Abstract
At present, there is a shortage of high-quality feedstock to produce widely used building materials—concretes. Depletion of natural resources and growing restrictions on their extraction, in connection with environmental protection, necessitate the search for an equivalent replacement for conventional raw materials. Magnesium–silicate rocks are a waste of the mining industry. We researched the possibility of using these rocks as coarse and fine aggregates in heavy concrete production. Following the requirements of the national standards, we studied the physical and mechanical characteristics of the obtained material. It was found that the strength of concrete, made of magnesium–silicate rock coarse aggregate, at the age of 28 days of hardening is within 28 MPa, while the strength of the control sample is 27.3 MPa. Replacing quartz sand with dunite sand also leads to an increase in concrete strength (~4%). Complete replacement of aggregates facilitates an increase in strength by 15–20% than the control sample. At the same time, the density of the obtained materials becomes higher. Concretes have a dense structure that affects their quality. Concrete water absorption is within 6%. The fluxing coefficient is 0.85–0.87. The application of magnesium–silicate rocks in concrete production enables the complete replacement of conventional aggregates with mining waste without reducing the quality of the obtained materials. Furthermore, the issues of environmental protection in mineral deposit development are being addressed.
1. Introduction
In the process of any mining, a huge number of overburden and host rocks is produced. Being in dumps negatively affects the whole environmental media [1,2,3]. At the present stage of civilization development, it is a topical problem to involve these rocks into the industrial turnover to produce new types of marketable products. Effective mining waste management will minimize its amount and solve the problems of environmental safety at mining enterprises [4,5].
The main industry that uses waste from mining enterprises is construction, for which they are high-quality raw materials. Production of concrete, consisting mainly of coarse and fine aggregates, is the first in this industry.
In the literature, there are works on using dump rocks as coarse aggregates [6,7]. At the same time, the type of crushed stone and its properties have a great influence on the quality of the obtained material [8,9]. Depending on the type of coarse aggregate, the concrete compressive strength may differ, practically, by 50% from the strength of the control sample [10].
River sand is conventionally used as fine aggregate. However, its shortage and increasing restrictions on the extraction of natural sand in connection with environmental protection necessitate the search for alternative sources of raw materials. Rock crushing sand, mine refuses of crude ore can act as this alternative [11,12]. Adding them to natural sand improves the physical properties of binary mixes [13]. At the same time, the quality of the obtained concretes depends on the shape of the particles, their surface, composition and content [14,15,16].
Particles of crushed rock sand have an angular shape and smooth surface. Concretes containing the optimal amount of this aggregate have higher water demand, a smaller air volume, higher density and strength [17].
According to physical and mechanical characteristics, silicate rocks, formed as a result of phosphate raw material extraction, are suitable for producing concrete B25 (25 MPa). The tests showed that materials made of them have high strength, and their quality is not inferior to the control sample [7].
Studies on using granite extraction and processing waste (granite sludge) showed that it could be used to produce self-compacting mortar. If the granite sludge is up to 40% of the aggregate mass, the physical and mechanical characteristics of the mortar correspond with the control sample [18].
During coal mining, there is a huge amount of waste produced, consisting mainly of aleurolites and sandstones. After preparatory works, they can be used as a substitute for natural sand in concrete production. To obtain materials of good quality, the amount of waste should not exceed 50% of the fine aggregate mass [19].
Keramzite from coal processing waste is the raw material for light concrete production. The frangibility of keramzite concrete coarse aggregate from carbon-veil rocks is stabilized by 28 days of hardening. The compressive strength of the obtained material is 39–42 MPa and corresponds with the strength of the control sample [20].
Fluorite sand waste is an alternative to natural sand. Replacing this with up to 70% of the conventional aggregate, it is possible to obtain concretes with high elastic modulus, tensile and compressive strength [21].
When using mine refuse as fine aggregate, it is necessary to consider that adding it into the concrete composition reduces its placeability. It is shown in [22] that the maximum content of mine refuses for the beneficiation of lead-zinc and copper ores in concrete should not exceed 30% of the fine aggregate mass since a higher percentage leads to a decrease in the strength characteristics of the obtained material. However, when using iron ore mine refuses, the optimal replacement percentage of natural sand varies within different limits. In the research [23], it is 20%, and in the research [24] is 35%. A further increase in the waste content deteriorates the physical and mechanical characteristics of concretes. For road construction, concrete mixes can contain up to 40% of iron ore mine refuses (complete sand replacement). At the same time, slag cement activated by alkalis is used as bonding material [25].
Copper mine refuses to have an increased content of fine fraction. Therefore, their content should not exceed 15% of the concrete composition. The obtained materials are suitable for paving stone and block production [26].
Among the mining waste, there is a great number of magnesium–silicate rocks. They are part of ultramafic–mafic massifs distributed throughout the world.
They include mineral deposits of various types: Ni-Cu and PGE [27], Cr [28], Fe-Ti-V [29], asbestos [30], jadeite and nephrite [31], magnesite, talc, vermiculite [32] et al. Kimberlite and lamproite pipes contain diamonds [33,34]. In their natural state, the complexes do not have a significant impact on the environment. However, they become a source of negative impact on nature in the process of mining. All these deposits contain a small proportion of valuable components. This is especially true for diamond and metal ore deposits. More than 90% of the extracted rock mass goes to dumps, taking into account dilution during mining.
While there are huge reserves, magnesium–silicate rocks are practically not used, although they are of good quality. Therefore, their application in the production of commercial products is an actual task. Let us examine the possibility of their application in the production of heavy concretes.
