | IIA | 2016.6.16 | Emerald deposit, Leckbachgraben, Nasenkopf, Habach Valley, Hohe Tauern, Salzburg | Emerald-bearing biotite-chlorite schists, intercalated between scheelite-bearing banded gneisses and amphibolites | Green-schist | 700 | 5000 | 31–21 | U-Pb apatite method |
| Colombia | IIB | 88296, 92642, 97472, 125052, 129266, 138646, 138646.1, 138646.2, 138646.3, 138646.7, 138646.8 | Muzo Mine, Mun. de Muzo, Vasquez-Yacopí mining district, Boyacá Department | Emerald hosted in a sedimentary basin of sandstone, limestone, black shale and evaporites and formed by hydrothermal brines and sulfate reduction in combination with organic-rich black shales | Low grade metamorphism | 290–360 | 100 | 35–38 | Muscovite, 40Ar/39Ar method |
| Colombia | IIB | 97019, 2016.6.5 | Mun. de Chivor, Guavió-Guatéque mining district, Boyacá Department | Emerald hosted in a sedimentary basin of sandstone, limestone, black shale and evaporites and formed by hydrothermal brines and sulfate reduction in combination with organic-rich black shales | Low grade metamorphism | 290–360 | 100 | 61 | Muscovite, 40Ar/39Ar method |
| USA | IIC | 124888 | Rist Mine, Hiddenite, Alexander Co., North Carolina | Emerald hosted in precambrian migmatitic schists and gneisses intruded by the medium-grained leucocratics. The emerald formation has been related to pegmatitic fluids as hydrothermal Alpine-type veins instead of pegmatites | Amphibolite | 230–290 | low pressure | 500–750 | Regional terranes |
| Egypt | IID | 2016.6.3 | Wadi, Sikait-Zabara region, Eastern Desert, Read Sea | Volcano-sedimentary sequence featuring an ophiolitic tectonic melange composed of metamorphosed M-UMR overlying biotite orthogneiss. Syntectonic intrusions of leucogranites and pegmatites occurred along the ductile shear-zone | Greenschist-amphibolite | 485–571 | 680–770 | 595 | Muscovite, 40Ar/39Ar method |
| Egypt | IID | 2018.13.2 | Gebel Zabara, Sikait-Zabara region, Eastern Desert, Read Sea | Volcano-sedimentary sequence featuring an ophiolitic tectonic melange composed of metamorphosed M-UMR overlying biotite orthogneiss. Syntectonic intrusions of leucogranites and pegmatites occurred along the ductile shear-zone | Greenschist-amphibolite | 485–571 | 680–770 | 595 | Muscovite, 40Ar/39Ar method |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Perez, R.; Day, J.M.D. Rare Earth Element and Incompatible Trace Element Abundances in Emeralds Reveal Their Formation Environments. Minerals 2021, 11, 513. https://doi.org/10.3390/min11050513
Alonso-Perez R, Day JMD. Rare Earth Element and Incompatible Trace Element Abundances in Emeralds Reveal Their Formation Environments. Minerals. 2021; 11(5):513. https://doi.org/10.3390/min11050513
Chicago/Turabian StyleAlonso-Perez, Raquel, and James M. D. Day. 2021. "Rare Earth Element and Incompatible Trace Element Abundances in Emeralds Reveal Their Formation Environments" Minerals 11, no. 5: 513. https://doi.org/10.3390/min11050513
APA StyleAlonso-Perez, R., & Day, J. M. D. (2021). Rare Earth Element and Incompatible Trace Element Abundances in Emeralds Reveal Their Formation Environments. Minerals, 11(5), 513. https://doi.org/10.3390/min11050513