The solution to using magnesium–silicate rocks is shown by the example of the Yoko–Dovyren dunite–troctolite–gabbro stratified massif, Northern Baikal region, Russia [35,36].
The Yoko–Dovyren intrusive is located in the southern folded margin of the Siberian craton, 60 km to the northeast of Lake Baikal. It lies subconcordantly in carbonate-terrigenous rocks (mainly black shale) of the Synnyr rift [37,38,39]. The Yoko–Dovyren massif is 26.0 × 3.5 km2 in size. It is part of the Synnyr–Dovyren volcanic-plutonic complex. The complex also includes underlying sills of plagioperidotites and dikes of gabbronorites [38,40] with similar dikes in the roof. The complex includes effusives of the Synnyr ridge, overlapping these bodies, highly titanian basalts of the Inyapuk suite, low-titanian andesites and basalts of the Synnyr suite [41]. The geochemical and isotope data indicate a genetic relationship between intrusive and low-titanian volcanic rocks [42]. U-Pb ages of zircons are 728.4 ± 3.4 Ma for the Yoko–Dovyren massif and 722 ± 7 Ma for the associating volcanites [43,44]. Using baddeleyite from pegmatoid gabbronorites in the roof, U-Pb dating gave 724.7 ± 2.5 Ma [45]. As a result of tectonic movements, the Yoko–Dovyren massif has an almost vertical position. The intrusive is composed of contact rocks (chilled gabbronorites and picrodolerites, further plagioclase lherzolites), there are five zones above them (bottom-up): dunite (Ol + Chr) → troctolite + plagiodunite (Ol + Pl. + Chr) → troctolite + olivine gabbro (Ol + Pl + Chr ± Cpx) → olivine gabbro (Pl + Ol + Cpx ± Chr) → olivine gabbronorite (Pl + Ol + Cpx ± Opx). Quartz gabbronorites and pigeonite-containing gabbro (Pl + Cpx ± Opx ± Pig) in the roof belong to the additional intrusion synchronously with the dikes of gabbronorites.
2. Materials and Methods
2.1. Materials
2.1.1. Magnesium–Silicate Rocks of the Yoko–Dovyren Massif
Magnesium–silicate rocks of the Yoko–Dovyren massif are dunites, wehrlites, troctolites and dunite sand of the following chemical composition (Table 1).

Table 1.
Chemical composition of magnesium–silicate rocks, wt %.
The studied rocks have mainly Mg-rich olivine close to forsterite in their composition. The dunites are almost 97% olivine. In addition to olivine, wehrlites contain diopside, troctolites-anorthite (Figure 1).
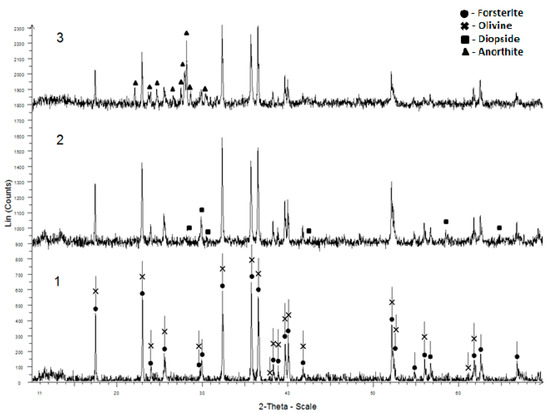
Figure 1.
X-ray diffraction pattern of magnesium–silicate rocks: 1—dunite, 2—wehrlite, 3—troctolite.
The dunite sand in the X-ray diffraction pattern is represented by olivine and serpentine (antigorite and chrysotile) (Figure 2).
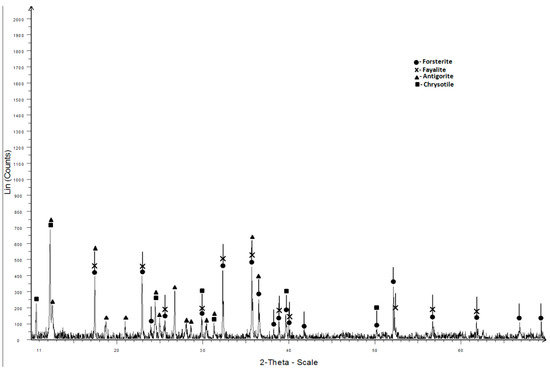
Figure 2.
X-ray diffraction pattern of dunite sand.
The granulometric composition of the studied rocks is found by sifting samples through sieves with different mesh sizes, and it is shown in Figure 3 and Figure 4.
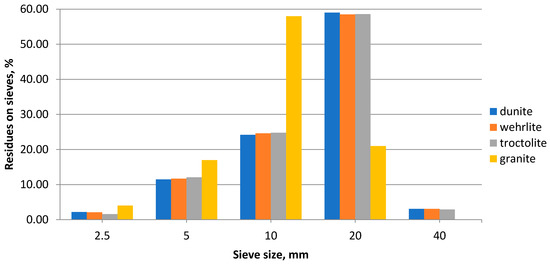
Figure 3.
Granulometric composition of the crushed stone.
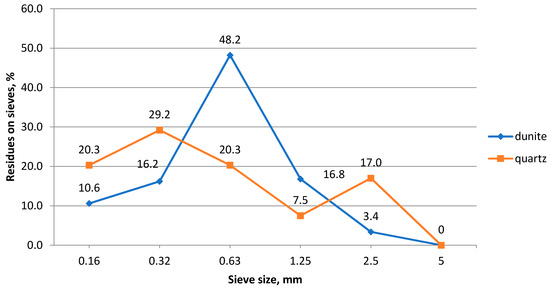
Figure 4.
Granulometric composition of the sand.
The analysis of graphical dependencies shows that crushed stone, 10–20 mm in size, makes up more than 80% of the studied sample. The number of grains, less than 5 mm in size, does not exceed 2%. It was found that dunite sand belongs to the group of coarse sands. Its composition contains 68.4% of particles larger than 0.63 mm. The fineness modulus (Mcr) of this sand is 2.72.
The physical and mechanical characteristics of the magnesium–silicate rocks are shown in Table 2 and Table 3.

Table 2.
Physical and mechanical characteristics of the crushed stone.

Table 3.
Physical and mechanical characteristics of the sand.
Dunites, wehrlites and troctolites are hard rocks. Grains of weak rocks were not found in their composition. They have a high grade of crushing (1200) and abrasion (II), and high specific gravity. They do not contain any impurities or harmful components. The magnesium–silicate rocks underwent freezing and thawing processes. Based on this test, it was concluded that they have an F400 frost resistance grade. The crushed stone from these rocks belongs to the I group of crushed stone because it lacks lamellar and needle-shaped grains. The studied rocks are not exposed to the environment. They do not interact with alkalis and are also resistant to all types of decomposition [35].
The crushed stone from magnesium–silicate rocks is of high quality and, following the requirements of GOST 8267-93, “Crushed stone and gravel of solid rocks for construction works. Specifications”(Russian Federation) [46], can be used as coarse aggregate in concrete production.
Dunite sand does not contain harmful components and impurities [35]. According to its characteristics, it meets the Interstate Standard of the Russian Federation GOST 8736–2014 “Sand for construction works. Specifications” [47] and can be used as fine aggregate in concrete production.
2.1.2. Other Materials
CEM II/A-P 32.5N Portland cement of the Timlyui Cement Plant (Timlyui Cement LLC, Russia, Republic of Buryatia) was used as a binder in the concrete preparation. This material was prepared following the technical documentation of the Russian Federation GOST 10178–85, “Portland cement and Portland blast furnace slag cement specifications” [48] and GOST 30515–2013, “Cements general specifications” [49]. The water meets the requirements of GOST 23732-2011, “Water for concrete and mortars specifications”(Russian Federation) [50].
During the research, the object of comparison was concrete, where granite crushed stone was used as coarse aggregate, and quartz sand was used as fine aggregate.
In the X-ray image of granite crushed stone (Russia, Republic of Buryatia), you can observe intense reflexes of quartz and microcline (Figure 5). The grain size composition of crushed stone is shown in Figure 3, and the main characteristics are shown in Table 2.
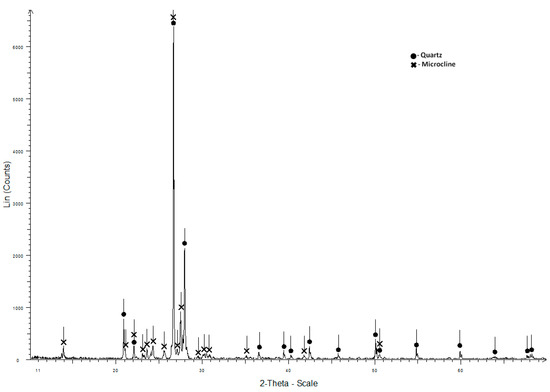
Figure 5.
X-ray diagram of the granite crushed stone.
The studied granite crushed stone meets the requirements of the technical documentation and can be used for the production of concrete, graded “100–400”.
Quartz sand (Russia, Republic of Buryatia) was used as fine aggregate. According to the mineralogical composition (Figure 6), it consists of quartz, orthoclase and muscovite.
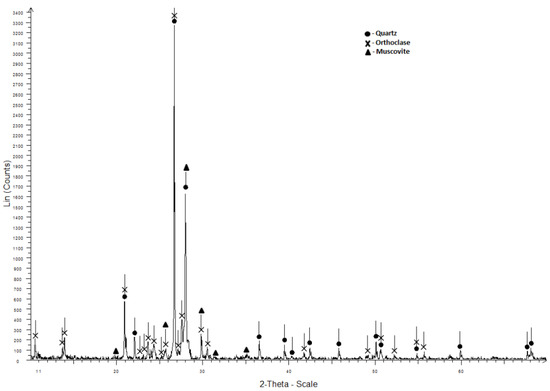
Figure 6.
X-ray diagram of the quartz sand.
The granulometric composition of quartz sand is shown in Figure 4. Its physical and mechanical characteristics are shown in Table 3.
The quartz sand fineness modulus Mcr is 2.5. According to this characteristic and the total sieve residue of 0.63, it is included in the group of medium sands. The physical and mechanical characteristics of the sand meet the requirements of the technical documentation (GOST 8736–2014), and this material can be used as fine aggregate in concrete production.
2.2. Methods
2.2.1. Concrete Sampling
The concrete mixes were prepared under the following conditions. Four types of crushed stone with grains 5–40 mm in size were used as the large aggregate. The volume of rubble remained constant. The amount of sand was 40% of the aggregate mass. The weight of the concrete was the same.
The proportions of the raw materials were the following: cement:sand:crushed stone:water = 1.00:1.50:2.25:0.55.
The prepared concrete mix was molded in cubes with a face size of 100 mm.
We prepared two series of samples: (1) crushed stone from magnesium–silicate rocks, used as coarse aggregate, quartz sand, used as fine aggregate; (2) crushed stone from magnesium–silicate rocks, used as coarse aggregate, dunite sand, used as fine aggregate. Concrete made of granite, crushed stone, and quartz sand is accepted as the reference standard. Moreover, there were samples made of granite crushed stone and dunite sand.
The samples were solidified in the air at a temperature of (20 ± 5) °C for 24 hours. Then, the samples were taken out of the mold and placed in a chamber with a temperature of (20 ± 2) °C and relative humidity of (95 ± 5)%. The samples were tested after 7, 14, 28 and 90 days of hardening.
The studies were carried out following the requirements of the national standards of the Russian Federation.
The data presented in the article is the average of three simultaneous test repetitions
2.2.2. Research Methods
The research methodology included chemical analysis, as well as physical and mechanical tests. The chemical analyses were performed by atomic absorption, spectrophotometric, gravimetric, titrimetric methods.
For spectrophotometric analysis, spectrophotometer PE–5300VI (ECROSKHIM, St. Petersburg, Russia) with a spectral range of 325–1000 nm and separable spectral range of 4 nm was used. For gravimetric analysis, electronic scale VSL–200/0.1A (VESSERVICE, St. Petersburg, Russia) with Max: 205 g, Min: 0.1 g, d = 0.0001 g, e = 0.001 g was used.
The chemical analysis was performed by atomic absorption spectroscopy on Unicam spectrophotometer SOLAAR–6 M (Thermo Electron, Franklin, MA, USA) with suitable software and gravimetry on electronic scale VSL–200/0.1A (Nevskiye Vesy, St. Petersburg, Russia).
The XRD analysis was performed on an X-ray diffractometer (Bruker AXS, D8–Advance, Bruker, Karlsruhe, Germany) with a Cu tube and scanning range from 5° to 70° 2theta, with a step of 0.02° and 0.2 s/step measuring time.
The microscopic analysis was performed on scanning election microscope SEM TM-1000 (Hitachi, Tokyo, Japan), magnification from 20 to 10,000, field depth 0.5 mm, resolution 60 nm.
The macrophotographs were taken on an MBS-9 stereoscopic microscope (Micromed, St. Petersburg, Russia), magnification 4.8, working distance 64 mm.
The mechanical tests were performed on PG–100 test hydraulic press (DEG, St. Petersburg, Russia) with a load range of up to 10 t and a plate movement speed of 10 ± 1 mm/min.
The abrasion capacity of the concrete was calculated by weight loss after four cycles of testing on laboratory abrasion wheel LAW–4 (RNPO RosPribor, Chelyabinsk, Russia), the rotation speed of the abrasive disc (30 ± 1) min−1, vertical load on the sample (300 ± 5) N.
Freeze-resistance tests were performed in the freezing chamber (FC) (Mayak, Yoshkar-Ola, Russia), operating temperature up to minus 30 °C. We applied the basic method for studying freeze resistance. The cycle included freezing samples (saturated with water) in the air (in a freezing chamber with a temperature of minus (18 ± 2) °C ), and then thawing them in water with a temperature of (20 ± 2) °C. The time of both freezing and thawing was 3 hours.
While studying the water absorption, the samples were immersed in water at a temperature of (20 ± 2) °C and weighed every 24 hours. The tests were carried out until the results of two successive weighings began to differ by more than 0.1%. The water absorption (by mass) Wmas is the ratio of the difference between the weight of the water-saturated and dry sample to the weight of the dry sample, expressed as a percentage.
To study the placeability of the concrete mixes, we used a steel cone 300 ± 2 mm high and internal diameters 100 ± 2 mm and 200 ± 2 mm. The cone was filled with a concrete mix in one step, the mix was compacted, and its excess was cut off. The cone was removed smoothly, and the mix slump was measured. By measuring the bottom diameter of the spreading cake, the spreading of the mix was found.
3. Results and Discussion
When making concretes from various aggregates, we determined the placeability of concrete mixes characterized by mobility values (cone flow and cone slump). The results are shown in Table 4.

Table 4.
Characteristics of concrete mixes.
The cone slump and the cone flow of all the presented compositions of the concrete mixes reach satisfactory values. However, it should be noted that adding magnesium–silicate aggregates reduces their fluidity. It is influenced by the size and shape of aggregates (Figure 7).

Figure 7.
Macro photographs of sand particles: (a) dunite; (b) quartz.
Quartz sand has a rounded shape without sharp corners. The shape of the dunite sand particles has a rough surface with numerous protrusions and depressions. This contributes to a tighter adhesion of the aggregates to the binder.
The average density of concrete mixes also depends on the type of aggregate and has the lowest value for the standard sample (2371 kg/m3).
The main characteristic of the concrete quality is compressive strength since, by its change, you can observe changes in the microstructure of the hardened stone. This characteristic depends on aggregates added in the concrete composition [10]. The compressive strength of the samples (P) at the age of 7, 14, 28 and 90 days (the average value of three cubes) is shown in Table 5. The variation of the change in strength is estimated by characteristic α, which represents the ratio of the strength of the studied sample to the strength of the control sample at the respective hardening age.

Table 5.
Compressive strength of the concrete samples.
As shown in the table, the mechanical characteristics of all samples increase over the hardening time. The main strength gain occurs by 14 days of concrete hardening and is more than 70% of the strength at 28 days of age. The mechanical characteristics depend on the type and the quality of the used aggregates. Magnesium–silicate aggregates facilitate an increase in strength of the studied samples. The greatest increase in strength is observed in concretes, where dunite sand is used as fine aggregate. It differs from quartz sand in composition, size and shape of the particles. As you know, these characteristics significantly affect the mechanical properties of the obtained materials [16,18,51,52].
We found the average density of hardened concrete; it is shown in Figure 8.
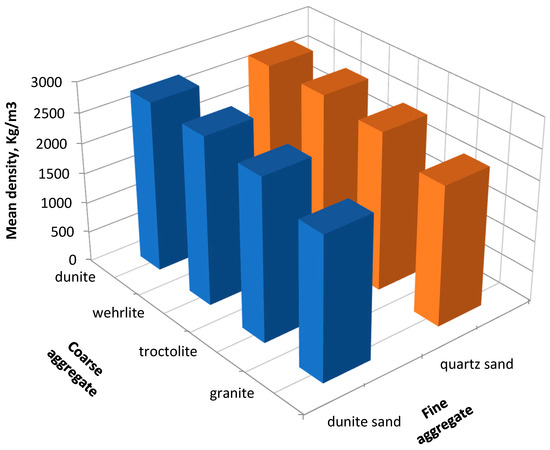
Figure 8.
Dependence of the average density of the concrete on the aggregate type.
The type of aggregate has an impact on the concrete density. The highest values are observed in samples where magnesium–silicate rocks are used as coarse aggregates, and dunite sand is used as fine aggregate. Simultaneously, the concrete density decreases depending on the type of coarse aggregate in the row dunite → wehrlite → troctolite → granite and has the following values 2800 kg/m3 → 2763 kg/m3 → 2666 kg/m3 → 2358 kg/m3. It can be explained by the high specific gravity of the raw materials and the packing density of the obtained materials.
In Figure 9, on the contact border aggregate-cement stone, cavities of small thickness are formed around the aggregate surface. The cavities have rounded edges, which indicates their formation during the hydration of the cement dough. In addition, the cement particles themselves have cavities and cracks of sufficiently small sizes that are inaccessible to water penetration. No deep cracks were found in the concrete structure; the cracks are caused by loose convergence of cement particles due to insufficient hydration.
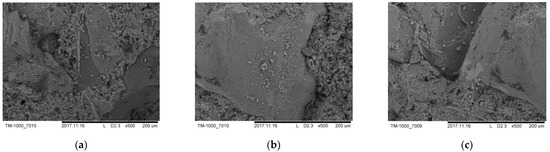
Figure 9.
Microstructure of concretes made of: (a) dunite, (b) wehrlite, (c) troctolite.
As a result of the research, it was found that new formations that crystallize in the cement with the addition of magnesium–silicate rocks lead to the strengthening of the structure of new types of concretes and an increase in the strength of the obtained materials.
When studying the physical and mechanical characteristics of the obtained concretes, we identified their water absorption. The study was conducted following the requirements of the Russian standard (GOST 12730.3-78, “Concretes. Method of determination of water absorption”) [53]. The results are shown in Figure 10.
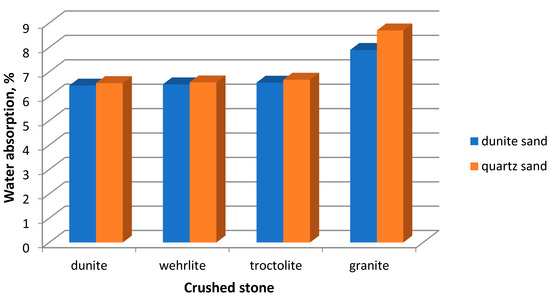
Figure 10.
Dependence of water absorption of the concrete samples on the aggregate type.
It is known that the water absorption of the concrete samples depends on the aggregate type [54]. Aggregates, made of magnesium–silicate rocks, have a positive effect on this characteristic. Adding them into concretes decreases the water absorption. The highest value of this characteristic is observed in the concretes made of granite crushed stone and quartz sand.
The water resistance of the concretes was calculated, which is characterized by softening coefficient Csoft, equal to the ratio of the strength of the water-saturated samples to the strength of the dry samples. In the work process, some of the samples were kept in water for two days, after which their strength values were measured. The conducted studies showed that magnesium–silicate rock concretes have softening coefficient equal to 0.85–0.87. For the control samples, the water resistance was 0.82.
The obtained data are consistent with the concrete frost resistance. The values are shown in Table 6.

Table 6.
Concrete frost resistance values.
After 75 freeze–thaw cycles, no damage was observed on the surface of the concrete samples. The samples withstood 50 freeze–thaw cycles without significant changes in weight. The loss of mass of the concrete samples with the addition of aggregates from dunite by 1.18%, from wehrlite—by 1.34%, from troctolite—by 1.67% was recorded. For the control sample, it was 1.83%. After 75 cycles, this characteristic was at the limit of acceptable values (2%). The frost resistance coefficient also reaches its limit values after 75 freeze–thaw cycles. Based on the obtained values, as well as the compressive strength values after the testing completion, the concrete was graded F50 for frost resistance.
The increased water absorption and frost resistance values in the concrete samples with the addition of magnesium–silicate rocks are explained by a denser structure than in the control sample. The decrease in open porosity reduces the amount of absorbed liquid, which helps to soften the structure of the obtained material.
Abrasion testing has shown that the weight loss of the samples, made of magnesium-containing aggregate, does not exceed the weight loss of the control sample (Figure 11).
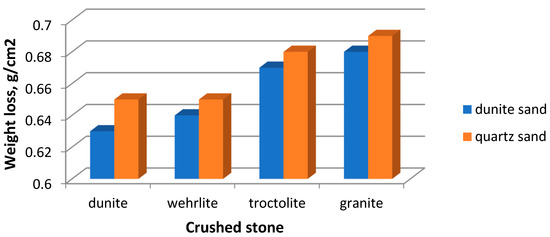
Figure 11.
Dependence of the weight loss of concrete samples during abrasion on the aggregate type.
Dunite concrete has the best characteristics (0.63 g/cm2). This can be explained by the quality of the raw material. Magnesium–silicate rocks do not contain grains of weak rocks. Concretes on them made of this material have increased density and strength, which also affects this characteristic. According to the abrasion capacity, the concretes have grade G1
Thus, concretes, made of magnesium–silicate rock aggregates, have high physical and mechanical properties and can produce load-bearing and special structures.
4. Conclusions
Among mining waste, there are many magnesium–silicate rocks. They are part of ultramafite–mafite complexes, which are found everywhere and include various mineral deposits. Having high physical and mechanical characteristics, they can be used as coarse and fine aggregates in heavy concrete production.
As a result of the studies, it was found that adding magnesium–silicate rocks to the composition of concrete mixes reduces their fluidity. The use of dunite sand helps to reduce the water demand of concrete mixes and makes a dense structure of the hardened material.
The compressive strength of the concretes made of magnesium–silicate rock coarse aggregate at the age of 28 days of hardening is within 28 MPa, while for the control sample, this characteristic is 27.3 MPa. Replacement of quartz sand with dunite sand also leads to an increase in the concrete strength (~4%). Complete replacement of coarse and fine aggregates with magnesium–silicate rocks increases the concrete strength by 15–20% than the control sample.
The concrete density depends on the type of aggregate as well. The samples containing dunite have the highest density values (2800 kg/m3), the reference samples have the lowest ones (2358 kg/m3). This can be explained by the high specific gravity of the used raw material and by the packing density of the obtained material.
The concrete water absorption is within 6%. The softening coefficient is 0.85–0.87. They are marked F50 for freeze resistance and G1 for abrasion capacity.
The obtained results showed that concretes made of magnesium–silicate rocks are a promising replacement for conventional concrete in building constructions. This will contribute to developing the concept of a closed-loop economy and will facilitate the environmental safety of the mining industry.
However, to determine the possibility of using the obtained materials in special structures, it is necessary to investigate the aspects of chemical decomposition of magnesium–silicate rock concretes in an aggressive environment.
Author Contributions
Conceptualization, L.I.K. and E.V.K.; Methodology, L.I.K.; Validation, L.I.K., I.Y.K. and P.L.P.; Formal Analysis, L.I.K. and I.Y.K.; Investigation, L.I.K., I.K. and P.L.P.; Resources, E.V.K.; Writing—Original Draft Preparation, L.I.K. and E.V.K.; Writing—Review & Editing, E.V.K.; Visualization, L.I.K.; Supervision, L.I.K.; Project Administration, L.I.K. and E.V.K.; Funding Acquisition, L.I.K. and E.V.K. All authors have read and agreed to the published version of the manuscript.
Funding
The study was carried out within the framework of the state assignment by Baikal Institute of Nature Management, SB RAS-Project No AAA-A21-121011890003-4 and Geological Institute, SB RAS-Project No AAA-A21-121011890029-4. The study was conducted using facilities of the Center for “Analytical center of mineralogical, geochemical and isotope Studies” at the Geological Institute, SB RAS, and equipment of the CCU BINM SB RAS, Ulan-Ude, Russia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The new data were created andr analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors wish to express their sincere thanks to the editorial board and 3 anonymous reviewers for their helpful remarks, which significantly improved the quality of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Emmanuel, A.Y.; Jerry, C.S.; Dzigbodi, D.A. Review of environmental and health impacts of mining in Ghana. J. Health Pollut. 2018, 8, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.K.; Das, A. Impact of mine waste leachates on aquatic environment: A review. Curr. Pollut. Rep. 2017, 3, 31–37. [Google Scholar] [CrossRef]
- Wellen, C.; Shatilla, N.J.; Carey, S.K. The influence of mining on hydrology and solute transport in the Elk Valley, British Columbia, Canada. Environ. Res. Lett. 2018, 13, 074012. [Google Scholar] [CrossRef]
- Tayebi-Khorami, M.; Edraki, M.; Corder, G.; Golev, A. Re-Thinking mining waste through an integrative approach led by circular economy aspirations. Minerals 2019, 9, 286. [Google Scholar] [CrossRef]
- Carvalho, F.P. Mining industry and sustainable development: Time for Change. Food Energy Secur. 2017, 6, 61–77. [Google Scholar] [CrossRef]
- Benarchid, Y.; Taha, Y.; Argane, R.; Tagnit-Hamou, A.; Benzaazoua, M. Concrete containing low-sulphide waste rocks as fine and coarse aggregates: Preliminary assessment of materials. J. Clean. Prod. 2019, 221, 419–429. [Google Scholar] [CrossRef]
- Machi, A.E.; Mabroum, S.; Taha, Y.; Tagnit-Hamou, A.; Benzaazoua, M.; Hakkou, R. Use of flint from phosphate mine waste rocks as an alternative aggregates for concrete. Constr. Build. Mater. 2021, 271, 121886. [Google Scholar] [CrossRef]
- Góra, J.; Piasta, W. Impact of mechanical resistance of aggregate on properties of concrete. Case Stud. Constr. Mater. 2020, 13, e00438. [Google Scholar] [CrossRef]
- Strzałkowski, J.; Garbalinska, H. The effect of aggregate shape on the properties of concretes with silica fume. Materials 2020, 13, 2780. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, J.; Karanjit, S. Effect of coarse aggregate sources on the compressive strength of various grade of nominal mixed concrete. Jpn. Soc. Civ. Eng. 2019, 7, 52–60. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Qiu, J.; Deng, W.; Xing, J.; Liang, J. Factors affecting brittleness behavior of coal-gangue ceramsite lightweigh aggregate concrete. Front. Mater. 2020, 7, 554718. [Google Scholar] [CrossRef]
- Gayana, B.C.; Chandar, K.R. Sustainable use of mine waste and tailings with suitable admixture as aggregates in concrete pavements—A review. Adv. Concr. Constr. 2018, 6, 221–243. [Google Scholar]
- Pilegis, M.; Gardner, D.; Lark, R. An investigation into the use of manufactured sand as a 100% replacement for fine aggregate in concrete. Materials 2016, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Tebbal, N.; Rahmouni, Z.E.A. Influence of local sand on the physicomechanical comportment and durability of high performance concrete. Adv. Civ. Eng. 2016, 2016, 3897064. [Google Scholar] [CrossRef]
- Tapkire, G.V.; Wagh, H.D.; Jade, Y.R. The effect of sand particle size and shape on compressive strength of cement. Int. J. Adv. Res. Sci. Eng. 2017, 6, 672–677. [Google Scholar]
- Belhadj, B.; Bederina, M.; Benguettache, K.; Queneudec, M. Effect of the type of sand on the fracture and mechanical properties of sand concrete. Adv. Concr. Constr. 2014, 2, 13–27. [Google Scholar] [CrossRef]
- Mavroulidou, M. Mechanical properties and durability of concrete with water cooled copper slag aggregate. Waste Biomass Valor. 2017, 8, 1841–1854. [Google Scholar] [CrossRef]
- Shen, W.; Liu, Y.; Wang, Z.; Cao, L.; Wu, D.; Wang, Y.; Ji, X. Influence of manufactured sand’s characteristics on its concrete performance. Constr. Build. Mater. 2018, 172, 574–583. [Google Scholar] [CrossRef]
- Lozano-Lunar, A.; Dubchenko, I.; Bashynskyi, S.; Rodero, A.; Fernández, J.M.; Jiménez, J.R. Performance of self-compacting mortars with granite sludge as aggregate. Constr. Build. Mater. 2020, 251, 118998. [Google Scholar] [CrossRef]
- Santos, C.R.; Tubino, R.M.C.; Schneider, I.A.H. Mineral processing and characterization of coal waste to be used as fine aggregates for concrete paving blocks. IBRACON Struct. Mater. J. 2015, 8, 14–24. [Google Scholar] [CrossRef]
- González, J.S.; Boadella, I.L.; Gayarre, F.L.; Pérez, C.L.-C.; López, M.S.; Stochino, F. Use of mining waste to produce ultra-high-performance fibre-reinforced concrete. Materials 2020, 13, 2457. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Zhou, L.; Then, N.W.Y. Utilization of tailings in cement and concrete: A review. Sci. Eng. Compos. Mater. 2019, 26, 449–464. [Google Scholar] [CrossRef]
- Ugama, T.I.; Ejeh, S.P.; Amartey, D.Y. Effect of iron ore tailing on the properties of concrete. Civ. Environ. Res. 2014, 6, 7–13. [Google Scholar]
- Tian, Z.X.; Zhao, Z.H.; Dai, C.Q.; Liu, S.J. Experimental study on the properties of concrete mixed with iron ore tailings. Adv. Mater. Sci. Eng. 2016, 2016, 8606505. [Google Scholar] [CrossRef]
- Krivenko, P.; Kovalchuk, O.; Boiko, O. Practical experience of construction of concrete pavement using non-conditional aggregates. IOP Conf. Ser. Mater. Sci. Eng. 2019, 708, 012089. [Google Scholar] [CrossRef]
- Lam, E.J.; Zetola, V.; Ramírez, Y.; Montofré, Í.L.; Pereira, F. Making paving stones from copper mine tailings as aggregates. Int. J. Environ. Res. Public Health 2020, 17, 2448. [Google Scholar] [CrossRef] [PubMed]
- Naldrett, A.J. Magmatic Sulfide Deposits: Geology, Geochemistry and Exploration; Springer: Berlin, Germany, 2004; p. 728. [Google Scholar]
- Stowe, C.W. Compositions and tectonic settings of chromite deposits through time. Econ. Geol. 1994, 89, 528–546. [Google Scholar] [CrossRef]
- Force, E.R. Geology of titanium-mineral deposits. Geol. Soc. Am. Spec. Pap. 1991, 259, 19–37. [Google Scholar]
- Virta, R.L. Asbestos: Geology, mineralogy, mining, and uses. In United States Geological Survey Open-File Report 02–149; USGS: Reston, VA, USA, 2002; p. 38. [Google Scholar]
- Harlow, G.E.; Sorensen, S.S. Jade (nephrite and jadeitite) and serpentinite: Metasomatic connections. Int. Geol. Rev. 2005, 47, 113–146. [Google Scholar] [CrossRef]
- Pohl, W. Economic Geology: Principles and Practice: Metals, Minerals, Coal and Hydrocarbons—Introduction to Formation and Sustainable Exploration of Mineral Deposits; Wiley-Blackwell: Hoboken, NJ, USA, 2011; p. 695. [Google Scholar]
- Gurney, J.J.; Helmstaedt, H.H.; Richardson, S.H.; Shirey, S.B. Diamonds through time. Econ. Geol. 2010, 105, 689–712. [Google Scholar] [CrossRef]
- Tappert, R.; Tappert, M.C. Diamonds in Nature: A Guide to Rough Diamonds; Springer: Berlin, Germany, 2011; p. 142. [Google Scholar]
- Kislov, E.V.; Khudyakova, L.I. Yoko–Dovyren Layered Massif: Composition, Mineralization, Overburden and Dump Rock Utilization. Minerals 2020, 10, 682. [Google Scholar] [CrossRef]
- Timofeeva, S.S.; Kislov, E.V.; Khudyakova, L.I. Yoko-Dovyren layered dunite-troctolite-gabbro massif, North Baikal region, Russia: Structure, composition and use of mineral raw materials. Earth Sci. Front. 2020, 27, 262–279. [Google Scholar]
- Konnikov, E.G. Differentiated Ultrabasic-Basic Complexes in the Precambrian Rocks of Transbaikalia; Publishing House of Science: Novosibirsk, Russia, 1986; p. 127. (In Russian) [Google Scholar]
- Kislov, E.V. The Yoko-Dovyren Layered Massif; RAS SD Buryat Scientific Centre Publishing House: Ulan-Ude, Russia, 1998; p. 265. (In Russian) [Google Scholar]
- Rytsk, E.Y.; Shalaev, V.S.; Rizvanova, N.G.; Krymskii, R.S.; Makeev, A.F.; Rile, G.V. The Olokit Zone of the Baikal Fold Region: New isotopic geochronological and geochemical data. Geotectonics 2002, 36, 24–35. [Google Scholar]
- Ariskin, A.; Danyushevsky, L.; Nikolaev, G.; Kislov, E.; Fiorentini, M.; McNeill, A.; Kostitsyn, Y.; Goemann, K.; Feig, S.; Malyshev, A. The Dovyren Intrusive Complex (Southern Siberia, Russia): Insights into dynamics of an open magma chamber with implications for parental magma origin, composition, and Cu-Ni-PGE fertility. Lithos 2018, 302, 242–262. [Google Scholar] [CrossRef]
- Manuilova, M.M.; Zarubin, V.V. Precambrian Vlcanogenic Rocks of the Northern Baikal Region; Publishing House of Science: Leningrad, Russia, 1981; p. 88. (In Russian) [Google Scholar]
- Ariskin, A.A.; Danyushevsky, L.V.; Konnikov, E.G.; Maas, R.; Kostitsyn, Y.A.; McNeill, A.; Meffre, S.; Nikolaev, G.S.; Kislov, E.V. The Dovyren Intrusive Complex (Northern Baikal region, Russia): Isotope-geochemical markers of contamination of parental magmas and extreme enrichment of the source. Russ. Geol. Geophys. 2015, 56, 411–434. [Google Scholar] [CrossRef]
- Ariskin, A.A.; Konnikov, E.G.; Danyushevsky, L.V.; Kislov, E.V.; Nikolaev, G.S.; Orsoev, D.A.; Barmina, G.S.; Bychkov, K.A. The Dovyren Intrusive Complex: Problems of petrology and Ni sulfide mineralization. Geochem. Int. 2009, 47, 425–453. [Google Scholar] [CrossRef]
- Ariskin, A.A.; Kostitsyn, Y.A.; Konnikov, E.G.; Danyushevsky, L.V.; Meffre, S.; Nikolaev, G.S.; McNeill, A.; Kislov, E.V.; Orsoev, D.A. Geochronology of the Dovyren Intrusive Complex, Northwestern Baikal area, Russia, in the Neoproterozoic. Geochem. Int. 2013, 51, 859–875. [Google Scholar] [CrossRef]
- Ernst, R.E.; Hamilton, M.A.; Söderlund, U.; Hanes, J.A.; Gladkochub, D.P.; Okrugin, A.V.; Kolotilina, T.; Mekhonoshin, A.S.; Bleeker, W.; LeCheminant, A.N.; et al. Long-lived connection between southern Siberia and northern Laurentia in the Proterozoic. Nat. Geosci. 2016, 9, 464–469. [Google Scholar] [CrossRef]
- GOST 8267–93 Crushed Stone and Gravel of Solid Rocks for Construction Works. In Specifications; Standartinform: Moscow, Russia, 2018; p. 27. (In Russian)
- GOST 8736–2014 Sand for Construction Works. In Specifications; Standartinform: Moscow, Russia, 2019; p. 16. (In Russian)
- GOST 10178–85 Portland Cement and Portland Blastfurnace Slag Cement. In Specifications; Standartinform: Moscow, Russia, 2008; p. 14. (In Russian)
- GOST 30515–2013 Cements. In General Specifications; Standartinform: Moscow, Russia, 2019; p. 39. (In Russian)
- GOST 23732-2011 Water for Concrete and Mortars. In Specifications; Standartinform: Moscow, Russia, 2012; p. 21. (In Russian)
- Li, B.X.; Ke, G.J.; Zhou, M.K. Influence of manufactured sand characteristics on strength and abrasion resistance of pavement cement concrete. Constr. Build. Mater. 2011, 25, 3849–3853. [Google Scholar] [CrossRef]
- Dilek, U. Effects of manufactured sand characteristics on water demand of mortar and concrete mixtures. J. Test. Eval. 2015, 43, 562–573. [Google Scholar] [CrossRef]
- GOST 12730.3-78 Concretes. In Method of Determination of Water Absorption; Standartinform: Moscow, Russia, 2007; p. 6. (In Russian)
- Apeagyei, A.K.; Grenfell, J.R.A.; Airey, G.D. Influence of aggregate absorption and diffusion properties on moisture damage in asphalt mixtures. Road Mater. Pavement Des. 2015, 16, 404–422. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).